Abstract
Increasing number of microRNAs (miRNAs) have been reported to play an important role in the development and progression of non-small cell lung cancer (NSCLC). In particular, microRNA-497-5p (miR-497-5p) has been proposed as a tumor suppressor miRNA in human cancers. However, the role of miR-497-5p and its potential molecular mechanism associated with NSCLC are less studied. Therefore, the role of miR-497-5p in the pathogenesis of NSCLC was investigated. In the present study, the expression of miR-497-5p was significantly downregulated in NSCLC. Moreover, overexpression of miR-497-5p inhibited the proliferation and invasion of NSCLC cells by suppressing FGF2. In addition, FGF2 was a downstream target of miR-497-5p in NSCLC. FGF2 was upregulated in NSCLC promoting cell proliferation and invasion. Overexpression of FGF2 impaired the inhibitory effect of miR-497-5p in NSCLC. Taken together, these results demonstrate that miR-497-5p is a tumor suppressor miRNA and demonstrate its potential for future use in the treatment of human NSCLC.
Keywords: miR-497-5p, proliferation, invasion, FGF2, non-small cell lung cancer
Introduction
Lung cancer is one of the most common malignant tumors in the world and has become a major cause of high mortality in Chinas urban population (1). Primary lung cancer includes small cell lung carcinoma (SCLC) and non-SCLC (NSCLC), and NSCLC accounts for ~85% of all lung cancer cases (2). Although treatments have improved significantly in recent years, the prognosis of patients with NSCLC remains poor, with a 5-year survival rate of only ~16% (3). Survival rates have been found to be closely associated with the lung cancer stage. Patients with stage I–III NSCLC can be treated surgically with a 5-year survival rate of 25–80% (4). Stage IV patients with NSCLC are often treated by chemotherapy to relieve symptoms. Even so, patients with stage IV NSCLC have a poor prognosis with a 5-year survival rate of only 2% (5). Unfortunately, NSCLC is often diagnosed in advanced stage. Therefore, finding effective screening strategies for early diagnosis is necessary. It would likely significantly reduce the morbidity and mortality of NSCLC.
It has been reported that microRNAs (miRNAs) inhibit expression of target genes by disrupting genes or impeding translation of mRNA (6). Especially, increased microRNAs have been identified as lung cancer biomarkers (7). For example, miR-215 inhibited the progression of NSCLC through targeting MMP-16 (8). Wei and Ran (9) reported that miR-20a promoted proliferation and invasion by directly targeting EGR2 in NSCLC. Recently, the specific function of miR-497 has attracted our attention. It was proven that miR-497 can be used as an effective biomarker to diagnose and predict prognosis of osteosarcoma (10). Abnormal expression and function of miR-497 have been identified in various types of human of cancer. For instance, miR-497 was downregulated and suppressed the development of renal cell carcinoma through targeting VEGFR-2 (11). Wu et al (12) proposed that miR-497 inhibited proliferation and induced apoptosis via the Bcl-2/Bax-caspase-9-caspase-3 pathway in HUVECs. It was also reported that miR-497 accelerated apoptosis of cervical cancer cells through negatively regulating the MAPK/ERK signaling pathway (13). However, it has been reported that miR-497 can also express high and enhanced metastasis by inhibiting SMAD7 in oral squamous cell carcinoma (14). These results led us to further explore the role of miR-497-5p in NSCLC.
As a member of FGF family, fibroblast growth factor 2 (FGF2) is involved in mitogenic and proliferative effect (15). FGF2 was reported to be upregulated in breast cancer and mediated cell migration and invasion (16). Moreover, FGF2 was found to facilitate cell invasion in pancreatic cancer (17). Joy et al (18) demonstrated that FGF2 could promote cell proliferation in human astrocytes and glioma. As a biomarker, FGF2 can predict the prognosis of patients with glioma (19). In addition, the interaction between miRNAs and FGF2 has been reported in human cancers, such as miR-15-16 (20). However, the regulatory mechanism between miR-497-5p and FGF2 is unclear and an in-depth study is desired urgently in NSCLC. In the present study, the expression of miR-497-5p and its regulatory mechanism in NSCLC were investigated. Moreover, the relationship between miR-497-5p and FGF2 was also explored in NSCLC.
Materials and methods
Clinical tissues
This experiment was approved by the Institutional Ethics Committee of People's Hospital of Rizhao (Rizhao, China) (Approval no. 2017-5, February 15, 2017). Informed consent was signed by all patients. In People's Hospital of Rizhao, 108 NSCLC tissues and adjacent normal tissues were obtained from patients. None of the patients with NSCLC received treatment before surgery. Tissues were then frozen in liquid nitrogen and stored in a −80°C refrigerator for further experiments.
Cell culture
Human H1299, A549 and SPC-A1 cell lines and non-tumorigenic bronchial epithelium cell line BEAS-2B (Homo sapiens) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). These cells were then incubated with 10% fetal bovine serum (FBS) in Dulbeccos modified Eagles medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA). They were placed in an incubator with 5% CO2 at 37°C.
RT-qPCR
Total RNA containing miRNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) to quantify the expression of miR-497-5p in NSCLC. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed on ABI 7500 Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientifc, Inc.) using SYBR-Green PCR Master Mix (Thermo Fisher Scientifc, Inc.). The primer sequences used for qPCR were: miR-497-5p 5′-CCTTCAGCAGCACACTGTGG-3′ (forward) and 5′-CAGTGCAGGGTCCGAGGTAT-3′ (reverse); U6 5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-AACGCTTCACGAATTTGCGT-3′ (reverse); FGF2 5′-ACTGGCTTCTAAATGTGTTACG-3′ (forward) and 5′-TTGGATCCAAGTTTATACTGCC-3′ (reverse); GAPDH 5′-TGGTATCGTGGAAGGACTCA-3′ (forward) and 5′-CCAGTAGAGGCAGGGATGAT-3′ (reverse). U6 and GAPDH were used as controls. Their expression was analyzed according to the 2−∆∆cq method (21).
Cell transfection
miR-497-5p mimic and inhibitor were used to mimic and inhibit miR-497-5p expression. FGF2 siRNA was used to inhibit FGF2 expression. miR-497-5p mimic and inhibitor, and FGF2 siRNA (si-FGF2) were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). They were then transferred to A549 cells with Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) based on the manufacturers protocols.
Cell proliferation
Cell proliferation was measured by the CCK-8 assay based on the manufacturers instructions. First, 4×104 cells were plated and incubated in 96-well plates for 0, 24, 48 and 72 h. They were placed in an incubator with 5% CO2 at 37°C. Next, 10 µl of CCK-8 reagent was added to each well for 2 h (Dojindo Laboratories, Kumamoto, Japan). Finally, they were detected using a microplate reader (Molecular Devices LLC, Sunnyvale, CA, USA) at an absorbance of 450 nm.
Cell invasion
Cell invasion ability was assessed by Transwell assay. First, 5×104 A549 cells in serum-free medium were seeded in a Matrigel-coated Transwell chambers (8-µm pore size; BD Biosciences, San Jose, CA, USA). A549 cells were then placed in the upper chamber and the lower chamber was filled with 10% FBS. The cells were then cultured at 37°C for 48 h to measure cell invasion. The cells were stained with 0.1% crystal violet. A light microscope (Olympus Corporation, Tokyo, Japan) was used to count invading cells.
Dual-Luciferase report assay
The 3′-UTR of wild or mutant type FGF2 was inserted into the pmirGLO plasmids (Shanghai GenePharma Co., Ltd.) for luciferase reporter assay. Then, the 3′-UTR of wild or mutant type FGF2 and miR-497-5p mimic were transfected into A549 cells. Luciferase activity was observed by a Dual-Luciferase assay system (Promega, Madison, WI, USA).
Western blotting
RIPA lysis buffer was used to extract the protein samples. Proteins were then separated through a 10% SDS-PAGE and incubated with 5% skim milk in polyvinylidene difluoride (PVDF) membranes at room temperature. Next we incubated the membranes with anti-FGF2 (dilution 1:1,000; rabbit polyclonal; cat. no. 208687; Abcam, Cambridge, MA, USA), anti-GAPDH (dilution 1:1,000; rabbit monoclonal; cat. no. ab9485; Abcam) antibodies at 4°C overnight, followed by incubation with goat polyclonal anti-rabbit IgG secondary antibodies (dilution 1:2,000; cat. no. ab6721; Abcam). Protein expression levels were then measured by ECL (Pierce; Thermo Fisher Scientific, Inc.).
Statistical analysis
Data were analyzed using SPSS 19.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as mean ± standard deviation (SD). Differences were calculated according to one-way analysis of variance (ANOVA) with the post hoc Tukeys test or Students t-test. The correlation between the miR-497-5p and FGF2 was analyzed through Spearman's two-tailed test. The association between the clinicopathological features of NSCLC and miR-497-5p or FGF2 was calculated by the Chi-squared test (χ2). The overall survival was analyzed by the Kaplan-Meier method and the log rank test. Significant differences were defined as P<0.05.
Results
Downregulation of miR-497-5p identified in NSCLC tissues and cell lines
First, mRNA expression of miR-497-5p was observed in NSCLC tissues. Downregulation of miR-497-5p was detected in NSCLC tissues compared to adjacent normal tissues (Fig. 1A). In the NSCLC cell lines, downregulation of miR-497-5p was also identified. The expression levels of miR-497-5p were significantly decreased in the H1299, A549 and SPC-A1 cell lines compared to BEAS-2B cells (Fig. 1B). In addition, the relationship between miR-497-5p abnormal expression and clinicopathological features of NSCLC patients was analyzed. Based on the expression of miR-497-5p, these cases were divided into a high miR-497-5p expression group and a low expression group, based on the median value of miR-497-5p expression levels in NSCLC patients as a cut-off point. We found that low miR-497-5p expression was associated with TNM stage (P=0.018) and distant metastases (P=0.02; Table I). Besides, low miR-497-5p expression predicted a poor prognosis, with NSCLC patients having a shorter overall survival (P=0.0078; Fig. 1C). Based on these results, we suspected that miR-497-5p was involved in the tumorigenesis of NSCLC.
Figure 1.
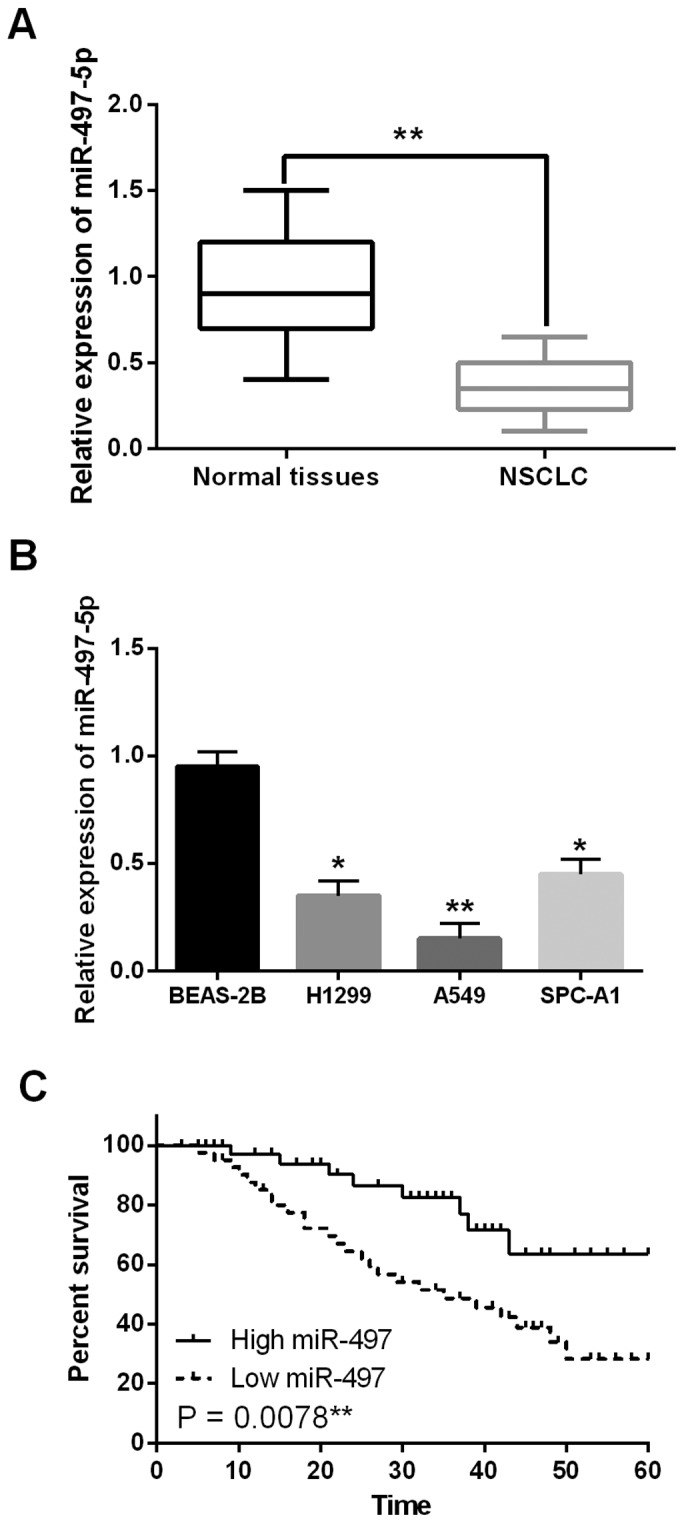
Downregulation of miR-497-5p was identified in NSCLC. (A) The expression of miR-497-5p in NSCLC tissues detected via qRT-PCR. (B) The miR-497-5p expression in H1299, A549, SPC-A1 and BEAS-2B cell lines. (C) Low miR-497-5p expression was correlated with shorter overall survival of NSCLC patients. *P<0.05, **P<0.01.
Table I.
Relationship between miR-497-5p expression and the clinicopathological characteristics of NSCLC patients.
| miR-497-5p | ||||
|---|---|---|---|---|
| Characteristics | No. of cases (n=108) | High | Low | P-value |
| Age (years) | 0.12 | |||
| ≥60 | 40 | 12 | 28 | |
| <60 | 68 | 28 | 40 | |
| Sex | 0.28 | |||
| Male | 48 | 20 | 28 | |
| Female | 60 | 22 | 38 | |
| Tumor size (cm) | 0.56 | |||
| <5 | 46 | 21 | 25 | |
| ≥5 | 62 | 22 | 40 | |
| TNM stage | 0.018a | |||
| I + II | 33 | 11 | 22 | |
| III + IV | 75 | 24 | 51 | |
| Distant metastases | 0.02a | |||
| Negative | 72 | 27 | 45 | |
| Positive | 36 | 13 | 23 | |
| Differentiation | 0.35 | |||
| I + II | 58 | 20 | 28 | |
| III + IV | 50 | 20 | 30 | |
Statistical analyses were performed by the χ2 test.
P<0.05 was considered significant.
Overexpression of miR-497-5p inhibited cell proliferation and invasion in NSCLC
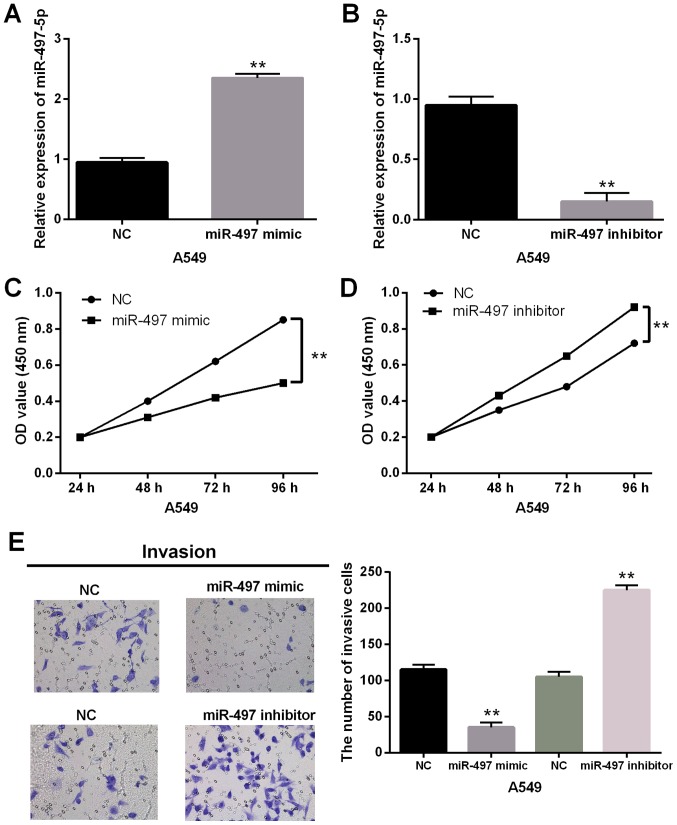
Next, miR-497-5p mimics or inhibitor was transfected into A549 cells to explore its role in NSCLC. The miR-497-5p mimics were found to enhance miR-497-5p expression (Fig. 2A) and miR-497-5p inhibitor reduced its expression (Fig. 2B). The ability of cell proliferation was then measured in transfected A549 cells. The CCK-8 assay indicated that upregulation of miR-497-5p suppressed proliferation of A549 cells (Fig. 2C). In contrast, knockdown of miR-497-5p promoted cell proliferation in NSCLC cells (Fig. 2D). In addition, the same effect of miR-497-5p was identified for cell invasion in NSCLC. miR-497-5p mimics significantly impaired cell invasive ability, while miR-497-5p inhibitor promoted invasion of A549 cells (Fig. 2E). Therefore, overexpression of miR-497-5p was found to suppress cell proliferation and invasion in NSCLC.
Figure 2.
Overexpression of miR-497-5p inhibits cell proliferation and invasion in NSCLC. (A and B) The miR-497-5p expression was examined in A549 cells with miR-497-5p mimics or inhibitor via RT-qPCR. (C and D) Cell proliferation was measured in cells with miR-497-5p mimics or inhibitor. (E) Cell invasion analysis in A549 cells with miR-497-5p mimics or inhibitor was detected. **P<0.01.
FGF2 is a downstream target of miR-497-5p in NSCLC
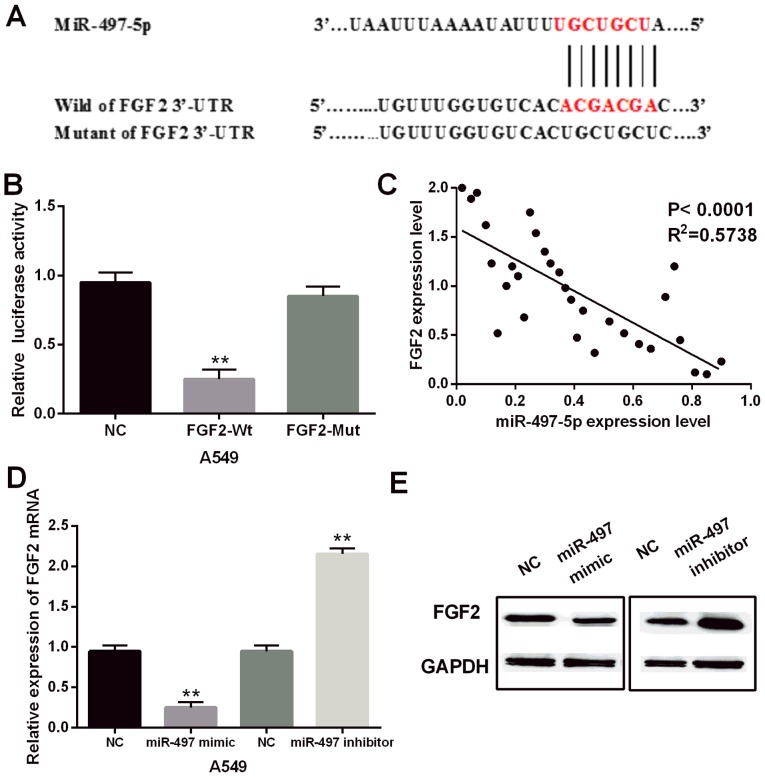
TargetScan (http://www.targetscan.org) was used to search for potential binding sites of the theoretical target gene with miR-497-5p. Among the predicted targets, the FGF2 gene was selected for further analysis because of its vital role in promoting tumor progression. The prediction of TargetScan showed that miR-497-5p had a binding site to the 3UTR of FGF2 (Fig. 3A). Luciferase assay was then performed to confirm the above prediction. The results showed that the miR-497-5p mimics significantly reduced the luciferase activity of wild-type FGF2. However, the luciferase activity of the mutant type FGF2 was not affected by the miR-497-5p mimics (Fig. 3B). In addition, a negative correlation between miR-497-5p and FGF2 expression was identified in NSCLC tissues (P<0.0001, R2=0.5738, Fig. 3C). To further confirm the relationship between them, the expression of FGF2 was detected in A549 cells with miR-497-5p mimics or inhibitor. The results indicated that miR-497-5p mimics obviously suppressed FGF2 expression, while the miR-497-5p inhibitor enhanced the expression level of FGF2 (Fig. 3D and E). FGF2 is a direct target of miR-497-5p, and miR-497-5p negatively regulated FGF2 expression in NSCLC.
Figure 3.
FGF2 is a downstream target of miR-497-5p in NSCLC. (A) The binding site of miR-497-5p on the 3′-UTR of FGF2. (B) Luciferase reporter assays. (C) The correlation between miR-497-5p and FGF2. (D and E) The expression of FGF2 was analyzed in cells with miR-497-5p mimics or inhibitor. **P<0.01.
Upregulation of FGF2 promotes cell proliferation and invasion in NSCLC
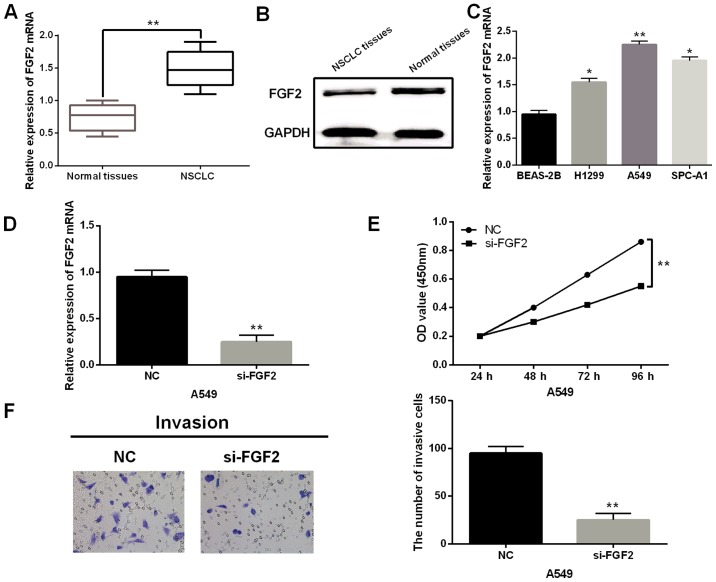
Then, FGF2 was identified to be upregulated in NSCLC tissues (Fig. 4A and B) and H1299, A549 and SPC-A1 cell lines (Fig. 4C). Moreover, high FGF2 expression was associated with TNM stage (P=0.009) and distant metastases (P=0.02; Table II). Next, the effect of FGF2 was investigated in NSCLC by transfecting FGF2 siRNA in A549 cells. First, we found that FGF2 siRNA apparently reduced the expression of FGF2 (Fig. 4D). Moreover, silencing of FGF2 prevented proliferation of A549 cells (Fig. 4E). Similarly, knockdown of FGF2 also inhibited cell invasion in A549 cells (Fig. 4F). In conclusion, it was confirmed that FGF2 functions as an oncogene in NSCLC by promoting cell proliferation and invasion.
Figure 4.
Upregulation of FGF2 promotes cell proliferation and invasion in NSCLC. (A and B) The mRNA and protein expression of FGF2 in NSCLC tissues was detected. (C) FGF2 expression in H1299, A549, SPC-A1 and BEAS-2B cell lines. (D) Expression of FGF2 was measured in cells with FGF2 siRNA. (E) Cell proliferation was measured in cells with FGF2 siRNA. (F) Cell invasion analysis in A549 cells with FGF2 siRNA was detected. *P<0.05, **P<0.01.
Table II.
Relationship between the FGF2 expression and the clinicopathological characteristics of NSCLC patients.
| FGF2 | ||||
|---|---|---|---|---|
| Characteristics | No. of cases (n=108) | High | Low | P-value |
| Age (years) | 0.26 | |||
| ≥60 | 35 | 24 | 11 | |
| <60 | 73 | 42 | 31 | |
| Sex | 0.08 | |||
| Male | 42 | 23 | 19 | |
| Female | 66 | 38 | 28 | |
| Tumor size (cm) | 0.63 | |||
| <5 | 50 | 37 | 13 | |
| ≥5 | 58 | 38 | 20 | |
| TNM stage | 0.009a | |||
| I + II | 34 | 21 | 13 | |
| III + IV | 74 | 45 | 29 | |
| Distant metastases | 0.02a | |||
| Negative | 78 | 48 | 30 | |
| Positive | 30 | 18 | 12 | |
| Differentiation | 0.053 | |||
| I + II | 61 | 35 | 26 | |
| III + IV | 47 | 28 | 19 | |
Statistical analyses were performed by the χ2 test.
P<0.05 was considered significant.
Upregulation of FGF2 impairs the inhibitory action of miR-497-5p in NSCLC
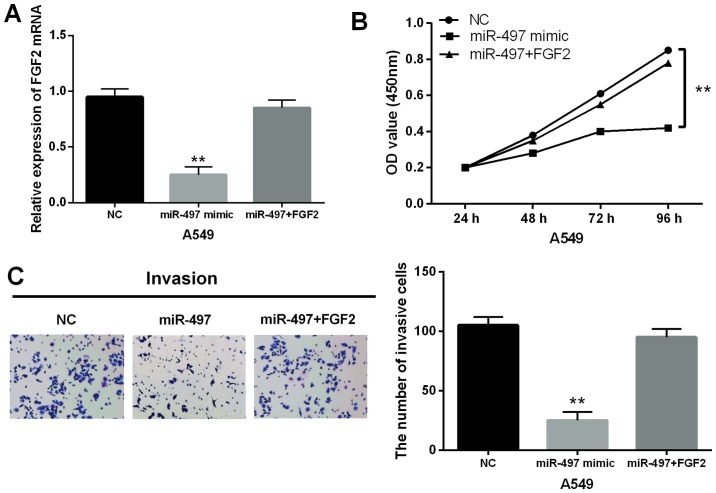
Finally, the interaction between miR-497-5p and FGF2 was explored through co-transfecting the miR-497-5p mimics and the FGF2 vector into A549 cells. A decrease in FGF2 expression induced by the miR-497-5p mimics was found to be restored by the FGF2 vector (Fig. 5A). Functionally, the CCK-8 assay indicated that upregulation of FGF2 impaired the inhibitory effect of miR-497-5p on cell proliferation in NSCLC (Fig. 5B). Moreover, the inhibitory effect of miR-497-5p on cell invasion was also reversed by upregulation of FGF2 in A549 cells (Fig. 5C). In conclusion, overexpression of FGF2 impaired the suppressive effect of miR-497-5p in NSCLC.
Figure 5.
Overexpression of FGF2 impairs the suppressive effect of miR-497-5p in NSCLC. (A) The expression of FGF2 was measured in A549 cells with FGF2 vector and miR-497-5p mimics. (B) Cell proliferation was measured in A549 cells with FGF2 vector and miR-497-5p mimics. (C) Cell invasion in A549 cells with FGF2 vector and miR-497-5p mimics was measured. **P<0.01.
Discussion
Various microRNAs (miRNAs) that can assess the risk of tumor progression and metastasis have been reported for use in non-small cell lung cancer (NSCLC) (22–24). In the present study, the expression of miR-497-5p was significantly reduced, and low expression of miR-497-5p was closely related to TNM stage and distant metastases of NSCLC. Similarly, miR-497 was reported to be downregulated and to inhibit tumor growth in NSCLC (25), which was consistent with our results. Besides, downregulation of miR-497 was also observed in osteosarcoma (26), thyroid (27) and colorectal cancer (28). These findings suggest that abnormal miR-497-5p expression is involved in the pathogenesis of NSCLC.
The effect of miR-497-5p on cell proliferation and invasion was also investigated in NSCLC. Li et al demonstrated that overexpression of miR-497 suppressed cell proliferation and invasion in human retinoblastoma (29). Similar inhibition of cell proliferation and invasion by miR-497-5p was also detected in NSCLC. Moreover, miR-497 has been found to regulate several target genes to mediate the development of human cancers. For example, miR-497 inhibited proliferation of hepatocellular carcinoma cells and induced cell apoptosis through targeting YAP1 (30). Ruan et al proposed that miR-497 inhibited proliferation, migration, and invasion of osteosarcoma cells via targeting AMOT (31). In the present study, FGF2 was shown to be a downstream and direct target of miR-497-5p in NSCLC.
Subsequently, the alteration of FGF2 expression and its effects were investigated, and the regulation mechanism of miR-497-5p in NSCLC was further elucidated. Upregulation of FGF2 was identified in NSCLC, which promoted proliferation and invasion of NSCLC cells. Similarly to our results, Cheng et al demonstrated that FGF2 expression was increased and knockdown of FGF2 inhibited proliferation and invasion of NSCLC cells (32). Besides, a negative correlation between miR-497-5p and FGF2 expression was revealed in NSCLC tissues. In addition, upregulation of FGF2 impaired the inhibitory effect of miR-497-5p in NSCLC. Similarly, previous studies have also shown that many miRNAs negatively regulated FGF2 expression, such as miR-195 (33), miR-205 (34) and miR-646 (35). Moreover, He et al implied that miR-16 targeting FGF2 inhibited proliferation and invasion of nasopharyngeal carcinoma cells (36). In the present study, miR-497-5p also inhibited proliferation and invasion of NSCLC cells by targeting FGF2.
In conclusion, in the present study, we revealed the inhibitory effect of miR-497-5p on the progression of NSCLC through targeting FGF2. This suggests that miR-497-5p acts as a novel tumor suppressor in NSCLC. In addition, FGF2 was a downstream target of miR-497-5p in NSCLC, indicating that FGF2 was involved in the regulatory mechanism of miR-497-5p. Further research is needed to investigate whether miR-497-5p is a useful biomarker and therapeutic target for NSCLC progression.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors contributions
XH wrote the manuscript and contributed to the conception of the study. LW performed the data analysis. WL contributed to the data acquisition and analysis, and revised the manuscript. FL worked on aspects of the study relating to NSCLC patients. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of People's Hospital of Rizhao (Rizhao, China). Signed informed consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Laskin JJ, Sandler AB. State of the art in therapy for non-small cell lung cancer. Cancer Invest. 2005;23:427–442. doi: 10.1081/CNV-67172. [DOI] [PubMed] [Google Scholar]
- 3.Ma L, Qiu B, Zhang J, Li QW, Wang B, Zhang XH, Qiang MY, Chen ZL, Guo SP, Liu H. Survival and prognostic factors of non-small cell lung cancer patients with postoperative locoregional recurrence treated with radical radiotherapy. Chin J Cancer. 2017;36:93. doi: 10.1186/s40880-017-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray N. Reality check for pemetrexed and maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol. 2014;32:482–483. doi: 10.1200/JCO.2013.53.3448. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson MK. Diagnosing and staging of non-small cell lung cancer. Hematol Oncol Clin North Am. 1990;4:1053–1068. [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Del Vescovo V, Grasso M, Barbareschi M, Denti MA. MicroRNAs as lung cancer biomarkers. World J Clin Oncol. 2014;5:604–620. doi: 10.5306/wjco.v5.i4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao Y, Shen H, Zhou Y, Yang Z, Hu T. MicroRNA-215 suppresses the proliferation, migration and invasion of non-small cell lung carcinoma cells through the downregulation of matrix metalloproteinase-16 expression. Exp Ther Med. 2018;15:3239–3246. doi: 10.3892/etm.2018.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei L, Ran F. MicroRNA-20a promotes proliferation and invasion by directly targeting early growth response 2 in non-small cell lung carcinoma. Oncol Lett. 2018;15:271–277. doi: 10.3892/ol.2017.7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang PC, Shi XY, Huang WL, Sun K. miR-497 as a potential serum biomarker for the diagnosis and prognosis of osteosarcoma. Eur Rev Med Pharmacol Sci. 2016;20:3765–3769. [PubMed] [Google Scholar]
- 11.Pengcheng S, Ziqi W, Luyao Y, Xiangwei Z, Liang L, Yuwei L, Lechen L, Wanhai X. MicroRNA-497 suppresses renal cell carcinoma by targeting VEGFR-2 in ACHN cells. Biosci Rep. 2017;37:37. doi: 10.1042/BSR20170270. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Wu R, Tang S, Wang M, Xu X, Yao C, Wang S. MicroRNA-497 induces apoptosis and suppresses proliferation via the Bcl-2/Bax-caspase9-caspase3 pathway and cyclin D2 protein in HUVECs. PLoS One. 2016;11:e0167052. doi: 10.1371/journal.pone.0167052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao L, Zhang CY, Guo L, Li X, Han NN, Zhou Q, Liu ZL. MicroRNA-497 accelerates apoptosis while inhibiting proliferation, migration, and invasion through negative regulation of the MAPK/ERK signaling pathway via RAF-1. J Cell Physiol. 2017;233:6578–6588. doi: 10.1002/jcp.26272. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Xu JF, Ge WL. MiR-497 enhances metastasis of oral squamous cell carcinoma through SMAD7 suppression. Am J Transl Res. 2016;8:3023–3031. [PMC free article] [PubMed] [Google Scholar]
- 15.Song KH, Cho H, Kim S, Lee HJ, Oh SJ, Woo SR, Hong SO, Jang HS, Noh KH, Choi CH, et al. API5 confers cancer stem cell-like properties through the FGF2-NANOG axis. Oncogenesis. 2017;6:e285. doi: 10.1038/oncsis.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khurana A, Liu P, Mellone P, Lorenzon L, Vincenzi B, Datta K, Yang B, Linhardt RJ, Lingle W, Chien J, et al. HSulf-1 modulates FGF2- and hypoxia-mediated migration and invasion of breast cancer cells. Cancer Res. 2011;71:2152–2161. doi: 10.1158/0008-5472.CAN-10-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman SJ, Chioni AM, Ghallab M, Anderson RK, Lemoine NR, Kocher HM, Grose RP. Nuclear translocation of FGFR1 and FGF2 in pancreatic stellate cells facilitates pancreatic cancer cell invasion. EMBO Mol Med. 2014;6:467–481. doi: 10.1002/emmm.201302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joy A, Moffett J, Neary K, Mordechai E, Stachowiak EK, Coons S, Rankin-Shapiro J, Florkiewicz RZ, Stachowiak MK. Nuclear accumulation of FGF-2 is associated with proliferation of human astrocytes and glioma cells. Oncogene. 1997;14:171–183. doi: 10.1038/sj.onc.1200823. [DOI] [PubMed] [Google Scholar]
- 19.Sooman L, Freyhult E, Jaiswal A, Navani S, Edqvist PH, Pontén F, Tchougounova E, Smits A, Elsir T, Gullbo J, et al. FGF2 as a potential prognostic biomarker for proneural glioma patients. Acta Oncol. 2015;54:385–394. doi: 10.3109/0284186X.2014.951492. [DOI] [PubMed] [Google Scholar]
- 20.Xue G, Yan HL, Zhang Y, Hao LQ, Zhu XT, Mei Q, Sun SH. c-Myc-mediated repression of miR-15-16 in hypoxia is induced by increased HIF-2α and promotes tumor angiogenesis and metastasis by upregulating FGF2. Oncogene. 2015;34:1393–1406. doi: 10.1038/onc.2014.82. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Yang CL, Zheng XL, Ye K, Ge H, Sun YN, Lu YF, Fan QX. MicroRNA-183 acts as a tumor suppressor in human non-small cell lung cancer by down-regulating MTA1. Cell Physiol Biochem. 2018;46:93–106. doi: 10.1159/000488412. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, Zhang Y, Zhang J, Cai H, Zhang C, Yang Z, Niu Y, Wang H, Wei X, Wang W, et al. MicroRNA-1253 suppresses cell proliferation and invasion of non-small-cell lung carcinoma by targeting WNT5A. Cell Death Dis. 2018;9:189. doi: 10.1038/s41419-017-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Wang Y, Sun D, Bu J, Ren F, Liu B, Zhang S, Xu Z, Pang S, Xu S. miR-455-5p promotes cell growth and invasion by targeting SOCO3 in non-small cell lung cancer. Oncotarget. 2017;8:114956–114965. doi: 10.18632/oncotarget.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Ma R, Yue J, Li N, Li Z, Qi D. MiR-497 suppresses YAP1 and inhibits tumor growth in non-small cell lung cancer. Cell Physiol Biochem. 2015;37:342–352. doi: 10.1159/000430358. [DOI] [PubMed] [Google Scholar]
- 26.Sun Z, Li A, Yu Z, Li X, Guo X, Chen R. MicroRNA-497-5p suppresses tumor cell growth of osteosarcoma by targeting ADP ribosylation factor-like protein 2. Cancer Biother Radiopharm. 2017;32:371–378. doi: 10.1089/cbr.2017.2268. [DOI] [PubMed] [Google Scholar]
- 27.Wang P, Meng X, Huang Y, Lv Z, Liu J, Wang G, Meng W, Xue S, Zhang Q, Zhang P, et al. MicroRNA-497 inhibits thyroid cancer tumor growth and invasion by suppressing BDNF. Oncotarget. 2017;8:2825–2834. doi: 10.18632/oncotarget.13747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Xu Y, Chen J, Gao C, Zhu D, Xu X, Wu C, Jiang J. MicroRNA-497 inhibits tumor growth through targeting insulin receptor substrate 1 in colorectal cancer. Oncol Lett. 2017;14:6379–6386. doi: 10.3892/ol.2017.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Zhang Y, Wang X, Zhao R. microRNA-497 overexpression decreases proliferation, migration and invasion of human retinoblastoma cells via targeting vascular endothelial growth factor A. Oncol Lett. 2017;13:5021–5027. doi: 10.3892/ol.2017.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Yu Z, Xian Y, Lin X. microRNA-497 inhibits cell proliferation and induces apoptosis by targeting YAP1 in human hepatocellular carcinoma. FEBS Open Bio. 2016;6:155–164. doi: 10.1002/2211-5463.12032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Ruan WD, Wang P, Feng S, Xue Y, Zhang B. MicroRNA-497 inhibits cell proliferation, migration, and invasion by targeting AMOT in human osteosarcoma cells. Onco 9Targets Ther. 2016;9:303–313. doi: 10.2147/OTT.S95204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng Z, Ma R, Tan W, Zhang L. MiR-152 suppresses the proliferation and invasion of NSCLC cells by inhibiting FGF2. Exp Mol Med. 2014;46:e112. doi: 10.1038/emm.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Xu J, Jiang T, Liu G, Wang D, Lu Y. MicroRNA-195 suppresses colorectal cancer cells proliferation via targeting FGF2 and regulating Wnt/β-catenin pathway. Am J Cancer Res. 2016;6:2631–2640. [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Y, Qiu Y, Yagüe E, Ji W, Liu J, Zhang J. miRNA-205 targets VEGFA and FGF2 and regulates resistance to chemotherapeutics in breast cancer. Cell Death Dis. 2016;7:e2291. doi: 10.1038/cddis.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun XH, Geng XL, Zhang J, Zhang C. miRNA-646 suppresses osteosarcoma cell metastasis by downregulating fibroblast growth factor 2 (FGF2) Tumour Biol. 2015;36:2127–2134. doi: 10.1007/s13277-014-2822-z. [DOI] [PubMed] [Google Scholar]
- 36.He Q, Ren X, Chen J, Li Y, Tang X, Wen X, Yang X, Zhang J, Wang Y, Ma J, et al. miR-16 targets fibroblast growth factor 2 to inhibit NPC cell proliferation and invasion via PI3K/AKT and MAPK signaling pathways. Oncotarget. 2016;7:3047–3058. doi: 10.18632/oncotarget.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.