Abstract
The expression of vascular endothelial growth factor (VEGF) and soluble fms-like tyrosine kinase-1 (sFLT-1) in the serum of patients with pregnancy induced hypertension (PIH) syndrome and its effects on the foetus was explored. A total of 105 cases of PIH pregnant women admitted to The First People's Hospital of Changzhou from March 2015 to February 2018 were divided into 3 groups according to the severity of the patients condition. Group A (n=35) was hypertension complicating pregnancy, group B (n=46) was mild preeclampsia and group C (n=24) was severe preeclampsia. In addition, 35 healthy pregnant women were selected as the control group. VEGF and sFLT-1 levels in serum were detected by enzyme linked immunosorbent assay, and the correlations between levels of VEGF and sFLT-1, neonatal weight and Apgar score were analyzed. Compared with group A, the level of serum VEGF was lower, while the level of sFLT-1 was higher in groups B and C (P<0.05). Compared with group B, the serum VEGF level in group C decreased significantly (P<0.05), while the serum level of sFLT-1 increased significantly (P<0.05). Compared with group A, neonatal weight and Apgar score in group C was significantly lower (P<0.05). There was a positive correlation between the serum VEGF level and neonatal weight and Apgar score (r=0.435, P<0.001. r=0.357, P<0.001). There was a negative correlation between the serum sFLT-1 level and neonatal weight and Apgar score (r=−0.351, P<0.001. r=−0.422, P<0.001). Therefore, we concluded that VEGF and sFlt-1 may be involved in the occurrence and development of PIH. The decrease of serum VEGF level and the increase of sFlt-1 level may be related to the inhibited fetal growth and development, which is of great significance in the clinical detection of PIH patients.
Keywords: pregnancy induced hypertension syndrome, VEGF, sFLT-1, neonatal weight, Apgar score
Introduction
Pregnancy-induced hypertension syndrome (PIH) is a common disease in women during pregnancy and is one of the important causes of maternal bleeding, infection and death. According to the World Health Organization (WHO), at least one woman dies of PIH complications every 7 min (1). PIH is divided into four categories: gestational hypertension, chronic hypertension with preeclampsia, preeclampsia and eclampsia. According to the clinical characteristics of the patients, preeclampsia can be divided into mild and severe preeclampsia (2). The main cause of maternal mortality is the wrong choice of PIH treatment methods and untimely treatment (3). PIH may cause a series of complications, such as chest pain, difficulty breathing, cortical blindness, fetal growth and development restriction, iatrogenic preterm delivery, fetal distress in uterus, and even organ failure and fetal death in severe cases (4).
At present, the pathogenesis of PIH has not been clarified, and the relationship between cytokines and the occurrence and development of PIH has attracted increasing attention. Some scholars believe that due to poor early development of the placenta, poor immunogenicity may cause extravillous trophoblasts to invade the parental spiral artery, resulting in poor remodeling of the blood vessels and reduced maternal blood supply, which in turn causes hypoxic and ischemic conditions in the placental tissue. Under this condition, trophoblasts produce anti-angiogenic factors such as soluble vascular endothelial growth factor receptor 1 (sFLT-1), and elevated sFlt-1 can cause endothelial dysfunction and hypoxia (5–7). Vascular endothelial growth factor (VEGF) is a multifunctional cytokine produced by vascular endothelial cells and macrophages (8). Previous studies have shown that the biological activity of VEGF is regulated by sFLT-1. sFLT-1 is an endogenous inhibitor of VEGF, and excess sFLT-1 can bind VEGF through high affinity, thereby neutralizing it. sFLT-1 is an endogenous inhibitor of VEGF, and excess sFLT-1 can bind VEGF to neutralize it. VEGF and sFLT-1 may play an important role in endothelial dysfunction (9). VEGF is mainly expressed on the surface of placental syncytiotrophoblasts and invasive chorionic trophoblast cells during pregnancy. VEGF is highly expressed in the blood vessels during early pregnancy and is abundant in trophoblast cells (10,11). It has been reported that sFLT-1 increases dramatically during pregnancy and then rapidly declines after delivery (12).
Previous studies have shown that VEGF and its receptor sFLT-1 in placenta may play an important role in the occurrence and development of PIH (13), but the relationship between serum VEGF and sFLT-1 expression in PIH and inhibited fetal growth and development is still unknown. The study examined serum VEGF and sFLT-1 levels in patients with PIH and explored their relationships with inhibited fetal growth and development.
Patients and methods
Patient information
A retrospective analysis of medical records of 105 PIH pregnant women admitted to The First People's Hospital of Changzhou (Changzhou, China) from March 2015 to February 2018, aged 21–39 years, with an average age of 26.47±3.15 years, 27–36 weeks of gestation with an average of 32.41±0.89 weeks, and 1–3 pregnancies with an average of 1.56±0.19 pregnancies was conducted. According to the severity of disease (14), patients were divided into 3 groups: group A (n=35) as the hypertension complicating pregnancy, group B (n=46) the mild preeclampsia and group C (n=24) the severe preeclampsia. Another 35 healthy pregnant women admitted to The First People's Hospital of Changzhou during the same period were selected as the control group. Participants in the control group were aged 21–35 years, with a mean age of 25.89±2.16 years, 28–35 weeks of gestation, average 33.15±0.79 weeks and 1–2 pregnancies, average 1.38±0.13 times.
Inclusion and exclusion criteria
Inclusion criteria were: The subjects were diagnosed in accordance with classification and diagnostic criteria of PIH (15); aged from 20 to 40, and with complete clinical data. This study was approved by the Ethics Committee of The First People's Hospital of Changzhou and all subjects were informed and agreed to participate in this clinical study and signed an informed consent. Exclusion criteria were: Patients with severe liver and kidney disease, diabetes, therioma, chronic inflammatory disease, hematopoietic dysfunction, immunological disease, psychosis or family history of mental illness.
Sample collection and detection
Venous blood was drawn under fasting condition, serum was separated by centrifugation at 3,000 × g for 10 min at 4°C and stored at −20°C in a cryogenic refrigerator. ELISA (16) was used to detect the levels of VEGF and sFLT-1 in serum. VEGF ELISA kit (Shanghai Kalang Biotechnology Co., Ltd., Shanghai, China) and sFLT-1 ELISA kit (Shanghai Jingyang Bioengineering Co., Ltd., Shanghai, China) were used. All operations were performed in strict accordance with the manufacturers instructions. No reagent was added into the blank control well. After adding samples, the plate was covered with film and incubated at 37°C for 1 h. Then, the plate was washed and 80 µl affinity chain enzyme was added into each well, followed by incubation at 37°C for 30 min. Substrate A and B (50 µl) were added, followed by incubation at 37°C for 10 min. Each well was added with 50 µl stop solution. An AMR-100 automatic enzyme marker analyzer (Hangzhou Allsheng Instrument Co., Ltd., Hangzhou, China) was used to detect the OD value of each well at the wavelength of 450 nm and to calculate the levels of VEGF and sFLT-1.
Observation index
According to the neonatal weight (17), 2.5–4.0 kg was classified as normal weight newborns, 1.5–2.5 kg were low weight newborns, <1.5 kg was very low weight newborns, and >4.0 kg is macrosomia. Apgar scores (18) were used to evaluate the degree of neonatal asphyxia and respiratory, muscular tension, heart rate, skin color and laryngeal reflex signs: 8–10 points for normal newborns, 4–7 points for mild asphyxia and 0–3 points for severe asphyxia. The lower the score, the more severe asphyxia was.
Statistical analysis
SPSS 18.0 (Yiyun Information Technology Co., Ltd., Shanghai, China) was used for statistical analysis. Measurement data were presented as mean ± standard deviation (mean ± SD). One way analysis of variance (ANOVA) was used for multigroup mean comparisons. Dunnetts t-test was used for pairwise comparison and Spearmans test for correlation analysis. P<0.05 indicates the difference was statistically significant.
Results
Baseline data
There was no significant difference in age, gestational age, gravida para, body mass index (BMI), hemoglobin (HB), red blood cell (RBC) count, or platelet (PLT) count among groups A, B, C and control group (P>0.05) (Table I).
Table I.
Baseline data of groups A, B, C and control group (mean ± SD).
| Classification | Group A (n=35) | Group B (n=46) | Group C (n=24) | Control group (n=35) | F | P-value |
|---|---|---|---|---|---|---|
| Age | 26.12±3.63 | 26.58±3.15 | 25.48±3.47 | 25.89±2.16 | 0.728 | 0.536 |
| Gestational age (week) | 32.65±0.91 | 32.65±0.84 | 32.87±0.97 | 33.15±0.79 | 1.811 | 0.148 |
| Gravida para | 1.48±0.16 | 1.49±0.25 | 1.45±0.18 | 1.38±0.13 | 2.494 | 0.062 |
| BMI (kg/m2) | 26.72±2.87 | 26.59±2.87 | 27.32±3.11 | 27.67±3.01 | 1.098 | 0.352 |
| Hb (g/l) | 123.58±9.47 | 128.58±11.37 | 126.19±10.08 | 127.58±10.37 | 1.637 | 0.183 |
| RBC (×1012/l) | 4.63±0.49 | 4.85±0.52 | 4.59±0.61 | 4.70±0.36 | 2.006 | 0.116 |
| PLT (×109/l) | 149.36±11.58 | 151.85±9.67 | 155.47±11.09 | 152.26±10.25 | 1.602 | 0.191 |
BMI, body mass index; Hb, hemoglobin; RBC, red blood cell; PLT, platelet.
Serum VEGF and sFLT-1 levels in four groups
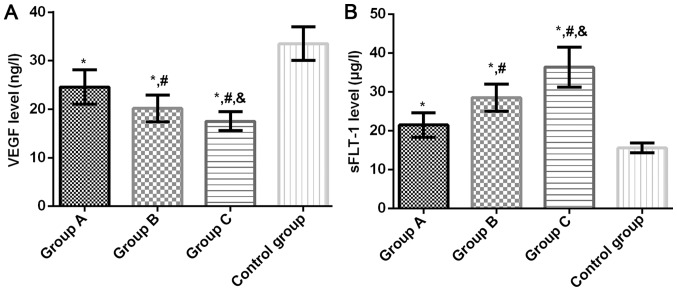
Serum VEGF levels in groups A, B, C and control group were 24.58±3.54, 20.17±2.74, 17.52±1.95 and 33.51±3.47 µg/l, respectively, and the sFLT-1 levels were 21.49±3.17, 28.52±3.48, 36.41±5.15 and 15.58±1.25 µg/l, respectively. Compared with the control group, the serum VEGF level in groups A, B and C was significantly lower (P<0.05). Compared with group A, the level of serum VEGF in groups B and C was significantly lower (P<0.05). Compared with group B, the serum VEGF level in group C decreased significantly (P<0.05). Compared with the control group, the serum sFLT-1 level in groups A, B and C was significantly higher (P<0.05). Compared with group A, the serum sFLT-1 level in groups B and C was significantly higher (P<0.05). Compared with group B, the serum sFLT-1 level in group C was significantly higher (P<0.05) (Fig. 1).
Figure 1.
(A) Comparison of serum VEGF level among four groups. Results of ELISA showed that serum VEGF level in groups A, B and C was significantly lower than that in the control group (P<0.05), the serum VEGF level in groups B and C was significantly lower than that in group A (P<0.05) and the serum VEGF level in group C was significantly lower than that in group B (P<0.05). (B) Comparison of serum sFLT-1 level among four groups. Results of ELISA showed that serum sFLT-1 level in groups A, B and C was significantly higher compared with the control group (P<0.05), the serum sFLT-1 level in groups B and C was significantly higher compared with group A (P<0.05). The serum sFLT-1 level in group C was significantly higher compared with group B (P<0.05). *P<0.05 compared with the control group; #P<0.05 compared with group A; &P<0.05 compared with group B.
Neonatal weight and Apgar score in four groups
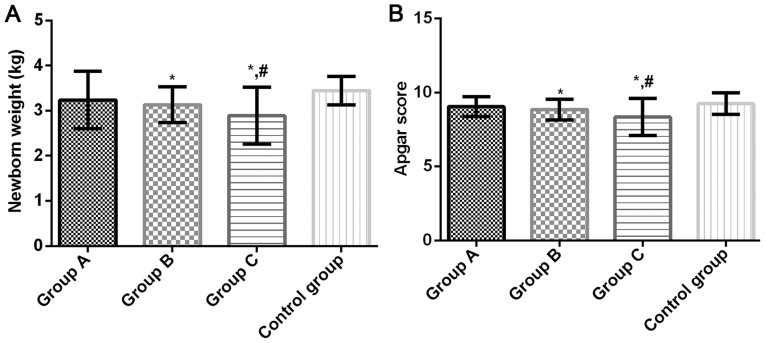
Neonatal weight of groups A, B, C and control group were 3.241±0.634, 3.137±0.398, 2.891±0.628 and 3.447±0.312 kg, respectively, and the Apgar scores were 9.05±0.68, 8.86±0.70, 8.36±1.25 and 9.25±0.73, respectively. Compared with the control group, neonatal weight and Apgar score in groups B and C were significantly lower (P<0.05). There was no significant difference in the neonatal weight and Apgar score between group A and the control group (P>0.05). Compared with group A, neonatal weight and Apgar score in group C were significantly lower (P<0.05). There was no significant difference between groups A and B (P>0.05) (Fig. 2).
Figure 2.
(A) Comparison of neonatal weight score among four groups. Neonatal weight in groups B and C decreased significantly compared with the control group (P<0.05). Neonatal weight in group C decreased significantly compared with group A (P<0.05). There was no significant difference in neonatal weight between groups A and B and the control group (P>0.05). (B) Comparison of Apgar score in four groups. Apgar score in groups B and C decreased significantly compared with the control group (P<0.05). Apgar score in group C decreased significantly compared with group A (P<0.05). There was no significant difference in Apgar score between groups A and B and the control group (P>0.05). *P<0.05 compared with the control group; #P<0.05 compared with group A.
Correlation between serum VEGF, sFLT-1 level and postpartum neonatal weight and Apgar score
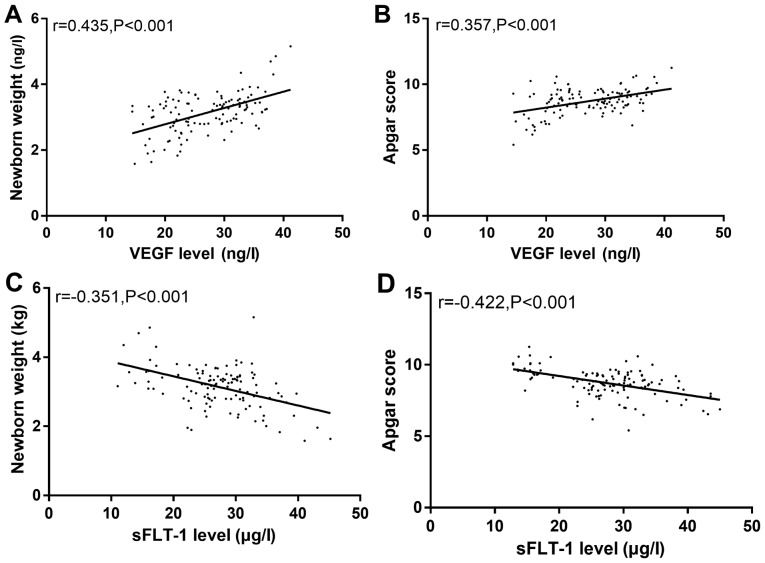
Spearmans test showed that there was a positive correlation between the serum VEGF level and neonatal weight and Apgar score (r=0.435, P<0.001; r=0.357, P<0.001) and a negative correlation between serum sFLT-1 level and neonatal weight and Apgar score (r=−0.351, P<0.001; r=−0.422, P<0.001) (Fig. 3).
Figure 3.
Correlation between serum VEGF, sFLT-1 level and postpartum neonatal weight and Apgar score. (A) Spearmans test showed that there was a positive correlation between serum VEGF level and neonatal weight (r=0.435, P<0.001). (B) VEGF level was positively correlated with Apgar score (r=0.357, P<0.001). (C) There was a negative correlation between serum sFLT-1 level and neonatal weight (r=−0.351, P<0.001). (D) Serum sFLT-1 level was negatively correlated with Apgar score (r=−0.422, P<0.001).
Discussion
Pregnancy-induced hypertension syndrome (PIH) is a disease in pregnant women and one of the main causes of maternal and perinatal mortality. At present, the specific physiological and pathological mechanism of PIH is not clear (19). Pathophysiological studies suggest that PIH is caused by insufficient trophoblastic infiltration due to reduced placental perfusion (20). Abnormal placenta is one of the main causes of preeclampsia. Due to abnormal placenta implantation, uterine and placental perfusion can cause oxidative stress in the body, eventually causing hypoxia and the release of anti-angiogenic factors. These anti-angiogenic factors can cause a wide range of endothelial dysfunction, which in turn leads to maternal hypertension and vasoconstriction (21).
VEGF is an important angiogenesis regulatory factor and a highly conserved glycoprotein in the human body. VEGF can accelerate angiogenesis and promote endothelial cell division, playing an important role in physiological or pathological angiogenesis (22). sFLT-1 can act as an effective anti-angiogenic molecule by binding to free VEGF in the blood. sFLT-1 can affect the infiltration of trophoblast cells by inhibiting the synthesis of trophoblast cells, which in turn leads to shallow implantation of placental villi and the development of preeclampsia (23). Results of this study showed that compared with the control group, the serum VEGF levels in groups A, B and C were significantly decreased, while sFLT-1 levels were significantly increased. VEGF levels were significantly decreased, while sFLT-1 levels were significantly increased with increasing severity of disease. These data suggest that VEGF and sFLT-1 may be involved in the occurrence and development of PIH. Consistently, Kleinrouweler et al (24) showed that VEGF levels are reduced and sFLT-1 levels are elevated in patients with preeclampsia during pregnancy. Maynard et al (25) suggested that excessive sFlt-1 production is the result of abnormal placental hypoxia. Gilbert et al (26) showed that hypertension caused by decreased intrauterine perfusion in pregnant rats is closely related to increased sFLT-1 levels. Pathological pregnancy may cause local tissue hypoxia-ischemia, and placenta will release toxic cytokines into the mothers blood circulation, resulting in endothelial cell damage and trophoblast cell proliferation and differentiation. Therefore, infiltration ability of trophoblast cells is reduced, causing a decrease in VEGF secretion and an increase in sFLT-1 levels.
Normal function of the placenta is an important factor in fetal growth and maintenance of the pregnancy. Placenta can provide oxygen and nutrients to the fetus, and can eliminate the metabolic waste generated by the fetus, so as to ensure the healthy growth of fetus (27). Normal pregnancy requires trophoblastic physiological invasion into the maternal uterine iris tissue, which promotes the exchange of blood circulation between the placenta and fetus. If this process fails, preeclampsia and fetal growth restriction in pregnancy will occur (28).
The basic lesions of PIH are hemodynamic changes and small arterial spasm, which can cause maternal placental thrombosis, atherosclerosis of the placental artery and poor blood supply and circulation. So the reserve capacity of the placenta is reduced, and the supply of nutrients to fetus from mother is hindered (25). Results of this study showed that compared with the control group, weight and Apgar scores of the newborns in groups B and C were significantly lower. Compared with group A, weight and Apgar scores of newborns in group C were significantly lower. Serum VEGF levels were positively correlated with neonatal weight and Apgar scores, and serum sFLT-1 levels were negatively correlated with neonatal weight and Apgar scores. It has been reported that (29) the expression of sFlt-1 mRNA and protein in placenta of severe intrauterine growth restriction was significantly upregulated compared to that in normal gestational age placenta, which is similar to the results of this study. Klein et al (30) considered that the risk of maternal and fetal adverse outcomes associated with preeclampsia increases with increasing sFlt-1/PlGF ratio and is the highest among women with sFlt-1/PlGF ratios of 85 and above. In the study of Zeisler et al (31), the sFlt-1: PlGF ratio is 38 or lower, which can be used to predict women with short-term suspected preeclampsia and PlGF and VEGF function similarly. Although serum levels of vascular endothelial growth factor were positively correlated with neonatal weight and Apgar score, and sFlt-1 level was negatively correlated with neonatal weight and Apgar score, this study did not investigate the correlation between VEGF and sFLT-1 levels and maternal and fetal adverse outcomes, so these data do not necessarily mean that VEGF and sFlt-1 will affect pregnancy outcomes, which is a limitation in our design. Further studies are required on this aspect.
In summary, VEGF and sFLT-1 may be involved in the occurrence and development of PIH. The decrease of serum VEGF level and the increase of sFLT-1 level may be related to fetal growth and development inhibition.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors contributions
YT analyzed the general data of patients. WY and XL helped with the sample collection and detection. YL and CY recorded and analyzed the Apgar score. JW was responsible for the statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of The First People's Hospital of Changzhou (Changzhou, China). Patients who participated in this research, signed an informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Susan ZS, Bulbul S, Ferdows JA, Nayeem A. Perinatal findings in pregnancy induced hypertension: A study in a Tertiary Teaching Hospital in Dhaka City. J Natl Inst Neurosci Bangladesh. 2017;2:10–13. doi: 10.3329/jninb.v2i1.32955. [DOI] [Google Scholar]
- 2.Kintiraki E, Papakatsika S, Kotronis G, Goulis DG, Kotsis V. Pregnancy-induced hypertension. Hormones (Athens) 2015;14:211–223. doi: 10.14310/horm.2002.1582. [DOI] [PubMed] [Google Scholar]
- 3.Liu FM, Zhao M, Wang M, Yang HL, Li L. Effect of regular oral intake of aspirin during pregnancy on pregnancy outcome of high-risk pregnancy-induced hypertension syndrome patients. Eur Rev Med Pharmacol Sci. 2016;20:5013–5016. [PubMed] [Google Scholar]
- 4.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, Magee LA, Audibert F, Bujold E, Côté A-M, Douglas MJ, et al. Canadian Hypertensive Disorders of Pregnancy Working Group Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: Executive summary. J Obstet Gynaecol Can. 2014;36:416–441. doi: 10.1016/S1701-2163(15)30588-0. [DOI] [PubMed] [Google Scholar]
- 5.Tateishi A, Ohira S, Yamamoto Y, Kanno H. Histopathological findings of pregnancy-induced hypertension: Histopathology of early-onset type reflects two-stage disorder theory. Virchows Arch. 2018;472:635–642. doi: 10.1007/s00428-018-2315-3. [DOI] [PubMed] [Google Scholar]
- 6.Steegers EAP, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 7.Roberts JM, Hubel CA. The two stage model of preeclampsia: Variations on the theme. Placenta. 2009;30(Suppl A):S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren Y, Wang H, Qin H, Yang J, Wang Y, Jiang S, Pan Y. Vascular endothelial growth factor expression in peripheral blood of patients with pregnancy induced hypertension syndrome and its clinical significance. Pak J Med Sci. 2014;30:634–637. doi: 10.12669/pjms.303.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang L, Wei Z, Li O, Huang R, Qin J, Chen H, Fan X, Chen ZJ. An increase in vascular endothelial growth factor (VEGF) and VEGF soluble receptor-1 (sFlt-1) are associated with early recurrent spontaneous abortion. PLoS One. 2013;8:e75759. doi: 10.1371/journal.pone.0075759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andraweera PH, Dekker GA, Laurence JA, Roberts CT. Placental expression of VEGF family mRNA in adverse pregnancy outcomes. Placenta. 2012;33:467–472. doi: 10.1016/j.placenta.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Demir R. Expression of VEGF receptors VEFGR-1 and VEGFR-2, angiopoietin receptors Tie-1 and Tie-2 in chorionic villi tree during early pregnancy. Folia Histochem Cytobiol. 2009;47:435–445. doi: 10.2478/v10042-009-0100-5. [DOI] [PubMed] [Google Scholar]
- 12.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- 13.Nadar SK, Karalis I, Al Yemeni E, Blann AD, Lip GY. Plasma markers of angiogenesis in pregnancy induced hypertension. Thromb Haemost. 2005;94:1071–1076. doi: 10.1160/TH05-03-0167. [DOI] [PubMed] [Google Scholar]
- 14.Myatt L, Roberts JM. Preeclampsia: Syndrome or disease? Curr Hypertens Rep. 2015;17:83. doi: 10.1007/s11906-015-0595-4. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Yamamoto T, Watanabe K, Yoshimatsu J, Matsubara K, Mimura K, Tanaka K, Nishizawa H, Makino S, Nohira T, et al. Home blood pressure measurement (HBPM) for the early detection of hypertensive disorders of pregnancy (HDP) in Japanese women: A multicenter prospective study. Hypertens Res Pregnancy. 2017;5:36–38. doi: 10.14390/jsshp.HRP2017-017. [DOI] [Google Scholar]
- 16.Prince HE, Lapé-Nixon M, Givens TS, Bradshaw T, Nowicki MJ. Elimination of falsely reactive results in a commercially-available West Nile virus IgM capture enzyme-linked immunosorbent assay by heterophilic antibody blocking reagents. J Immunol Methods. 2017;444:24–28. doi: 10.1016/j.jim.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Simmons MA, Laughon MM. 50 years ago in the Journal of Pediatrics: A practical classification of newborn infants by weight and gestational age. J Pediatr. 2017;187:33. doi: 10.1016/j.jpeds.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 18.Cnattingius S, Norman M, Granath F, Petersson G, Stephansson O, Frisell T. Apgar score components at 5 minutes: Risks and prediction of neonatal mortality. Paediatr Perinat Epidemiol. 2017;31:328–337. doi: 10.1111/ppe.12360. [DOI] [PubMed] [Google Scholar]
- 19.Shirasuna K, Karasawa T, Usui F, Kobayashi M, Komada T, Kimura H, Kawashima A, Ohkuchi A, Taniguchi S, Takahashi M. NLRP3 deficiency improves angiotensin II-induced hypertension but not fetal growth restriction during pregnancy. Endocrinology. 2015;156:4281–4292. doi: 10.1210/en.2015-1408. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal PK, Chandel N, Jain V, Jha V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J Hum Hypertens. 2012;26:236–241. doi: 10.1038/jhh.2011.29. [DOI] [PubMed] [Google Scholar]
- 21.McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. Kidney disease after preeclampsia: A systematic review and meta-analysis. Am J Kidney Dis. 2010;55:1026–1039. doi: 10.1053/j.ajkd.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 22.Ogunleye O, Campo B, Herrera D, Post Uiterweer ED, Conrad KP. Relaxin confers cytotrophoblast protection from hypoxia-reoxygenation injury through the phosphatidylinositol 3-kinase-Akt/protein kinase B cell survival pathway. Am J Physiol Regul Integr Comp Physiol. 2017;312:R559–R568. doi: 10.1152/ajpregu.00306.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton GJ. Intrauterine devices, the endometrium and the risk of pre-eclampsia. BJOG. 2016;123:796–796. doi: 10.1111/1471-0528.13470. [DOI] [PubMed] [Google Scholar]
- 24.Kleinrouweler CE, Wiegerinck MMJ, Ris-Stalpers C, Bossuyt PM, van der Post JA, von Dadelszen P, Mol BW, Pajkrt E, EBM CONNECT Collaboration Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: A systematic review and meta-analysis. BJOG. 2012;119:778–787. doi: 10.1111/j.1471-0528.2012.03311.x. [DOI] [PubMed] [Google Scholar]
- 25.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 27.Ganguly A, Tamblyn JA, Finn-Sell S, Chan SY, Westwood M, Gupta J, Kilby MD, Gross SR, Hewison M. Vitamin D, the placenta and early pregnancy: Effects on trophoblast function. J Endocrinol. 2018;236:R93–R103. doi: 10.1530/JOE-17-0491. [DOI] [PubMed] [Google Scholar]
- 28.Carreras-Badosa G, Prats-Puig A, Puig T, Vázquez-Ruíz M, Bruel M, Mendoza E, de Zegher F, Ibáñez L, López-Bermejo A, Bassols J. Circulating fatty acid synthase in pregnant women: Relationship to blood pressure, maternal metabolism and newborn parameters. Sci Rep. 2016;6:24167. doi: 10.1038/srep24167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nevo O, Many A, Xu J, Kingdom J, Piccoli E, Zamudio S, Post M, Bocking A, Todros T, Caniggia I. Placental expression of soluble fms-like tyrosine kinase 1 is increased in singletons and twin pregnancies with intrauterine growth restriction. J Clin Endocrinol Metab. 2008;93:285–292. doi: 10.1210/jc.2007-1042. [DOI] [PubMed] [Google Scholar]
- 30.Klein E, Schlembach D, Ramoni A, Langer E, Bahlmann F, Grill S, Schaffenrath H, van der Does R, Messinger D, Verhagen-Kamerbeek WD, et al. Influence of the sFlt-1/PlGF ratio on clinical decision-making in women with suspected preeclampsia. PLoS One. 2016;11:e0156013. doi: 10.1371/journal.pone.0156013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.