Abstract
Fatigue is one of the most common and disabling nonmotor symptoms seen in Parkinson's disease (PD) and is also commonly seen in healthy older adults. Our understanding of the etiology of fatigue in older adults with or without PD is limited and it remains unclear whether fatigue in PD is specifically related to PD pathology. The objective of this study was thus to determine whether fatigue in PD was associated with structural changes in gray or white matter and explore whether these changes were similar in older adults without PD. Magnetic resonance imaging (T1 weighted) and diffusion tensor imaging were performed in 60 patients with PD (17 females; age = 67.58 ± 5.51; disease duration = 5.67 ± 5.83 years) and 41 age- and sex- matched healthy controls. FSL image processing was used to measure gray matter volume, fractional anisotropy, and leukoariosis differences. Voxel-based morphometry confirmed gray matter loss across the dorsal striatum and insula in the PD patient cohort. PD patients with fatigue had reduced gray matter volume in dorsal striatum relative to PD patients without fatigue (P < 0.05 False Discovery Rate corrected). No significant fatigue-related structural atrophy was found in controls. There were no areas of significant fractional anisotropy differences between high and low fatigue subjects in either the PD or non-PD groups. Control participants with high fatigue, but not PD, showed significantly greater total leukoariosis volumes (p = 0.03). Fatigue in PD is associated with unique structural changes in the caudate and putamen suggesting fatigue in PD is primarily related to PD pathology, particularly in the dorsal striatum, and not simply a consequence of aging.
Keywords: Parkinson's disease, Parkinson's disease imaging, Arousal, Neuropsychiatry: Imaging, Neuroanatomy
Abbreviations: AAL, Anatomical Automatic Labeling; ANTS, Advanced Normalization Tools; BDI, Beck Depression Inventory; DSC, Dice Similarity Coefficient; DSM, Diagnostic and Statistical Manual of Mental Disorders; FLIRT, FMRIB (Functional MRI of the Brain) Linear Image Registration Tool; FSL, FMRIB (Functional MRI of the Brain) Software Library; ICBM, International Consortium for Brain Mapping; MNI, Montreal Neurological Institute; MR, Magnetic Resonance; PD, Parkinson's Disease; SPECT, Single-photon Emission Computed Tomography; SPSS, Statistical Package for the Social Sciences; TBSS, Tract-based Spatial Statistics
Graphical abstract
Areas of reduced gray matter in the analysis of Parkinson's disease with high fatigue compared with Parkinson's disease with low fatigue in Axial and Coronal slices (controlling for total intracranial volume, BDI, Age, Sleep Duration and Mini-Mental State Examination, False Discovery Rate corrected P < 0.05).
Highlights
-
•
Fatigue is a disabling symptom in Parkinson's disease (PD) and healthy older adults.
-
•
We studied structural correlates of fatigue using MRI morphometry.
-
•
PD patients with high fatigue had caudate atrophy.
-
•
Healthy older adults with fatigue had increased burden of leukoariosis.
-
•
Patterns of structural brain changes were distinct between PD and healthy group.
1. Introduction
Fatigue affects approximately half of all Parkinson's disease (PD) patients and is reported by one-third to be their single most disabling symptom (Friedman et al., 2007a). Fatigue in PD is also associated with diminished activity levels, quality of life, and physical function (Abrantes et al., 2012; Elbers et al., 2014; Kluger et al., 2014). Despite the undeniable impact of fatigue on persons living with PD, there has been relatively little study of its etiology and currently available treatments are minimally effective. Studies to date generally do not find significant associations between disease duration, disease stage, or motor symptoms with fatigue suggesting the involvement of nonmotor networks (Friedman et al., 2007b). Indeed, two prior functional imaging studies on PD-related fatigue suggested potential involvement of prefrontal cortex using SPECT perfusion (Abe et al., 2000) and serotonergic dysfunction in ventral striatum and thalamus and dopaminergic dysfunction in the caudate and insula using PET ligands (Pavese et al., 2010). Regarding other potential secondary causes of fatigue in PD, fatigue is often associated with depression (Friedman et al., 2007a). However, depression and fatigue frequently exist independently and fatigue may persist following successful treatment of depression (Alves et al., 2004). Fatigue in PD is also inconsistently associated with sleep disorders and sleep quality and is dissociable from daytime somnolence (van Hilten et al., 1993; Valko et al., 2010; Stocchi et al., 2014).
Fatigue is common in many diseases and complaints may be driven by dysfunction in the peripheral nervous system (e.g. myasthenia gravis), CNS (e.g. multiple sclerosis), or other organ systems (e.g. heart failure) (Kluger et al., 2013). Although this may seem to support the claim that fatigue is a nonspecific symptom, there are important differences in the phenomenology of fatigue in these diverse disorders and treatments targeted to the specific pathophysiological mechanism (e.g. anemia in cancer fatigue) are more effective than generic treatments (e.g. stimulants) (Minton et al., 2008). Regarding CNS causes of fatigue, Chaudhuri and Behan proposed a neuroanatomic model in which disruption of cortical-subcortical loops important for internal drive (ventral striatum, anterior cingulate cortex, thalamus) causes fatigue by altering one's ability to maintain consistent effort (Chaudhuri and Behan, 2000, Chaudhuri and Behan, 2004). Lesion analysis in both stroke and multiple sclerosis support this model and argue that fatigue is indeed due to focal network dysfunctions and not global factors (Rocca et al., 2014). While the high prevalence of fatigue in PD has been used to support this model (particularly the contributions of basal ganglia dysfunction to fatigue) it has not been directly tested in PD and does not address why approximately half of PD patients do not report significant fatigue.
As PD is associated with structural changes in cortical, subcortical, and white matter structures, we hypothesized that morphometric variability may be used to improve our understanding of the pathogenesis of fatigue as has been done with other nonmotor symptoms (Bohnen and Albin, 2011; Kostic and Filippi, 2011; Cruz Gomez et al., 2013; Gama et al., 2014; Huang et al., 2014; Rocca et al., 2014; Tanner et al., 2015; Price et al., 2016). Thus, our objectives in the current study were to utilize MR-morphometry and diffusion tensor imaging to identify cortical, subcortical, and white matter changes associated with fatigue in PD while controlling for potential confounds (e.g. sleep and depression) with the goal of improving our understanding of the pathogenesis of PD-related fatigue. We specifically hypothesized PD-related fatigue would be associated with anterior cingulate cortex, striatum, and insula volume. To address the question of whether fatigue in PD is primarily related to PD pathology or more generic factors related to aging, we also examined structural correlates of fatigue in healthy older adults.
2. Methods
2.1. Participants
This study was approved by the Institutional Review Board of the University of Florida, required individual consent form signature, and followed the Declaration of Helsinki. Recruitment involved a combination of: 1) brochure mailings to individuals identified through a research database within the University of Florida Center for Neurorestoration and Movement Disorder Center; 2) University of Florida Center for Neurorestoration and Movement Disorder Center direct neurology referrals; 3) advertisement at different PD support symposiums. All individuals were screened via telephone or in person and then completed a baseline cognitive testing to ensure they met cognitive screening criteria.
All participants had to be right-handed and speak fluent English. Individuals with PD were diagnosed by a movement disorder fellowship trained neurologist, met criteria outlined by the United Kingdom Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria (Hughes et al., 1992), and had a Hoehn and Yahr scale (Hoehn and Yahr, 1967) ranging from 1 to 3. All participants were tested while on-medication. Medical exclusions included having any underlying medical disease likely to limit lifespan or cause fatigue independent of PD: cancer requiring treatment in past five years (exception: non-melanoma skin cancer), serious infectious diseases (e.g., self-reported HIV), myocardial infarction or cerebrovascular accident in the last six months, congestive heart failure, chronic hepatitis, history of organ transplantation, seizure disorders, head trauma resulting in intensive care, or any other chronic medical condition likely to limit lifespan. Surgical exclusion included having undergone Deep Brain Stimulation Surgery. Neurodegenerative exclusions included evidence of secondary or atypical Parkinsonism as suggested by the presence of any of the following: 1) history of major stroke(s) associated with cognitive sequelae; 2) exposure to toxins or neuroleptics; 3) history of encephalitis; 4) neurological signs of upper motor neuron disease, cerebellar involvement, supranuclear palsy, or significant orthostatic hypertension. Patients were excluded if they presented with signs of a dementia as indicated by the neurological/ neuropsychological assessment, DSM-IV criteria, and Mattis Dementia Rating Scale corrected scale score < 8 (please see Price et al., 2016 for full details of neuropsychological testing). Psychiatric exclusions included having a major psychiatric disorder as assessed by the psychiatric and neurological team with the Structured Clinical Interview for DSM-IV. Per patient record, those with histories of major depression were excluded. We did not exclude patients reporting mild depression or anxiety for many PD patients report such symptoms. Conditions or behaviors likely to affect cognitive testing included claustrophobia, non-medical bodily metal, pace-maker device, less than five years of normal education, inability to read or write, and self-reported hearing difficulty that interferes with standardized test administration. Data from select participants have been published elsewhere (Schwab et al., 2015; Tanner et al., 2015; Price et al., 2016; Tanner et al., 2016; Crowley et al., 2018).
2.2. Fatigue severity
Fatigue severity was measured using the Fatigue Severity Scale which is widely used, validated, and recommended for use in PD, including by the Movement Disorders Society Fatigue Task Force (Krupp et al., 1989; Grace et al., 2007; Friedman et al., 2010). The Fatigue Severity Scale is a 9-item questionnaire that asks patients to rate the severity and impact of fatigue on various aspects of daily life on a 1 to 7 scale (strongly disagree to strongly agree). We used the suggested cut-off point of 4 (9-item average) to identify individuals with clinically significant (moderate to severe) fatigue (Krupp et al., 1989).
2.3. Sleep and depression
To control for the potentially confounding effects of sleep and depression we quantified sleep duration based on self-reported average number of hours of sleep per night. Data were acquired from a routine health screening completed as part of the parent investigation and within 24 h of the brain MRI. Depressive symptoms were measured using the total score from the BDI-II (Beck et al., 1996).
2.4. Parkinson's disease motor severity and levodopa equivalency dose
PD motor severity on medication was quantified using part III of the Unified Parkinson Disease Rating Scale (S Fahn, 1987) and then scored by trained and reliable raters. A database containing all participants' medication and vitamins was recorded and reviewed by two separate raters. Medication containing L-dopa was converted to a common metric (Levodopa Equivalency Dose) (Tomlinson et al., 2010) in which the drugs are compared in relation to the immediate release L-dopa dose (400 mg of the immediate release L-dopa dose is equivalent to 400 mg Levodopa Equivalency Dose).
2.5. MRI data acquisition
Neuroimaging data were prospectively acquired with a Siemens 3 T Verio scanner using an 8-channel head coil. For gray and white matter analyses we acquired two T1-weighted scans (176 contiguous slices, 1 mm3 voxels, TR/TE = 2500/3.77 ms). For diffusion analyses we acquired two separate single-shot EPI diffusion weighted images with gradients applied along six directions (b = 100 s/mm2) and 64 directions (b = 1000 s/mm2) respectively. Diffusion imaging parameters were set at 73 contiguous axial slices with 2 mm3 voxels and TR/TE = 17,300/81 ms. For skull segmentation, we acquired T2-weighted images with the following parameters: 176 contiguous slices, 1 mm3 voxels, TR/TE = 3200/409 ms.
2.6. Total intracranial volume
Total intracranial volume was measured by exporting the inner surface of the skull from FSL version 4.1 Brain Extraction Tool (Smith, 2002). These initial intracranial masks were then manually cleaned by expert raters to fill the enclosure within the inner surface of the skull. The inferior portion of the mask terminated on a straight line between the bottom of the occipital bone and the clivus. Reliability was high (intra-rater and inter-rater reliability DSC > 0.99). The final variable of interest was total intracranial volume in mm3.
2.7. Cortical gray, subcortical gray, white matter segmentation
Cortical reconstruction and volumetric segmentation was performed with the Freesurfer image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications (Dale and Sereno, 1993; Dale et al., 1999; Fischl et al., 1999a; Fischl et al., 1999b; Fischl and Dale, 2000; Fischl et al., 2001; Fischl et al., 2002; Fischl et al., 2004a; Fischl et al., 2004b; Segonne et al., 2004; Han et al., 2006; Jovicich et al., 2006). This processing includes removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (Fischl et al., 2002; Fischl et al., 2004a), intensity normalization (Sled et al., 1998), tessellation of the gray matter white matter boundary, automated topology correction (Fischl et al., 2001; Segonne et al., 2007), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale and Sereno, 1993; Dale et al., 1999; Fischl and Dale, 2000). Parcellation of the cerebral cortex into units based on gyral and sulcal structure was also performed (Fischl et al., 2004b; Desikan et al., 2006). Freesurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and across field strengths (Han et al., 2006).
2.8. Gray matter volume differences: voxel based morphometry
We performed a Voxel Based Morphometry analysis on T1 structural images to investigate voxel-wise gray matter differences between PD patients and control participants. An optimized Voxel Based Morphometry protocol was carried out, using FSL software package version 5.0. First, structural images were reoriented to match the MNI 152 standard space orientation. Brains of the reoriented structural images were extracted using FSL's Brain Extraction Tool. Second, brain-extracted images were segmented into gray matter, white matter, and cerebral spinal fluid to create study specific gray matter template. All gray matter images were registered to gray matter ICBM-152 template with non-linear registration and averaged across all participants. The averaged image was flipped along the x-axis to create a left-right symmetric, first pass gray matter template. The native gray matter images were re-registered to this first pass gray matter template using non-linear registration, averaged, and flipped along the x-axis. The mirror images were averaged to create the final study-specific gray matter template. All native gray matter images were non-linearly registered to the study-specific gray matter template and “modulated” by compensating for the contraction or enlargement due to non-linear component of spatial transformation. Modulated gray matter images were spatially smoothed with isotropic Gaussian kernel (sigma = 3 mm). Finally, voxel-wise general linear model analysis was applied on the smoothed images, using permutation-based nonparametric testing with multiple comparison correction across space (Mechelli et al., 2005).
From the AAL template (Tzourio-Mazoyer et al., 2002), we extracted caudate, putamen, insula, and anterior cingulate cortex. Dorsolateral prefrontal cortex was located at Brodmann area 9 and 46 by atlas of Talairach and Toumoux (Talairach and Tournoux, 1988). For nucleus accumbens, two 5-mm-radius spheres were generated at MNI coordinates (±10, 10, −8) according to previous publication (Collins et al., 1994; Tanabe et al., 2007; Abdo et al., 2009; Heldmann et al., 2012). These regions of interest are schematically visualized in Fig. 1.
Fig. 1.
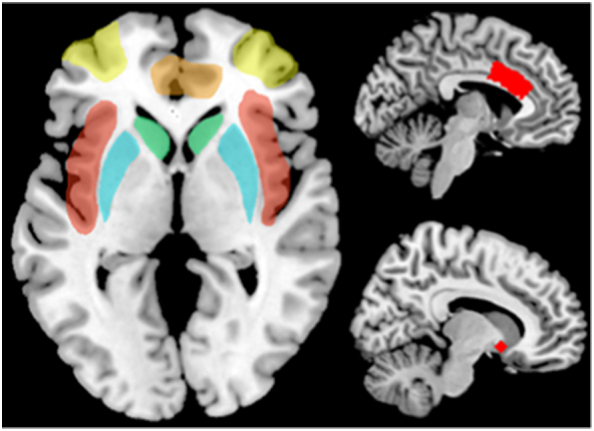
The schematic map of regions of interest. Yellow: dorsolateral prefrontal cortex. Red: insula. Green: caudate. Orange: anterior cingulate cortex. Cyan: putamen. Right top: Caudal anterior cingulate cortex. Right bottom: nucleus accumbens. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.9. White matter integrity: fractional anisotropy and leukoaraiosis
In order to assess group differences in white matter fractional anisotropy values, we utilized TBSS, a method that provides a robust, voxelwise analysis of diffusion data (Smith et al., 2006). We calculated fractional anisotropy maps using dtifit after correcting for eddy current distortions with eddy_correct; both tools are part of the FSL (http://www.fmrib.ox.ac.uk/fsl/) (Smith et al., 2004). We created a study-specific fractional anisotropy template by averaging representative fractional anisotropy maps from four individuals registered into MNI152 space and then using ANTS (Avants et al., 2011) to produce a final template from 60 PD fractional anisotropy maps and 41 non-PD peer fractional anisotropy maps. The fractional anisotropy maps from all participants were then aligned to the template image using an automated method implemented in the TBSS pipeline, which applies a non-linear registration of each fractional anisotropy map to the template. A diffusion MRI template was then generated from the average of the normalized fractional anisotropy maps, non-linearly aligned to the target image.
Voxel-wise statistical analysis of the fractional anisotropy data was carried out using TBSS (Smith et al., 2006). After alignment of the fractional anisotropy maps to the study specific template, a mean fractional anisotropy skeleton was created; this fractional anisotropy skeleton represents the centers of all tracts common to the group. This skeleton was thresholded at a fractional anisotropy level of >0.2 and voxel-wise statistics were used with randomize to test for differences in fractional anisotropy between 1) PD with high fatigue and PD with low fatigue and 2) non-PD peers with high fatigue and non-PD peers with low fatigue using a two tailed t-test. A statistical threshold of p < 0.05 was applied after family-wise error correction for multiple comparisons following threshold-free cluster enhancement (Smith and Nichols, 2009).
2.10. Leukoaraiosis
The significance of leukoariosis in PD is debated. Leukoariosis is one marker of small vessel vascular disease that could contribute to processing speed and working memory impairments (Jones et al., 2017 Thus, leukoariosis was measured for both PD and non-PD and examined as a possible vascular covariate to white matter integrity. One reliable rater using a locally developed macro for ImageJ (Abràmoff et al., 2004; http://rsbweb.nih.gov/ij/docs/index.html) measured the leukoariosis for all brains. Rater reliability was established with another expert rater using 15 MRIs representing a wide range of lesion severity that were measured in a blind pseudo-random order on three separate occasions with non-consecutive repeats. Leukoariosis spatial overlap was high in all inter- and intrarater reliability comparisons (Inter-rater range: DCS = 0.84–0.93; Intra-rater range = 0.80–0.83; DSC mean ± s.d. = 0.84 ± 0.12; obtaining a DSC value >0.7 indicates an excellent agreement between two raters; Zijdenbos et al., 1994).
2.11. Statistical analysis approaches
Statistical calculations above were performed using a commercially available software package (SPSS, version 19; SPSS, Inc). Variables were confirmed for normality and statistical assumptions prior to all analyses. Parametric analyses were used to complete demographic analyses and to examine cognitive, mood, fatigue, and region of interest neuroanatomical group differences. Due to violations of normality, Mann-Whitney U tests were used to compare Unified Parkinson's disease Rating Scale scores. Linear regression analyzed the contribution of the putamen, caudate, insula, anterior cingulate cortex, dorsolateral prefrontal cortex, and nucleus accumbens on the fatigue severity scale treated as a continuous variable. Whole brain analyses (Voxel Based Morphometry, TBSS) were used to examine group differences between high and low fatigue cohorts and are discussed in references and subsections above. To account for potential differences between high and low fatigue groups, we added covariates to regression models to control for differences in total intracranial volume, BDI, Age, sleep duration and Mini-Mental State Examination.
3. Results
Of 105 people enrolled, 63 individuals with PD and 42 non-PD peers completed the study. Four participants (three PD, one control) could not complete MRI scans (claustrophobia, metal artifact) and were excluded from the current analyses. Final analyses were conducted with 60 PD participants and 41 normal controls, totaling 101 subjects.
3.1. Parkinson's disease and non-Parkinson's disease peer demographic, general cognitive and motor characteristics
As shown in Table 1, PD and control participants were statistically similar in age, education, ratio of male to female, and health comorbidities (all p values > .05). Motor: Individuals with PD were had predominantly unilateral tremor and low disease severity (70% Hoehn and Yahr ≤ 1.5), and 83% had <10 years of disease duration (two participants self-reported >20 years duration but had Hoehn and Yahr of 1 and 1.5). One control participant was taking 40 mg of levodopa-based medication for restless leg syndrome. General cognition: Groups were significantly different in general cognition as measured by the Mini-Mental State Examination (p < .05; Table 1) although PD and non-PD individuals both reported independence in instrumental activities of daily living that involved telephone use, housekeeping, and financial management. All but one PD individual independently managed his/her medications. Mood: Relative to the non-PD peers, individuals with PD reported greater more symptoms of depression, apathy, and anxiety as measured by the BDI-II (p < .00). Mini-Mental State Examination, BDI-II, total intracranial volume, and age were included in the general linear model as additional covariates to remove the effects of these parameters. Findings indicate the PD and non-PD groups are well matched demographically, demonstrating expected higher rates of motor abnormalities, but with slightly lower rates of general cognition as well as increased rates of depression, apathy, and state anxiety in the PD group. Table 2 presents data on demographic and disease characteristics of high and low fatigue groups with PD and Table 3 presents high and low fatigue demographic and clinical characteristics for non-PD controls. Patients were well-matched with the exception of high fatigue patients having greater depression (BDI-II 11.14 ± 6.0 vs. 6.32 ± 4.4, p = 0.001). High and low fatigue controls did not differ in terms of demographic, mood or cognitive measures.
Table 1.
Demographic and neuropsychological information for participants.
| Non-Parkinson's disease (n = 41) |
Parkinson's disease (n = 60) |
P-value | |
|---|---|---|---|
| Male:Female | 43:7 | 34:7 | 0.143 |
| Age (years) | 68.29 ± 5.07 | 67.58 ± 5.51 | 0.514 |
| Education (years) | 16.80 ± 2.25 | 16.40 ± 2.81 | 0.444 |
| Sleep Duration | 7.44 ± 0.74⁎ | 6.85 ± 1.25 | 0.005 |
| Levodopa Equivalency | 0.976 ± 6.25⁎⁎ | 573.65 ± 341.12 | 0.001 |
| Unified Parkinson Disease Rating Scale-IIIa | 3.36 ± 3.82 | 18.32 ± 9.73 | <0.001 |
| Disease duration (years) | – | 5.67 ± 5.83 | – |
| Mini-Mental State Examination | 29.22 ± 0.96⁎⁎⁎ | 28.57 ± 1.31 | 0.008 |
| BDI II | 2.98 ± 5.14 | 8.73 ± 5.57 | 0.000 |
| Fatigue Severity Scale | 3.04 ± 1.32 | 3.70 ± 1.39 | 0.019 |
| Leukoariosisb | 4194.45 ± 3768.96 | 3856.14 ± 5022.30 | 0.716 |
| total intracranial volume | 1655418 ± 120427 | 1688759 ± 158329 | 0.257 |
| Leukoariosisb/total intracranial volume | 0.0025 ± 0.0023 | 0.0023 ± 0.0029 | 0.601 |
On medication.
Raw voxel volume.
Sleep duration = self-report average number of hours of sleep nightly (Non-PD n = 40; PD = 58).
Levodopa Equivalency = dosage in non- PD peer due to restless leg syndrome.
PD min = 24; max = 30; non-PD min = 25 max = 30.
Table 2.
Demographic and neuropsychological information for Parkinson's disease High Low Fatigue.
| Parkinson's disease High (n = 29) |
Parkinson's disease Low (n = 31) |
P-value | |
|---|---|---|---|
| Male:Female | 24:5 | 19:12 | 0.065 |
| Age (years) | 67.00 ± 6.02 | 68.10 ± 5.06 | 0.447 |
| Education (years) | 16.10 ± 2.88 | 16.68 ± 2.76 | 0.434 |
| Sleep Duration | 6.96 ± 1.09 | 6.75 ± 1.41 | 0.529 |
| Levodopa Equivalency | 626.63 ± 372.17 | 524.10 ± 307.13 | 0.256 |
| Unified Parkinson Disease Rating Scale-IIIb | 20.67 ± 10.63 | 16.20 ± 8.47 | 0.157 |
| Disease duration (years) | 8.72 ± 5.68 | 6.32 ± 4.94 | 0.085 |
| Mini-Mental State Examinationa | 28.55 ± 1.15 | 28.58 ± 1.46 | 0.933 |
| BDI II | 11.14 ± 5.97 | 6.32 ± 4.40 | 0.001 |
| Fatigue Severity Scale | 43.64 ± 8.60 | 23.77 ± 6.76 | 0.000 |
| Leukoariosis | 2762.56 ± 4004.63 | 4949.72 ± 5730.06 | 0.098 |
| total intracranial volume | 1710059 ± 158,270 | 1668833 ± 15834 | 0.318 |
| Leukoariosis/total intracranial volume | 0.002 ± 0.002 | 0.003 ± 0.003 | 0.072 |
On medication.
Raw voxel volume.
Table 3.
Demographic and neuropsychological information for non-Parkinson's disease High Low Fatigue.
| Non-Parkinson's disease High (n = 19) |
Non-Parkinson's disease Low (n = 22) |
P-value | |
|---|---|---|---|
| Male:Female | 15:4 | 19:3 | 0.529 |
| Age (years) | 68.68 ± 5.97 | 68.05 ± 4.28 | 0.693 |
| Education (years) | 17.05 ± 2.30 | 16.59 ± 2.24 | 0.519 |
| Mini-Mental State Examination* | 29.00 ± 1.20 | 29.41 ± 0.67 | 0.178 |
| BDI II | 4.21 ± 6.97 | 2.09 ± 3.37 | 0.212 |
| Fatigue Severity Scale | 35.79 ± 11.84 | 19.59 ± 6.08 | 0.000 |
| Leukoariosis | 5568.51 ± 4595.59 | 3007.77 | 0.028 |
| total intracranial volume | 1666888 ± 128,851 | 1645513 ± 114767 | 0.577 |
| Leukoariosis/total intracranial volume | 0.003 ± 0.003 | 0.002 ± 0.001 | 0.035 |
3.2. Fatigue severity scale and regions of interest analysis for Parkinson's disease and controls
Data were analyzed using regression models for regions of interest including bilateral caudate, putamen, insula, anterior cingulate cortex, dorsolateral prefrontal cortex, and nucleus accumbens. These regions of interest are schematically visualized in Fig. 1.
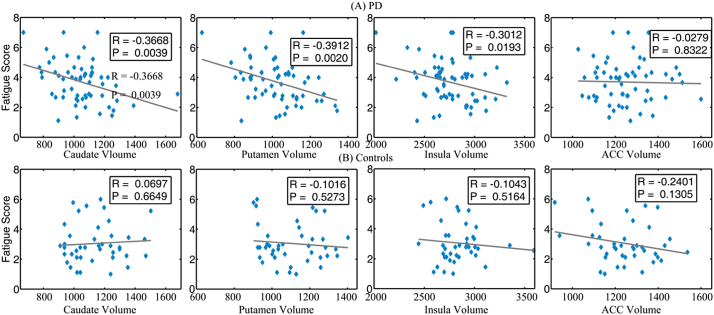
Table 4 and Fig. 2 show coefficients between Fatigue Severity Scale and regions of interest volumes. For PD patients, gray matter volumes in striatal regions (caudate and putamen) and in insula were moderately negatively correlated with the Fatigue Severity Scale (p < 0.05) (Table 4 and Fig. 2A); while for normal controls, gray matter volumes in anterior cingulate cortex and dorsolateral prefrontal cortex suggested an association with the Fatigue Severity Scale at a trend level (p = 0.13 and p = 0.078 respectively) (Table 4 and Fig. 2B). When including total intracranial volume, BDI, age, sleep duration, and Mini-Mental State Examination as covariates, only gray matter volumes in caudate and putamen negatively correlated with Fatigue Severity Scale for PD patients; control analyses were unchanged.
Table 4.
The correlation of Fatigue score and regions of interest volume.
| Sig. P | Parkinson's disease |
Non-Parkinson's disease |
||
|---|---|---|---|---|
| Fatigue Severity Scale_total | Fatigue Severity Scale Controlled total intracranial volume, BDI-II, Age, Mini-Mental State Examination |
Fatigue Severity Scale_total | Fatigue Severity Scale Controlled total intracranial volume, BDI, Age, Mini-Mental State Examination |
|
| Caudate | 0.004⁎⁎ | 0.002⁎⁎ | 0.665 | 0.920 |
| Insula | 0.019⁎ | 0.081 | 0.516 | 0.392 |
| Putamen | 0.002⁎⁎ | 0.004⁎⁎ | 0.527 | 0.534 |
| anterior cingulate cortex | 0.832 | 0.602 | 0.131 | 0.180 |
| Caudal anterior cingulate cortex | 0.842 | 0.462 | 0.302 | 0.405 |
| Nucleus accumbens | 0.338 | 0.904 | 0.986 | 0.659 |
| Dorsolateral prefrontal cortex | 0.973 | 0.739 | 0.078 | 0.052 |
P < 0.05.
P < 0.005.
Fig. 2.
Scatter plot of correlation between Fatigue Severity Scale and volume of different Regions of Interest. (A) Parkinson's disease patients and (B) Non- Parkinson's disease peers.
3.3. High vs. low fatigue participants: gray matter differences
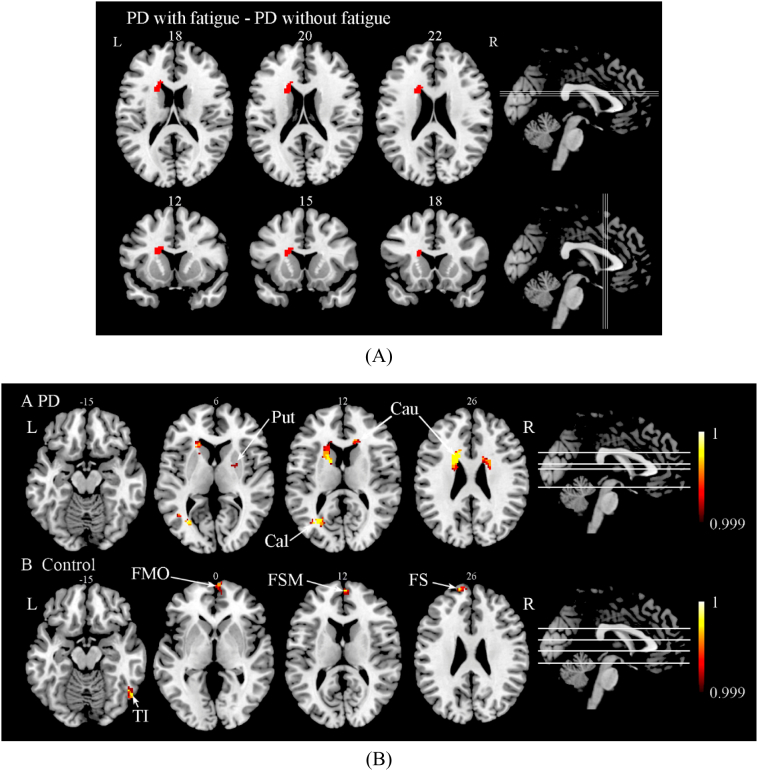
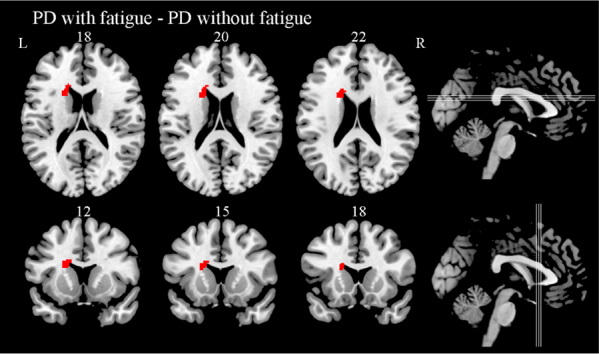
PD with high fatigue (Fatigue Severity Scale ≥ 4, 29 subjects) and low fatigue (Fatigue Severity Scale < 4, 31 subjects) were not statistically different in age, education, sleep duration, or Mini-Mental State Examination. PD with high fatigue had reduced gray matter volume in left caudate relative to PD with low fatigue (p<0.05 False Discovery Rate corrected; Table 5A, Fig. 3A). When relaxing the threshold to uncorrected p < 0.001, a reduction in gray matter volumes in the bilateral caudate, left calcarine, right putamen, and left angular gyrus was found when comparing the fatigue group to the non-fatigue group (Fig. 3B and Table 5B).
Table 5A.
Anatomical location of areas of reduced gray matter in Parkinson's disease patients with fatigue compared with Parkinson's disease patients without fatigue.
| Pcorrected | Cluster (k) | MNI coordinates |
Location | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 0.017 | 97 | −16 | 14 | 22 | L Caudate |
A threshold of P < 0.001 (uncorrected) was used to identify the most significant peaks. Coordinates (x, y, z) refer to standard MNI. Results are listed by cluster size as indicated the number of voxels in a particular cluster. Numbers refer to Brodmann areas (location). L = left; R = right.
Fig. 3.
(A) Areas of reduced gray matter in the analysis of Parkinson's disease with high fatigue compared with Parkinson's disease with low fatigue in Axial and Coronal slices (controlling for total intracranial volume, BDI, Age, Sleep Duration and Mini-Mental State Examination, False Discovery Rate corrected P < 0.05). (B) Areas of reduced gray matter in the analysis of Parkinson disease patients (A) and normal controls (B) with fatigue compared with subjects without fatigue (controlling for total intracranial volume, BDI, Age, Sleep Duration, Mini-Mental State Examination, Uncorrected P < 0.001).
Table 5B.
Anatomical location of areas of reduced gray matter in Parkinson’s disease patients with fatigue compared with Parkinson’s disease patients without fatigue.
| Puncorrected | Cluster (voxels) | MNI coordinates |
Location | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 0.000 | 656 | −14 | 4 | 12 | L Caudate |
| 243 | 12 | 22 | 16 | R Caudate | |
| 170 | −27 | −65 | 11 | L Calcarine 18 | |
| 0.001 | 30 | 24 | −2 | 2 | R Putamen |
A threshold of P < 0.001 (uncorrected) was used to identify the most significant peaks. Coordinates (x, y, z) refer to standard MNI. Results are listed by cluster size as indicated the number of voxels in a particular cluster. Numbers refer to Brodmann areas (location). L = left; R = right.
Non-PD controls with high fatigue score (Fatigue Severity Scale ≥ 2.89, 19 subjects) and low fatigue score (Fatigue Severity Scale ≤ 2.78, 22 subjects) were not statistically different on any demographics. We found no areas where high fatigue controls had more gray matter atrophy than controls with low fatigue score. However as shown in Fig. 3C and Table 5C, there were reduced gray matter density in the inferior temporal gyrus and right superior temporal gyrus on the right side when applying uncorrected (p < 0.001) threshold.
Table 5C.
Anatomical location of areas of reduced gray matter in controls with high fatigue compared with controls with low fatigue.
| Puncorrected | Cluster (voxels) | MNI coordinates |
Location | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 0.000 | 111 | 54 | −64 | −16 | R Temporal_Inf 37 |
| 58 | −14 | 62 | 26 | L Frontal_Sup 10 | |
| 49 | 6 | 66 | 0 | R Frontal_Med_Orb 10 | |
| 31 | 4 | 60 | 12 | R Frontal_Sup_Medial 10 | |
A threshold of P < 0.001 (uncorrected) was used to identify the most significant peaks. Coordinates (x, y, z) refer to standard MNI. Results are listed by cluster size as indicated the number of voxels in a particular cluster. Numbers refer to Brodmann areas (location). L = left; R = right.
3.4. High vs. low fatigue participants: white matter differences
For both PD and non-PD high/low fatigue TBSS analyses there were no areas of significant fractional anisotropy differences. There were no group differences in leukoaraiosis for individuals with PD. In non-PD controls, high fatigue individuals had a greater total volume of leukoariosis than low fatigue individuals (p = 0.035; Table 3).
4. Discussion
Here we report the first study to examine structural correlates of self-reported fatigue in patients with PD and healthy older adults. Using both a priori regions of interest analyses and data-driven approaches we found that PD patients with high fatigue complaints demonstrate atrophy in the dorsal striatum, specifically the caudate. There were no white matter correlates nor any association found with leukoariosis. In contrast, older adults without fatigue experiencing high fatigue demonstrated a higher burden of leukoariosis but had no other specific structural abnormalities. Notably, these findings remained significant even after controlling for total intracranial volume and important potential clinical confounds such as general cognitive function, depression, and sleep that were expectedly more impaired in the PD cohort (but not different between high and low PD patients).
The association of caudate atrophy with fatigue in PD patients is consistent with Chaudhuri and Behan's model of basal ganglia dysfunction in central fatigue which hypothesized that dorsal striatal areas and cortical-subcortical networks contribute to perceptions of fatigue due to disruption of internally generated effort (Chaudhuri and Behan, 2000, Chaudhuri and Behan, 2004). Although the high incidence of fatigue in PD was hypothesized to support this theory, our results provide the first empirical support for this theory in a PD cohort. These data are also consistent with prior lesion-analysis studies in stroke and volumetric studies in multiple sclerosis which similarly show strong associations between self-reported fatigue and caudate damage even when controlling for involvement of other brain areas and clinical factors (Tang et al., 2013; Damasceno et al., 2016).
More recently, several authors have conceptualized fatigue in terms of an ongoing assessment balancing the costs of ongoing activity with the potential awards of that activity (Boksem and Tops, 2008). There is accumulating evidence that alterations in reward processing may underlie fatigue perception in both healthy and clinical populations (Boksem and Tops, 2008; Pardini et al., 2010; Dobryakova et al., 2013). The caudate has been associated with reward motivation in multiple imaging (Aupperle et al., 2015) and animal studies (Yamada et al., 2004), and future studies on the mechanisms and phenomenology of fatigue in PD should investigate associations with both effort and reward. The insula is similarly involved in reward processing and was also identified in our volumetric analyses as associated with fatigue in our PD cohort although it did not survive correction for other confounds. Notably, one prior study of PD fatigue utilizing PET scans found reduced 18F-dopa uptake in the caudate and insula in high versus low fatigue individuals with PD (Pavese et al., 2010) and the insula is suspected to contrbute to many non-motor symptoms (Christopher et al., 2014). Other brain areas associated with fatigue in PD do not clearly fit in this theoretical framework, namely the angular gyrus and left calcarine cortex. Increased angular gyrus functional connectivity was previously reported in patients with chronic fatigue syndrome, suggesting it may play a role in compensating for fatigue (Boissoneault et al., 2016). The calcarine cortex has not been shown to be involved in fatigue in other conditions to our knowledge but may relate to possible associations between cognitive dysfunction and fatigue in the PD population which tend to involve visuospatial areas (Friedman et al., 2016).
Older adults without PD did not demonstrate any overlap in terms of brain areas associated with fatigue compared to PD patients suggesting that one or more aspects of PD pathology rather than aging or other non-specific factors is driving fatigue in this cohort. In our non-PD control group a strong correlation was found between fatigue and total leukoariosis. This association may either reflect a central mechanism for fatigue in this population, possibly disruption of cortical subcortical circuits. It is also possible an artifact of an association between cerebrovascular and cardiovascular disease or possibly even a reflection of reverse causation with more fatigued subjects developing cerebrovascular disease due to reduced activity. Similar to our findings in non-PD older adults, atrophy in right-sided temporal regions has also been reported to be associated with fatigue in traumatic brain injury and disruptions in the right arcuate fasciculus have been associated with chronic fatigue syndrome (Zeineh et al., 2015; Schonberger et al., 2016). This association may due to the role of these right hemisphere structures in attention and arousal (Krall et al., 2015). As our non-PD group may have had fatigue from a more heterogeneous set of causes it is possible that stronger correlations may have been found in this group if patients were specifically selected for certain traits and/or potential secondary causes of fatigue were excluded.
Limitations of the current study include the absence of objective or subjective measures of sleep quality or daytime sleepiness which could confound reports of fatigue and may underlie some fatigue complaints. We also controlled only for general cognitive function (MMSE). Although there were no differences in other cognitive domains between high and low fatigue in this cohort it is possible we may have detected subtle cognitive differences with a larger cohort that could impact fatigue (Kluger et al., 2017). Although there were no significant differences between groups in levodopa or dopamine agonist use, we did not capture use of all medications (e.g. stimulants, antidepressants) which should be examined in future studies.
5. Conclusions
In conclusion, we found fatigue in PD to be associated with a specific pattern of brain atrophy including the caudate and insula and that this association was not seen in age-matched adults with high fatigue but without PD. These results suggest that PD-related fatigue is a neurobiologically distinct syndrome and that further research is merited to better understand its phenomenology and pathophysiology with the goal of developing better treatments for this common and debilitating symptom.
Acknowledgments
Acknowledgements
We are most grateful for the participants and their time. The authors also acknowledge Aisha Stevens for her work with levodopa equivalent dose calculations and Chuck Crew and Cassie Catania for assistance with the sleep data.
Funding
This work was supported by the National Institute for Neurological Disorders and Stroke (C.C.P; K23NS060660, R01NS082386; B.M.K; K02NS080885), the National Institute of Aging (MD & BMK; R21AG044862) and in part by the University of Florida's Center for Movement Disorders and Neurorestoration, as well as the National Institutes of Health/National Center for Advancing Translational Sciences (NIH/NCATS) and Clinical and Translational Science Award to the University of FloridaUL1TR000064.
Contributor Information
Benzi M. Kluger, Email: benzi.kluger@ucdenver.edu.
Catherine Price, Email: cep23@phhp.ufl.edu.
References
- Abdo A.A., Ackermann M., Ajello M., Atwood W.B., Axelsson M., Baldini L. Detection of high-energy gamma-ray emission from the globular cluster 47 Tucanae with Fermi. Science. 2009;325(5942):845–848. doi: 10.1126/science.1177023. [DOI] [PubMed] [Google Scholar]
- Abe K., Takanashi M., Yanagihara T. Fatigue in patients with Parkinson's disease. Behav. Neurol. 2000;12(3):103–106. doi: 10.1155/2000/580683. [DOI] [PubMed] [Google Scholar]
- Abràmoff M.D., Magalhães P.J., Ram S.J. Image processing with ImageJ. Biophoton. Int. 2004;11:36–42. [Google Scholar]
- Abrantes A.M., Friedman J.H., Brown R.A., Strong D.R., Desaulniers J., Ing E. Physical activity and neuropsychiatric symptoms of Parkinson disease. J. Geriatr. Psychiatry Neurol. 2012;25(3):138–145. doi: 10.1177/0891988712455237. [DOI] [PubMed] [Google Scholar]
- Alves G., Wentzel-Larsen T., Larsen J.P. Is fatigue an independent and persistent symptom in patients with Parkinson disease. Neurology. 2004;63(10):1908–1911. doi: 10.1212/01.wnl.0000144277.06917.cc. [DOI] [PubMed] [Google Scholar]
- Aupperle R.L., Melrose A.J., Francisco A., Paulus M.P., Stein M.B. Neural substrates of approach-avoidance conflict decision-making. Hum. Brain Mapp. 2015;36(2):449–462. doi: 10.1002/hbm.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. 2nd ed. Psychological Corp.; Harcourt Brace; San Antonio, Tex. Boston: 1996. BDI-II, Beck Depression Inventory : Manual. [Google Scholar]
- Bohnen N.I., Albin R.L. White matter lesions in Parkinson disease. Nat. Rev. Neurol. 2011;7(4):229–236. doi: 10.1038/nrneurol.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissoneault J., Letzen J., Lai S., O'Shea A., Craggs J., Robinson M.E. Abnormal resting state functional connectivity in patients with chronic fatigue syndrome: an arterial spin-labeling fMRI study. Magn. Reson. Imaging. 2016;34(4):603–608. doi: 10.1016/j.mri.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksem M.A., Tops M. Mental fatigue: costs and benefits. Brain Res. Rev. 2008;59(1):125–139. doi: 10.1016/j.brainresrev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Behan P.O. Fatigue and basal ganglia. J. Neurol. Sci. 2000;179(S 1–2):34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Behan P.O. Fatigue in neurological disorders. Lancet. 2004;363(9413):978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- Christopher L., Koshimori Y., Lang A.E., Criaud M., Strafella A.P. Uncovering the role of the insula in non-motor symptoms of Parkinson's disease. Brain. 2014 Apr 15;137(8):2143–2154. doi: 10.1093/brain/awu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D.L., Neelin P., Peters T.M., Evans A.C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Crowley S., Huang H., Tanner J., Zho Q., Schwab N., Hizel L. Considering total intracranial volume and other nuisance variances in brain voxel based morphometry in idiopathic PD. Brain Imag. Behav. 2018 Feb 1;12(1):1–2. doi: 10.1007/s11682-016-9656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Gomez A.J., Ventura Campos N., Belenguer A., Avila C., Forn C. Regional brain atrophy and functional connectivity changes related to fatigue in multiple sclerosis. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Sereno M.I. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J. Cogn. Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damasceno A., Damasceno B.P., Cendes F. Atrophy of reward-related striatal structures in fatigued MS patients is independent of physical disability. Mult. Scler. 2016;22(6):822–829. doi: 10.1177/1352458515599451. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dobryakova E., DeLuca J., Genova H.M., Wylie G.R. Neural correlates of cognitive fatigue: cortico-striatal circuitry and effort-reward imbalance. J. Int. Neuropsychol. Soc. 2013;19(8):849–853. doi: 10.1017/S1355617713000684. [DOI] [PubMed] [Google Scholar]
- Elbers R.G., van Wegen E.E., Verhoef J., Kwakkel G. Impact of fatigue on health-related quality of life in patients with Parkinson's disease: a prospective study. Clin. Rehabil. 2014;28(3):300–311. doi: 10.1177/0269215513503355. [DOI] [PubMed] [Google Scholar]
- Fahn S. Macmillan; New York: 1987. MotUDC. Unified Parkinson's Disease Rating Scale. [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Liu A., Dale A.M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., van der Kouwe A.J., Makris N., Segonne F., Quinn B.T. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl. 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Friedman J.H., Brown R.G., Comella C., Garber C.E., Krupp L.B., Lou J.S. Fatigue in Parkinson's disease: a review. Mov. Disord. 2007;22(3):297–308. doi: 10.1002/mds.21240. [DOI] [PubMed] [Google Scholar]
- Friedman J.H., Brown R.G., Comella C., Garber C.E., Krupp L.B., Lou J.S. Fatigue in Parkinson's disease: a review. Mov. Disord. 2007;22(3):297–308. doi: 10.1002/mds.21240. [DOI] [PubMed] [Google Scholar]
- Friedman J.H., Alves G., Hagell P., Marinus J., Marsh L., Martinez-Martin P. Fatigue rating scales critique and recommendations by the Movement Disorders Society task force on rating scales for Parkinson's disease. Mov. Disord. 2010;25(7):805–822. doi: 10.1002/mds.22989. [DOI] [PubMed] [Google Scholar]
- Friedman J.H., Beck J.C., Chou K.L., Clark G., Fagundes C.P., Goetz C.G. Fatigue in Parkinson's disease: report from a multidisciplinary symposium. NPJ Parkinson's Dis. 2016;2016(2):15025. doi: 10.1038/npjparkd.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama R.L., Bruin V.M., Tavora D.G., Duran F.L., Bittencourt L., Tufik S. Structural brain abnormalities in patients with Parkinson's disease with visual hallucinations: a comparative voxel-based analysis. Brain Cogn. 2014;87:97–103. doi: 10.1016/j.bandc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Grace J., Mendelsohn A., Friedman J.H. A comparison of fatigue measures in Parkinson's disease. Parkinsonism Relat. Disord. 2007;13(7):443–445. doi: 10.1016/j.parkreldis.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Heldmann M., Berding G., Voges J., Bogerts B., Galazky I., Muller U. Deep brain stimulation of nucleus accumbens region in alcoholism affects reward processing. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Huang P., Xu X., Gu Q., Xuan M., Yu X., Luo W. Disrupted white matter integrity in depressed versus non-depressed Parkinson's disease patients: a tract-based spatial statistics study. J. Neurol. Sci. 2014;346(1–2):145–148. doi: 10.1016/j.jns.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Hughes A.J., Ben-Shlomo Y., Daniel S.E., Lees A.J. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;42(6):1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- Jones J., Tanner J.J., Okun M., Price C.C., Bowers D. Are Parkinson's patients more vulnerable to the effects of cardiovascular risk: a neuroimaging and neuropsychological study. J. Int. Neuropsychol. Soc. 2017 Apr;23(4):322–331. doi: 10.1017/S1355617717000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J., Czanner S., Greve D., Haley E., van der Kouwe A., Gollub R. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kluger B.M., Krupp L.B., Enoka R.M. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80(4):409–416. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger B.M., Brown R.P., Aerts S., Schenkman M. Determinants of objectively measured physical functional performance in early to mid-stage Parkinson disease. PM&R: J. Inj, funct. Rehabil. 2014;6(11):992–998. doi: 10.1016/j.pmrj.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger B.M., Pedersen K.F., Tysnes O.B., Ongre S.O., Øygarden B., Herlofson K. Is fatigue associated with cognitive dysfunction in early Parkinson's disease? Parkinsonism Relat. Disord. 2017 Apr 1;37:87–91. doi: 10.1016/j.parkreldis.2017.02.005. [DOI] [PubMed] [Google Scholar]
- Kostic V.S., Filippi M. Neuroanatomical correlates of depression and apathy in Parkinson's disease: magnetic resonance imaging studies. J. Neurol. Sci. 2011;310(1–2):61–63. doi: 10.1016/j.jns.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Krall S.C., Rottschy C., Oberwelland E., Bzdok D., Fox P.T., Eickhoff S.B. The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Struct. Funct. 2015;220(2):587–604. doi: 10.1007/s00429-014-0803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp L.B., LaRocca N.G., Muir-Nash J., Steinberg A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Mechelli A., Price C.J., Friston K.J., Ashburner J. Voxel-based morphometric of the human brain: methods and applications. Curr. Med. Imag. Rev. 2005;1(2):105–113. [Google Scholar]
- Minton O., Richardson A., Sharpe M., Hotopf M., Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J. Natl. Cancer Inst. 2008;100(16):1155–1166. doi: 10.1093/jnci/djn250. [DOI] [PubMed] [Google Scholar]
- Pardini M., Krueger F., Raymont V., Grafman J. Ventromedial prefrontal cortex modulates fatigue after penetrating traumatic brain injury. Neurology. 2010;74(9):749–754. doi: 10.1212/WNL.0b013e3181d25b6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese N., Metta V., Bose S.K., Chaudhuri K.R., Brooks D.J. Fatigue in Parkinson's disease is linked to striatal and limbic serotonergic dysfunction. Brain. 2010;133(11):3434–3443. doi: 10.1093/brain/awq268. [DOI] [PubMed] [Google Scholar]
- Price C.C., Tanner J., Nguyen P.T., Schwab N.A., Mitchell S., Slonena E. Gray and white matter contributions to cognitive frontostriatal deficits in non-demented Parkinson's disease. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Parisi L., Pagani E., Copetti M., Rodegher M., Colombo B. Regional but not global brain damage contributes to fatigue in multiple sclerosis. Radiology. 2014;273(2):511–520. doi: 10.1148/radiol.14140417. [DOI] [PubMed] [Google Scholar]
- Schonberger M., Reutens D., Beare R., O'Sullivan R., Rajaratnam S.M., Ponsford J. Brain lesion correlates of fatigue in individuals with traumatic brain injury. Neuropsychol. Rehabil. 2016:1–15. doi: 10.1080/09602011.2016.1154875. [DOI] [PubMed] [Google Scholar]
- Schwab N.A., Tanner J.J., Nguyen P.T., Schmalfuss I.M., Bowers D., Okun M. Proof of principle: transformation approach alters caudate nucleus volume and structure-function associations. Brain Imag. Behav. 2015;9(4):744–753. doi: 10.1007/s11682-014-9332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F., Pacheco J., Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging. 2007;26(4):518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1) doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Stocchi F., Abbruzzese G., Ceravolo R., Cortelli P., D'Amelio M., De Pandis M.F. Prevalence of fatigue in Parkinson disease and its clinical correlates. Neurology. 2014;83(3):215–220. doi: 10.1212/WNL.0000000000000587. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme Medical Publishers; New York: 1988. Co-Planar Steorotaxic Atlas of the Human Brain: Three Dimensional Proportional System - an Approach to Cerebral Imaging. [Google Scholar]
- Tanabe J., Thompson L., Claus E., Dalwani M., Hutchison K., Banich M.T. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Hum. Brain Mapp. 2007;28(12):1276–1286. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.K., Liang H.J., Chen Y.K., Chu W.C., Abrigo J., Mok V.C. Poststroke fatigue is associated with caudate infarcts. J. Neurol. Sci. 2013;324(1–2):131–135. doi: 10.1016/j.jns.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Tanner J.J., Mareci T.H., Okun M.S., Bowers D., Libon D.J., Price C.C. Temporal lobe and frontal-subcortical dissociations in non-demented Parkinson's disease with verbal memory impairment. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J.J., Levy S.A., Schwab N.A., Hizel L.P., Nguyen P.T., Okun M.S. Marked brain asymmetry with intact cognitive functioning in idiopathic Parkinson's disease: a longitudinal analysis. Clin. Neuropsychol. 2016:1–22. doi: 10.1080/13854046.2016.1251973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Valko P.O., Waldvogel D., Weller M., Bassetti C.L., Held U., Baumann C.R. Fatigue and excessive daytime sleepiness in idiopathic Parkinson's disease differently correlate with motor symptoms, depression and dopaminergic treatment. Eur. J. Neurol. 2010;17(12):1428–1436. doi: 10.1111/j.1468-1331.2010.03063.x. [DOI] [PubMed] [Google Scholar]
- van Hilten J.J., Weggeman M., van der Velde E.A., Kerkhof G.A., van Dijk J.G., Roos R.A. Sleep, excessive daytime sleepiness and fatigue in Parkinson's disease. J. Neural Transm. Park. Dis. Dement. Sect. 1993;5(3):235–244. doi: 10.1007/BF02257678. [DOI] [PubMed] [Google Scholar]
- Yamada H., Matsumoto N., Kimura M. Tonically active neurons in the primate caudate nucleus and putamen differentially encode instructed motivational outcomes of action. J. Neurosci. 2004;24(14):3500–3510. doi: 10.1523/JNEUROSCI.0068-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh M.M., Kang J., Atlas S.W., Raman M.M., Reiss A.L., Norris J.L. Right arcuate fasciculus abnormality in chronic fatigue syndrome. Radiology. 2015;274(2):517–526. doi: 10.1148/radiol.14141079. [DOI] [PubMed] [Google Scholar]
- Zijdenbos A.P., Dawant B.M., Margolin R.A., Palmer A.C. Morphometric analysis of white matter lesions in MR images: method and validation. IEEE Trans. Med. Imaging. 1994;13(4):716–724. doi: 10.1109/42.363096. [DOI] [PubMed] [Google Scholar]