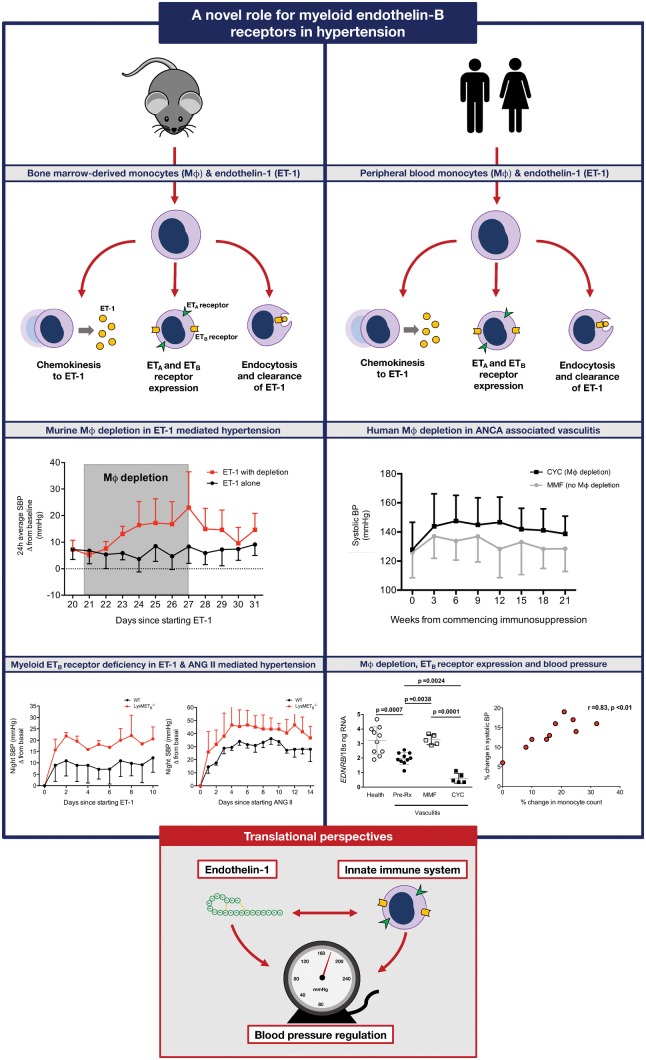
Abstract
Aims
Hypertension is common. Recent data suggest that macrophages (Mφ) contribute to, and protect from, hypertension. Endothelin-1 (ET-1) is the most potent endogenous vasoconstrictor with additional pro-inflammatory properties. We investigated the role of the ET system in experimental and clinical hypertension by modifying Mφ number and phenotype.
Methods and results
In vitro, Mφ ET receptor function was explored using pharmacological, gene silencing, and knockout approaches. Using the CD11b-DTR mouse and novel mice with myeloid cell-specific endothelin-B (ETB) receptor deficiency (LysMETB−/−), we explored the effects of modifying Mφ number and phenotype on the hypertensive effects of ET-1, angiotensin II (ANG II), a model that is ET-1 dependent, and salt. In patients with small vessel vasculitis, the impacts of Mφ depleting and non-depleting therapies on blood pressure (BP) and endothelial function were examined. Mouse and human Mφ expressed both endothelin-A and ETB receptors and displayed chemokinesis to ET-1. However, stimulation of Mφ with exogenous ET-1 did not polarize Mφ phenotype. Interestingly, both mouse and human Mφ cleared ET-1 through ETB receptor mediated, and dynamin-dependent, endocytosis. Mφ depletion resulted in an augmented chronic hypertensive response to both ET-1 and salt. LysMETB−/− mice displayed an exaggerated hypertensive response to both ET-1 and ANG II. Finally, in patients who received Mφ depleting immunotherapy BP was higher and endothelial function worse than in those receiving non-depleting therapies.
Conclusion
Mφ and ET-1 may play an important role in BP control and potentially have a critical role as a therapeutic target in hypertension.
Keywords: Myeloid cell, Endothelin, Hypertension
Translational perspective
Hypertension is a costly global health problem, and an important risk factor for the development and progression of chronic kidney disease. Its aetiology remains unclear in most adults. Here, the data provided suggest that the immune and endothelin systems play important roles in blood pressure regulation and provide a rational basis for further investigation into the modulation of these pathways. These studies may encourage industry to take a lead in this relatively orphan area, potentially resulting in a more rational prescribing of endothelin receptor antagonists for hypertension that has developed as part of a multi-system inflammatory disease, with these agents potentially affording broader cardiovascular protection. These studies may also help inform the design of novel antihypertensive therapies.
Introduction
Arterial hypertension is a major risk factor for atherosclerosis, coronary artery disease, stroke, and chronic kidney disease, and is a prominent contributor to death worldwide.1 It is estimated that a quarter of the world’s adult population is hypertensive and this number is projected to rise to nearly 30% by 2025.2 By age 70 years, 70% of the US population have hypertension. However, despite the frequency of hypertension, its cause in the majority of adults is unknown.
Hypertension is complex, with no single mechanism—sodium retention, renin release, and increased vascular tone—entirely explaining the blood pressure (BP) rise. The past 50 years have seen growing evidence implicating the immune system.3 Recent data suggest that macrophages (Mφ) contribute to, and protect from, hypertension. Early studies in the spontaneously hypertensive rat4 found a correlation between the distribution of sub-endothelial Mφ and endothelial function, and that treatment with an angiotensin converting enzyme inhibitor improved endothelial function and reduced the number of vascular Mφ. Furthermore, many models of hypertension—angiotensin II (ANG II), high salt—are associated with renal accumulation of Mφ.5,6 Despite these and many other observational studies, few have attempted to modify Mφ phenotype/number to examine their role in hypertension.7–10
Endothelin-1 (ET-1) is the most potent endogenous vasoconstrictor.11 Its production is triggered by multiple stimuli including ANG II and pro-inflammatory cytokines.12,13 ET-1 acts by binding to two distinct receptors, the endothelin-A (ETA) and the endothelin-B (ETB) receptors.14,15 ETA and ETB receptors on vascular smooth muscle cell (VSMC) mediate the vasoconstrictor effects of ET-1.16 ETB receptors are also found on vascular endothelial cells (EC) where their activation results in dilation.17,18 Production of vascular ET-1 is increased in some but not all animal models of hypertension.19 Interestingly, ET antagonism can blunt BP elevation in ANG II infused rats suggesting that in this model, ET-1 largely mediates the hypertensive effects of ANG II.20,21 Importantly, a number of clinical studies have gone on to show the effectiveness of both selective ETA and mixed ETA/B antagonism in lowering BP in hypertension.22–24
ET-1 is considered to be pro-inflammatory. ETA receptor activation is crucial in mediating the ANG II-induced infiltration of renal cortical T cells.25 In both models of aldosterone-induced hypertension and diabetic nephropathy, selective ETA receptor antagonism reduced Mφ infiltration,26,27 and in the diabetic model also reduced fibrosis.27 However, the effects of ET-1 on Mφ biology are not well studied. In the current studies, we explored the role of Mφ and monocytes in mediating the pro-hypertensive effects of ET-1 and ANG II.
Methods
Results
Macrophages possess endothelin receptors but are not polarized by endothelin-1
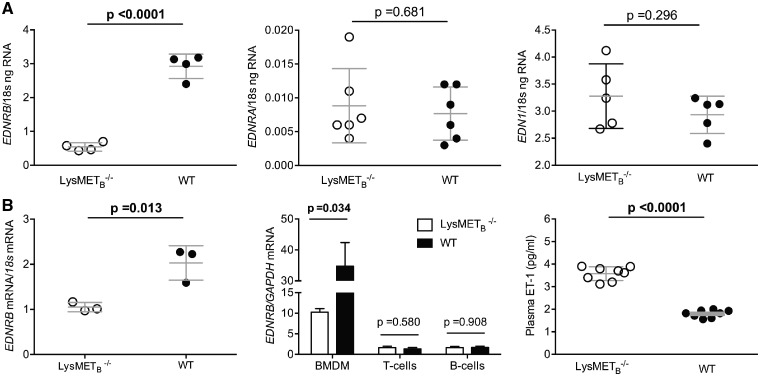
We first found that mouse bone marrow-derived macrophages (BMDM) possess both ETA and ETB receptors (ETB > ETA) although in relatively lower amounts than seen in VSMC and EC, respectively (see Supplementary material online, Figure S1). To examine the role of the Mφ ETB receptor in greater detail, we generated novel mice deficient in ETB on myeloid cells alone (LysMETB−/−). These mice showed no differences in baseline vascular function or circulating immune cells compared with littermate controls (see Figure 1 and Supplementary material online, Figures S2–S4).
Figure 1.
Characterization of the endothelin system in LysMETB−/−. (A) qRT-PCR analysis of EDNRB (endothelin-B), EDNRA (endothelin-A), and EDN1 (endothelin-1) expression in bone marrow-derived Mφ. (B) EDNRB (endothelin-B) expression in peritoneal Mφ (left panel), bone marrow-derived Mφ, T and B cells (middle panel), and plasma endothelin-1 (right panel). Data are expressed as mean ± standard deviation and comparisons were made with unpaired t-tests and for (B) middle panel, Holm–Sidak was used to correct for multiple comparisons; adjusted P-values are shown.
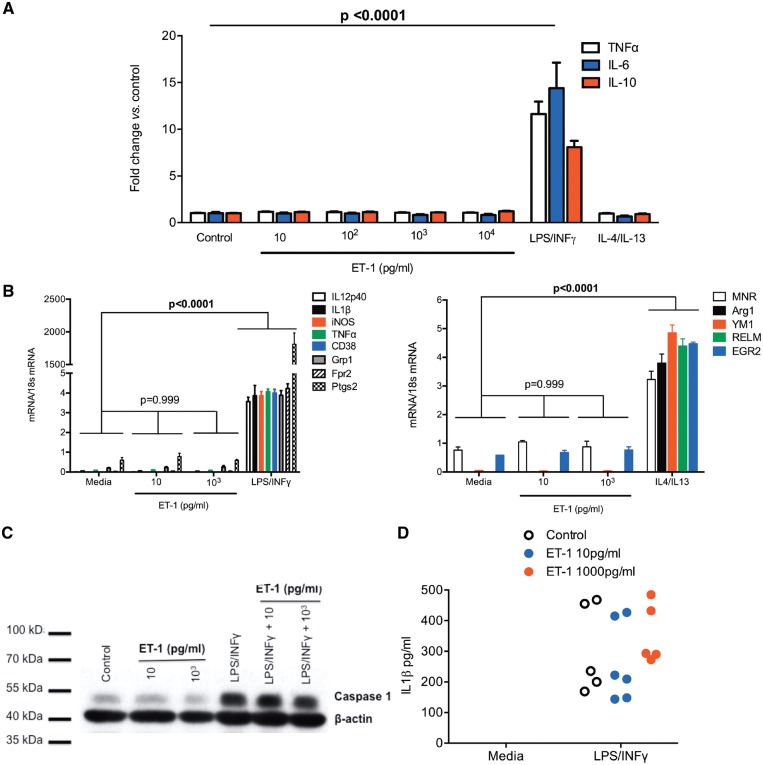
We then explored the ability of ET-1 to polarize BMDM. Increasing concentrations of ET-1 (10–104 pg/mL) were unable to polarize BMDM to a classical (M1) or alternative (M2) phenotype either alone or in combination (see Figure 2 and Supplementary material online, Figure S5). Furthermore, neither co-stimulation with LPS and ET-1 nor LPS stimulation following ET-1 priming, in the presence or absence of ET receptor blockade, augmented the BMDM response to LPS (see Supplementary material online, Figure S6).
Figure 2.
In vitro stimulation with endothelin-1 does not polarize Mφ to a classical or alternative phenotype. (A) Bone marrow-derived Mφ production of TNFα, IL-6, and IL-10 after 24 h stimulation with endothelin-1 (10–104 pg/mL), LPS/INFγ, IL-4/IL-13, and in untreated controls. Data (mean ± standard deviation; n = 4 per group) were compared by two-way analysis of variance, with main effects of treatment (P < 0.0001), production (P = 0.186), and the interaction (P = 0.0006). Multiple comparisons were made to compare the effect of each treatment against control (media alone), with a family P-value of 0.01; an adjusted P-value is shown. (B) Bone marrow-derived Mφ mRNA production of a range of M1 (left) and M2 (right) markers following 24 h stimulation with endothelin-1 (10 pg/mL and 104 pg/mL). Data (mean ± standard deviation; n = 4 per group) were compared by two-way analysis of variance (main effects of treatment, of gene product and the interaction were all P < 0.0001). Multiple comparisons were made to compare the effect of each treatment on mRNA production against control (media), with a family P-value of 0.01. Adjusted P-values are shown. (C) Western blot analysis demonstrating endothelin-1, either alone or in combination with LPS/INFγ, did not stimulate production of caspase 1 in mouse Mφ. (D) Endothelin-1 alone (media) or in combination with LPS/INFγ, did not stimulate production of IL-1β by mouse Mφ.
BMDM stimulation with LPS/INFγ (but not IL-4/IL-13) increased the media concentration of ET-1 at 24 h (see Supplementary material online, Figure S7), an effect that was blocked by phosphoramidon, an inhibitor of endothelin converting enzyme (ECE) which catalyzes the conversion of big ET-1 to the mature peptide. Thus, this increase in ET-1 likely represents de novo production.
Macrophages demonstrate chemokinesis to endothelin-1
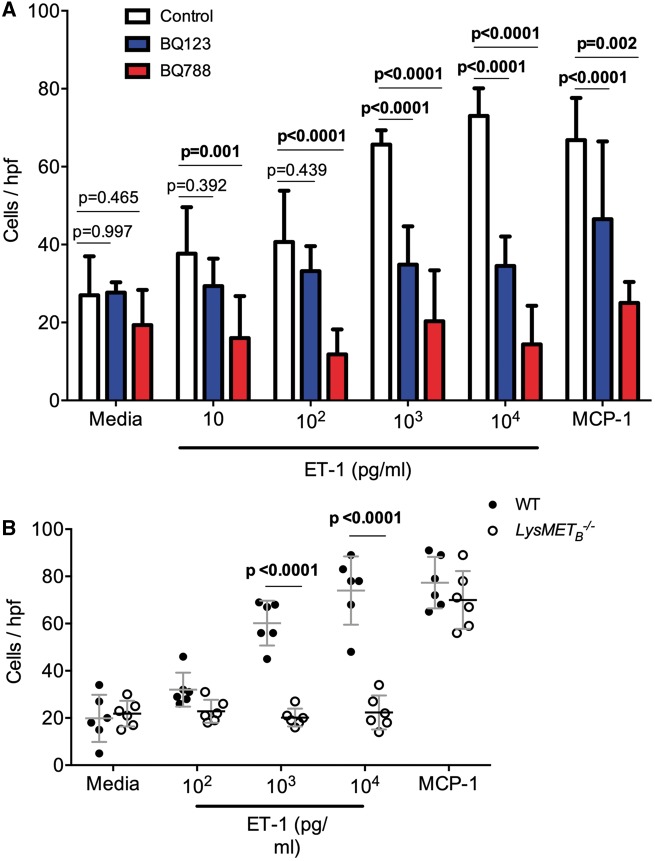
Despite the lack of polarization by ET-1, BMDM demonstrated chemokinesis to ET-1. This effect was more apparent at higher concentrations of ET-1 and no different to MCP-1 at ET-1 103 pg/mL and 104 pg/mL. BMDM chemokinesis to ET-1 was blocked by both selective ETA (BQ123) and selective ETB (BQ788) receptor antagonism (Figure 3A). In terms of chemokinetic ability, LysMETB−/− BMDM had a blunted response to incremental concentrations of ET-1 compared with control BMDM, although the response to MCP-1 was maintained (Figure 3B).
Figure 3.
Macrophage demonstrate chemokinesis towards endothelin-1. (A) Bone marrow-derived Mφ chemokinesis in response to increasing doses of endothelin-1 in the presence or absence of selective endothelin-A (BQ123) or endothelin-B (BQ788) antagonism; MCP-1 was used as a positive control. The number of Mφ per high powered field (mean ± standard deviation; n = 6 mice per group) was compared by two-way analysis of variance, with main effects of endothelin-1 or MCP-1 treatment (P < 0.0001) and receptor antagonism (P < 0.0001) and the interaction (P < 0.0001). Effects within rows were compared, using one family per row and a family P-value of 0.01. Adjusted P-values are shown. (B) Chemokinesis of bone marrow-derived Mφ in response to endothelin-1 or MCP-1. Bone marrow-derived Mφ were isolated from LysMETB−/− (open circles; n = 6) and control (closed circles; n = 6) mice. Data were compared by two-way analysis of variance, with main effects of endothelin-1 or MCP-1 treatment (P < 0.0001) and genotype (P < 0.0001) and the interaction (P < 0.0001). Between genotype comparisons were made and adjusted P-values are shown.
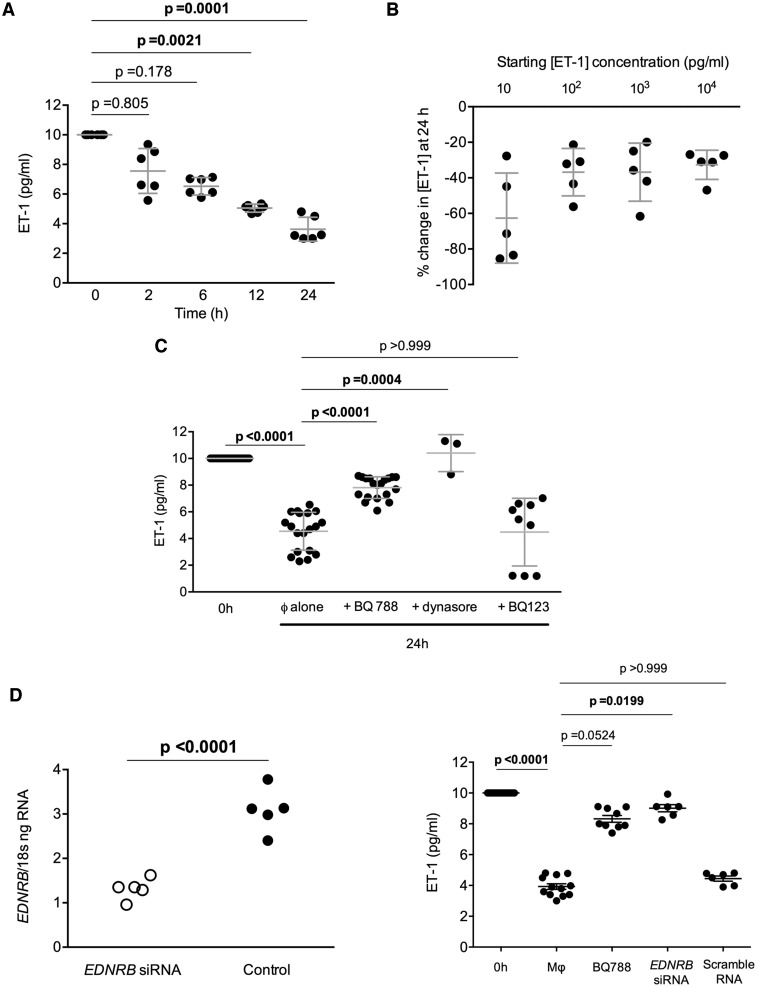
Macrophages demonstrate endothelin-B-mediated endothelin-1 uptake
BMDM were then exposed to ET-1 10 pg/mL in their media. Serial assay of the media for ET-1 showed a gradual reduction over a 24 h period with the concentration at 24 h being approximately 60% that at baseline (Figure 4A). This reduction in media ET-1 was seen when BMDM were exposed to increasing concentrations of ET-1 (Figure 4B) and was not explained by degradation of the peptide over the 24 h period (see Supplementary material online, Figure S8). The fall in ET-1 was prevented by both selective antagonism of the ETB receptor (but not ETA) as well as inhibition of dynamin-dependent endocytosis (see Figure 4C and Supplementary material online, Figure S9), supporting BMDM uptake of ET-1 through ETB receptor-mediated endocytosis. Neutral endopeptidase (NEP) inhibition had no effect. Additionally, mixed ETA/B receptor antagonism was no different to selective ETB blockade alone (see Supplementary material online, Figure S10).
Figure 4.
Macrophages clear endothelin-1 through endothelin-B receptor-mediated endocytosis. Endothelin-1 concentration in cell culture supernatant from bone marrow-derived Mφ (A) over 24 h after incubation with a starting endothelin-1 concentration of 10 pg/mL, (B) at 24 h following incubation with endothelin-1 (10–104 pg/mL), (C) after incubation with an endothelin-A (BQ123) or endothelin-B (BQ788) antagonist or dynasore, an inhibitor of dynamin-dependent endocytosis receptor antagonism, and (D) following bone marrow-derived Mφ endothelin-B receptor knockdown with siRNA (efficiency of knockdown left panel). Kruskal–Wallis tests were used with Dunn’s test for multiple planned comparisons. Adjusted P-values are shown. For (D) left panel an unpaired t-test was used; the two-tailed P-value is shown.
Figure 4.
(E) Vascular smooth muscle cells do not remove endothelin-1 from their media in vitro. Data from individual experiments are shown and were compared by Kruskal–Wallis; with Dunn’s test for multiple planned comparisons as shown. (F) Wild type (white circles) and LysMETB−/− (black circles) bone marrow-derived Mφ were exposed to endothelin-1 10 pg/mL in vitro in the presence or absence of BQ788, an endothelin-B receptor antagonist. Endothelin-1 was measured in the supernatant at 24 h. One-way analysis of variance (P = 0.0003) was used and adjusted P-values for multiple comparisons are shown. (G) Uptake of fluorescent endothelin-1 by wild type or LysMETB−/− bone marrow-derived Mφ in the presence or absence of BQ788. One-way analysis of variance (P < 0.0001) was used and adjusted P-values for planned comparisons, as shown. (H) Rapid uptake of endothelin-1 by bone marrow-derived Mφ.
Further data to support the role of the Mφ ETB receptor in clearing ET-1 were provided using an ETB gene silencing approach. Mφ EDNRB knockdown again prevented ET-1 uptake by BMDM, an effect that was similar in magnitude to that seen with pharmacological ETB receptor antagonism (Figure 4D). As ETB receptors are also present on VSMC, we assessed their ability to remove ET-1 in vitro. VSMC did not remove ET-1 from their surrounding media (Figure 4E). BMDM from LysMETB−/− and controls were also exposed to ET-1 for 24 h. As previously seen, control BMDM removed ET-1 from their medium, an effect that was blocked by BQ788. Medium from LysMETB−/− BMDM showed no difference in ET-1 concentration at 24 h compared with baseline and there was no effect of pre-treatment with BQ788 (Figure 4F). To further support our hypothesis of Mφ ET-1 uptake, we exposed BMDM to fluorescent ET-1. Our results suggested a statistically significant (P < 0.01) increase in BMDM intracellular fluorescence following treatment with ET-1 (Figure 4G). Uptake of fluorescence was blocked by selective blockade of the ETB receptor and absent, as expected, in BMDM from LysMETB−/− mice. Furthermore, this uptake process occurred rapidly (Figure 4H) and reached a steady state within 60 min.
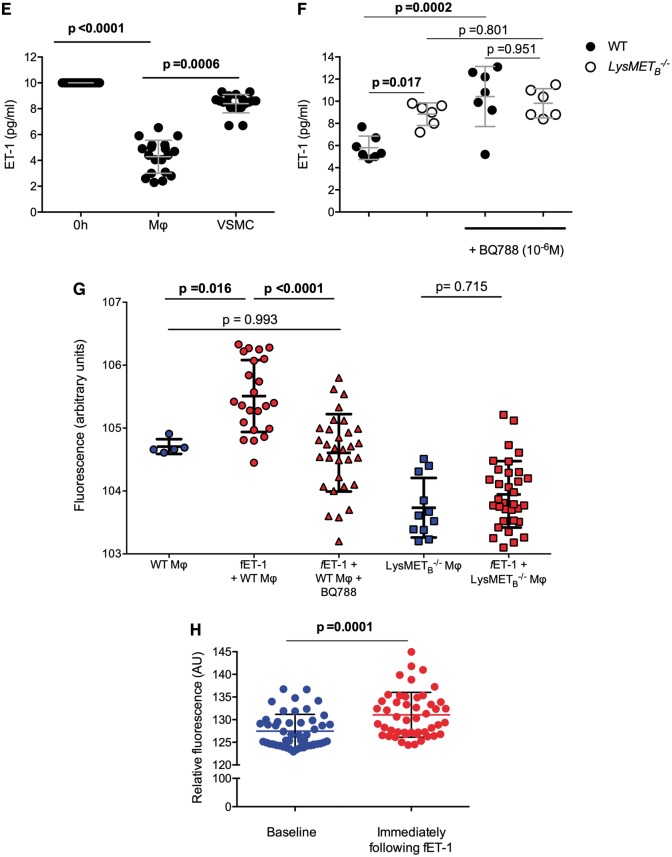
Macrophages modify vascular contractility
The functional importance of Mφ ETB receptor-mediated ET-1 uptake is demonstrated in Figure 5. Here, we infused Mφ into the myography bath 15 min prior to infusing ET-1. Increasing number of Mφ significantly attenuated the vasoconstrictor actions of ET-1 (P < 0.001 vs. control); this effect was lost when the Mφ were pre-treated with selective ETB receptor antagonism or when LysMETB−/− BMDM were infused. There was no effect of pre-treating Mφ with a selective ETA receptor antagonist. Mφ pre-treatment did not alter the contractile response to KCl. Again, these findings are in keeping with a rapid binding of ET-1 to Mφ ETB that blunts its functional effects. Additionally, and as expected, ET-1 increased oxidative stress in mesenteric vessels; this effect was attenuated by Mφ and attenuation was dependent on an unblocked ETB receptor.
Figure 5.
Macrophages modify vascular contraction and oxidative stress in response to endothelin-1. (A) Contraction of mesenteric artery segments to increasing (endothelin-1) (Control, black line, n = 10) and following pre-incubation with varying number of Mφ (n = 6 per group). (B) Vascular responses to 107 Mφ (red line, n = 6), Mφ + BQ123 (dotted red line, n = 4), Mφ from LysMETB−/− mice (blue line, n = 4), and Mφ + BQ788 (dotted blue line, n = 6). Data (mean ± standard deviation) were compared by two-way analysis of variance, with main effects of endothelin-1, pre-treatment and the interaction (all P < 0.0001). Adjusted P-values for planned comparisons are shown and only the comparisons where P < 0.05 at 107 endothelin-1 are shown. (C) The effects of Mφ on oxidative stress. Mean ± standard deviation were compared by one-way analysis of variance (P = 0.0002), using Sidak correction for multiple comparisons, with adjusted P-values are shown.
Human and mouse macrophages show similar responses to endothelin-1
As in mouse BMDM, human Mφ showed expression of both the ETA and ETB receptor (see Supplementary material online, Figure 11A). Similarly, human Mφ were not polarized to a classical or alternative phenotype by ET-1 (see Supplementary material online, Table S1 and Figure S11B) but showed evidence of ETB receptor-mediated ET-1 uptake and chemokinesis to ET-1 and (see Supplementary material online, Figure S11C and D).
Take home figure.
A novel role for myeloid endothelin-B receptors in hypertension.
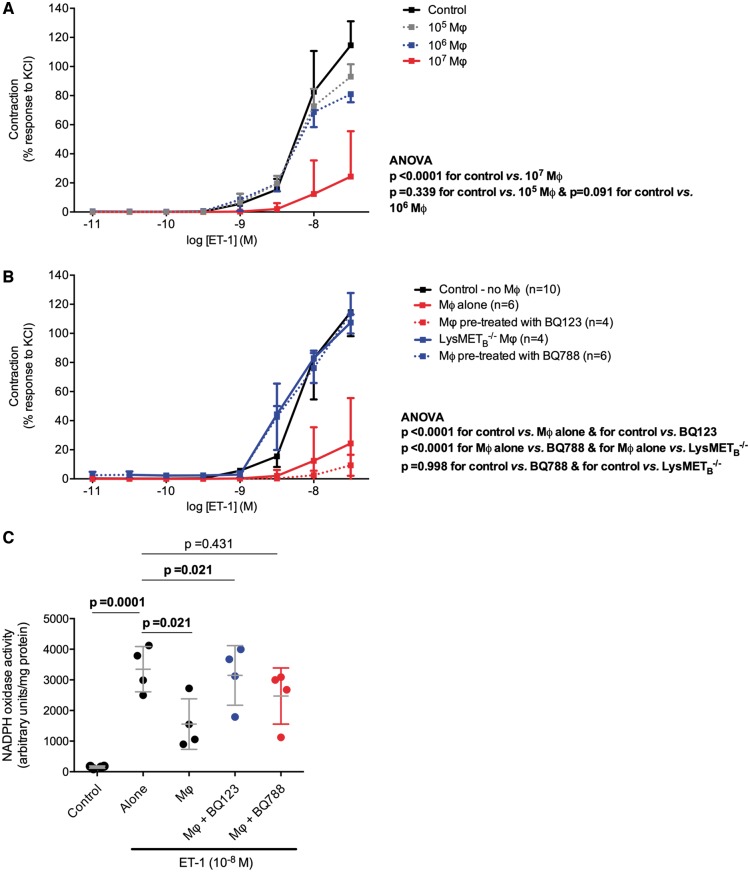
Macrophage depletion augments the pressor response to endothelin-1 but not angiotensin II
To understand the role of Mφ in the pressor response to ET-1, we administered incremental doses of intravenous (i.v.) ET-1 to CD11b-DTR mice given diphtheria toxin (DT) and to controls. Mφ depletion per se was not associated with a difference in baseline mean arterial pressure (MAP) (Figure 6A) or a shift in vasoconstrictor-vasodilator capacity of conduit or resistance vessels (see Supplementary material online, Figure S12). Administration of ET-1 following Mφ depletion resulted in an exaggerated hypertensive response compared with controls (Figure 6B) with a greater maximal change in mean arterial pressure (MAP) in Mφ-deficient mice (Figure 6C). At a dose of ET-1 1 nmol/kg the maximal change in MAP was approximately two-fold greater in Mφ deficient mice compared with control groups. Notably, Mφ depletion did not affect the acute pressor response to ANG II (Figure 6D).
Figure 6.
Role of Mφ in the pressor response to endothelin-1. Acute blood pressure response to incremental doses of endothelin-1 following acute depletion of circulating monocytes and resident Mφ (DTR+DT+), and in controls [those with the diphtheria toxin receptor (DTR) construct but given saline, DTR+DT−, and mice given diphtheria toxin (DT), DTR−DT+; n = 10 mice/group]. (A) Baseline blood pressure. (B) Example of the differences seen among the three groups in the acute pressor response to endothelin-1. Intravenous endothelin-1 administration is defined by the black arrow. The dotted and dashed lines represent baseline and maximal mean arterial pressure, respectively. (C) Maximal change in mean arterial pressure, compared by two-way analysis of variance (main effect of genotype, of endothelin-1 dose and the interaction all P = 0.0001); Holm–Sidak planned comparisons were made and adjusted P-values are shown. (D) Acute blood pressure response to ANG II (1 nmol/kg) following depletion of circulating monocytes and resident Mφ. (E) Baseline mean arterial pressure and (F) maximal change in mean arterial pressure in response to endothelin-1 in LysMETB−/− and littermate control mice (n = 6 mice per group), compared by two-way analysis of variance [main effect of genotype P = 0.0128, of endothelin-1 dose (P = 0.0001) and the interaction P = 0.0004]; Holm–Sidak planned comparisons were made and adjusted P-values are shown.
To specifically explore the role of the Mφ ETB receptor in the pressor response to ET-1, we administered i.v. ET-1 to LysMETB−/− and control mice. Baseline MAP did not differ between the two groups (Figure 6E). Similar to the response seen following systemic Mφ depletion, LysMETB−/− mice demonstrated an exaggerated pressor response to ET-1 (Figure 6F). At a dose of ET-1 0.1 nmol/kg the maximal change in MAP was approximately two-fold that seen in control animals.
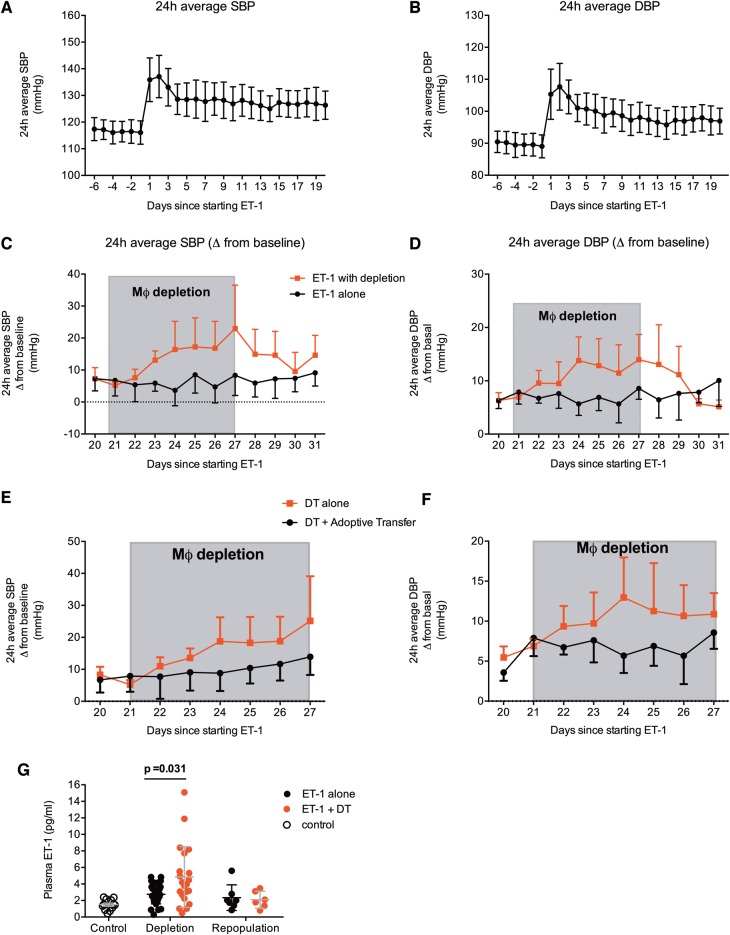
Macrophage depletion augments the chronic hypertensive response to endothelin-1 and adoptive transfer of wild type monocytes prevents this
A 3-week infusion of ET-1 led to sustained increases in systolic and diastolic BP in CD11b-DTR mice (Figure 7A and B). Both systolic and diastolic BP increased by approximately 10–12 mmHg above baseline. DT was used to deplete Mφ over a period of 7–10 days resulting in gradual increases in both systolic and diastolic BP (Figure 7C and D). As the effects of DT weaned, and Mφ repopulated, BP gradually returned to pre-depletion levels. The maximal increase in systolic BP was approximately 20 mmHg and for diastolic BP this was approximately 15 mmHg. In the negative control arm, CD11b-DTR mice were injected with phosphate buffered saline, and here, BP remained stable throughout the experiment.
Figure 7.
Effects of chronic Mφ depletion on endothelin-1-mediated hypertension. Effects on systolic and diastolic blood pressure of endothelin-1 infusion (10 pmol/kg/min administered via minipump) for 3 weeks (mean ± standard deviation, n = 12 mice, A and B). From Day 21, half the mice (n = 6) underwent Mφ depletion over the period shown in grey. Groups were compared by two-away analysis of variance, assessing the main effect of Mφ depletion, duration in days and the interaction. The effect of Mφ depletion was P < 0.0001 for (C) systolic blood pressure and (D) diastolic blood pressure. Effects of diphtheria toxin alone or diphtheria toxin with adoptive transfer of CD11b+Gr1+ monocytes at Day 21 and Day 24 on systolic and diastolic blood pressure are shown in (E) and (F). Two-way analysis of variance reported a significant (P = 0.0003) effect of group. (G) The effects of Mφ depletion and repopulation on plasma endothelin-1 (n = 5 mice per group), assessed by one-way analysis of variance (P < 0.0001), with the adjusted P-values for planned comparisons are shown.
In keeping with an important role for circulating monocytes in these effects, adoptive transfer of wild type monocytes prevented the rise in BP seen with DT (Figure 7E and F). As might be expected, Mφ depletion was associated with a rise in circulating ET-1 with a fall to pre-depletion levels with Mφ repopulation (Figure 7G).
Macrophage depletion augments the chronic hypertensive response to a high salt diet
Next, we explored the role of Mφ in a second model of hypertension that associated with a high salt diet. High salt led to rises in both systolic and diastolic BP of approximately 10 mmHg above baseline (see Supplementary material online, Figure S13A). Mφ depletion led to further rises in both and, as previously seen, these effects diminished with Mφ repopulation (see Supplementary material online, Figure S13B and C).
Myeloid ETB receptor deficiency augments the chronic hypertensive response to endothelin-1 and angiotensin II
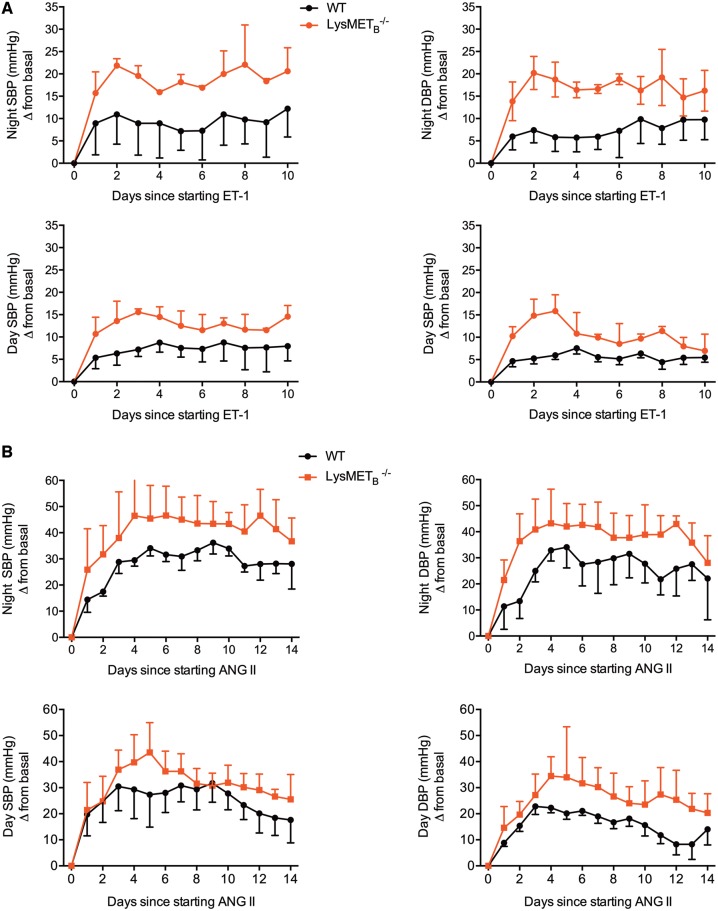
In line with an important role for the myeloid ETB receptor in protecting from the deleterious effects of ET-1, ET-1 administration into LysMETB−/− mice led to a two- to three-fold exaggerated hypertensive response to ET-1 compared with littermate control mice (Figure 8A). To confirm our findings, we exposed LysMETB−/− and littermate control mice to a 2-week infusion of ANG II, a model of hypertension that is ET-1 dependent.20,21 Baseline systolic and diastolic BP were similar between the two groups of animals during both the active (night) and inactive (day) phases. LysMETB−/− mice had a significantly exaggerated hypertensive response to ANG II compared with their controls. For example, at night, systolic BP rose on average by approximately 25 mmHg in control mice but by approximately 40 mmHg in knockouts. For diastolic BP, these corresponding figures were approximately 25 mmHg and approximately 35 mmHg (Figure 8B).
Figure 8.
Effects of myeloid endothelin-B receptor deficiency on endothelin-1 and angiotensin II-mediated hypertension. Night-time and daytime telemetry systolic and diastolic blood pressure in LysMETB−/− and wild type controls receiving 2 weeks of endothelin-1 (5 pmol/kg/min; n = 8 mice per group) (A) or angiotensin II (1 µg/kg/min; n = 12 mice/group) (B). Data are mean ± standard deviation and two-way analysis of variance compared the main effects of genotype, treatment (endothelin-1 or angiotensin II) and the interaction. In all cases, there was a significant (P<0.0001) effect of genotype.
In patients with anti-neutrophil cytoplasmic antibody vasculitis different immunotherapies variably affect blood pressure and the endothelin system
Cyclophosphamide (CYC) and mycophenolate mofetil (MMF) are standard therapies for patients with small vessel vasculitis associated with autoantibodies to neutrophil cytoplasmic antigens (ANCA). Cyclophosphamide not only depletes B and T cells but also effectively depletes circulating and tissue Mφ28; MMF suppresses T- and B-cell function but does not deplete Mφ. Thus, we investigated changes in BP and the ET system following treatment in 20 patients with ANCA vasculitis: 10 received CYC as their immunosuppressive therapy whereas the other 10 received MMF. Demographics and other treatments, including corticosteroid dose, antihypertensive treatment, and the use of plasmapharesis, were similar between groups (see Supplementary material online, Table S2).
Baseline systolic and diastolic BP, plasma and urine ET-1 did not differ between the two groups (see Supplementary material online, Table S2). Interestingly, plasma ET-1 was higher in patients with ANCA vasculitis than in healthy volunteers (3.74 ± 0.38 pg/mL vs. 1.21 ± 1.2 pg/mL, P = 0.02). Whereas CYC reduced mean circulating monocyte count MMF did not (baseline vs. week 6: CYC: 0.87 ± 0.15 vs. 0.26 ± 0.17 × 109, P < 0.05; MMF: 0.77 ± 0.25 vs. 0.69 ± 0.29 × 109, P = 0.756). Cyclophosphamide treatment was associated with a greater increase in BP compared with treatment with MMF (Figure 9A and B, P < 0.01 for CYC vs. MMF). There was a positive correlation between the change in peripheral monocyte count and the extent to which BP rose in those patients receiving CYC (Figure 9C and D). Plasma ET-1 fell following treatment with MMF (Figure 9E, P < 0.001 for both weeks 0 vs. 6 and 6 vs. 12), whereas CYC treatment only led to an initial fall in plasma ET-1 (P < 0.05 for weeks 0 vs. 6). Plasma ET-1 was lower at week 12 in those treated with MMF (Figure 9E). Urine ET-1 fell with both CYC and MMF (Figure 9F, P < 0.01 for CYC and P < 0.0001 for MMF for weeks 0 vs. 12) but to a greater degree with MMF (P < 0.01 for MMF vs. CYC). Flow-mediated dilation (FMD) of the brachial artery was similar at baseline between the two groups (6.7 ± 0.2 for MMF vs. 6.6 ± 0.1% for CYC, P = 0.589). At 12 weeks, FMD had not changed in those patients receiving MMF treatment) whereas those patients receiving CYC had lower FMD in keeping with worse endothelial function (−1.2%, P < 0.05 vs. baseline).
Figure 9.
Effects of treatment with cyclophosphamide and mycophenolate mofetil in patients with autoantibodies to neutrophil cytoplasmic antigens vasculitis. (A and B) Systolic and diastolic blood pressure, with two-way analysis of variance showing a significant effect of treatment (P = 0.001). (C and D) The relationship between change in circulating monocyte count and changes in systolic and diastolic blood pressure is shown. (E) Plasma endothelin-1 (effect of treatment P = 0.0225) and (F) urine endothelin-1 (effect of treatment P = 0.2053). (G and H) Monocyte expression of endothelin-A (P = 0.1310) and endothelin-B receptors (P = 0.0001) in patients with autoantibodies to neutrophil cytoplasmic antigens vasculitis, compared by one-way analysis of variance with adjusted P-values are shown.
In these same patients, we characterized Mφ expression of ETA and ETB receptors and compared this to health (see Supplementary material online, Table S2 and Figure S9G and H). There were no differences in expression of the ETA receptor between the groups and in keeping with our previous data its expression was lower than that of the ETB receptor. Interestingly, patients presenting with ANCA vasculitis demonstrated reduced expression of the ETB receptor on their Mφ compared with levels seen in health. This was normalized by treatment with MMF but further reduced following treatment with CYC.
Discussion
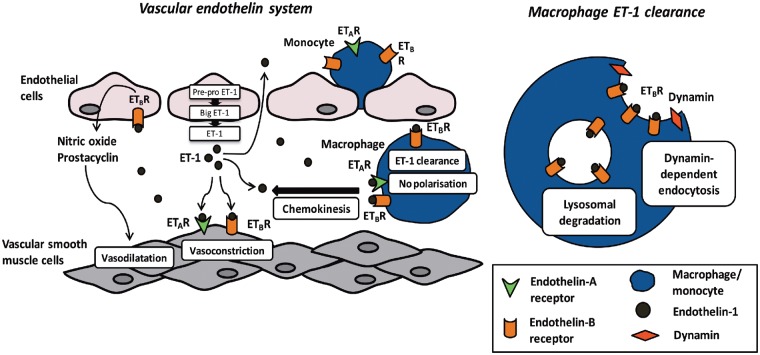
For the first time, we have demonstrated that the Mφ ETB receptor in both mouse and humans provides a novel clearance mechanism for ET-1. Its functional importance in vivo is demonstrated by the exaggerated pro-hypertensive effect of ET-1 and ANG II in mice with a deletion of the Mφ ETB receptor or following systemic Mφ depletion. Interestingly, and unexpectedly, we found no evidence that ET-1 was able to polarize mouse or human Mφ towards a classical pro-inflammatory or alternative anti-inflammatory phenotype but both displayed chemokinesis towards ET-1. Overall, these data provide us with new knowledge, and a clarification of mechanisms underlying the pathological basis of hypertension in relation to the immune and ET systems (Figure 10).
Figure 10.
Macrophage-endothelin system interplay. Endothelin-1 is produced predominantly by vascular endothelial cells. EDN1 gene transcription produces pre-pro endothelin-1 which is cleaved to big endothelin-1 and then endothelin-1. Endothelin-1 is largely secreted abluminally where binding to endothelin-A and endothelin-B receptors on vascular smooth muscle cells causes vasoconstriction. Endothelin-B receptor activation on endothelial cells results in the release of prostacyclin and NO and consequently vasodilatation. Macrophages express both endothelin-A and endothelin-B receptors and display chemokinesis towards endothelin-1. However, endothelin-1 does not polarize Mφ to a pro-inflammatory or anti-inflammatory phenotype. Our data suggest that Mφ clear endothelin-1 through endothelin-B receptor-mediated uptake. Binding of endothelin-1 to endothelin-B receptor on Mφ results in dynamin-dependent endocytosis. This endothelin-B receptor bound endothelin-1 is then transported to the lysosomes for degradation.
To date, many of the studies investigating the role of the innate immune system in the pathogenesis of, and response to, hypertension have focused on the role of T cells in relation to ANG II-mediated hypertension.29–31 Few have examined the role of Mφ and only one study relates to ET-1-mediated vascular injury.32 Recent studies have explored the effects of altering the phenotype of bone marrow-derived cells9 or Mφ10 on hypertension and its complications, whereas others have elegantly depleted neutrophils and Mφ8 to this end. The results of these studies are often contradictory but, nevertheless, suggest that Mφ may contribute to, and protect from, hypertension. The study by Machnik et al.7 showed that salt loading increases Mφ accumulation in the subcutaneous space. These Mφ are stimulated by the hypertonic environment to produce vascular endothelial growth factor C. This leads to proliferation of lymphatics. This is protective, because clodronate-mediated Mφ depletion prevents the lymphatic proliferation and leads to hypertension in response to salt loading. These landmark findings support a role for Mφ acting as a buffering mechanism, protecting against the development of hypertension. Our study extends beyond these, providing a mechanistic understanding of the interaction between Mφ and local vascular contractility and the impact on BP.
The Mφ system comprises a spectrum of cell types, and depletion strategies vary in their specificity and efficacy.33 Hence, we used two depletion models here, the CD11b-DTR (reduction in Mφ number) and lysozyme M (LysM; alternation in Mφ phenotype)-Cre systems. DT administration on CD11b-DTR mice achieved 90% ablation of circulating monocytes. In terms of resident cells, there was a significant reduction in renal Mφ but less of an effect in the liver and spleen. Analysis of the main monocyte subsets (Gr1+CCR2+CX3CR1− and Gr1− CCR2− CX3CR1+) demonstrated that both are equally depleted over the time course of our studies. DT administration did not deplete neutrophils. These findings are in keeping with earlier studies.34,35 Using the LysM-Cre system, there is functional depletion (80–100%) of mature Mφ but also neutrophils. Additionally, this system may partially (16–20%) deplete dendritic cells (DCs),36 an effect that is more pronounced in the CD11b-DTR mice.35
Both CD11b-DTR mice administered DT and LysMETB−/− mice were more sensitive to the pressor effects of ET-1. Taken together it is likely these effects were due to an absence of circulating monocytes and/or vascular Mφ. Given the acute nature of the response, we hypothesize that in control mice circulating monocytes remove the intravenously administered ET-1 to some extent thus attenuating the amount reaching the target VSMC. In contrast, the absence of this ET-1 clearance in Mφ-deplete mice allows more to be available to act on these cells promoting an exaggerated vasoconstrictor and so hypertensive response. There may be a contribution from DCs and neutrophils to these effects. Although there is currently no evidence that murine or human DCs are able to regulate ET-1, by its clearance or degradation, one study has shown that DCs are able to synthesize ET-1 in response to inflammatory stimuli37 although another has suggested the opposite.38 Interestingly, the few data on neutrophils and ET-1 suggest that these cells can both produce and degrade ET-1.39
Our data also demonstrate that Mφ are important in hypertension. We used three models of hypertension—chronic ET-1 and ANG II infusion, which are both dependent on the ET system,20,21,40,41 and a high salt diet. Mφ depletion with repeated doses of DT resulted in gradual increases in both systolic and diastolic BP in chronic ET-1 and salt-dependent hypertension. Interestingly, once the DT administration was stopped (and Mφ were allowed to repopulate) as well as monocyte adoptive transfer resulted in both systolic and diastolic BP returning to pre-depletion levels. This suggests that the importance of both circulating and/or organ-based Mφ and future work should focus on discriminating which of these is more important here. The importance of the ETB receptor in chronic hypertension is provided by data from our mice genetically deficient for ETB on Mφ alone. Here, two separate models of hypertension, chronic ET-1 and chronic ANG II infusion, elicited exaggerated rises in both systolic and diastolic BP in knockout animals compared with littermate controls.
ET-1 is considered to be pro-inflammatory, so it was surprising that it was unable to polarize Mφ phenotype, at least in vitro. This was true at a range of ET-1 concentrations (10–10 000 pg/mL; mean plasma ET-1 in mice 2–3 pg/mL42), whether the ET-1 was administered prior to, following or concomitantly with classical (LPS/INFγ) or alternative (IL-4/IL-13) stimulation. The few data supporting a pro-inflammatory effect of ET-1 on Mφ used immortalized Mφ tumour cell lines and lacked robust methodology.43,44 Interestingly, and in keeping with earlier studies,37,45 LPS/INFγ stimulation of mouse Mφ did increase ET-1 concentrations in the supernatant at 24 h and this was completely blocked by an inhibitor of ECE suggesting that this increase in immunoreactive ET-1 is a result of de novo production by Mφ.
Mouse BMDM demonstrated chemokinesis towards ET-1 and this was reduced by selective ETA antagonism and completely abrogated by selective ETB blockade. Two recent studies support our findings46,47 but both found that the ability of Mφ to move towards ET-1 was more dependent on the ETA receptor than the ETB. Of note, both studies investigated that the role of Mφ and the ET system in the setting of cancer (bladder and breast) where there may well be several different Mφ phenotypes with a different balance of ETA:ETB receptors. In support of ETB-mediated chemokinesis, BMDM from LysMETB−/− displayed no migration towards ET-1. This was not due to an inability to move as they retained their chemokinetic response to MCP-1. Our data showing that Mφ produce ET-1 in response to an inflammatory stimulus allows us to postulate that this may in turn lead to recruitment of further Mφ to the area of inflammation as a mechanism to propagate or regulate the response of the innate immune system.
Thus, although Mφ are not activated by ET-1 they migrate towards it and clear the peptide through ETB receptor-mediated uptake providing a novel clearance mechanism for the peptide. In EC, the ETB receptor resides within caveolae. Binding of ET-1 to endothelial ETB stimulates rapid budding and internalization of the caveolae containing the ETB receptor bound ET-1.48 This mechanism is dynamin-dependent. In our in vitro studies, both mouse and human Mφ removed ET-1 from their surrounding media, an effect that was significantly reduced by selective antagonism (or knockdown) of the ETB receptor but unaffected by ETA blockade. In keeping with ET-1 clearance by caveolar ETB receptors, inhibiting dynamin GTPase activity with dynasore completely prevented ET-1 removal by Mφ. Mφ are multi-functional cells and are able to degrade peptides through the secretion of proteases as well as through the activity of the cell surface metalloprotease, NEP.49 However, broad protease and NEP inhibition did not affect Mφ ET-1 uptake.
To demonstrate the clinical relevance of our findings, we studied patients with ANCA-associated vasculitis, a potentially life-threatening autoimmune condition. Circulating ET-1 was higher in those with vasculitis than in health, probably contributed to by systemic inflammation and endothelial dysfunction. Both MMF and CYC are standard therapies for this condition but they differ in their mechanisms of action. Whereas, MMF inhibits T- and B-cell proliferation and function,50 CYC is directly cytotoxic and depletes not only these cells but also circulating and tissue Mφ.28 In keeping with this action, we demonstrate that CYC has a tendency to reduce the circulating monocyte count by approximately 50% whereas MMF does not. Although this is an accepted measure of circulating monocytes51 it does not account for tissue-based Mφ. Nevertheless, we show here that those patients receiving CYC have a greater increase in BP and deterioration in endothelial function than those receiving MMF. In part, this may be due to loss of Mφ-ET regulation. This is supported by the greater fall in plasma ET-1 in the MMF, but not CYC group, and the down-regulation of the Mφ ETB receptor with CYC but not MMF. Thus, hypertension in CYC-treated patients may respond well to ET antagonists. This hypothesis should be explored in future clinical studies as there are few data that relate to the impact of vasculitis and its treatment on BP or vascular function—which is important because ET antagonism may have broader cardiovascular benefits.52–55
In summary, our study has identified a new interaction between Mφ and the endothelin system, whereby Mφ are drawn to ET-1, without any evident effect on polarization of phenotype, and clear ET-1 from the surrounding milieu. In vivo, this cellular action has a significant impact on acute vascular function and BP; importantly this pathway exerts a restraining effect on BP in chronic hypertension. Our final study strongly suggests that this system is operational in humans and therefore represents an intriguing opportunity to modulate BP and reduce cardiovascular risk in multi-system inflammatory conditions. In future, studies such as ours might lead to newer and currently available BP lowering treatments being used in a more rational way for specific groups of patients, such as those with cancer, as outlined in recent guidelines.56
Supplementary Material
Acknowledgements
The authors would like to thank Anthony Davenport for the antisera against the endothelin-A and endothelin-B receptors.
Funding
This work was supported by the British Heart Foundation [FS/11/78/29328; FS/13/30/29994; FS/16/54/32730], Kidney Research UK [IN10/2010], and the Institut National de Santé et de la Recherche Médicale (INSERM) and research grant from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013)/ ERC grant agreement no 107037 (to Dr. Tharaux).
Conflict of interest: none declared.
References
- 1. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R.. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J.. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 3. Verlohren S, Muller DN, Luft FC, Dechend R.. Immunology in hypertension, preeclampsia, and target-organ damage. Hypertension 2009;54:439–443. [DOI] [PubMed] [Google Scholar]
- 4. Clozel M, Kuhn H, Hefti F, Baumgartner HR.. Endothelial dysfunction and subendothelial monocyte macrophages in hypertension. Effect of angiotensin converting enzyme inhibition. Hypertension 1991;18:132–141. [DOI] [PubMed] [Google Scholar]
- 5. Franco M, Martinez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodriguez-Iturbe B.. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol 2007;293:R251–R256. [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez-Iturbe B, Vaziri ND, Johnson RJ.. Inflammation, angiotensin II, and hypertension. Hypertension 2008;52:e135; author reply e136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J.. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 2009;15:545–552. [DOI] [PubMed] [Google Scholar]
- 8. Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T.. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 2011;124:1370–1381. [DOI] [PubMed] [Google Scholar]
- 9. Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P.. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 2010;55:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ.. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension 2009;54:537–543. [DOI] [PubMed] [Google Scholar]
- 11. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T.. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332:411–415. [DOI] [PubMed] [Google Scholar]
- 12. Boesen EI, Sasser JM, Saleh MA, Potter WA, Woods M, Warner TD, Pollock JS, Pollock DM.. Interleukin-1beta, but not interleukin-6, enhances renal and systemic endothelin production in vivo. Am J Physiol 2008;295:F446–F453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sprague AH, Khalil RA.. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 2009;78:539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S.. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 1990;348:730–732. [DOI] [PubMed] [Google Scholar]
- 15. Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T.. Cloning of a cDNA encoding a non-isopeptide selective subtype of the endothelin receptor. Nature 1990;348:732–735. [DOI] [PubMed] [Google Scholar]
- 16. Davenport AP, O’Reilly G, Molenaar P, Maguire JJ, Kuc RE, Sharkey A, Bacon CR, Ferro A.. Human endothelin receptors characterized using reverse transcriptase-polymerase chain reaction, in situ hybridization, and subtype-selective ligands BQ123 and BQ3020: evidence for expression of ETB receptors in human vascular smooth muscle. J Cardiovasc Pharmacol 1993;22:S22–S25. [DOI] [PubMed] [Google Scholar]
- 17. Verhaar MC, Strachan FE, Newby DE, Cruden NL, Koomans HA, Rabelink TJ, Webb DJ.. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation 1998;97:752–756. [DOI] [PubMed] [Google Scholar]
- 18. Newby DE, Strachan FE, Webb DJ.. Abnormal endothelin B receptor vasomotor responses in patients with Hirschprung’s disease. QJM 2002;95:159–163. [DOI] [PubMed] [Google Scholar]
- 19. Schiffrin EL. State-of-the-Art lecture. Role of endothelin-1 in hypertension. Hypertension 1999;34:876–881. [DOI] [PubMed] [Google Scholar]
- 20. Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG.. Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension 1997;30:29–34. [DOI] [PubMed] [Google Scholar]
- 21. Moreau P, d’Uscio LV, Shaw S, Takase H, Barton M, LüScher TF.. Angiotensin II increases tissue endothelin and induces vascular hypertrophy. Reversal by ETA-receptor antagonist. Circulation 1997;96:1593–1597. [DOI] [PubMed] [Google Scholar]
- 22. Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V.. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. N Engl J Med 1998;338:784–790. [DOI] [PubMed] [Google Scholar]
- 23. Nakov R, Pfarr E, Eberle S.. Darusentan: an effective endothelin A receptor antagonist for treatment of hypertension. Am J Hypertens 2002;15:583–589. [DOI] [PubMed] [Google Scholar]
- 24. Weber MA, Black H, Bakris G, Krum H, Linas S, Weiss R, Linseman JV, Wiens BL, Warren MS, Lindholm LH.. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet 2009;374:1423–1431. [DOI] [PubMed] [Google Scholar]
- 25. Boesen EI, Krishnan KR, Pollock JS, Pollock DM.. ETA activation mediates angiotensin II-induced infiltration of renal cortical T cells. J Am Soc Nephrol 2011;22:2187–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tostes RC, Touyz RM, He G, Ammarguellat F, Schiffrin EL.. Endothelin A receptor blockade decreases expression of growth factors and collagen and improves matrix metalloproteinase-2 activity in kidneys from stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol 2002;39:892–900. [DOI] [PubMed] [Google Scholar]
- 27. Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS.. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 2007;18:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santosuosso M, Divangahi M, Zganiacz A, Xing Z.. Reduced tissue macrophage population in the lung by anticancer agent cyclophosphamide: restoration by local granulocyte macrophage-colony-stimulating factor gene transfer. Blood 2002;99:1246–1252. [DOI] [PubMed] [Google Scholar]
- 29. Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL.. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 2011;57:469–476. [DOI] [PubMed] [Google Scholar]
- 30. Zhang JD, Patel MB, Song YS, Griffiths R, Burchette J, Ruiz P, Sparks MA, Yan M, Howell DN, Gomez JA, Spurney RF, Coffman TM, Crowley SD.. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res 2012;110:1604–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG.. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007;204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Javeshghani D, Barhoumi T, Idris-Khodja N, Paradis P, Schiffrin EL.. Reduced macrophage-dependent inflammation improves endothelin-1-induced vascular injury. Hypertension 2013;62:112–117. [DOI] [PubMed] [Google Scholar]
- 33. Chow A, Brown BD, Merad M.. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol 2011;11:788–798. [DOI] [PubMed] [Google Scholar]
- 34. Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP.. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 2005;115:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TM, Marson LP, Kluth DC, Hughes J.. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int 2012;82:928–933. [DOI] [PubMed] [Google Scholar]
- 36. Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I.. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgen Res 1999;8:265–277. [DOI] [PubMed] [Google Scholar]
- 37. Spirig R, Potapova I, Shaw-Boden J, Tsui J, Rieben R, Shaw SG.. TLR2 and TLR4 agonists induce production of the vasoactive peptide endothelin-1 by human dendritic cells. Mol Immunol 2009;46:3178–3182. [DOI] [PubMed] [Google Scholar]
- 38. Slobodin G, Pavlotzky E, Panov J, Rosner I, Kessel A, Toubi E.. Endothelin-1 does not change the function of monocyte-derived dendritic cells grown from patients with systemic sclerosis. Immunol Invest 2008;37:841–848. [DOI] [PubMed] [Google Scholar]
- 39. Sessa WC, Kaw S, Zembowicz A, Anggard E, Hecker M, Vane JR.. Human polymorphonuclear leukocytes generate and degrade endothelin-1 by two distinct neutral proteases. J Cardiovasc Pharmacol 1991;17:S34–S38. [DOI] [PubMed] [Google Scholar]
- 40. Elmarakby AA, Loomis ED, Pollock JS, Pollock DM.. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension 2005;45:283–287. [DOI] [PubMed] [Google Scholar]
- 41. Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM.. Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension 2010;56:942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bagnall AJ, Kelland NF, Gulliver-Sloan F, Davenport AP, Gray GA, Yanagisawa M, Webb DJ, Kotelevtsev YV.. Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension 2006;48:286–293. [DOI] [PubMed] [Google Scholar]
- 43. Ruetten H, Thiemermann C.. Endothelin-1 stimulates the biosynthesis of tumour necrosis factor in macrophages: ET-receptors, signal transduction and inhibition by dexamethasone. J Physiol Pharmacol 1997;48:675–688. [PubMed] [Google Scholar]
- 44. Wilson SH, Simari RD, Lerman A.. The effect of endothelin-1 on nuclear factor kappa B in macrophages. Biochem Biophys Res Commun 2001;286:968–972. [DOI] [PubMed] [Google Scholar]
- 45. Ehrenreich H, Anderson RW, Fox CH, Rieckmann P, Hoffman GS, Travis WD, Coligan JE, Kehrl JH, Fauci AS.. Endothelins, peptides with potent vasoactive properties, are produced by human macrophages. J Exp Med 1990;172:1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Said N, Smith S, Sanchez-Carbayo M, Theodorescu D.. Tumor endothelin-1 enhances metastatic colonization of the lung in mouse xenograft models of bladder cancer. J Clin Invest 2011;121:132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen CC, Chen LL, Hsu YT, Liu KJ, Fan CS, Huang TS.. The endothelin-integrin axis is involved in macrophage-induced breast cancer cell chemotactic interactions with endothelial cells. J Biol Chem 2014;289:10029–10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oh P, Horner T, Witkiewicz H, Schnitzer JE.. Endothelin induces rapid, dynamin-mediated budding of endothelial caveolae rich in ET-B. J Biol Chem 2012;287:17353–17362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schreiter A, Gore C, Labuz D, Fournie-Zaluski MC, Roques BP, Stein C, Machelska H.. Pain inhibition by blocking leukocytic and neuronal opioid peptidases in peripheral inflamed tissue. FASEB J 2012;26:5161–5171. [DOI] [PubMed] [Google Scholar]
- 50. Ransom JT. Mechanism of action of mycophenolate mofetil. Ther Drug Monitor 1995;17:681–684. [DOI] [PubMed] [Google Scholar]
- 51. Barr LC, Brittan M, Morris AC, McAuley DF, McCormack C, Fletcher AM, Richardson H, Connell M, Patel D, Wallace WA, Rossi AG, Davidson DJ, Manson L, Turner M, Hirani N, Walsh TS, Anderson NH, Dhaliwal K, Simpson AJ.. A randomized controlled trial of peripheral blood mononuclear cell depletion in experimental human lung inflammation. Am J Resp Crit Care Med 2013;188:449–455. [DOI] [PubMed] [Google Scholar]
- 52. Dhaun N, Goddard J, Kohan DE, Pollock DM, Schiffrin EL, Webb DJ.. Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension 2008;52:452–459. [DOI] [PubMed] [Google Scholar]
- 53. Dhaun N, Goddard J, Webb DJ.. The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol 2006;17:943–955. [DOI] [PubMed] [Google Scholar]
- 54. Dhaun N, Johnston NR, Goddard J, Webb DJ.. Chronic selective endothelin a receptor antagonism reduces serum uric Acid in hypertensive chronic kidney disease. Hypertension 2011;58:e11–e12. [DOI] [PubMed] [Google Scholar]
- 55. Dhaun N, MacIntyre IM, Kerr D, Melville V, Johnston NR, Haughie S, Goddard J, Webb DJ.. Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension 2011;57:772–779. [DOI] [PubMed] [Google Scholar]
- 56. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.