Abstract
Background:
The data needed to understand the characteristics and outcomes, over time, of adolescents enrolling in HIV care in East Africa are limited.
Setting:
Six HIV care programs in Kenya, Tanzania, and Uganda.
Methods:
This retrospective cohort study included individuals enrolling in HIV care as younger adolescents (10–14 years) and older adolescents (15–19 years) from 2001–2014. Descriptive statistics were used to compare groups at enrollment and antiretroviral therapy (ART) initiation over time. The proportion of adolescents was compared with the total number of individuals aged 10 years and older enrolling over time. Competing-risk analysis was used to estimate 12-month attrition after enrollment/pre-ART initiation; post-ART attrition was estimated by Kaplan-Meier method.
Results:
A total of 6344 adolescents enrolled between 2001 and 2014. The proportion of adolescents enrolling among all individuals increased from 2.5% (2001–2004) to 3.9% (2013–2014, P< 0.0001). At enrollment, median CD4 counts in 2001–2004 compared with 2013–2014 increased for younger (188 vs. 379 cells/ mm3, P< 0.0001) and older (225 vs. 427 cells/mm3, P< 0.0001) adolescents. At ART initiation, CD4 counts increased for younger (140 vs. 233 cells/mm3, P< 0.0001) and older (64 vs. 323 cells/ mm3, P< 0.0001) adolescents. Twelve-month attrition also increased for all adolescents both after enrollment/pre-ART initiation (4.7% vs. 12.0%, P< 0.001) and post-ART initiation (18.7% vs. 31.2%, P< 0.001).
Conclusions:
Expanding HIV services and ART coverage was likely associated with earlier adolescent enrollment and ART initiation but also with higher attrition rates before and after ART initiation. Interventions are needed to promote retention in care among adolescents.
Keywords: HIV, adolescents, antiretroviral therapy, East Africa, retention
INTRODUCTION
Adolescents living with HIV are a priority population in addressing the global HIV epidemic.1,2 The major burden of HIV among adolescents exists in sub-Saharan Africa, where 85% of the estimated 2 million global HIV-infected adolescents live, and where 92% of global AIDS-related adolescent deaths occur.3–5 Although new HIV infections and AIDS- related deaths have declined overall in sub-Saharan Africa, HIV remains the leading cause of death among adolescents 10–19 years of age in the region.3,6 Furthermore, the World Health organization (WHo) estimated a 50% increase in the incidence of AIDS-related deaths among adolescents from 2005 to 2012, compared with a 32% decrease among other age groups.4,7 Despite the broad scale-up of HIV services that includes expanded antiretroviral therapy (ART) coverage and eligibility that occurred over this timeframe, few studies describe how the characteristics of adolescents enrolling in HIV care and initiating ART have changed over the years.6
Understanding these characteristics is important because adolescents face multiple challenges in navigating the HIV care and treatment cascade. Limited awareness of HIV infection, inadequate access to testing and counseling services, and low uptake of these services lead to delayed care entry and more advanced disease stage at enrollment in care.1,8,11 Multiple studies in sub-Saharan Africa have reported higher attrition for adolescents both before and after ART initiation when compared with adults, and poor adherence to ART and attrition have both been associated with worse virologic outcomes and higher mortality compared with adults.11–22Many of these studies report aggregate data or are limited by small sample sizes.4 As such, they do not allow us to address the distinct developmental and social differences between younger and older adolescents, who may have different modes of HIV acquisition and risks of adverse outcomes. Moreover, many studies are cross-sectional and limited in their ability to extrapolate epidemiologic trends to evaluate and contextualize public health interventions, or sample from few geographic locations with potentially limited generalizability.4
As of 2014, Kenya, Tanzania, and Uganda together accounted for 33% of HIV-infected adolescents aged 10–19% and 31% of AIDS-related adolescent deaths in Eastern and Southern Africa.24 The population of HIV-infected adolescents in these and other global regions is expected to grow over the coming years, given the increasing survival of perinatally infected children, the contribution of new sexually transmitted infections, and the projected increase in the absolute number of adolescents in living in low-income countries.4,25 Achieving the Joint United Nations Programme on HIV/AIDS 90–90-90 targets for this vulnerable population will require robust data concerning the epidemiologic patterns of adolescents enrolling in HIV care and initiating ART.6,26 The objective of this study is to understand the characteristics and outcomes over time of adolescents enrolling in HIV care and initiating ART in Kenya, Tanzania, and Uganda using data from the East Africa (EA) International Epidemiology Databases to Evaluate AIDS (IeDEA) Consortium.
METHODS
Study Design
This retrospective cohort used data of prospectively enrolled HIV-infected adolescents attending HIV care programs affiliated with the EA-IeDEA Consortium. The study was approved under the EA-IeDEA by the Indiana University IRB and all local regulatory bodies affiliated with each participating site. Patient-level consent was waived by all regulatory bodies because the data were collected as part of routine clinical care and were deidentified before data transfer and analysis.
Study Setting
This study was conducted at 6 HIV care programs in Kenya, Tanzania, and Uganda that participate in EA-IeDEA, an NIH-funded consortium that pools and harmonizes patient data collected in the context of routine care. Descriptions of the study sites can be found elsewhere.27,28 All facilities provided standard-of-care HIV treatment services as per their respective local and national guidelines. None of the facilities during the period of study offered Adolescent Clinics. With the exception of Moi Teaching and Referral Hospital at the Academic Model Providing Access to Healthcare (AM- PATH) program in Kenya, all children were seen in the same clinic as adults; however, at times, there was a dedicated clinic day for children when a consultant might be present to support the regular providers, and care was not transitioned. At this clinic, adolescents were commonly seen in the pediatric clinic into their 20s. The study population included all adolescents who were between 10 and 19 years of age, inclusive, at the time of enrollment in HIV care at one of the participating programs between 2001 and 2014.
Data Management
At each facility, demographic and clinical data were collected on paper-based forms completed by clinicians during the course of routine clinical care. Forms were then transcribed into the local electronic medical record system by trained data entry clerks. Data quality assessments were performed according to local protocols for each facility. Data were transferred biannually to the IeDEA-EA Regional Data Center where they were merged, and additional data quality assessments were undertaken before data were submitted for analysis.
Data Analysis
Adolescence was defined as ages 10–19 years to be consistent with the WHO and United Nations definition of adolescence.3,29 Adolescents were stratified into 2 groups based on age at the time of enrollment in HIV care: younger adolescents (10–14 years) and older adolescents (15–19 years). Patient-level, independent variables included the following: (1) demographic characteristics: sex, age, and pregnancy status at enrollment, and (2) clinical characteristics: WHO clinical stage at enrollment and at ART initiation, CD4 count at enrollment and at ART initiation, receipt of pneumocystis pneumonia prophylaxis, or isoniazid prophylaxis for tuberculosis. For all post-enrollment variables, the follow-up time was constructed such that younger adolescents were censored on reaching age 15, and older adolescents were censored on their 20th birthday. Outcome variables included incident pregnancy and TB diagnosis, which excluded prevalent pregnancy and TB cases, and were reported in person-year incidence rates to account for differences in observation time between adolescents in each group. The first available WHO stage within 90 days after enrollment was recorded as the WHO stage at enrollment. If no WHO stage was given within this window and the next available WHO stage was stage 1, then the WHO stage at enrollment was assumed to be stage 1. Subjects with no WHO stage within the 90-day window and no WHO stage 1 beyond this window were categorized as having missing WHO stage at enrollment. The maximum WHO stage before ART initiation was taken to be the WHO stage at ART initiation. If no stage was available before ART initiation, the first stage within 60 days after ART initiation was used. If no WHO stage was given within this 60-day window and the next available was stage 1, then the WHO stage was assumed to be stage 1. Otherwise, WHO stage at ART initiation was recorded as missing.
To assess the changes in characteristics and retention for younger and older adolescents at enrollment and ART initiation over time, the study timeframe was divided into 4 enrollment periods: 2001–2004, 2005–2009, 2010–2012, and 2013–2014. The first period occurred before the introduction of the United States President’s Emergency Plan for AIDS Relief (PEPFAR) program while the remaining periods align with the WHO guideline changes for ART eligibility.30,31 The characteristics of younger and older adolescents enrolling within each of these periods were summarized with standard descriptive analyses. The proportion of enrolling adolescents was compared with the total number of individuals aged 10 years and older enrolling during each period. The Cochran- Armitage linear trend test was used for categorical variables to assess the presence of association and detect trends across periods for younger and older adolescents; the Kruskal- Wallis test was used for continuous variables. All P values were two-tailed with a value <0.05 considered statistically significant.
Program attrition at 12 months after enrollment but before ART initiation, and at 12 months after ART initiation, was analyzed in each enrollment period. For this analysis, younger adolescents who reached their 15th birthday and older adolescents who reached their 20th birthday but who had not attained 12 months of follow-up were not censored at their date of birth. Attrition was defined as the composite outcome of death and loss to follow-up (LTFU). LTFU was defined as having no clinic visits for 90 days after the last scheduled clinic appointment and not having been recorded as dead or transferred out before the 12-month endpoint.27 Competing-risk estimation was used to estimate attrition incidence after enrollment but before ART initiation, where ART initiation was treated as a competing risk. Cumulative incidence of attrition was calculated by the Aalen-Johansen estimator. Attrition after ART initiation was calculated using the Kaplan-Meier method. Cumulative incidence of attrition was estimated as one minus the Kaplan-Meier estimate of the probability of retention. Cumulative incidences were compared between groups using the Gray test.32 In both cases, 95% confidence intervals were produced by these methods. Landmark values of attrition at 12 months after enrollment and ART initiation, with attendant 95% confidence intervals, were also produced. Follow-up time was determined by the Kaplan-Meier method with end of follow-up being the event of interest and program attrition considered a censoring event.
RESULTS
Characteristics at Enrollment and ART Initiation
A total of 6344 adolescents aged 10–19 years were enrolled into care from November 27, 2001, through December 10, 2014 (Table 1). The total number of adolescents enrolled across programs ranged from 142 adolescents (2.2% of total cohort) in Rakai, Uganda, to 3845 adolescents (60.6% of total cohort) at AMPATH, Kenya. Overall, 3241 (51.1%) were younger adolescents, and younger adolescent enrollment exceeded older adolescent enrollment in over half of the facilities. Two-thirds (67.2%) of all adolescents were female, with girls accounting for 55.4% of younger and 79.5% of older adolescents. The prevalence of tuberculosis at enrollment was 3.5% for younger and 1.4% for older adolescents. Among those with available WHO stage at enrollment, the majority (71.2%) had WHO stage 1 or 2, including 63.0% of younger and 78.9% of older adolescents. Among those with available WHO stage at ART initiation, 46.2% and 64.2% of younger and older adolescents, respectively, had WHO stage 1 or 2 at ART initiation.
TABLE 1.
Characteristics at Enrollment at Study Facilities in Kenya, Tanzania, and Uganda From 2001 to 2014
| Total |
Younger Adolescents |
Older Adolescents |
|
|---|---|---|---|
| n (%) |
10–14 yrs, n (%) |
15–19 yrs, n (%) |
|
| Characteristic | n = 6344 | n = 3241 | n = 3103 |
| East African IeDEA site | |||
| AMPATH, Kenya | 3845 (60.6) | 2266 (69.9) | 1579 (50.9) |
| FACES, Kenya | 1198 (18.9) | 270 (8.3) | 928 (29.9) |
| Morogoro, Tanzania | 285 (4.5) | 188 (5.8) | 97 (3.1) |
| Tumbi, Tanzania | 221 (3.5) | 125 (3.9) | 96 (3.1) |
| Masaka, Uganda | 653 (10.3) | 312 (9.6) | 341 (11.0) |
| Rakai, Uganda | 142 (2.2) | 80 (2.5) | 62 (2.0) |
| Sex | |||
| Male | 2082 (32.8) | 1447 (44.7) | 635 (20.5) |
| Female | 4262 (67.2) | 1794 (55.4) | 2468 (79.5) |
| Pregnant at enrollment* | 534 (12.5) | 15 (0.8) | 519 (21.0) |
| Tuberculosis at | 156 (2.5) | 114 (3.5) | 42 (1.4) |
| enrollment | |||
| WHO stage at enrollment | |||
| Stage 1 and 2 | 3077 (48.5) | 1320 (40.7) | 1757 (56.6) |
| Stage 3 and 4 | 1244 (19.6) | 775 (23.9) | 469 (15.1) |
| Missing | 2023 (31.9) | 1146 (35.4) | 877 (28.3) |
| WHO stage at ART | |||
| initiation † | |||
| Stage 1 and 2 | 1803 (39.9) | 842 (32.9) | 961 (49.1) |
| Stage 3 and 4 | 1518 (33.6) | 981 (38.3) | 537 (27.4) |
| Missing | 1199 (26.5) | 739 (28.8) | 460 (23.5) |
Percentage expressed among female adolescent population only.
A total of 4520 adolescents initiated ART.
FACES, Family AIDS Care and Education Services.
Clinical Events while Enrolled
The median follow-up period for younger adolescents was 5.0 years (95% confidence interval: 4.6 to 5.4) and 2.1 years (95% confidence interval: 1.9 to 2.3) for older adolescents estimated by Kaplan-Meier analysis. The incidence of first pregnancy after enrollment was 6 cases per 100,000 person-years for younger and 100 per 100,000 person-years for older female adolescents. The incidence of tuberculosis was 28 and 12 cases per 100,000 person-years for younger and older adolescents, respectively.
Characteristics Over Time
From 2001 to 2014, the proportion of adolescents among all individuals aged 10 years and older enrolling at the study facilities increased from 2.5% in 2001–2004% to 3.9% in 2013–2014 (Fig. 1; P< 0.0001). During the same period, the proportion of older adolescents enrolling into care increased from 0.9% to 2.6% (P < 0.0001). The proportion of female adolescents also increased over time for younger (47.1%−60.8%, P = 0.003) and older (70.7%−82.7%, P = 0.001) adolescents (Table 2). The percentage of younger and older adolescents who received pneumocystis pneumonia prophylaxis increased over time, from 83.0% to 98.3% for younger (P < 0.0001) and 56.1%−99.9% for older (P < 0.0001) adolescents from 2001–2004 to 2013–2014, respectively. Over the same periods, the percentage who received isoniazid prophylaxis for tuberculosis decreased for younger (10.5%−2.6%, P< 0.0001) and older (29.3%−3.6%, P< 0.0001) adolescents.
FIGURE 1.
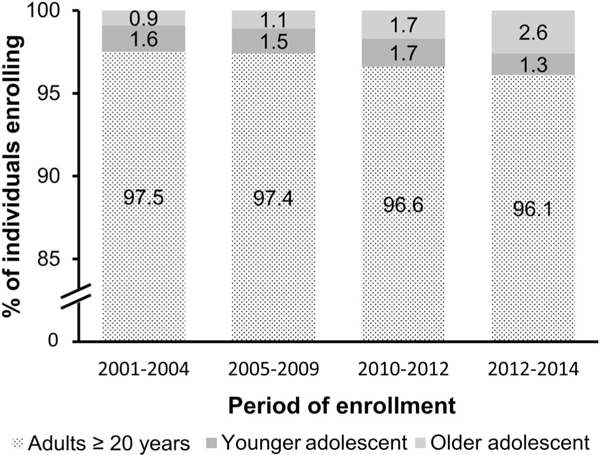
Proportion of adolescents among all individuals aged 10 years and older enrolling at the study facilities from 2001 to 2014. *The total number of individuals aged 10 years and older enrolling at the study facilities during each period was 2001–2004 (n = 9320);2005–2009 (n = 110,488);2010– 2012 (n = 62,838);and 2013–2014 (n = 27,223). †Pro-portions were significantly different from one another across periods for younger (P < 0.0001) and older (P < 0.0001) adolescents.
TABLE 2.
Characteristics of Adolescents Enrolling at Study Facilities in Kenya, Tanzania, and Uganda Over Four Periods Between 2001 and 2014
| Characteristic Younger Adolescents |
Period of Enrollment |
P | |||
|---|---|---|---|---|---|
| 2001–2004 |
2005–2009 |
2010–2012 |
2013–2014 |
||
| n (%) |
n (%) |
n (%) |
n (%) |
||
| n = 153 | n = 1672 | n = 1074 | n = 342 | ||
| Female sex | 72 (47.1) | 907 (54.3) | 607(56.5) | 208 (60.8) | 0.003 |
| Receipt of PCP prophylaxis | 127 (83.0) | 1527 (91.3) | 1041(96.9) | 336 (98.3) | <0.0001 |
| Receipt of isoniazid for TB prophylaxis | 16 (10.5) | 81 (4.8) | 24(2.2) | 9 (2.6) | <0.0001 |
| CD4 at enrollment, median (IQR) | 188 (52-444) | 262 (93-471) | 307(117-550) | 379 (144-662) | <0.0001 |
| CD4 at ART initiation, median (IQR) | 140 (33-329) | 172 (56-298) | 221(74-385) | 233 (73-479) | <0.0001 |
| WHO stage at enrollment* | |||||
| Stage 1 and 2 | 41 (26.8) | 722 (43.2) | 472(44) | 85 (24.9) | — |
| Stage 3 and 4 | 49 (32.0) | 466 (27.9) | 243(22.6) | 17 (5.0) | — |
| Missing | 63 (41.2) | 484 (29.0) | 359(33.4) | 240 (70.2) | — |
| WHO stage at ART initiation*† | |||||
| Stage 1 and 2 | 21 (14.4) | 432 (32.7) | 341(39.5) | 48 (20.6) | — |
| Stage 3 and 4 | 82 (56.2) | 603 (45.7) | 281(32.6) | 15 (6.4) | — |
| Missing | 43 (29.5) | 285 (21.6) | 241(27.9) | 170 (73.0) | — |
| Older Adolescents | n = 82 | n = 1234 | n= 1069 | n = 718 | |
| Female sex | 58 (70.7) | 959 (77.7) | 857(80.2) | 594 (82.7) | 0.0013 |
| Receipt of PCP prophylaxis | 46 (56.1) | 907 (73.5) | 1021(95.5) | 717 (99.9) | <0.0001 |
| Receipt of isoniazid for TB prophylaxis | 24(29.3) | 124(10.1) | 34(3.2) | 26 (3.6) | <0.0001 |
| CD4 at enrollment, median (IQR) | 225 (25-520) | 322 (134-529) | 398(178-612) | 427 (261 -646) | <0.0001 |
| CD4 at ART initiation, median (IQR) | 64 (13-246) | 153 (57-258) | 219(63-358) | 323 (157-480) | <0.0001 |
| WHO stage at enrollment* | |||||
| Stage 1 and 2 | 35 (42.7) | 643 (52.1) | 611(57.2) | 468 (65.2) | — |
| Stage 3 and 4 | 17 (20.7) | 234 (19.0) | 136(12.7) | 82 (11.4) | — |
| Missing | 30 (36.6) | 357 (28.9) | 322(30.1) | 168 (23.4) | — |
| WHO stage at ART initiation*† | |||||
| Stage 1 and 2 | 18 (28.6) | 303 (42.7) | 340(49.9) | 300 (59.5) | — |
| Stage 3 and 4 | 24 (38.1) | 246 (34.7) | 183(26.8) | 84 (16.7) | — |
| Missing | 21 (33.3) | 160 (22.6) | 159(23.3) | 120 (23.8) | — |
| Total Adolescents | n = 235 | n = 2906 | n= 2143 | n = 1060 | |
| Female sex | 130 (55.3) | 1866 (64.2) | 1464(68.3) | 802 (75.7) | <0.0001 |
| Receipt of PCP prophylaxis | 202 (86.0) | 2618 (90.1) | 2096(97.8) | 1057 (99.7) | <0.0001 |
| Receipt of isoniazid for TB prophylaxis | 47 (20.0) | 238 (8.2) | 69(3.2) | 37 (3.5) | <0.0001 |
| CD4 at enrollment, median (IQR) | 197 (45-444) | 281 (105-491) | 352(143-588) | 419 (207-652) | <0.0001 |
| CD4 at ART initiation, median (IQR) | 126 (20-320) | 164 (56-280) | 221(71-371) | 309 (124-480) | <0.0001 |
| WHO stage at enrollment* | |||||
| Stage 1 and 2 | 76 (32.3) | 1365 (47.0) | 1083(50.5) | 553 (52.2) | — |
| Stage 3 and 4 | 66 (28.1) | 700 (24.1) | 379(17.7) | 99 (9.3) | — |
| Missing | 93 (39.6) | 841 (28.9) | 681(31.8) | 408 (38.5) | — |
| WHO stage at ART initiation*† | |||||
| Stage 1 and 2 | 39 (18.7) | 735 (36.2) | 681(44.1) | 348 (47.2) | — |
| Stage 3 and 4 | 106 (50.7) | 849 (41.8) | 464(30.0) | 99 (13.4) | — |
| Missing | 64 (30.6) | 445 (21.9) | 400(25.9) | 290 (39.4) | — |
Statistical tests of significance were not performed for WHO stages given the high proportions of missing data.
Percentages expressed among total number of adolescents who initiated ART (n = 4520).
PCP, pneumocystis pneumonia; TB, tuberculosis.
Overall, both younger and older adolescents had less advanced HIV disease at enrollment and at ART initiation in more recent periods (Table 2 and Fig. 2). At enrollment, median CD4 counts increased for younger adolescents from 188 cells/mm3 in 2001–2004 to 379 cells/mm3 in 2013–2014 (P < 0.0001), and also increased among older adolescents, from 225 cells/mm3 to 427 cells/mm3 (P < 0.0001) over the same periods. At ART initiation, median CD4 counts also increased for younger adolescents (140 vs. 233 cells/mm3, P< 0.0001) and older adolescents (64 vs. 323 cells/mm3, P<0.0001) over these periods. The proportions of younger adolescents, among the various enrollment periods, with missing WHO stage ranged from 29.0% to 70.2% at enrollment and 21.6%−73.0% at ART initiation across periods (Table 2). For older adolescents, the proportions with missing WHO stage ranged from 23.4% to 36.6% at enrollment and 22.6%−33.3% at ART initiation. Statistical analysis for WHO stage differences between groups and over time was not performed given the high proportion of missing data.
FIGURE 2.
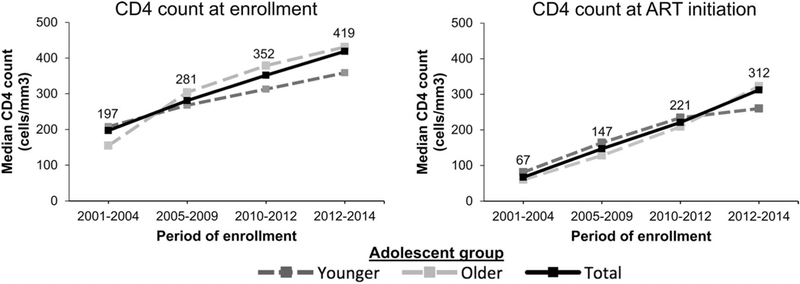
Median CD4 count for younger, older, and total (labeled) adolescents at enrollment and ART initiation across periods. All values were significantly different from one another across periods (P < 0.0001).
The cumulative incidence estimates of 12-month attrition after enrollment/pre-ART initiation significantly increased over time among all adolescents, from 2.6% in 2001–2004 to 8.7% in 2013–2014 (P < 0.001; Table 3). After ART initiation, 12-month attrition among all adolescents also increased over the same periods, from 18.7% to 31.2% (P < 0.001). Attrition estimates after enrollment/pre-ART initiation and after ART initiation were significantly higher for older compared with younger adolescents in all periods except 2013–2014.
TABLE 3.
Attrition After Enrollment and After ART Initiation for Younger, Older, and all Adolescents Enrolling at Study Facilities in Kenya, Tanzania, and Uganda From 2001 to 2014
| Age Group | Period of Enrollment |
P* | |||
|---|---|---|---|---|---|
| 2001-2004 |
2005-2009 |
2010-2012 |
2013-2014 |
||
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | ||
| Attrition at 12 mo after enrollment | |||||
| Younger adolescents | 2.6 (0.9 to 6.2) | 12.1 (10.6 to 13.8) | 9.9 (8.2 to 11.8) | 8.7 (5.9 to 12.1) | <0.001 |
| Older adolescents | 8.5 (3.7 to 15.9) | 25.4 (23.0 to 27.8) | 21.5 (19.1 to 24.0) | 13.6 (11.0 to 16.4) | <0.001 |
| Total adolescents | 4.7 (2.5 to 7.9) | 17.7 (16.4 to 19.2) | 15.7 (14.1 to 17.3) | 12.0 (10.1 to 14.2) | <0.001 |
| P value† | 0.041 | <0.001 | <0.001 | 0.076 | |
| Attrition rate at 12 mo after ART initiation | |||||
| Younger adolescents | 15.1 (9.8 to 21.4) | 15.4 (13.5 to 17.4) | 19.8 (17.2 to 22.6) | 25.3 (18.8 to 32.3) | <0.001 |
| Older adolescents | 27.0 (16.7 to 38.4) | 24.5 (21.4 to 27.7) | 31.8 (28.2 to 35.4) | 34.3 (28.7 to 40.0) | 0.016 |
| Total adolescents | 18.7 (13.7 to 24.2) | 18.6 (16.9 to 20.3) | 25.1 (22.9 to 27.3) | 31.2 (26.9 to 35.6) | <0.001 |
| P value† | 0.035 | <0.001 | <0.001 | 0.124 | |
Compares the cumulative incidence curves by enrollment periods within adolescent age groups
Compares the cumulative incidence curves by adolescent age groups within enrollment periods using the Gray test.
CI, confidence interval.
DISCUSSION
This study presents the trends over time for adolescents enrolling in HIV care and initiating ART at HIV care programs in Kenya, Tanzania, and Uganda. We found that the proportion of older adolescents enrolling among all enrollees aged 10 years and older significantly increased from 0.9% in 2001–2004% to 2.6% in 2013–2014. Although adolescents accounted for a low proportion of individuals aged 10 years and older enrolling overall, the proportion of older adolescents increased nearly threefold over the study timeframe, whereas the proportion of younger adolescents was stable or slightly declined over the same periods. This finding highlights the importance of disaggregating adolescent data by age to better understand programmatic and epidemiologic trends in the population of adolescents living with HIV. Ongoing tracking of age- and sex-disaggregated enrollment data for adolescents in East Africa will be needed to contextualize the potential impact of HIV prevalence- modifying influences in the region [eg, prevention of mother- to-child transmission (PMTCT) program expansion, increasing HIV knowledge and prevention behaviors, and increasing HIV testing and counseling programs].24
Our study also suggests that the proportion of adolescents enrolling in care and initiating ART with advanced HIV disease has decreased markedly over time. It has been reported elsewhere that progressive decentralization and expansion of HIV testing and counseling, including home- based testing and enrollment through PMTCT services, have likely contributed to earlier enrollment of adolescents.8, 33–35 In another EA-IeDEA analysis, pregnant women aged 13 years and older were found to comprise an increasing proportion of those initiating ART from 2004–2014, even before the expansion of ART eligibility through Option B+.36 However, overall decreases in the proportion of individuals starting treatment with WHO stage 3/4 disease over time were primarily attributable to nonpregnant women and men rather than pregnant women. Earlier ART initiation may also reflectearlier enrollment, as well as higher national CD4-based ART eligibility thresholds, better treatment of sicker individuals, and increasing care capacity and ART access for HIV programs in the region over time.11,35,37,38 Our data, which are largely descriptive in nature, cannot directly address these issues, but the observed trends are consistent with the aforementioned studies and add to the growing volume of studies showing the association of ART scale-up and contemporary patient trends in our region.39 The increase in median CD4 count at enrollment further suggests that horizontally infected adolescents are seeking care earlier, which may also be contributing to the increasing enrollment of older adolescents. This may also include younger adolescents with horizontally acquired infections, as population data from Kenya, Tanzania, and Uganda indicate 9%−12% of boys and 12%−22% of girls have had sex before the age of 15.5 Importantly, median CD4 counts at ART initiation throughout our study remained below 350 cells per microliter for younger and older adolescents despite the 2009 recommendation that ART be initiated at this CD4 threshold irrespective of clinical symptoms.31 Continued increases in CD4 counts at enrollment and ART initiation are anticipated in the future with broader HIV testing and adoption of universal treatment guidelines.
Finally, we found that attrition significantly increased over time for all adolescents and was nearly twice as high among older compared with younger adolescents in all periods except 2013–2014. These findings emphasize the importance of addressing adolescent attrition, particularly among older adolescents, within HIV programs in our region. Notably, attrition before ART initiation markedly increased among younger and older adolescents in 2005–2009 compared with 2001–2004. This coincides with the major expansion of HIV care and treatment capacity in the region under the US President’s Emergency Plan for AIDS Relief (PEPFAR) after 2005. During the ensuing increase in the number of adolescents enrolling in HIV care, programs may not have been adequately equipped to manage attrition before ART initiation, when the CD4 criterion for ART initiation was ≤350 cells/mm3. Changing programmatic or facility characteristics (including implementation of pediatric-focused clinics and service delivery), treatment guidelines, poor patient tracing amidst increasing adolescent enrollment, or changing demographic and clinical characteristics (eg, HIV disease stage) of adolescents enrolling in HIV care, may also account for the estimated increases in attrition over time in our study. That attrition was higher for older compared with younger adolescents, is consistent with other studies, and likely reflects the increased autonomy experienced by older adolescents during their transition to adulthood as well as differences in care and treatment approaches by providers as adolescent age.11,21–23,34 Within this study, we are unable to address the issues surrounding transition during adolescents because the clinics represented during the study period did not have standardized practices for transition. However, we have an ongoing study of transitioning and will be able to address this in a future article. Transition of care for adolescents and youth has emerged as a key issue that involves increasing roles and responsibilities for adolescents as they navigate developmental and psychosocial changes, as well as changes in health care providers and care structure.40 Data to understand transition outcomes and optimal models of transition are limited in sub-Saharan Africa. Adolescent- focused services and staff that include peer and psychosocial support are likely required, and existing transition policies need to be implemented.40–44 Reducing attrition among adolescents enrolling in HIV care in our region will also require a better understanding of LTFU (ie, as unidentified mortality, silent transfer, or complete disengagement from care), as well as a deeper grasp of the individual-, social-, and facility-level factors that influence attrition.27,43
Our data have several strengths but also significant limitations. A strength of this study is its longitudinal focus on adolescent care across multiple high- and low-volume facilities serving adolescents in East Africa. Our study provides a broad picture of the evolving HIV epidemic among adolescents that may serve as a benchmark against which future research can be evaluated. The use of routine program data to understand adolescent characteristics at enrollment and post-ART initiation phases of care is both a strength, as our data is likely to reflect typical care environments in East Africa, and limitation, as reliance on routine program data resulted in missing WHO stage in a high proportion of adolescents. This limitation is consistent with other adolescent studies using routinely collected data in sub- Saharan Africa.11,21,23,35 Our study also included a limited number of HIV facilities across East Africa, which may not be generalizable to the region as a whole, particularly given the variability in resources and HIV services that exist within and between countries. Finally, our attrition results may have been affected by overestimation of LTFU (eg, because of undocumented transfer).45 Although, LTFU among patients with generally less advanced HIV disease suggests that most of the program attrition is caused by disengagement from care rather than death. This limitation underscores the importance of understanding the causes of LTFU to accurately understand program attrition.46
Finally, it is important to recognize that our findings are not disaggregated by sex, and there remains a paucity of gender-disaggregated retention data and for adolescents in sub-Saharan Africa.47–50 Two studies did not identify sex differences in adolescent LTFU, whereas 1 in South Africa identified higher retention among male adolescents attending adolescent-friendly clinics.51–53 This contrasts with multiple studies among adults in sub-Saharan Africa that have found higher LTFU among male adolescents compared with female adolescents.54–56 Given the significant developmental and psychosocial changes that occur during adolescence, which can affect male and female adolescents differently, research in this area is critically needed. Stigma, disclosure, depression, and other stressors may be experienced and coped with differently by each sex.57–59 Pregnancy may also jeopardize retention among female adolescents. In a related EA-IeDEA study, the risk of LTFU was nearly twice as high for pregnant compared with nonpregnant female adolescents.60 Moreover, attrition during PMTCT follow-up is alarmingly com- mon.57,61 Future studies are needed to explore the sex- specific factors that influence retention and adherence, both to more sharply define and track the adolescent HIV epidemic, and to develop more appropriately targeted interventions in program design, policy, and advocacy for adolescents.
CONCLUSIONS
Earlier enrollment and ART initiation occurred over time for adolescents in East Africa, likely influenced by the scale-up of HIV testing, ART coverage, and national guidelines recommending earlier ART initiation in the region. However, attrition also increased over time for younger and older adolescents, emphasizing the need for additional efforts to address and define the factors and interventions that promote retention among adolescents, particularly for those aged 15 years and older. Future longitudinal analyses will be needed to track the evolving HIV epidemic among adolescents in East Africa.
ACKNOWLEDGMENTS
The authors thank the following programs, site principal investigators and data managers who contributed data to this study and made this analysis possible: in Kenya at the Academic Model Providing Access to Healthcare (AMPATH) Dr. Samuel Ayaya, Dr. Lameck Diero, and Mr. Edwin Sang; at Family AIDS Care and Education Services (FACES) Dr. Elizabeth Bukusi, Mr. Charles Kibaara, and Ms. Elisheba Mutegi; in Tanzania at Morogoro Regional Hospital Dr. Rita Lyamuya and Mr. Francis Mayanga; at the Tumbi Regional Hospital Dr. Kapella Nyonyani and Mr. Jerome Lwali; in Uganda at Masaka Regional hospital HIV Care Clinic Dr. John Ssali and Mr. Matthew Ssemakadde, and at Rakai Health Sciences Program (RHSP) Dr. Fred Nalugoda, Dr. Steve Reynolds, and Mr. Francis Wasswa.
Supported by the National Institute Of Allergy And Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Drug Abuse (NIDA), National Cancer Institute (NCI), and the National Institute of Mental Health (NIMH), in accordance with the regulatory requirements of the National Institutes ofHealth under Award Number U01AI069911East Africa IeDEA Consortium. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Presented at ninth IAS Conference on HIV Science; July 23–26, 2017; Paris, France.
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.World Health Organization; Global Update on HIV Treatment 2013: Results, Impact and Opportunities. Geneva, Switzerland: WHO; 2013. Available at: http://www.who.int/hiv/pub/progressreports/update2013/en/. Accessed April 10, 2017. [Google Scholar]
- 2.Republic of Kenya Ministry of Health. Kenya’s Fast Track to End HIV Amongst Adolescents and Young People. National AIDS Control Council; 2015. Available at: http://www.lvcthealth.org/online-library?format=raw&task=download&fid=55. Accessed April 10, 2017. [Google Scholar]
- 3.United Nations Children’s Fund. The AIDS Epidemic Continues to Take a Staggering Toll, Especially in Sub-Saharan Africa. New York, USA: UNICEF; 2016. Available at: http://data.unicef.org/hiv-aids/global-trends.html. Accessed September 11, 2016. [Google Scholar]
- 4.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(suppl 2):S144–S153. [DOI] [PubMed] [Google Scholar]
- 5.United Nations Children’s Fund. Global HIV Database. New York, NY: UNICEF; 2014. Available at: http://data.unicef.org/topic/hivaids/global-regional-trends/. Accessed September 11, 2016. [Google Scholar]
- 6.Joint United Nations Programme on HIV/AIDS. Global AIDS Update 2016. Geneva, Switzerland: UNAIDS; 2016 Available at: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf. Accessed April 10, 2017. [Google Scholar]
- 7.Kasedde S, Luo C, McClure C, et al. Reducing HIV and AIDS in adolescents: opportunities and challenges. Curr HIV/AIDS Rep. 2013;10: 159–168. [DOI] [PubMed] [Google Scholar]
- 8.Sam-Agudu NA, Folayan MO, Ezeanolue EE. Seeking wider access to HIV testing for adolescents in sub-Saharan Africa. Pediatr Res. 2016;79:838–845. [DOI] [PubMed] [Google Scholar]
- 9.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS. 2014;28:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobat R, Archary M, Lawler M. An update on the HIV treatment cascade in children and adolescents. Curr Opin HIV AIDS. 2015;10:411–419. [DOI] [PubMed] [Google Scholar]
- 11.Koech E, Teasdale CA, Wang C, et al. Characteristics and outcomes of HIV-infected youth and young adolescents enrolled in HIV care in Kenya. AIDS. 2014;28:2729–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS. 2008;22: 1463–1473. [DOI] [PubMed] [Google Scholar]
- 13.Nachega JB, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. JAcquirImmune Defic Syndr. 2009; 51:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulage L, Ssewanyana I, Nankabirwa V, et al. Factors associated with virological non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, August 2014-July 2015. BMC Infect Dis. 2017; 17:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakanda C, Birungi J, Mwesigwa R, et al. Survival of HIV-infected adolescents on antiretroviral therapy in Uganda: findings from a nationally representative cohort in Uganda. PLoS One. 2011;6:e19261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weigel R, Estill J, Egger M, et al. Mortality and loss to follow-up in the first year of ART: Malawi national ART programme. AIDS. 2012;26: 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wandeler G, Keiser O, Pfeiffer K, et al. Outcomes of antiretroviral treatment programs in rural Southern Africa. J Acquir Immune Defic Syndr. 2012;59:e9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekouevi DK, Balestre E, Ba-Gomis FO, et al. Low retention of HIV- infected patients on antiretroviral therapy in 11 clinical centres in West Africa. Trop Med Int Health. 2010;15 (suppl 1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyles TH, Wilkinson LS, Leisegang R, et al. Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PLoS One. 2011;6:e19201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jani IV, Sitoe NE, Alfai ER, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011; 378:1572–1579. [DOI] [PubMed] [Google Scholar]
- 21.Bygrave H, Mtangirwa J, Ncube K, et al. Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PLoS One. 2012;7: e52856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans D, Menezes C, Mahomed K, et al. Treatment outcomes of HIV- infected adolescents attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retrovir. 2013;29:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, et al. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS. 2014;28:559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United Nations Children’s Fund. Turning the Tide against AIDS Will Require More Concentrated Focus on Adolescents and Young People. New York, NY: UNICEF; 2015. Available at: http://data.unicef.org/hiv-aids/adolescents-young-people.html. Accessed May 10, 2018. [Google Scholar]
- 25.Eaton JW, Garnett GP, Takavarasha FR, et al. Increasing adolescent HIV prevalence in Eastern Zimbabwe—evidence of long-term survivors of mother-to-child transmission? PLoS One. 2013;8:e70447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joint United Nations Programme on HIV/AIDS 90–90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva, Switzerland: UNAIDS; 2014. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/90-90-90_en.pdf Accessed May 10, 2018. [Google Scholar]
- 27.Geng EH, Odeny TA, Lyamuya R, et al. Retention in care and patient- reported reasons for undocumented transfer or stopping care among HIV- infected patients on antiretroviral therapy in eastern Africa: Application of a sampling-based approach. Clin Infect Dis. 2016;62:935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Epidemiologic Databases to Evaluate AIDS—East Africa. Regional Partners 2016. Available at: https://www.iedea-ea.org. Accessed May 10, 2018. [Google Scholar]
- 29.World Health Organization; Maternal, Newborn, Child and Adolescent Health: Adolescent Development. Geneva, Switzerland: WHO; 2016. Available at: http://www.who.int/maternal_child_adolescent/topics/adolescence/dev/en/. Accessed May 12, 2018. [Google Scholar]
- 30.World Health Organization; WHO Issues New HIV Recommendations Calling for Earlier Treatment. Geneva, Switzerland: WHO; 2013. Available at: http://www.who.int/mediacentre/news/releases/2013/new_hiv_recommendations_20130630/en/. Accessed May 12, 2018. [Google Scholar]
- 31.World Health Organization; Antiretroviral Therapy for HIV Infection in Infants and Children: Recommendations for a Public Health Approach. Geneva, Switzerland: WHO; 2010. Available at: http://apps.who.int/iris/bitstream/10665/44379/1/9789241599764_eng.pdf. Accessed May 12, 2018. [PubMed] [Google Scholar]
- 32.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 33.Wachira J, Kimaiyo S, Ndege S, et al. What is the impact of home-based HIV counseling and testing on the clinical status of newly enrolled adults in a large HIV care program in Western Kenya? Clin Infect Dis. 2012;54: 275–281. [DOI] [PubMed] [Google Scholar]
- 34.Ssali L, Kalibala S, Birungi J, et al. Retention ofAdolescents Living with HIV in Care, Treatment, and Support Programs in Uganda, HIVCore Final Report. Washington, DC: USAID|Project Search: HIVCore; 2014. [Google Scholar]
- 35.Lahuerta M, Wu Y, Hoffman S, et al. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006–2011: findings from four sub-saharan African countries. Clin Infect Dis. 2014; 58:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmes CB, Yiannoutsos CT, Elul B, et al. Increased prevalence of pregnancy and comparative risk of program attrition among individuals starting HIV treatment in East Africa. PLoS One. 2018:13:e0190828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chi BH, Adler MR, Bolu O, et al. Progress, challenges, and new opportunities for the prevention of mother-to-child transmission of HIV under the US President’s Emergency Plan for AIDS Relief. J Acquir Immune Defic Syndr. 2012;60(suppl 3):S78–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lahuerta M, Ue F, Hoffman S, et al. The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved. 2013;24:359–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grabowski MK, Serwadda DM, Gray RH, et al. HIV prevention efforts and incidence of HIV in Uganda. N Engl J Med. 2017;377:2154–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahourou DL, Gautier-Lafaye C, Teasdale CA, et al. Transition from paediatric to adult care of adolescents living with HIV in sub-Saharan Africa: challenges, youth-friendly models, and outcomes. J Int AIDS Soc. 2017;20(suppl 3):21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mark D, Armstrong A, Andrade C, et al. HIV treatment and care services for adolescents: a situational analysis of 218 facilities in 23 sub-Saharan African countries. J Int AIDS Soc. 2017;20(suppl 3):21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vreeman RC, McCoy BM, Lee S. Mental health challenges among adolescents living with HIV. J Int AIDS Soc. 2017;20(suppl 3):21497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teasdale CA, Alwar T, Chege D, et al. Impact of youth and adolescent friendly services on retention of 10–24-year-olds in HIV care and treatment programs in Nyanza, Kenya. J Acquir Immune Defic Syndr. 2016;71:e56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mark D, Taing L, Cluver L, et al. What is it going to take to move youth- related HIV programme policies into practice in Africa? J Int AIDS Soc. 2017;20(suppl 3):21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geng EH, Odeny TA, Lyamuya RE, et al. Estimation ofMortality among HIV-infected people on antiretroviral therapy treatment in east Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV. 2015;2:e107–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rachlis B, Bakoyannis G, Easterbrook P, et al. Facility-level factors influencing retention of patients in HIV care in East Africa. PLoS One. 2016:11:e0159994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joint United Nations Programme on HIV/AIDS. A Progress Report: All in to End the Adolescent AIDS Epidemic. 2016. Geneva, Switzerland: UNAIDS; 2016 Available at: http://www.unaids.org/sites/default/files/media_assetZALLIN2016ProgressReport_en.pdf. Accessed May 12, 2018. [Google Scholar]
- 48.United Nations Children’s Fund. Collecting and Reporting of Sex- and Age-disaggregated Data on Adolescents at the Sub-national Level. New York, NY: UNICEF; 2016. Available at: https://data.unicef.org/wp-content/uploads/2016/11/Data-Abstraction-Guide-November-2016.pdf. Accessed May 10, 2018. [Google Scholar]
- 49.Sherwood J, Sharp A, Cooper B, et al. HIV/AIDS National Strategic Plans of Sub-Saharan African countries: an analysis for gender equality and sex-disaggregated HIV targets. Health Policy Plan. 2017;32: 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joint United Nations Programme on HIV/AIDS. All in to End Adolescent AIDS. Geneva, Switzerland: UNAIDS; 2015. Available at: http://www.unaids.org/en/resources/documents/2015/20150217_ALL_IN_brochure. Accessed May 10, 2018. [Google Scholar]
- 51.ojwang Vo, Penner J, Blat C, et al. Loss to follow-up among youth accessing outpatient HIV care and treatment services in Kisumu, Kenya. AIDS Care. 2016;28:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shroufi A, Gunguwo H, Dixon M, et al. HIV-infected adolescents in southern Africa can achieve good treatment outcomes: results from a retrospective cohort study. AIDS. 2013;27:1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zanoni BC, Sibaya T, Cairns C, et al. Higher retention and viral suppression with adolescent-focused HIV clinic in South Africa. PLoS One. 2017:12:e0190260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takarinda KC, Harries AD, Shiraishi RW, et al. Gender-related differences in outcomes and attrition on antiretroviral treatment among an HIV-infected patient cohort in Zimbabwe: 2007–2010. Int J Infect Dis. 2015;30:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Onoka CA, Uzochukwu BS, Onwujekwe OE, et al. Retention and loss to follow-up in antiretroviral treatment programmes in southeast Nigeria. Pathog Glob Health. 2012;106:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hawkins C, Chalamilla G, Okuma J, et al. Sex differences in antiretroviral treatment outcomes among HIV-infected adults in an urban Tanzanian setting. AIDS. 2011;25:1189–1197. [DOI] [PubMed] [Google Scholar]
- 57.Phillips T, Thebus E, Bekker LG, et al. Disengagement of HIV-positive pregnant and postpartum women from antiretroviral therapy services: a cohort study. J Int AIDS Soc. 2014;17:19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim MH, Mazenga AC, Yu X, et al. Factors associated with depression among adolescents living with HIV in Malawi. BMC Psychiatry. 2015;15:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanmogne GD, Qiu F, Ntone FE, et al. Depressive symptoms in HIV- infected and seronegative control subjects in Cameroon: effect of age, education and gender. PLoS One. 2017:12:e0171956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nuwagaba-Biribonwoha H, Yiannoutsos C, Musick B, et al. Teenage Pregnancy: A Critical Barrier to Retention on Antiretroviral Therapy. Conference on Retroviruses and Opportunistic Infections; 13–16 February 2017; Seattle, WA. [Google Scholar]
- 61.Chi BH, Bolton-Moore C, Holmes CB. Prevention of mother-to-child HIV transmission within the continuum of maternal, newborn, and child health services. Curr Opin HIV AIDS. 2013;8:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]


