Abstract
Despite advances in heart failure treatment, advanced heart failure affects 5–10% of people with the condition and is associated with poor prognosis. Selection for heart transplantation and left ventricular assist device implantation is a rigorous and validated process performed by specialised heart failure teams. This entails comprehensive assessment of complex diagnostic tests and risk scores, and selecting patients with the optimal benefit-risk profile. In contrast, referral for advanced heart failure evaluation is an arbitrary and poorly studied process, performed by generalists, and patients are often referred too late or not at all. The study elaborates on the differences between selection and referral and proposes some simple strategies for optimising timely referral for advanced heart failure evaluation.
Keywords: Advanced heart failure, selection, referral, heart transplantation, left ventricular assist device, palliative care
Heart failure (HF) is associated with poor quality of life, high risk of death and is the leading cause of hospitalisation.[1] With an ageing population and improved care for cardiovascular diseases, the prevalence of HF is increasing. Despite advances in HF therapies, 1–10% of the population with HF progress to an advanced stage of the disease.[2,3] In the US, an estimated 250,000–300,000 patients younger than 75 years suffer from advanced HF; extrapolated, this would yield approximately 500,000 patients in the EU.[4] Prognosis in advanced heart failure is poor, with 1-year mortality rates of 25–50%[2,2]
Heart transplantation (HTx) remains the gold standard treatment for severe HF refractory to medical and device therapy, with a 1-year survival of almost 90%.[7,8] However, since access to organs is limited, durable left ventricular assist devices (LVADs) are increasingly being implanted in these patients either as bridge to transplantation or destination therapy.[9]
There have been remarkable advances in mechanical assist device therapy over the past decade and current data indicate a survival after LVAD implantation of around 80% at 1 year and 70% at 2 years.[10,11] Patients are, however, believed to be underserved regarding advanced HF therapies.[12,13] While the main explanation for HTx is organ shortage, important reasons for underuse of LVADs are most likely a lack of awareness among clinicians caring for patients with HF and difficulty in assessing the need for advanced therapy.
Accurate prognostication in HF is challenging. The transition from stable, chronic HF to advanced HF is often gradual and no single test or imaging modality is capable of identifying this change in severity. For general practitioners or cardiologists who do not deal with advanced HF on a daily basis, it is difficult to identify patients who may benefit from HTx or LVAD therapy. Patients are often referred too late, when end-stage organ failure that disqualifies them for advanced treatment is already present.[14] While HF teams and advanced HF referral centres follow rigorous selection criteria and guidelines when selecting patients for HTx and LVAD implantation, there are no guidelines or criteria to serve the GP or cardiologist in deciding when to refer patients for advanced HF assessment and potential selection for advanced therapy.[15–17]
Referral to an Advanced Heart Failure Centre
Before advanced therapy is considered, evidence-based HF therapy should be optimised. Medication must be uptitrated to maximum tolerated doses and patients should receive cardiac resynchronisation therapy and/or implantable defibrillation therapy (ICD) as indicated according to current guidelines.[18]
No validated criteria or cut-off values for referral to an advanced HF clinic or HF specialist exist. The Heart Failure Association of the European Society of Cardiology position statement lists triggers for referral (Table 1).[19] The variables listed include clinical, laboratory, imaging and risk score data; they are all relevant prognostic variables, but many are non-specific and/or subjective. The variables should perhaps be seen as general markers of deterioration rather than distinct referral criteria.
Table 1: Triggers for Referral for Advanced Therapy.
| Clinical | Laboratory | Imaging | Risk Score Data |
|---|---|---|---|
|
|
|
|
6MWT = 6-min walk test; CPET = cardiopulmonary exercise test; CRT = cardiac resynchronization therapy; eGFR = estimated glomerular filtration rate; GDMT = guideline-directed medical therapy; Hb = haemoglobin; HF = heart failure; IVC = inferior vena cava; K = potassium; KCCQ = Kansas City Cardiomyopathy Questionnaire; LVEF = left ventricular ejection fraction; MAGGIC = Meta-Analysis Global Group in Chronic Heart Failure; MLHFQ = Minnesota Living with Heart Failure Questionnaire; Na = sodium; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; PA = pulmonary artery; RV = right ventricular; SBP = systolic blood pressure; SCr = serum creatinine; SHFM = Seattle Heart Failure Model. Source: Crespo-Leiro et al. 2018.19 Reproduced with permission from John Wiley and Sons.
Articles in which referral for advanced HF therapy is discussed tend to focus on selection criteria for HTx or LVAD implantation rather than referral criteria.[14] Cardiopulmonary exercise testing, 6-minute-walk test, assessment of prognosis using comprehensive risk scores, evaluation of end-stage organ failure, assessment of cardiac index and intracardiac pressures measured by right heart catheterisation are all part of a complete patient eligibility assessment performed by the interdisciplinary HF team at specialised HF centres when evaluating potential candidates for advanced HF treatment.[2] It should be noted that, for referral to a HF centre, a complete assessment of the patient is not required. The general cardiologist or primary care physician does need, however, to identify that the disease is progressing toward a stage of advanced HF, and this may be challenging.
Systematic screening of certain patient categories has been suggested as a way of improving referral for advanced therapy. A pilot study suggested that screening patients receiving cardiac resynchronisation therapy for possible HTx or LVAD indication identified otherwise neglected candidates.[21]. The Screening for Advanced Heart Failure Treatment (SEE-HF) study showed that actively screening patients with cardiac resynchronisation therapy and/or an ICD in an outpatient setting found few patients were candidates for advanced therapy. However, when selecting patients with an ejection fraction (EF) <40% and New York Heart Association (NYHA) class III–IV, 26% were found to have an unrecognised need for advanced therapy (HTx and/or LVAD).[22] More studies are needed to evaluate the effectiveness of screening to identify candidates for advanced therapy.
Clinical decision supports (CDS) may be of value in identifying patients eligible for advanced therapies. Evans, et al. developed a computer application that, by automatically extracting information from the patient’s integrated electronic health record, could monitor their HF status and alert the treating physician if the criteria for advanced HF were met.[23] More patients were referred to specialised heart facilities when CDS were used than in the year before CDS were introduced. However, if information cannot be abstracted automatically — which is the case in many healthcare systems — it would be time consuming to use the application and possibly of less help in busy daily practice. Most likely, simpler tools are needed to ensure timely referral. In a study by the Swedish Heart Failure Registry, five risk factors were suggested as triggers for referral to an advanced HF centre.[24] For patients with NYHA class III–IV HF and EF <40% with one or more of the defined risk factors present, 1-year survival was worse than for HF patients post-HTx or post-LVAD implantation (Figure 1). One or more risk factors is therefore reason to refer to a HF centre. The five risk factors were: systolic blood pressure <90 mmHg; creatinine >160 µmol/l, haemoglobin <120 g/l; no renin-angiotensin system antagonist and no beta-blocker. These risk factors are easily identified in daily clinical practice and reflect disease severity. The focus of the study was not on optimal biological discrimination (in which glomerular filtration rate rather than creatinine would be used, and discrimination would be formally assessed with e.g. areas under the receiver operating characteristic curves), but rather on simple, memorable and distinct criteria suitable for busy clinicians. In a recent review article, it was suggested that if a patient was highly symptomatic (NYHA III–IV) despite optimal HF treatment, this should prompt for referral to a HF centre.[4] More than one hospitalisation despite good medical therapy indicates disease is severe, as do intolerance to HF medication and hyponatremia.[12,25]
Figure 1: Survival by Number of Risk Factors for New York Heart Association Class III–IV, with Ejection Fraction <40%.
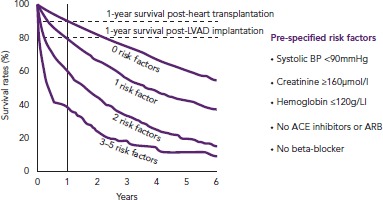
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; LVAD, left ventricular assist device. Source: Adapted from Thorvaldsen, et al. 2014,[24] with permission from Elsevier.
A pragmatic approach, such as using the five risk factors or a patient being highly symptomatic despite the best care as referral criteria could increase the number of referrals. By no means does this imply that the majority of these patients would benefit from or be eligible for HTx or LVAD implantation; it means only that they deserve at least one expert assessment by a HF specialist. Additionally, underuse of intermediate-level HF interventions such as cardiac resynchronisation and ICD therapy has been reported previously, so a more liberal referral to a HF specialist seems motivated by a wish to optimise evidence-based treatments.[26–28]
Palliative programmes have been shown to reduce readmissions and improve symptoms in patients with end-stage HF.[29] However, palliative care is substantially less implemented for patients with HF than in those with cancer, and is often initiated too late.[30,31] The benefits of palliative care have been recognised by the American Heart Association and the body of literature focusing on the integration of palliative care in HF management is increasing.[32] Therefore, even for patients with a heavy comorbidity burden or those who are presumed to be too old for advanced HF therapy, a referral to an advanced HF centre is justified for considering different treatment options and initiating palliative care if appropriate.
Table 2 shows the important differences between the comprehensive assessment of a potential candidate for LVAD and/or HTx and suggested criteria for referring a patient to an advanced HF centre discussed in this article.
Table 2: Assessment of Eligibility for Advanced Heart Failure Treatment Versus Indications for Referral to a Specialised Heart Failure Centre.
| Assessment of Eligibility for Advanced Heart Failure Therapy | Indications for Referral to a Specialised Heart Failure Centre |
|---|---|
|
|
EF = ejection fraction; HF = heart failure; INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; NT-proBNP = N-Terminal-pro brain natriuretic peptide; NYHA = New York Heart Association; VO2 = volume oxygen.
Conclusion
Mortality in advanced HF remains high. Identifying patients in need of advanced therapy starts with referral to a heart failure centre. Timely referral for evaluation for HTx and LVAD therapy is crucial for the success of these treatments. In contrast to the complex criteria for selection for LVAD and HTx therapy, indication for evaluation to a HF specialist should simply be deterioration despite optimal HF care.
References
- 1.Ambrosy AP, Fonarow GC, Butler J et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–33. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Xanthakis V, Enserro DM, Larson MG et al. Prevalence, neurohormonal correlates, and prognosis of heart failure stages in the community. JACC Heart Fail. 2016;4:808–15. doi: 10.1016/j.jchf.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjork JB, Alton KK, Georgiopoulou VV et al. Defining advanced heart failure: a systematic review of criteria used in clinical trials. J Card Fail. 2016;22:569–77. doi: 10.1016/j.cardfail.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail. 2017;19:595–602. doi: 10.1002/ejhf.779. [DOI] [PubMed] [Google Scholar]
- 5.Metra M, Eichhorn E, Abraham WT et al. Effects of low-dose oral enoximone administration on mortality, morbidity, and exercise capacity in patients with advanced heart failure: the randomized, double-blind, placebo-controlled, parallel group ESSENTIAL trials. Eur Heart J. 2009;30:3015–26. doi: 10.1093/eurheartj/ehp338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindenfeld J, Feldman AM, Saxon L et al. Effects of cardiac resynchronization therapy with or without a defibrillator on survival and hospitalizations in patients with New York Heart Association class IV heart failure. Circulation. 2007;115:204–12. doi: 10.1161/CIRCULATIONAHA.106.629261. [DOI] [PubMed] [Google Scholar]
- 7.Lund LH, Edwards LB, Dipchand AI et al. The registry of the International Society for Heart and Lung Transplantation: thirty-third adult heart transplantation report — 2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35:1158–69. doi: 10.1016/j.healun.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Lund LH. Optimizing outcomes after heart transplantation. Eur J Heart Fail. 2018;20:395–7. doi: 10.1002/ejhf.1026. [DOI] [PubMed] [Google Scholar]
- 9.Ciarka A, Edwards L, Nilsson J et al. Trends in the use of mechanical circulatory support as a bridge to heart transplantation across different age groups. Int J Cardiol. 2017;231:225–7. doi: 10.1016/j.ijcard.2016.10.049. [DOI] [PubMed] [Google Scholar]
- 10.Kirklin JK, Pagani FD, Kormos RL et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–6. doi: 10.1016/j.healun.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Kirklin JK, Cantor R, Mohacsi P et al. First annual IMACS report: a global international society for heart and lung transplantation registry for mechanical circulatory support. J Heart Lung Transplant. 2016;35:407–12. doi: 10.1016/j.healun.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Miller LW. Left ventricular assist devices are underutilized. Circulation. 2011;123((14)):1552–8. doi: 10.1161/CIRCULATIONAHA.110.958991. [DOI] [PubMed] [Google Scholar]
- 13.Miller LW, Guglin M. Patient selection for ventricular assist devices: a moving target. J Am Coll Cardiol. 2013;61:1209–21. doi: 10.1016/j.jacc.2012.08.1029. [DOI] [PubMed] [Google Scholar]
- 14.Fanaroff AC, DeVore AD, Mentz RJ et al. Patient selection for advanced heart failure therapy referral. Crit Pathw Cardiol. 2014;13:1–5. doi: 10.1097/HPC.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehra MR, Canter CE, Hannan MM et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10–year update. J Heart Lung Transplant. 2016;35:1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Sartipy U, Goda A, Yuzefpolskaya M et al. Utility of the Seattle Heart Failure Model in patients with cardiac resynchronization therapy and implantable cardioverter defibrillator referred for heart transplantation. Am Heart J. 2014;168:325–31. doi: 10.1016/j.ahj.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Lund LH, Matthews J, Aaronson K. Patient selection for left ventricular assist devices. Eur J Heart Fail. 2010;12:434–43. doi: 10.1093/eurjhf/hfq006. [DOI] [PubMed] [Google Scholar]
- 18.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 19.Crespo-Leiro MG, Metra M, Lund LH et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:1505–35. doi: 10.1002/ejhf.1236. [DOI] [PubMed] [Google Scholar]
- 20.Goda A, Williams P, Mancini D, Lund LH. Selecting patients for heart transplantation: comparison of the Heart Failure Survival Score (HFSS) and the Seattle heart failure model (SHFM) J Heart Lung Transplant. 2011;30:1236–43. doi: 10.1016/j.healun.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Zabarovskaja S, Gadler F, Gabrielsen A et al. Identifying patients for advanced heart failure therapy by screening patients with cardiac resynchronization therapy or implantable cardioverter-defibrillator: a pilot study. J Heart Lung Transplant. 2013;32:651–4. doi: 10.1016/j.healun.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Lund LH, Trochu JN, Meyns B et al. Screening for heart transplantation and left ventricular assist system: results from the ScrEEning for advanced Heart Failure treatment (SEE-HF) study. Eur J Heart Fail. 2018;20:152–60. doi: 10.1002/ejhf.975. [DOI] [PubMed] [Google Scholar]
- 23.Evans RS, Kfoury AG, Horne BD et al. Clinical decision support to efficiently identify patients eligible for advanced heart failure Therapies. J Card Fail. 2017;23:719–26. doi: 10.1016/j.cardfail.2017.08.449. [DOI] [PubMed] [Google Scholar]
- 24.Thorvaldsen T, Benson L, Stahlberg M et al. Triage of patients with moderate to severe heart failure: who should be referred to a heart failure center? J Am Coll Cardiol. 2014;63:661–71. doi: 10.1016/j.jacc.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Trochu JN, Leprince P, Bielefeld-Gomez M et al. Left ventricle assist device: when and which patients should we refer? Arch Cardiovasc Dis. 2012;105:114–21. doi: 10.1016/j.acvd.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Thorvaldsen T, Benson L, Dahlstrom U et al. Use of evidence-based therapy and survival in heart failure in Sweden 2003–2012. Eur J Heart Fail. 2016;18:503–11. doi: 10.1002/ejhf.496. [DOI] [PubMed] [Google Scholar]
- 27.Bank AJ, Gage RM, Olshansky B. On the underutilization of cardiac resynchronization therapy. J Card Fail. 2014;20:696–705. doi: 10.1016/j.cardfail.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Bradfield J, Warner A, Bersohn MM. Low referral rate for prophylactic implantation of cardioverter-defibrillators in a tertiary care medical center. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S194–7. doi: 10.1111/j.1540-8159.2008.02281.x. [DOI] [PubMed] [Google Scholar]
- 29.Wong FK, Ng AY, Lee PH et al. Effects of a transitional palliative care model on patients with end-stage heart failure: a randomised controlled trial. Heart. 2016;102:1100–8. doi: 10.1136/heartjnl-2015-308638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setoguchi S, Glynn RJ, Stedman M et al. Hospice, opiates, and acute care service use among the elderly before death from heart failure or cancer. Am Heart J. 2010;160:139–44. doi: 10.1016/j.ahj.2010.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakitas M, Macmartin M, Trzepkowski K et al. Palliative care consultations for heart failure patients: how many, when, and why? J Card Fail. 2013;19::193–201. doi: 10.1016/j.cardfail.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diop MS, Rudolph JL, Zimmerman KM et al. Palliative care interventions for patients with heart failure: a systematic review and meta-analysis. J Palliat Med. 2017;20:84–92. doi: 10.1089/jpm.2016.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]


