Abstract
The aim of the present study was to assess the genetic diversity of the B-cell receptor (BCR) in kidney transplant recipients with acute rejection. A total of three patients with acute rejection after kidney transplantation were examined by performing a composition and diversity analysis of the BCR immunoglobulin heavy chain (IGH) complementarity-determining region 3 (H-CDR3) repertoire. The peripheral blood mononuclear cells of patients were collected at 1 day prior to (Pre1), as well as 1 day (Post1) and 7 days (Post7) after the transplantation, and DNA was extracted. High-throughput sequencing technology was applied to determine the BCR repertoire. Raw sequences in FASTQ format were analyzed with the Basic Local Alignment Search Tool. The diversity of the BCR repertoire was assessed by calculating Shannon entropy, Simpson's diversity index, the Gini coefficient and highly expanded clone distributions. The diversity of the BCR repertoire at Pre1 was greater than that at Post1 or Post7. The diversity of the BCR repertoire was the lowest at Post1 and increased at Post7 but failed to reach the pre-transplantation levels. Patients exhibited the loss of seven IGH variable (IGHV)3 family genes, while five new genes were expressed at a low frequency. Furthermore, five IGHV-IGH joining (IGHJ) gene pairings, including IGHJ6-IGHV3-11, were detected in the patients. Up- and downregulated genes were assessed by calculating the expression frequencies of the IGH diversity and IGHV gene families at Post1 and Post7. The results of the H-CDR3 length distribution and H-CDR3 amino acid (AA) usage analyses indicated that in Case 1 and 2, the AA length was similar at mostly 14–18 AA, while that in Case 3 was relatively stable at 12–16 AA. In conclusion, the present results illustrate the diversity of H-CDR3 in patients with acute rejection after kidney transplantation may provide novel ideas, methods and means of monitoring and analyzing the immune status of patients under physiological and pathological conditions.
Keywords: kidney transplantation, acute rejection, B-cell receptor, immunoglobulin heavy chain complementarity-determining region 3, high-throughput sequencing
Introduction
The number of patients suffering from chronic kidney disease is rapidly growing worldwide, with increasing incidence, prevalence and progressiveness resulting in end-stage renal disease (ESRD) (1). Kidney transplantation is the most effective treatment for ESRD (2). In 1954, a team headed by Murray ushered in the modern era of organ transplantation with the first successful human organ transplantation between two identical twins (3). Scholars then began to explore kidney transplantation and experiments revealed that successful kidney transplantation may significantly improve the quality of life for patients with ESRD. Advances in immunosuppression over the last three decades have led to vast improvements in the control of acute rejection and short-term graft survival in kidney transplant patients (4). However, no significant improvements in long-term outcomes have been achieved, and concerns over the morbidity of lifelong regimens of immunosuppressive drugs remain. Analysis using the Kaplan-Meier method indicated a cumulative probability of graft survival at 1, 3, 5, 7 and 10 years of 99.1, 97.7, 94.3, 85.7 and 62.1%, respectively (5). Acute rejection is the major cause of kidney transplant loss and an independent risk factor affecting kidney function and long-term survival, which are dependent on immune rejection and the side effects of immunosuppressive regimens (6). Following kidney transplantation, immunosuppressants are used to prevent graft rejection, which greatly enhances the probability of survival of the transplant (7). However, since the rejection process is difficult to reverse once the typical clinical symptoms occur, the prevention and reduction of early acute rejection is conducive to transplant recipients.
The rejection of kidney transplants is essentially an immune response. Cellular immunity is the core factor for transplant immunization. The immune cells of the recipient attack the transplanted kidney directly or indirectly by releasing a large amount of various cytokines. Concerning the particular cell types involved, several studies have discussed the role of T-cell receptors (TCRs) in transplant rejection. Velásquez et al (8) suggested that differential expression of particular TCR β chain variable families and high levels of circulating CD4(+) CD25 (high) T cells in long-term surviving renal transplant patients contribute to an active and specific state of immunologic suppression. Matsutani et al (9) indicated that the skew in TCR usage was correlated with the levels of clonal T-cell expansion, indicating that the expanding T cells were responsible for the skew in TCR usage. These results demonstrate that clonal T-cell expansion in the periphery has a negative impact on long-term graft function. In addition, a previous study by our group on TCR after kidney transplantation indicated that the TCR repertoire diversity of transplantation groups was relatively lower compared with that in the NC group (10). The diversity of TCRs, B-cell receptors (BCRs) and secreted antibodies makes up the core of the complex immune system, and they serve as pivotal defensive components to protect the body against invading pathogens, including viruses and bacteria (11). However, the changes in B cells and BCRs in patients with acute rejection after kidney transplantation at the molecular level remain to be determined and their role in the underlying pathological process requires to be investigated. BCR antigens (Ags) are formed through the rearrangement of the immunoglobulin (Ig) heavy chain (IGH) variable (IGHV), IGH diversity (IGHD) and IGH joining (IGHJ) gene segments of the complementarity-determining region 3 (H-CDR3) of the BCR. CDR3 is the most hyper-variable region in the BCR and the most important structure in Ag recognition, as it determines the fate of developing and responding lymphocytes (12), which provides the most important structural basis for Ag binding. CDR3 is the product of multiple VDJ gene rearrangements and multiple non-coding N nucleotide insertions.
In the present study, the IGH of the CDR3 region of BCR was assessed in peripheral blood mononuclear cells (PBMCs) of 3 cases of typical acute rejection after kidney transplantation by using high-throughput sequencing. Comparative CDR3 diversity and length distribution analyses were performed and the IGHD, IGHJ and IGHV gene family expression as well as the IGHV-IGHJ family distribution were assessed. In addition, the Shannon entropy (SE), highly expanded clone (HEC) distributions, Simpson's diversity (SD) index and the Gini coefficient for the diversity and molecular expression of the IGH of the CDR3 region of BCR were calculated and analyzed. The present study enhances the current understanding of the balance between immunodeficiency and immune oversuppression in kidney transplant recipients. It provides knowledge to guide the individualized and rational use of immunosuppressive agents for preventing acute rejection or infection in renal transplant recipients.
Patients and methods
Patients and controls
With the widespread use of various novel immunosuppressive agents, the incidence of acute rejection has decreased in recent years, therefore, 3 cases encountered over 1.5 years were included in the current study (13). The study assessed 3 patients with typical acute rejection after kidney transplantation. Following obtainment of informed consent and in accordance with a protocol approved by the Ethics Committee of Guangxi Key Laboratory of Metabolic Disease Research (Guilin, China), a total of 3.5 ml peripheral blood was drawn from 3 typical patients [post-operative renal function and normal recovery within two weeks of acute rejection; pathological examination results and diagnostic criteria in line with the Baff classification of acute rejection (14)] at Guilin No. 924 Hospital (Guilin, China) between October 2014 and June 2016. Samples were collected 1 day prior to kidney transplantation (Pre1), 1 day after kidney transplantation (Post1) and 7 days after kidney transplantation (Post7). These samples were collected from one female patient aged 51 years (Case 1; weight, 54 kg), one female patient aged 47 years (Case 2; weight, 51 kg) and one male patient aged 61 years (Case 3; weight, 63 kg). The clinicopathological characteristics of the patients are listed in Table I. The treatment of each of the patients prior to and after transplantation/rejection is summarized in Table II.
Table I.
Clinicopathological characteristics of the patients.
| Case no. | Sex | Age (years) | Time-point | Temperature (°C) | Urinary volume (ml) | Urine protein (g/l) | Creatinine (µmol/l) | Lymphocytes (109/l) | Tacrolimus (ng/ml) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pre1 | 36.4 | 6,710 | 1+ | 223 | 0.05 | / | ||
| Female | 51 | Post1 | 37.2 | 4,300 | 1+ | 112 | 0.09 | / | |
| Post7 | 36.8 | 3,700 | 1+ | 108 | 0.07 | 4.3 | |||
| 2 | Pre1 | 36.0 | 2,050 | / | 454 | 1.59 | / | ||
| Female | 47 | Post1 | 36.0 | 6,560 | 1+ | 549 | 0.14 | / | |
| Post7 | 36.5 | 2,500 | +/− | 156 | 0.14 | 9.0 | |||
| 3 | Pre1 | 36.5 | 4,260 | 1+ | 1,925 | 1.16 | / | ||
| Male | 61 | Post1 | 36.0 | 5,820 | +/− | 530 | 0.23 | / | |
| Post7 | 36.3 | 3,980 | +/− | 81 | / | 6.3 |
Pre1, 1 day prior to transplantation; post7, 7 days post transplantation.
Table II.
Response to therapy in the patients.
| Case no. | Pre-operative management of renal dysfunction | Post-operative and prior to acute rejection medication intravenous drip | Oral medication (post-operative and prior to acute rejection) | Following acute rejection medication administration via intravenous drip |
|---|---|---|---|---|
| 1 | Hemodialysis | Anti-human T Lymphocyte Porcine Immunoglobulin | Tacrolimus (3.5 mg, 2 times/day) | Methylprednisolone (500 mg/day, 2 days) |
| (Surgery and post1-2, 500 mg/day; post3-7, 250 mg/day) | Mycophenolate sodium enteric-coated | Anti-human T Lymphocyte Porcine | ||
| Methylprednisolone (Surgery and post1-2, 500 mg/day) | tablets (360 mg, 2 times/day) | Immunoglobulin (500 mg/day, 5 days) | ||
| Mycophenolate mofetil (Post1-2, 1,000 mg, 2 times/day) | Methylprednisolone (8 mg/day) | |||
| 2 | Hemodialysis | Anti-human T Lymphocyte Rabbit Immunoglobulin | Tacrolimus (3.0 mg morning, 3.5 mg night) | Anti-human T Lymphocyte Rabbit |
| (Surgery and post1-2, 500 mg/day; post3-7, 250 mg/day) | Mycophenolate mofetil (750 mg, 2 times/day) | Immunoglobulin (500 mg/day, 4 days) | ||
| Methylprednisolone (Surgery and post1-2, 500 mg/day) | Methylprednisolone (16 mg/day) | |||
| Mycophenolate mofetil (Post1-2, 1,000 mg/day) | ||||
| 3 | Hemodialysis | Anti-human T Lymphocyte Porcine Immunoglobulin | Tacrolimus (3.0 mg, 2 times/day) | Methylprednisolone (500 mg/day, 3 days) |
| (Surgery and post1-2, 500 mg/day; post3-7, 250 mg/day) | Mycophenolate mofetil (750 mg, 2 times/day) | |||
| Methylprednisolone (Surgery and post1-2, 500 mg/day) | Methylprednisolone (16 mg/day) | |||
| Mycophenolate mofetil (Post1-2, 1,000 mg, 2 times/day) |
Pre2, 2 days prior to transplantation.
B-cell isolation and DNA extraction
Ficoll lymphocyte separation medium (cat. no. MD-YSZ707; Mei De Biotechnology Co., Ltd., Tianjin, China) and density gradient centrifugation were used to separate peripheral mononuclear cells from the blood obtained at different time points (Pre1, Post1 and Post7). The PBMCs of each patient were separated from the blood obtained at the different time-points. A QIAamp DNA Mini kit (cat. no. 51304; Qiagen, Hilden, Germany) was used to extract genomic DNA from 9 peripheral blood samples of the 3 patients, which was stored at −20°C.
Multiplex polymerase chain reaction (PCR) amplification of the BCR H-CDR3 region
DNA samples (500 ng; minimum concentration of no less than 35 ng/µl) were obtained from each patient. Specifically designed V-region primers and J-region primers were used. Multiplex PCR was performed with the Qiagen multiplex PCR kit (cat. no. Y5-206145; Qiagen). The 12 forward primers and 4 reverse primers utilized were used for multiplex PCR to amplify the rearranged H-CDR3 region (Table III). High fidelity enzyme was used for multiplex PCR (Qiagen Multiplex PCR kit; Qiagen). For each sample, the same amount of DNA was used for multiplex PCR. The PCR conditions were set as 95°C for 15 min, followed by 25 cycles of 94°C for 15 sec and 60°C for 3 min, with a final extension at 72°C for 10 min. PCR was performed using the ProFlex™ PCR machine (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the PCR products were purified by AMPure XP beads (Beckman Coulter, Brea, CA, USA) to remove primer sequences. A second round of PCR was performed to add a sequencing index to each sample. The PCR conditions were set as 98°C for 1 min, followed by 25 cycles of 98°C for 20 sec, 65°C for 30 sec and 72°C for 30 sec, with a final extension at 72°C for 5 min. The library was separated on agarose gel and the target region was isolated and cleaned using QIAquick Gel Extraction kits (cat. no. Y5-28704; Qiagen). A total of 5 µl DNA sample was mixed with the appropriate amount of bromophenol blue indicator spotting buffer for electrophoresis. Subsequently, 3 µl DNA marker and 0.7% agarose gel were added. Electrophoresis was performed for 40 min at 150 volts (large electrophoresis tank); electrophoresis to bromophenol blue migrated for a suitable distance in the gel. The gel was removed and the ethidium bromide color was checked, followed by capturing of images under ultraviolet light.
Table III.
Multiplex-PCR amplification primers of the IGH complementarity-determining region 3 region.
| Primer name | Sequence (5′-3′) |
|---|---|
| IGHV1-18 | CAGACGTGTGCTCTTCCGATCTAGAGAGTCACCATGACCACAGAC |
| IGHV1-2/1-46 | CAGACGTGTGCTCTTCCGATCTAGAGAGTCACCAKKACCAGGGAC |
| IGHV1-24 | CAGACGTGTGCTCTTCCGATCTAGAGAGTCACCATGACCGAGGAC |
| IGHV1-3/1–45 | CAGACGTGTGCTCTTCCGATCTAGAGAGTCACCATTACYAGGGAC |
| IGHV1-69/1-f | CAGACGTGTGCTCTTCCGATCTAGAGAGTCACGATWACCRCGGAC |
| IGHV1-8 | CAGACGTGTGCTCTTCCGATCTAGAGAGTCACCATGACCAGGAAC |
| IGH2-70/26/5 | CAGACGTGTGCTCTTCCGATCTAGACCAGGCTCACCATYWCCAAGG |
| IGHV3 | CAGACGTGTGCTCTTCCGATCTAGGGCCGATTCACCATCTCMAG |
| IGH4 | CAGACGTGTGCTCTTCCGATCTAGCGAGTCACCATRTCMGTAGAC |
| IGHV5-51 | CAGACGTGTGCTCTTCCGATCTAGCAGCCGACAAGTCCATCAGC |
| IGHV6-1 | CAGACGTGTGCTCTTCCGATCTAGAGTCGAATAACCATCAACCCAG |
| IGHV7-NEW | CAGACGTGTGCTCTTCCGATCTAGGACGGTTTGTCTTCTCCTTG |
| IGHJ-Rev1 | CTACACGACGCTCTTCCGATCTCTGAGGAGACRGTGACCAGGGTG |
| IGHJ-Rev2 | CTACACGACGCTCTTCCGATCTCTGAAGAGACGGTGACCATTGTC |
| IGHJ-Rev3 | CTACACGACGCTCTTCCGATCTCTGAGGAGACGGTGACCAGGGT |
| IGHJ-Rev4 | CTACACGACGCTCTTCCGATCTTGAGGAGACGGTGACCGTGGTC |
IGHV, immunoglobulin heavy chain variable; IGHJ, IGH joining; f, forward; Rev, reverse; Annexing primer bases: R=A or G, Y=C or T, K=G or T M=A or C, W=A or T.
Electrophoresis was performed to recover 100–190 bp multiplex PCR products using a QIAquick Gel Extraction kit (Qiagen). End Repair Mix (Sangon Biotech Co., Ltd., Shanghai, China) was added and the terminal repair reaction was performed at 20°C, in accordance with the manufacturer's protocol, followed by purification (cat. no. Y5-28106; QIAquick PCR Purification kit; Qiagen). The DNA fragment obtained after the terminal repair was attached to the ‘A’ base at its 3′ end. The adapter was ligated with Adapter oligo mix and DNA ligase. The linker-modified DNA fragment was enriched by PCR and subsequently, the PCR products were subjected to agarose gel electrophoresis. After the target fragment was excised from the gel, the QIAquick Gel Extraction kit (Qiagen) was used for gel purification and the fragment containing the PCR product dissolved in Elution Buffer (provided by the Gel Extraction kit). The library was then constructed.
High-throughput sequencing
High-throughput sequencing approaches to study BCR repertoires may be used to measure the diversity of B-cell populations (15). For high-throughput sequencing, each sample must be prepared in the same manner, containing 2 µg total DNA. Multiplex PCR with a mixture of primers targeted the rearranged V and J segments to represent receptor diversity (16). Multiplex PCR amplification was performed to amplify rearranged CDR3 sequences. An upstream primer and downstream primer in the IGHV functional gene region and IGHJ functional gene region were designed. Each primer was specific for a particular site of the H-CDR3 of BCR. Using the Illumina HiSeq 2000 sequencing platform (Illumina, Inc., San Diego, CA, USA) to perform the sequencing of PCR products, NaOH was added and samples were diluted to a certain concentration according to the expected amount of data obtained with the machine. The library was added to FlowCell (part of the Illumina Hiseq 2000 platform) after denaturation and dilution. PCR-bridged amplification was performed using the reagent TruSeq PE Cluster kit v3-cBot-HS (cat. no. PE-401-3001; Illumina, Inc.) on the cluster generation platform cBot. Finally, the prepared Flow Cell was sequenced with the HiSeq 2000 sequencing system and the reagent TruSeq SBS KIT-HS v3 (FC-401-3001; both Illumina, Inc.).
Bioinformatics analysis
Raw sequences in the FASTQ format were processed using the Basic Local Alignment Search Tool (BLAST) online software (http://blast.ncbi.nlm.nih.gov/Blast.cgi). PCR products were sequenced using an Illumina Genome Analyzer and the sequences were scored and filtered with the subsequent formula. Firstly the sequences with connectors were filtered out and the reads with an average quality score <15 (Illumina 0–40 quality system) were removed. For unknown bases (N bases), the quantity was ≤5%. Regarding the sequences at the tail with low quality, those with a score <10 were removed to ensure the average quality score of sequences was >15 and the length of remaining sequences were >60 nt following filtration. With the above filtration steps, clean data was generated. Two Pair-end (PE) sequences from clean data were merged into a contig sequence in two steps: i) By aligning the tail parts of two sequences and assessing the identity [COPEv1.1.3 software; Bureau Gravimetrique International (BGI), Toulouse, France] with at least 10 bases of overlap required and the overlapping section having 90% base match; ii) as different primers may result in sequences of different lengths, the short certain products (<100 bp) were merged by aligning the head part of the sequence (FqMerger software; BGI). Using this method, the merged contig sequences and the length of the distribution plot were obtained.
Statistical analysis
The statistical analyses were performed with GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA), and the Student's t-test was used with Bonferroni correction. P<0.05 was considered to indicate a statistically significant difference.
Results
Sequencing
Using high-throughput sequencing (Illumina Genome Analyzer), BCR H-CDR3 repertoires were sequenced from B cells isolated from the PBMCs of 3 kidney transplant patients with acute rejection by obtaining an average of 401967.4 total raw reads per sample. After filtering and removing adaptor sequences, contamination and low-quality reads, an average of 369,389.9 reads that met the quality requirements were collected. The sequence statistics for each sample after comparison and statistical analysis are provided in Table IV.
Table IV.
Sequence statistics for each sample.
| Case/time-point | Total reads (n) | Immune sequences (n) | In-frame sequences (n) | Out-of-frame sequences (n) | Total CDR3 sequences (n) | Unique CDR3 nt sequences (n) | Unique CDR3 AA sequences (n) |
|---|---|---|---|---|---|---|---|
| Case 1 | |||||||
| Pre1 | 435,064 | 431,037 | 340,034 | 90,817 | 285,020 | 39,406 | 32,744 |
| Post1 | 496,537 | 486,442 | 268,278 | 217,707 | 182,009 | 18,828 | 15,331 |
| Post7 | 403,593 | 398,636 | 220,842 | 177,542 | 150,370 | 23,895 | 20,310 |
| Case 2 | |||||||
| Pre1 | 572,795 | 480,031 | 404,703 | 75,068 | 352,553 | 51,913 | 43,978 |
| Post1 | 650,859 | 356,884 | 242,488 | 114,047 | 191,181 | 21,340 | 17,431 |
| Post7 | 588,477 | 323,629 | 253,726 | 69,720 | 209,382 | 29,920 | 25,090 |
| Case 3 | |||||||
| Pre1 | 922,607 | 313,193 | 240,813 | 72,173 | 202,578 | 26,384 | 21,771 |
| Post1 | 130,4368 | 393,937 | 300,335 | 93,207 | 257,911 | 28,326 | 22,837 |
| Post7 | 770,650 | 383,720 | 271,055 | 112,466 | 224,214 | 24,027 | 19,428 |
Pre1, 1 day prior to transplantation; post7, 7 days post transplantation; CDR3, complementarity-determining region 3; nt, nucleotide; AA, amino acid.
Diversity evaluation of the BCR repertoire
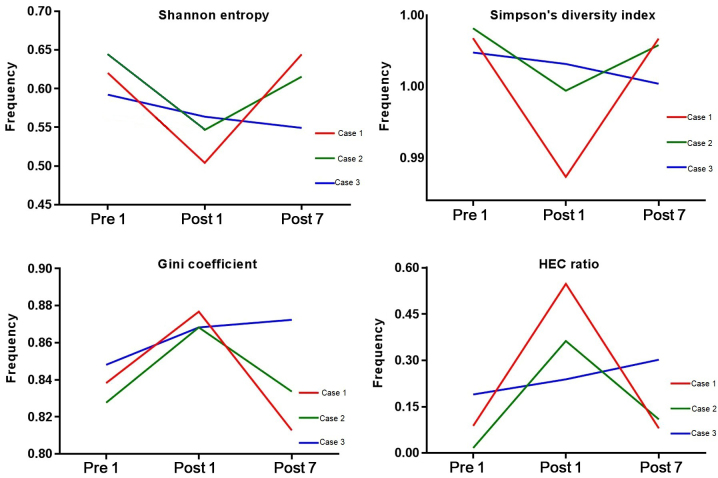
To evaluate the diversity of the BCR repertoire, the SE, SD, the Gini coefficient and HEC distributions were determined at the three different time-points (Fig. 1). SE is a measure of diversity in the immune repertoire, with a value closer to 1 indicating a higher diversity. The SE for Case 1, 2 and 3 was 0.620, 0.645 and 0.592, respectively, at Pre1, 0.504, 0.547 and 0.563, respectively, at Post1, and 0.644, 0.615 and 0.549, respectively, at Post7. The SE at Pre1 was significantly lower than that at Post1 (P=0.0267). The SD index may be used to describe the diversity of the clone type of the BCR H-CDR3 in each patient at different time-points, which a larger index indicating a greater diversity. The SD index for Case 1, 2 and 3 at Pre1 was 0.9984, 0.9991 and 0.9974, respectively, that at Post1 was 0.9885, 0.9947 and 0.9966, respectively and that at Post7 was 0.9984, 0.9979 and 0.9952 respectively. The Gini coefficient is used to measure the heterogeneity of different clone types, with 1 indicating only one clone and 0 indicating the same frequency of all clones in the samples. At Pre1, the Gini coefficient for Case 1, 2 and 3 was 0.838, 0.828 and 0.848, respectively, that at Post1 was 0.877, 0.868 and 0.868, respectively, and that at Post7 was 0.813, 0.834 and 0.872, respectively. The Gini coefficient at Post1 was significantly lower than that at Pre1 (P=0.0164). The HEC ratio is defined as the expression of a certain CDR3 sequence exceeding 0.5% of the total CDR3 sequence. At Pre1, the HEC ratio for Case 1, 2 and 3 was 0.088, 0.017 and 0.190, respectively, that at Post1 was 0.547, 0.363 and 0.239, respectively and that at Post7 was 0.080, 0.109 and 0.302, respectively. Fig. 1 indicates that the initial diversity of the BCR repertoire of Case 1 and 2 was higher at Pre1, decreased at Post1 and then increased at Post7. That of Case 3 was lower at Pre1 and then decreased after transplantation. From the results, the current study hypothesized that the pre-operative induction drugs and surgical trauma increased the HEC number and reduced the immune diversity of CDR3. With the recovery of immune function after transplantation, or the assumed lack of withdrawal immunosuppression, the number and ratio of HECs decreased in the patients and then increased.
Figure 1.
Shannon entropy, Simpson's diversity index, Gini coefficient and HEC distribution at three different time-points. HEC, highly expanded clone; Pre1, 1 day prior to transplantation; post7, 7 days post transplantation.
Analysis of IGHD, IGHJ and IGHV gene families
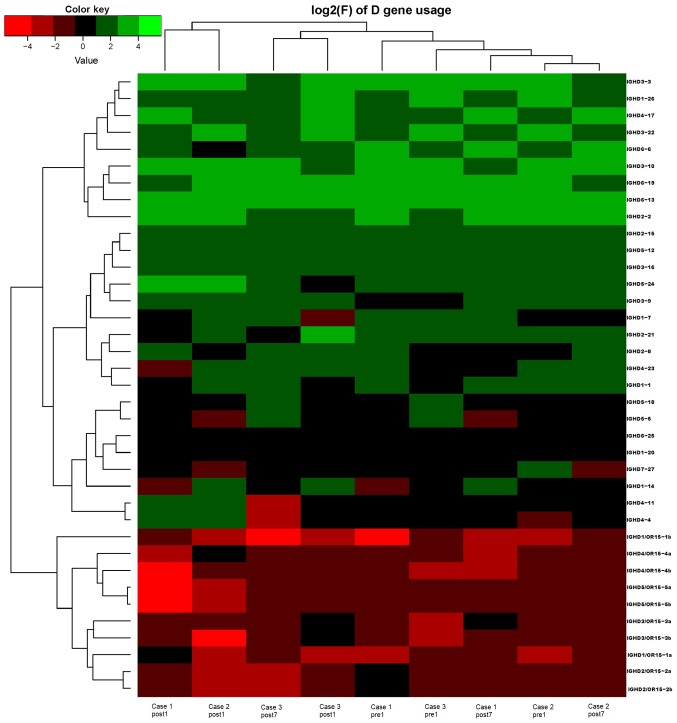
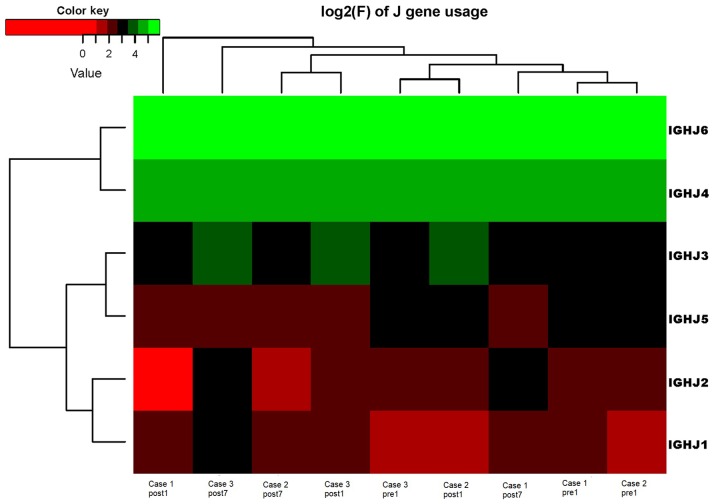
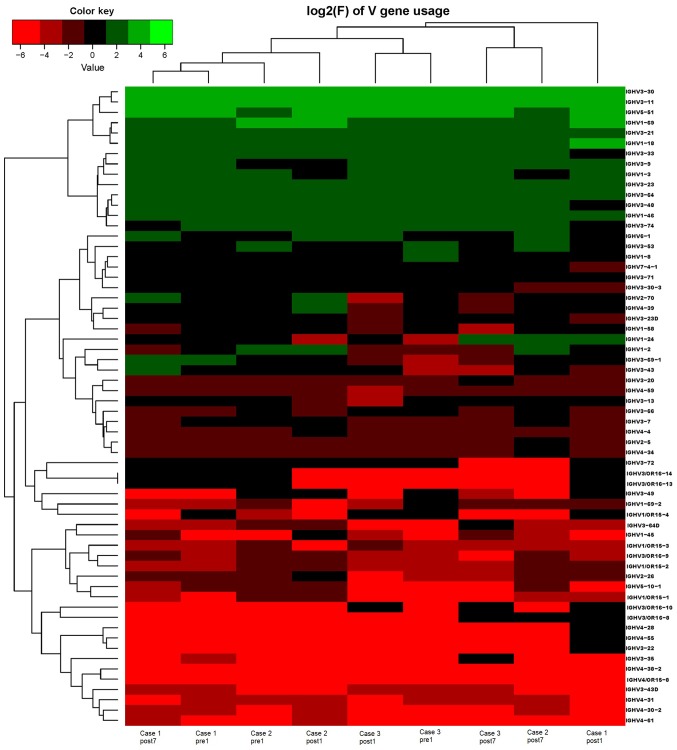
Ig gene sequences have recently gained significance in clinical decision-making. To evaluate changes in the IGHD, IGHJ and IGHV gene frequencies of the three patients over time, the frequencies were calculated at different time-points. As indicated in Figs. 2, 3 and 4, respectively, the IGHD, IGHJ and IGHV gene family distributions of the patients have different expressions levels at each time-point. Fig. 5 indicates that the BCR repertoire of the IGHV gene family was altered after transplantation. For instance, Case 1 lost IGHV3-22, IGHV3-49, IGHV3/OR16-10, IGHV4-28 and IGHV4-55 after transplantation. Case 2 gained new IGHV usage at a low frequency, including IGHV3-64D and IGHV3-72. Case 3 gained new IGHV usage, IGHV1-69-2 at Post1 and IGHV1/OR15-4 and IGHV3-72 at Post7, while he lost IGHV3-64D and IGHV3/OR16-8 at Post7. Of note, these gene changes were all within the IGHV3 family. The usage frequency of the IGHD and IGHV gene families was also analyzed, and it was assessed which genes were upregulated and downregulated. At Post1, IGHV3/OR was significantly upregulated (P=0.0474), while IGHD3-9 was significantly downregulated (P=0.0437). At Post7, IGHV3-21 was significantly upregulated (P=0.0328), and IGHD2-15 and IGHV1-45 were significantly downregulated (P=0.0456 and 0.0143, respectively). Heat maps detailing the differential expression of the genes among the different patients at different time-points are presented in Fig. 6, while those for IGHJ are provided in Fig. 7 and those for the IGHV gene family in Fig. 8.
Figure 2.
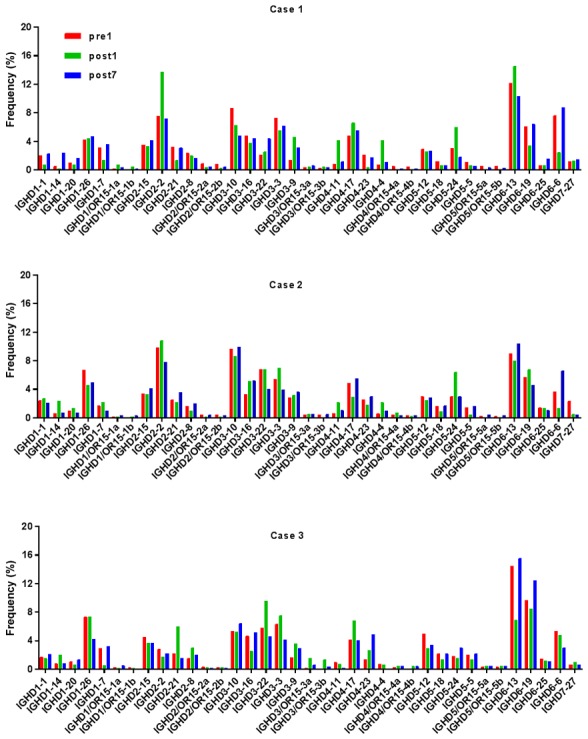
Frequencies of IGHD at different time-points. IGHD, immunoglobulin heavy chain diversity; Pre1, 1 day prior to transplantation; post7, 7 days post transplantation.
Figure 3.
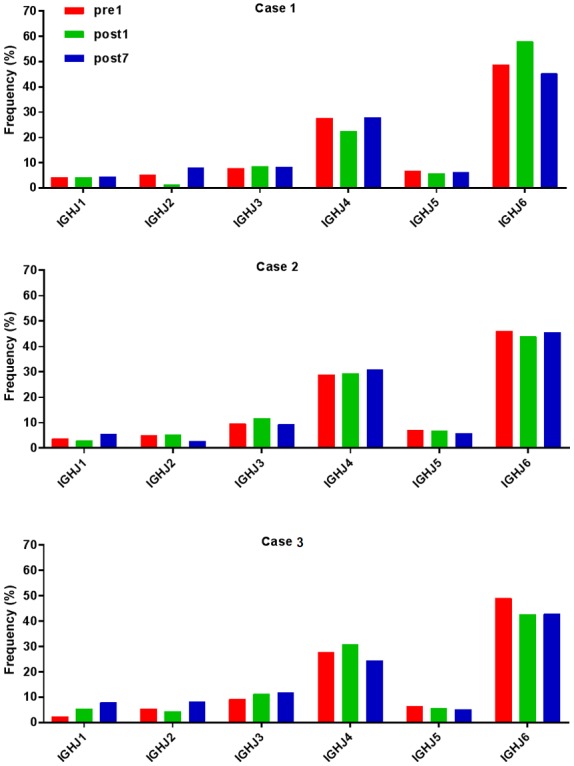
Frequencies of IGHJ at different time-points. IGHJ, immunoglobulin heavy chain joining; Pre1, 1 day prior to transplantation; post7, 7 days post transplantation.
Figure 4.
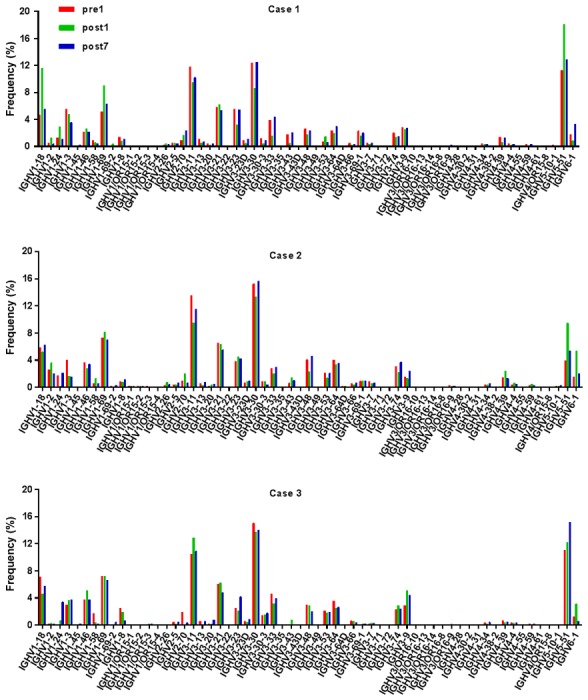
Frequencies of IGHV at different time-points. IGHV, immunoglobulin heavy chain variable; Pre1, 1 day prior to transplantation; post7, 7 days post transplantation.
Figure 5.
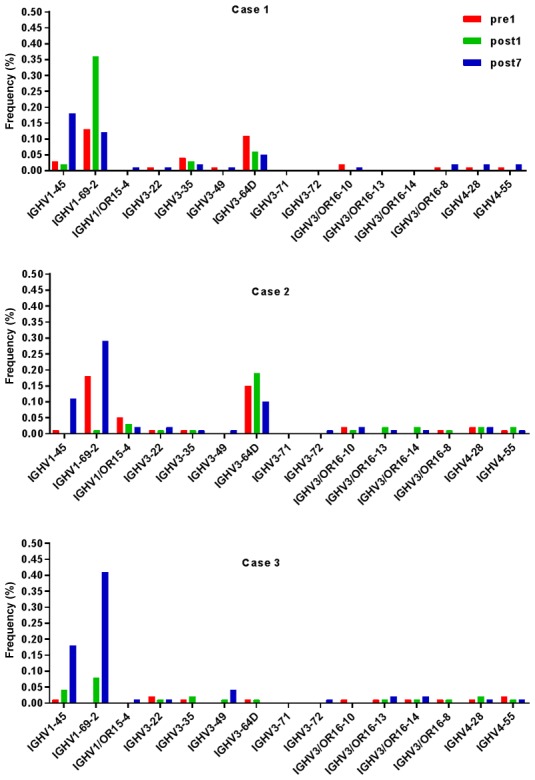
BCR repertoires of the IGHV gene family were altered after transplantation. IGHV, immunoglobulin heavy chain variable; Pre1, 1 day prior to transplantation; post7, 7 days post transplantation.
Figure 6.
Frequencies of the correlation coefficients for the log2 values of the corresponding differences in the frequency of IGHD gene family changes. IGHD, immunoglobulin heavy chain diversity.
Figure 7.
Frequencies of the correlation coefficients for the log2 values of the corresponding differences in the frequency of IGHJ gene family changes. IGHJ, immunoglobulin heavy chain joining.
Figure 8.
Frequencies of the correlation coefficients for the log2 values of the corresponding differences in the frequency of IGHV gene family changes. IGHV, immunoglobulin heavy chain variable.
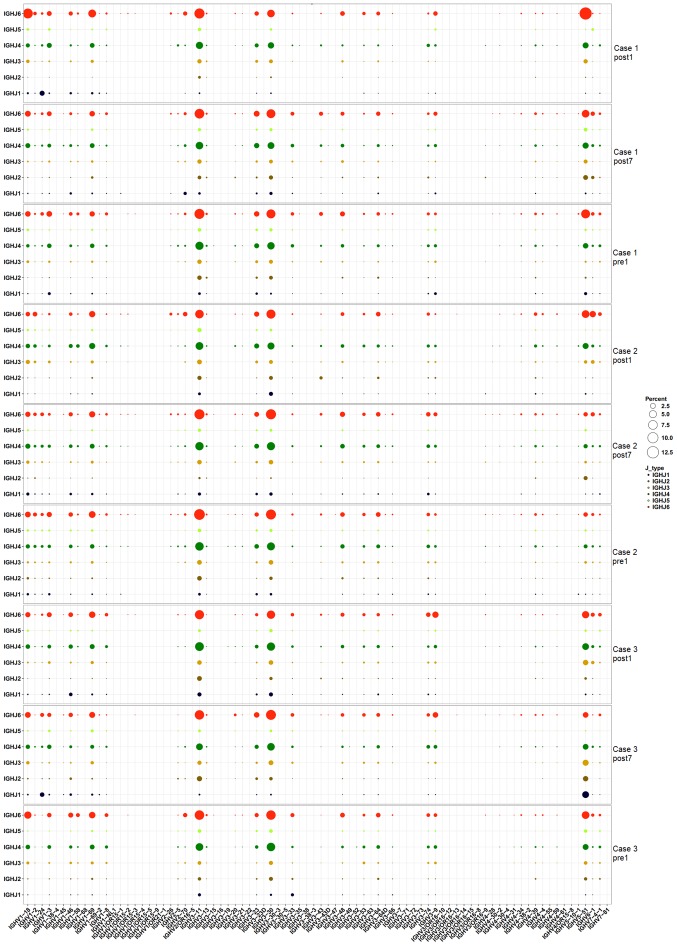
Pairing of IGHV-IGHJ genes
To assess changes in IGHV-IGHJ gene pairing over time, the frequency of IGHV-IGHJ pairing was calculated for the three time-points. The heat map in Fig. 9 indicates that IGHV-IGHJ gene pairing in the three patients was not markedly changed at the three time-points. It is worth noting that all 3 cases had a similar dominant pairing. Different colors represent different IGHJ genes, and the size of the circles represents the level of expression. The genes that exhibited high expression levels included IGHJ6-IGHV3-11, IGHJ4-IGHV3-11, IGHJ6-IGHV3-30, IGHJ4-IGHV3-30 and IGHJ6-IGHV5-51.
Figure 9.
IGHV-IGHJ gene pairing in the three patients had little change at the three time-points. It is worth noting that the three cases had a similar dominant pairing. IGHJ/V, immunoglobulin heavy chain joining/variable.
Length distribution of H-CDR3
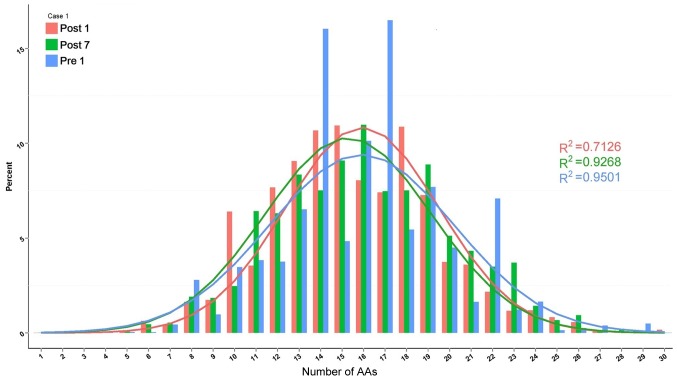
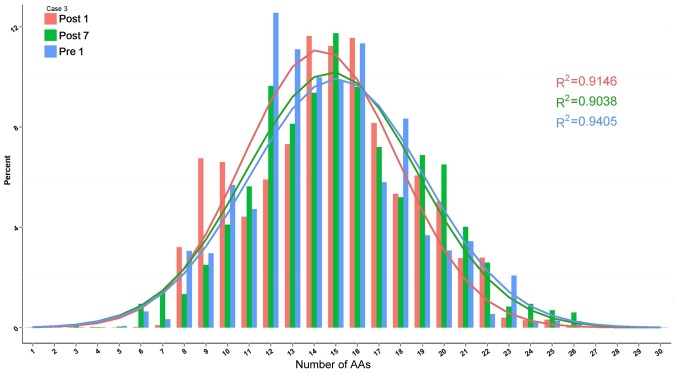
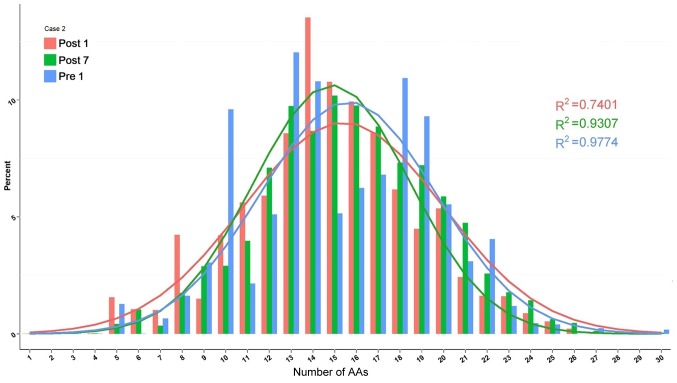
The change in composition of unique H-CDR3 over time, including H-CDR3 length distribution and H-CDR3 amino acid (AA) usage, was then assessed. The change in H-CDR3 length distribution in the 3 patients is provided in Figs. 10–12. The length of CDR3 in healthy people should be similar to the gaussian distribution. Where R2 is the fitting value of gaussian distribution, R2 is the closer to 1 and the value is the closer to the gaussian distribution. For the H-CDR3 length distribution of Case 1, the R2 value at Pre1, Post1 and Post7 was 0.9501, 0.7126 and 0.9268, respectively. The AA usage of unique H-CDR3 in Case 1 was 17 and 14 AA at Pre1, 14, 15 and 18 AA at Post1 and 16 AA at Post7 (Fig. 10). For the H-CDR3 length distribution of Case 2, the R2 value at Pre1, Post1 and Post7 was 0.9774, 0.7401 and 0.9307, respectively (Fig. 11). The AA usage of unique H-CDR3 for Case 2 was 13, 14 and 18AA at Pre1, 14 and 15 AA at Post1 and 13, 15 and 16 AA at Post7. The CDR3 length distribution of Case 1 was similar to that of Case 2. The diversity of the BCR repertoire was the highest at Pre1 and the lowest at Post1, and was then increased again at Post7, but not to the pre-operative level. For the H-CDR3 length distribution of Case 3, the R2 value at Pre1, Post1 and Post7 was 0.9405, 0.9146 and 0.9038 (Fig. 12). The AA usage of unique H-CDR3 for Case 3 was 12 and 13AA at Pre1, 14–16 AA at Post1 and 15AA at Post7. The diversity of the BCR repertoire of Case 3 did not change significantly.
Figure 10.
Change in the composition of unique H-CDR3 over time, including H-CDR3 length distribution and H-CDR3 AA usage of Case 1. H-CDR3, immunoglobulin heavy chain complementarity-determining region 3; AA, amino acids; Pre1, 1 day prior to transplantation; post7, 7 days post transplantation.
Figure 12.
Change in the composition of unique H-CDR3 over time, including H-CDR3 length distribution and H-CDR3 AA usage of Case 3. H-CDR3, immunoglobulin heavy chain complementarity-determining region 3; AA, amino acids; Pre1, 1 day prior to transplantation; post7, 7 days post transplantation.
Figure 11.
Change in the composition of unique H-CDR3 over time, including H-CDR3 length distribution and H-CDR3 AA usage of Case 2. H-CDR3, immunoglobulin heavy chain complementarity-determining region 3; AA, amino acids; Pre1, 1 day prior to transplantation; post7, 7 days post transplantation.
Discussion
B cells represent a major component of the cellular immune system. Acute rejection after kidney transplantation is mainly caused by cellular immunity and is reversible in a large proportion of cases. When rejection occurs, the patient produces a large number of cytotoxic T cells, which then kill their target cells directly, and furthermore, the patient's T cells help to activate lymphokines to then promote the differentiation of B-cells, which produce antibodies against the graft, ultimately leading to acute rejection after kidney transplantation (17). Certain studies conclude that B-cell diversity exhibits a marked decrease with age, and B-cell immune frailty is also a marker of general frailty (18). Patients with Omenn syndrome have a high proportion of class-switched IHC transcripts and an increased somatic hypermutation rate, suggesting in vivo activation of these B cells (19). The BCR is an indispensable functional receptor for B cells and its diversity ensures that B cells are able to respond to a variety of different Ags. B cells also participate in the anti-inflammatory response and tolerance induction (20). Studies have indicated that in addition to its association with the clinical and phenotypic presentation, renal allograft tolerance is highly associated with the B-cell signature. Newell et al (21) demonstrated that the regulation of cellular immunity is a critical role for B cells and provided a set of candidate genes for the wider-scale screening of kidney transplant recipients.
Characterization of Ag-specific BCR repertoires is essential for understanding disease mechanisms involving humoral immunity. A study by Ma et al (22) suggests that the mechanism of high-frequency CDR3 generation is associated with the maturation of IgG affinity (somatic hypermutation) during the recombinant HBV vaccine-induced B-lymphocyte responses. In the present study, using high-throughput sequencing, the BCR H-CDR3 region was analyzed in the DNA from PBMCs of 3 kidney transplant patients with typical acute rejection after kidney transplantation. The results suggested that the diversity of the BCR repertoire in patients was the highest at Pre1, while it was the lowest at Post1, which may be due to the induction of immunosuppressive agents and the effect of surgical trauma. The diversity increased again at Post7 but failed to achieve pre-transplantation levels before acute rejection occurred. It may be hypothesized that the number of Ig subtypes increased in patients after kidney transplantation.
The present study comprehensively analyzed the IGHD, IGHJ and IGHV gene family distribution and the frequency of IGHV-IGHJ gene pairing. The results suggested that the IGHV3-22, IGHV3-49, IGHV3/OR16-10, IGHV4-28, IGHV4-55, IGHV3-64D and IGHV3/OR16-8 genes were lost from the IGHV gene family. In addition, the newly formed IGHV3-72, IGHV1-69-2 and IGHV1/OR15-4 genes were expressed at low levels. Among the lost genes identified in the present study, the IGHV gene family was also reported in other studies. A study by Roy et al (23) revealed features of a disease- and Ag-specific autoantibody repertoire with preferred paired H chain V region and L chain V region usage and pairings, limited mutations, molecular dominance and selection of particular CDR3 sequences. According to individual gene segments of kidney transplantation patients divided into different groups, blood group ABO-incompatible patients with accommodation (ABOiA), ABO-compatible stable patients (ABOcS), and ABO-incompatible patients with biopsy-proven acute antibody-mediated rejection (ABOiR), gene segments of IGHV3, IGKV1, IGLV2 and IGLJ3 were most frequently used in all groups, while IGHV3-7, IGHV3-15, IGHV4-59, IGKV3-11, IGLV1-44, IGLV2-14, IGLV4-69 and IGLV7-46 were more frequently used in the ABOcS group than in the other groups, and IGKV3-7 was more frequently used in the ABOiR group than in the other groups. IGLV5-52 and IGLV7-43 were more frequently used in the ABOiA group than in the ABOcS group (24). The BCR repertoires of systemic lupus erythematosus patients lost a certain amount of IGHV gene usage after high-dose glucocorticoid treatment (25). IGHV genes from the IGHV3 superfamily encode antibodies against the D Ag (26). It is presumed that B-cell expansion against allogeneic Ags prior to the occurrence of acute rejection leads to the production of these abnormally expressed genes, with highly expanded clones disrupting the diversity of the CDR3 region. The three patients of the present study had a similar dominant IGHV-IGHJ gene pairing (IGHJ6-IGHV3-11, IGHJ4-IGHV3-11, IGHJ6-IGHV3-30, IGHJ4-IGHV3-30 and IGHJ6-IGHV5-51), suggesting that the rejection in the patients may be associated with the auto-Ag that is induced. A previous study by our group on the T-cell repertoire following kidney transplantation determined by high-throughput sequencing revealed that that the length of CDR3 varied from 16 to 106 nt, and the 5 most frequently observed CDR3 lengths were 42, 45, 39, 36 and 48 nt. The 5 most frequently observed VD indel lengths were 0, 2, 3, 1 and 4 nt. The 5 most commonly observed DJ indel lengths were 4, 0, 3, 1 and 2 nt (10). The results of the length distribution analysis of H-CDR3 suggested a similar length (14–18 AA) among the three patients. The length distribution at Pre1 and Post7 tended to be consistent with a normal distribution pattern. However, at Post1, a skewed distribution was observed. The length distribution of CDR3 in Case 3 was stable at 12–16 AA and tended to exhibit a normal distribution pattern. These results suggest that the length distribution of H-CDR3 may change after kidney transplantation, but the specific AA sites require to be determined. Whether these observed changes in IGHV genes and IGHD genes may serve as better markers in B-cell immunology requires further investigation. The study by Beausang et al (27) suggested that subjects that responded to de-sensitization therapy had pre-treatment repertoires composed of a larger fraction of class-switched (IgG and IgA) isotypes compared to non-responding candidates, indicating that the measurement of B-cell repertoires may be applied to identify candidates that respond to de-sensitization therapy. In the present study, three patients with acute rejection after kidney transplantation were assessed for the purpose of performing a composition and diversity analysis of the BCR H-CDR3 repertoire. The next step will be to assess the association of the repertoire with clinical conditions. The study of de-sensitization therapy for renal transplant patients is a promising field.
Due to the limited sample size of the present study, most differences were not observed to reach statistical significance. The small sample size is a limitation of the present study, and therefore, more samples should be included in follow-up studies to reach statistical significance. The association of the variation of the BCR H-CDR3 repertoire with clinical outcomes in kidney transplantation patients should also be explored. This may offer potential guidance for individualized treatment with immunosuppressive agents and contribute to the improvement of the short- and long-term survival of kidney transplant recipients.
Traditional sequencing and cloning methods only generate a small amount of sequence information on BCR dominant clones; it is difficult to obtain individual BCR sequence library information, and it is impossible to evaluate the immune status. Using high-throughput sequencing, a large number of BCR sequences in kidney transplant patients may be obtained from a small amount of peripheral blood, and the BCR dominant clones as well as quantitative indicators, including the SE and the diversity of the BCR, may be determined. Furthermore, changes in BCR CDR3 diversity may be observed in patients with acute rejection after renal transplantation in order to investigate its potential clinical application for early detection of acute rejection. In future studies, the utility of dynamic monitoring of BCR CDR3 should be verified by prospective analyses and comparisons. BCR CDR3 diversity helps to determine the patient's immune status following transplantation and is instructive for applying the correct treatment regimen.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guangxi Natural Science Foundation (grant no. 2017GXNSFAA198185), the National Natural Science Foundation of China (grant nos. 81671596 and 31700795), The Science and Technology Plan of Shenzhen (grant no. JCYJ20160422164313440) and the Science and Technology Plan of Guilin (grant no. 20170117-1).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
LL, XZ, QY and YD conceived the study and designed the experiments. LL, HC and WS performed the experiments. DT, JZ and YL analyzed the data. LL, XZ and YD wrote the manuscript and QY, YD and XZ revised it. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Informed consent was obtained from the patients. The protocol was approved by the Ethics Committee of Guangxi Key Laboratory of Metabolic Disease Research under the reference no. 160125-2.
Patient consent for publication
Consent was obtained from all patients prior to publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Liyanage T, Ninomiya T, Perkovic V, Woodward M, Stirnadel-Farrant H, Matsushita K, Iseki K, Seong HL, Monaghan H, Jha V. Chronic kidney disease in Asia: Protocol for a collaborative overview. Nephrology (Carlton) 2017;22:456–462. doi: 10.1111/nep.12821. [DOI] [PubMed] [Google Scholar]
- 2.Roodnat JI, Hilbrands LB, Hené RJ, de Sévaux RG, Smak Gregoor PJ, Kal-van Gestel JA, Konijn C, van Zuilen A, van Gelder T, Hoitsma AJ, Weimar W. 15-year follow-up of a multicenter, randomized, calcineurin inhibitor withdrawal study in kidney transplantation. Transplantation. 2014;98:47–53. doi: 10.1097/01.TP.0000442774.46133.71. [DOI] [PubMed] [Google Scholar]
- 3.Ota K. Organ transplantation-approching the 21st century. Nihon Geka Gakkai Zasshi. 1998;99:110–112. (In Japanese) [PubMed] [Google Scholar]
- 4.Yilmaz S. Chronic allograft nephropathy (chronic allograft damage): Can it be avoided? Curr Transpl Rep. 2014;1:91–99. doi: 10.1007/s40472-014-0009-6. [DOI] [Google Scholar]
- 5.Shahbazi F, Ranjbaran M, Karamifar S, Soori H, Manesh HJ. Graft survival rate of renal transplantation during a period of 10 years in Iran. J Res Med Sci. 2015;20:1046–1052. doi: 10.4103/1735-1995.172814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.San Segundo D, Rodrigo E, Kislikova M, Ruiz JC, Fernandez-Fresnedo G, Asensio E, Arias M, Lopez-Hoyos M. Frequencies of circulating B-cell subpopulations before kidney transplantation identify patients at risk of acute rejection. Transplant Proc. 2015;47:54–56. doi: 10.1016/j.transproceed.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Ming M, Zhao B, Qiang L, He YY. Effect of immunosuppressants tacrolimus and mycophenolate mofetil on the keratinocyte UVB response. Photochem Photobiol. 2015;91:242–247. doi: 10.1111/php.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velásquez SY, Arias LF, García LF, Alvarez CM. T cell receptor beta chain (TCR-Vbeta) repertoire of circulating CD4(+) CD25(−), CD4(+) CD25(low) and CD4(+) CD25(high) T cells in patients with long-term renal allograft survival. Transpl Int. 2010;23:54–63. doi: 10.1111/j.1432-2277.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsutani T, Ohashi Y, Yoshioka T, Tsuruta Y, Doi H, Satomi S, Suzuki R. Skew in T-cell receptor usage and clonal T-cell expansion in patients with chronic rejection of transplanted kidneys. Transplantation. 2003;75:398–407. doi: 10.1097/01.TP.0000043927.00113.29. [DOI] [PubMed] [Google Scholar]
- 10.Lai L, Wang L, Chen H, Zhang J, Yan Q, Ou M, Lin H, Hou X, Chen S, Dai Y, Sui W. T cell repertoire following kidney transplantation revealed by high-throughput sequencing. Transpl Immunol. 2016;39:34–45. doi: 10.1016/j.trim.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Du Y, Su Z, Wang C, Zeng X, Zhang R, Hong X, Nie C, Wu J, Cao H, et al. IMonitor: A robust pipeline for TCR and BCR repertoire analysis. Genetics. 2015;201:459–472. doi: 10.1534/genetics.115.176735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zan H, Casali P. Editorial: Epigenetics of B cells and antibody responses. Front ImmunoL. 2015;6:656. doi: 10.3389/fimmu.2015.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bican Demir A, Erer Özbek S, Bora I, Hakyemez B, Tırnova I, Kaya E. Two cases with developing neurologic complications after liver transplant. Exp Clin Transplant. 2016;14:685–687. doi: 10.6002/ect.2014.0204. [DOI] [PubMed] [Google Scholar]
- 14.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 15.Galson JD, Pollard AJ, Trück J, Kelly DF. Studying the antibody repertoire after vaccination: Practical applications. Trends Immunol. 2014;35:319–331. doi: 10.1016/j.it.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Pickman Y, Dunn-walters D, Mehr R. BCR CDR3 length distributions differ between blood and spleen and between old and young patients, and TCR distributions can be used to detect myelodysplastic syndrome. Phys Biol. 2013;10:056001. doi: 10.1088/1478-3975/10/5/056001. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Yang H, Liu X, Zhang J, Han Z, Tao J, Zhao C, Ju X, Tan R, Gu M. Role of B and T lymphocyte attenuator in renal transplant recipients with biopsy-proven acute rejection. Med Sci Monit. 2018;24:387–396. doi: 10.12659/MSM.905752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, Nilsson BO, Wikby A, Kipling D, Dunn-Walters DK. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YN, Frugoni F, Dobbs K, Tirosh I, Du L, Ververs FA, Ru H, Ott de Bruin L, Adeli M, Bleesing JH, et al. Characterization of T and B cell repertoire diversity in patients with RAG deficiency. Sci Immunol. 2016;1:eaah6109. doi: 10.1126/sciimmunol.aah6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berthelot JM, Jamin C, Amrouche K, Le Goff B, Maugars Y, Youinou P. Regulatory B cells play a key role in immune system balance. Joint Bone Spine. 2013;80:18–22. doi: 10.1016/j.jbspin.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, Burlingham WJ, Marks WH, Sanz I, Lechler RI, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma L, Wang X, Bi X, Yang J, Shi B, He X, Ma R, Ma Q, Yao X. Characteristics peripheral blood IgG and IgM heavy chain complementarity determining region 3 repertoire before and after immunization with recombinant HBV vaccine. PLoS One. 2017;12:e170479. doi: 10.1371/journal.pone.0170479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy B, Neumann RS, Snir O, Iversen R, Sandve GK, Lundin KEA, Sollid LM. High-throughput single-cell analysis of B cell receptor usage among autoantigen-specific plasma cells in celiac disease. J Immunol. 2017;199:782–791. doi: 10.4049/jimmunol.1700169. [DOI] [PubMed] [Google Scholar]
- 24.Jeon HJ, Fang T, Lee JG, Jang JY, Kim K, Choi S, Yan JJ, Ryu JH, Koo TY, Ahn C, Yang J. VDJ gene usage of B cell receptors in peripheral blood of ABO-incompatible kidney transplantation patients. Transplant Proc. 2018;50:1056–1062. doi: 10.1016/j.transproceed.2018.01.047. [DOI] [PubMed] [Google Scholar]
- 25.Shi B, Yu J, Ma L, Ma Q, Liu C, Sun S, Ma R, Yao X. Short-term assessment of BCR repertoires of SLE patients after high dose glucocorticoid therapy with high-throughput sequencing. Springerplus. 2016;5:75. doi: 10.1186/s40064-016-1709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dohmen SE, Muit J, Ligthart PC, Verhagen OJ, van der Schoot CE. Anti-e found in a case of hemolytic disease of the fetus and newborn make use of the IGHV3 superspecies genes. Transfusion. 2008;48:194–195. doi: 10.1111/j.1537-2995.2007.01559.x. [DOI] [PubMed] [Google Scholar]
- 27.Beausang JF, Fan HC, Sit R, Hutchins MU, Jirage K, Curtis R, Hutchins E, Quake SR, Yabu JM. B cell repertoires in HLA-sensitized kidney transplant candidates undergoing desensitization therapy. J Transl Med. 2017;15:9. doi: 10.1186/s12967-017-1118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.