Abstract
The associations between human papillomavirus (HPV) infection or hormonal exposure and cervical cancer risk are well established. However, to the best of our knowledge, the association between high endogenous estradiol levels in conjunction with HPV/HPV16 infection and the risk of cervical squamous cell carcinoma remains unknown. To investigate this, the current study conducted a matched case-control study in Shanxi Province, China, in which clinical samples were obtained from 74 females with newly diagnosed uterine cervix squamous cell carcinoma and 74 matched healthy females who were selected from 582 healthy females according to age, place of residence, marital status and menopausal status. From all participants, DNA was extracted from cells obtained from a cervical smear and serum was separated from venous blood withdrawn during days 5–8 of the menstrual cycle. HPV/HPV16 DNA and estradiol expression levels in the serum were measured by general polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. Significant differences were identified in the positive HPV and HPV16 DNA expression rates between patients and controls, with odds ratios (95% confidence interval) of 3.74 (1.84–7.59) and 4.04 (1.97–8.28), respectively. Expression levels of estradiol in patients were significantly higher compared with the controls (P<0.001), however, this was only identified when the HPV16 E2 or E6 oncogene status was negative. Considering 40 ng/ml as the cut-off estradiol level, 78.38% of patients exhibited high estradiol levels, which was significantly higher than the percentage of controls (P<0.001). An additive interaction pattern was revealed between estradiol expression levels and HPV/HPV16 infection. The results suggest that among the various types of HPV, HPV16 may be most likely to cause uterine cervix squamous cell carcinoma and an abnormally high level of endogenous estradiol may further increase this risk. Therefore, estradiol therapy may represent a new treatment strategy for cases of cervical cancer associated with HPV infection.
Keywords: uterine cervix squamous cell carcinoma, human papillomavirus, estradiol, additive interaction pattern
Introduction
Cervical cancer is the fourth most common type of cancer in females worldwide (1), with >52,000 new cases diagnosed annually and a morbidity rate that is markedly high in the Shanxi Province of China (2). Cervical cancer has a complicated etiology. Infection with human papillomavirus (HPV), particularly high-risk HPV, has been implicated in cervical squamous cell carcinoma (3,4), however, not all females with HPV infection develop cervical cancer (5,6), which indicates that other factors are responsible for inducing cervical carcinogenesis. The HPV oncogenes E2 and E6 exhibit differential effects during carcinogenesis (7,8). In our previous study (9), it was identified that both the expression levels of HPV16 E6 and E2 were significantly higher in patients with cervical cancer compared with healthy controls and the expression level of E6 was higher compared with E2 in each group. The cervix is the narrow portion of the uterus at the point that joins with the top of the vagina. The majority of cervical cancer cases are squamous cell carcinomas, which occur in the squamous epithelial cells that line the cervix (10,11). This transformation zone, where the majority of cervical cancer cases arise, is the most estrogen-sensitive region of the cervix (12). DNA damage by estrogen metabolites may also contribute to cervical carcinogenesis (12,13). Furthermore, females taking oral contraceptives are at an increased risk of developing cervical cancer (14,15), which suggests an association between hormonal exposure and cancer risk. Parity has also been demonstrated to be a cofactor, along with HPV, in increasing the risk of developing cervical cancer (16). Similarly, lesions have been observed in mice that were perinatally exposed to diethylstilbestrol (17,18).
The role of female hormones in cervical carcinogenesis has traditionally been studied using cervical cell lines and HPV-16 transgenic mice (19,20), however, the role of estradiol in the development of precancerous and cancerous lesions, particularly the association between estradiol and HPV infection, remains unclear (21). Periodic changes in the levels of endogenous hormones are under tight regulation, however, this regulation may be disrupted in certain conditions, including disease and certain environmental exposure. Therefore, further studies are required to investigate the association and mechanism between endogenous hormones and the development of cervical cancer. Therefore, we conducted a case-control study to examine the association between estradiol level and the risk of cervical cancer, and to determine the potential synergistic effect of estradiol and HPV on the progression of cervical cancer.
Materials and methods
Subjects
The present study was conducted with females that were engaged in agriculture and mining in Shanxi Province, located in the north of China. Cervical cancer mortality rates in this population are relatively high (22). Participants were assigned to either the case group or the control group according to their pathologic diagnosis. According to our pilot study, the HPV infective rate among all normal individuals was 28%, with an odds ratio (OR) of 3. The following sample size calculation formula was used to determine that a sample size of 74 for each group was required: n={Z1-α/2*[(2*ρ*(1-ρ)]1/2+ Zβ* [P1*(1- P1)+ P0 *(1- P0)]1/2}2/ (P1- P0)2, P1=(OR*P0)/(1- P0+ OR*P0), ρ= (P1+P0)/2, where α = 0.05 and β = 0.10 (Z: critical value of standard normal distribution, P: HPV exposure rate). Therefore, a total of 74 patients with cervical cancer that were newly diagnosed by histologic assessment were recruited, and 74 healthy controls were selected at random among 582 healthy females who were diagnosed without cervical intraepithelial neoplasia, invasive cancer or other gynecologic diseases during a routine physical examination between January 2016 and December 2016 at Shanxi Province Tumor Hospital (Taiyuan, China). The controls were matched to patients according to age, place of residence, marital status and menopausal status. Pregnant females, females with ovarian disease or other types of cancer, and those who had been treated with steroid hormones in the prior six months were excluded. Written informed consent was obtained from each participant prior to study initiation. The mean ages of women included in the case and control groups were 47.32 years (range, 25–75 years) and 47.42 years (range, 29–77 years), respectively.
Clinical information was obtained by a questionnaire following ethical institutional approval. All subjects were interviewed according to the questionnaire, which focused on demographic characteristics, lifestyle, personal hygiene behavior and reproductive factors. Cervical tissue specimens were surgically obtained from all females in the case group, and cells were obtained from a cervical smear from females in both the case and control groups. In addition, venous blood was collected during days 5–8 of the menstrual cycle from all participants and the serum was separated for subsequent analysis of estradiol levels. Informed consent forms were signed by all individuals who agreed to participate in the study. The study was approved by the Science Research Ethics Committee of Shanxi Medical University (Taiyuan, China).
General polymerase chain reaction (PCR) analysis
Genomic DNA was extracted from cervical tissues and cells using the standard phenol-chloroform method (23). HPV DNA was detected by PCR with the following consensus primers from the L1 regions of HPV type 16, 18, 33, 6, 11 and 31: Forward, 5′-CGTAAACGTTTTCCCTATTTTTTT-3′ and reverse, 5′-TACCCTAAATACTCTGTATTG-3′ (24). High-risk HPV16 DNA was detected using multiple PCR to amplify the E6 and E2 gene sequences with the following primers: HPV16 E2 forward, 5′-AAGGGCGTAACCGAAATCGGT-3′ and reverse, 5′-CATATACCTCACGTCGCAG-3′); and HPV16 E6 forward, 5′-CTTGGGCACCGAAGAAACC-3′ and reverse, 5′-TTGGTCACGTTGCCATTCAC-3′ (25). PCR was performed using 50-µl samples, containing 100 ng template DNA, 25 mM MgCl2, 10 mM each of deoxyadenosine triphosphate, deoxythymidine triphosphate, deoxycytidine triphosphate and deoxyguanosine triphosphate, 25 pM of each consensus forward and reverse primer, and 1 U of Jump-Start Taq DNA polymerase (cat no. D0089: Bio Basic Inc., Markham, ON, Canada). The following conditions were used: 30 cycles at 95°C for 30 sec, 55°C for 60 sec, 72°C for 60 sec and a final extension at 72°C for 10 min. 10 µl PCR products and 2 µl ethidium bromide were mixed and then subjected to electrophoresis on 2% agarose gels. A 253-base pair (bp) fragment represented HPV-positive cases, and two bands of 351 and 208 bp, which were produced by the HPV16 E2 and E6 genes, respectively, represented HPV16-positive cases.
Enzyme-linked immunosorbent assay (ELISA)
Estradiol levels in the serum were measured using an Estradiol enzyme immunoassay (EIA) kit (cat no. BC-1111; Biocheck Inc., San Francisco, CA, USA), according to the manufacturer's protocol.
Statistical analysis
SPSS 19.0 statistical software (IBM Corp., Armonk, NY, USA) was used to analyze the data. Demographic and clinical variables are presented as means ± standard deviations, with ranges for continuous variables, and frequencies and percentages for categorical variables. Each variable was compared between the case group and the control group. For continuous variables, a two-sample t-test was used, while categorical variables were compared with a chi-square test to identify significant differences in HPV/HPV16 infection and estradiol levels between the two groups, and to obtain maximum-likelihood estimates of the OR and corresponding 95% confidence intervals (CIs). Effect modification between estradiol and HPV/HPV16 positivity in cervical cancer cases was measured according to additive interaction patterns (26), Additive interaction is a type of biological interaction which means the effect of two (or more) factors acting on a disease is not equal to the sum of the independent effects of these factors separately (27). Furthermore, the extent of additive interaction with the relative excess risk of interaction (RERI), the attributable proportion of interaction (API) and synergy index (S) were estimated. If there was no association, RERI and API were equal to 0 and S was equal to 1. P<0.05 was considered to indicate a statistically significant difference.
Results
Demographic characteristics of the subjects
There was no significant difference between the ages of the case and control groups (t=0.06; P=0.952). In addition, no significant differences were identified between the groups with respect to education level, frequency of vaginal cleaning, marital status and menopausal status (all P>0.05). However, a significantly higher number of women in the case group were identified to have an occupation as a farmer compared with the control group (P<0.05). The full demographic characteristics are summarized in Table I.
Table I.
Logistic regression analysis comparing characteristics of individuals in the case group and control group.
| Variables | Patients, n (%) | Controls, n (%) | χ2 | P-value | OR (95%CI) |
|---|---|---|---|---|---|
| Education level (high school or above) | 25 (33.78) | 29 (39.19) | 2.77 | 0.096 | 0.47 (0.19–1.15) |
| Occupation (farmer) | 70 (94.59) | 62 (83.78) | 3.98 | 0.046 | 3.67 (1.02–13.14) |
| Frequency of vaginal cleaning (≥3 times a week) | 21 (28.38) | 29 (39.19) | 2.14 | 0.144 | 0.46 (0.16–1.31) |
| Marital status (married) | 49 (66.22) | 45 (60.81) | 1.02 | 0.312 | 0.77 (0.47–1.28) |
| Menopausal status (post-menopause) | 29 (39.19) | 19 (25.68) | 3.20 | 0.074 | 0.50 (0.23–1.07) |
| Number of parity (≥3 times) | 26 (35.14) | 12 (16.22) | 6.96 | 0.008 | 2.22 (1.23–4.03) |
OR, odds ratio; CI, confidence interval.
HPV and HPV16 infection in the case and control groups
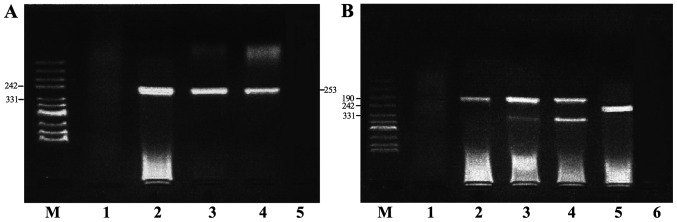
HPV and HPV16 E6/E2 DNA were detected by semi-quantitative PCR (Fig. 1). The HPV16 E2 gene was absent in certain HPV16-positive samples, therefore, HPV16 E6-positive samples were selected to represent HPV16-positive samples. The positivity rates of HPV and HPV16 in the case group (77.03 and 52.70%, respectively) were significantly higher compared with those in the control group (47.30 and 21.62%, respectively), with OR values of 3.74 (95% CI, 1.84–7.59) and 4.04 (95% CI, 1.97–8.28), respectively (Table II). To prevent any deviation caused by differences in tissue and cell specimens, both types of specimens were analyzed consistently for HPV and HPV16 in 24 cervical cancer cases. The results demonstrated that the consistency among the percentage of HPV- and HPV16-positive cases was 91.67% (κ=0.78) and 87.50% (κ=0.75), respectively (Table III).
Figure 1.
Semi-quantitative PCR. (A) HPV DNA detection by PCR. Lane M contains pUC mix Marker 8. Lane 1 contains a HPV negative control. Lanes 2 and 4 represent positive samples. Lane 3 contains a HPV positive control. Lane 5 contains a blank control. (B) HPV 16 E2/E6 DNA detection by PCR amplification. Lane M contains a pUC mix Marker 8. Lane 1 contains a HPV negative control. Lane 2 represents positive and negative HPV16 E6. Lanes 3 and 4 represent positive samples. Lane 5 contains a HPV positive control. Lane 6 contains a blank control. PCR, polymerase chain reaction; HPV, human papillomavirus.
Table II.
HPV and HPV16 infection in the case group and control group.
| Infection | Case group (n=74) Positive, n (%) | Control group (n=74) Positive, n (%) | χ2 | P-value | OR (95% CI) |
|---|---|---|---|---|---|
| HPV | 57 (77.03) | 35 (47.30) | 13.904 | <0.001 | 3.74 (1.84–7.59) |
| HPV16 E6 | 39 (52.70) | 16 (21.62) | 15.306 | <0.001 | 4.04 (1.97–8.28) |
| HPV16 E2 | 31 (41.90) | 13 (17.60) | 10.479 | 0.001 | 3.38 (1.59–7.21) |
HPV, human papillomavirus; OR, odds ratio; CI, confidence interval.
Table III.
Consistency analysis of tissue and cell specimens for HPV and HPV16.
| Variables | HPV, n (%) | HPV16, n (%) |
|---|---|---|
| Tissue specimens/Cell specimens | ||
| +/+ | 17 (70.83) | 10 (41.67) |
| +/− | 1 (4.17) | 2 (8.33) |
| −/+ | 1 (4.17) | 1 (4.17) |
| −/− | 5 (20.83) | 11 (45.83) |
| Total | 24 (100.00) | 24 (100.00) |
| Consistency rate (%) | 91.67 | 87.50 |
| κ | 0.78 | 0.75 |
HPV, human papillomavirus; κ, Kappa.
Estradiol levels in the case and control groups
Estradiol expression levels in the case group (45.98±11.48 pg/ml) were significantly higher compared with the control group (26.96±8.31 pg/ml; Table III). This significant difference between the case and control groups was maintained regardless of the HPV status (Fig. 2A). However, it was identified that estradiol expression level was only significantly higher in the case group compared with the control when the HPV16 E2 or E6 status was negative (Fig. 2B and C).
Figure 2.
Estradiol expression levels in samples either positive of negative for (A) HPV, (B) HPV16 E2 and (C) HPV16 E6 status. Data are presented as the mean ± standard deviation. *P<0.05.
The lowest concentration of estradiol (40 pg/ml) detected in healthy females during the follicular period was defined as the cut-off value; therefore, estradiol levels >40 pg/ml were considered as abnormally high. Accordingly, estradiol levels in 78.38% (58/74) of the cervical cancer cases were revealed to be abnormally high, whereas the estradiol level was identified as abnormally high in only 27.03% (20/74) of the controls, which indicates a significant difference between the two groups (Table IV).
Table IV.
Association between estradiol level and cervical cancer occurrence.
| Group | n | Mean ± SD, pg/ml | Estradiol >40 pg/ml, n (%) | χ2 | P-value | OR (95% CI) |
|---|---|---|---|---|---|---|
| Case | 74 | 45.98±11.48 | 58 (78.38) | 39.14 | <0.001 | 9.79 (4.60–20.82) |
| Control | 74 | 26.96±8.31 | 20 (27.03) |
SD, standard deviation; OR, odds ratio; CI, confidence interval.
Association between estradiol level and HPV or HPV16 infection
The risk of cervical cancer in HPV- or HPV16-positive females with abnormally high levels of estradiol was higher compared with females who were either HPV/HPV16-positive or exhibited abnormally high levels of estradiol. According to a method previously described by Knol and VanderWeele (26), the present study quantitatively analyzed the association between estradiol and HPV/HPV16 infection. and estimated the parameters RERI, API and S (Table V). The results revealed an additive interaction pattern between estradiol levels and HPV/HPV16 infection.
Table V.
Additive interaction between estradiol level and HPV or HPV16 infection.
| A, Additive interaction between estradiol level and HPV infection | ||||||||
|---|---|---|---|---|---|---|---|---|
| HPV | Estradiol, pg/ml | Case group, n | Control group, n | OR (95% CI) | RERI | API | S | |
| + | ≥40 | 44 | 11 | 41.33 (10.64–160.53) | 18.67 | 0.45 | 1.86 | |
| + | <40 | 13 | 24 | 5.60 (1.06–8.56) | ||||
| − | ≥40 | 14 | 8 | 18.08 (4.16–78.60) | ||||
| − | <40 | 3 | 31 | 1.00 | ||||
| B, Additive interaction between estradiol level and HPV16 infection | ||||||||
| HPV16 | Estradiol, pg/ml | Case group, n | Control group, n | OR (95% CI) | RERI | API | S | |
| + | ≥40 | 28 | 8 | 32.90 (9.80–110.48) | 5.20 | −0.16 | 0.86 | |
| + | <40 | 11 | 8 | 12.93 (3.54–47.23) | ||||
| − | ≥40 | 30 | 11 | 25.64 (8.10–81.13) | ||||
| − | <40 | 5 | 47 | 1.00 | ||||
HPV, human papillomavirus; OR, odds ratio; CI, confidence interval; RERI, relative excess risk of interaction; API, attributable proportion of interaction; S, synergy index.
Discussion
HPV infection is relatively common among females (28,29). Persistent HPV infection may cause intraepithelial pre-neoplastic lesions and invasive cervical cancer (30,31). The present study revealed that the positivity rates of HPV and HPV16 in patients with cervical cancer were 77.03 and 52.07%, respectively, which were significantly higher compared with the controls. Among the HPV-positive cases, >68% were HPV16-positive. The results of previous studies, which also investigated HPV infection and cervical cancer in females of Shanxi Province (32,33), in combination with the present results suggest that cervical cancer is closely associated with HPV infection, particularly the high-risk type HPV16, which is considered to be a primary cause of cervical cancer.
To the best of our knowledge, the etiological role of HPV in the development of uterine cervix squamous cell carcinoma remains unclear. Cervical carcinogenesis is a multistep process initiated by HPV, however, infection alone is insufficient to induce malignancy (34). HPV leads to the development of intraepithelial lesions in the presence of other cofactors (35). One such cofactor that has been reported to initiate neoplasia in cervical cancer is prolonged exposure to sex hormones (14,15). Therefore, the potential association between uterine cervix squamous cell carcinoma and sex hormones has been extensively studied (36,37). A meta-analysis has demonstrated that controlled ovarian hyperstimulation (COH) for in vitro fertilization (IVF) could elevate the levels of hormones, however an increased risk of cervical cancer was not identified, which indicates a protective role of IVF (38). However, females undergoing IVF are considered to have stable sexual relationships and a high socioeconomic status, and may be treated for cervical lesions prior to IVF. In addition, HPV infection has been revealed to be significantly lower in females undergoing IVF compared with controls (39). All these factors may reduce the risk of hormone levels on cervical cancer development. By contrast, long-term use of oral contraceptives has been demonstrated to increase the risk of cervical cancer (40). Elevated hormone levels were revealed to induce the proliferation and apoptosis of cervical adenocarcinoma HeLa cells in vitro (19). The biological effects of COH or oral contraceptives in the human body may be influenced by numerous factors, such as drug type, dosage, time of taking medicin and so on (40). A number of studies investigating endogenous steroid hormones have demonstrated an association between endogenous hormone levels and cervical cancer based on the general population (41–43). Estradiol is the most important steroid hormone in the normal female endocrine system (44); the present study identified that endogenous estradiol levels in patients with cervical cancer were higher compared with controls, which suggests that high endogenous estradiol levels are associated with an elevated risk of cervical cancer. Estrogen receptor-α binds to the forkhead box P3 promoter and modulates regulatory T-cell function in human cervical cancer (45). Therefore, sex hormones may have an influence on the immune system. In summary, this evidence suggests estrogen exposure may be associated with hyperplasia and squamous differentiation of reserve cells.
Notably, the present study obtained different conclusions according to the expression of the HPV16 oncogene. A significant difference in HPV positivity was identified between patients and controls, however, estradiol levels in patients were significantly higher compared with controls only when HPV16 E2 or E6 status was negative. HPV16 E6 protein is required for cell transformation, while the HPV E2 protein is required for viral replication and gene expression (46). Estrogen has been demonstrated to upregulate the transcription of HPV E6 oncogenes in vitro (47). A number of studies involving HPV-positive transgenic mice that expressed HPV16 oncogenes in their basal keratinocytes have demonstrated that chronic exposure to estradiol is required for the induction of cervical tumors (47–49). Furthermore, based on biological interaction analysis, the present study revealed an additive interaction between endogenous estradiol level and HPV/HPV16 occurrence. In addition, it was identified that the risk of cervical cancer in females with both a high endogenous estradiol level and HPV infection was 18.67-fold greater compared with the risk associated with other factors, accounting for 45% of the risk, and was 1.86-fold greater compared with the sum of risks associated with endogenous estradiol level and HPV infection. A similar interaction was also observed between the endogenous estradiol level and HPV16 infection, with an RERI of −5.20, API of −0.16 and S of 0.86. These results indicate that the additive effect of HPV/HPV16 infection and high estradiol level is greater compared with their individual effects on cancer risk.
The present study possessed a number of limitations. Firstly, analysis of HPV positivity and high endogenous estradiol levels at a single point in a female's lifetime are difficult to interpret as these could represent either a recent change of a long-term status. Secondly, a case-control study can only suggest associations and cannot determine causal associations. In addition, given the complicated association between endogenous estradiol level and HPV infection in cervical cancer, there are numerous other influencing factors that should be investigated.
Despite these limitations, the cervix epithelium is a hormone-dependent epithelium, and the present study provides a strong indication that abnormally high endogenous estradiol levels may be associated with increased risk of cervical cancer and estradiol may serve as a cofactor along with HPV infection in the development of cervical cancer. Further studies, including a prospective cohort study should be performed to provide etiological evidence that may clarify the mechanism of the association between endogenous hormone level and HPV infection in cervical carcinogenesis. In addition, further studies are required to evaluate whether an increased risk of cervical cancer is associated with other factors. Furthermore, the results of the present study suggest that estradiol may represent a new treatment strategy in cases of cervical cancer due to HPV infection, however, this requires further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81703313, 81473060 and 81702583).
Availability of data and materials
The datasets used or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
LD performed data analyses, drafted the manuscript, finalized and submitted the manuscript. LD, and MF contributed to perform the DNA extraction, PCR experiments and ELISA experiments. CL and QZ contributed to the data collection and quality control. JW and LD conceived the idea, designed and led the project. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Science Research Ethics Committee of Shanxi Medical University (Taiyuan, China) and all participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Skinner RS, Wheeler CM, Romanowski B, Castellsagué X, Lazcano-Ponce E, Del Rosario-Raymundo RM, Vallejos C, Minkina G, Pereira Da Silva D, McNeil S, et al. Progression of HPV infection to detectable cervical lesions or clearance in adult women: Analysis of the control arm of the VIVIANE study. Int J Cancer. 2016;138:2428–2438. doi: 10.1002/ijc.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madeleine MM, Anttila T, Schwartz SM, Saikku P, Leinonen M, Carter JJ, Wurscher M, Johnson LG, Galloway DA, Daling JR. Risk of cervical cancer associated with Chlamydia trachomatis antibodies by histology, HPV type and HPV cofactors. Int J Cancer. 2007;120:650–655. doi: 10.1002/ijc.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi YJ, Park JS. Clinical significance of human papillomavirus genotyping. J Gynecol Oncol. 2016;7:e21. doi: 10.3802/jgo.2016.27.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egawa N, Egawa K, Griffin H, Doorbar J. Human papillomaviruses: epithelial tropisms, and the development of neoplasia. Viruses. 2015;7:3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellanger S, Tan CL, Xue YZ, Teissier S, Thierry F. Tumor suppressor or oncogene? A critical role of the human papillomavirus (HPV) E2 protein in cervical cancer progression. Am J Cancer Res. 2011;1:373–389. [PMC free article] [PubMed] [Google Scholar]
- 8.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25(Suppl 1):S2–S23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JT, Ding L, Gao ES, Cheng YY. Analysis on the expression of human papillomavirus type 16 E2 and E6 oncogenes and disruption of E2 in cervical cancer. Zhonghua Liu Xing Bing Xue Za Zhi. 2007;28:968–971. (In Chinese) [PubMed] [Google Scholar]
- 10.Missaoui N, Trabelsi A, Landolsi H, Jaidaine L, Mokni M, Korbi S, Hmissa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among Tunisian women. Asian Pac J Cancer Prev. 2010;11:777–780. [PubMed] [Google Scholar]
- 11.Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer. 2004;100:1035–1044. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- 12.Auborn KJ, Woodworth C, DiPaolo JA, Bradlow HL. The interaction between HPV infection and estrogen metabolism in cervical carcinogenesis. Int J Cancer. 1991;49:867–869. doi: 10.1002/ijc.2910490611. [DOI] [PubMed] [Google Scholar]
- 13.Newfield L, Bradlow HL, Sepkovic DW, Auborn K. Estrogen metabolism and the malignant potential of human papillomavirus immortalized keratinocytes. Proc Soc Exp Biol Med. 1998;217:322–326. doi: 10.3181/00379727-217-44239. [DOI] [PubMed] [Google Scholar]
- 14.Moodley M, Moodley J, Chetty R, Herrington CS. The role of steroid contraceptive hormones in the pathogenesis of invasive cervical cancer: A review. Int J Gynecol Cancer. 2003;13:103–110. doi: 10.1046/j.1525-1438.2003.13030.x. [DOI] [PubMed] [Google Scholar]
- 15.Salazar EL, Sojo-Aranda I, Lopez R, Salcedo M. The evidence for an etiological relationship between oral contraceptive use and dysplastic change in cervical tissue. Gynecol Endocrinol. 2001;15:23–28. doi: 10.1080/gye.15.1.23.28. [DOI] [PubMed] [Google Scholar]
- 16.Muñoz N, Franceschi S, Bosetti C, Moreno V, Herrero R, Smith JS, Shah KV, Meijer CJ, Bosch FX; International Agency for Research on Cancer (IARC) Multicentric Cervical Cancer Study Group: Role of parity and human papillomavirus in cervical cancer: The IARC multicentric case-control study. Lancet. 2002;359:1093–1101. doi: 10.1016/S0140-6736(02)08151-5. [DOI] [PubMed] [Google Scholar]
- 17.Plapinger L, Bern HA. Adenosis-like lesions and other cervicovaginal abnormalities in mice treated perinatally with estrogen. J Natl Cancer Inst. 1979;63:507–518. [PubMed] [Google Scholar]
- 18.McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–3999. [PubMed] [Google Scholar]
- 19.Liu Y, Tian LB, Yang HY, Zhang HP. Effects of estradiol and progesterone on the growth of HeLa cervical cancer cells. Eur Rev Med Pharmacol Sci. 2017;21:3959–3965. [PubMed] [Google Scholar]
- 20.Brake T, Lambert PF. Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc Natl Acad Sci USA. 2005;102:2490–2495. doi: 10.1073/pnas.0409883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.den Boon JA, Pyeon D, Wang SS, Horswill M, Schiffman M, Sherman M, Zuna RE, Wang Z, Hewitt SM, Pearson R, et al. Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling. Proc Natl Acad Sci USA. 2015;112:E3255–E3264. doi: 10.1073/pnas.1509322112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang SK, Kang LN, Chang IJ, Zhao FH, Hu SY, Chen W, Shi JF, Zhang X, Pan QJ, Li SM, et al. The natural history of cervical cancer in Chinese women: results from an 11-year follow-up study in china using a multistate model. Cancer Epidemiol Biomarkers Prev. 2014;23:1298–1305. doi: 10.1158/1055-9965.EPI-13-0846. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Russell DW. Purification of nucleic acids by extraction with phenol:chloroform. CSH Protoc. 2006;2006(pii) doi: 10.1101/pdb.prot4455. pdb.prot4455. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikawa H, Kawana T, Kitagawa K, Mizuno M, Yoshikura H, Iwamoto A. Detection and typing of multiple genital human papillomaviruses by DNA amplification with consensus primers. Jpn J Cancer Res. 1991;82:524–531. doi: 10.1111/j.1349-7006.1991.tb01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badaracco G, Venuti A, Sedati A, Marcante ML. HPV16 and HPV18 in genital tumors: Significantly different levels of viral integration and correlation to tumor invasiveness. J Med Virol. 2002;67:574–582. doi: 10.1002/jmv.10141. [DOI] [PubMed] [Google Scholar]
- 26.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41:514–520. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman KJ, Greenland S, Lash TL. In: Modern epidemiology. Lippincott Williams & Wilkins; 2008. Chapter 5 Concepts of Interaction. [Google Scholar]
- 28.Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Ning L, Killeen J, Kamemoto L, Hernandez BY. Acquisition of anal human papillomavirus (HPV) infection in women: The Hawaii HPV Cohort study. J Infect Dis. 2008;197:957–966. doi: 10.1086/529207. [DOI] [PubMed] [Google Scholar]
- 29.Shvetsov YB, Hernandez BY, McDuffie K, Wilkens LR, Zhu X, Ning L, Killeen J, Kamemoto L, Goodman MT. Duration and clearance of anal human papillomavirus (HPV) infection among women: The Hawaii HPV cohort study. Clin Infect Dis. 2009;48:536–546. doi: 10.1086/596758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen HC, Schiffman M, Lin CY, Pan MH, You SL, Chuang LC, Hsieh CY, Liaw KL, Hsing AW, Chen CJ, et al. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst. 2011;103:1387–1396. doi: 10.1093/jnci/djr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byun JM, Jeong DH, Kim YN, Jung EJ, Lee KB, Sung MS, Kim KT. Persistent HPV-16 infection leads to recurrence of high-grade cervical intraepithelial neoplasia. Medicine (Baltimore) 2018;97:e13606. doi: 10.1097/MD.0000000000013606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai M, Bao YP, Li N, Clifford GM, Vaccarella S, Snijders PJ, Huang RD, Sun LX, Meijer CJ, Qiao YL, Franceschi S. Human papillomavirus infection in Shanxi Province, People's Republic of China: A population-based study. Br J Cancer. 2006;95:96–101. doi: 10.1038/sj.bjc.6603208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J, Zhao F, Liu R, Mu Y. Prevalence and type distribution of human papillomavirus infection in women from Datong, China. Scand J Infect Dis. 2010;42:72–75. doi: 10.3109/00365540903311169. [DOI] [PubMed] [Google Scholar]
- 34.Briolat J, Dalstein V, Saunier M, Joseph K, Caudroy S, Prétet JL, Birembaut P, Clavel C. HPV prevalence, viral load and physical state of HPV-16 in cervical smears of patients with different grades of CIN. Int J Cancer. 2010;121:2198–2204. doi: 10.1002/ijc.22959. [DOI] [PubMed] [Google Scholar]
- 35.Luhn P, Walker J, Schiffman M, Zuna RE, Dunn ST, Gold MA, Smith K, Mathews C, Allen RA, Zhang R, et al. The role of co-factors in the progression from human papillomavirus infection to cervical cancer. Gynecol Oncol. 2013;128:265–270. doi: 10.1016/j.ygyno.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno V, Bosch FX, Munoz N, Meijer CJ, Shah KV, Walboomers JM, Herrero R, Franceschi S; International Agency for Research on Cancer. Multicentric Cervical Cancer Study Group: Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: The IARC multicentric case-control study. Lancet. 2002;359:1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 37.Santos Filho MV, Gurgel AP, Lobo CD, Freitas AC, Silva-Neto JC, Silva LA. Prevalence of human papillomavirus (HPV), distribution of HPV types, and risk factors for infection in HPV-positive women. Genet Mol Res. 2016;15 doi: 10.4238/gmr.15028315. [DOI] [PubMed] [Google Scholar]
- 38.Siristatidis C, Sergentanis TN, Kanavidis P, Trivella M, Sotiraki M, Mavromatis I, Psaltopoulou T, Skalkidou A, Petridou ET. Controlled ovarian hyperstimulation for IVF: Impact on ovarian, endometrial and cervical cancer-a systematic review and meta-analysis. Hum Reprod Update. 2013;19:105–123. doi: 10.1093/humupd/dms051. [DOI] [PubMed] [Google Scholar]
- 39.Lundqvist M, Westin C, Lundkvist O, Simberg N, Strand A, Andersson S, Wilander E. Cytologic screening and human papilloma virus test in women undergoing artificial fertilization. Acta Obstet Gynecol Scand Oct. 2002;81:949–953. doi: 10.1034/j.1600-0412.2002.811009.x. [DOI] [PubMed] [Google Scholar]
- 40.International Collaboration of Epidemiological Studies of Cervical Cancer. Appleby P, Beral V, Berrington de González A, Colin D, Franceschi S, Goodhill A, Green J, Peto J, Plummer M, Sweetland S. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609–1621. doi: 10.1016/S0140-6736(07)61684-5. [DOI] [PubMed] [Google Scholar]
- 41.Rinaldi S, Plummer M, Biessy C, Castellsagué X, Overvad K, Krüger Kjær S, Tjønneland A, Clavel-Chapelon F, Chabbert-Buffet N, Mesrine S, et al. Endogenous sex steroids and risk of cervical carcinoma: results from the EPIC study. Cancer Epidemiol Biomarkers Prev. 2011;20:2532–2540. doi: 10.1158/1055-9965.EPI-11-0753. [DOI] [PubMed] [Google Scholar]
- 42.Shields TS, Falk RT, Herrero R, Schiffman M, Weiss NS, Bratti C, Rodriguez AC, Sherman ME, Burk RD, Hildesheim A. A case-control study of endogenous hormones and cervical cancer. Br J Cancer. 2004;90:146–152. doi: 10.1038/sj.bjc.6601514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spurgeon ME, den Boon JA, Horswill M, Barthakur S, Forouzan O, Rader JS, Beebe DJ, Roopra A, Ahlquist P, Lambert PF. Human papillomavirus oncogenes reprogram the cervical cancer microenvironment independently of and synergistically with estrogen. Proc Natl Acad Sci USA. 2017;114:E9076–E9085. doi: 10.1073/pnas.1712018114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuhl H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric. 2005;8(Suppl 1):S3–S63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- 45.Adurthi S, Kumar MM, Vinodkumar HS, Mukherjee G, Krishnamurthy H, Acharya KK, Bafna UD, Uma DK, Abhishekh B, Krishna S, et al. Oestrogen receptor-α binds the FOXP3 promoter and modulates regulatory T-cell function in human cervical cancer. Sci Rep. 2017;7:17289. doi: 10.1038/s41598-017-17102-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blachon S, Demeret C. The regulatory E2 proteins of human genital papillomaviruses are pro-apoptotic. Biochimie. 2003;85:813–819. doi: 10.1016/j.biochi.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Riley RR, Duensing S, Brake T, Münger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63:4862–4871. [PubMed] [Google Scholar]
- 48.Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67:1626–1635. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci USA. 1996;93:2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the present study are available from the corresponding author on reasonable request.