Abstract
Introduction
Sleep disruption is a characteristic of Alzheimer's disease (AD) that may exacerbate disease progression. This study tested whether a dual orexin receptor antagonist (DORA) would enhance sleep and attenuate neuropathology, neuroinflammation, and cognitive deficits in an AD-relevant mouse model, 5XFAD.
Methods
Wild-type (C57Bl6/SJL) and 5XFAD mice received chronic treatment with vehicle or DORA-22. Piezoelectric recordings monitored sleep and spatial memory was assessed via spontaneous Y-maze alternations. Aβ plaques, Aβ levels, and neuroinflammatory markers were measured by immunohistochemistry, enzyme-linked immunosorbent assay, and real-time polymerase chain reaction, respectively.
Results
In 5XFAD mice, DORA-22 significantly increased light-phase sleep without reducing Aβ levels, plaque density, or neuroinflammation. Effects of DORA-22 on cognitive deficits could not be determined because the 5XFAD mice did not exhibit deficits.
Discussion
These findings suggest that DORAs may improve sleep in AD patients. Further investigations should optimize the dose and duration of DORA-22 treatment and explore additional AD-relevant animal models and cognitive tests.
Keywords: Sleep-wake cycles, Sleep fragmentation, Orexin, Dual orexin receptor antagonist, Amyloid β, Neuroinflammation, Alzheimer's disease
Highlights
-
•
Daily DORA-22 treatment at light onset for 5 weeks increased sleep in 5XFAD mice.
-
•
DORA-22 mainly increased sleep during the light (inactive) phase.
-
•
Chronic DORA-22 treatment did not affect cortical amyloid β levels or plaques.
-
•
Chronic DORA-22 treatment did not alter cortical neuroinflammation.
1. Introduction
Sleep disruption, including fragmentation and loss of sleep, affects 25% to 40% of patients with Alzheimer's disease (AD) [1], [2], [3] and often precedes overt cognitive impairments and diagnosis of AD by a decade or more [4]. Accumulating evidence suggests that sleep disruption—long recognized as a challenge for caregivers—may contribute to the progression of AD neuropathology and cognitive impairment. Sleep is essential for brain function because it stimulates removal of neurotoxic waste products, such as amyloid β (Aβ) [5], and enhances learning-dependent synapse formation and maintenance [6], [7]. Humans with shorter sleep duration or lower sleep quality have higher levels of Aβ in the hippocampus and cortex [8]. Similarly, sleep deprivation strongly increases hippocampal interstitial Aβ levels [9] and reduces Aβ clearance [5] in AD-relevant mouse models. Sleep disruption may contribute to memory impairments in mice and in patients with AD, because sleep promotes memory consolidation [10] whereas sleep deprivation attenuates this process [11], [12]. Sleep fragmentation is associated with earlier cognitive decline and greater risk of incident AD in older humans [13]. In rodents and humans, sleep deprivation or fragmentation impairs performance on many cognitive measures [11], [12], [14], [15], [16], [17], [18], [19], [20], [21]. Moreover, experimentally induced sleep disruption stimulates inflammation and reactive glia response [22], [23], which could be cellular and molecular contributors to accumulation of neurotoxic waste products and cognitive deterioration. These associations of sleep disruption with AD neuropathology led to the following question: Can sleep enhancement attenuate the progression of AD by reducing Aβ accumulation, restoring the homeostatic balance of glia, and reducing cognitive decline?
In this study, we tested the hypothesis that chronic administration of a dual orexin receptor antagonist (DORA) to 5XFAD mice would lead to sleep enhancement that would be associated with attenuation of cognitive deficits, Aβ accumulation, and neuroinflammation. 5XFAD mice were chosen for study because they exhibit AD-like sleep disruptions as well as AD-like neuropathology and cognitive deficits [27], [28], [29]. To improve sleep in 5XFAD mice, DORA-22 was used. In contrast to traditional insomnia medications (e.g., zolpidem [Ambien] and eszopiclone [Lunesta]), which tend to impair memory, DORAs such as DORA-22 exhibit a wide therapeutic margin between sleep induction and cognitive disruption [27]. Furthermore, DORAs restore natural sleep patterns by increasing both rapid eye movement (REM) and non-REM sleep, whereas eszopiclone and related nonbenzodiazepine hypnotics only increase non-REM sleep and decrease REM sleep [28]. The diminution of REM sleep, such as that resulting from traditional insomnia medications, interferes with spatial memory [25], [26]. The 5XFAD mice were selected for testing the therapeutic effects of sleep enhancement because they exhibit not only the prominent neuropathologic features [24] but also sleep alterations resembling those seen in patients with AD including reduced total sleep and shorter sleep bouts [25]. Moreover, the 5XFAD mice exhibit increased neuroinflammation and impairments in spatial memory around the age at which they show sleep disruption [27], [29]; thus, the 5XFAD mice provide a useful preclinical model to test our hypothesis.
2. Methods
2.1. Experimental animals
Male and female 5XFAD hemizygous and wild-type (WT) mice, 4 to 4.5 months old, were used. The 5XFAD mouse is an early onset, aggressive amyloid model that has five distinct human mutations associated with familial early onset AD in two genes encoding the amyloid precursor protein (APP) and presenilin 1 (PS1); these mutations are engineered into two transgenes driven by a neuron-specific promoter (murine Thy1) [24]. The mice were obtained from a breeding colony established at the University of Kentucky using breeders from Jackson Laboratories (hemizygous male 5XFAD [B6SJL-Tg(APPSwFlLon, PSEN1*M146 L*L286V)6799Vas/Mmjax (MMRRC)] and WT female mice [C57B6/SJL]). Three matings were conducted to produce three cohorts of mice (≥32 mice per cohort). After weaning, tissue from ear punches was used for genotyping (Transnetyx). The pups were identified by tattoo. Until the age of 4 months, the mice were group-housed (three to five per cage) with littermates (except when aggression occurred), exposed to an alternating 14 hour light (L):10 hour dark (D) cycle (standard for breeding colonies at our institution), and were weighed monthly. The mice were gently handled three to four times a week for 2 weeks before experimentation began. One week before the study onset, the mice were singly housed and transferred to a separate room dedicated to this study, with a 12L:12D cycle (lights on at 9:15 am, Eastern Standard Time) because 12L:12D is a standard photoperiod for rodent sleep studies. Mouse chow and water were available ad libitum throughout the study.
2.2. Experimental design
All procedures were performed according to the American Association for Accreditation of Laboratory Animal Care Guide and were preapproved by the University of Kentucky Institutional Animal Care and Use Committee. The study was conducted sequentially on three cohorts (32–36 mice per cohort) using the basic experimental design described briefly here, with more details presented subsequently. Before treatment, short-term working spatial memory was assessed at zeitgeber time (ZT) 7 to 9 (ZT 0 = lights on), using the Y-maze test (described in Section 2.4). After resting in their home cages for 3 to 5 days, baseline sleep was assessed for 4 days (Section 2.5). Body weight was recorded and daily oral gavage was conducted at ZT 0 for 5 weeks, with either vehicle (VEH; Vitamin E [d-alpha-tocopheryl polyethylene glycol 1000 succinate], Sigma Aldrich) or DORA-22, 100 mg/kg, a dose that increases sleep in WT mice [28]). The treatment groups (N = 10–12 each) were (1) WT, male, VEH; (2) WT, male, DORA-22; (3) WT, female, VEH; (4) WT, female, DORA-22; (5) 5XFAD, male, VEH; (6) 5XFAD, male, DORA-22; (7) 5XFAD, female, VEH; and (8) 5XFAD, female, DORA-22. Y-maze testing was conducted during the fourth treatment week, and sleep recording was conducted the following week for 3 to 5 days. Sleep recording was continued for 48 hours after the administration of the last dose of DORA-22 or VEH. Body weight was monitored daily during the first week of dosing and at least once a week thereafter and remained stable throughout the study.
2.3. Drug treatment
DORA-22 (100 mg/kg, Merck & Co, Inc) or VEH was administered daily by oral gavage for 5 weeks. Dosing occurred at the time of lights on, that is, at the beginning of the daily inactive phase for mice to mimic a translationally relevant treatment regimen. In a previous study of male C57BL6NTc mice, this dose of DORA-22, although given at dark onset, significantly suppressed wake time for 2 hours after administration while increasing light sleep and REM sleep for ∼5 hours and delta sleep for 1.5 hours [28]. On the basis of the instructions from Merck, the DORA-22 solution was prepared the afternoon before dosing began and was stirred at room temperature thereafter; a fresh solution was prepared every 12 to 14 days.
2.4. Memory testing
Short-term working spatial memory was assessed using the spontaneous alternation Y-maze test described previously [27], [31]. The test was administered 7 to 9 hours after lights on to assess performance at least 1 hour after the end of any acute sleep promoting effect of DORA-22 as determined previously [28]. During testing, the investigators were naïve with respect to the genotype and treatment of each mouse. Each mouse was placed at the end of one arm of a symmetrical Y-maze and allowed to explore the maze freely for 8 minutes. After each session, the Y-maze was cleaned with Quatricide PV (second generation, Pharmacal Research Laboratories). The sequence and total number of arms entered were recorded by the Noldus EthoVision XT 8.0 system. The percentage alternation was defined as the number of triads containing entries into all three arms/maximum possible alternations (the total number of arms entered − 2) × 100.
2.5. Sleep monitoring
A sleep recording system using piezoelectric films that detect pressure changes induced by movements, including small respiratory movements, was used as described for 5XFAD mice [25] and other strains [32], [33]. On the basis of the electroencephalography and human observations, the piezoelectric system has a classification accuracy of >90% [34], [35], [36]. This noninvasive method is more feasible than polysomnography for monitoring sleep in 96 mice. In this study, sleep and wake states were assessed for at least three consecutive days, after 1 day of acclimation to the sleep recording cages. The sleep parameters studied included average total sleep percent for more than 24 hours, average sleep percent at night, average sleep percent during the day, average sleep bout length for more than 24 hours, average sleep bout length at night, and average sleep bout length during the day. Durations of consecutive sleep states were used to compute mean sleep bout lengths, as reported previously [25]. To eliminate the impact of short and ambiguous arousals on the bout length statistics, a bout length count is initiated when a 30-second interval contains greater than 50% sleep and terminates when a 30-second interval has less than 50% sleep.
2.6. Brain tissue collection
Approximately 50 hours after the last dose, the mice were anesthetized with isoflurane and perfused intracardially with ice-cold phosphate-buffered saline (PBS) to exsanguinate. Each brain was bisected in the sagittal plane and one-half was fixed in paraformaldehyde, sucrose embedded, and frozen before subsequent coronal sectioning for immunohistochemistry. The other brain half was microdissected into hippocampal and cortical samples that were frozen and stored (−80°C) for analysis of inflammatory markers and Aβ40 and Aβ42 using enzyme-linked immunosorbent assay (Section 2.7).
2.7. Tissue histologic and biochemical analysis
For each analysis, tissues from all mice were processed simultaneously to reduce variability. Immunohistochemistry was conducted to identify Aβ plaques, as described previously [37], [38]. Briefly, coronal sections (30 μm) were made using a sliding microtome and a freezing stage. Staining procedures were conducted on free-floating brain sections using every 10th section of the entire left hemisphere. Mouse anti-Aβ 6E10 (Covance, catalog #SIG-39340, 1:3000) monoclonal antibody was used to stain for Aβ. The Aperio ScanScope was used to quantify Aβ plaques in the subiculum, hippocampus, and cortex, as previously described [37], [38], [39], [40]. The entire tissue section was scanned using a ×20 objective and converted into a single high-resolution digital image. The Aperio-positive pixel count algorithm (version 9) quantified the amount of specific staining in the region. The number of positive pixels was normalized to the area of the brain regions sampled. The resulting color markup of the analysis was confirmed for each slide. Personnel (D.-H.Y. and A.D.B.) blind to the experimental conditions performed all quantifications.
Brain homogenates for Aβ protein levels were made as previously described [37]. Levels of Aβ1-40 and Aβ1-42 were measured by V-Plex enzyme-linked immunosorbent assay from Meso Scale Discovery as previously described [38]. RNA was isolated using RNeasy minicolumns on dissected neocortical tissues stored at −80°C. cDNA was made using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, catalog #4368814) according to the manufacturer's protocol. Real-time polymerase chain reaction was performed using TaqMan Array Microfluidic Cards (Applied Biosystems, catalog no. 4342253) according to the manufacturer's instructions on a QuantStudio 7 Flex Real-Time Polymerase Chain Reaction System (Applied Biosystems). The following TaqMan probes (Applied Biosystems) were used: C1qa-Mm00432142_m1, C1qb-Mm01179619_m1, C1qc-Mm00776126_m1, C3-Mm01232779_m1, Il1b-Mm00434228_m1, Il6-Mm00446190_m1, Igf1-Mm00439560_m1, Tgfb1-Mm01178820_m1, Ccl3-Mm00441259_g1, Ccl4-Mm00443111_m1, Ccl6-Mm01302419_m1, Cxcl10-Mm00445235_m1, Cd68-Mm03047343_m1, Clec7a-Mm01183349_m1, Cst7-Mm00438351_m1, Itgax-Mm00498701_m1, Gfap-Mm01253033_m1, Lcn2-Mm01324470_m1, Ptx3-Mm00477268_m1, and S100b-Mm00485897_m1. Relative gene expression was calculated by the 2−ΔΔCT method. Hprt-Mm03024075_m1 was used as the normalizing gene. Personnel (A.D.B.) blind to the experimental conditions performed all quantifications.
2.8. Statistical analyses
Statistical analyses were completed in SAS 9.4 (SAS Institute Inc, Cary, NC, USA). The significance level was set at .05. Repeated measures analysis of covariance (ANCOVA), adjusting for cohort, sex, and the interaction of sex with time, were performed. The arcsine of the square root of all percent data and the square root of total alternations and total arms entered were used to satisfy assumptions of the ANCOVA. If the repeated measures ANCOVA revealed significant effects, post hoc least square difference tests were performed. Also, to investigate the interaction and main effects of sex and drug treatment, postdosing data were analyzed by two-way analysis of variance.
3. Results
3.1. Sleep monitoring
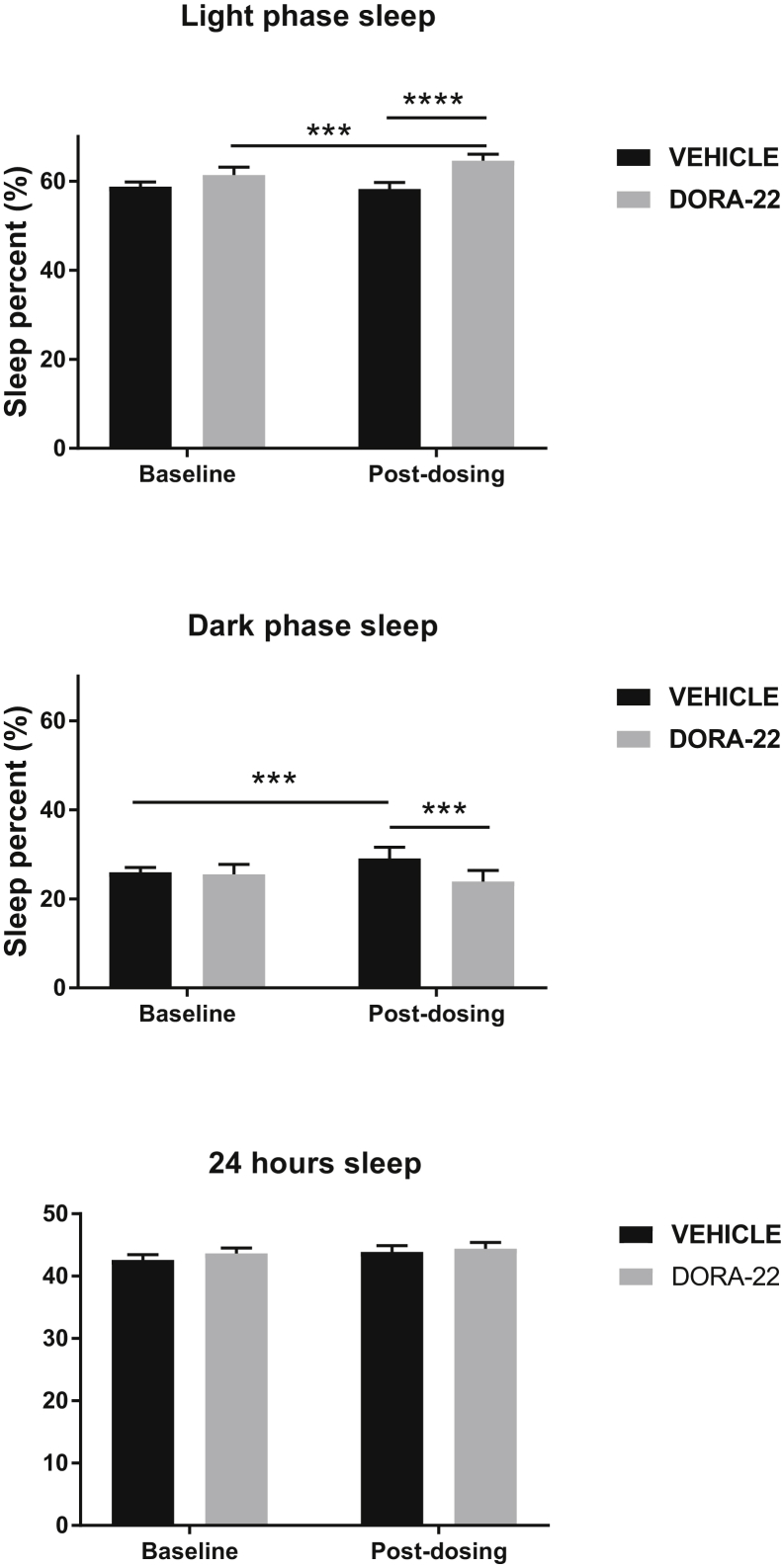
Sleep was disrupted in 5XFAD mice, as reported [25], and was improved by chronic DORA-22 treatment. Representative 24-hour sleep-wake profiles are shown in Supplementary Figs. 1 and 2. Repeated measures ANCOVA revealed that compared with WT mice, the 5XFAD mice exhibited lower percentages of sleep and shorter sleep bout lengths; these effects were observed during the light phase, the dark phase, and across the entire 24-hour period (Table 1). Also, repeated measures ANCOVA showed significant effects of DORA-22 treatment collapsed across genotypes (Fig. 1). DORA-22 increased the light phase sleep percent compared with either the baseline period (t(89) = −3.83; P = .0002) or VEH administration during the treatment period (t(87) = 6.14; P < .0001). Sleep percent during the dark phase was lower during treatment with DORA-22 than VEH (t(89) = −3.04; P = .0031), whereas VEH treatment led to a small increase in dark phase sleep compared with baseline sleep (t(89) = −2.95; P = .0041). Sleep percent during the 24-hour period was not significantly affected by DORA-22 treatment. Unlike sleep percent, sleep bout length in 5XFAD mice (during the light phase, dark phase, or 24-hour period) was not affected by DORA-22 treatment (data not shown).
Table 1.
Genotype effects on sleep
| Sleep parameter | Genotype | Least-squared mean (95% confidence interval) | Test statistic (degrees of freedom) | P value | Average % change, 5XFAD versus WT |
|---|---|---|---|---|---|
| Sleep percent | |||||
| Light phase sleep | 5XFAD | 59.7 (58.3, 61.1) | t(89) = −2.23 | .029 | −3.4 |
| WT | 61.8 (60.6, 63.2) | ||||
| Dark phase sleep | 5XFAD | 24.2 (22.2, 26.3) | t(89) = −2.60 | .011 | −13.6 |
| WT | 28.0 (25.9, 31.1) | ||||
| Total 24-h sleep | 5XFAD | 42.9 (41.7, 44.2) | t(87) = −3.13 | .002 | −6.0 |
| WT | 45.6 (44.4, 46.8) | ||||
| Bout length (s) | |||||
| Light phase bouts | 5XFAD | 61.3 (58.1, 64.4) | t(89) = −2.40 | .018 | −8.0 |
| WT | 66.6 (63.4, 69.7) | ||||
| Dark phase bouts | 5XFAD | 33.8 (30.5, 37.2) | t(87) = −2.41 | .018 | −14.4 |
| WT | 39.5 (36.2, 42.8) | ||||
| Total 24-h bouts | 5XFAD | 49.7 (46.9, 52.5) | t(87) = −2.05 | .043 | −7.6 |
| WT | 53.8 (51.0, 56.7) |
Abbreviation: WT, wild type.
Fig. 1.
Comparison of sleep percent before and after chronic treatment with DORA-22 (100 mg/kg per day for 5 weeks). Bars represent the least-squared mean and the upper limit of the 95% confidence interval for all mice (collapsed across genotypes). ∗∗∗P < .005; ∗∗∗∗P < .0001 (N = 22–24 per group). Abbreviation: DORA, dual orexin receptor antagonist.
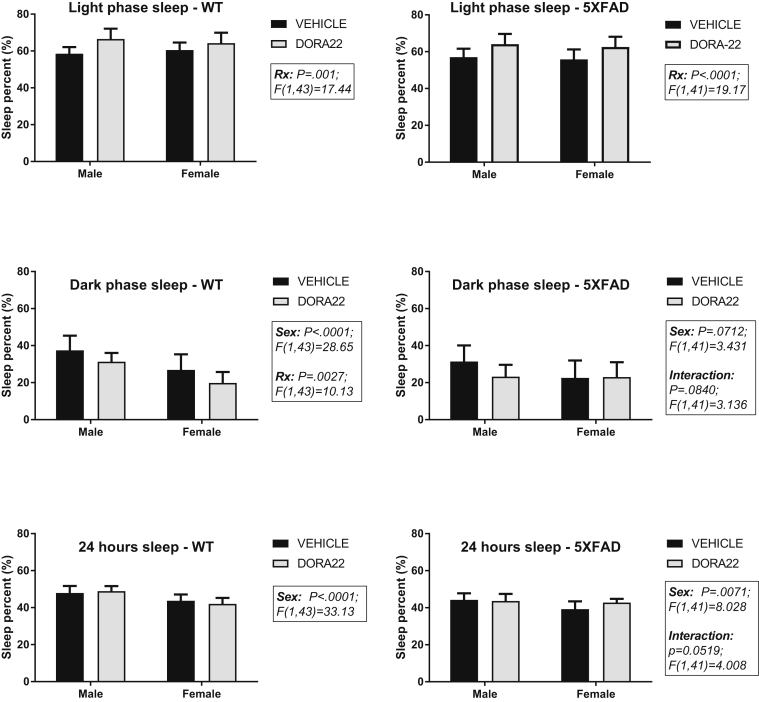
In view of sex differences in cortical Aβ accumulation in the 5XFAD mice and the interactions between Aβ and sleep, two-way analysis of variance was conducted to assess the effects of sex and DORA-22 treatment on sleep percent in each genotype (Fig. 2). In the 5XFAD mice, sleep percent during the light phase was significantly increased by DORA-22 treatment (F [1, 41] = 19.17; P < .0001) but not by sex or by an interaction of sex and treatment. Dark phase sleep percent in 5XFAD mice showed nonsignificant trends toward an effect by sex (F [1, 41] = 3.431; P = .0712) and an interaction effect of sex and treatment (F [1, 41] = 3.136; P = .0840). Sleep percent during the 24-hour period was significantly affected by sex (F [1, 41] = 8.028; P = .0071) but not by DORA-22, and there was a trend toward an interaction effect (F [1, 41] = 4.008; P = .0519). The sleep percent in WT mice showed similar effects of DORA-22 treatment and sex (Fig. 2). Light phase sleep was significantly increased by DORA-22 (F [1, 43] = 17.44; P = .001) but not by sex or an interaction of sex and DORA-22 in WT mice. Dark phase sleep in WT mice was significantly decreased by DORA-22 (F [1, 43] = 10.13; P = .0027) and was lower in females (F [1, 43] = 28.65; P = .0001). Sleep percent over the 24-hour period also was lower in females (F [1, 43] = 28.65; P < .0001) but was not affected by DORA-22 or by an interaction between sex and DORA-22 in WT mice.
Fig. 2.
Effects of sex and chronic DORA-22 treatment (100 mg/kg per day for 5 weeks) on sleep percent in WT and 5XFAD mice. Bars represent the mean + SD (N = 11–12 per group). Significant main effects of two-way ANOVA are shown. RX = treatment with DORA-22. Abbreviations: ANOVA, analysis of variance; DORA, dual orexin receptor antagonist; SD, standard deviation; WT, wild type.
3.2. Memory testing
The percent alternation in the Y-maze was not significantly affected by genotype, sex, or treatment, but was affected by study time point (i.e., baseline vs. during treatment; P < .0001; Table 2). The percent alternation was lower during treatment (mean 0.48; confidence interval, 0.45–0.51) than at baseline (0.56; confidence interval, 0.54–0.59). The total arms entered showed a significant interaction effect between treatment and study time point. Post hoc tests revealed that DORA-22–treated mice entered fewer arms during treatment than before treatment (P = .0275) and showed fewer arm entries than VEH-treated mice either before (P = .0037) or during treatment (P = .0011) (Table 2), possibly indicating residual sleepiness.
Table 2.
Y-maze performance
| Group | % Alternation |
# Arms entered |
||
|---|---|---|---|---|
| Baseline | During Rx | Baseline | During Rx | |
| WT | ||||
| Male, vehicle | 61.7 ± 3.34 | 47.7 ± 5.41 | 28.7 ± 2.60 | 32.7 ± 4.27 |
| Male, DORA-22 | 56.4 ± 5.00 | 48.8 ± 3.99 | 28.9 ± 2.61 | 24.9 ± 1.70 |
| Female, vehicle | 55.2 ± 4.16 | 54.3 ± 4.10 | 31.4 ± 2.84 | 31.8 ± 3.38 |
| Female, DORA-22 | 53.7 ± 2.71 | 52.9 ± 2.81 | 27.8 ± 2.72 | 29.1 ± 3.06 |
| 5XFAD | ||||
| Male, vehicle | 59.2 ± 1.76 | 46.7 ± 3.68 | 29.5 ± 2.88 | 27.1 ± 1.60 |
| Male, DORA-22 | 58.9 ± 2.99 | 48.4 ± 3.30 | 28.6 ± 1.33 | 22.2 ± 1.94 |
| Female, vehicle | 54.9 ± 3.84 | 45.3 ± 3.47 | 33.2 ± 2.73 | 35.4 ± 3.15 |
| Female, DORA-22 | 55.6 ± 2.95 | 46.7 ± 4.68 | 29.5 ± 8.94 | 25.1 ± 2.30 |
Abbreviations: WT, wild type; SEM, standard error of the mean.
NOTE. Values represent the mean ± SEM (n = 10–14 per group). Rx = treatment. The percent alternation was not significantly affected by genotype, sex, or treatment, but was affected by study time point (t(91) = 4.55, P < .0001). The percent alternation was lower during the treatment than at baseline. The total arms entered showed a significant interaction effect between treatment and study time point. Post hoc tests revealed that DORA-22–treated mice entered fewer arms during treatment than before treatment (t(90) = 2.24, P = .0275) and that they showed fewer arm entries compared with vehicle-treated mice either before (t(90) = −2.98, P = .0037) or during treatment (t(90) = −3.36, P = .0011).
3.3. Neuroinflammation and Aβ plaques
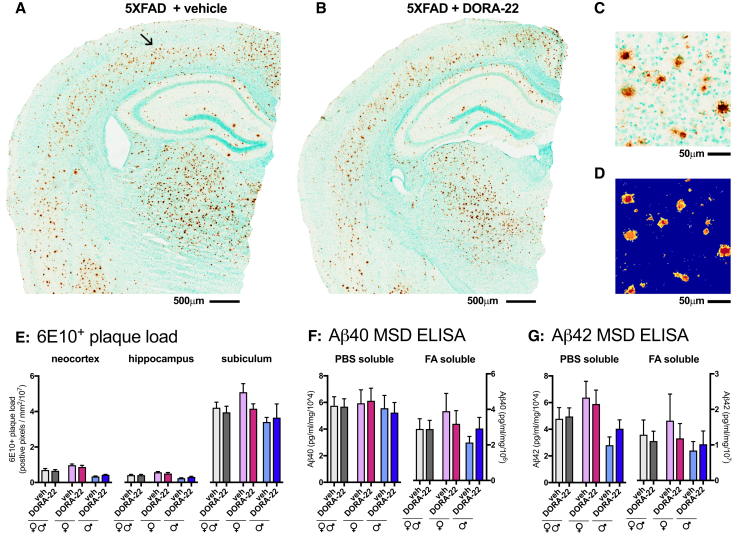
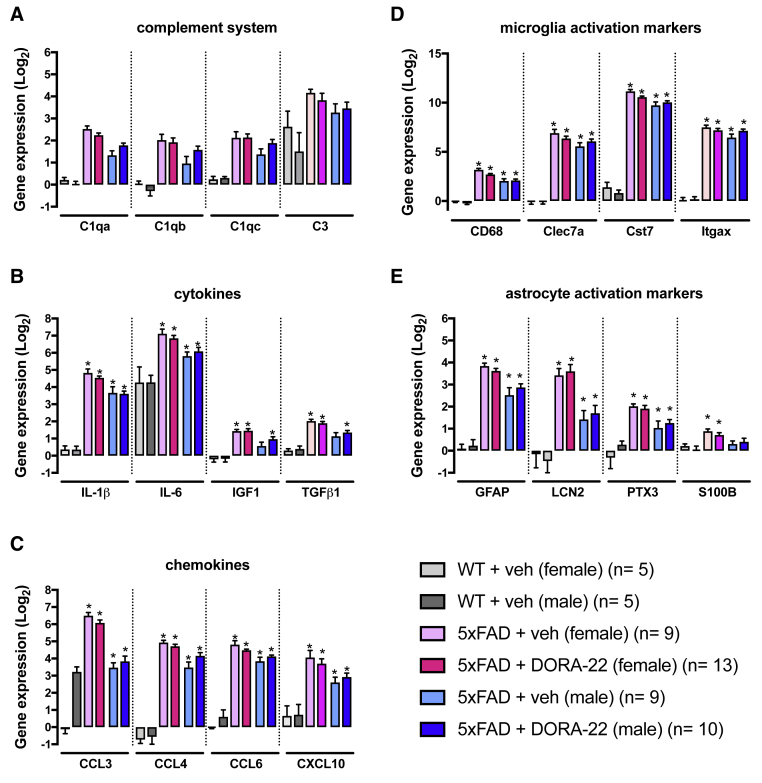
The cortical levels of PBS soluble, and insoluble Aβ1-40 and Aβ1-42, and the density of Aβ (6E10+) plaques in the neocortex, hippocampus, and subiculum of 5XFAD mice were not affected by DORA-22 treatment (Fig. 3). Compared with WT mice, the 5XFAD mice showed significantly higher cortical expressions of all the investigated neuroinflammatory markers (Fig. 4). These markers represented five distinct classes of neuroinflammatory responses including the complement system, cytokines, chemokines, microglial reactivity, and astrocytic reactivity, which have all been implicated in the progression of AD neuropathology. Only females showed overexpression of S100β whereas both sexes showed overexpression of all other neuroinflammatory markers investigated. DORA-22 did not affect the expression of any of the neuroinflammatory markers.
Fig. 3.
Effect of chronic DORA-22 treatment (100 mg/kg per day for 5 weeks) on amyloid β plaques in 5XFAD mice. Representative photomicrograph of amyloid β plaque staining with 6E10 antibody is shown in the brown staining from a female 5XFAD mouse treated with vehicle (A) or treated with DORA-22 (B). Tissue sections are counterstained with methyl green. An arrow in (A) indicates the location of higher magnification view of the staining shown in (C). (D) The color markup of the positive pixel algorithm (Aperio Image Scope software) demonstrates the ability of the algorithm to quantify the 6E10 immunohistochemical staining accurately. The blue color in the markup indicates negative (unstained) pixels. The yellow, orange, and red color in the markup indicates positive (stained) pixels of increasing intensity, respectively. Orange and red color positive pixels were used in the quantification. Results of the area fraction quantification for the neocortex, hippocampus, and subiculum identified according to the Allen Institute mouse brain atlas (mouse.brain-map.org) on the digital neuropathologic slides, is shown in (E). Levels of PBS soluble and FA soluble Aβ1-40 (F) and Aβ1-42 (G) were measured in cortex homogenates by enzyme-linked immunosorbent assay. Data are plotted as the mean ± SEM (N = 10–12 per group). Abbreviations: FA, formic acid; DORA, dual orexin receptor antagonist; PBS, phosphate-buffered saline; SEM, standard error of the mean.
Fig. 4.
Cortical gene expression of neuroinflammatory markers in 5XFAD mice treated with DORA-22 (100 mg/kg per day for 5 weeks). Gene expression analysis was conducted using custom TaqMan Microfluidic Arrays on RNA isolated from neocortical tissue. The inflammatory markers chosen were selected to represent five discrete classes of neuroinflammatory responses based on the presumed function of the markers. The categories included (A) complement system, (B) cytokines, (C) chemokines, (D) reactive microglia markers, and (E) reactive astrocyte markers. Data are presented as log2 fold change from WT + vehicle (veh). ∗P < .05 compared with WT + vehicle (FDR corrected). Bars represent the mean + SEM. Abbreviations: CCL, chemokine (C-C motif ligand); CXCL, chemokine (C-X-C motif) ligand; DORA, dual orexin receptor antagonist; FDR, false discovery rate; GFAP, glial fibrillary acidic protein; IGF, insulin growth factor; IL, interleukin; LCN, lipocalin 2; PTX, pentraxin; TGF, transforming growth factor; SEM, standard error of the mean; WT, wild type.
4. Discussion
Sleep disruption is a common characteristic of AD that impairs quality of life for patients and caregivers and is strongly associated with the progression of neuropathologic changes and cognitive decline. In spite of the prevalence of sleep problems, strategies to improve sleep in patients with AD have received relatively little attention. The traditional sleep-inducing medications, which modify GABAergic activity, tend to impair memory, and thus are not appropriate for patients with AD. Therefore, we tested DORA-22 with a broad therapeutic window between its sleep-inducing and memory-impairing effects [27] for its ability to improve sleep in 5XFAD mice, which display disrupted sleep patterns [28], [41]. In this study, 5XFAD mice exhibited less total 24-hour sleep, dark phase sleep, and light phase sleep than WT mice. The 5XFAD mice also showed shorter sleep bouts over the 24-hour period, during the dark phase, and during the light phase. Chronic daily DORA-22 treatment at lights on increased sleep during the light phase in both 5XFAD and WT mice. Although the magnitude of the DORA-22 enhancement of light phase sleep was small (3.2% increase over baseline sleep), it essentially overcame the light phase sleep deficit (3.4%) compared with WT mice. Prior studies administered DORA-22 in WT mice during the active phase for the mice (i.e., at lights off) to demonstrate that DORA-22 can increase sleep during a time when the percentage of sleep is usually low [28]. In contrast, the present study administered DORA-22 during the inactive phase (i.e., at lights on). This dosing protocol was done to be translationally relevant; however, it does limit the overall magnitude of the effect of DORA-22 treatment as it aims to restore homeostatic levels of sleep in the 5XFAD mice and not necessarily to improve unimpaired sleep in WT mice. The results for the present study support the ability of DORA-22 to restore physiological levels of sleep in the 5XFAD mice. In addition, the results suggest that DORA-22 does not have a major impact on increasing homeostatic drive for sleep above physiological levels in WT mice.
It was not surprising that DORA-22 did not increase sleep during the dark phase that began approximately 11.5 to 12 hours after dosing because a previous study showed that DORA-22 (100 mg/kg per os) was biologically active in male C57Bl6J mice for only 5 to 6 hours [28] (although in that study DORA-22 was administered at lights off). In WT mice, DORA-22 decreased dark phase sleep, suggesting that the homeostatic drive for nighttime sleep is lower after DORA-22 administration during day. In 5XFAD mice, there was not a significant effect of DORA-22 on dark phase sleep, although there was a trend toward an interaction between sex and DORA-22 with lower levels in males. A decrease in sleep during the inactive phase would be desirable in patients with AD who exhibit excessive sleepiness during the daytime [42], [43]. It is important to note that unlike humans, mice normally exhibit >25% of their daily sleep during the “active” phase, and thus administering DORA-22 at night as well as during the day might have been worthwhile. Finally, DORA-22 treatment did not affect sleep bout length, which was shorter in 5XFAD mice than in WT mice, suggesting that DORA-22 does not attenuate sleep fragmentation in mice.
This study has some limitations, including the investigation of only one dose, route, and duration of treatment for DORA-22. The present study showed that chronic daily administration of DORA-22 (100 mg/kg) for 5 weeks to 5XFAD mice increased sleep percent during the inactive phase but did not attenuate neuroinflammation or reduce the density of amyloid plaques. Stress associated with chronic oral gavage might have reduced some of the protective effects of the DORA-22–improved sleep, although this stress did not prevent sleep enhancement. However, future studies using a different route of administration or longer treatment may be warranted. In a previous study, 8 weeks of daily intraperitoneal injections of another DORA compound, almorexant, reduced cortical plaque burden in APPswe/PS1sE9 mice [9]. Whether sleep enhancement with DORA-22 ameliorates cognitive deficits remains to be tested because the 5XFAD mice in the present study did not exhibit a deficit compared with WT control subjects in spontaneous alternations in the Y-maze. The reason for the absence of a deficit in Y-maze spontaneous alternation performance in the 5XFAD mice compared with WT mice in the present study, unlike two previous reports [27], [28], [29], is unclear. In the present study, the WT mice performed poorly, with a percent alternation similar to that in 5XFAD mice in previous studies [27], [29]. Because the WT and the 5XFAD mice in the present study were singly housed for at least a week before Y-maze testing, chronic social isolation may have impaired their performance. Another factor that may have contributed to the discrepancy between the current and previous findings is a potential difference in the time of day of testing, as this factor influences the spontaneous alternations in the T-maze and Y-maze tests [44], [45]. The test time was late in the light phase for the present study but was not reported in previous studies of 5XFAD mice. Finally, as noted in the limitations, the stress associated with the chronic oral gavage may have reduced Y-maze performance.
The 5XFAD mice showed robust increases in cortical expression of a range of neuroinflammatory markers, including cytokines, chemokines, components of the complement system, microglia activation markers, and astrocyte activation markers. Neuroinflammation contributes to the progression of AD pathology and cognitive impairment [37], [45]. These findings support and extend previous findings that revealed increased expression of many inflammation-associated genes in 5XFAD mice [45], [46], [47]. In contrast to earlier studies, both sexes were included in the present study. In general, female 5XFAD mice showed higher expression of neuroinflammatory markers, consistent with observations that females exhibit higher levels of Aβ than males of this transgenic strain [24]. Because inflammation and the reactive glia response are increased by sleep disruption [22], [23], this study tested whether sleep enhancement with DORA-22 administration would attenuate these phenomena, but no attenuation was observed. DORA-22 also did not reduce the density of Aβ plaques. As the 5XFAD is a very aggressive model, longer treatment with DORA-22 and a less stressful route of administration may be necessary to elicit a change in neuroinflammation or Aβ plaques. Alternatively, a less aggressive AD-relevant model could be used to test the effects of DORA-22 on neuroinflammation or Aβ plaques, assuming the model shows sleep abnormalities.
In conclusion, DORA-22 increased sleep in 5XFAD mice, suggesting that treatment with a DORA such as Belsomra (Merck & Co) may be feasible for improving sleep in patients with AD, as recently reported in a pilot study [48]. Furthermore, additional research is necessary to determine optimal dosing and therapeutic window for DORA-22, and to test this drug in additional AD-relevant animal models and cognitive tests.
Research in Context.
-
1.
Systematic review: The literature was reviewed using PubMed and abstracts and presentations at national meetings, including those of the Society for Neuroscience and the Society for Research on Biological Rhythms. Relevant articles are cited appropriately.
-
2.
Interpretation: Review of the literature revealed that Alzheimer's disease is associated with sleep disruption, and sleep disruption impairs cognition and brain clearance of amyloid β (Aβ) and increases neuroinflammation. These findings led to the hypothesis that sleep enhancement would attenuate memory deficits, Aβ accumulation, and neuroinflammation in an AD mouse model. Treatment with a dual orexin receptor antagonist (DORA-22) increased sleep, but did not reduce Aβ or neuroinflammation. The 5XFAD mice did not show the expected memory deficit.
-
3.
Future directions: To further test the hypothesis, several directions for further studies are proposed, including further exploring: (1) the therapeutic window of DORA-22 delivery; (2) cognitive effects of DORA; and (3) additional animal models of AD.
Acknowledgments
This research was supported by a contract from Merck (MIS #201607071502 [M.J.D. and B.F.O.]), pilot funds from National Institutes of Health (NIH) grant NIH-5P30AG02838 (L. Van Eldik, PI of grant; M.J.D. and A.D.B., MPIs of pilot study), NIH R00 AG044445 (A.D.B.), and pilot funds from the University of Kentucky, Department of Neuroscience (M.J.D. and A.D.B.).
Footnotes
Conflicts of interest: The authors declare no competing financial arrangements or connections and no nonfinancial conflicts of interest.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.01.003.
Supplementary data
References
- 1.Prinz P.N., Peskind E., Vitaliano P.P., Raskind M., Eisdorfer C., Zemcuznikov N. Changes in the sleep and waking EEGs in non-demented and demented elderly subjects. J Am Geriatr Soc. 1982;30:86–93. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 2.Bliwise D.L., Mercaldo N.D., Boever B.F., Greer S.A., Kukull W.A. Sleep disturbance in dementia with Lewy bodies and Alzheimer's disease: A multicenter analysis. Dement Geriatr Cogn Disord. 2011;31:239–246. doi: 10.1159/000326238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bliwise D.L. Sleep disorders in Alzheimer's disease and other dementias. Clin Cornerstone. 2004;6:S16–S28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- 4.Vitiello M.V., Borson S. Sleep disturbances in patients with Alzheimer's disease epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15:777–796. doi: 10.2165/00023210-200115100-00004. [DOI] [PubMed] [Google Scholar]
- 5.Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tononi G. Slow wave homeostasis and plasticity. J Clin Sleep Med. 2009;15:S15–S19. [PMC free article] [PubMed] [Google Scholar]
- 7.Vyazovskiy V.V., Faraguna U. Sleep and synaptic homeostasis. Curr Top Behav Neurosci. 2015;25:91–121. doi: 10.1007/7854_2014_301. [DOI] [PubMed] [Google Scholar]
- 8.Spira A.P., Gamaldo A.A., An Y., Wu M.N., Simonsick E.M., Bilgel M. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang J.E., Lim M.M., Bateman R.J., Lee J.J., Smyth L.P., Liam P. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker M.P., Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 11.Cai D.J., Shuman T., Gorman M.R., Sage J.R., Anagnostaras S.G. Sleep selectively enhances hippocampus-dependent memory in mice. Behav Neurosci. 2009;123:713–719. doi: 10.1037/a0016415. [DOI] [PubMed] [Google Scholar]
- 12.Ward C.P., McCoy J.G., McKenna J.T., Connolly N.P., McCarley R.W., Strecker R.E. Spatial learning and memory deficits following exposure to 24 h of sleep fragmentation or intermittent hypoxia in a rat model of obstructive sleep apnea. Brain Res. 2009;1294:128–137. doi: 10.1016/j.brainres.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim A.S.P., Kowigier M., Yu L., Buchman A.S., Bennett D.A. Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta S., Mavanji V., Ulloor J., Patterson E.H. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2004;24:1416–1427. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J.R., Wang T.J., Huang H.Y., Chen L.J., Huang Y.S., Wang Y.J. Fatigue reversibly reduced cortical and hippocampal dendritic spines concurrent with compromise of motor endurance and spatial memory. Neuroscience. 2009;161:1104–1113. doi: 10.1016/j.neuroscience.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Palchykova S., Crestani F., Meerlo P., Tobler I. Sleep deprivation and daily torpor impair object recognition in Djungarian hamsters. Physiol Behav. 2006;87:44–53. doi: 10.1016/j.physbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara M., Iaria G., De Gennaro L., Guariglia C., Curcio G., Tempesta D. The role of sleep in the consolidation of route learning in humans: A behavioural study. Brain Res Bull. 2006;71:4–9. doi: 10.1016/j.brainresbull.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara M., Iaria G., Tempesta D., Curcio G., Moroni F., Marzano C. Sleep to find your way: The role of sleep in the consolidation of memory for navigation in humans. Hippocampus. 2008;18:844–851. doi: 10.1002/hipo.20444. [DOI] [PubMed] [Google Scholar]
- 19.Feng L., Wu H., Song G., Lu C., Qu L., Chen S. Chronical sleep interruption-induced cognitive decline assessed by a metabolomics method. Behav Brain Res. 2016;302:60–68. doi: 10.1016/j.bbr.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 20.Tartar J.L., Ward C.P., McKenna J.T., Thakkar M., Arigoni E., McCarley R.W. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downey R., Bonnet M.H. Performance during frequent sleep disruption. Sleep. 1987;10:354–363. doi: 10.1093/sleep/10.4.354. [DOI] [PubMed] [Google Scholar]
- 22.Ramesh V., Nair D., Zhang S.X.L., Hakim F., Kaushal N., Kayali F. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-alpha pathway. J Neuroinflamm. 2012;9:91. doi: 10.1186/1742-2094-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll J.E., Cole S.W., Seeman T.E., Breen E.C., Witaramaet T., Arevalo J.M.G. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav Immun. 2016;51:223–229. doi: 10.1016/j.bbi.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oakley H., Cole S.L., Logan S., Maus E., Shao P., Craft J. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sethi M., Joshi S.S., Webb R.L., Beckett T.L., Donohue K.D., Murphy M.P. Increased fragmentation of sleep-wake cycles in the 5XFAD mouse model of Alzheimer's disease. Neuroscience. 2015;290:80–89. doi: 10.1016/j.neuroscience.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devi L., Ohno M. Mitochondrial dysfunction and accumulation of the beta-secretase-cleaved C-terminal fragment of APP in Alzheimer's disease transgenic mice. Neurobiol Dis. 2012;45:417–424. doi: 10.1016/j.nbd.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uslaner J.M., Tye S.J., Eddins D.M., Wang X., Fox S.V., Savitz A.T. Orexin receptor antagonists differ from standard sleep drugs by promoting sleep at doses that do not disrupt cognition. Sci Transl Med. 2013;5:179ra44. doi: 10.1126/scitranslmed.3005213. [DOI] [PubMed] [Google Scholar]
- 28.Gotter A.L., Garson S.L., Stevens J., Munden R.L., Fox S.V., Tannenbaum P.L. Differential sleep-promoting effects of dual orexin receptor antagonists and GABAA receptor modulators. BMC Neurosci. 2014;15:109. doi: 10.1186/1471-2202-15-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang R.H., Hu S.-J., Wang Y., Zhang W.-B., Luo W.-J., Chen J.-Y. Paradoxical sleep deprivation impairs spatial learning and affects membrane excitability and mitochondrial protein in the hippocampus. Brain Res. 2018;1230:224–232. doi: 10.1016/j.brainres.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 30.Sil'Kis I.G. Paradoxical sleep as a tool for understanding the hippocampal mechanisms of contextual memory. Neurosci Behav Physiol. 2012;40:5–19. doi: 10.1007/s11055-009-9230-7. [DOI] [PubMed] [Google Scholar]
- 31.Ohno M., Sametsky E.A., Younkin L.H., Oakley H., Youkin S.F., Citron M. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer's disease. Neuron. 2004;41:27–33. doi: 10.1016/s0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 32.Jiang P., Franklin K.M., Duncan M.J., O'Hara B.F., Wisor J.P. Distinct phase relationships between suprachiasmatic molecular rhythms, cerebral cortex molecular rhythms and behavioral rhythms in early runner (CAST/EiJ) and nocturnal (C57Bl6) mice. Sleep. 2012;35:1385–1394. doi: 10.5665/sleep.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan M.J., Smith J.T., Franklin K.M., Murphy M.P., St Clair D., Striz M. Effects of aging and genotype on circadian rhythms, sleep, and clock gene expression in APPxPS1 knock-in mice, a model for Alzheimer's disease. Exp Neurol. 2012;236:249–258. doi: 10.1016/j.expneurol.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Flores A.E., Flores J.E., Deshpande H., Picazo J.A., Xie X.M.S., Francken P. Pattern recognition of sleep in rodents using piezoelectric signals generated by gross body movements. IEEE Trans Biomed Eng. 2007;54:225–233. doi: 10.1109/TBME.2006.886938. [DOI] [PubMed] [Google Scholar]
- 35.Donohue K.D., Medonza D.C., Crane E.R., O'Hara B.F. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed Eng Online. 2008;7:1–13. doi: 10.1186/1475-925X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mang G.M., Nicod J., Emmenegger Y., Donohue K.D., O'Hara B.F. Evaluation of a piezoelectric system as an alternative to electroencephalogram/elctromyogram recording in mouse sleep studies. Sleep. 2014;37:1383–1392. doi: 10.5665/sleep.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachstetter A.D., Norris C.M., Sompol P., Wilcock D.M., Goulding D., Neltner J.H. Early stage drug treatment that normalizes proinflammatory cytokine production attenuates synaptic dysfunction in a mouse model that exhibits age-dependent progression of Alzheimer's disease-related pathology. J Neurosci. 2012;32:10201–10210. doi: 10.1523/JNEUROSCI.1496-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster S.J., VanEldik L.J., Watterson D.M., Bachstetter A.D. Closed head injury in an age-related Alzheimer mouse model leads to an altered neuroinflammatory response and persistent cognitive impairment. J Neurosci. 2015;35:6554–6569. doi: 10.1523/JNEUROSCI.0291-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samaroo H.D., Opsahl A.C., Schreiber J., O'Neill S.M., Marconi M., Qian J. High throughput object-based image analysis of beta-amyloid plaques in human and transgenic mouse brain. J Neurosci Methods. 2012;204:179–188. doi: 10.1016/j.jneumeth.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Neltner J.H., Abner E.L., Schmitt F.A., Denison S.K., Anderson S., Patel E. Digital pathology and image analysis for robust high-throughput quantitative assessment of Alzheimer disease neuropathologic changes. J Neuropathol Exp Neurol. 2012;71:1075–1085. doi: 10.1097/NEN.0b013e3182768de4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider F., Baldauf K., Wetzel W., Reymann K.G. Behavioral and EEG changes in male 5XFAD mice. Physiol Behav. 2014;135:25–33. doi: 10.1016/j.physbeh.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 42.Bliwise D.L. Sleep in normal aging and dementia. Sleep. 1993;161:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 43.Bonanni E., Maestri M., Tognoni G., Fabbrini M., Nucciarone B., Manca M.L. Daytime sleepiness in mild and moderate Alzheimer's disease and its relationship with cognitive impairment. J Sleep Res. 2005;14:311–317. doi: 10.1111/j.1365-2869.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 44.Ruby N.F., Fernandez F., Garrett A., Klima J., Zhang P., Sapolsky H. Spatial memory and long-term object recognition are impaired by circadian arrhythmia and restored by the GABA-A antagonist pentylenetetrazole. PLoS One. 2013;8:e72433. doi: 10.1371/journal.pone.0072433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ardestani P.M., Evans A.K., Yi B., Nguyen T., Cotellier L., Shamloo M. Modulation of neuroinflammation and pathology in the 5XFAD mouse model of Alzheimer's disease using a biased and selective beta-1 adrenergic receptor partial agonist. Neuropharmacology. 2017;116:371–386. doi: 10.1016/j.neuropharm.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landel V., Baranger K., Virard I., Loriod B., Khrestchatisky M., Rivera S. Temporal gene profiling of the 5XFAD transgenic mouse model highlights the importance of microglial activation in Alzheimer's disease. Mol Neurodegen. 2014;9:33. doi: 10.1186/1750-1326-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouter Y., Kacprowski T., Weissmann R., Dietrich K., Borgers H., Brauss A. Deciphering the molecular profile of plaques, memory decline and neuron loss in two mouse models for Alzheimer's disease by deep sequencing. Front Aging Neurosci. 2014;6:75. doi: 10.3389/fnagi.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamuro A., Honda M., Wakamura Y. Suvorexant for the treatment of insomnia in patients with Alzheimer's disease. Aust N Z J Psych. 2018;52:207–208. doi: 10.1177/0004867417747402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.