Abstract
Zolpidem (N,N-Dimethyl-2-[6-methyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]acetamide) is a well-known drug for the treatment of sleeping disorders. Recent literature reports on positive effects of zolpidem therapy on improving renal damage after cisplatin and on reducing akinesia without sleep induction. This has been ascribed to the antioxidant and neuroprotective capacity of this molecule, and tentatively explained according to a generic structural similarity between zolpidem and melatonin. In this work, we investigate in silico the antioxidant potential of zolpidem as scavenger of five ROSs, acting via hydrogen atom transfer (HAT) mechanism; computational methodologies based on density functional theory are employed. For completeness, the analysis is extended to six metabolites. Thermodynamic and kinetic results disclose that indeed zolpidem is an efficient radical scavenger, similarly to melatonin and Trolox, supporting the biomedical evidence that the antioxidant potential of zolpidem therapy may have a beneficial effect against oxidative injury, which is emerging as an important etiopathogenesis in numerous severe diseases, including psychiatric disorders.
Keywords: Zolpidem, Oxidative stress, Psychiatric disorders, Antioxidant activity, Radical scavenging, DFT calculations
Abbreviations: DFT, Density Functional Theory; HAT, Hydrogen Atom Transfer (mechanism); M06-2X, Minnesota Hybrid functional with 54% Hartree-Fock exchange; NBO, Natural Bond Orbitals; NPA, Natural Population Analysis; PC, Product Complex; RAF, Radical Adduct Formation (mechanism); RC, Reactant Complex; ROS, Reactive Oxygen Species; SMD, Solvation Model based on Density; TS, Transition State
Graphical Abstract
1. Introduction
The etiopathogenesis of psychiatric disorders suggests a complex picture with many social, physical, chemical exposures and genetic factors that contribute to the onset of mental illness [1]. The exact delineation of etiology is very difficult to detect because of a multitude of variables that influences each other. Therefore, the importance of better understanding pathopshysiological pathways and their reciprocal actions could provide a broadly applicable framework and subsequent means of therapeutic intervention. Neurochemistry, psychoneuroendocrinology and psychoneuroimmunology are fundamental biomedical fields that study the underlying mechanisms, but, in particular, neurochemistry informs most of the current biological treatments. In a similar context, oxidation biology is emerging as a promising avenue of investigation and has been actively pursued also in other areas of medicine [2].
The brain with its high oxygen consumption and a lipid-rich environment is very vulnerable to oxidative stress [3]. Oxidative stress, defined by Gingrich in 2005 as the “new stress” [4], consists in a serious alteration in the prooxidant-antioxidant balance in favor of the prooxidants, a condition in which elevated levels of intracellular reactive oxygen species (ROS) contribute to tissue damage. When this balance is altered, an overproduction of ROS and/or a deficit of the antioxidant defense mechanisms occurs with many effects as protein oxidation, loss of sulfhydryl groups and modifications of amino acids that render proteins non-functional, causing peroxidative damage to lipids with a consequent cell degeneration. Therefore, the fact that oxidative stress is implicated in the pathophysiology of several mental disorders as schizophrenia, bipolar disorder, major depressive disorder and anxiety, has not to surprise [[5], [6], [7]].
Recent data show that lowered quality of life in mood disorders could be in part associated to an increased neuro oxidative stress [8]. There are psychopharmacological treatments that have anti-inflammatory and antioxidant effects as for example lithium, that is the most effective medication for the prevention of long-term relapse in bipolar mood disorders, and N-acetyl-cysteine, a glutathione based redox modulator, anti-inflammatory agent and mitochondrial modulator, that decreases symptoms of depression and mania in bipolar disorders [9]. However, established agents have tolerability issues and efficacy limitations; therefore, more novel therapeutic strategies are needed. In the attempt to find alternative approaches to better care these patients, researchers have embarked on using antioxidant treatment as therapy for psychiatric disorders. Evidence from clinical, pre-clinical and epidemiological studies suggest that antioxidant therapy could enhance neuroprotection [10]. Several antioxidant compounds could be used, like vitamin E, vitamin C, Omega-3 fatty acids, coenzyme Q10, NAC, GSH, rutin, Ginkgo biloba, melatonin, hydroxytyrosol, caffeic acid phenethyl ester, resveratrol, quercetin and lycopene [9,11]. Metal ions such as zinc and manganese are also useful enforcing antioxidant defense [9]. Nevertheless, it is important to make clear that though oxidative stress has been found associated with several acute and chronic diseases, its role seems still secondary, since the sole antioxidants interventions have not been able to cure any disease.
A recent study has demonstrated a significant positive effect of the hypnotic drug zolpidem (Scheme 1) on the improvement of renal damage caused by cisplatin [12]. Zolpidem exhibits this effect by reducing oxidative stress, increasing the activity of the endogenous antioxidant system, including superoxide dismutase, catalase and glutathione peroxidase and preventing apoptosis in renal cells [12]. An interesting case report has demonstrated improved akinesia after taking zolpidem without sleep induction or consciousness changes [13]. There are many zolpidem binding receptors in the globus pallidus interna, substantia nigra pars reticulata and subthalamic nucleus and zolpidem's antioxidant and neuroprotective effects might prevent oxidative damage to the brain and consequently certain abnormal movement disorders [13].
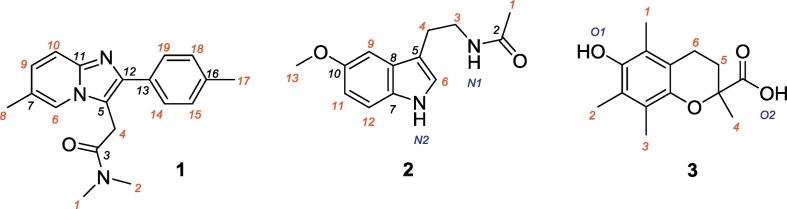
Scheme 1.
Zolpidem (1), melatonin (2) and Trolox (3). The red numbers indicate the HAT sites.
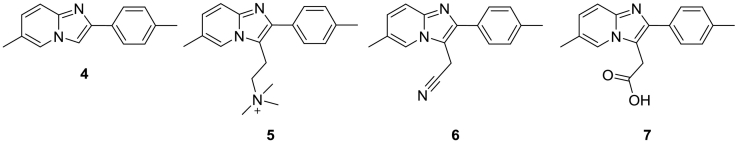
Garcia-Santos et al. inspired by a certain degree of structural similarity of zolpidem with melatonin, discussed for the first time the potential antioxidant activity of zolpidem [14]. The authors investigated its direct antioxidant and neuroprotective properties and those of some synthetic intermediates (Scheme 2). Although it has been proved that the stimulation of the peripheral benzodiazepine receptors promotes some protection against oxidants' cytotoxicity, it is clear that zolpidem does not target these receptors [15]. Thus, the authors focused on the influence of zolpidem 1 and of its analogues 4–7 on intracellular ROS levels, lipid peroxidation, protein protection against metal-catalyzed oxidative damage and their neuroprotective effects. In general, compounds 1, 4 and 5 present superior performances in the above cited biological tests. Although this pioneering work suggests for the first time the possibility to use zolpidem and its derivatives as antioxidants, more studies are needed to clarify the mechanism of action of these compounds and the role played by the imidazopyridine substituents.
Scheme 2.
Synthetic intermediates of zolpidem with antioxidant capacity.
Bishnoi et al. studied the influence of zolpidem on superoxide dismutase (SOD) and catalase activity in striatum of rats [16]. Discussing the results of their biochemical study on neuroprotective mechanisms of zolpidem in typical anti-psychotic-induced orofacial dyskinesia, the authors stated that “zolpidem dose dependently reversed the increase in oxidative damage […] induced by haloperidol and chloropromazine” [16].
A radical mechanism can be invoked to explain the antioxidant activity of zolpidem, very much like what is proposed for melatonin and Trolox (Scheme 1), and several natural substances [[17], [18], [19], [20], [21], [22], [23]]. Particularly, in this work, we have investigated the radical scavenging activity of zolpidem via hydrogen atom transfer (HAT, Scheme 3) from all its available sites and those of selected metabolites. Using a computational approach, based on density functional theory (DFT) methodologies, Gibbs free reaction and activation energies for the process were computed. Quantum chemistry calculations are a powerful tool to screen and rationalize the reactivity properties of molecular compounds, and have been successfully applied to numerous antioxidant systems, including natural substances acting as radical scavengers [24] and enzyme-inspired artificial compounds, for example GPx mimics [[25], [26], [27]].
Scheme 3.
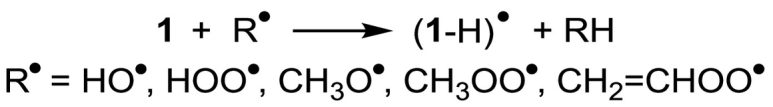
HAT mechanism in zolpidem (1).
We demonstrate that indeed zolpidem and its most important metabolites have antioxidant capacity similarly to Trolox and melatonin.
2. Methods
All calculations were carried out using Density Functional Theory (DFT) methodologies. The M06-2X functional was used [28], having been preliminarily benchmarked by Kozuch et al. [29] and by us on Trolox and melatonin (our data are reported in the Supporting Information file). Gas-phase full geometry optimizations of the reactants, products, and transition states were carried out at M06-2X/6-31G(d) level of theory, as implemented in Gaussian16 [30]. Spin contamination was checked to ascertain the correctness of the wavefunction for the radical doublets and was found negligible. Afterwards, single point energy calculations were computed on the optimized structures at M06-2X/6‐311+G(d,p) level to have a more accurate estimate of the energies. To verify the stationary nature of the minima (no imaginary frequency), to confirm the correctness of the transition states (only one imaginary frequency, which was analyzed to ensure that the right vibrational mode was found), and to obtain thermodynamic corrections at 298 K and 1 atm, frequency calculations were carried out at M06-2X/6-31G(d). The SMD continuum model [31,32] was adopted to take into account solvation effects in single point calculations performed on the gas-phase optimized structures with the larger basis sets (level of theory: SMD-M06-2X/6‐311+G(d,p)//M06-2X/6-31G(d)). Water and benzene were chosen to mimic a polar and a non-polar environment, respectively [19]. In gas-phase, a reactant complex (RC) and a product complex (PC) were located on the potential energy surface, before and after the transition state (TS), respectively. In all cases, both species are destabilized in energy with respect to the free reactants and products; thus, energy barriers were calculated respect to the sum of the energies of the free reactants. The same approach was used in solvent [33].
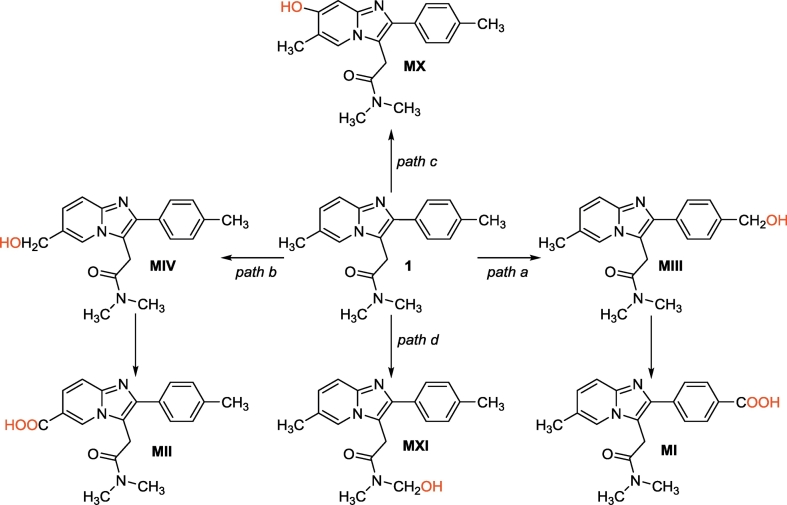
Natural spin densities and atomic charges were calculated with the natural population analysis (NPA) for C4 sites in 1 and its metabolites (Scheme 4) [34]. The electron spin density surfaces related to the localized natural bond orbitals (NBO) were also drawn for selected structures with Multiwfn [35] with a high-quality grid and the isodensity value of 0.003.
Scheme 4.
Metabolic paths of zolpidem; the sites which may be involved in HAT and are not present in 1 are in red.
3. Results and Discussion
The radical scavenging capacity of zolpidem (1) was investigated considering the hydrogen atom transfer (HAT) mechanism from all the available sites (Scheme 1)2.
These ROSs were considered: HO•, which is the most reactive and electrophilic oxygen-centered radical; HOO• and CH3OO•, which are less reactive and thus capable to reach remote cellular locations; CH2=CHOO•, mimic of larger unsaturated peroxyl radicals, and CH3O•, which has a reactivity intermediate between HO• and peroxyl radicals. Thermodynamically as well as kinetically, HAT resulted the most relevant mechanism with these radicals also in the cases of Trolox and melatonin [19,21].
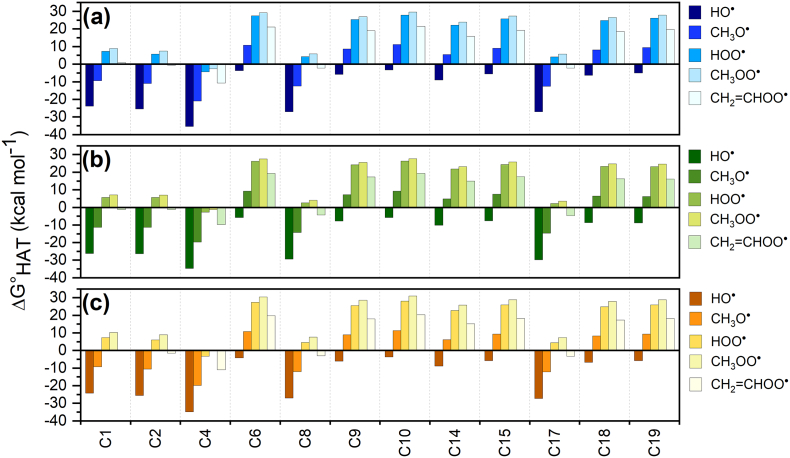
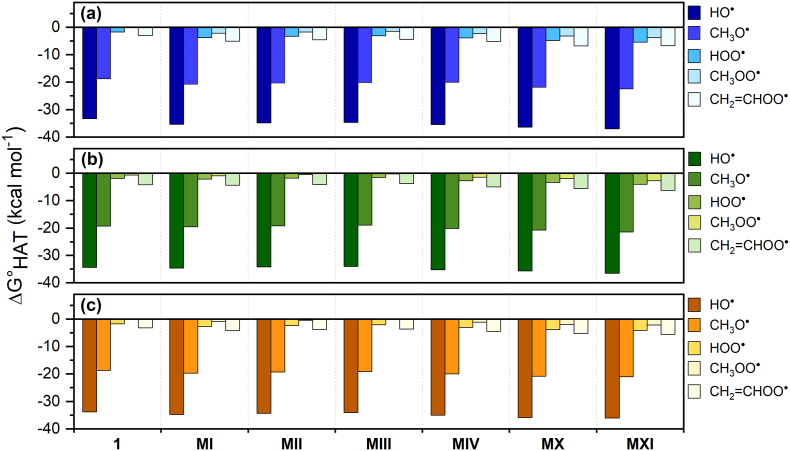
The thermodynamic data, which reveal the feasibility of the reactions, are presented and discussed first. Gibbs free reaction energies (ΔG°HAT) computed in gas-phase and in two different solvents, i.e. water and benzene, are reported in Table S1 and Fig. 1. When considering the same ROS, they reflect the stability of the (1-H)• radical. By inspecting the data in gas-phase (Fig. 1a), all reactions with HO• are neatly and in most cases strongly exergonic, as expected, with ΔG°HAT values in the range − 33.3 to −1.2 kcal mol−1. Conversely, all reactions with the hydroperoxyl and methyl peroxyl radicals are endergonic, and thus thermodynamically disfavored, with the exception of HAT from C4, for which ΔG°HAT values are −1.7 and − 0.1 kcal mol−1, respectively; in this latter case, the reaction can be considered isoergonic. The HATs to the alkoxyl radical are endergonic only when considering sites on the rings' C atoms. The scavenging activity of zolpidem toward CH2=CHOO• is thermodynamically disfavored, with values similar to those computed for the hydroperoxyl radical: HAT is endergonic from all sites except C4 from which it results thermodynamically favored (ΔG°HAT = −3.0 kcal mol−1). Notably, HAT from C4 is exergonic for all the considered radicals, indicating that the unpaired electron on this methylene carbon atom corresponds to an energetically very favored situation. The HAT from C10 is in all cases the least favored process, with ΔG°HAT ranging from −1.2 kcal mol−1 (HO•) to 31.9 kcal mol−1 (CH3OO•). The stability of the HAT products can be inferred by inspecting the electron spin density surfaces shown in Fig. 2, showing a larger delocalization when HAT occurs from C4 than from C10.
Fig. 1.
ΔG°HAT (kcal mol−1) in gas-phase (a), in water (b) and in benzene (c) for the scavenging of HO•, CH3O•, HOO•, CH3OO• and CH2=CHOO• through HAT from all the available sites of 1. Level of theory: (SMD)-M06-2X/6‐311+G(d,p)//M06-2X/6-31G(d).
Fig. 2.
Spin densities on (1-H)• when HAT occurs from C4 (a) and C10 (b) and on (MX-H)• when HAT occurs from C4 (c) and from the OH group linked to C9 (d).
The trends in gas-phase are maintained in the presence of solvent. In water (Fig. 1b), an appreciable stabilization of the products is observed, while in benzene (Fig. 1c), the effect on ΔG°HAT is neatly weaker and the values are very close to those computed in the gas-phase.
The pharmacokinetics of zolpidem prompted us to extend our analysis to other molecular compounds. Zolpidem shows rapid and quantitative absorption in humans [36]. Its pharmacological effect is consequently characterized by a quick onset, while the elimination of the compound occurs with a half-life of 2.4 h [36,37]. In fact, zolpidem is substrate of extensive metabolic reactions mediated by CYP3A, CYP1A2 and CYP2D6, and the unchanged compound can barely be detected in urine, feces and bile (0.5%) [38]. In humans, the biotransformation takes place through three main different paths, where redox reactions play a crucial role (Scheme 4) [37]. The first metabolic path leads to the hydroxylation of the methyl group of the phenyl moiety (path a, metabolite MIII) and, after a further oxidation, to the corresponding carboxylic acid (metabolite MI). This last compound represents the main metabolite detected in urine (72–86% of the administered dose). In the second path (path b), the hydroxylation and subsequent oxidation reactions take place on the methyl group of the imidazopyridine moiety (metabolites MIV and MII respectively). Metabolite MII accounts for 10% of the administered dose. Hydroxylation on the imidazopyridine (path c, metabolite MX) represents the third metabolic path (10% of the administered dose). A minor path is based on the hydroxylation of one of the methyl groups of the substituted amide (path d, metabolite MXI). Interestingly, none of the formed metabolites is pharmacologically active, but they might be possibly involved in the antioxidant activity.
More recent reports on zolpidem metabolism can be found in references [38, 39]. The six metabolites of 1, which are shown in Scheme 4, were included in this study. We focused on the HAT mechanism from C4; in addition, HAT from OH and COOH groups present in the metabolites, which are expected to be reactive sites, was analyzed too. The first set of results, referring to C4 site, are shown in Fig. 3 and Table S2.
Fig. 3.
ΔG°HAT (kcal mol−1) in gas-phase (a), in water (b) and in benzene (c) for the scavenging of HO•, CH3O•, HOO•, CH3OO• and CH2=CHOO• through HAT from C4 site of 1 (included as reference) and of its metabolites. Level of theory: (SMD)-M06-2X/6‐311+G(d,p)//M06-2X/6-31G(d).
In gas-phase (Fig. 3a), all HATs are exergonic and the ΔG°HAT values become progressively less negative in the order HO•, CH3O•, CH2=CHOO•, CH3OO• and HOO•, the reaction with HO• being the most favored. This reflects the reactivity of the ROSs, or equivalently the O—H bond strength of the neutral species which form as products [40]. No significant differences can be noticed for the HAT from C4 among the different metabolites when considering the same radical: in all six cases, the values span a rather narrow range of approximately 4 kcal mol−1. Interestingly, the largest negative ΔG°HAT values are found for MX and MXI. For example, the stability of (MX-H)• can be inferred by the delocalization of the electron spin density in this radical when HAT occurs from C4 and compared to that of (1-H)•, shown in Fig. 2c and a, respectively.
In solvent, the ΔG°HAT values become more negative than in gas-phase and increase in absolute value with increasing polarity, i.e. when going from benzene to water. This can be explained with a stabilization of the products of the HATs occurring from C4 sites in the metabolites, analogous to what computed for 1. Also, when considering the different ROSs, the trends remain identical to those illustrated for the gas-phase. Importantly, the exergonicity of these reactions in the case of 1 and of its metabolites, although limited to a single site (C4), is a remarkable result since, even for Trolox, positive Gibbs free reaction energies were computed for HATs to peroxyl radicals [21]. For a quantitative comparison, data for Trolox were recalculated at (SMD)-M06-2X/6‐311+G(d,p)//M06-2X/6-31G(d) level of theory and are reported in Table S3. By comparing the reactivity of the C sites of Trolox with HAT from C4 in 1, we do not find in the former all negative ΔG°HAT values for the studied set of radicals. HAT from C6, which results the most reactive C site, is associated to a neatly positive ΔG°HAT when CH3OO• is considered (1.8 kcal mol−1 in gas-phase). Conversely, the same HAT from C4 in 1 is isoergonic in gas-phase and becomes exergonic in water (−0.7 kcal mol−1). In Trolox, all negative and the largest negative ΔG°HAT values are computed when HAT occurs from the OH group on the phenyl ring.
It is interesting also to compare our results with the reaction energies of ROS scavenging via HAT reported for melatonin whose antioxidant activity has been clearly established [41,42]. For a quantitative comparison, we have recomputed (level of theory: (SMD)-M06-2X/6‐311+G(d,p)//M06-2X/6-31G(d)) the ΔG°HAT for the HATs from C4 sites of the two lowest energy conformers of melatonin to the studied five ROSs (Table S4). The reactivity of site C4 was reported to be the highest one [19]. In gas-phase, the trend of the reaction Gibbs free energies reflects the reactivity of the radicals. Melatonin is a scavenger for HO• and CH3O•, but is not efficient for peroxyl radicals. Nevertheless, the ΔG°HAT are always smaller (absolute value) than those computed for HAT from C4 in 1. In addition, the HAT from C4 of zolpidem with CH2 = CHOO• and HOO• is favored, while the reaction with CH3OO• is isoergonic.
A possible enhancement of the antioxidant activity found in the zolpidem metabolites might directly involve the group added during metabolism in a ROS scavenging reaction. Therefore, calculations were conducted to see if the HAT from the carboxylic or hydroxyl groups might be a feasible pathway. Our results show that, in most of the cases, the HATs from the O sites of the metabolites are thermodynamically unfavored compared to those from C4, in gas as well as in condensed phase (Table S5). The only exception is MX for which the HAT from the OH group linked directly to the aromatic C9 carbon has a ΔG°HAT value more negative than ΔG°HAT for HAT occurring at C4 in the same metabolite, and slightly exceeding also ΔG°HAT for HAT from C4 in 1, (Table 1). For example, referring to the data in gas-phase and to HO•, ΔG°HAT values are −37.3 kcal mol−1 (HAT from OH linked to C9) and − 36.4 kcal mol−1 (HAT from C4), while ΔG°HAT for HAT from C4 in 1 is −33.3 kcal mol−1. The stability of the radical (MX-H)• when the HAT occurs from the OH group linked to C9 can be inferred by the large delocalization of the electron spin density shown in Fig. 2 (d).
Table 1.
ΔG°HAT (kcal mol−1) and ΔG‡HAT (kcal mol−1) in gas-phase, water and benzene, for the scavenging of HO•, HOO•, CH3O•, CH3OO• and CH2=CHOO• through HAT from the OH group linked to C9 in metabolite MX (see Scheme 4). Level of theory: (SMD)-M06-2X/6‐311+G(d,p)//M06-2X/6-31G(d).
| ΔG°HAT | ΔG‡HAT | ΔG°HAT,water | ΔG‡HAT,water | ΔG°HAT,benzene | ΔG‡HAT,benzene | |
|---|---|---|---|---|---|---|
| HO• | −37.3 | 4.0 | −40.8 | 5.6 | −38.6 | 5.3 |
| HOO• | −5.7 | 12.9 | −8.3 | 14.3 | −6.5 | 14.8 |
| CH3O• | −22.8 | 8.7 | −25.7 | 16.1 | −23.5 | 10.2 |
| CH3OO• | −4.1 | 19.8 | −7.1 | 15.1 | −4.7 | 21.4 |
| CH2 = CHOO• | −7.0 | 12.7 | −10.6 | 10.4 | −8.0 | 14.3 |
As further step to assess the activity of 1 and its metabolites as radical scavenging agents, we calculated the activation energies ΔG‡HAT for the most thermodynamically favored HATs, i.e. those from C4 site, in gas as well as in condensed phase. The results are shown in Table 2.
Table 2.
ΔG‡HAT (kcal mol−1 for the scavenging of HO•, HOO•, CH3O•, CH3OO• and CH2=CHOO• radicals through HAT from C4 for 1 and its metabolites. Level of theory: (SMD)-M06-2X/6‐311+G(d,p)//M06-2X/6-31G(d).
| ΔG‡HAT |
ΔG‡HAT,water |
ΔG‡HAT,benzene |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HO• | HOO• | CH3O• | CH3OO• | CH2 = CHOO• | HO• | HOO• | CH3O• | CH3OO• | CH2 = CHOO• | HO• | HOO• | CH3O• | CH3OO• | CH2 = CHOO• | |
| 1 | 5.4 | 16.9 | 9.4 | 19.3 | 14.1 | 7.8 | 19.3 | 11.4 | 21.7 | 15.3 | 7.2 | 19.4 | 11.9 | 22.9 | 17.1 |
| MI | 4.4 | 16.6 | 8.8 | 18.9 | 14.3 | 7.0 | 19.0 | 10.8 | 21.5 | 15.6 | 6.3 | 19.1 | 11.3 | 22.5 | 17.3 |
| MII | 4.9 | 16.7 | 8.9 | 20.6 | 15.8 | 7.6 | 19.6 | 11.3 | 23.4 | 17.6 | 6.9 | 19.4 | 11.6 | 24.3 | 19.0 |
| MIII | 5.4 | 17.6 | 9.7 | 19.4 | 14.7 | 7.8 | 19.5 | 11.6 | 21.8 | 15.8 | 7.2 | 19.9 | 12.1 | 22.9 | 17.7 |
| MIV | 3.9 | 15.0 | 8.0 | 19.3 | 13.7 | 6.8 | 18.6 | 10.8 | 21.2 | 14.8 | 6.0 | 17.8 | 10.8 | 22.7 | 16.8 |
| MX | 4.7 | 17.6 | 8.4 | 17.9 | 13.2 | 7.2 | 19.4 | 10.0 | 19.9 | 13.7 | 6.5 | 19.8 | 10.9 | 21.4 | 16.1 |
| MXI | 2.2 | 12.9 | 5.2 | 13.9 | 10.4 | 6.0 | 18.1 | 10.4 | 18.3 | 13.7 | 4.6 | 16.2 | 8.5 | 18.0 | 14.1 |
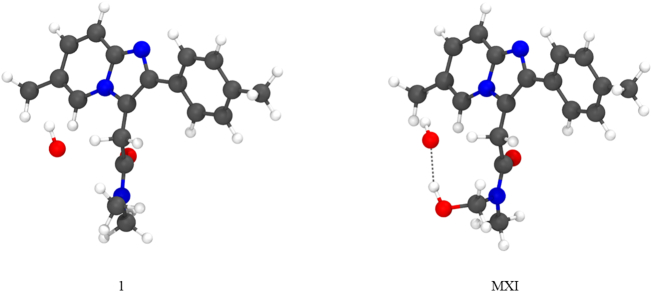
Focusing on the values in gas-phase, ΔG‡HAT for 1 and all the metabolites increases in all cases with decreasing the reactivity of the ROS, with a remarkable variation when passing from CH3O• to HOO• that can be explained considering the very different half-life of alkoxyl and peroxyl radicals, i.e. 10−9 s for HO• [43] and seconds for the HOO• [44]. The trend in activation energies found for the different radicals mirrors the trend reported for ΔG°HAT, i.e. the reactions with the largest (negative) ΔG°HAT display also the lowest activation energies for a given metabolite. However, comparing the reactivity of the different metabolites, it can be seen how those with the most favorable reaction energies (i.e. MI, MIV, MX or MXI) do not exactly match to those with the lowest activation barriers as there is only one structure that stands out among the metabolites with an activation energy appreciably lower than 1, i.e. MXI. The reason behind the lowering of the energy barrier in the case of MXI is evident when inspecting the structures of the transition states (Fig. 4). In fact, the modification that leads from 1 to MXI involves the hydroxylation of C2 which is close enough to C4, so that a hydrogen bond between the hydroxyl group and the reacting free radical forms. In this way, the transition states receive a further stabilization that is not possible in the other metabolites due to the distance of the other modified sites, which is too large for the establishment of any kind of weak interaction with C4. Consequently, the other five metabolites have energy barriers that are found within ±1.5 kcal mol−1 from that of 1, suggesting that the biomodification prevents any strong effect on the energy barriers of HATs from C4.
Fig. 4.
Fully optimized transition state geometries of HAT from C4 to HO• in 1 and its metabolite MXI. Level of theory: M06-2X/6-31G(d).
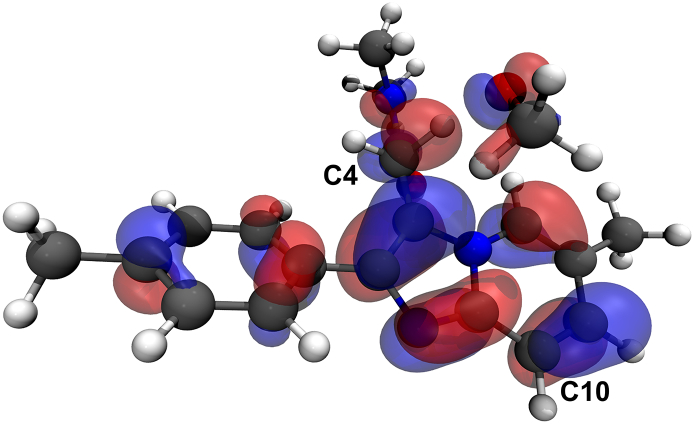
When solvent is added, the resulting transition states are destabilized with respect to those in the gas-phase, an effect which increases with increasing solvent polarity, i.e. from benzene to water for HO•. In fact, the opposite holds true for most of the reactions with HOO•, CH3O• and CH3OO•, for which the transition states in water lie at lower energies than those in benzene (Table 2). However, differences between the reaction barriers in the two solvents are below 1 kcal mol−1 in the case of HO•, HOO• and CH3O• meaning that the change in dielectric constant does not affect too much the kinetics of these radical reactions. The lowest energy barriers are computed for MXI in water and benzene too, but differences with the other metabolites and with 1 are found to be smaller than in the gas-phase. The availability of the transition states' electronic structures allowed us to assess that indeed the HAT mechanism is operative from the site C4. In fact, the singly occupied molecular orbital (SOMO) corresponding to this mechanism must possess significant density in the atomic orbitals oriented along the transition vector donor⋯H⋯acceptor, as we found. The SOMO of the transition state of HAT from C4 in 1 is shown as example in Fig. 5.
Fig. 5.
SOMO of the transition state of HAT from C4 site in 1 to CH3O• computed with an isodensity value of 0.003. Level of theory: M06-2X/6‐311+G(d,p)//M06-2X/6-31G(d).
The activation energies were computed for selected radicals also for HATs from the OH or COOH sites of zolpidem metabolites (Table S6). Hence, among all the HATs from the modified groups added to 1 during metabolism, HAT from the OH group of MX is the only one showing even a superior scavenging activity than 1 itself, in gas as well as in condensed phase, for HO• and HOO•. This is not surprising as studies on Trolox itself reveal that phenolic groups are very active in radical scavenging and the topology of this OH group in MX closely resembles the alcoholic group in Trolox [21]. For the other metabolites and radicals different from HO•, quite high activation energies are computed, ranging from 15 to 40 kcal mol−1 (Table S6).
4. Conclusions
In this work, we have analyzed with DFT based methodologies the radical scavenging activity via HAT of the well-known and largely diffuse hypnotic drug zolpidem and its six identified metabolites, toward five ROSs, i.e. HO•, HOO•, CH3O•, CH3OO• and CH2=CHOO•.
Our computational study discloses that when the HAT occurs from the C4 site in zolpidem, ΔG°HAT is negative for all the studied radicals, in polar as well as in non-polar environment, an important outcome if compared to the reactivity of melatonin, which is rather ineffective for scavenging peroxyl radicals.
Calculations on the metabolites for the HAT from the C4 site lead in three cases, i.e. MI, MII and MXI, to similar or larger (negative) Gibbs reaction free energies.
The trend of ΔG°HAT values to establish the thermodynamically feasibility of radical scavenging reflects the reactivity of the ROSs, i.e. CH3OO• < HOO• < CH2=CHOO• < CH3O• < HO•.
The calculation of the activation barriers for these processes expands the insights gained by the thermodynamic analysis. It emerges that the reactions with the largest (negative) ΔG°HAT have also the smallest activation barriers, with barriers that are below 10 kcal mol−1 in the case of HO• and CH3O•. For the other radicals, the barriers range from 10 to approximatively 20 kcal mol−1. This suggests that zolpidem and its metabolites have comparable activation energies for HAT from C4, except in the case of MXI, for which the TS is peculiarly stabilized by an intramolecular H-bond.
Last, the reactivity of the metabolites in the radical scavenging process was investigated considering the HAT from the group added during metabolism, i.e. OH or COOH. Our results reveal a less thermodynamically favored situation and higher activation energies for all the metabolites with the sole exception of MX for which HAT from OH linked to C9 has larger (negative) ΔG°HAT and smaller activation energies than those of zolpidem, in most of the studied reactions.
Strategies to ameliorate oxidative injury and thereby improve clinical symptoms are of considerable importance, despite the sole antioxidant treatments have not been able to cure any disease. But a molecule that can treat either the primary cause of disease and at the same time reduce the oxidative stress associated to the disease might be more efficient than a drug that treats only the primary cause of disease. We have proved that indeed zolpidem has antioxidant capacity similarly to Trolox and melatonin. In this respect, the disclosure of antioxidant capacity in commercial drugs may be valuable or, at least, may help to better interpret clinical evidence during therapy.
Acknowledgements
This work was financially supported by Università degli Studi di Padova thanks to the P-DiSC (BIRD2018-UNIPD) project MAD3S (Modeling Antioxidant Drugs: Design and Development of computer-aided molecular Systems); P.I. L.O. All the calculations were carried out on Marconi (CINECA: Casalecchio di Reno, Italy) thanks to the ISCRA Grant REBEL (REdox state role in Bio-inspired ELementary reactions), P.I.: L.O.; M.D.T. is grateful to Fondazione CARIPARO for financial support (Ph.D. grant).
Footnotes
RAF (Radical Adduct Formation), Involving the formation of a σ bond between the radical and the rings’ C atoms via electrophilic addition, was excluded because in most cases it leads to endoergonic or weakly isoergonic reactions, as reported for melatonin and Trolox [19,21], In all cases, the RAF path always remains less thermodynamically favored than HAT.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2019.02.004.
Appendix A. Supplementary data
Supplemenatry material
References
- 1.Uher R., Zwicker A. Etiology in psychiatry: embracing the reality of poly-gene-environmental causation of mental illness. World Psychiatry. 2017;16:121–129. doi: 10.1002/wps.20436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng F., Berk M., Dean O., Bush A.I. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 3.Cobley J.N., Fiorello M.L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gingrich J.A. Oxidative stress is the new stress. Nat Med. 2005;11:1281–1282. doi: 10.1038/nm1205-1281. [DOI] [PubMed] [Google Scholar]
- 5.Salim S. Oxidative stress and psychological disorders. Curr Neuropharmacol. 2014;12:140–147. doi: 10.2174/1570159X11666131120230309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan W., Noreen H., Castro-Gomes V., Mohammadzai I., Landeira-Fernandez J.B.T. Association of oxidative stress with psychiatric disorders. Curr Pharm Des. 2016;22:2960–2974. doi: 10.2174/1381612822666160307145931. [DOI] [PubMed] [Google Scholar]
- 7.Tsaluchidu S., Cocchi M., Tonello L., Puri B.K. Fatty acids and oxidative stress in psychiatric disorders. BMC Psychiatry. 2008;8:S5. doi: 10.1186/1471-244X-8-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunes C.S., Maes M., Roomruangwong C., Moraes J.B., Bonifacio K.L., Vargas H.O. Lowered quality of life in mood disorders is associated with increased neuro-oxidative stress and basal thyroid-stimulating hormone levels and use of anticonvulsant mood stabilizers. J Eval Clin Pract. 2018;24:869–878. doi: 10.1111/jep.12918. [DOI] [PubMed] [Google Scholar]
- 9.Pandya C.D., Howell K.R., Pillai A. Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:214–223. doi: 10.1016/j.pnpbp.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillai A. Brain-derived neurotropic factor/TrkB signaling in the pathogenesis and novel pharmacotherapy of schizophrenia. Neurosignals. 2008;16:183–193. doi: 10.1159/000111562. [DOI] [PubMed] [Google Scholar]
- 11.Bošković M., Vovk T., Kores Plesničar B., Grabnar I. Oxidative stress in schizophrenia. Curr Neuropharmacol. 2011;9:301–312. doi: 10.2174/157015911795596595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasanvand A., Pirzadroozbahani N., Ahmadizar F., Kharazmkia A., Mir S., Amanolahi Baharvand P. Evaluation of the antioxidant effects of zolpidem in the rat model of cisplatin-induced nephrotoxicity. J Renal Inj Prev. 2018;7:235–239. [Google Scholar]
- 13.Kim M.J. The Effects of Zolpidem on Akinesia in a Parkinson Disease Patient: a Case Report. J Korean Geriatr Soc. 2015;19:241–243. [Google Scholar]
- 14.García-Santos G., Herrera F., Martín V., Rodriguez-Blanco J., Antolín I., Fernández-Marí F. Antioxidant activity and neuroprotective effects of zolpidem and several synthesis intermediates. Free Radic Res. 2004;38:1289–1299. doi: 10.1080/10715760400017343. [DOI] [PubMed] [Google Scholar]
- 15.Carayon P., Portier M., Dussossoy D., Bord A., Petitpretre G., Canat X. Involvement of peripheral benzodiazepine receptors in the protection of hematopoietic cells against oxygen radical damage. Blood. 1996;87:3170–3178. [PubMed] [Google Scholar]
- 16.Bishnoi M., Chopra K., Kulkarni S.K. Possible anti-oxidant and neuroprotective mechanisms of zolpidem in attenuating typical anti-psychotic-induced orofacial dyskinesia—a biochemical and neurochemical study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1130–1138. doi: 10.1016/j.pnpbp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Galano A., Tan D.X., Reiter R.J. Melatonin and related compounds: chemical insights into their protective effects against oxidative stress. Curr Org Chem. 2017;21:2077–2095. [Google Scholar]
- 18.Galano A., Martínez A. Capsaicin, a tasty free radical scavenger: mechanism of action and kinetics. J Phys Chem B. 2012;116:1200–1208. doi: 10.1021/jp211172f. [DOI] [PubMed] [Google Scholar]
- 19.Galano A. On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys Chem Chem Phys. 2011;13:7178. doi: 10.1039/c0cp02801k. [DOI] [PubMed] [Google Scholar]
- 20.Marino T., Galano A., Russo N. Radical scavenging ability of gallic acid toward oh and ooh radicals. Reaction mechanism and rate constants from the density functional theory. J Phys Chem B. 2014;118:10380–10389. doi: 10.1021/jp505589b. [DOI] [PubMed] [Google Scholar]
- 21.Alberto M.E., Russo N., Grand A., Galano A. A physicochemical examination of the free radical scavenging activity of Trolox: mechanism, kinetics and influence of the environment. Phys Chem Chem Phys. 2013;15:4642–4650. doi: 10.1039/c3cp43319f. [DOI] [PubMed] [Google Scholar]
- 22.Galano A., Álvarez-Diduk R., Ramírez-Silva M.T., Alarcón-Ángeles G., Rojas-Hernández A. Role of the reacting free radicals on the antioxidant mechanism of curcumin. Chem Phys. 2009;363:13–23. [Google Scholar]
- 23.Treml J., Šmejkal K. Flavonoids as potent scavengers of hydroxyl radicals. Compr Rev Food Sci Food Saf. 2016;15:720–738. doi: 10.1111/1541-4337.12204. [DOI] [PubMed] [Google Scholar]
- 24.Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. [Google Scholar]
- 25.Orian L., Toppo S. Organochalcogen peroxidase mimetics as potential drugs: a long story of a promise still unfulfilled. Free Radic Biol Med. 2014;66:65–74. doi: 10.1016/j.freeradbiomed.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Wolters L.P., Orian L. Peroxidase activity of organic selenides: Mechanistic insights from quantum chemistry. Curr Org Chem. 2016;20:189–197. [Google Scholar]
- 27.Dalla Tiezza M., Ribaudo G., Orian L. Organodiselenides: Organic catalysis and drug design learning from glutathione peroxidase. Curr Org Chem. 2018 [Google Scholar]
- 28.Zhao Y., Truhlar D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. 2008;120:215–241. [Google Scholar]
- 29.Fogueri U.R., Kozuch S., Karton A., Martin J.M.L. The Melatonin Conformer Space: benchmark and Assessment of Wave Function and DFT Methods for a Paradigmatic Biological and Pharmacological Molecule. J Phys Chem A. 2013;117:2269–2277. doi: 10.1021/jp312644t. [DOI] [PubMed] [Google Scholar]
- 30.Gaussian 16 rev. B.01. Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ, Wallingford, CT, 2016.
- 31.Marenich A.V., Cramer C.J., Truhlar D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B. 2009;113:6378–6396. doi: 10.1021/jp810292n. [DOI] [PubMed] [Google Scholar]
- 32.Antony J., Sure R., Grimme S. Using dispersion-corrected density functional theory to understand supramolecular binding thermodynamics. Chem Commun. 2015;51:1764–1774. doi: 10.1039/c4cc06722c. [DOI] [PubMed] [Google Scholar]
- 33.Bortoli M., Wolters L.P., Orian L., Bickelhaupt F.M. Addition–Elimination or Nucleophilic Substitution? Understanding the Energy Profiles for the Reaction of Chalcogenolates with Dichalcogenides. J Chem Theory Comput. 2016;12:2752–2761. doi: 10.1021/acs.jctc.6b00253. [DOI] [PubMed] [Google Scholar]
- 34.NBO 6.0. Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Landis CR, Weinhold F, Theoretical Chemistry Institute, University of Wisconsin, Madison (2013).
- 35.Lu T., Chen F. Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- 36.Garrigou-Gadenne D., Burke J.T., Durand A., Depoortere H., Thénot J.P., Morselli P.L. Pharmacokinetics, brain distribution and pharmaco-electrocorticographic profile of zolpidem, a new hypnotic, in the rat. J Pharmacol Exp Ther. 1989;248:1283–1288. [PubMed] [Google Scholar]
- 37.Pichard L., Gillet G., Bonfils C., Domergue J., Thénot J.P., Maurel P. Oxidative metabolism of zolpidem by human liver cytochrome P450S. Drug Metab Dispos. 1995;23:1253–1262. [PubMed] [Google Scholar]
- 38.von Moltke L.L., Greenblatt D.J., Granda B.W., Duan S.X., Grassi J.M., Venkatakrishnan K. Zolpidem metabolism in vitro: responsible cytochromes, chemical inhibitors, and in vivo correlations. Br J Clin Pharmacol. 1999;48:89–97. doi: 10.1046/j.1365-2125.1999.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krylova E.A., Kataev S.S., Khomov Y.A. Determination of zolpidem and its metabolites by chromatography-mass spectrometry. J Anal Chem. 2013;68:722–729. [Google Scholar]
- 40.Blanksby S.J., Ellison G.B. Bond Dissociation Energies of Organic Molecules. Acc Chem Res. 2003;36:255–263. doi: 10.1021/ar020230d. [DOI] [PubMed] [Google Scholar]
- 41.Galano A., Medina M.E., Tan D.X., Reiter R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physicochemical analysis. J Pineal Res. 2015;58:107–116. doi: 10.1111/jpi.12196. [DOI] [PubMed] [Google Scholar]
- 42.Reiter R.J., Rosales-Corral S., Tan D.X., Jou M.J., Galano A., Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell Mol Life Sci. 2017;74:3863–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Draganić I.G., Draganić Z.D., editors. The radiation chemistry of water. Physical chemistry. Vol. 26. Academic Press; New York: 1971. pp. 1–242. [Google Scholar]
- 44.Pryor W.A. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annu Rev Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemenatry material