Abstract
Polymer conjugation is a clinically proven approach to generate long acting protein drugs with decreased immune responses. Although poly(ethylene glycol) (PEG) is one of the most commonly used conjugation partners due to its unstructured conformation, its therapeutic application is limited by its poor biodegradability, propensity to induce an anti-PEG immune response, and the resultant accelerated blood clearance (ABC) effect. Moreover, the prevailing preference of unstructured polymers for protein conjugation still lacks strong animal data support with appropriate control reagents. By using two biodegradable synthetic polypeptides with similar structural compositions (l-P(EG3Glu) and dl-P(EG3Glu)) for site-specific protein modification, in the current study, we systematically investigate the effect of the polymer conformation on the in vivo pharmacological performances of the resulting conjugates. Our results reveal that the conjugate l20K-IFN, interferon (IFN) modified with the helical polypeptide l-P(EG3Glu) shows improved binding affinity, in vitro antiproliferative activity, and in vivo efficacy compared to those modified with the unstructured polypeptide analogue dl-P(EG3Glu) or PEG. Moreover, l20K-IFN triggered significantly less antidrug and antipolymer antibodies than the other two. Importantly, the unusual findings observed in the IFN series are reproduced in a human growth hormone (GH) conjugate series. Subcutaneously infused l20K-GH, GH modified with l-P(EG3Glu), evokes considerably less anti-GH and antipolymer antibodies compared to those modified with dl-P(EG3Glu) or PEG (dl20K-GH or PEG20K–GH). As a result, repeated injections of dl20K-GH or PEG20K-GH, but not l20K-GH, result in a clear ABC effect and significantly diminished drug availability in the blood. Meanwhile, immature mouse bone marrow cells incubated with the helical l20K-GH exhibit decreased drug uptake and secretion of proinflammatory cytokines compared to those treated with one of the other two GH conjugates bearing unstructured polymers. Taken together, the current study highlights an urgent necessity to systematically reassess the pros and cons of choosing unstructured polymers for protein conjugation. Furthermore, our results also lay the foundation for the development of next-generation biohybrid drugs based on helical synthetic polypeptides.
Short abstract
The covalent attachment of a helical polypeptide dramatically enhances the efficacy and reduces the unwanted immune response of protein drugs, which may suggest a new paradigm shift of drug design.
Introduction
Therapeutic proteins are important biologics that frequently exhibit high potency and selectivity. However, their clinical use has been hampered by their rapid renal clearance, susceptibility to proteolysis, and strong immunogenicity.1−3 Particularly, the generation of antidrug antibodies (ADAs) has been a serious hurdle for many protein drugs.4 One proven strategy to overcome these limitations is to covalently conjugate the protein of interest to polymers such as poly(ethylene glycol) (PEG), a process known as PEGylation, which can lead to significantly increased hydrodynamic volume, in vivo stability, and circulation half-life.5−10 However, there is mounting evidence that PEGylated proteins tend to show poorer binding affinity and biological activity than their unconjugated equivalents.11,12 Furthermore, although one of the initial purposes of PEGylation is for reduced ADA generation, PEG is known to elicit anti-PEG antibodies that adversely accelerate the blood clearance of the PEGylated proteins or nanoparticles, known as the ABC effect. As evidence, reduction in the therapeutic efficacy of many PEGylated proteins, such as uricase, asparaginase, and interferon (IFN), has been found to strongly correlate with the occurrence of the anti-PEG immune response that they induce.13 More worrisome is the fact that the percentage of healthy adults carrying pre-existing anti-PEG antibodies has increased sharply from 0.2% to 42% during the past three decades, likely because of their daily exposure to PEG-containing commodities.13 Thus, a pressing need in this field is seeking new polymers beyond PEGylation.
In recent years, researchers have investigated a wide range of alternative conjugation partners,14 including zwitterionic polymers,15,16 polyglycerol,17 glycopolymers,18 and oligo-EGylated poly(meth)acrylates,19,20 with varying degrees of success. Despite the potential of these methods, the lack of biodegradability has remained a central problem.5 Synthetic polypeptides have been increasingly considered as a biodegradable and biocompatible alternative to PEG with great clinical promise.21−25 There has been evidence that the genetic fusion of therapeutic proteins/peptides to intrinsically disordered polypeptides, such as XTEN, PAS, and elastin-like polypeptides (ELP), can lead to improved pharmacological performance in vivo.26−32 We envisage that the chemical modification of proteins by synthetic polypeptides, which we call PEPylation, could open up enormous possibilities.33−35 Particularly, the chemical diversity of synthetic polypeptides has been greatly expanded by incorporating noncanonical amino acids via ring-opening polymerization (ROP) of α-amino acid N-carboxyanhydrides (NCA) and utilizing d-amino acids.20 Notably, during the preparation of this manuscript, Jiang et al. reported the nonspecific grafting of zwitterionic polypeptides to uricase,36 which showed extraordinarily low immunogenicity and outstanding safety profile in vivo. Their work underscored the exceptional clinical potential of PEPylation.
When surveying the aforementioned polymers for protein modification, one can easily draw the conclusion that unstructured and flexible polymers (e.g., PEG) have long been the preferred conjugation partners due to their ability to augment the hydrodynamic volume of the modified protein and provide an excellent stealth effect that minimizes renal filtration and immune attack. Following the same principle, elementary amino acids are carefully selected in the design of XTEN to ensure an unstructured conformation and absence of helical structures.26 However, it is surprising that there have been very few studies that attempt to investigate whether the conformation, particularly the helix, of the polymer has any effect on the in vivo performance of the protein that it modifies. One practical challenge resides in the difficulty of generating protein conjugates that only differ in the conformation of the attached polymers to ensure a fair comparison. We reason that synthetic polypeptides offer an ideal solution to this problem as their secondary conformations (e.g., helix and coil) can be easily manipulated by switching the chirality of the monomers without altering the overall chemical composition.37,38
Results
Synthesis and Characterization of Different IFN–Polymer Conjugates
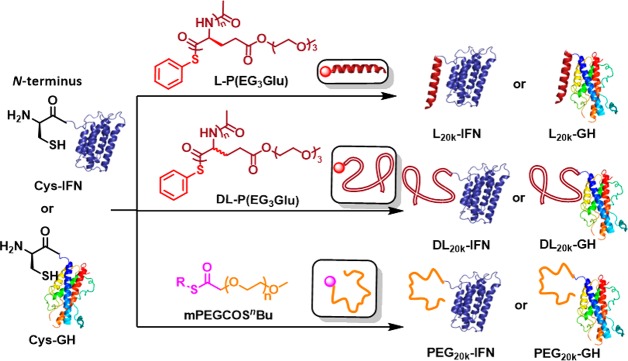
Recombinant IFN, an antiviral and antitumor cytokine, was selected as our first model drug. For a fair comparison, we synthesized two chemically similar but conformationally varied polypeptides (Scheme 1).34,35 Specifically, monomer γ-(2-(2-(2-methoxyethoxy)ethoxy)ethyl l-glutamate NCA39 (l-EG3GluNCA) was polymerized by trimethylsilyl phenylsulfide (PhS-TMS) to yield phenyl thioester-functionalized l-P(EG3Glu) (Scheme 1). Similarly, dl-P(EG3Glu) was produced from a racemic mixture of dl-EG3GluNCA. The molecular weights (MW) of both polymers were carefully controlled to be ∼20 kDa, in line with many clinically approved PEG conjugates. Gel permeation chromatography (GPC) indicated that the two polymers had a similar MW ≈ 22–23 kDa and narrow dispersity (D̵) below 1.05 (Figure S1). 1H NMR spectroscopy showed that the two polymers differed in the chemical shift of the α-H due to the different α-C chirality (Figure S2). As expected, circular dichroism (CD) spectroscopy revealed that α-helices constituted more than 90% of l-P(EG3Glu), whereas dl-P(EG3Glu) was unstructured as design (Figure S3). Subsequently, we conjugated each synthetic polypeptide to an IFN mutant bearing a N-terminal cysteine (Cys-IFN) via native chemical ligation, thereby forming two PEPylated IFNs denoted as l20K-IFN and dl20K-IFN (Scheme 1). We also generated PEG20K-IFN as a positive control by attaching a thioester-functionalized PEG (MW ≈ 20 kDa) to IFN via the same method (Scheme 1 and Figure S4).
Scheme 1. Site-Specific Conjugation of Synthetic Polypeptides or PEG To Engineer Therapeutic Proteins (IFN and GH) via Native Chemical Ligation.
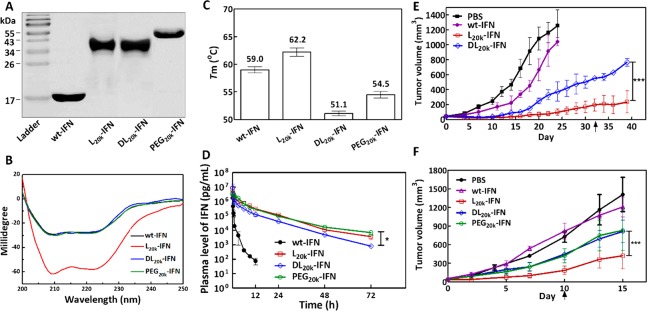
All purified IFN conjugates exhibited a narrow size distribution based on SDS-PAGE analysis (Figure 1A). l20K-IFN and dl20K-IFN shared an almost identical apparent MW, whereas PEG20K-IFN appeared to electrophoresize slightly slower than its PEPylated counterparts but was still comparable (Figure 1A). CD spectroscopy suggested that PEG20K-IFN and dl20K-IFN were similar in helicity as wt-IFN, whereas l20K-IFN produced a stronger helical signal intensity (Figure 1B). A thermofluoro assay39 indicated that l20K-IFN possessed a higher Tm, and therefore greater thermostability, than both dl20K-IFN and PEG20k–IFN (Figure 1C). All conjugates were shown to be significantly more resistant to proteolysis than wt-IFN in trypsin digestion assays (Figure S5). Surface plasmon resonance (SPR) found the KD values for the binding of l20K-IFN, dl20K-IFN, and PEG20K–IFN to human IFNAR2 were 5.8, 19.6, and 15.9 nM, respectively (Table 1 and Figure S6). Thus, l20K-IFN appeared to be ∼3–4 fold more efficient in its receptor interaction than dl20K-IFN or PEG20K–IFN. Consistently, an in vitro viability assay demonstrated that the IC50 values of l20K-IFN, dl20K-IFN, and PEG20K–IFN against Daudi cells, an IFN-sensitive human cancer cell line, were 36, 160, and 190 pg/mL, respectively (Table 1). This implied that l20K-IFN could induce a significantly more potent antitumor effect than dl20K-IFN or PEG20K–IFN does.
Figure 1.
Characterization and in vivo pharmacological performances of various IFN conjugates. (A) SDS-PAGE gel, stained by Coomassie blue. (B) Circular dichroism (CD) spectroscopy. (C) Melting temperature (Tm) measured by thermofluoro assay. (D) In vivo pharmacokinetics (i.v. injection) of wt-IFN (n = 6), l20K-IFN (n = 6), and dl20K-IFN (n = 6), and PEG20K–IFN (n = 3). (E–F) Tumor growth inhibition curves. BALB/C-nu mice bearing s.c. OVCAR-3 xenograft (E) or patient-derived xenograft (PDX) tumors (F) were i.v. injected with PBS saline or one of the IFN-based drugs (n = 7 each); treatments began on day 0, and the black arrows indicate ending of the treatments. The total injection numbers are six in E and three in F. Data are expressed as mean ± SD. P value is determined by two-way ANOVA (Bonferroni post-test) analysis: *p < 0.05, **p < 0.01, ***p < 0.001.
Table 1. In Vitro Binding, Anti-Proliferative Activity, and in Vivo Pharmacokineticsa of wt-IFN and Various IFN Conjugates.
| sample | IC50 (pg/mL) | KD (nM) | elimination half-life (h)b | AUC0-t ((μg/mL)*h)c | Vd (mL)d | CI (mL/h) |
|---|---|---|---|---|---|---|
| wt-IFN | 8.5 ± 1.4 | 1.0 | 0.5 ± 0.1 | 0.4 ± 0.1 | 125 ± 21.8 | |
| L20k-IFN | 36.0 ± 1.3 | 5.8 | 9.6 ± 0.6 | 15.5 ± 2.2 | 167 ± 49 | 3.2 ± 0.6 |
| DL2ok-IFN | 160 ± 4 | 19.6 | 7.8 ± 0.3 | 8.6 ± 0.7 | 239 ± 49 | 5.8 ± 0.7 |
| PEG20k-IFN | 190 ± 10 | 15.9 | 9.8 ± 1.9 | 17.0 ± 3.0 | 161 ± 25 | 2.9 ± 0.4 |
Dose: 50 μg/rat on IFN base.
Elimination half-life: Time points used to calculate t1/2β are 3–12 h (wt IFN), 12–72 h (all conjugates).
AUC calculated by logarithmic trapezoidal rule up to 12 h (wt-IFN),72 h (l20K-IFN, dl20K-IFN, and PEG20K–IFN).
Vd calculated at 12 h after intravenous injection.
Data are expressed as mean ± SD.
In Vivo Pharmacological Performances of IFN Conjugates
We next measured the pharmacokinetic parameters of the IFN variants in female Sprague−Dawley rats. As shown in Figure 1D and Table 1, the elimination half-lives (t1/2β) of l20K-IFN, dl20K-IFN, and PEG20K–IFN were 9.6, 7.8, and 9.8 h, respectively, all significantly longer than the 0.5 h t1/2β of wt-IFN. Interestingly, l20K-IFN was slightly but consistently longer-lived than dl20K-IFN (P value < 0.05; reproducible in at least two independent experiments with different batches of materials). This was further evidenced by the greater AUC of l20K-IFN than that of dl20K-IFN (Table 1). The in vivo efficacy of the conjugates was further evaluated in two murine models, one bearing OVCAR-3 tumor xenografts and the other xenografts derived from a prostate cancer patient (PDX) (see Materials and Methods). In both cases, administration of l20K-IFN, which carried the helical l-P(EG3Glu), led to significantly slower tumor growth (Figure 1A). The superior antitumor efficacy was further confirmed by the reduced tumor cell proliferation activity according to Ki-67 staining (Figure S7). No body weight loss was observed in either model during the treatment with l20K-IFN, suggesting that the drug was well tolerated under the regimen that we employed (Figure S8).
Antibody Generation Triggered by IFN Conjugates
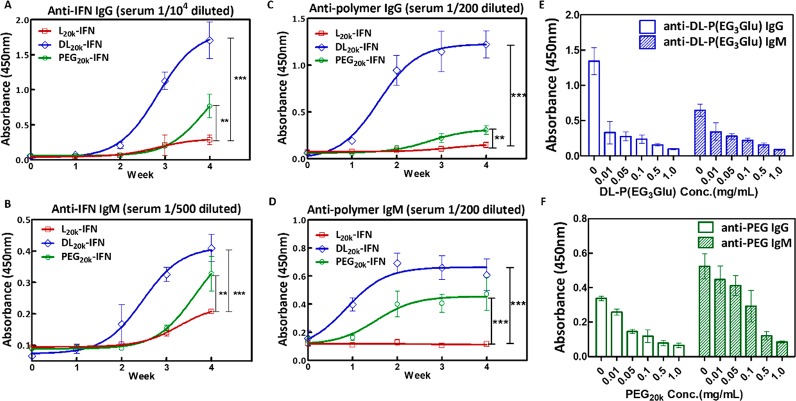
To investigate the immune response of the conjugates, Sprague–Dawley rats were randomly grouped and subcutaneously administrated with l20K-IFN, dl20K-IFN, or PEG20K–IFN at a weekly dose of 0.2 mg/kg. Interestingly, sera from the mice immunized with l20K-IFN showed significantly lower levels of anti-IFN IgG and IgM than those receiving dl20K-IFN or PEG20K–IFN (Figure 2A–B). Serial dilution of sera from week 4 revealed that l20K-IFN produced ∼50–100 fold lower anti-IFN IgG and ∼5–10 fold lower IgM titers than those immunized with dl20K-IFN or PEG20K–IFN (Figure S9). In addition, injection with dl20K-IFN or PEG20K–IFN appeared to also induce a detectable amount of antipolymer antibodies, particularly IgM (Figure 2C–D). The specificity of the antipolymer antibodies in dl20K-IFN and PEG20K–IFN sera was further validated by the corresponding polymer competition (Figure 2E–F). Strikingly, we discovered that l20K-IFN exhibited almost no detectable effect on the serum level of antipolymer IgG or IgM in the immunized rats.
Figure 2.
In vivo immune responses triggered by IFN conjugates. (A–B) Anti-IFN IgG (A) and IgM (B) contents in the sera measured by ELISA; the plates were coated with wt-IFN and then incubated with 104-fold (for IgG) or 500-fold (for IgM) prediluted sera in PBS. (C–D) Antipolymer IgG (C) and IgM (D) contents in the sera immunized with various polymer-IFN conjugates; for each polymer-of-interest, the ELISA plates were coated with the corresponding polymer-GH conjugate. (E–F) Antipolymer ELISA assays using free dl-P(EG3Glu) (E) or PEG (F) as the competition agent; sera immunized with dl20K-IFN or PEG20K–IFN (week 4) were prediluted 200-fold and incubated with the corresponding free polymer at gradient concentrations. Immunization protocol: rats were s.c. infused with l20K-IFN, dl20K-IFN, or PEG20K–IFN at a weekly dose 0.2 mg/kg for 4 weeks; sera were drawn from the rats (n = 3) every week starting from week 0. For ELISA analysis, after sera incubation and washing, all plates were incubated with antimouse IgG-HRP or IgM-HRP, and analyzed by TMB solution (CWBIO). TWEEN was excluded from the buffers in all antipolymer ELISA studies. Data are expressed as mean ± SD P value is determined by two-way ANOVA (Bonferroni post-test) analysis: *p < 0.05, **p < 0.01, ***p < 0.001.
Synthesis of and Immune Responses Triggered by Different GH–Polymer Conjugates
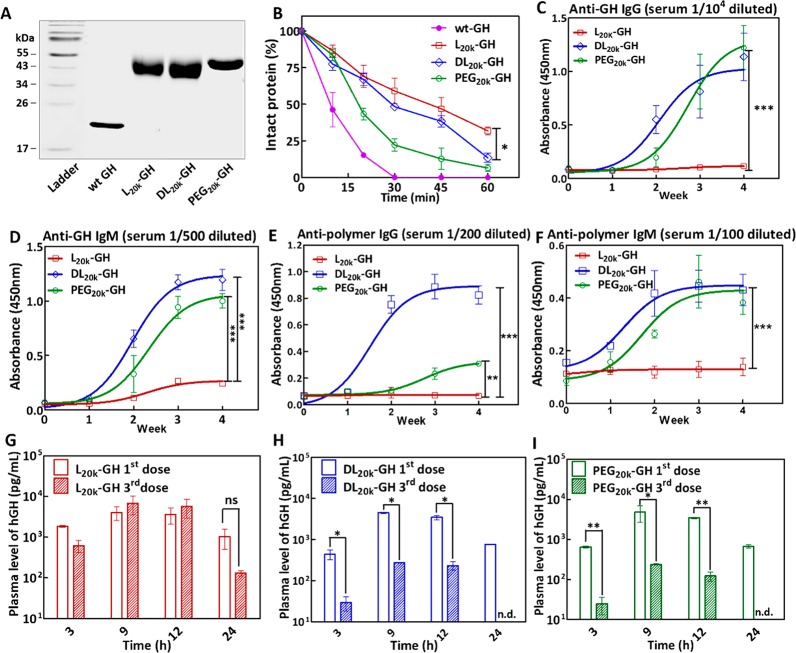
To test whether our findings observed in the IFN conjugates were also applicable to other therapeutic proteins, we selected human growth hormone (GH)41,42 as our second example and engineered the protein with a N-terminal cysteine (Cys-GH), similar to that in Cys-IFN. We next covalently tethered l-P(EG3Glu), dl-P(EG3Glu), and PEG separately to Cys-GH to generate three conjugates denoted as l20K-GH, dl20K-GH, and PEG20K–GH, respectively (Scheme 1 and Figure 3A). Trypsin digestion revealed that l20K-GH was significantly more resistant to proteolysis than dl20K-GH and PEG20K-GH (Figure 3B). Furthermore, injection with l20K-GH provoked substantially less production of anti-GH IgG and IgM antibodies in rats from week 2, compared to treatment with dl20K-GH or PEG20K-GH (Figure 3C–D). Serial dilution of sera from week 4 revealed that l20K-GH produced ∼100 fold lower anti-GH IgG and ∼20-fold lower IgM titers than those immunized with dl20K-GH or PEG20K–GH (Figure S10). The same trend was observed when we measured the levels of antipolymer IgG and IgM following the immunization (Figure 3E–F and Figure S11). To examine the ABC effect, we measured the blood concentration of GH at selected time points after the first and third injection of each conjugate. The results demonstrated that infusions of l20K-GH led to very similar blood levels of GH during the first 12 h and generated almost no ABC effect in 24 h (Figure 3G, statistically insignificant). In sharp contrast, both dl20K-GH and PEG20K–GH caused a characteristic ABC effect after the third injection (Figure 3H–I). In fact, our ELISA kit failed to detect blood GH at 24 h following the administration of dl20K-GH or PEG20K–GH (Figure 3H–I). As a result, the AUC0–24h of l20K-GH were comparable after the first and third injection (100% vs 112%), whereas the AUC0–24h of both dl20K-GH and PEG20K–GH after the third infusion were only ∼6% of those after the first drug infusion (Table S1).
Figure 3.
In vivo immune responses triggered by GH conjugates. (A) SDS-PAGE gel analysis. (B) Trypsin degradation curves. (C–D) Anti-GH IgG (C) and IgM (D) contents in the sera measured by ELISA; the plates were coated with wt-GH and then incubated with 104-fold (for IgG) or 500-fold (for IgM) prediluted sera in PBS. (E–F) Antipolymer IgG (E) and IgM (F) contents in the sera immunized with polymer-GH conjugates; for each polymer of interest, the ELISA plates were coated with the corresponding polymer–IFN conjugate and then incubated with the 200-fold prediluted sera. Immunization protocol: rats were s.c. infused with l20K-GH, dl20K-GH, or PEG20K-GH at a weekly dose 0.2 mg/kg for 4 weeks; sera were drawn from the rats (n = 3) every week starting from week 0. For ELISA analysis, after sera incubation and washing, all plates were incubated with antimouse IgG-HRP or IgM-HRP, and analyzed by TMB solution (CWBIO). TWEEN was excluded from the buffers in all antipolymer ELISA studies. (G–I) Blood GH contents at selected time points, measured by ELISA, after the first and third s.c. injections of l20K-GH (G), dl20K-GH (H), or PEG20K–GH (I). Data are expressed as mean ± SD. P value is determined by two-way ANOVA (Bonferroni post-test) analysis: *p < 0.05, **p < 0.01, ***p < 0.001.
BMDC Uptake and Activation
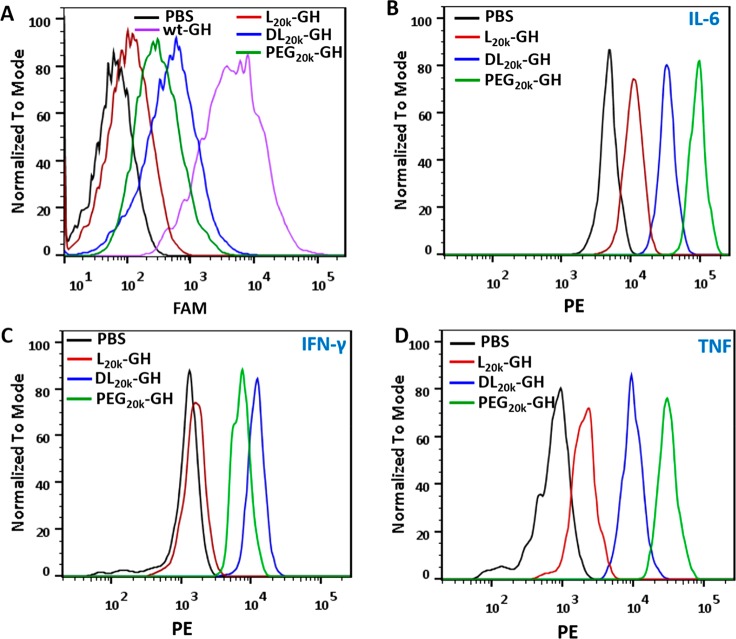
During antibody production, the antigens are usually internalized, fragmented in lysosome, and displayed on the cell surface by dendritic cells (DCs) to trigger downstream T cell and B cell response. To understand the different antibody responses triggered by the conjugates, we sought to examine the very first DC internalization step. For this, we incubated the GH conjugates with freshly induced immature mouse bone marrow-derived dendritic cells (BMDCs), which are widely used for the assessment of antigen presenting.43 Flow cytometric analysis found clear evidence for the internalization of dl20K-GH and PEG20K–GH into BMDCs after 12 h of incubation, whereas the uptake level of l20K-GH was considerably lower (Figure 4A). Consistently, treatment of BMDCs with l20K-GH resulted in appreciably less secretion of proinflammatory cytokines, including interleukine-6 (IL-6, Figure 4B), interferon-γ (IFN-γ, Figure 4C), and tumor necrosis factor (TNF, Figure 4D), compared to the other two GH conjugates carrying unstructured polymers.
Figure 4.
BMDC internalization and activation. (A) Flow cytometry analysis of BMDC internalization of various FAM-labeled GH conjugates. (B–D) Flow cytometry analysis of proinflammatory cytokines secretion: IL-6 (B), IFN-γ (C), and TNF (D). Freshly separated naïve BMDCs were ex vivo incubated in 24-well plate (5 × 105 cells/well) for 6 days and treated with conjugates for 12 h (A) or 24 h (B–D) at 37 °C. The cytokines in the medium were measured with CBA Mouse Inflammation kit following manufacturer’s protocol. The PBS-treated BMDCs were served as controls.
Discussion
The conjugation of polymers to a protein has been demonstrated to extend its half-time by increasing its hydrodynamic volume and mitigating the ADA generation.1 However, the role that the secondary conformation of a polymer plays in the resultant protein conjugate has been very rarely investigated, as unstructured polymers have been the heavily favored choice in past studies. Notably, the polypeptide–uricase conjugate reported by Jiang focused on the zwitterionic side chain without studying the secondary conformation effect.36 We speculated that peptide-based drugs and biomaterials covalently modified with α-helical polypeptides could exhibit improved proteolytic and thermal stability, binding, as well as other biological functions over those conjugated with disordered polymers.37,44−47 To ascertain whether this is the case, however, one needs to employ polymers that only differ in conformation. Gratifyingly, controlled NCA ROP and chemoselective labeling collaboratively enabled us to generate protein conjugates that shared almost identical modification sites and MWs, and were attached to nearly the same number of polypeptides with highly similar chemical compositions.34 As a result, the secondary conformation of the tethered polypeptides became the only major variable. This was corroborated by the GPC curves of the polymers and the narrow size distributions of the resultant conjugates on the SDS-PAGE gel (Figure S1, Figures 1A and 3A). Of note, due to the distinct chemical structures of PEG and our P(EG3-Glu), the migration of those conjugates in SDS-PAGE gel might not completely correlate their MWs, which is often observed for other polymer modified proteins.
Our results found the helical polypeptide-bearing l20K-IFN to have higher binding affinity and antiproliferative activity in vitro than dl20K-IFN and PEG20K–IFN, both of which were attached to unstructured polymers (Table 1). This could be partially attributed to the less steric hindrance imparted by the rigid helical polypeptides. Moreover, l20K-IFN exhibited significant improvement in circulation half-life and in vivo efficacy compared to dl20K-IFN (Figure 1D). Taken together, these data suggested that the conjugation of a rigid helical polypeptide could improve the blood retention of the modified protein drug without significantly affecting its binding affinity or potency, thereby offering a viable solution to the well-known “PEG dilemma”.11
Some of the greatest controversies of PEGylation include the insufficient protection of the conjugated proteins from immune recognition and the generation of anti-PEG antibodies.13,48 In the clinic, the anti-IFN neutralizing antibodies has previously been observed in nonresponding patients and believed to be the major reason for their development of resistance.49 In this regard, it was remarkable that the administration of l20K-IFN provoked substantially lower production of anti-IFN, as well as antipolymer IgG and IgM, than dl20K-IFN or PEG20K–IFN (Figure 2). Importantly, similar results were also obtained from the GH conjugates, indicating that the benefits we observed were independent of the modified protein (Figure 3). We also synthesized a left-handed helical polypeptide d-P(EG3Glu) (∼23 kDa) and produced two conjugates, d20K-IFN and d20K-GH (data not shown). We discovered that both d20K-IFN and d20K-GH, similar to l20K-IFN or l20K-GH, showed almost no antibody response after repeated administration (data not shown). The results lent further evidence to the generality of the helix effect. Moreover, the above study help ruling out the possibility of d-amino acid-induced antibody production in the cases of dl20K-IFN and dl20K-GH. Although the exact mechanistic role of helicity remains insufficiently explored, a number of reasons may count for the unexpected findings. First of all, the helical l-P(EG3Glu) seems to provide better antifouling property than dl-P(EG3Glu) and PEG, and thus minimizing nonspecific internalization with cells and proteins. Our initial investigation provided preliminary evidence of conformation-dependent internalization and activation of immature BMDCs for those examined protein–polymer conjugates. In fact, this helical conformation enhanced antifouling and anticell adhesion was also observed when the polypeptides were anchored on gold surfaces.50 Second, helical polypeptides are well-known more proteolytic stable (Figure 3B) than those unstructured peptidyl analogues, which may lead to inefficient fragmentation and MHC presentation after BMDC internalization. More rigorous experimental and modeling studies are currently ongoing to fully uncover the molecular mechanism of the unusual helical conformation effect.
Conclusions
In conclusion, we generated synthetic polypeptides that only differed in conformation and compared their effects on the in vivo therapeutic and immunological properties of the protein drugs to which they were conjugated. Compared with the unstructured dl-P(EG3Glu) or PEG, the covalent attachment of the helical l-P(EG3Glu) to therapeutic proteins (namely, IFN and GH) led to substantial improvement in a variety of pharmacological properties, such as binding affinity, stability, and in vivo efficacy. Most interestingly, the helical l-P(EG3Glu)-conjugated IFN and GH elicited a significantly milder immune response and exhibited a much weaker ABC effect than those modified with unstructured polymers. Thus, the helical nonfouling polypeptides that we employed could be excellent alternatives to PEG for mitigating the antibody response to repeatedly administrated therapeutic proteins, though whether similar benefits apply to more immunogenic foreign proteins requires further validation. Moreover, our results suggested that the helical conformation of the synthetic nonfouling polypeptides played an important role in minimizing/delaying this antibody response. Taken together, the current study highlighted an urgent necessity to systematically reassess the pros and cons of choosing unstructured polymers for protein conjugation. Furthermore, our results also lay the foundation for the development of next-generation biohybrid drugs based on helical synthetic polypeptides.
Acknowledgments
We thank Prof. Demin Zhou for sharing the plasmid encoding the wt-GH, Prof. Wei Wei for the help of BMDC culturing, and Prof. Richard Lerner, Prof. Xing Chen, Prof. Feng Wang for helpful discussion.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.8b00548.
GPC curves, 1H NMR, CD spectra, trypsin degradation curves, SPR binding curves, Ki-67 stained images of tumor, relative body weight of mice, antibody titer curves, AUC0–24h of GH-polymer conjugates at first and third dose (PDF)
This work was financially supported by National Key Research and Development Program of China (2016YFA0201400). We acknowledge grants from National Natural Science Foundation of China (21474004 and 21722401). H.L. acknowledges the startup funding from Youth Thousand-Talents Program of China.
The authors declare no competing financial interest.
Supplementary Material
References
- Kontos S.; Hubbell J. A. Drug development: longer-lived proteins. Chem. Soc. Rev. 2012, 41 (7), 2686–2695. 10.1039/c2cs15289d. [DOI] [PubMed] [Google Scholar]
- Zelikin A. N.; Ehrhardt C.; Healy A. M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8 (11), 997–1007. 10.1038/nchem.2629. [DOI] [PubMed] [Google Scholar]
- Liu T.; Du J. J.; Luo X. Z.; Schultz P. G.; Wang F. Homogeneously modified immunoglobulin domains for therapeutic application. Curr. Opin. Chem. Biol. 2015, 28, 66–74. 10.1016/j.cbpa.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Krishna M.; Nadler S. G. Immunogenicity to biotherapeutics – the role of anti-drug immune Complexes. Front. Immunol. 2016, 7, 21. 10.3389/fimmu.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegri-O’Day E. M.; Lin E. W.; Maynard H. D. Therapeutic protein-polymer conjugates: advancing beyond PEGylation. J. Am. Chem. Soc. 2014, 136 (41), 14323–14332. 10.1021/ja504390x. [DOI] [PubMed] [Google Scholar]
- Cobo I.; Li M.; Sumerlin B. S.; Perrier S. Smart hybrid materials by conjugation of responsive polymers to biomacromolecules. Nat. Mater. 2015, 14 (2), 143–159. 10.1038/nmat4106. [DOI] [PubMed] [Google Scholar]
- White C. J.; Bode J. W. PEGylation and dimerization of expressed proteins under near equimolar conditions with potassium 2-pyridyl acyltrifluoroborates. ACS Cent. Sci. 2018, 4 (2), 197–206. 10.1021/acscentsci.7b00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P.; Nicolas J.; Haddleton D. M.. Polymer–protein/peptide bioconjugates. In Chemistry of Organo-Hybrids; John Wiley & Sons, Inc.: Hoboken, NJ, 2014; p 466. [Google Scholar]
- Dumas A.; Spicer C. D.; Gao Z.; Takehana T.; Lin Y. A.; Yasukohchi T.; Davis B. G. Self-liganded Suzuki-Miyaura coupling for site-selective protein PEGylation. Angew. Chem., Int. Ed. 2013, 52 (14), 3916–3921. 10.1002/anie.201208626. [DOI] [PubMed] [Google Scholar]
- Pasut G.; Veronese F. M. State of the art in PEGylation: the great versatility achieved after forty years of research. J. Controlled Release 2012, 161 (2), 461–472. 10.1016/j.jconrel.2011.10.037. [DOI] [PubMed] [Google Scholar]
- Fishburn C. S. The pharmacology of PEGylation: Balancing PD with PK to generate novel therapeutics. J. Pharm. Sci. 2008, 97 (10), 4167–4183. 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- Gauthier M. A.; Klok H. A. Polymer-protein conjugates: an enzymatic activity perspective. Polym. Chem. 2010, 1 (9), 1352–1373. 10.1039/c0py90001j. [DOI] [Google Scholar]
- Zhang P.; Sun F.; Liu S. J.; Jiang S. Y. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Controlled Release 2016, 244, 184–193. 10.1016/j.jconrel.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop K.; Hoogenboom R.; Fischer D.; Schubert U. S. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem., Int. Ed. 2010, 49 (36), 6288–6308. 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- Liu S. J.; Jiang S. Y. Chemical conjugation of zwitterionic polymers protects immunogenic enzyme and preserves bioactivity without polymer-specific antibody response. Nano Today 2016, 11 (3), 285–291. 10.1016/j.nantod.2016.05.006. [DOI] [Google Scholar]
- Keefe A. J.; Jiang S. Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nat. Chem. 2012, 4 (1), 59–63. 10.1038/nchem.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey H.; Haag R. Dendritic polyglycerol: a new versatile biocompatible-material. Rev. Mol. Biotechnol. 2002, 90 (3–4), 257–267. 10.1016/S1389-0352(01)00063-0. [DOI] [PubMed] [Google Scholar]
- Mancini R. J.; Lee J.; Maynard H. D. Trehalose glycopolymers for stabilization of protein conjugates to environmental stressors. J. Am. Chem. Soc. 2012, 134 (20), 8474–8479. 10.1021/ja2120234. [DOI] [PubMed] [Google Scholar]
- Liu M.; Johansen P.; Zabel F.; Leroux J. C.; Gauthier M. A. Semi-permeable coatings fabricated from comb-polymers efficiently protect proteins in vivo. Nat. Commun. 2014, 5, 5526. 10.1038/ncomms6526. [DOI] [PubMed] [Google Scholar]
- Gao W. P.; Liu W. G.; Mackay J. A.; Zalutsky M. R.; Toone E. J.; Chilkoti A. In situ growth of a stoichiometric PEG-like conjugate at a protein’s N-terminus with significantly improved pharmacokinetics. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (36), 15231–15236. 10.1073/pnas.0904378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming T. J. Synthesis of side-chain modified polypeptides. Chem. Rev. 2016, 116 (3), 786–808. 10.1021/acs.chemrev.5b00292. [DOI] [PubMed] [Google Scholar]
- Talelli M.; Vicent M. J. Reduction sensitive poly(l-glutamic acid) (PGA)-protein conjugates designed for polymer masked-unmasked protein therapy. Biomacromolecules 2014, 15 (11), 4168–4177. 10.1021/bm5011883. [DOI] [PubMed] [Google Scholar]
- Lu Y. J.; Mbong G. N. N.; Liu P.; Chan C.; Cai Z. L.; Weinrich D.; Boyle A. J.; Reilly R. M.; Winnik M. A. Synthesis of polyglutamide-based metal-chelating polymers and their site-specific conjugation to trastuzumab for auger electron radioimmunotherapy. Biomacromolecules 2014, 15 (6), 2027–2037. 10.1021/bm500174p. [DOI] [PubMed] [Google Scholar]
- Song Z.; Han Z.; Lv S.; Chen C.; Chen L.; Yin L.; Cheng J. Synthetic polypeptides: from polymer design to supramolecular assembly and biomedical application. Chem. Soc. Rev. 2017, 46 (21), 6570–6599. 10.1039/C7CS00460E. [DOI] [PubMed] [Google Scholar]
- Kramer J. R.; Onoa B.; Bustamante C.; Bertozzi C. R. Chemically tunable mucin chimeras assembled on living cells. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (41), 12574–12579. 10.1073/pnas.1516127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberger V.; Wang C. W.; Geething N. C.; Spink B. J.; Campbell A.; To W.; Scholle M. D.; Yin Y.; Yao Y.; Bogin O.; Cleland J. L.; Silverman J.; Stemmer W. P. C. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nat. Biotechnol. 2009, 27 (12), 1186–1155. 10.1038/nbt.1588. [DOI] [PubMed] [Google Scholar]
- Hu J.; Wang G. L.; Liu X. Y.; Gao W. P. Enhancing pharmacokinetics, tumor accumulation, and antitumor efficacy by elastin-like polypeptide fusion of interferon alpha. Adv. Mater. 2015, 27 (45), 7320–7324. 10.1002/adma.201503440. [DOI] [PubMed] [Google Scholar]
- Luginbuhl K. M.; Schaal J. L.; Umstead B.; Mastria E. M.; Li X.; Banskota S.; Arnold S.; Feinglos M.; D’Alessio D.; Chilkoti A. One-week glucose control via zero-order release kinetics from an injectable depot of glucagon-like peptide-1 fused to a thermosensitive biopolymer. Nat. Biomed. Engin. 2017, 1, 0078. 10.1038/s41551-017-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G. K.; Glassman M. J.; Lam C. N.; Chang D.; Schaible E.; Hexemer A.; Olsen B. D. Topological effects on globular protein-ELP fusion block copolymer self-assembly. Adv. Funct. Mater. 2015, 25 (5), 729–738. 10.1002/adfm.201403453. [DOI] [Google Scholar]
- Petitdemange R.; Garanger E.; Bataille L.; Dieryck W.; Bathany K.; Garbay B.; Deming T. J.; Lecommandoux S. Selective tuning of elastin-like polypeptide properties via methionine/oxidation. Biomacromolecules 2017, 18 (2), 544–550. 10.1021/acs.biomac.6b01696. [DOI] [PubMed] [Google Scholar]
- Gomes S.; Leonor I. B.; Mano J. F.; Reis R. L.; Kaplan D. L. Natural and genetically engineered proteins for tissue engineering. Prog. Polym. Sci. 2012, 37 (1), 1–17. 10.1016/j.progpolymsci.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlapschy M.; Binder U.; Borger C.; Theobald I.; Wachinger K.; Kisling S.; Haller D.; Skerra A. PASylation: a biological alternative to PEGylation for extending the plasma half-life of pharmaceutically active proteins. Protein Eng., Des. Sel. 2013, 26 (8), 489–501. 10.1093/protein/gzt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.; Zhou Y.; Wang H.; Wang R.; Yuan J.; Hu Y.; Sheng K.; Feng J.; Yang S.; Lu H. Macrocyclization of interferon-poly(alpha-amino acid) conjugates significantly improves the tumor retention, penetration, and antitumor efficacy. J. Am. Chem. Soc. 2018, 140 (3), 1170–1178. 10.1021/jacs.7b13017. [DOI] [PubMed] [Google Scholar]
- Yuan J.; Sun Y.; Wang J.; Lu H. Phenyl trimethylsilyl sulfide-mediated controlled ring-opening polymerization of alpha-amino acid N-carboxyanhydrides. Biomacromolecules 2016, 17 (3), 891–896. 10.1021/acs.biomac.5b01588. [DOI] [PubMed] [Google Scholar]
- Hou Y.; Yuan J.; Zhou Y.; Yu J.; Lu H. A Concise approach to site-specific topological protein-poly(amino acid) conjugates enabled by in-situ generated functionalities. J. Am. Chem. Soc. 2016, 138 (34), 10995–11000. 10.1021/jacs.6b05413. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Jain P.; Tsao C.; Yuan Z.; Li W.; Li B.; Wu K.; Hung H. C.; Lin X.; Jiang S. Polypeptides with high zwitterion density for safe and effective therapeutics. Angew. Chem., Int. Ed. 2018, 57 (26), 7743–7747. 10.1002/anie.201802452. [DOI] [PubMed] [Google Scholar]
- Xiong M.; Lee M. W.; Mansbach R. A.; Song Z.; Bao Y.; Peek R. M.; Yao C.; Chen L. F.; Ferguson A. L.; Wong G. C. L.; Cheng J. J. Helical antimicrobial polypeptides with radial amphiphilicity. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (43), 13155–13160. 10.1073/pnas.1507893112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.; Wang J.; Bai Y.; Lang J. W.; Liu S.; Lin Y.; Cheng J. Ionic polypeptides with unusual helical stability. Nat. Commun. 2011, 2, 206. 10.1038/ncomms1209. [DOI] [PubMed] [Google Scholar]
- Chen C. Y.; Wang Z. H.; Li Z. B. Thermoresponsive polypeptides from pegylated poly-L-glutamates. Biomacromolecules 2011, 12 (8), 2859–2863. 10.1021/bm200849m. [DOI] [PubMed] [Google Scholar]
- Popp M. W.; Dougan S. K.; Chuang T. Y.; Spooner E.; Ploegh H. L. Sortase-catalyzed transformations that improve the properties of cytokines. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (8), 3169–3174. 10.1073/pnas.1016863108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Chen J. X.; Wu Y. M.; Zhang B.; Cai X. C.; Zhang Z. W.; Wang Y.; Si L. L.; Xu H.; Zheng Y. X.; Zhang C. L.; Liang C. G.; Li J.; Zhang L.; Zhang Q.; Zhou D. M. Precise and combinatorial PEGylation generates a low-immunogenic and stable form of human growth hormone. J. Controlled Release 2017, 249, 84–93. 10.1016/j.jconrel.2017.01.029. [DOI] [PubMed] [Google Scholar]
- Cho H.; Daniel T.; Buechler Y. J.; Litzinger D. C.; Maio Z.; Putnam A. M.; Kraynov V. S.; Sim B. C.; Bussell S.; Javahishvili T.; Kaphle S.; Viramontes G.; Ong M.; Chu S.; Becky G. C.; Lieu R.; Knudsen N.; Castiglioni P.; Norman T. C.; Axelrod D. W.; Hoffman A. R.; Schultz P. G.; DiMarchi R. D.; Kimmel B. E. Optimized clinical performance of growth hormone with an expanded genetic code. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (22), 9060–9065. 10.1073/pnas.1100387108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Y.; Wei W.; Yue H.; Ni D. Z.; Yue Z. G.; Wang S.; Fu Q.; Wang Y. Q.; Ma G. H.; Su Z. G. Nanoparticles-based multi-adjuvant whole cell tumor vaccine for cancer immunotherapy. Biomaterials 2013, 34 (33), 8291–8300. 10.1016/j.biomaterials.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Chang Y. S.; Graves B.; Guerlavais V.; Tovar C.; Packman K.; To K. H.; Olson K. A.; Kesavan K.; Gangurde P.; Mukherjee A.; Baker T.; Darlak K.; Elkin C.; Filipovic Z.; Qureshi F. Z.; Cai H. L.; Berry P.; Feyfant E.; Shi X. G. E.; Horstick J.; Annis D. A.; Manning A. M.; Fotouhi N.; Nash H.; Vassilev L. T.; Sawyer T. K. Stapled alpha-helical peptide drug development: a potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (36), E3445–E3454. 10.1073/pnas.1303002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. E.; Cornejo M.; Davis T. N.; Del Bianco C.; Aster J. C.; Blacklow S. C.; Kung A. L.; Gilliland D. G.; Verdine G. L.; Bradner J. E. Direct inhibition of the NOTCH transcription factor complex. Nature 2009, 462 (7270), 182–188. 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky L. D.; Bird G. H. Hydrocarbon-stapled peptides: principles, practice, and progress. J. Med. Chem. 2014, 57 (15), 6275–6288. 10.1021/jm4011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida Y.; Cabral H.; Miura Y.; Albertini F.; Fukushima S.; Osada K.; Nishiyama N.; Kataoka K. Bundled assembly of helical nanostructures in polymeric micelles loaded with platinum drugs enhancing therapeutic efficiency against pancreatic tumor. ACS Nano 2014, 8 (7), 6724–6738. 10.1021/nn500498t. [DOI] [PubMed] [Google Scholar]
- Kierstead P. H.; Okochi H.; Venditto V. J.; Chuong T. C.; Kivimae S.; Frechet J. M. J.; Szoka F. C. The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes. J. Controlled Release 2015, 213, 1–9. 10.1016/j.jconrel.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eijk A. A.; Vrolijk J. M.; Haagmans B. L. Antibodies neutralizing peginterferon alfa during retreatment of hepatitis C. N. Engl. J. Med. 2006, 354 (12), 1323–1324. 10.1056/NEJMc052880. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Yuan J.; Lu J.; Hou Y.; Xiong W.; Lu H. From neutral to zwitterionic poly(alpha-amino acid) nonfouling surfaces: Effects of helical conformation and anchoring orientation. Biomaterials 2018, 178, 728–737. 10.1016/j.biomaterials.2018.01.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.