Abstract
A mosquito needs to ingest at least one male and one female gametocyte to become infected with malaria. The sex of Plasmodium falciparum gametocytes can be determined microscopically but recent transcriptomics studies paved the way for the development of molecular methods that allow sex-ratio assessments at much lower gametocyte densities. These sex-specific gametocyte diagnostics were recently used to examine gametocyte dynamics in controlled and natural infections as well as the impact of different antimalarial drugs. It is currently unclear to what extent sex-specific gametocyte diagnostics obviate the need for mosquito feeding assays to formally assess transmission potential. Here, we review recent and historic assessments of gametocyte sex ratio in relation to host and parasite characteristics, treatment, and transmission potential.
Keywords: gametocytes, sex ratio, transmission, Plasmodium falciparum, diagnosis, commitment to gametocytes
Highlights
Recent RNA sequencing studies have uncovered a number of P. falciparum gametocyte sex-specific targets and provided new insights in gametocyte biology.
After decades when gametocyte sex-ratio research was restricted to nonhuman malarias or in vitro experiments, molecular tools for assessing gametocyte sex ratio are now increasingly available for use in natural P. falciparum infections.
Evidence that gametocyte sex ratio is influenced by total gametocyte density and antimalarial treatment, and improves predictions of transmission potential, highlight the relevance of understanding the gametocyte sex ratio during natural infections.
The finding that the most widely used P. falciparum gametocyte marker Pfs25 is expressed predominantly by female gametocytes and has non-negligible levels of background expression in asexual parasites necessitates a re-evaluation of existing gametocyte data.
Sexual Commitment and First Appearance of Male and Female P. falciparum Gametocytes
The transmission of malaria from human to mosquito depends on the presence of gametocytes (see Glossary), sexual-stage Plasmodium parasites, in the peripheral blood. The development of gametocytes of P. falciparum takes 8–12 days and it involves transitions from committed rings, via early and intermediate stage gametocytes that are sequestered in the bone marrow and spleen (stages I–IV) 1, 2, 3, to mature male and female stage V gametocytes [4]. In other Plasmodium species, such as P. vivax, P. chabaudi, P. vinckei, and P. gallinaceum, gametocytes develop much more rapidly, typically within 3 days [5]. In controlled human malaria infections (CHMIs) less than 10% of all asexual P. falciparum parasites commit to form gametocytes 6, 7, and mature gametocytes typically comprise less than 5% of the circulating parasite biomass in natural infections [8]. Sexual commitment happens before the stage of schizogony 9, 10, 11, 12, and all merozoites derived from one schizont develop into either microgametocytes (males) or macrogametocytes (females) 13, 14. The lack of sex chromosomes in haploid Plasmodium hampers our understanding of the commitment to sexual differentiation and the timing of sex determination [15]. Sex determination could occur at the same moment when commitment to sexual differentiation is determined [13], or alternatively via a two-stage process in which sex determination happens after the decision on commitment to sexual differentiation. At the molecular level, the nuclear protein P. falciparum gametocyte development 1 (GDV1) triggers the first known part of the molecular cascade of commitment and acts as the upstream regulator of the DNA-binding protein AP2-G (PF3D7_1222600), by antagonizing heterochromatin protein 1 repression of AP2-G transcription [16]. Sufficient activation of AP2-G, the master transcriptional regulator of gametocytogenesis, represents the ‘point of no return’ in commitment to both male and female gametocytes 11, 17, 18. The molecular basis of sex determination, and its timing during development, is currently unknown.
Here, we summarize historic and recent estimates of gametocyte sex ratio in natural infections and the limitations of older estimates that relied on microscopy. We present the merits and limitations of molecular tools for quantifying gametocyte sex ratio and review evidence for a sex-specific effect of antimalarial drugs on circulating gametocytes and the implications for malaria transmission potential.
Microscopy Is an Imperfect Tool for Quantifying Gametocyte Sex Ratio
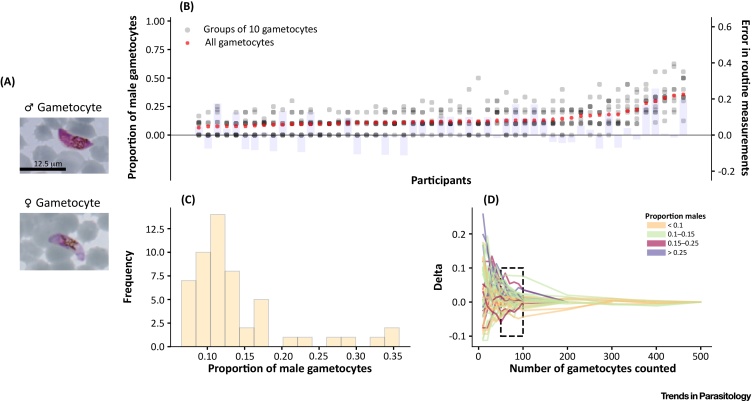
In natural infections of P. falciparum, the sex ratio of circulating gametocytes is mostly female biased [19]. Only a few epidemiological studies have quantified female and male gametocytes separately with sex ratios ranging from a mean of ∼three to five females to one male (Table 1) 20, 21, 22, 23. Previously reported sex ratios are based mainly on microscopic examination of Giemsa-stained thick or thin blood smears, relying on subtle morphological differences between mature (stage V) macrogametocytes and microgametocytes [24] (Figure 1). Gametocyte sex determination by microscopy is challenging due to the typically low gametocyte densities and the difficulty of allocating a sex to the sparsely observed gametocytes 22, 25, 26, 27, 28. As an illustration of their sparseness, in a recent detailed study on gametocyte carriage in Burkina Faso, only 20% (95% confidence interval [CI] 14–27%) of patent asexual parasite carriers had microscopically detected gametocytes, and only 7% (95% CI 4–12%) had more than two gametocytes observed whilst enumerating against a conventional number of 500 white blood cells (∼0.06 μl of blood microscopically screened) [29]. The implications of low observed gametocyte counts for sex ratio precision are presented in Figure 1, indicating that observations based on 500 white blood cells can rarely lead to accurate gametocyte sex ratio estimates. Only by observing 50–100 gametocytes can a reproducible sex ratio be calculated (Figure 1D) 8, 19, and this is hardly ever attainable by routine microscopy. Microscopic investigation of gametocyte sex can be improved by concentrating gametocytes with magnetic enrichment [30], assuming it enriches male and female gametocytes equally, allowing more gametocytes to be examined. The accuracy of differentiating male and female gametocytes can be further improved by targeting proteins or transcripts that are preferentially expressed in a specific sex using immunofluorescence or probe-based hybridization assays, respectively. Assays that target stage-specific expression of transcripts, such as Pf77 (PF3D7_0621400) and Pfg377 (PF3D7_1250100), both enriched in female gametocytes 31, 32 and Pfg27 (PF3D7_1302100, detecting both sexes) 33, 34 have been reported previously. Immunofluorescence assays based on antibodies that bind proteins specific for early gametocytes such as Pfs16 (PF3D7_0406200) 14, 35, male gametocytes (α-tubulin II, PF3D7_0422300) 13, 14, 36, or female gametocytes (Pfg377, PF3D7_1250100) [13] have also been used. Insights into sex differences in the transcriptional and proteomic makeup are summarized in Box 1. These immunofluorescent assays alleviate common problems that complicate microscopic examination but their reliance on fluorescence microscopy greatly affects their deployment in the field. The recent development of molecular assays to quantify male and female gametocytes may allow more robust sex ratio determination at low gametocyte densities and easier application in field settings.
Table 1.
Summary of Studies That Evaluated Sex Ratio in Natural Infections
| Setting | Population | Proportion malea | Tool | Refs |
|---|---|---|---|---|
| Mali, Burkina Faso, Cameroon | Asymptomatic gametocyte carriers | 0.14–0.51 | qRT-PCR | [23] |
| The Netherlands | Controlled human malaria infection volunteers | 0.29 | qRT-PCR | [7] |
| Mali | Asymptomatic gametocyte carriers | 0.30 | qRT-PCR | [62] |
| Australia | Controlled human malaria infection volunteers | 0.20 | qRT-PCR | [6] |
| Kenya, Mali | Asymptomatic gametocyte carriers | 0.36 | qRT-PCR | [34] |
| Nigeria | Symptomatic malaria patients | 0.21 | Microscopy | [75] |
| Nigeria | Symptomatic children | 0.34 | Microscopy | [87] |
| Nigeria | Symptomatic malaria patients | 0.22 | Microscopy | [88] |
| Nigeria | Symptomatic malaria patients | 0.75 | Microscopy | [76] |
| India | Symptomatic malaria patients | 0.31 | Microscopy | [89] |
| Nigeria | Symptomatic malaria patients | 0.20 | Microscopy | [78] |
| Nigeria | Symptomatic malaria patients | 0.05 | Microscopy | [90] |
| Nigeria | Symptomatic malaria patients | 0.14 | Microscopy | [91] |
| Senegal | Symptomatic children | 0.15 | Microscopy | [92] |
| Senegal | Total population | 0.35 | Microscopy | [93] |
| Nigeria | Asymptomatic children | 0.42 | Microscopy | [77] |
| Tanzania | Not applicableb | 0.34 | Indirect: calculated from inbreeding coefficient | [94] |
| The Gambia | Not applicablec | 0.22 | Indirect: calculated from inbreeding coefficient | [94] |
| Sudan | Not applicabled | 0.07 | Indirect: calculated from inbreeding coefficient | [94] |
| Cameroon | Symptomatic malaria patients | 0.22 | Microscopy | [22] |
| Papua New Guinea | Not applicableb | 0.04 | Indirect: calculated from inbreeding coefficient | [95] |
| Papua New Guinea | Not indicated | 0.18 | Microscopy | [96] |
Proportion male is defined as the proportion of all gametocytes that is male, [male gametocytes/(male + female gametocytes)].
Inbreeding coefficients calculated directly from oocyst selfing rates from mosquitoes.
Inbreeding coefficients calculated from allele frequencies in blood-stage infections, but sex ratios not determined in these patients. Patients were symptomatic children.
Inbreeding coefficients calculated from allele frequencies in blood-stage infections, but sex ratios not determined in these patients. Patients were symptomatic children and adults.
Figure 1.
Intensive Microscopy-Based Quantification of Gametocyte Sex Ratio. In this figure, we use data generated by one of the authors (C.D.) in 1992–1993 to illustrate the likely error in gametocyte sex ratio estimation when determining the sex of only a few gametocytes per sample as routinely done in epidemiological studies. Briefly, 100–500 gametocytes were sexed per blood smear in 53 samples from 43 individuals living in malaria-endemic regions in The Gambia, and sex-specific counts were recorded in groups of 10 gametocytes. Light microscopy images of male and female gametocytes stained with Giemsa’s stain are shown in (A) for illustration. Female gametocytes are blue/violet (as opposed to pink males), more crescent-shaped, and have more compact nuclei and more centrally located pigment. In (B), x coordinates represent data from different samples, partially transparent to allow visualization of overlapping data points: red crosses (left y axis) correspond to the proportions of gametocytes identified as male-based on data from groups of 10 gametocytes or the total number of gametocytes, respectively; light blue bars represent the difference (right y axis) between the proportion of male gametocytes when considering only the first 10 gametocytes observed in each smear versus the proportion calculated based on the total number of gametocytes. This difference exemplifies the error that might occur in routine measurements that quantify only a limited number of gametocytes. In (C), the distribution of the proportion of male gametocytes in the different thick smears analyzed, based on the total number of gametocytes, is shown. Panel (D) presents the progressive reduction in error as the number of gametocytes counted increases. In this panel, the x axis corresponds to the cumulative number of gametocytes sexed, and the y axis corresponds to the difference in the proportion of male gametocytes relative to the same proportion when estimated based on all gametocytes observed in the smear. Each line represents a different sample, and colors relate to the overall proportion of male gametocytes in the smear. The rectangle delimited by the dashed lines encloses error values between −0.1 and 0.1 when 50–100 gametocytes were counted. Only data from thick smears were used in this figure.
Box 1. The Development of Male and Female Gametocytes.
Molecular mechanisms underlying the differentiation switch towards becoming male or female gametocytes remain largely unknown. Candidate genes that may be associated with sex-specific differentiation include the AP2-G2 in P. berghei [18], the Puf family of translational repressors in P. falciparum 97, 98, and MAPK1 and MAPK2 as potential regulators of female and male gametocytogenesis in P. falciparum, respectively [63].
Male gametocytes are terminally differentiated forms with one last task ahead; producing motile microgametes upon activation. Proteomic and transcriptomic data reflect this, showing upregulation of genes involved in nucleic acid metabolism, DNA replication, and axoneme formation. Female gametocytes, on the other hand, contain many proteins and transcripts intended for longer term sustainment of postfertilization stages, including genes involved in protein biosynthesis, degradation, transport, and metabolic activity. Another important female gametocyte-specific mechanism is translational repression in which stored transcripts support cellular processes immediately after fertilization, when transcription is absent for multiple hours 99, 100. The deletion of genes involved in the maintenance of translationally repressed mRNA transcripts, such as DOZI [101] and CITH [102] in P. berghei, results in full developmental arrest during ookinete formation, although fertilization is successful. The first indication that translational repression is also active in P. falciparum was obtained through deletion of Puf2, which resulted in altered expression of genes known to be translationally repressed, such as Pfs25 and Pfs28 103, 104. More recent proteomics and transcriptomics data have uncovered a large-scale translational repression of hundreds of transcripts in P. falciparum female gametocytes [41].
Alt-text: Box 1
New Molecular Techniques Improve Sex Ratio Quantification
Gametocyte detection has been improved in the last decade with the introduction and wider use of molecular detection tools. Baker et al. reported the first molecular assay for an indirect detection of gametocyte sexes based on in situ hybridization using a Pf77 RNA probe that targeted female gametocytes [31]. Assays were subsequently developed targeting the Pfs25 (PF3D7_1031000) transcript, which was considered highly specific for mature gametocytes, using qRT-PCR 37, 38 or real-time quantitative nucleic acid sequence-based amplification (QT-NASBA) [39]. Whilst highly sensitive, with an estimated sensitivity around 0.1 gametocytes per μl in 50–100 μl blood samples, this marker (Pfs25) has recently been shown to be expressed predominantly by female gametocytes 40, 41. Drew and Reece reported the first molecular approach to gametocyte sex ratio determination [28] in the rodent malaria parasite Plasmodium chabaudi using targets that were previously described in a proteomics study in Plasmodium berghei [42], based on the quantification of total gametocytes by common gametocyte gene 1 (CG1 or PSOP1: PCHAS_0620900) RNA, and male gametocytes by the male gametocyte-specific gene 1 (MG1, a putative dynein heavy chain: PCHAS_0417000). Schneider and colleagues [40] reported the first qRT-PCR assays that quantify female and male gametocytes in P. falciparum through quantification of a combination of female-enriched (Pfs25) and male-enriched (Pfs230p) transcripts. The higher limit of detection for male (1.8 male gametocytes/μl) compared to female gametocytes (0.3 female gametocytes/μl) in this assay, and the typical female bias in natural infections, may affect the sensitivity of sex ratio determination at low gametocyte densities. Recently, additional male gametocyte-enriched transcripts (Pf13, PF3D7_1311100 and PfMGET, PF3D7_1469900) were identified that improved the detection limit of male gametocytes to the level comparable with female gametocytes 34, 43. Details of all currently used transcripts for quantifying male and female gametocytes are provided in Box 2. Of note, none of the gametocyte markers are exclusively expressed in gametocytes of one sex 34, 43, and low levels of ‘gametocyte transcripts’ are also detectable in asexual-stage parasites [44]. The latter has implications for earlier statements on the high prevalence of gametocytes in P. falciparum-infected individuals. Whilst it remains plausible that (nearly) all infections produce gametocytes 6, 7, the initial wave of high asexual parasitemia that is observed in clinical malaria cases may coincide with false-positive gametocyte signals. This may result in an overestimation of gametocyte prevalence at the moment of sampling, in particular in clinical malaria cases [19].
Table I.
Gametocyte- and Sex-Specific Transcripts Currently Used in Molecular Detection Methods
| Gene ID name | Limit of detection | Description/putative function | FG:MG ratioa | Remarks | Refs |
|---|---|---|---|---|---|
| PF3D7_1031000 Pfs25 |
0.01 FG/μl | Zygote and ookinete surface protein, necessary for infectivity, transmission-blocking vaccine candidate | 35.58 | 37, 112 | |
| PF3D7_0630000 No alias |
0.3 gametocytes/μl | CPW-WPC family protein | 47.83 | Contains nine introns | [111] |
| PF3D7_1351600 PfGK |
0.3 FG/μl | Glycerol kinase | 41.41 | [43] | |
| PF3D7_0903800 CCp4 |
0.1 FG/μl | LCCL domain-containing protein, sexual-stage adhesion | 38.49 | Contains one intron | [44] |
| PF3D7_0208900 Pfs230p |
1.8 MG/μl | Gamete surface protein, knock out has no effect on fertilization | 0.02 | [40] | |
| PF3D7_1469900 PfMGET |
0.01 MG/μl | Conserved Plasmodium protein, unknown function | 0.02 | Contains two introns | [34] |
| PF3D7_1311100 Pf13 |
22 MG/μl | Meiosis-specific nuclear structural protein | 0.005 | [43] | |
| PF3D7_1319800 Pfg17 |
0.1 gametocytes/μl | Conserved Plasmodium protein, unknown function | 0.60 | Total gametocyte marker | [113] |
Ratio of transcript expression as detected by RNAseq in sex-sorted gametocytes, expressed as FPKM (fragments per kilobase million) values in female over male gametocytes [41].
Box 2. Marker Transcripts for Molecular Detection of Male and Female Gametocytes.
Mature male and female gametocytes can be distinguished on the basis of light microscopy on Giemsa-stained thin smears [24], by electron microscopy 10, 105, by an immunofluorescence assay based on female- or male-enriched proteins and monoclonal antibodies 14, 35, and by sex-enriched transcripts 31, 32. Sex is not determined chromosomally, since haploid clone lines from a single haploid parasite can generate both male and female gametocytes 16, 106. Gametocyte identification is thus solely based on quantification of stage-specific expression of transcripts.
Suitable targets for gametocyte diagnostics require high and stable expression of the gene in the stage and sex of interest. In Table I, the characteristics of the most important targets for gametocyte detection and quantification are summarized. Putative gametocyte sex markers rely on abundant transcript expression at the gametocyte stage and not in the asexual blood stage, with expression at other life stages being irrelevant. Importantly, Pfs25 expression in asexual blood-stage parasites is not completely absent 43, 44, 107 but approximately 100 000-fold reduced. This estimate is similar for other reported markers [44], and hence low gametocyte transcript numbers may be derived from asexual parasites. Gametocyte prevalence thus needs to be interpreted with caution, especially at higher parasite burdens (above 1000 parasites/μl). The high rate of expression of some male markers (Pf13 and Pf230p) [43] in mature trophozoites raises questions on the utility of these transcripts as differential sex-specific markers. During the dynamics of sequestration in the bone marrow and release into the peripheral blood, transcripts derived from these stages may be detected and could affect sex-specific quantification.
Differential P. falciparum [41] and P. berghei [108] gene expression studies per sex are resources for identification of potential candidates and may be particularly informative when integrated with asexual expression levels [109]. These studies have uncovered an increasing number of ‘sex-specific transcripts’ (Table I) that are, however, not sex-specific in the strict sense but rather sex-enriched. The commonly used (female) gametocyte marker Pfs25 shows lower expression in male gametocytes compared to females, ranging from 35-fold lower as detected by RNAseq [41] to 200-fold lower using qRT-PCR [44]. Of note, spurious transcript expression in asexual parasites cannot be linked to (functional) protein expression, and so the biological relevance of transcripts of ‘gametocyte genes’ in asexual parasites remains unknown. For any mRNA target, qRT-PCR specificity can be enhanced if an intron-spanning region is targeted, as this ensures amplification of DNA derived from transcripts only, rather than genomic DNA, and avoids the need for DNase treatment that may decrease sensitivity of the assay through RNA damage [110]. The reliability of sex ratio determination can be further enhanced by multiplexing targets with the above features in one assay. For example, a multiplexed qRT-PCR that targets female gametocyte-specific (PF3D7_0630000) and total parasite (18S rRNA) transcripts, with no DNase treatment step, was introduced recently [111]. Similarly, a multiplex assay for male and female gametocytes with intron-spanning primers for both male (PfMGET) and female (CCp4) targets was recently proposed as a more scalable and robust approach to molecular gametocyte sex ratio assessments.
Alt-text: Box 2
Sex Ratio Plasticity in Response to a Changing Environment
Recently, molecular methods to accurately sex gametocytes have been used to assess the first appearance of mature male and female gametocytes after experimental infections. Gametocyte appearance is estimated to occur 10 days post blood-stage inoculum [6] or 8.5–12 days after the first detection of asexual parasites in the peripheral blood [7], supporting previous suggestions of gametocyte commitment during the first erythrocytic cycle of asexual parasites 45, 46. During infections, malaria parasites may respond to environmental cues to alter their investment into the transmission stages (reviewed in [19]). In addition to an overall increased investment in gametocytes 47, 48, malaria parasites may adjust their sex ratio to maximize the transmission success when under stress, such as that induced by immunity developing as the infection progresses. In an early observation of an individual deliberately infected with P. falciparum, the sex ratio changed from female biased to equal sexes over 13 days [49].
Data from the avian parasite Plasmodium gallinaceum and rodent malaria Plasmodium vinckei further suggest that the sex ratio in Plasmodium infections varies substantially during the course of an infection 50, 51, possibly in response to the host’s immune response, anemia, and gametocyte density 50, 52, 53. A change in concentration of erythropoietin, a hormone that controls erythrocyte production, causes an increase in the proportion of male gametocytes in P. vinckei and increases overall gametocyte density in P. gallinaceum [54] and P. chabaudi [55].
Competition between parasites of different genotype can also affect sex ratio. Hamilton’s theory of ‘local mate competition’ (LMC) [56] states that female-biased sex ratios are optimal when genetically-related males compete for mates; the optimal sex ratio for malaria parasites depends on the rate of self-fertilization (inbreeding rate), where sex ratio (here: proportion of male gametocytes) = (1 − f)/2, and f is Wright’s inbreeding coefficient [57]. When individuals are infected with a single or a low number of distinct parasite genotypes, inbreeding will be high and a female-biased sex ratio is predicted. As inbreeding levels fall with increased diversity of parasite genotypes within the infection, the optimal sex ratio will approach 0.5. In an experimental test of this theory, using the rodent malaria P. chabaudi where genetic diversity was adjusted deliberately, sex ratio became more male-biased as the number of genotypes present increased [52].
The effect of sex ratio on transmission success may depend on total gametocyte density [23]. In low-density infections, a larger investment in male gametocytes is favorable to increase the chance that all females are fertilized 58, 59. As male gametocytes can produce up to eight microgametes upon activation, female-biased gametocyte sex ratios contribute to a more balanced number of macro- and microgametes in the mosquito midgut [51]. At high gametocyte densities, a male-biased sex ratio leads to less efficient transmission [60], favoring a less male-biased ratio. Recently, molecular gametocyte sex ratio assays supported the density-dependency of sex ratio with a higher proportion of male gametocytes in low-density infections [23]. The same study also suggested that quantifying male and female gametocytes allowed a better prediction of mosquito infection rates as compared to previous estimates that quantified female gametocytes only [61], and that the number of male gametocytes may become a limiting factor in determining transmission success at low densities. These findings require replication, but potentially have important implications for gametocyte diagnostics. If male gametocytes are indeed a limiting factor for transmission, diagnostics that quantify male gametocytes might be better indicators of transmission potential than female gametocyte diagnostics that are currently most widely used.
Differences in the longevity of male and female gametocytes can also contribute to sex ratio differences. Two studies have also hinted towards a shorter circulation time of male gametocytes upon clearance of the asexual progenitors 7, 62, although a third study observed identical circulation times [62]. An early study on induced P. vivax malaria also reported a shorter lifespan of male P. vivax gametocytes [49]. The different circulation time estimates may be influenced by differences in the ability to detect male and female gametocytes (including a lower sensitivity of male markers 6, 34) but are potentially highly important to understand the duration of infectiousness of gametocyte carriers. There is currently no published evidence on differences between male and female gametocytes in maturation or longevity in vitro. Longitudinal studies are needed to understand the dynamics of male and female gametocyte densities during natural infections and their impact on transmissibility.
If Plasmodium can indeed alter sex ratio in response to environmental cues, an essential outstanding question is what governs sex allocation. Candidate genes that may be associated with sex-specific regulation (other than or in addition to AP2-G) include MAPK1 (PF3D7_1431500) as potential regulator of female gametocytogenesis, and MAPK2 (PF3D7_1113900) as possible regulator of male gametocytogenesis [63]. Given the 10–12 day maturation time in P. falciparum infections and early commitment to the sexual stage (and plausibly gametocyte sex), any response to environmental triggers must involve significant ‘planning ahead' and cross-talk between potentially very low numbers of gametocytes or precursors. Even if environmental signals are as rapidly translated into elevated gametocyte conversion rates as recently shown for P. chabaudi [64], it will only have an effect after full maturation. One speculative option that would allow P. falciparum to rapidly respond to environmental triggers is the bone marrow reservoir for gametocytes. It is conceivable that mature gametocytes may not all be immediately released upon completion of maturation but may be in part retained for release upon environmental stimuli.
Our understanding of environmental factors that influence gametocyte sex ratio is incomplete. Moreover, some of the ‘known’ stimuli for overall investment in gametocyte production appear uncertain when assessed with current methodologies [65] and thus require re-examination. Useful markers to examine stimuli for gametocyte production and sex allocation include parasite AP2-G transcripts 11, 17, 18, the host factor lysophosphatidylcholine [65] and molecular markers to distinguish early gametocytes (GEXP5, PF3D7_0936600 66, 67), immature gametocytes (Pfs16, PF14_0748) [35], and mature gametocytes of different sexes (Table 1) 68, 69, 70. Potential stimuli include anemia, antimalarial treatment, host immunity, and coinfections with other Plasmodium parasites or clones [19].
Antimalarials Influence Gametocyte Sex Ratio
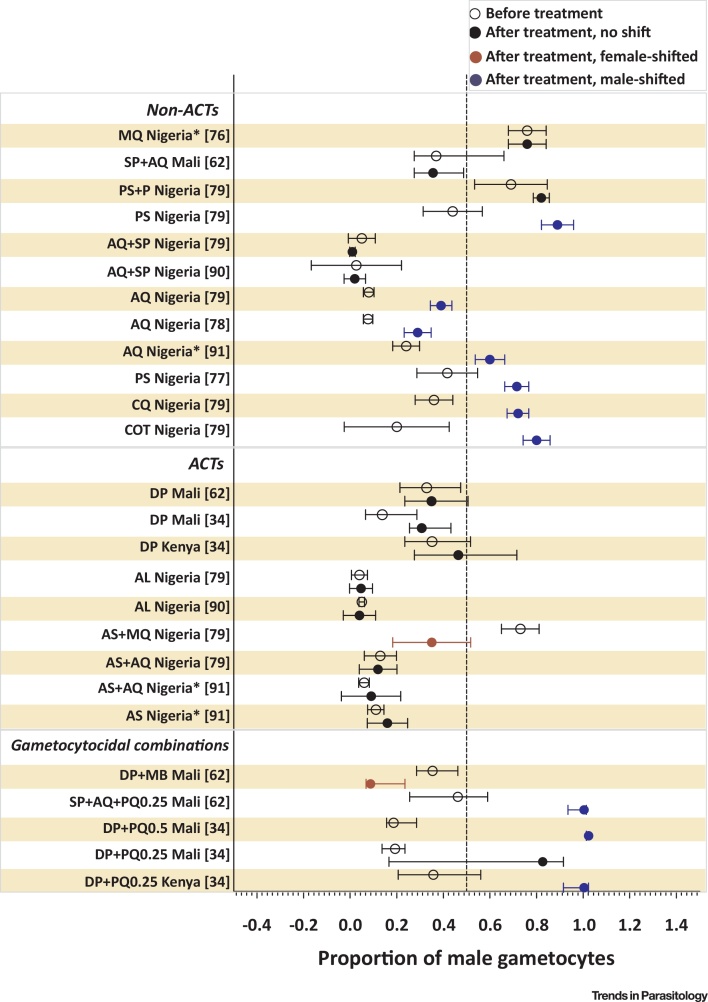
Malaria parasites may alter their investment in asexual replication and transmission in response to drug pressure. Treatment with subcurative doses of antimalarial drugs has been shown to increase the rate of gametocytogenesis in the rodent malaria parasite P. chabaudi [71] and in laboratory strains of P. falciparum [72]. P. falciparum parasites may respond to their proliferation state rather than directly to the presence of drugs. Drug pressure may thus lead to an initial increased investment in asexual proliferation (aimed towards survival within the infected host) until survival is unlikely and a terminal investment in transmission occurs 64, 73. A direct effect of antimalarial drugs on a preferential production of male or female gametocytes has not been reported. By comparison, there is increasing evidence that antimalarial drugs may influence sex ratio through preferential clearance of gametocytes of either sex. In vitro drug tests suggest that male gametocytes are more sensitive to a range of antimalarial drugs compared to female gametocytes, with impaired male gametocyte fitness (reduction in exflagellation) at drug concentrations that do not affect female gametocyte fitness (as measured by activation into gametes) [20]. Antifolates, for example, may disproportionately affect males by inhibiting the folate-mediated pyrimidine synthesis required for DNA replication during exflagellation [20]. Although a sex-specific effect of antimalarial drugs has been proposed as an explanation for the early sterilizing effect of transmission-blocking antimalarials [74], gametocyte sex ratio assessments are not commonly incorporated in antimalarial drug efficacy clinical trials. Despite initial reports of a reduced duration of male gametocyte carriage [75], and a relative decrease in the proportion of male gametocytes [76] following artemisinin-combination treatment (ACT), no obvious pattern of a sex-specific effect of ACT emerges when all available data are examined (Figure 2). On the other hand, most available (microscopy-based) data suggest that treatment with non-ACTs (such as sulphadoxine-pyrimethamine, amodiaquine and chloroquine) results in a male-biased sex ratio shortly after treatment 77, 78, 79 (Figure 2). Whilst a single trial with molecular gametocyte sexing tools did not confirm this effect for sulphadoxine-pyrimethamine plus amodiaquine [62], it is intriguing to consider if this explains earlier observations on enhanced infectivity early after treatment with non-ACTs [80]. Recent evidence suggests that adding a single dose of the gametocytocidal drug primaquine to ACT is associated with male-biased sex ratios in the first 14 days after treatment, followed by a normalization to a female-biased ratio 34, 62 that may be a consequence of gametocyte release, ongoing gametocyte production, or a longer circulation time of female gametocytes. The most pronounced sex-specific effect may be exerted by the gametocytocidal drug methylene blue that appears to preferentially clear male gametocytes 62, 81, 82.
Figure 2.
Forest Plot on Proportion of Male Gametocytes before and after Treatment. This plot summarizes available clinical trial data on the effect of antimalarials on gametocyte sex ratio. Indicated are the proportion of gametocytes that were male before treatment (open symbols) and after treatment (closed symbols; at day 7 post-treatment – if earlier, indicated with asterisks). Study drugs are indicated on the y axis and grouped by nonartemisinin-based combination therapies (non-ACTs), ACTs, and drug combinations with a gametocytocidal compound. Blue symbols indicate a shift towards males, and red indicates a shift towards more females surviving; based on nonoverlapping confidence intervals. In most cases estimates indicate the mean and 95% confidence interval; for two studies 34, 62 it is median and interquartile range. Abbreviations: ACT, artemisinin-combination treatment; AL, artemether-lumefantrine; AQ, amodiaquine; AS, artesunate; COT, cotrimoxazole; CQ, chloroquine; DP, dihydroartemisinin-piperaquine; MQ, mefloquine; P, probenecid; PQ, primaquine; PS, Pyrimethamine–sulfadoxine; SP, sulfalene–pyrimethamine. See also 76, 77, 78, 79, 90, 91.
One important outstanding question is whether transmission potential is predictable after treatment based on gametocyte density and sex ratio, or whether mosquito feeding assays remain essential to predict transmission potential after treatment 74, 83. The strong association between gametocyte density and mosquito infection rates that is observed before treatment 23, 84 may be retained 62, 85 or lost after treatment [84]. If the quantification of gametocyte sex ratio allows mosquito infection rates to be predicted with acceptable precision, this may obviate the need for mosquito feeding assays in antimalarial drug trials. Another scenario is that drugs may sterilize gametocytes, either of both sexes, or preferentially affecting one sex, without immediately affecting their circulation time. Under this scenario mosquito transmission assays will continue to be essential to estimate the effects of antimalarial drugs on transmission potential.
Concluding Remarks
The transmission of P. falciparum to mosquitoes represents a developmental bottleneck in terms of parasite numbers; at the same time it is a very efficient process that may even increase in efficiency once transmission intensity declines [86]. For successful fertilization in the mosquito midgut, sufficient numbers of male and female gametocytes need to be generated during infections. Quantifying both male and female gametocytes therefore plausibly allows a better prediction of infectivity than the total gametocyte biomass or simple measurement of (the more abundant) female gametocytes [23]. Quantifying the densities of male and female gametocytes may also assist in the evaluation of the transmission-blocking properties of antimalarial drugs but at present cannot replace mosquito feeding assays to provide definitive evidence of post-treatment transmission potential (see Outstanding Questions). Changes in total parasite density or gametocyte density by antimalarial drugs or other environmental factors may promote malaria parasites to adjust their investment in transmission stages; this may be by increasing commitment to gametocytes or by increasing the proportion of male gametocytes to maximize transmission success [23]. Whilst of clear value in this respect, previously published assessments of gametocyte sex ratio are affected by the limited sensitivity of microscopy for gametocyte detection and sexing. With the recent development of molecular tools to enumerate male and female gametocytes, presumed environmental stimuli for sex ratio adjustment should be reconsidered. For such studies to provide reliable estimates, it is essential to acknowledge the presence of low levels of male and female gametocyte transcripts in both sexes and in asexual parasites, which necessitates a careful interpretation of gametocyte prevalence estimates in samples from patients with high total parasite burden and a careful interpretation of extreme sex ratios.
Outstanding Questions.
What are the sensing mechanisms and stimuli that allow malaria parasites to adjust their sex ratio?
Do male and female gametocytes differ in longevity or the duration of infectivity?
What range of gametocyte sex ratio allows transmission under different conditions?
Do different clones within the same infection vary in gametocyte sex ratio, and how does this affect their transmission?
Do antimalarial drugs prevent transmission by means of gametocyte sterilization rather than gametocyte clearance?
Can robust quantification of male and female gametocytes replace mosquito feeding assays?
Acknowledgments
We would like to thank Sarah Reece and two anonymous reviewers for the critical comments on the manuscript. F.G.T., L.M.K., C.D., and T.B. are supported by the Bill and Melinda Gates Foundation (INDIE OPP1173572). T.B. is further supported by a grant from The Netherlands Organization for Scientific Research (Vidi fellowship NWO project number 016.158.306) and a fellowship from the European Research Council [ERC-2014-StG 639776]. F.G.T. is further supported by The Netherlands organization for international cooperation in higher education (Nuffic) [grant number NFP-PhD.14/150]. Research in L.R.C.’s laboratory on transmission and sex ratios was supported by the Wellcome Trust (078749) and the BBSRC (PhD studentship).
Glossary
- Controlled human malaria infection (CHMI)
deliberate infection of a healthy noninfected human volunteer with malaria under highly standardized conditions. Infection happens either by intravenous inoculation of Plasmodium-infected erythrocytes or through the bite of sporozoite-infected mosquitoes. Upon reaching blood-stage infection, the volunteer receives treatment. In CHMI models aiming to study gametocyte development, treatment aims to eliminate asexual parasite replication while allowing gametocytes to develop and mature.
- Gametes
sexually dimorphic parasite forms that develop from gametocytes activating in the mosquito gut. Female gametocytes give rise to a single female gamete (macrogamete), male gametocytes give rise to up to eight motile microgametes; each female gamete may be fertilized by a male microgamete.
- Gametocyte
the sexual stage malaria parasite capable of reproduction in the mosquito. Female and male gametocytes circulate in the human peripheral blood, where they may be ingested by blood-feeding Anopheles mosquitoes and continue sexual development (see Gametes).
- Mosquito feeding assay
an assay for determining the infectiousness of Plasmodium gametocytes to Anopheles mosquitoes. Mosquito feeding assay may refer to an assay in which mosquitoes are allowed to feed on the patient’s blood (skin feeding or direct membrane feeding assay after venous blood was drawn) or on cultured gametocytes (standard membrane feeding assay).
- Proportion of male gametocytes
the proportion or percentage of all gametocytes that is male .
- Proteomics
studies that capture the entirety of proteins of a cell or organism, often at a certain life stage. Proteomics is usually based on mass spectrometry.
- Schizogony/erythrocytic schizogony
creation of, on average, 16–32 merozoites in an infected red blood cell during the schizont stage of asexual replication. Schizogony is a rapid process in P. falciparum (∼48 h), and should not be confused with liver-stage (exoerythrocytic) schizogony in hepatocytes, which takes 6–7 days and produces thousands of merozoites.
- Sex determination
used to describe the ‘decision’ to become either a male or female gametocyte, plausibly determined at the same time when sexual commitment happens.
- Sex ratio
the ratio of male to female gametocytes . This term is used inconsistently in the literature, referring to either sex, dependent on context. In the current review, we instead use the proportion of male gametocytes.
- Sexual commitment
the route to the differentiation into a gametocyte, thereby leaving the asexual replication cycle. The exact timing of commitment is not entirely understood, but all merozoites derived from one committed schizont will develop into gametocytes of one sex.
- Transcriptomics
studies that capture the entirety of transcripts of a cell or organism, often at a certain life stage. Transcriptomics is usually based on microarrays or RNA sequencing.
- Translational repression
a phenomenon in which mRNA transcripts are not (yet) translated into proteins but accumulated and protected from degradation. Stored transcripts frequently support cellular processes immediately after fertilization when translation is needed but transcription is low or absent.
References
- 1.Farfour E. The extravascular compartment of the bone marrow: a niche for Plasmodium falciparum gametocyte maturation? Malar. J. 2012;11:285. doi: 10.1186/1475-2875-11-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar R. Severity of anaemia is associated with bone marrow haemozoin in children exposed to Plasmodium falciparum. Br. J. Haematol. 2014;164:877–887. doi: 10.1111/bjh.12716. [DOI] [PubMed] [Google Scholar]
- 3.Joice R. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3008882. 244re5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinden R. Malaria, sexual development and transmission: retrospect and prospect. Parasitology. 2009;136:1427–1434. doi: 10.1017/S0031182009990667. [DOI] [PubMed] [Google Scholar]
- 5.Meibalan E., Marti M. Biology of malaria transmission. Cold Spring Harb. Perspect. Med. 2017;7 doi: 10.1101/cshperspect.a025452. a025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins K.A. A controlled human malaria infection model enabling evaluation of transmission-blocking interventions. J. Clin. Invest. 2018;128:1551–1562. doi: 10.1172/JCI98012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reuling I.J. A randomized feasibility trial comparing four antimalarial drug regimens to induce Plasmodium falciparum gametocytemia in the controlled human malaria infection model. eLife. 2018;7 doi: 10.7554/eLife.31549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor L., Read A. Why so few transmission stages? Reproductive restraint by malaria parasites. Parasitol. Today. 1997;13:135–140. doi: 10.1016/s0169-4758(97)89810-9. [DOI] [PubMed] [Google Scholar]
- 9.Eichner M. Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malaria therapy data. Trans. R. Soc. Trop. Med. Hyg. 2001;95:497–501. doi: 10.1016/s0035-9203(01)90016-1. [DOI] [PubMed] [Google Scholar]
- 10.Sinden R. Sexual development of malarial parasites. Adv. Parasitol. 1983;22:153–216. doi: 10.1016/s0065-308x(08)60462-5. [DOI] [PubMed] [Google Scholar]
- 11.Poran A. Single-cell RNA sequencing reveals a signature of sexual commitment in malaria parasites. Nature. 2017;551:95–99. doi: 10.1038/nature24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce M.C. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100:191–200. doi: 10.1017/s0031182000061199. [DOI] [PubMed] [Google Scholar]
- 13.Silvestrini F. Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology. 2000;121:465–471. doi: 10.1017/s0031182099006691. [DOI] [PubMed] [Google Scholar]
- 14.Smith T.G. Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology. 2000;121:127–133. doi: 10.1017/s0031182099006265. [DOI] [PubMed] [Google Scholar]
- 15.Smith T.G. Sexual differentiation and sex determination in the Apicomplexa. Trends Parasitol. 2002;18:315–323. doi: 10.1016/s1471-4922(02)02292-4. [DOI] [PubMed] [Google Scholar]
- 16.Filarsky M. GDV1 induces sexual commitment of malaria parasites by antagonizing HP1-dependent gene silencing. Science. 2018;359:1259–1263. doi: 10.1126/science.aan6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kafsack B.F. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507:248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha A. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature. 2014;507:253–257. doi: 10.1038/nature12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bousema T., Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin. Microbiol. Rev. 2011;24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delves M.J. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob. Agents Chemother. 2013;57:3268–3274. doi: 10.1128/AAC.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talman A.M. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar. J. 2004;3:24. doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert V. Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Trans. R. Soc. Trop. Med. Hyg. 1996;90:621–624. doi: 10.1016/s0035-9203(96)90408-3. [DOI] [PubMed] [Google Scholar]
- 23.Bradley J. Predicting the likelihood and intensity of mosquito infection from sex specific Plasmodium falciparum gametocyte density. eLife. 2018;7 doi: 10.7554/eLife.34463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter R., Miller L.H. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull. World Health Organ. 1979;57:37–52. [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider P. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am. J. Trop. Med. Hyg. 2007;76:470–474. [PubMed] [Google Scholar]
- 26.Coleman R.E. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J. Med. Entomol. 2004;41:201–208. doi: 10.1603/0022-2585-41.2.201. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet S. Comparison of artificial membrane feeding with direct skin feeding to estimate infectiousness of Plasmodium falciparum gametocyte carriers to mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 2000;94:103–106. doi: 10.1016/s0035-9203(00)90456-5. [DOI] [PubMed] [Google Scholar]
- 28.Drew D.R., Reece S.E. Development of reverse-transcription PCR techniques to analyse the density and sex ratio of gametocytes in genetically diverse Plasmodium chabaudi infections. Mol. Biochem. Parasitol. 2007;156:199–209. doi: 10.1016/j.molbiopara.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goncalves B.P. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat. Commun. 2017;8:1133. doi: 10.1038/s41467-017-01270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karl S. Enhanced detection of gametocytes by magnetic deposition microscopy predicts higher potential for Plasmodium falciparum transmission. Malar. J. 2008;7:66. doi: 10.1186/1475-2875-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker D.A. Sexual-stage-specific RNA expression of a new Plasmodium falciparum gene detected by in situ hybridisation. Mol. Biochem. Parasitol. 1995;72:193–201. doi: 10.1016/0166-6851(95)00073-a. [DOI] [PubMed] [Google Scholar]
- 32.Alano P. COS cell expression cloning of Pfg377, a Plasmodium falciparum gametocyte antigen associated with osmiophilic bodies. Mol. Biochem. Parasitol. 1995;74:143–156. doi: 10.1016/0166-6851(95)02491-3. [DOI] [PubMed] [Google Scholar]
- 33.Olivieri A. The Plasmodium falciparum protein Pfg27 is dispensable for gametocyte and gamete production, but contributes to cell integrity during gametocytogenesis. Mol. Microbiol. 2009;73:180–193. doi: 10.1111/j.1365-2958.2009.06762.x. [DOI] [PubMed] [Google Scholar]
- 34.Stone W. A molecular assay to quantify male and female Plasmodium falciparum gametocytes: results from 2 randomized controlled trials using primaquine for gametocyte clearance. J. Infect. Dis. 2017;216:457–467. doi: 10.1093/infdis/jix237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruce M.C. Cellular location and temporal expression of the Plasmodium falciparum sexual stage antigen Pfs16. Mol. Biochem. Parasitol. 1994;65:11–22. doi: 10.1016/0166-6851(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 36.Rawlings D.J. α-Tubulin II is a male-specific protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 1992;56:239–250. doi: 10.1016/0166-6851(92)90173-h. [DOI] [PubMed] [Google Scholar]
- 37.Babiker H.A., Schneider P. Application of molecular methods for monitoring transmission stages of malaria parasites. Biomed. Mater. 2008;3 doi: 10.1088/1748-6041/3/3/034007. [DOI] [PubMed] [Google Scholar]
- 38.Babiker H.A. Gametocytes: insights gained during a decade of molecular monitoring. Trends Parasitol. 2008;24:525–530. doi: 10.1016/j.pt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider P. Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification. Mol. Biochem. Parasitol. 2004;137:35–41. doi: 10.1016/j.molbiopara.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Schneider P. Quantification of female and male Plasmodium falciparum gametocytes by reverse transcriptase quantitative PCR. Mol. Biochem. Parasitol. 2015;199:29–33. doi: 10.1016/j.molbiopara.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Lasonder E. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic Acids Res. 2016;44:6087–6101. doi: 10.1093/nar/gkw536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan S.M. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell. 2005;121:675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 43.Santolamazza F. Detection of Plasmodium falciparum male and female gametocytes and determination of parasite sex ratio in human endemic populations by novel, cheap and robust RTqPCR assays. Malar. J. 2017;16:468. doi: 10.1186/s12936-017-2118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meerstein-Kessel L. A multiplex assay for the sensitive detection and quantification of male and female Plasmodium falciparum gametocytes. Malar. J. 2018;17:441. doi: 10.1186/s12936-018-2584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shute P.G., Maryon M. A study of gametocytes in a West African strain of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1951;44:421–438. doi: 10.1016/s0035-9203(51)80020-8. [DOI] [PubMed] [Google Scholar]
- 46.Ciuca M. Contribution à l’étude de l’ infection Expérimentale au Plasmodium falciparum. Festchrift Institute Bernard Nocht. 1937:81–101. [Google Scholar]
- 47.Rono M.K. Adaptation of Plasmodium falciparum to its transmission environment. Nat. Ecol. Evol. 2018;2:377. doi: 10.1038/s41559-017-0419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gadalla A.A. Associations between season and gametocyte dynamics in chronic Plasmodium falciparum Infections. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.James S. Some general results of a study of induced malaria in England. Trans. R. Soc. Trop. Med. Hyg. 1931;24:5. [Google Scholar]
- 50.Reece S.E. Plastic parasites: sophisticated strategies for survival and reproduction? Evol. Appl. 2009;2:11–23. doi: 10.1111/j.1752-4571.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paul R.E. Plasmodium sex determination and transmission to mosquitoes. Trends Parasitol. 2002;18:32–38. doi: 10.1016/s1471-4922(01)02122-5. [DOI] [PubMed] [Google Scholar]
- 52.Reece S.E. Sex ratio adjustment and kin discrimination in malaria parasites. Nature. 2008;453:609–614. doi: 10.1038/nature06954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramiro R.S. Sex and death: the effects of innate immune factors on the sexual reproduction of malaria parasites. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paul R.E. Sex determination in malaria parasites. Science. 2000;287:128–131. doi: 10.1126/science.287.5450.128. [DOI] [PubMed] [Google Scholar]
- 55.Reece S.E. Host cell preference and variable transmission strategies in malaria parasites. Proc. Biol. Sci. 2005;272:511–517. doi: 10.1098/rspb.2004.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamilton W.D. Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- 57.Wright S. Coefficients of inbreeding and relationship. Am. Nat. 1922;56:330–338. [Google Scholar]
- 58.West S.A. Fertility insurance and the sex ratios of malaria and related hemospororin blood parasites. J. Parasitol. 2002;88:258–263. doi: 10.1645/0022-3395(2002)088[0258:FIATSR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 59.Gardner A. Even more extreme fertility insurance and the sex ratios of protozoan blood parasites. J. Theor. Biol. 2003;223:515–521. doi: 10.1016/s0022-5193(03)00142-5. [DOI] [PubMed] [Google Scholar]
- 60.Mitri C. Density-dependent impact of the human malaria parasite Plasmodium falciparum gametocyte sex ratio on mosquito infection rates. Proc. Biol. Sci. 2009;276:3721–3726. doi: 10.1098/rspb.2009.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Churcher T.S. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. eLife. 2013;2 doi: 10.7554/eLife.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dicko A. Efficacy and safety of primaquine and methylene blue for prevention of Plasmodium falciparum transmission in Mali: a phase 2, single-blind, randomised controlled trial. Lancet Infect. Dis. 2018;18:627–639. doi: 10.1016/S1473-3099(18)30044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walzer K.A. Single-cell analysis reveals distinct gene expression and heterogeneity in male and female Plasmodium falciparum gametocytes. mSphere. 2018;3 doi: 10.1128/mSphere.00130-18. e00130-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schneider P. Adaptive plasticity in the gametocyte conversion rate of malaria parasites. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brancucci N.M.B. Lysophosphatidylcholine regulates sexual stage differentiation in the human malaria parasite Plasmodium falciparum. Cell. 2017;171 doi: 10.1016/j.cell.2017.10.020. 1532–1544.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tibúrcio M. Specific expression and export of the Plasmodium falciparum Gametocyte EXported Protein-5 marks the gametocyte ring stage. Malar. J. 2015;14:334. doi: 10.1186/s12936-015-0853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farid R. Initiation of gametocytogenesis at very low parasite density in Plasmodium falciparum infection. J. Infect. Dis. 2017;215:1167–1174. doi: 10.1093/infdis/jix035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor B.J. A direct from blood reverse transcriptase polymerase chain reaction assay for monitoring falciparum malaria parasite transmission in elimination settings. Am. J. Trop. Med. Hyg. 2017;97:533–543. doi: 10.4269/ajtmh.17-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang H.H. Persistence of Plasmodium falciparum parasitemia after artemisinin combination therapy: evidence from a randomized trial in Uganda. Sci. Rep. 2016;6:26330. doi: 10.1038/srep26330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joice R. Inferring developmental stage composition from gene expression in human malaria. PLoS Comput. Biol. 2013;9 doi: 10.1371/journal.pcbi.1003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buckling A.G. Adaptive changes in Plasmodium transmission strategies following chloroquine chemotherapy. Proc. Biol. Sci. 1997;264:553–559. doi: 10.1098/rspb.1997.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buckling A. Chloroquine increases Plasmodium falciparum gametocytogenesis in vitro. Parasitology. 1999;118:339–346. doi: 10.1017/s0031182099003960. [DOI] [PubMed] [Google Scholar]
- 73.Reece S.E. Stress, drugs and the evolution of reproductive restraint in malaria parasites. Proc. Biol. Sci. 2010;277:3123–3129. doi: 10.1098/rspb.2010.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White N.J. Assessment of therapeutic responses to gametocytocidal drugs in Plasmodium falciparum malaria. Malar. J. 2014;13:483. doi: 10.1186/1475-2875-13-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gbotosho G.O. Plasmodium falciparum gametocyte carriage, sex ratios and asexual parasite rates in Nigerian children before and after a treatment protocol policy change instituting the use of artemisinin-based combination therapies. Mem. Inst. Oswaldo Cruz. 2011;106:685–690. doi: 10.1590/s0074-02762011000600007. [DOI] [PubMed] [Google Scholar]
- 76.Sowunmi A. Effects of mefloquine and artesunate mefloquine on the emergence, clearance and sex ratio of Plasmodium falciparum gametocytes in malarious children. Malar. J. 2009;8:297. doi: 10.1186/1475-2875-8-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sowunmi A., Fateye B.A. Gametocyte sex ratios in children with asymptomatic, recrudescent, pyrimethamine-sulfadoxine-resistant, Plasmodium falciparum malaria. Ann. Trop. Med. Parasitol. 2003;97:671–682. doi: 10.1179/000349803225002381. [DOI] [PubMed] [Google Scholar]
- 78.Sowunmi A. Plasmodium falciparum gametocyte sex ratios in children with acute, symptomatic, uncomplicated infections treated with amodiaquine. Malar. J. 2008;7:169. doi: 10.1186/1475-2875-7-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sowunmi A. Plasmodium falciparum gametocyte sex ratios in symptomatic children treated with antimalarial drugs. Acta Trop. 2009;109:108–117. doi: 10.1016/j.actatropica.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 80.Targett G. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 2001;183:1254–1259. doi: 10.1086/319689. [DOI] [PubMed] [Google Scholar]
- 81.Adjalley S.H. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc. Natl. Acad. Sci. U. S. A. 2011;108:E1214–E1223. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coulibaly B. Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: a randomised controlled trial. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karunajeewa H.A., Mueller I. How important is gametocyte clearance after malaria therapy? BMC Med. 2016;14:93. doi: 10.1186/s12916-016-0641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dicko A. Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect. Dis. 2016;16:674–684. doi: 10.1016/S1473-3099(15)00479-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robert V. Gametocytemia and infectivity to mosquitoes of patients with uncomplicated Plasmodium falciparum malaria attacks treated with chloroquine or sulfadoxine plus pyrimethamine. Am. J. Trop. Med. Hyg. 2000;62:210–216. doi: 10.4269/ajtmh.2000.62.210. [DOI] [PubMed] [Google Scholar]
- 86.Churcher T.S. Human-to-mosquito transmission efficiency increases as malaria is controlled. Nat. Commun. 2015;6 doi: 10.1038/ncomms7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gbotosho G.O. Plasmodium falciparum gametocyte carriage, emergence, clearance and population sex ratios in anaemic and non-anaemic malarious children. Mem. Inst. Oswaldo Cruz. 2011;106:562–569. doi: 10.1590/s0074-02762011000500008. [DOI] [PubMed] [Google Scholar]
- 88.Sowunmi A. Population structure of Plasmodium falciparum gametocyte sex ratios in malarious children in an endemic area. Parasitol. Int. 2009;58:438–443. doi: 10.1016/j.parint.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kar P. Plasmodium falciparum gametocytaemia with chloroquine chemotherapy in persistent malaria in an endemic area of India. Indian J. Med. Res. 2009;129:299–305. [PubMed] [Google Scholar]
- 90.Sowunmi A. Activities of artemether-lumefantrine and amodiaquine-sulfalene-pyrimethamine against sexual-stage parasites in falciparum malaria in children. Chemotherapy. 2008;54:201–208. doi: 10.1159/000140463. [DOI] [PubMed] [Google Scholar]
- 91.Sowunmi A. Activities of amodiaquine, artesunate, and artesunate-amodiaquine against asexual- and sexual-stage parasites in falciparum malaria in children. Antimicrob. Agents Chemother. 2007;51:1694–1699. doi: 10.1128/AAC.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Talman A.M. Influence of chemotherapy on the Plasmodium gametocyte sex ratio of mice and humans. Am. J. Trop. Med. Hyg. 2004;71:739–744. [PubMed] [Google Scholar]
- 93.Robert V. Sex ratio of Plasmodium falciparum gametocytes in inhabitants of Dielmo, Senegal. Parasitology. 2003;127:1–8. doi: 10.1017/s0031182003003299. [DOI] [PubMed] [Google Scholar]
- 94.Babiker H.A. Genetic structure and dynamics of Plasmodium falciparum infections in the Kilombero region of Tanzania. Trans. R. Soc. Trop. Med. Hyg. 1999;93:11–14. doi: 10.1016/s0035-9203(99)90321-8. [DOI] [PubMed] [Google Scholar]
- 95.Paul R.E. Mating patterns in malaria parasite populations of Papua New Guinea. Science. 1995;269:1709–1711. doi: 10.1126/science.7569897. [DOI] [PubMed] [Google Scholar]
- 96.Read A.F. Gametocyte sex ratios as indirect measures of outcrossing rates in malaria. Parasitology. 1992;104:387–395. doi: 10.1017/s0031182000063630. [DOI] [PubMed] [Google Scholar]
- 97.Fan Q. Characterization of PfPuf2, member of the Puf family RNA-binding proteins from the malaria parasite Plasmodium falciparum. DNA Cell Biol. 2004;23:753–760. doi: 10.1089/dna.2004.23.753. [DOI] [PubMed] [Google Scholar]
- 98.Miao J. The Puf-family RNA-binding protein PfPuf2 regulates sexual development and sex differentiation in the malaria parasite Plasmodium falciparum. J. Cell Sci. 2010;123:1039–1049. doi: 10.1242/jcs.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paton M.G. Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol. Biochem. Parasitol. 1993;59:263–275. doi: 10.1016/0166-6851(93)90224-l. [DOI] [PubMed] [Google Scholar]
- 100.Thompson J., Sinden R.E. In situ detection of Pbs21 mRNA during sexual development of Plasmodium berghei. Mol. Biochem. Parasitol. 1994;68:189–196. doi: 10.1016/0166-6851(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 101.Mair G.R. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313:667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mair G.R. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Turque O. Translational repression in malaria sporozoites. Microb. Cell. 2016;3:227–229. doi: 10.15698/mic2016.05.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muller K. The Puf-family RNA-binding protein Puf2 controls sporozoite conversion to liver stages in the malaria parasite. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sinden R.E. Gametocytogenesis of Plasmodium falciparum in vitro: ultrastructural observations on the lethal action of chloroquine. Ann. Trop. Med. Parasitol. 1982;76:15–23. doi: 10.1080/00034983.1982.11687500. [DOI] [PubMed] [Google Scholar]
- 106.Downs W.G. Infections of chicks with single parasites of Plasmodium gallinaceum Brumpt. Am. J. Hyg. 1947;46 doi: 10.1093/oxfordjournals.aje.a119153. 41–41. [DOI] [PubMed] [Google Scholar]
- 107.Painter H.J. Genome-wide real-time in vivo transcriptional dynamics during Plasmodium falciparum blood-stage development. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-04966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yeoh L.M. Comparative transcriptomics of female and male gametocytes in Plasmodium berghei and the evolution of sex in alveolates. BMC Genomics. 2017;18:734. doi: 10.1186/s12864-017-4100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meerstein-Kessel L. Probabilistic data integration identifies reliable gametocyte-specific proteins and transcripts in malaria parasites. Sci. Rep. 2018;8 doi: 10.1038/s41598-017-18840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wampfler R. Strategies for detection of Plasmodium species gametocytes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanron A.E. Multiplex, DNase-free one-step reverse transcription PCR for Plasmodium 18S rRNA and spliced gametocyte-specific mRNAs. Malar. J. 2017;16:208. doi: 10.1186/s12936-017-1863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Babiker H.A. Detection of low level Plasmodium falciparum gametocytes using reverse transcriptase polymerase chain reaction. Mol. Biochem. Parasitol. 1999;99:143–148. doi: 10.1016/s0166-6851(98)00175-3. [DOI] [PubMed] [Google Scholar]
- 113.Essuman E. A novel gametocyte biomarker for superior molecular detection of the Plasmodium falciparum infectious reservoirs. J. Infect. Dis. 2017;216:1264–1272. doi: 10.1093/infdis/jix442. [DOI] [PubMed] [Google Scholar]