Abstract
One of the major roles of professional phagocytes is the removal of dead cells in the body. We know less about the clearance of necrotic cells than apoptotic cell phagocytosis, despite the fact that both types of dead cells need to be cleared together and necrotic cells appear often in pathological settings. In the present study, we examined phagocytosis of heat‐ or H2O2‐killed necrotic and apoptotic thymocytes by mouse bone marrow‐derived macrophages (BMDMs) in vitro and found that the two cell types are engulfed at equal efficiency and compete with each other when added together to BMDMs. Phagocytosis of both apoptotic and necrotic thymocytes was decreased by (a) blocking phosphatidylserine on the surface of dying cells; (b) inhibition of Mer tyrosine kinase, Tim‐4, integrin β3 receptor signaling, or Ras‐related C3 botulinum toxin substrate 1 activity; or (c) using BMDMs deficient for transglutaminase 2. Stimulation of liver X, retinoid X, retinoic acid or glucocorticoid nuclear receptors in BMDMs enhanced not only apoptotic, but also necrotic cell uptake. Electron microscopic analysis of the engulfment process revealed that the morphology of phagosomes and the phagocytic cup formed during the uptake of dying thymocytes is similar for apoptotic and necrotic cells. Our data indicate that apoptotic and necrotic cells are cleared via the same mechanisms, and removal of necrotic cells in vivo can be facilitated by molecules known to enhance the uptake of apoptotic cells.
Keywords: apoptosis, macrophages, phagocytosis, phosphatidylserine, primary necrosis
Abbreviations
- 9cRA
9‐cis retinoic acid
- ATRA
all‐trans retinoic acid
- BMDM
bone marrow‐derived macrophage
- CD
cluster of differentiation
- CFDA‐SE
carboxyfluorescein diacetate succinimidyl ester
- CMTMR
5‐(and‐6)‐(((4‐chloromethyl)benzoyl)amino)tetramethylrhodamine
- GR
glucocorticoid receptor
- LXR
liver X receptor
- MerTK
Mer tyrosine kinase
- MFG‐E8
milk fat globule‐EGF factor 8 protein
- PS
phosphatidylserine
- Rac1
Ras‐related C3 botulinum toxin substrate 1
- RAR
retinoic acid receptor
- RGD
arginylglycylaspartic acid
- RXR
retinoid X receptor
- TAM
Tyro3, Axl, Mer
- TG2
transglutaminase 2
- Tim‐4
T‐cell immunoglobulin mucin receptor 4
Every day billions of damaged or senescent cells die in our body and are replaced with new cells 1. One of the physiological cell death types is apoptosis characterized by detachment and shrinkage of the cell, condensation and fragmentation of nuclear content 2, maintenance of membrane integrity and display of ‘eat me’ signals such as phosphatidylserine (PS) 3, or disappearance of so‐called ‘don't eat me’ signals, such as cluster of differentiation (CD) 47 on the apoptotic cell surface 4. Apoptosis can be activated by a wide range of stimuli, which trigger either the cell death receptor or the mitochondrial pathway of apoptosis 5, 6. Apoptosis is considered an immunologically silent process, since not only do apoptotic cells fail to induce inflammation, but uptake of apoptotic cells was shown to actively suppress the inflammatory program in engulfing macrophages 7, 8. In contrast to apoptosis, necrosis is characterized by swelling of the cell and early membrane rupture 9 leading to release of the intracellular content, which can damage the surrounding tissues and initiate local inflammation 10, 11, 12. Several conditions can result in necrosis, such as exposure of cells to high temperature in burns, physical damage, hypoxia, viral infection or in the case of programmed necroptosis, cell death receptor ligation 13. Necrotic cells were also shown to display PS on their outer membrane leaflet, which is used for their uptake 14, 15. Similar to apoptotic cells, engagement of PS receptors on the surface of macrophages elicits an anti‐inflammatory response, but this effect is overridden by the noxious cell content released during cell necrosis 14, 16, 17. Efficient clearance of necrotic cells in the organism helps to resolve the wounded area and the initiated inflammation. Apoptotic cells can also lose membrane integrity and undergo secondary necrosis in cases in which they are not cleared from the tissues properly 18. In this case, the accumulating secondary necrotic cells initiate local inflammation, which can lead to the development of autoimmune diseases in the long term 19, 20.
Macrophages are considered to be the primary phagocytes responsible for clearing dead cells in most organs. Macrophages are equipped with a battery of receptors to recognize, bind and engulf apoptotic cells. Among others, these receptors include the direct PS receptor T‐cell immunoglobulin mucin receptor 4 (Tim‐4), stabilin‐2, brain‐specific angiogenesis inhibitor 1, and receptors, such as Mer tyrosine kinase (MerTK), integrin αvβ3 and its co‐receptor transglutaminase 2 (TG2), that bind to PS through various bridging molecules 21. Interestingly, the CD36–αvβ3 integrin receptor complex was reported to recognize and bind externalized PS during necrotic cell uptake as well 16. The phagocytic receptors participating in the uptake of apoptotic cells activate two evolutionarily conserved signaling pathways, both of which trigger the activity of the small G protein Rac that regulates actin reorganization and lamellipodia formation during phagocytosis 22. Despite the shared integrin receptors considerable differences were found in the uptake of the two cell types. On one hand, it was suggested that necrotic cells compete with apoptotic ones much more efficiently than the other way around 14, though surprisingly apoptotic cells are engulfed more quickly than necrotic ones 17. On the other, in sharp contrast, it was indicated by another laboratory that macrophages have distinct modes for apoptotic and necrotic cell recognition, and they do not compete with each other upon simultaneous exposure 23. While uptake of apoptotic cells takes place via well‐defined portals in macrophages, where phagocytic receptors assemble together to mediate efficient uptake of a number of dead cells into tight‐fitting phagosomes 24, necrotic cell phagocytosis seems to take place via a macropinocytosis‐like process generating spacious macropinosomes and an abundant number of pseudopods 25. Activation of nuclear receptor liver X (LXR), retinoic acid (RAR), retinoid X (RXR) 26 and glucocorticoid (GR) receptors 26, 27, 28 in macrophages leads to upregulation of several of the above‐mentioned apopto‐phagocytic genes and results in enhanced apoptotic cell clearance.
While we consider apoptosis and necrosis as two different cell death forms, under pathological conditions they very often appear together and have to be cleared simultaneously 29, 30, 31, 32, 33, 34. Thus in this study, we decided to reinvestigate the mechanism and preference in the uptake of apoptotic and necrotic cells by studying the engulfment of serum‐starved apoptotic and heat‐ or H2O2‐killed necrotic thymocytes by bone marrow‐derived macrophages (BMDMs). Our data indicate that apoptotic and heat‐ or H2O2‐treated necrotic thymocytes are cleared by macrophages via similar PS‐dependent mechanisms and this clearance can be enhanced by nuclear receptor agonists known to induce apoptotic cell phagocytosis. In addition, the necrotic and apoptotic cells are engulfed at equal efficiency and compete with each other suggesting similar uptake mechanisms.
Materials and methods
Reagents
All reagents were obtained from Sigma‐Aldrich (Budapest, Hungary) except when indicated otherwise.
Experimental animals
The experiments were carried out with 4‐week‐old or 2‐ to 4‐month‐old C57B6 mice. In some experiments, TG2+/+ and TG2−/− mice were used. Mice were maintained in specific pathogen‐free conditions in the Central Animal Facility and all animal experiments were approved by the Animal Care and Use Committee of University of Debrecen (DEMÁB).
BMDM cell culture and treatment
Bone marrow progenitors were obtained from the femurs of 2‐ to 4‐month‐old mice. Femurs were washed with sterile physiological saline. Cells were allowed to differentiate for 6 days in DMEM supplemented with 10% FBS, 10% conditioned medium derived from L929 cells as a source of macrophage colony‐stimulating factor, 2 mm glutamine, 100 U·mL−1 penicillin and 100 mg·mL−1 streptomycin at 37 °C in 5% CO2. Non‐adherent cells were washed away after 3 days. BMDMs were treated with the RAR agonist all‐trans retinoic acid (ATRA; 1 μm), the RXR agonist 9‐cis retinoic acid (9cRA; 1 μm), the LXR agonist GW3965 (Tocris, Bioscience, Bristol, UK; 1 μm) or the GR agonist dexamethasone acetate (1 μm) to activate nuclear receptors, or vehicle (0.5% DMSO) for 24 h. In the inhibitory experiments, 1 μm BMS777607 Tyro3, Axl, Mer (TAM) receptor tyrosine kinase inhibitor or 100 μm NSC23766 small GTP binding protein Ras‐related C3 botulinum toxin substrate 1 (Rac1) inhibitor (Tocris) was used for 24 h. For blocking integrins, either 2 mg·mL−1 arginylglycylaspartic acid (RGD) peptide or 4 μg·mL−1 fluorescein isothiocyanate hamster anti‐mouse CD61 antibody (BD Biosciences, Franklin Lakes, NJ, USA) was used for 1 h before addition of the apoptotic or necrotic cells. Four micrograms per milliliter Tim‐4 monoclonal antibody [54 (RMT4‐54)], phycoerythrin (Thermo Fisher Scientific, Waltham, MA, USA) was used for 1 h to block the PS receptor Tim‐4 on the surface of BMDMs.
Apoptosis and necrosis induction and PS masking on thymocytes
Thymi were collected from 4‐week‐old C57B6 mice, and thymocytes were isolated and cultured for 24 h (107 cells·mL−1) in DMEM supplemented with 2 mm glutamine, 100 U·mL−1 penicillin, and 100 mg·mL−1 streptomycin. To avoid potential effects of apoptosis inducers on the uptake of apoptotic cells, apoptosis of thymocytes was induced by growth factor withdrawal 35 by incubating the cells in serum‐free medium for 24 h, as we described previously 36. To generate necrotic target cells, thymocytes were incubated either at 55 °C for 20 min 23 or with 1 mm H2O2 for 24 h 37. Since isolated thymocytes show spontaneous cell death after 24 h of incubation 38, freshly isolated thymocytes were used as living cell controls in the experiments. Propidium iodide/annexin V labeling was used to determine the percentage of apoptotic and necrotic cells and transmission electron microscopy was used to confirm the cell death mechanism (Fig. 1A–D). Ten micromolar carboxyfluorescein diacetate succinimidyl ester (CFDA‐SE; Thermo Fisher Scientific) or 0.5 μm Cell Tracker deep red dye (Thermo Fisher Scientific) was used to label apoptotic and necrotic cells as indicated below. PS was blocked by Alexa Fluor® 647‐conjugated annexin V (Thermo Fisher Scientific) on the surface of apoptotic and necrotic thymocytes.
Figure 1.
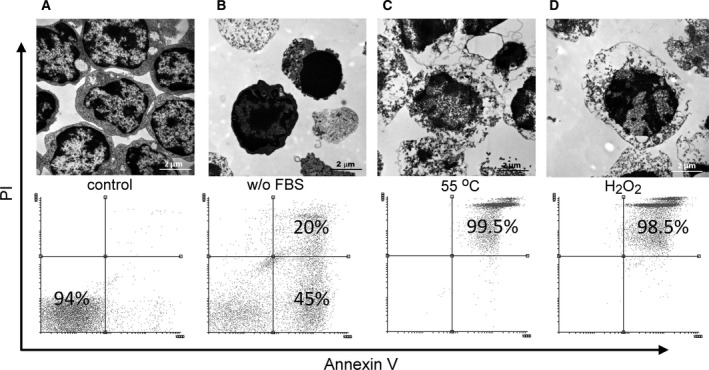
Morphology and viability of target cells. Transmission electron microscopic images and propidium iodide/annexin V staining of target cells. (A) Thymocytes immediately after isolation are considered as viable cells. (B) Apoptosis induced by serum deprivation for 24 h is characterized with condensed nuclei. According to the propidium iodide staining, both early and late apoptotic cells are included. (C,D) Incubation at 55 °C for 20 min (C) or with 1 mm H2O2 for 24 h (D) induced necrosis with early swelling and propidium iodide positivity. Scale bar: 2 μm.
In vitro phagocytosis assay
BMDMs were plated in 24‐well plates (3 × 105/well) and co‐incubated with fluorescently stained apoptotic or necrotic cells (CFDA‐SE, deep red). Thymocytes were added to the BMDMs at the indicated (dead cells per macrophage) ratios. Since 100% of heat‐ or H2O2‐treated cells were necrotic, but in the apoptotic samples 30–40% cells were living, before the phagocytosis experiments we verified the viability stage of the apoptotic and necrotic cell population and normalized the target cell number to have the same amount of apoptotic and necrotic cells during phagocytosis assays. Phagocytosis was allowed to proceed for the indicated time periods at 37 °C. After coculture, thymocytes were washed away extensively and macrophages were detached by trypsinization. Percentage of macrophages engulfing dead cells was determined on a BD Biosciences FACSCalibur flow cytometer. In competition experiments differently stained apoptotic (CFDA‐SE) and necrotic (deep red) thymocytes were added together at different ratios (1 : 1, 1 : 3, 1 : 6, 1 : 9) to macrophages. For determining phagocytic preference, macrophages were exposed to stained (CFDA‐SE) apoptotic or necrotic thymocytes for 30 min, and then were further exposed to labeled apoptotic or necrotic cells. After coculture, thymocytes were washed away extensively and macrophages were detached by trypsinization. Percentage of macrophages engulfing dead cells was analyzed on a FACSCalibur flow cytometer.
Laser scanning cytometry
BMDMs were plated into (IBIDI, Madison, WI, USA) eight‐well chamber slides (30 000 macrophages per well) and were stained with Hoechst 33342. Fluorescently labelled apoptotic (CFDA‐SE) and necrotic (deep red) thymocytes were added into the BMDMs immediately before the measurement. During the measurement, cells were kept in an IBIDI incubator at 37 °C with 5% CO2 air and 90% humidity. Images were made using an Olympus (Tokyo, Japan) IX‐71 inverted microscope and a video file was generated from these images using imagej software (NIH, Bethesda, MD, USA).
Confocal microscopy
Prior to measurement BMDMs were plated into IBIDI eight‐well chamber slides (30 000 macrophages per well) and were stained with (5‐(and‐6)‐(((4‐chloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR); Thermo Fisher Scientific). Fluorescently labelled apoptotic (CFDA‐SE) and necrotic (deep red) thymocytes were added into the BMDMs immediately before the measurement. Time‐lapse movies were made using Zeiss (Oberkochen, Germany) LSM510 confocal laser scanning microscope. Images were taken every 10 s at 140 nm per pixel resolution. The region of interest was extracted and exported with 16 frames·s−1 speed, yielding a compressed video 160 times the actual speed of the process of phagocytosis.
Transmission electron microscopy
For transmission electron microscopy apoptotic and necrotic thymocytes were collected and subsequently fixed with 1% glutaraldehyde in 0.1 m cacodylate buffer, and postfixed with 2% OsO4 in the same buffer. For analysis of phagocytosis, 5‐day‐old BMDMs on a glass slide were fed with apoptotic and necrotic thymocytes for 30 min and subsequently fixed with 1% glutaraldehyde in 0.1 m cacodylate buffer, and postfixed with 2% OsO4 in the same buffer. Slide samples were then dehydrated and stained with uranyl acetate and lead citrate and observed under a Zeiss EM900 electron microscope.
Statistical analyses
All the data are representative of at least three independent experiments. Values are expressed as mean ± SD. Statistical analysis was performed using an unpaired Student's t test.
Results and Discussion
The uptake of both apoptotic and necrotic cells is PS‐, MerTK‐, Tim‐4‐, integrin β3‐ and TG2‐dependent
The exposure of PS on the surface of apoptotic cells is an important primary signal recognized by phagocytes. Our results using the high‐affinity PS‐binding protein annexin V labeling confirmed the results from others that PS appeared on the surface of both heat‐killed and H2O2‐exposed thymocytes (Fig. 1). Thus, we decided to test whether the uptake of apoptotic and necrotic thymocytes is dependent on PS via performing blocking experiments with annexin V. Masking of PS on the surface of necrotic and apoptotic thymocytes by recombinant annexin V treatment significantly decreased the uptake of both cell types (Fig. 2A) indicating a role of PS in the phagocytosis of necrotic and apoptotic cells. Previously, we detected the expression of several phagocytic receptors (integrin αv, β1, β3, β5, MerTK, Tim‐4, Stabilin‐2, CD14, and CD36) in mouse BMDMs 26. Among these, thrombospondin–CD36–αvβ3 complex, CD14, and CD36 were already demonstrated to participate in the necrotic cell uptake as well 20.
Figure 2.
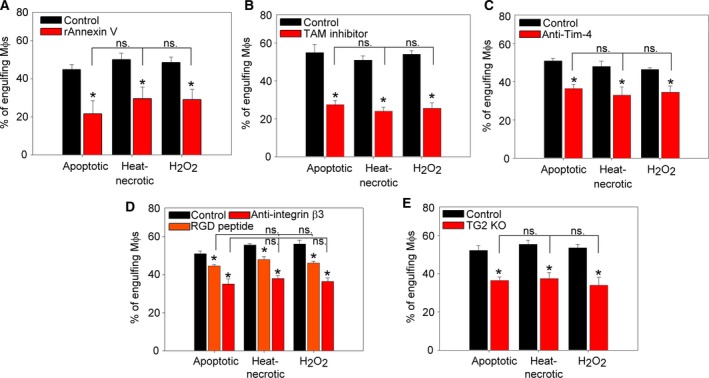
PS‐dependent mechanisms participate in the uptake of both apoptotic and primary necrotic cells. (A) Phagocytosis of control or recombinant annexin V‐exposed fluorescently labeled apoptotic or necrotic thymocytes by BMDMs added in 5 : 1 target cell : macrophage ratio. (B–E) Phagocytosis of fluorescently labelled apoptotic or necrotic thymocytes added to BMDMs in 5 : 1 target cell : macrophage ratio after treating macrophages with 1 μm BMS777607 TAM kinase inhibitor (B) for 24 h, or in the presence of 4 μg·mL−1 anti‐Tim‐4 antibody (C), 4 μg·mL−1 anti‐integrin β3 antibody or 2 mg·mL−1 RGD peptide (D). (E) Phagocytosis by macrophages derived from TG2 knockout mice was also tested. Apoptosis and necrosis were induced as described in Materials and methods. Phagocytosis was allowed for 60 min. Results are expressed as mean ± SD of three independent experiments (*significantly different from its own control, P < 0.05 determined by unpaired Student's t test). ns: not significant; MΦ?s: macrophages.
In our experiments, we aimed to test the requirement for the other PS‐dependent receptors in the necrotic cell uptake. MerTK is a member of the TAM family of receptor tyrosine kinases and can bind the PS indirectly via bridging molecules (e.g. growth arrest specific 6, protein S) 39, 40. Inhibition of MerTK by using the BMS777607 TAM receptor tyrosine kinase inhibitor in BMDMs resulted in 50% reduction of both necrotic and apoptotic thymocyte uptake (Fig. 2B). We also tested the involvement of the direct PS receptor Tim‐4 in necrotic cell uptake by blocking the receptor with an anti‐Tim‐4 antibody and observed a similarly decreased necrotic and apoptotic thymocyte uptake (Fig. 2C). αvβ3 integrin receptors also can recognize PS on the surface of apoptotic and necrotic cells in an indirect way with the help of bridging molecules [e.g. milk fat globule‐EGF factor 8 protein (MFG‐E8)] 41. Blockade of receptor αvβ3 function by RGD peptides or by an anti‐mouse CD61 antibody resulted in an effective inhibition of the uptake of both apoptotic and necrotic thymocytes by BMDMs (Fig. 2D). Previously we have reported that TG2 can act as a β3 integrin co‐receptor in the context of MFG‐E8 binding; thus, it can contribute to the proper phagocytosis of apoptotic cells 24. Using BMDMs derived from TG2−/− mice, we again observed a similar reduction in the uptake of apoptotic and necrotic cells (Fig. 2E). Altogether, our observations indicate that the same PS‐recognizing receptors participate in the uptake of apoptotic and primary necrotic cells.
Triggering of various nuclear receptors enhances the phagocytosis of both apoptotic and necrotic cells
Previously we have demonstrated that during engulfment the lipid content of apoptotic cells triggers the LXR receptor of the macrophage which, in response, upregulates the expression of MerTK and retinaldehyde dehydrogenases leading to retinoid synthesis, which then contributes to the upregulation of further phagocytic receptors and finally results in enhanced apoptotic cell engulfment 26. The retinoid‐dependent phagocytic receptors included TG2, Tim‐4, and stabilin‐2 in BMDMs. In addition, it was also demonstrated that activation of the GR also leads to an enhanced apoptotic cell uptake mainly via MerTK‐dependent mechanisms 26, 27. That is why we decided to compare how triggering these nuclear receptors affects the uptake of apoptotic and necrotic thymocytes by BMDMs. For this purpose, macrophages were treated with 9cRA (RXR agonist; Fig. 3A), ATRA (RAR agonist; Fig. 3B), GW3965 (LXR agonist; Fig. 3C) or dexamethasone (GR agonist; Fig. 3D) for 24 h before exposing them to apoptotic or necrotic thymocytes. According to our results triggering of all these nuclear receptors in BMDMs enhances the uptake of both apoptotic and necrotic thymocytes suggesting that nuclear receptor ligation‐induced phagocytosis enhancement is not restricted only to apoptotic cell uptake.
Figure 3.
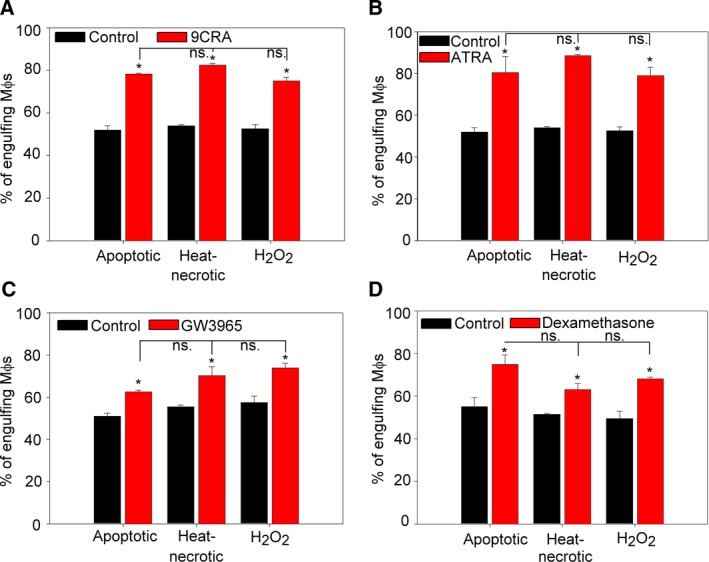
Stimulation of nuclear receptors increases the uptake of both apoptotic and necrotic thymocytes by BMDMs. BMDMs were treated with the RXR agonist 9cRA (A), with the RAR agonist ATRA (B), with the LXR agonist GW3965 (C) or with the GR agonist dexamethasone acetate (D) all in 1 μm concentrations for 24 h. Apoptotic or necrotic thymocytes were added to BMDMs in 5 : 1 target cell : macrophage ratio. Apoptosis and necrosis were induced as described in Materials and methods and phagocytosis was allowed for 60 min. Results are expressed as mean ± SD of three independent experiments (*significantly different from its own control, P < 0.05 determined by unpaired Student's t test). ns: not significant.
Apoptotic and necrotic thymocytes compete with each other during in vitro phagocytosis
If the same receptors participate in the uptake of both apoptotic and necrotic cells, uptake of the two cell types should compete with each other. To investigate this possibility, we performed short‐term phagocytosis competition assays for 30 min in which mouse BMDMs were fed with differently stained apoptotic and/or necrotic thymocytes in various apoptotic–necrotic cell ratios. As shown in Fig. 4A,B, necrotic thymocytes were engulfed as efficiently as apoptotic ones, and they competed for the uptake equally well when added together to macrophages. In contrast, viable cells are not phagocytosed and they do not compete with dead cell phagocytosis (Fig. 4C). During the flow cytometry measurements, we observed, however, that only a small percentage of macrophages engulfed both apoptotic and necrotic cells when added together (Fig. 4D). Therefore, we went on to determine whether uptake of one cell type influences the further phagocytic preference of BMDMs.
Figure 4.
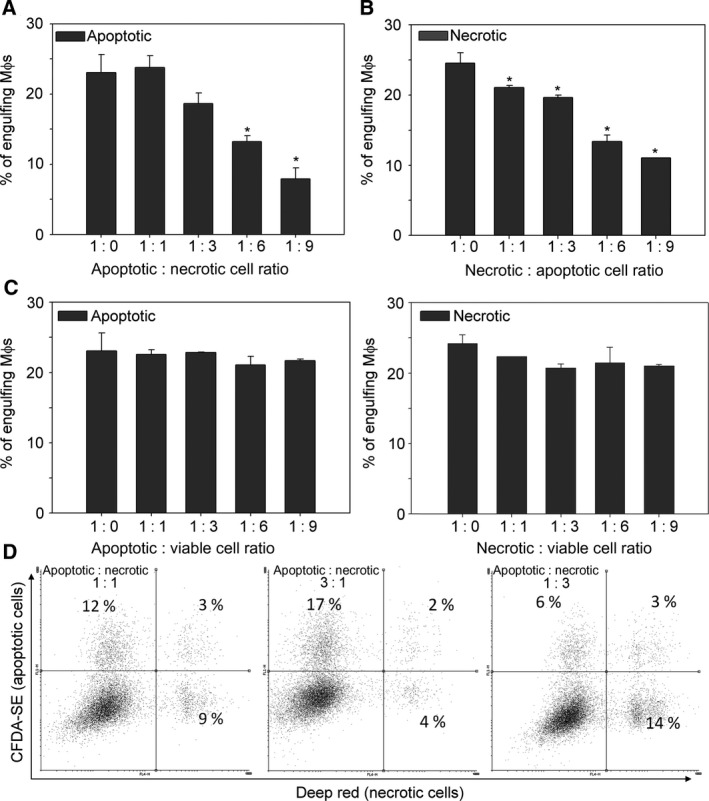
Apoptotic and necrotic thymocytes compete with each other for uptake by BMDMs. (A) Uptake of apoptotic cells in the presence of increasing amounts of necrotic cells. (B) Uptake of necrotic cells in the presence of increasing amounts of apoptotic cells. (C) Uptake of apoptotic and necrotic cells in the presence of increasing amounts of viable cells. BMDMs were exposed to differently stained apoptotic (CFDA‐SE), heat‐necrotic (deep red) and non‐stained viable thymocytes at the indicated ratios. (D) Representative flow cytometry scatter plots of BMDMs engulfing differently stained target cells at the indicated apoptotic : necrotic cell ratios. The initial number of apoptotic or necrotic cells was fixed to 1 million and only the amount of the other cell type was increased. Apoptosis and necrosis were induced as described in Materials and methods. Phagocytosis was allowed for 30 min to make increased phagocytosis detectable even in the presence of significantly enhanced target cell number. Results are expressed as mean ± SD of four independent experiments (*significantly different from its own control, P < 0.05 determined by unpaired Student's t test).
Macrophages use the same phagocytic portal for engulfing apoptotic and necrotic cells
During engulfment the phagocytic receptors cluster laterally in the cell membrane forming phagocytic portals that can be used for the uptake of several apoptotic cells 24, 42, 43. Our observation could indicate that macrophages form a different phagocytic portal for the apoptotic and necrotic cells, and once the portal formation is induced, it will prefer the uptake of the related cell type. To investigate this possibility, macrophages were exposed first to fluorescently labeled either apoptotic or necrotic thymocytes for 30 min. Then they were washed and exposed to either apoptotic or necrotic cells stained differently for an additional 30 min. As shown in Fig. 5A, we found that within those macrophages that already engulfed a target cell, the percentage of the uptake of the second cell type was independent of the previously engulfed cells indicating that the preassembled phagocytic portals for the two target cell type may be similar or the same. Interestingly, the uptake of necrotic cells, when added following the prefeeding, was slightly lower as compared to apoptotic cell phagocytosis, but this lower uptake was independent of the first cell type taken up. Thus we concluded that the low simultaneous uptake of apoptotic and necrotic cells observed in Fig. 4D might be related to the fact that during the short phagocytic uptake period mostly only one target cell was taken up by macrophages.
Figure 5.
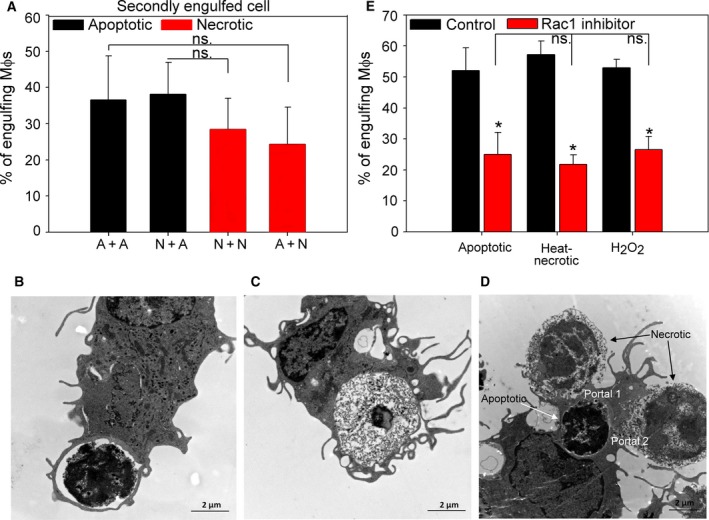
Apoptotic and necrotic thymocytes are engulfed via the same phagocytic portal and their uptake is Rac1‐dependent. (A) BMDMs were exposed first to either apoptotic or necrotic thymocytes for 30 min, and then these cells were washed away and phagocytosis was followed with the same amount, but differently stained apoptotic or necrotic cells for an additional 30 min. Apoptosis and necrosis were induced as described in Materials and methods. The graph shows the fraction of the BMDMs that engulfed the secondly added cells as the percentage of macrophages that phagocytosed the firstly added target cells. Results are expressed as mean ± SD of four independent experiments. (B–D) Representative transmission electron microscopic images of BMDMs engulfing apoptotic (B), heat‐killed necrotic (C) cells, or both types of dead cells simultaneously (D). (E) BMDMs were treated with the NSC23766 Rac1 inhibitor (100 μm) for 24 h and subsequently exposed to apoptotic or necrotic thymocytes in 5 : 1 target cell : macrophage ratio. Apoptosis and necrosis were induced as described in Materials and methods and phagocytosis was allowed for 60 min. Results are expressed as mean ± SD of four independent experiments (*significantly different from its own control, P < 0.05 determined by unpaired Student's t test). ns: not significant. Scale bar: 2 μm.
In order to characterize the internalization mechanisms used by macrophages to engulf apoptotic and necrotic cells, we studied the morphological characteristics of BMDMs during phagocytosis by transmission electron microscopy. During the individual uptake of both apoptotic and necrotic thymocytes, the engulfing pseudopods similarly followed tightly the contour of the target particle (Fig. 5B–C and Fig. S1). Moreover, the two types of dead cells can be taken up via the same phagocytic portal (Fig. 5D, Videos [Link], [Link], and Fig. S1A–C) suggesting that similar preassembled receptor clusters might play a role in their phagocytosis. Previously, experiments using electron microscopic visualization of early apoptotic and necrotic L929 fibroblast cell phagocytosis demonstrated two different uptake mechanisms for the uptake of these cells 25. The apparent contradictions might be resolved by taking the size of the targets into consideration. L929 cells were shown to form small apoptotic bodies that were surrounded by pseudopods and internalized into tight‐fitting phagosomes while large necrotic cells are engulfed piece‐by‐piece via a macropinocytosis‐like process. In our case, the small size of thymocytes, as compared to L929 cells, might enable the utilization and formation of similar pseudopods and phagosomes during apoptotic and necrotic cell uptake.
A key participant of phagocytosis of apoptotic cells is the small GTPase Rac1, which regulates the redistribution of actin to the membrane ruffles and is activated by all the phagocytic signaling pathways 21. Inhibition of Rac1 by the NSC23766 small GTP binding protein Rac1 inhibitor inhibited the uptake of necrotic cells as well (Fig. 5E) indicating that not only the phagocytic receptors, but the signaling pathways activated by them must be the same in the uptake of apoptotic and necrotic cells. Altogether our data indicate that both heat‐ and H2O2‐treated necrotic thymocytes express PS and are taken up by PS‐dependent mechanisms, as was indicated previously 22. We identified several PS‐dependent phagocytic receptors, such as MerTK, integrins, TG2, and Tim‐4, which are known to participate in the uptake of apoptotic cells, as participating in necrotic cell uptake as well. Nuclear receptor stimulation, known to induce the expression of these phagocytic receptors 26, 27, 28 and consequently the uptake of apoptotic cells, also induced the engulfment of both types of necrotic cells further proving the involvement of the same phagocytic receptors. Prevention of the necrotic cell uptake by Rac1 inhibition indicated that not only the receptors, but also the signaling pathways of apoptotic and necrotic cell uptake are shared. Moreover, the electron microscopic images also showed that the same phagocytic portals are opened for the uptake of both apoptotic and necrotic cells. In accordance, our results also demonstrate that necrotic cells are engulfed as efficiently as apoptotic ones and they compete for uptake equally well when added together to macrophages. What is more, the uptake of the first cell type (apoptotic or necrotic) does not decide the preference for the second cell type taken up. These observations indicate that under in vivo pathological settings, when very often both apoptotic and necrotic cells can be detected in the affected tissue, both cell types will be cleared with similar efficiency. Effective clearance of dead cells plays a central role in the prevention of long‐term pathological events 44, 45. We have recently reviewed the therapeutic possibilities, such as that LXR, peroxisome proliferator‐activated receptor gamma, GR receptor ligation or macrolide antibiotic administration can enhance the uptake of apoptotic cells 46. Our present observations indicate that many of these approaches might be effective in stimulating the necrotic cell clearance as well. Dead cells can be removed not only by professional phagocytes, but by neighboring non‐professional phagocytes as well. Interestingly, these cells utilize the same mechanisms for efferocytosis that macrophages use 47. To support our findings, administration of MFG‐E8, a bridging molecule that links dead cell surface PS to the αvβ3 integrin and its co‐receptor TG2 on the phagocytes 24, 41, accelerated the recovery after myocardial infarction 48, a condition where necrotic and apoptotic cells were shown to be engulfed by neighboring cardiac myofibroblast 32.
Conclusions
Altogether our results indicate that primary necrotic cell phagocytosis seems morphologically similar to apoptotic cell uptake and the two processes involve TIM‐4, MerTK, integrin avβ3, TG2, and Rac1 molecules on the site of macrophages and PS on the dead cells surface. GR, RAR or LXR receptor activation augments both necrotic and apoptotic cell clearance, and therefore clinical therapies targeting apoptotic cell clearance might also enhance necrotic cell uptake, which can contribute to the success of these strategies.
Conflict of interests
The authors declare no conflict of interest.
Author contributions
ZB performed the majority of experiments, analyzed the data, and participated in the writing of the manuscript; L‐NU, MA, ZB, CB, and GNK participated in the microscopic experiments; ZS and ZS designed and coordinated the research, and participated in the writing of the manuscript. All authors read and approved the final manuscript.
Supporting information
Fig. S1. (A–C) Representative transmission electron microscopic images of BMDMs engulfing apoptotic and heat‐killed necrotic thymocytes at the same site. (D–E) Representative transmission electron microscopic images showing that BMDMs form tight‐fitting phagosomes around both the engulfed apoptotic and heat‐killed necrotic thymocytes. Scale bar: 5 μm (A, B) or 2 μm (C–E).
Video S1. Fluorescence live‐cell imaging of apoptotic and necrotic cell engulfing BMDMs by laser scanning microscopy. Apoptotic and necrotic thymocytes were added to BMDMs in 5 : 1 target cell : macrophage ratio. Apoptosis and necrosis were induced as described in Materials and methods. Apoptotic thymocytes are labeled with green, necrotic thymocytes with red and the nuclei of BMDMs with blue colors. Arrows point to macrophages that took up an apoptotic and a necrotic cell at the same site.
Video S2. Fluorescence live‐cell imaging of apoptotic and necrotic cell engulfing BMDMs by confocal microscopy. Apoptotic and necrotic thymocytes were added to BMDMs in 5 : 1 target cell : macrophage ratio. Apoptosis and necrosis were induced as described in Materials and methods. Apoptotic thymocytes are labeled with green, necrotic thymocytes with blue and BMDMs with red colors. In the middle there is a macrophage that took up firstly an apoptotic then a necrotic cell at the same site. Note that apoptotic and necrotic cells interact at several sites with macrophages but uptake happens only at one site.
Acknowledgements
This study was supported by the National Research, Development and Innovation Office (124244), by the GINOP‐2.3.2‐15‐2016‐00006 project and EFOP‐3.6.3‐VEKOP‐16‐2017‐00009 (co‐financed by the European Union and the European Regional Development Fund), and University of Debrecen (RH/751/2015). Zsolt Sarang was a recipient of Lajos Szodoray fellowship given by the University of Debrecen.
Zsuzsa Szondy and Zsolt Sarang contributed equally to the design of the experiments and writing of the paper.
References
- 1. Renehan AG, Booth C and Potten CS (2001) What is apoptosis, and why is it important? BMJ 322, 1536–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kerr JF, Wyllie AH and Currie AR (1972) Apoptosis: a basic biological phenomenon with wide‐ranging implications in tissue kinetics. Br J Cancer 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Depraetere V (2000) “Eat me” signals of apoptotic bodies. Nat Cell Biol 2, e104. [DOI] [PubMed] [Google Scholar]
- 4. Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy‐Ullrich JE, Bratton DL, Oldenborg PA, Michalak M and Henson PM (2005) Cell‐surface calreticulin initiates clearance of viable or apoptotic cells through trans‐activation of LRP on the phagocyte. Cell 123, 321–334. [DOI] [PubMed] [Google Scholar]
- 5. Sartorius U, Schmitz I and Krammer PH (2001) Molecular mechanisms of death‐receptor‐mediated apoptosis. ChemBioChem 2, 20–29. [DOI] [PubMed] [Google Scholar]
- 6. Shi Y (2001) A structural view of mitochondria‐mediated apoptosis. Nat Struct Biol 8, 394–401. [DOI] [PubMed] [Google Scholar]
- 7. Savill J (1997) Apoptosis in resolution of inflammation. J Leukoc Biol 61, 375–380. [DOI] [PubMed] [Google Scholar]
- 8. Szondy Z, Sarang Z, Kiss B, Garabuczi É and Köröskényi K (2017) Anti‐inflammatory mechanisms triggered by apoptotic cells during their clearance. Front Immunol 8, 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buja LM, Eigenbrodt ML and Eigenbrodt EH (1993) Apoptosis and necrosis. Basic types and mechanisms of cell death. Arch Pathol Lab Med 117, 1208–1214. [PubMed] [Google Scholar]
- 10. Li M, Carpio DF, Zheng Y, Bruzzo P, Singh V, Ouaaz F, Medzhitov RM and Beg AA (2001) An essential role of the NF‐kappa B/Toll‐like receptor pathway in induction of inflammatory and tissue‐repair gene expression by necrotic cells. J Immunol 166, 7128–7135. [DOI] [PubMed] [Google Scholar]
- 11. Scaffidi P, Misteli T and Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195. [DOI] [PubMed] [Google Scholar]
- 12. Fadok VA, Bratton DL, Guthrie L and Henson PM (2001) Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol 166, 6847–6854. [DOI] [PubMed] [Google Scholar]
- 13. Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B and Tschopp J (2000) Fas triggers an alternative, caspase‐8‐independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1, 489–495. [DOI] [PubMed] [Google Scholar]
- 14. Hirt UA and Leist M (2003) Rapid, noninflammatory and PS‐dependent phagocytic clearance of necrotic cells. Cell Death Differ 10, 1156–1164. [DOI] [PubMed] [Google Scholar]
- 15. Li Z, Venegas V, Nagaoka Y, Morino E, Raghavan P, Audhya A, Nakanishi Y and Zhou Z (2015) Necrotic cells actively attract phagocytes through the collaborative action of two distinct PS‐exposure mechanisms. PLoS Genet 11, e1005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Böttcher A, Gaipl US, Fürnrohr BG, Herrmann M, Girkontaite I, Kalden JR and Voll RE (2006) Involvement of phosphatidylserine, alphavbeta3, CD14, CD36, and complement C1q in the phagocytosis of primary necrotic lymphocytes by macrophages. Arthritis Rheum 54, 927–938. [DOI] [PubMed] [Google Scholar]
- 17. Brouckaert G, Kalai M, Krysko DV, Saelens X, Vercammen D, Ndlovu MN, Haegeman G, D'Herde K and Vandenabeele P (2004) Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol Biol Cell 15, 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cejna M, Fritsch G, Printz D, Schulte‐Hermann R and Bursch W (1994) Kinetics of apoptosis and secondary necrosis in cultured rat thymocytes and S.49 mouse lymphoma and CEM human leukemia cells. Biochem Cell Biol 72, 677–685. [DOI] [PubMed] [Google Scholar]
- 19. Potter PK, Cortes‐Hernandez J, Quartier P, Botto M and Walport MJ (2003) Lupus‐prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. J Immunol 170, 3223–3232. [DOI] [PubMed] [Google Scholar]
- 20. Gaipl US, Kuhn A, Sheriff A, Munoz LE, Franz S, Voll RE, Kalden JR and Herrmann M (2006) Clearance of apoptotic cells in human SLE. Curr Dir Autoimmun 9, 173–187. [DOI] [PubMed] [Google Scholar]
- 21. Poon IK, Lucas CD, Rossi AG and Ravichandran KS (2014) Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 14, 166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kinchen JM, Cabello J, Klingele D, Wong K, Feichtinger R, Schnabel H, Schnabel R and Hengartner MO (2005) Two pathways converge at CED‐10 to mediate actin rearrangement and corpse removal in C. elegans . Nature 434, 93–99. [DOI] [PubMed] [Google Scholar]
- 23. Cocco RE and Ucker DS (2001) Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol Biol Cell 12, 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tóth B, Garabuczi E, Sarang Z, Vereb G, Vámosi G, Aeschlimann D, Blaskó B, Bécsi B, Erdõdi F, Lacy‐Hulbert A et al (2009) Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol 182, 2084–2092. [DOI] [PubMed] [Google Scholar]
- 25. Krysko DV, Brouckaert G, Kalai M, Vandenabeele P and D'Herde K (2003) Mechanisms of internalization of apoptotic and necrotic L929 cells by a macrophage cell line studied by electron microscopy. J Morphol 258, 336–345. [DOI] [PubMed] [Google Scholar]
- 26. Sarang Z, Joós G, GarabucziÉ Rühl R, Gregory CD and Szondy Z (2014) Macrophages engulfing apoptotic cells produce nonclassical retinoids to enhance their phagocytic capacity. J Immunol 192, 5730–5738. [DOI] [PubMed] [Google Scholar]
- 27. Zahuczky G, Kristóf E, Majai G and Fésüs L (2011) Differentiation and glucocorticoid regulated apopto‐phagocytic gene expression patterns in human macrophages. Role of MerTK in enhanced phagocytosis. PLoS ONE 6, e21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McColl A, Bournazos S, Franz S, Perretti M, Morgan BP, Haslett C and Dransfield I (2009) Glucocorticoids induce protein S‐dependent phagocytosis of apoptotic neutrophils by human macrophages. J Immunol 183, 2167–2175. [DOI] [PubMed] [Google Scholar]
- 29. Yurdagul A Jr, Doran AC, Cai B, Fredman G and Tabas IA (2018) Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front Cardiovasc Med 4, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khan HA, Ahmad MZ, Khan JA and Arshad MI (2017) Crosstalk of liver immune cells and cell death mechanisms in different murine models of liver injury and its clinical relevance. Hepatobiliary Pancreat Dis Int 16, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Radak D, Katsiki N, Resanovic I, Jovanovic A, Sudar‐Milovanovic E, Zafirovic S, Mousad SA and Isenovic ER (2017) Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol 15, 115–122. [DOI] [PubMed] [Google Scholar]
- 32. Prabhu SD and Frangogiannis NG (2016) The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119, 91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sciorati C, Rigamonti E, Manfredi AA and Rovere‐Querini P (2016) Cell death, clearance and immunity in the skeletal muscle. Cell Death Differ 23, 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cao Q, Harris DC and Wang Y (2015) Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 30, 183–194. [DOI] [PubMed] [Google Scholar]
- 35. McConkey DJ, Jondal M and Orrenius S (1994) The regulation of apoptosis in thymocytes. Biochem Soc Trans 22, 606–610. [DOI] [PubMed] [Google Scholar]
- 36. Köröskényi K, Duró E, Pallai A, Sarang Z, Kloor D, Ucker D, Beceiro S, Castrillo A, Chawla A, Ledent CA et al (2011) Involvement of adenosine A2A receptors in engulfment‐dependent apoptotic cell suppression of inflammation. J Immunol 186, 7144–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rello S, Stockert JC, Moreno V, Gámez A, Pacheco M, Juarranz A, Cañete M and Villanueva A (2005) Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis 10, 201–208. [DOI] [PubMed] [Google Scholar]
- 38. Purton JF, Boyd RL, Cole TJ and Godfrey DI (2000) Intrathymic T cell development and selection proceeds normally in the absence of glucocorticoid receptor signaling. Immunity 13, 179–186. [DOI] [PubMed] [Google Scholar]
- 39. Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H and Mizuno K (1996) Identification of the product of growth arrest‐specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 271, 30022–30027. [DOI] [PubMed] [Google Scholar]
- 40. Hall MO, Obin MS, Heeb MJ, Burgess BL and Abrams TA (2005) Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp Eye Res 81, 581–591. [DOI] [PubMed] [Google Scholar]
- 41. Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A and Nagata S (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187. [DOI] [PubMed] [Google Scholar]
- 42. Niedergang F and Grinstein S (2018) How to build a phagosome: new concepts for an old process. Curr Opin Cell Biol 50, 57–63. [DOI] [PubMed] [Google Scholar]
- 43. Nakaya M, Kitano M, Matsuda M and Nagata S (2008) Spatiotemporal activation of Rac1 for engulfment of apoptotic cells. Proc Natl Acad Sci USA 105, 9198–9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aprahamian T, Rifkin I, Bonegio R, Hugel B, Freyssinet JM, Sato K, Castellot JJ and Walsh K (2004) Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J Exp Med 199, 1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fond AM and Ravichandran KS (2016) Clearance of dying cells by phagocytes: mechanisms and implications for disease pathogenesis. Adv Exp Med Biol 930, 25–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szondy Z, Garabuczi E, Joós G, Tsay GJ and Sarang Z (2014) Impaired clearance of apoptotic cells in chronic inflammatory diseases: therapeutic implications. Front Immunol 5, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arandjelovic S and Ravichandran KS (2015) Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 16, 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakaya M, Watari K, Tajima M, Nakaya T, Matsuda S, Ohara H, Nishihara H, Yamaguchi H, Hashimoto A, Nishida M et al (2017) Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction. Clin Invest 127, 383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. (A–C) Representative transmission electron microscopic images of BMDMs engulfing apoptotic and heat‐killed necrotic thymocytes at the same site. (D–E) Representative transmission electron microscopic images showing that BMDMs form tight‐fitting phagosomes around both the engulfed apoptotic and heat‐killed necrotic thymocytes. Scale bar: 5 μm (A, B) or 2 μm (C–E).
Video S1. Fluorescence live‐cell imaging of apoptotic and necrotic cell engulfing BMDMs by laser scanning microscopy. Apoptotic and necrotic thymocytes were added to BMDMs in 5 : 1 target cell : macrophage ratio. Apoptosis and necrosis were induced as described in Materials and methods. Apoptotic thymocytes are labeled with green, necrotic thymocytes with red and the nuclei of BMDMs with blue colors. Arrows point to macrophages that took up an apoptotic and a necrotic cell at the same site.
Video S2. Fluorescence live‐cell imaging of apoptotic and necrotic cell engulfing BMDMs by confocal microscopy. Apoptotic and necrotic thymocytes were added to BMDMs in 5 : 1 target cell : macrophage ratio. Apoptosis and necrosis were induced as described in Materials and methods. Apoptotic thymocytes are labeled with green, necrotic thymocytes with blue and BMDMs with red colors. In the middle there is a macrophage that took up firstly an apoptotic then a necrotic cell at the same site. Note that apoptotic and necrotic cells interact at several sites with macrophages but uptake happens only at one site.


