Abstract
Up to ∼20% of HIV-infected individuals eventually develop broadly neutralizing antibodies (bnAbs), and many of these antibodies (∼40%) target a region of dense high-mannose glycosylation on gp120 of the HIV envelope protein, known as the “high-mannose patch” (HMP). Thus, there have been numerous attempts to develop glycoconjugate vaccine immunogens that structurally mimic the HMP and might elicit bnAbs targeting this conserved neutralization epitope. Herein, we report on the immunogenicity of glycopeptides, designed by in vitro selection, that bind tightly to anti-HMP antibody 2G12. By analyzing the fine carbohydrate specificity of rabbit antibodies elicited by these immunogens, we found that they differ from some natural human bnAbs, such as 2G12 and PGT128, in that they bind primarily to the core structures within the glycan, rather than to the Manα1 → 2Man termini (2G12) or to the whole glycan (PGT128). Antibody specificity for the glycan core may result from extensive serum mannosidase trimming of the immunogen in the vaccinated animals. This finding has broad implications for vaccine design aiming to target glycan-dependent HIV neutralizing antibodies.
Short abstract
Vaccines that elicit antibodies against oligomannose carbohydrates may protect against HIV, but carbohydrate trimming in serum is a critical factor that may have thwarted successful vaccine attempts.
Introduction
Despite decades of effort, no HIV vaccine candidates tested so far elicit substantial breadth of protection against the diverse viral strains in circulation.1 However, over the last ∼20 years, a vast amount of data has accumulated about broadly neutralizing antibodies (bnAbs), which are found in up to 20% of infected individuals.2 These antibodies neutralize diverse strains of HIV, are often protective in animal models of infection, and provide clues for vaccine design.
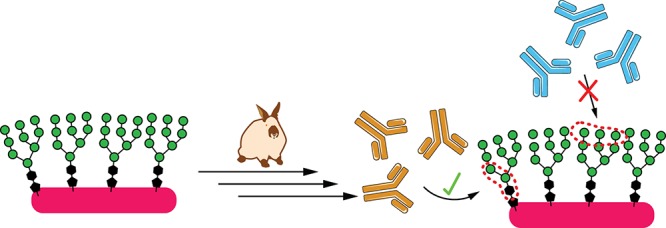
Structural studies of bnAbs in complex with the HIV envelope (Env) glycoproteins gp120 and gp41 reveal which epitopes can be targeted by antibodies to achieve broad neutralization. This information can then be used for “epitope-focused” vaccine design,3−11 in which whole or truncated Env, or even glycopeptide fragments thereof,12−22 are engineered to maximize presentation of the epitope, while minimizing or excluding “distracting” epitopes that may lead to development of non-neutralizing or strain-specific antibodies (Figure 1a).
Figure 1.
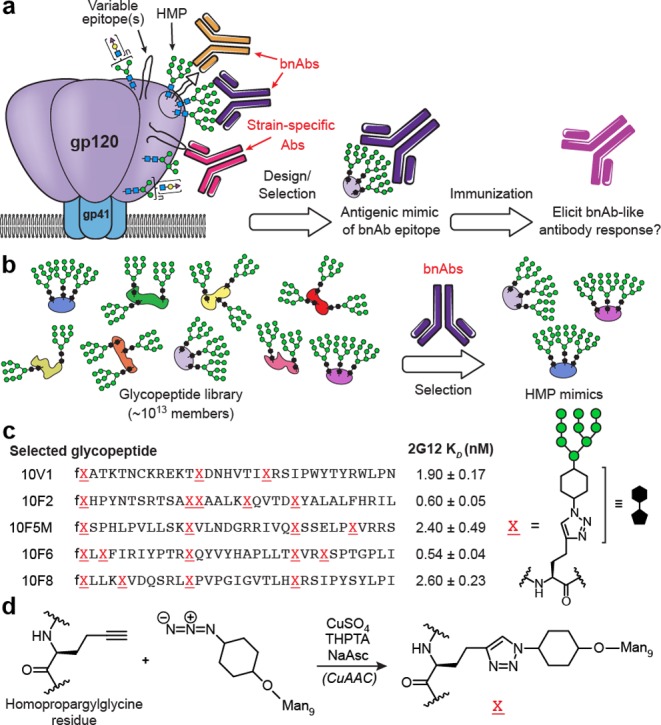
Selection-based design of 2G12-targeted HMP mimetic glycopeptides used in this study. (a) “Epitope-focused” vaccine design: many broadly neutralizing antibodies (bnAbs) bind to particular configurations of glycans in the “high-mannose patch” (HMP) on HIV gp120, usually in combination with conserved polypeptide residues (shown as a triangle). (b) In previous work,44,47 we used our laboratory’s glycopeptide mRNA display technique to evolve carbohydrate cluster HIV antigens. Libraries of ∼1013 peptide backbones were tagged with their encoding mRNAs and glycosylated with Man9 using alkyne/azide “click” chemistry.49 HMP-binding bnAb 2G12 was then used as an affinity reagent to select HMP epitope mimics from the libraries. (c) Sequences of selected glycopeptide immunogens tested in this study. KD values were previously reported,44,47 for glycopeptides appended with a GSGSLGHHHHHHRDYKDDDDK C-terminal tag. Synthetic glycopeptides used in the present study all contain a GSGSGCA C-terminal tag, in which cysteine is either StBu disulfide-protected (for crystallographic studies), linked to biotin (for BLI), or conjugated to carrier. (d) “Click” chemistry attachment of oligomannose-cyclohexyl-azide to homopropargylglycine residues of peptides, and structure of resulting linkage.
In particular, the dense array of glycans on HIV Env has been of great interest in vaccine design23 because most bnAbs whose structures have been determined bind to epitopes containing glycans,24 and around 60% of elite neutralizers’ sera exhibit glycan-dependent neutralization.25 Although the glycans serve to block much of the polypeptide surface from antibody recognition,5 they frequently become a target of recognition themselves.24,26−35 In a study of ∼60 patients with broad neutralizing sera, over half exhibited glycan-dependent neutralization, with 38% targeting the “high-mannose patch” (HMP) on Env protein gp120.25 The HMP is a cluster of high-mannose glycans centered on the N332 residue36−38 and recognized by bnAbs such as the PGT121 and PGT128 families,28,39 as well as 2G12.27 2G12 was among the first HIV bnAbs to be discovered,40 and contains an unusual domain-swapped dimer of Fabs that recognizes a solely carbohydrate epitope on the HMP. Our laboratory has created directed evolution-based techniques for design of glycan clusters that may mimic such epitopes (Figure 1b), enabling us to discover structures with high antigenicity (low nM KD) for 2G12.41−46 Herein, we report structural studies and immunogenicity of these synthetically glycosylated peptides in rabbits.
Results
Antigen Design by in Vitro Selection
The in vitro selection and synthesis of our highly antigenic 2G12-binding glycopeptides have been described in detail previously.44,47 In summary, we generated random libraries of ∼1013 Man9-decorated glycopeptides, covalently fused to their encoding mRNAs.48 The library fraction that bound to 2G12 was isolated, then amplified by PCR. The PCR product was then used to produce a new library, and this process was repeated for 10 cycles, yielding tight 2G12 binders (low nM KD). Importantly, the attachment of glycans to such highly diverse libraries was facilitated by the use of copper-assisted alkyne–azide cycloaddition (CuAAC) “click” chemistry.49 Thus, Man9 units bearing an azidocyclohexyl linker were attached to the alkyne side chains of homopropargylglycine (HPG) residues in the peptides.50 The resulting glycan–peptide linkages differ from the GlcNAc2–Asn linkages present in natural N-linked glycosylation; however, despite the unnatural core, these glycopeptide libraries provided highly antigenic binders for 2G12, which binds to the Man1α–2Man nonreducing termini, and not the core, of glycans.27
Pilot Immunogenicity Study
To recruit T cell help that would result in high-affinity class-switched IgG responses to our carbohydrate immunogens, we conjugated our glycopeptides to the carrier protein CRM197.51 This carrier is a nontoxic mutant of diphtheria toxin (DT) and is used in commercialized polysaccharide vaccines to elicit T-cell-dependent antibody responses against bacterial pathogens such as Haemophilus influenzae type b (“Hib”).52 We opted to use Adjuplex adjuvant, which has been tested in rabbit immunizations with other glycosylated immunogens.8 To verify that high antigen-specific titers could be obtained using this carrier/adjuvant combination and to determine the optimum dose, we conducted a pilot study in which small groups of rabbits (n = 3) were given doses of conjugate containing 10, 50, or 100 μg of glycopeptide.
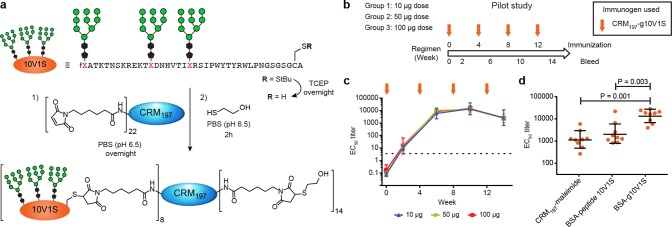
For the pilot study, we chose 10V1, a glycopeptide clone that was selected from our libraries and binds to 2G12 IgG with a KD of 1.9 ± 0.2 nM (Figure 1c).44 We mutated the unpaired cysteine within the 10V1 sequence to serine and introduced a C-terminal linker containing a cysteine to be used in conjugation to maleimide-functionalized CRM197. This “10V1S” mutant exhibited a 2G12 KD that was similar to 10V1 (SI, Table S1), and conjugation to the carrier protein proceeded well using methods that we have reported previously (Figure 2a).47
Figure 2.
Pilot immunogenicity study and time course of serum IgG response to glycopeptide 10V1S. (a) Conjugation of glycopeptide 10V1S to maleimide-activated CRM197. (b) Groups of New Zealand rabbits (n = 3) each were immunized with CRM197–glycopeptide 10V1S conjugate in 10, 50, or 100 μg doses with Adjuplex adjuvant. (c) Graph shows time course of EC50 ELISA IgG titers binding to glycopeptide 10V1S conjugated to BSA. Arrows indicate immunization time points, and the horizontal dotted line indicates the lowest serum dilution tested. (d) Comparison of dose 3 (week 10) serum IgG ELISA against three coating antigens: CRM197+linker, peptide 10V1S–BSA, and glycopeptide 10V1S–BSA. Data from low-, medium-, and high-dose groups were combined for analysis and presented with geometric mean and geometric standard deviation. Statistical significance was determined by one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons.
Immunization of New Zealand white rabbits at 4 week intervals resulted in IgG ELISA EC50 titers for glycopeptide immunogen that reached a maximum of ∼20 000 after three doses (Figure 2c). Titers were measured against glycopeptide conjugated to BSA via a linker different from that in the immunogen (SI, Figure S1) to detect antibodies specific for glycopeptide and not CRM197 or linker. Glycopeptide-specific titers were consistently in the ∼104 range, and no difference was observed between groups receiving 10, 50, or 100 μg doses. Importantly, titers against glycopeptide were significantly higher than those against the unglycosylated peptide or the carrier protein itself (Figure 2d), giving us reason to proceed with this carrier/adjuvant combination in more detailed studies.
Structural Studies of Glycopeptide–2G12 Binding Interactions
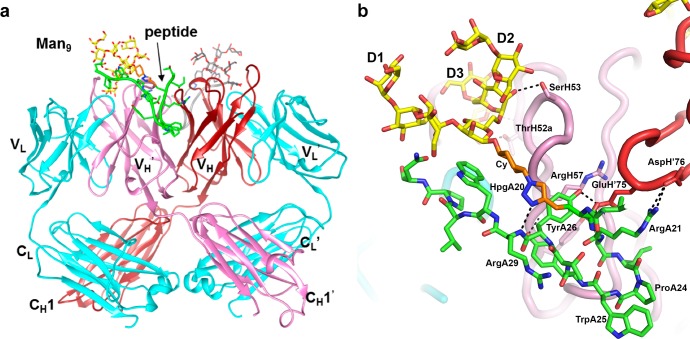
Having verified that glycopeptide 10V1S–CRM197 conjugates are immunogenic and elicit high glycopeptide-specific titers, we next wished to design a larger study to evaluate and compare several different glycopeptide immunogens derived from our in vitro selection experiments.44 Those selections had yielded 2G12-binding glycopeptides of which many, including 10V1, contained a family of related peptide motifs, SIPxYTY (x = variable amino acid). During the design of this larger study, a crystal structure was determined for the 10V1S glycopeptide (40 amino acid residues with three Man9 glycans covalently attached by a linker as shown in Figure 1c,d) in complex with the 2G12 domain-swapped dimer (Fab)2,27 revealing a 2:1 stoichiometry with one glycopeptide occupying each side of the Fab dimer (Figure 3a). Ordered electron density was observed for 16 peptide residues (18–33; TIXRSIPWYTYRWLPN, where X is the click-glycosylated homopropargylglycine residue), and one Man9. For each glycopeptide, the D3 arm of Man9 binds in the primary glycan binding pocket of the Fab, whereas the 21RSIPWYTYRW30 sequence of the peptide forms a hairpin structure that occupies the secondary glycan binding pocket27,53 at the Fab dimer interface. 2G12 recognition of the D3 arm in glycopeptide–Man9 contrasts with recognition of free Man9GlcNAc2, where a cocrystal structure shows the D1 arm in the primary binding pocket (PDB 1OP5). However, an analogous structure with free Man8 (PDB 6MNF) shows a D3 arm in one primary binding pocket and a D1 arm in the other, illustrating the flexibility of 2G12 to bind either arm of the glycan. The D3 arm of 10V1S binds to this pocket through contacts that are essentially identical to those observed in 6MNF (see the SI, Figure S14). In the peptide moiety of 10V1S, Pro24 and Trp25 are the i + 1 and i + 2 residues of a type VIII reverse turn at the tip of the hairpin, with Pro24 adopting a cis-peptide conformation. Two hydrogen bonds are formed within the peptide hairpin, one between Arg21 O and Thr27 NH, and the other across the hairpin from the backbone NH of Arg29 to the triazole moiety of residue 20. The hydrophobic indole of the glycopeptide Trp30 makes contact with the hydrophobic α face of the reducing-terminal core mannose (Figure 3b). The Fab–glycopeptide interface is extensive, with 854 and 884 Å2 buried on the glycopeptide and Fab, respectively (SI, Tables S2 and S3), and 60% of the glycopeptide contribution is from the peptide residues. Although all of the mannose moieties of the bound glycan are visible in the electron density, the additional two Man9 glycans and 24 peptide residues are not observed, and likely do not participate in the interaction with 2G12.
Figure 3.
The 10V1S-2G12 crystal structure and binding interaction. (a) Overall view of domain-swapped (Fab)2 2G12 in complex with glycopeptide 10V1S. Two glycopeptides bind per Fab dimer. There is no symmetry-related glycan binding at the VH–VH′ interface, although the peptide component binds at this interface. 2G12 and peptide are shown as ribbons, and the glycan portion of the glycopeptide in a stick representation. The two light chains are shown in cyan, and two heavy chains in red and pink. One glycopeptide is colored yellow for the glycan, green for the peptide portion and orange for the glycan linkers, whereas the other glycopeptide is colored gray. (b) Close-up view of one of the 10V1S glycopeptides bound to (Fab)2 2G12. The glycopeptide is shown as sticks, with hydrogen bonds shown as dotted lines.
In contrast with 10V1S, the structure of glycopeptide 10F5M (40 amino acids with 4 Man9 glycans (Figure 1c) lacking the SIPWYTY motif of 10V1) in complex with 2G12 revealed a multivalent glycan interaction, with Man9 glycans bound to the two canonical and two noncanonical VH–VH′ interface glycan binding sites in the Fab dimer (Figure 4). Although the structure is of low to moderate resolution (3.6 Å), with no visible electron density for the peptide, there is clear density for all of the mannose residues, and the glycan–antibody interactions are identical to those observed for the Man9GlcNAc2/2G12 complex.27,53 Incubation of 10F5M in crystallization buffer resulted in no detectable cleavage of glycans from the peptide (SI, Figure S15), suggesting that the peptide is present but disordered in the crystal. There is also a large void above the combining site in the crystal that could presumably accommodate the disordered peptide, or alternatively, more than one peptide bridging multiple 2G12 molecules.
Figure 4.
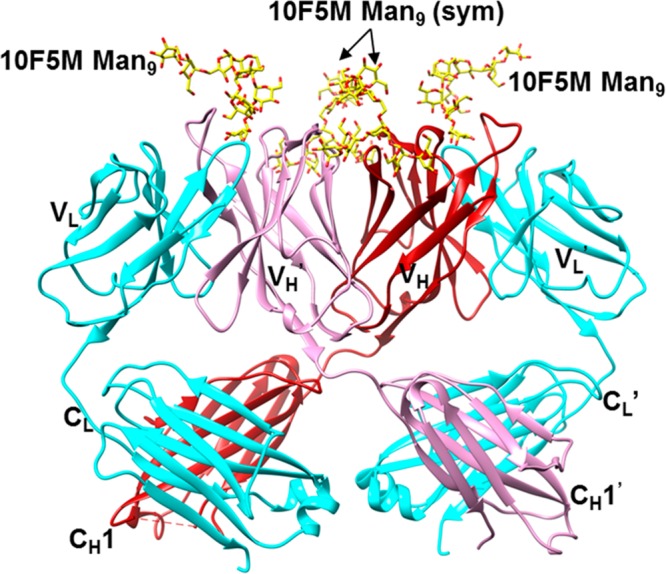
Overall view of crystal structure of (Fab)2 2G12 in complex with glycopeptide 10F5M. Ordered electron density was only visible for the glycan portion of the glycopeptide. Coloring is as in Figure 3. Similar to the previously published structure of 2G12 in complex with Man9GlcNAc2 (PDB 1OP5), there is one Man9 binding at each canonical Fab binding site, and two Man9 moieties (“sym”) binding at the VH–VH′ interface, that bridge to symmetry-related 2G12 molecules in the crystal.
Multi-Immunogen Study
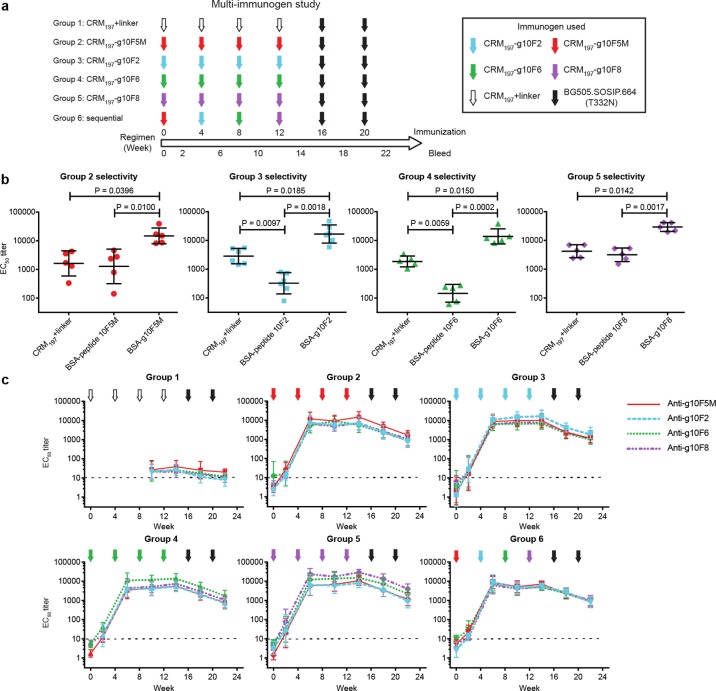
Because glycopeptides containing the SIPxYTY sequence bind 2G12 via both glycan and peptide contacts, distinct from the glycan-only contacts with gp120,27,54 we opted to conduct subsequent immunogenicity studies primarily on glycopeptides lacking that sequence (10F5M, 10F2, 10F6), with just one glycopeptide containing that sequence for comparison (10F8). In this experiment, rabbits in each of four groups received 50 μg doses of one glycopeptide (conjugated to CRM197) at weeks 0, 4, 8, and 12. A control group received linker-functionalized CRM197 at the same time points, and a sixth group received a sequence of all four glycopeptides (Figure 5a). For the sixth group, we reasoned that sequential immunization with four glycopeptides that share similar antigenic presentations of glycans on differing scaffolds might focus the antibody response on the glycans, without boosting antibodies that bind to any of the peptide scaffold structures.
Figure 5.
Immunogenicity of four glycopeptide conjugates. (a) Immunization regimen. Groups of rabbits (n = 6) were immunized with linker-functionalized carrier or glycopeptide conjugates. Groups 2–5 repeatedly received 50 μg of glycopeptides 10F5M, 10F2, 10F6, and 10F8 (conjugated to CRM197 carrier), respectively, at weeks 0, 4, 8, and 12. Group 1 received CRM197+linker, and Group 6 received all four glycopeptide conjugates sequentially. All groups received two “boost” immunizations with native-like Env trimer BG505.SOSIP.664 (T332N) at weeks 16 and 20. Sera were collected 2 weeks after each immunization. (b) Comparison of dose 4 serum IgG ELISA EC50 titers against CRM197+linker vs peptide–BSA vs glycopeptide–BSA. Statistical significance was determined by matched one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. (c) Time course of rabbit serum IgG binding to autologous and heterologous glycopeptides (geometric mean ELISA EC50 titers). Arrow colors indicate the glycopeptide used for immunization, while the color of each line represents the glycopeptide–BSA. The horizontal dotted line represents the lowest serum dilution tested.
Consistent with the pilot study, all glycopeptide-immunized groups produced autologous glycopeptide-reactive IgG antibodies with EC50 titers in the range ∼10 000–20 000 following the third immunization (Figure 5b). Dose 4 sera from all glycopeptide-immunized groups exhibited 10–100-fold selectivity in mean serum reactivity for glycopeptide over peptide alone, and most exhibited 6–9-fold selectivity for binding to glycopeptide over CRM197 carrier (Figure 5b); however, the sequentially immunized Group 6 exhibited slightly lower selectivity for glycopeptides vs peptides or CRM197, as glycopeptide-specific titers were slightly lower than in other groups (SI, Figure S2).
For each group immunized repeatedly with a single glycopeptide (Groups 2–5), a high degree of cross-reactivity was evident between glycopeptides: sera from each group bound almost as strongly to any of the three glycopeptides used to immunize the other groups (Figure 5c). As sera of all groups bound 10–100-fold more weakly to peptides without glycans (Figure 5b) and still lower to the BSA and linker in the coating antigens (SI, Figure S1), these data suggest that this cross-reactivity is due to antibodies that bind the glycan, or possibly to a common glycan presentation. While sera were mostly cross-reactive for all glycopeptides, Groups 2, 4, and 5 exhibited modest (∼1.8–3-fold), but statistically significant (p < 0.05; SI, Table S8), serum selectivity for the particular glycopeptide used to immunize that group. As expected, rabbits that were sequentially immunized with all glycopeptides (Group 6) exhibited no apparent difference in titers to the four glycopeptide immunogens.
Boosting with Env SOSIP Trimers
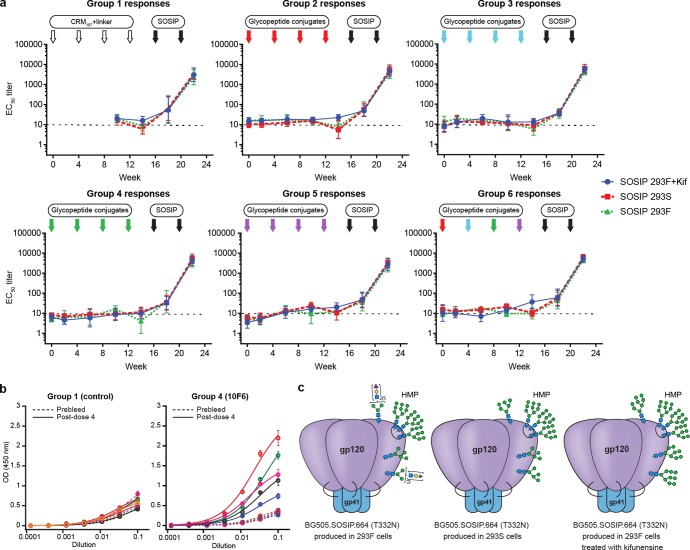
Since the immunogenicity data suggested elicitation of antibodies that bind to oligomannose moieties, we next tested whether these sera would bind to native-like soluble trimeric HIV Env protein (BG505.SOSIP.664 (T332N)), which has been optimized for near-native folding and glycosylation, and is highly decorated with oligomannose.55,56 Env-specific ELISA titers were close to background, when measured at the 12 ng/well coating concentration used for screening all sera (Figure 6a, dose 0–4 time points). However, when a higher 200 ng/well coating concentration of SOSIP trimer was used in the ELISA of dose 4 sera, above-background binding was observed for several animals in Groups 3 and 4 (Figure 6b and SI, Figure S16). Nevertheless, since the glycopeptide-elicited Env-specific titers were modest and sporadic, we next tested whether the Env-binding response could be boosted by Env immunizations. Thus, at weeks 16 and 20, all animals were boosted with 50 μg doses of BG505.SOSIP.664 (T332N) in Adjuplex adjuvant (Figure 5a). Two boosts were sufficient to elicit ELISA EC50 titers of ∼103–104 for autologous Env trimer (Figure 6a, green). Anti-Env titers were similar for Group 1 compared with Groups 2–6, indicating that glycopeptide priming did not influence development of antibodies against the SOSIP immunogen. To test for Env-specific antibodies that bind to oligomannose glycans, we also measured binding to SOSIP trimers grown in the presence of kifunensine (bearing only Man8–9 glycans) or grown in HEK293S cells (GnTI–/– cells producing only Man5–9 glycans) (Figure 6a, blue and red). Unlike monoclonal antibodies PGT128, 2G12, or DH501, which exhibit 10- to 40-fold binding enhancements to hypermannosylated Env variants,57 the vaccinated rabbit sera bound with equal titers to Env variants with all three levels of mannosylation. Moreover, serum binding to Env was not particularly affected by addition of 500 mM mannose competitor, in comparison with control antibodies (SI, Table S9). These data suggest that the serum antibodies primarily responsible for the observed Env titers are not heavily dependent on binding to mannose motifs.
Figure 6.
Env binding of immunized rabbit sera. (a) Time course of rabbit serum IgG binding to three glycosylation variants of soluble trimeric Env BG505.SOSIP.664 (T332N) before and after boost with trimeric Env (measured at 12 ng SOSIP/well coating concentration). Arrows indicate immunization time points; horizontal lines indicate the lowest serum dilution tested. (b) ELISA of post-dose 4 sera binding to 293F SOSIP trimer at higher coating concentration (200 ng/well). Groups 1 (CRM197+linker control) and 4 (10F6) are shown in the figure. Colors represent individual rabbits, with solid lines for postdose 4 sera and dotted lines for prebleed sera. Data for all groups are presented in SI, Figure S16, with individual rabbit identifier numbers. (c) Schematic of glycosylation patterns on Env constructs grown in 293F cells (native, used in immunization), 293S cells (GnTI–/–), and in the presence of kifunensine. Data in (a) and (b) are averages of three replicates.
We also performed a neutralization screen on a small panel of test viruses (SI, Tables S5a,b). Neutralization of the sensitive Tier 1 clade C strain MW965.26 was observed for nearly all rabbits after the second SOSIP boost (dose 6) and weakly detected for two rabbits from Groups 3 and 4 after immunizations with glycopeptide conjugate alone (dose 4). Post-dose 6 neutralization of autologous Tier 2 clade A BG505 (T332N) strain was detected in only a few animals; albeit one rabbit also weakly neutralized heterologous Tier 2 strain JR-FL. More consistent autologous Tier 2 neutralization has previously been detected in rabbits immunized with three doses of the same SOSIP construct, but neutralization in that study was similarly sporadic after the second dose.7 To assay for mannose-dependent neutralization, heterologous Tier 2 JR-FL was tested in comparison with the same strain grown in GnTI–/– cells (in which complex glycans are replaced with Man5GlcNAc2). Whereas only one animal’s serum neutralized WT JR-FL, all sera neutralized GnTI–/– JR-FL, possibly suggesting that mannose- or Man5-binding antibodies are responsible for neutralizing activity; however, enhanced neutralization of GnTI–/– virus was observed with sera from all groups, with and without a glycopeptide prime. Therefore, mannose-binding anti-Env antibodies, if present after dose 6, do not result from the glycopeptide prime. The enhanced neutralization of GnTI–/– virus may be due to increased exposure of protein epitopes when complex glycans are replaced with Man5.58
Fine Carbohydrate Specificity of the Immune Response
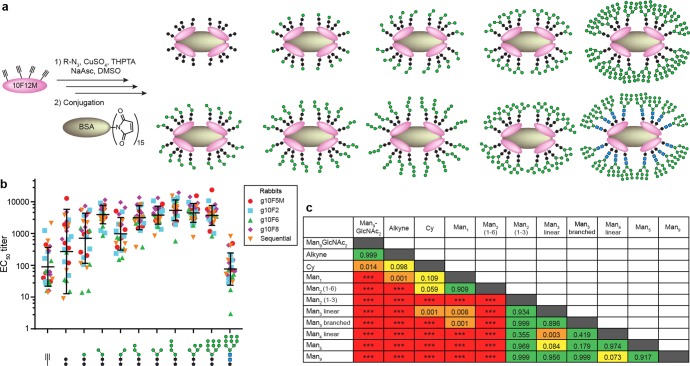
Since our glycopeptide-elicited sera bound to glycans in our glycopeptides, but did not exhibit strong evidence of mannose-dependent Env binding, we performed further experiments to determine the fine carbohydrate specificity. To detect antibodies specific for different parts of the glycan, but not the peptide scaffold, we produced a panel of ELISA coating antigens in which various glycan fragments (Figure 7) were attached to 10F12M (Figure 8a), a peptide backbone that differs from those present in the immunogens. Man1, Man2, and Man3 isomers, Man4, Man5, and Man9, were attached to peptide via the cyclohexyl triazole linker (as in the immunogens used); however, we also tested a Man9GlcNAc2–triazole variant, 10F12M peptide alone, and 10F12M bearing the cyclohexyl triazole linker only. For all glycopeptide-immunized groups, serum reactivity (Figure 8b) to Man9GlcNAc2–triazole peptide was low, about equal to that for 10F12M peptide alone, indicating the absence of antibodies that can bind to the mannose residues alone or to the triazole alone. ELISA against various oligomannose fragments revealed a general trend: in all glycopeptide-immunized groups, most sera bound with statistically indistinguishable titers (Figure 8c, p = 0.4–1, green cells) to all glycans containing two or more mannose residues (Man2(1 → 3), Man3, Man4, Man5, Man9) linked via the cyclohexyl core (Cy) present in the immunogens. An exception was Man2(1 → 6)Cy, which was recognized with an intermediate avidity. In the aggregate of Groups 2–5, binding clearly decreased in the order Man2-Cy > Man1-Cy > HO-Cy (p < 0.0001 and p = 0.011, respectively; SI, Table S6). Among individual groups, this trend was less statistically significant. Taken together, these results suggest that the majority of serum reactivity to the Man9Cy structure in the immunogens results from antibodies focused on its reducing-terminal “core” (Figure 9a); this includes the reducing-terminal mannose residue of the D1 arm, the core mannose, and the cyclohexyl linker. Very little binding was observed to glycopeptides containing Man9GlcNAc2 (natural GlcNAc2 core instead of Cy), and was highly variable toward the Cy linker alone (Figure 8b); thus, the cyclohexyl group is a necessary, but not always a sufficient, binding determinant of the elicited sera.
Figure 7.
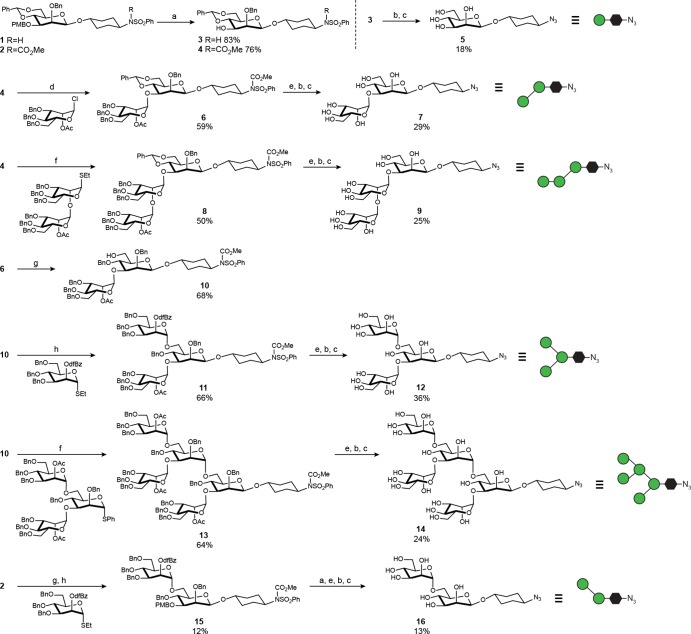
Synthesis of glycan fragments for fine specificity studies. Reagents: (a) DDQ, CH2Cl2/H2O, 0 °C; (b) Na0, THF, –78 °C, NH3(l); (c) TfN3, K2CO3, CuSO4, H2O/MeOH/CH2Cl2, rt; (d) AgOTf, 4 Å MS, di-tert-butylpyridine, toluene, –20 °C; (e) NaOMe/MeOH, rt; (f) Sinaÿ Reagent ((4–Br-C6H4)3N+SbF6−), 4 Å MS, MeCN, 0 °C; (g) Et3SiH, PhBCl2, –78 °C; and (h) 4 Å MS, CH2Cl2, AgOTf, N-iodosuccinimide, –20 °C.
Figure 8.
Glycan microspecificity of glycopeptide-elicited sera. (a) “Click” attachment of glycans to 10F12M peptide and conjugation to BSA. 10F12M (XSYVTVIPAXNXPEARLGIVSHXPGIRRGKALYGSGSGC(StBu)A, X = Man9-HPG, see Figure 1) is a derivative of evolved 2G12-binding glycopeptide 10F12, containing a pair of C → S mutations (in bold) that weaken 2G12 binding from 0.77 nM44 to >20 nM KD (SI, Figure S17). (b) ELISA EC50 IgG titers of dose 4 sera against different glycans clustered on 10F12M. Bars represent geometric means and geometric standard deviations. Data from all glycopeptide-immunized groups (2–5) are pooled for analysis. (c) Differences in serum binding to various glycans, represented by p values of pairwise comparisons of the data shown in part b. p values are determined by matched one-way ANOVA, followed by Tukey’s post-hoc test for multiple comparisons. *** denotes p < 0.0001. Green cells represent pairs of glycan antigens to which serum binding is statistically indistinguishable, whereas red cells represent glycan antigen pairs to which serum binding is well differentiated (p < 0.0001). Intermediate colors represent intermediate p values (orange, p = 0.001–0.05; yellow, p = 0.05–0.15).
Figure 9.
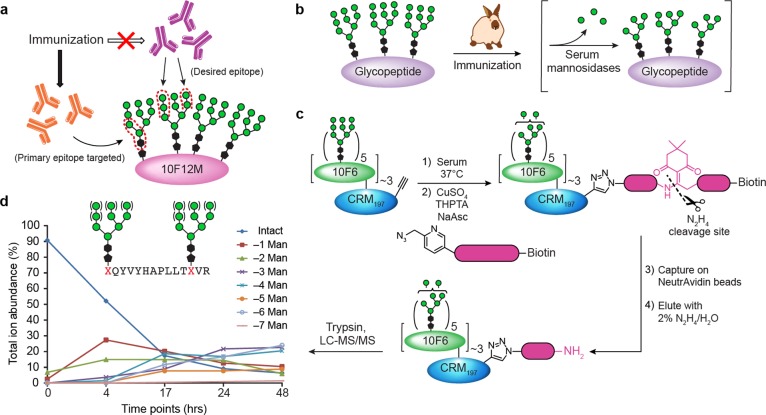
Serum mannosidase activity may account for preferential targeting of glycan core by elicited antibodies. (a) Depiction of glycan residues preferentially targeted by the glycopeptide-elicited response. Antibodies elicited in this study bind preferentially to the cyclohexyl and core mannose residues of the glycopeptide immunogens, but not to the Manα1 → 2Man termini. (b) Hypothesis that serum mannosidase trimming of Manα1 → 2Man in vivo leads to lack of antibodies elicited against Manα1 → 2Man. Similar trimming of Man9GlcNAc2 on kif-Rituximab has been measured in vivo.69 (c, d) Experiment to assess mannosidase trimming of 10F6 glycopeptide conjugate in serum. An alkyne-tagged version of the 10F6–CRM197 glycoconjugate was incubated in serum, and aliquots were “quenched” by addition of mannosidase inhibitors kifunensine and swainsonine. Conjugate was retrieved from samples by “click” biotinylation, affinity capture on NeutrAvidin beads, and elution by dilute hydrazine cleavage of the linker. Trypsin digestion followed by nanoUPLC-MS and MS/MS enabled identification and relative quantification of glycoforms of 10F6 tryptic glycopeptides, showing cleavage of ca. 3 mannose residues per glycan after 2 days. Similar data for a different tryptic glycopeptide can be found in the SI, Figure S18 and Table S10.
Discussion
This study has demonstrated that Man9-functionalized glycopeptide–CRM197 conjugates elicit robust antibody titers against the attached glycans. However, antibody binding is focused on the reducing-terminal residues of the oligomannose structure, including the hydrophobic linker present in the immunogen, rather than the Manα1 → 2Man termini of the glycans. This finding has implications for HIV carbohydrate vaccine studies because prototypical HMP-targeting antibodies (2G12, PGT128, PGT122) bind, at least in part, to Manα1 → 2Man termini.27,28,38 Although various oligomannose structures have previously been tested as immunogens,17,21−23,59−61 only a few studies describe the fine carbohydrate specificity of the elicited sera.57,62−66 Manα1 → 2Man binding antibodies with 2G12-blocking activity have been elicited by a 3 year immunization regimen with both DNA and protein Env constructs.57 Previously, vaccination with hypermannosylated yeast and yeast glycoproteins elicited sera in which modest Manα1 → 2Man binding was detected in enriched fractions of IgG.64,66 In studies most comparable to ours, BSA or Qβ phage particles were functionalized with Man9 or Man4 containing an α-linked n-pentyl linker, and the resulting vaccinated rabbit sera were characterized on a glycan array;62,63 binding was observed to numerous synthetic oligomannose glycans containing an α-linked n-alkyl linker analogous to the immunogen, but not to high-mannose glycans containing the natural GlcNAc2 core.
In those reports, it was proposed that the hydrophobic linker alters the conformation of the oligomannose moiety so as to render it antigenically distinct from Man9GlcNAc2. While this may be the case, the data reported here, together with the previous studies,62,63 are also consistent with a simpler interpretation, that antibodies are preferentially generated against the core residues of the glycan, including the linker, rather than against the terminal Manα1 → 2Man “tips” (Figure 9a). If this is true, then why should the “tips” of the oligomannose moiety fail to elicit antibodies? A conventional explanation is lack of immunogenicity: B cells whose antibody receptors are specific for Manα1 → 2Man moiety may be rare or nonexistent, or inactivated by tolerance mechanisms; the desired specificity exhibited by 2G12 and PGT128 might arise only after extensive affinity maturation, sometimes including domain exchange,27,67,68 which may not be sufficiently driven by this vaccination regimen. An alternative hypothesis consistent with the data is that the vaccine pharmacokinetics alter the immune response: the lack of antibodies to Manα1 → 2Man, particularly in synthetic immunogens, might reflect rapid mannosidase trimming of these structures in the vaccinated animal, on a time scale that is competitive with the development of the antibody response (Figure 9b). Thus, germinal centers would be presented primarily with truncated glycan immunogens, resulting in affinity maturation that favors antibodies focused on the glycan core structures.
Supporting this hypothesis, serum mannosidase activity has been observed in several species, including rabbits, both in vivo and ex vivo.69−72 In an antibody pharmacokinetic study in mice,69 Man9GlcNAc2 on the Rituximab Fc was found to be truncated in vivo to Man5/6 with a half-life of ∼6 h, and nearly complete conversion was observed within ∼2 days, well before the establishment of mature germinal centers (∼7 days).73 To assess whether our glycoconjugates are similarly trimmed, we prepared an alkyne-labeled version of the 10F6 glycoconjugate that could be retrieved for mass spectrometric analysis after incubation in serum ex vivo (Figure 9c). At various time points, conjugate in each serum sample was selectively tagged by “click” with an azide biotin tag, then captured on NeutrAvidin resin and specifically eluted by hydrazine cleavage of the linker. Trypsin digests of the retrieved conjugate were analyzed by nanoUPLC-MS and MS/MS to identify and determine relative quantities of truncated 10F6 glycoforms. Significant trimming was observed within 48 h, with up to ∼24% of glycans trimmed to Man6 (Figure 9d; SI, Figure S18 and Table S10).
Thus, mannosidase trimming of our vaccine glycans occurs on a time scale that may affect the glycan microspecificity of vaccine-elicited antibodies. In natural HIV infection as well, analogous mannosidase trimming of the virus may contribute to the heterogeneity of HIV glycosylation and similarly retard the development of Manα1 → 2Man-specific antibodies; bnAb responses would thus be more likely to begin against conserved peptide motifs and the GlcNAc2 core common to the glycans. Over years, persistent exposure to intact Man9GlcNAc2 on freshly produced viral Env, especially the most sterically protected glycans in the HMP, may favor evolution of bnAbs that accommodate or depend on Manα1 → 2Man motifs. Similarly, in very long immunization regimens with Env protein or DNA,57 or with hypermannosylated yeast or glycoproteins that contain dense configurations of mannose,64,66 exposure to intact Manα1 → 2Man motifs may be more prolonged and more likely to stimulate mannose-binding antibodies. In light of these considerations, it is important to routinely evaluate the glycan microspecificity of sera elicited by glycosylated immunogens, and to test alternative immunization regimens and strategies including sustained release of immunogen74,75 or the use of mannosidase inhibitors.
Conclusion
This study has demonstrated that evolved Man9–glycopeptide immunogens, conjugated to CRM197 carrier, elicit a glycan-dependent IgG immune response. However, these antibodies preferentially target the reducing-terminal structures within the glycans of the immunogen, including a hydrophobic linker moiety, readily providing an explanation for low reactivity to Man9GlcNAc2 glycans on HIV Env. Mannosidase trimming of the immunogen glycan “tips” was observed in serum and may contribute to the generation of antibodies focused on the glycan core. This study provides insight into the design of vaccines and immunization strategies targeting the glycan shield of HIV.
Methods
Synthetic glycan azides and peptides were synthesized, coupled, and characterized by LC-ESI-MS as detailed in the SI, Materials and Methods. Glycopeptides were conjugated to CRM197 (for immunizations) or to BSA (for ELISA antigens) using distinct linkers, and loading was characterized by MALDI-TOF MS, as detailed in the SI, Materials and Methods. Serum mannosidase trimming of conjugates was monitored by nanoUPLC-MS and MS/MS, as detailed in the SI, Materials and Methods. New Zealand white rabbits were housed at Prosci, Inc., and immunized with glycopeptide–CRM197 conjugates or SOSIP Env trimers, with Adjuplex adjuvant according to Brandeis University IACUC-approved protocols, as detailed in the SI, Materials and Methods. ELISAs were performed in triplicate, and statistical analyses were performed in Graphpad Prism on log-transformed EC50 titer values using ANOVA with Tukey’s post-hoc test for multiple comparisons, as detailed in the SI, Materials and Methods. Crystal structure determination methods are detailed in the SI, Materials and Methods, and coordinates for 2G12 + 10V1S and 2G12 + 10F5M have been deposited to the worldwide Protein Data Bank76 with IDs 6CXG and 6CXL, respectively.
Acknowledgments
I.J.K. gratefully acknowledges the support of the NIH (R01 AI090745 and R01 AI113737), and I.A.W. acknowledges NIH UM1 AI100663 and the IAVI/Scripps CAVD supported by the Bill and Melinda Gates Foundation. Andrew Lees and Fina Biosolutions (Rockville, MD) are acknowledged for the supply of CRM197. The Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393). Neutralizing antibody assays were supported by Contract HHSN27201100016C (to D.C.M.) from NIAID/NIH. C.E.C. acknowledges the support of NIH Grants P41 GM104603 and S10 OD021728. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS, NIAID, or NIH.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.8b00588.
Additional data and figures including synthesis schemes, chromatographic, MS and NMR data of glycans, glycopeptides and glycopeptide conjugates, crystal structure of 2G12–glycopeptide complexes, neutralization assay results, ELISA binding data, and statistical analysis (PDF)
The authors declare no competing financial interest.
Notes
Data availability: the data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request
Notes
Safety statement: all experiments were conducted in accordance with institutional chemical, biological, and animal safety protocols. No unexpected or unusually high safety hazards were encountered.
Supplementary Material
References
- Burton D. R.; Ahmed R.; Barouch D. H.; Butera S. T.; Crotty S.; Godzik A.; Kaufmann D. E.; McElrath M. J.; Nussenzweig M. C.; Pulendran B.; Scanlan C. N.; Schief W. R.; Silvestri G.; Streeck H.; Walker B. D.; Walker L. M.; Ward A. B.; Wilson I. A.; Wyatt R. A Blueprint for HIV Vaccine Discovery. Cell Host Microbe 2012, 12 (4), 396–407. 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. R.; Mascola J. R. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat. Immunol. 2015, 16 (6), 571–576. 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia B. E.; Bates J. T.; Loomis R. J.; Baneyx G.; Carrico C.; Jardine J. G.; Rupert P.; Correnti C.; Kalyuzhniy O.; Vittal V.; Connell M. J.; Stevens E.; Schroeter A.; Chen M.; MacPherson S.; Serra A. M.; Adachi Y.; Holmes M. A.; Li Y.; Klevit R. E.; Graham B. S.; Wyatt R. T.; Baker D.; Strong R. K.; Crowe J. E.; Johnson P. R.; Schief W. R. Proof of principle for epitope-focused vaccine design. Nature 2014, 507 (7491), 201–206. 10.1038/nature12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R.; Bottomley M. J.; D’Oro U.; Finco O.; De Gregorio E. Reverse vaccinology 2.0: Human immunology instructs vaccine antigen design. J. Exp. Med. 2016, 213 (4), 469–481. 10.1084/jem.20151960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy L. E.; van Gils M. J.; Ozorowski G.; Messmer T.; Briney B.; Voss J. E.; Kulp D. W.; Macauley M. S.; Sok D.; Pauthner M.; Menis S.; Cottrell C. A.; Torres J. L.; Hsueh J.; Schief W. R.; Wilson I. A.; Ward A. B.; Sanders R. W.; Burton D. R. Holes in the Glycan Shield of the Native HIV Envelope Are a Target of Trimer-Elicited Neutralizing Antibodies. Cell Rep. 2016, 16 (9), 2327–2338. 10.1016/j.celrep.2016.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine J. G.; Kulp D. W.; Havenar-Daughton C.; Sarkar A.; Briney B.; Sok D.; Sesterhenn F.; Ereño-Orbea J.; Kalyuzhniy O.; Deresa I.; Hu X.; Spencer S.; Jones M.; Georgeson E.; Adachi Y.; Kubitz M.; deCamp A. C.; Julien J.-P.; Wilson I. A.; Burton D. R.; Crotty S.; Schief W. R. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 2016, 351 (6280), 1458–1463. 10.1126/science.aad9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. W.; van Gils M. J.; Derking R.; Sok D.; Ketas T. J.; Burger J. A.; Ozorowski G.; Cupo A.; Simonich C.; Goo L.; Arendt H.; Kim H. J.; Lee J. H.; Pugach P.; Williams M.; Debnath G.; Moldt B.; van Breemen M. J.; Isik G.; Medina-Ramírez M.; Back J. W.; Koff W. C.; Julien J.-P.; Rakasz E. G.; Seaman M. S.; Guttman M.; Lee K. K.; Klasse P. J.; LaBranche C.; Schief W. R.; Wilson I. A.; Overbaugh J.; Burton D. R.; Ward A. B.; Montefiori D. C.; Dean H.; Moore J. P. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 2015, 349 (6244), aac4223. 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingale J.; Tran K.; Kong L.; Dey B.; McKee K.; Schief W.; Kwong P. D.; Mascola J. R.; Wyatt R. T. Hyperglycosylated Stable Core Immunogens Designed To Present the CD4 Binding Site Are Preferentially Recognized by Broadly Neutralizing Antibodies. J. Virol. 2014, 88 (24), 14002–14016. 10.1128/JVI.02614-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell A. J.; Jiang X.; Totrov M.; Powell R.; Hioe C.; Haigwood N. L.; Kong X.-P.; Zolla-Pazner S. V1V2 multivalent scaffolds induce focused antibody responses with functional activity, prolonged durability, and modest neutralization potency. J. Immunol. 2017, 198 (1S), 225.14. [Google Scholar]
- Zolla-Pazner S.; Kong X. P.; Jiang X. Q.; Cardozo T.; Nadas A.; Cohen S.; Totrov M.; Seaman M. S.; Wang S. X.; Lu S. Cross-Clade HIV-1 Neutralizing Antibodies Induced with V3-Scaffold Protein Immunogens following Priming with gp120 DNA. J. Virol. 2011, 85 (19), 9887–9898. 10.1128/JVI.05086-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T. Q.; Zhu J.; Yang Y. P.; Gorman J.; Ofek G.; Srivatsan S.; Druz A.; Lees C. R.; Lu G.; Soto C.; Stuckey J.; Burton D. R.; Koff W. C.; Connors M.; Kwon P. D. Transplanting Supersites of HIV-1 Vulnerability. PLoS One 2014, 9 (7), e99881 10.1371/journal.pone.0099881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M.; Shahzad-ul-Hussan S.; Doria-Rose N. A.; McLellan J. S.; Bailer R. T.; Dai K. F.; Loesgen S.; Louder M. K.; Staupe R. P.; Yang Y. P.; Zhang B. S.; Parks R.; Eudailey J.; Lloyd K. E.; Blinn J.; Alam S. M.; Haynes B. F.; Amin M. N.; Wang L. X.; Burton D. R.; Koff W. C.; Nabel G. J.; Mascola J. R.; Bewley C. A.; Kwong P. D. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat. Struct. Mol. Biol. 2013, 20 (7), 804–813. 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M. N.; McLellan J. S.; Huang W.; Orwenyo J.; Burton D. R.; Koff W. C.; Kwong P. D.; Wang L. X. Synthetic glycopeptides reveal the glycan specificity of HIV-neutralizing antibodies. Nat. Chem. Biol. 2013, 9 (8), 521–526. 10.1038/nchembio.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toonstra C.; Amin M. N.; Wang L. X. Site-Selective Chemoenzymatic Glycosylation of an HIV-1 Polypeptide Antigen with Two Distinct N-Glycans via an Orthogonal Protecting Group Strategy. J. Org. Chem. 2016, 81 (15), 6176–6185. 10.1021/acs.joc.6b01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Orwenyo J.; Guenaga J.; Giddens J.; Toonstra C.; Wyatt R. T.; Wang L. X. Synthetic multivalent V3 glycopeptides display enhanced recognition by glycan-dependent HIV-1 broadly neutralizing antibodies. Chem. Commun. 2017, 53 (39), 5453–5456. 10.1039/C7CC02059G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwenyo J.; Cai H.; Giddens J.; Amin M. N.; Toonstra C.; Wang L. X. Systematic Synthesis and Binding Study of HIV V3 Glycopeptides Reveal the Fine Epitopes of Several Broadly Neutralizing Antibodies. ACS Chem. Biol. 2017, 12 (6), 1566–1575. 10.1021/acschembio.7b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Orwenyo J.; Giddens J. P.; Yang Q.; Zhang R. S.; LaBranche C. C.; Montefiori D. C.; Wang L. X. Synthetic Three-Component HIV-1 V3 Glycopeptide Immunogens Induce Glycan-Dependent Antibody Responses. Cell. Chem. Biol. 2017, 24 (12), 1513–1522. 10.1016/j.chembiol.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S. M.; Aussedat B.; Vohra Y.; Ryan Meyerhoff R.; Cale E. M.; Walkowicz W. E.; Radakovich N. A.; Anasti K.; Armand L.; Parks R.; Sutherland L.; Scearce R.; Joyce M. G.; Pancera M.; Druz A.; Georgiev I. S.; Von Holle T.; Eaton A.; Fox C.; Reed S. G.; Louder M.; Bailer R. T.; Morris L.; Abdool-Karim S. S.; Cohen M.; Liao H.-X.; Montefiori D. C.; Park P. K.; Fernández-Tejada A.; Wiehe K.; Santra S.; Kepler T. B.; Saunders K. O.; Sodroski J.; Kwong P. D.; Mascola J. R.; Bonsignori M.; Moody M. A.; Danishefsky S.; Haynes B. F. Mimicry of an HIV broadly neutralizing antibody epitope with a synthetic glycopeptide. Sci. Transl. Med. 2017, 9 (381), eaai7521 10.1126/scitranslmed.aai7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aussedat B.; Vohra Y.; Park P. K.; Fernández-Tejada A.; Alam S. M.; Dennison S. M.; Jaeger F. H.; Anasti K.; Stewart S.; Blinn J. H.; Liao H.-X.; Sodroski J. G.; Haynes B. F.; Danishefsky S. J. Chemical Synthesis of Highly Congested gp120 V1V2 N-Glycopeptide Antigens for Potential HIV-1-Directed Vaccines. J. Am. Chem. Soc. 2013, 135 (35), 13113–13120. 10.1021/ja405990z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S. M.; Dennison S. M.; Aussedat B.; Vohra Y.; Park P. K.; Fernández-Tejada A.; Stewart S.; Jaeger F. H.; Anasti K.; Blinn J. H.; Kepler T. B.; Bonsignori M.; Liao H.-X.; Sodroski J. G.; Danishefsky S. J.; Haynes B. F. Recognition of synthetic glycopeptides by HIV-1 broadly neutralizing antibodies and their unmutated ancestors. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (45), 18214–18219. 10.1073/pnas.1317855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce J. G.; Krauss I. J.; Song H. C.; Opalka D. W.; Grimm K. M.; Nahas D. D.; Esser M. T.; Hrin R.; Feng M. Z.; Dudkin V. Y.; Chastain M.; Shiver J. W.; Danishefsky S. J. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (41), 15684–15689. 10.1073/pnas.0807837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss I. J.; Joyce J. G.; Finnefrock A. C.; Song H. C.; Dudkin V. Y.; Geng X.; Warren J. D.; Chastain M.; Shiver J. W.; Danishefsky S. J. Fully Synthetic Carbohydrate HIV Antigens Designed on the Logic of the 2G12 Antibody. J. Am. Chem. Soc. 2007, 129 (36), 11042–11044. 10.1021/ja074804r. [DOI] [PubMed] [Google Scholar]
- Horiya S.; MacPherson I. S.; Krauss I. J. Recent strategies targeting HIV glycans in vaccine design. Nat. Chem. Biol. 2014, 10 (12), 990–999. 10.1038/nchembio.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispin M.; Ward A. B.; Wilson I. A. Structure and Immune Recognition of the HIV Glycan Shield. Annu. Rev. Biophys. 2018, 47, 499–523. 10.1146/annurev-biophys-060414-034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landais E.; Huang X.; Havenar-Daughton C.; Murrell B.; Price M. A.; Wickramasinghe L.; Ramos A.; Bian C. B.; Simek M.; Allen S.; Karita E.; Kilembe W.; Lakhi S.; Inambao M.; Kamali A.; Sanders E. J.; Anzala O.; Edward V.; Bekker L.-G.; Tang J.; Gilmour J.; Kosakovsky-Pond S. L.; Phung P.; Wrin T.; Crotty S.; Godzik A.; Poignard P. Broadly Neutralizing Antibody Responses in a Large Longitudinal Sub-Saharan HIV Primary Infection Cohort. PLoS Pathog. 2016, 12 (1), e1005369 10.1371/journal.ppat.1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss I. J. Antibody recognition of HIV and dengue glycoproteins. Glycobiology 2016, 26 (8), 813–819. 10.1093/glycob/cww031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarese D. A.; Scanlan C. N.; Zwick M. B.; Deechongkit S.; Mimura Y.; Kunert R.; Zhu P.; Wormald M. R.; Stanfield R. L.; Roux K. H.; Kelly J. W.; Rudd P. M.; Dwek R. A.; Katinger H.; Burton D. R.; Wilson I. A. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 2003, 300 (5628), 2065–2071. 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- Pejchal R.; Doores K. J.; Walker L. M.; Khayat R.; Huang P.-S.; Wang S.-K.; Stanfield R. L.; Julien J.-P.; Ramos A.; Crispin M.; Depetris R.; Katpally U.; Marozsan A.; Cupo A.; Maloveste S.; Liu Y.; McBride R.; Ito Y.; Sanders R. W.; Ogohara C.; Paulson J. C.; Feizi T.; Scanlan C. N.; Wong C.-H.; Moore J. P.; Olson W. C.; Ward A. B.; Poignard P.; Schief W. R.; Burton D. R.; Wilson I. A. A Potent and Broad Neutralizing Antibody Recognizes and Penetrates the HIV Glycan Shield. Science 2011, 334 (6059), 1097–1103. 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan J. S.; Pancera M.; Carrico C.; Gorman J.; Julien J. P.; Khayat R.; Louder R.; Pejchal R.; Sastry M.; Dai K. F.; O’Dell S.; Patel N.; Shahzad-ul-Hussan S.; Yang Y. P.; Zhang B. S.; Zhou T. Q.; Zhu J.; Boyington J. C.; Chuang G. Y.; Diwanji D.; Georgiev I.; Do Kwon Y.; Lee D.; Louder M. K.; Moquin S.; Schmidt S. D.; Yang Z. Y.; Bonsignori M.; Crump J. A.; Kapiga S. H.; Sam N. E.; Haynes B. F.; Burton D. R.; Koff W. C.; Walker L. M.; Phogat S.; Wyatt R.; Orwenyo J.; Wang L. X.; Arthos J.; Bewley C. A.; Mascola J. R.; Nabel G. J.; Schief W. R.; Ward A. B.; Wilson I. A.; Kwong P. D. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 2011, 480 (7377), 336–343. 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien J.-P.; Sok D.; Khayat R.; Lee J. H.; Doores K. J.; Walker L. M.; Ramos A.; Diwanji D. C.; Pejchal R.; Cupo A.; Katpally U.; Depetris R. S.; Stanfield R. L.; McBride R.; Marozsan A. J.; Paulson J. C.; Sanders R. W.; Moore J. P.; Burton D. R.; Poignard P.; Ward A. B.; Wilson I. A. Broadly Neutralizing Antibody PGT121 Allosterically Modulates CD4 Binding via Recognition of the HIV-1 gp120 V3 Base and Multiple Surrounding Glycans. PLoS Pathog. 2013, 9 (5), e1003342 10.1371/journal.ppat.1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivatare V. S.; Shivatare S. S.; Lee C.-C. D.; Liang C.-H.; Liao K.-S.; Cheng Y.-Y.; Saidachary G.; Wu C.-Y.; Lin N.-H.; Kwong P. D.; Burton D. R.; Wu C.-Y.; Wong C.-H. Unprecedented Role of Hybrid N-Glycans as Ligands for HIV-1 Broadly Neutralizing Antibodies. J. Am. Chem. Soc. 2018, 140 (15), 5202–5210. 10.1021/jacs.8b00896. [DOI] [PubMed] [Google Scholar]
- Mouquet H.; Scharf L.; Euler Z.; Liu Y.; Eden C.; Scheid J. F.; Halper-Stromberg A.; Gnanapragasam P. N. P.; Spencer D. I. R.; Seaman M. S.; Schuitemaker H.; Feizi T.; Nussenzweig M. C.; Bjorkman P. J. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (47), E3268–E3277. 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garces F.; Sok D.; Kong L.; McBride R.; Kim H. J.; Saye-Francisco K. F.; Julien J. P.; Hua Y. Z.; Cupo A.; Moore J. P.; Paulson J. C.; Ward A. B.; Burton D. R.; Wilson I. A. Structural Evolution of Glycan Recognition by a Family of Potent HIV Antibodies. Cell 2014, 159 (1), 69–79. 10.1016/j.cell.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner C.; Lee J. H.; Sliepen K.; Derking R.; Falkowska E.; de la Peña A. T.; Cupo A.; Julien J.-P.; van Gils M.; Lee P. S.; Peng W.; Paulson J. C.; Poignard P.; Burton D. R.; Moore J. P.; Sanders R. W.; Wilson I. A.; Ward A. B. Structural Delineation of a Quaternary, Cleavage-Dependent Epitope at the gp41-gp120 Interface on Intact HIV-1 Env Trimers. Immunity 2014, 40 (5), 669–680. 10.1016/j.immuni.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M.; Shahzad-ul-Hussan S.; Doria-Rose N. A.; McLellan J. S.; Bailer R. T.; Dai K.; Loesgen S.; Louder M. K.; Staupe R. P.; Yang Y.; Zhang B.; Parks R.; Eudailey J.; Lloyd K. E.; Blinn J.; Alam S. M.; Haynes B. F.; Amin M. N.; Wang L.-X.; Burton D. R.; Koff W. C.; Nabel G. J.; Mascola J. R.; Bewley C. A.; Kwong P. D. Structural basis for diverse N-glycan recognition by HIV-1–neutralizing V1–V2–directed antibody PG16. Nat. Struct. Mol. Biol. 2013, 20 (7), 804–813. 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomelli C.; Doores K. J.; Dunlop D. C.; Thaney V.; Dwek R. A.; Burton D. R.; Crispin M.; Scanlan C. N. The Glycan Shield of HIV Is Predominantly Oligomannose Independently of Production System or Viral Clade. PLoS One 2011, 6 (8), e23521 10.1371/journal.pone.0023521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doores K. J.; Bonomelli C.; Harvey D. J.; Vasiljevic S.; Dwek R. A.; Burton D. R.; Crispin M.; Scanlan C. N. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (31), 13800–13805. 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L.; Lee J. H.; Doores K. J.; Murin C. D.; Julien J.-P.; McBride R.; Liu Y.; Marozsan A.; Cupo A.; Klasse P.-J.; Hoffenberg S.; Caulfield M.; King C. R.; Hua Y.; Le K. M.; Khayat R.; Deller M. C.; Clayton T.; Tien H.; Feizi T.; Sanders R. W.; Paulson J. C.; Moore J. P.; Stanfield R. L.; Burton D. R.; Ward A. B.; Wilson I. A. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat. Struct. Mol. Biol. 2013, 20 (7), 796–803. 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. M.; Huber M.; Doores K. J.; Falkowska E.; Pejchal R.; Julien J.-P.; Wang S.-K.; Ramos A.; Chan-Hui P.-Y.; Moyle M.; Mitcham J. L.; Hammond P. W.; Olsen O. A.; Phung P.; Fling S.; Wong C.-H.; Phogat S.; Wrin T.; Simek M. D.; Principal Investigators P. G.; Koff W. C.; Wilson I. A.; Burton D. R.; Poignard P. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011, 477 (7365), 466–470. 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trkola A.; Purtscher M.; Muster T.; Ballaun C.; Buchacher A.; Sullivan N.; Srinivasan K.; Sodroski J.; Moore J. P.; Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 1996, 70 (2), 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiya S.; Bailey J. K.; Krauss I. J. Directed Evolution of Glycopeptides Using mRNA Display. Methods Enzymol. 2017, 597, 83–141. 10.1016/bs.mie.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme J. S.; Krauss I. J. SELMA: Selection with Modified Aptamers. Curr. Protoc Chem. Biol. 2015, 7, 73–92. 10.1002/9780470559277.ch140233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme J. S.; MacPherson I. S.; DeCourcey J. F.; Krauss I. J. High Temperature SELMA: Evolution of DNA-Supported Oligomannose Clusters Which Are Tightly Recognized by HIV bnAb 2G12. J. Am. Chem. Soc. 2014, 136 (5), 1726–1729. 10.1021/ja411212q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiya S.; Bailey J. K.; Temme J. S.; Guillen Schlippe Y. V.; Krauss I. J. Directed evolution of multivalent glycopeptides tightly recognized by HIV antibody 2G12. J. Am. Chem. Soc. 2014, 136 (14), 5407–5415. 10.1021/ja500678v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme J. S.; Drzyzga M. G.; MacPherson I. S.; Krauss I. J. Directed evolution of 2G12-targeted nonamannose glycoclusters by SELMA. Chem. - Eur. J. 2013, 19 (51), 17291–17295. 10.1002/chem.201303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson I. S.; Temme J. S.; Habeshian S.; Felczak K.; Pankiewicz K.; Hedstrom L.; Krauss I. J. Multivalent glycocluster design through directed evolution. Angew. Chem., Int. Ed. 2011, 50 (47), 11238–11242. 10.1002/anie.201105555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. K.; Nguyen D. N.; Horiya S.; Krauss I. J. Synthesis of multivalent glycopeptide conjugates that mimic an HIV epitope. Tetrahedron 2016, 72 (40), 6091–6098. 10.1016/j.tet.2016.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. H.; Barrick J. E.; Szostak J. W.; Roberts R. W. Optimized synthesis of RNA-protein fusions for in vitro protein selection. Methods Enzymol. 2000, 318, 268–293. 10.1016/S0076-6879(00)18058-9. [DOI] [PubMed] [Google Scholar]
- Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective ″ligation″ of azides and terminal alkynes. Angew. Chem., Int. Ed. 2002, 41 (14), 2596–2599. . [DOI] [PubMed] [Google Scholar]
- Hartman M. C. T.; Josephson K.; Lin C.-W.; Szostak J. W. An Expanded Set of Amino Acid Analogs for the Ribosomal Translation of Unnatural Peptides. PLoS One 2007, 2 (10), e972 10.1371/journal.pone.0000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-L.; Hung J.-T.; Cheung S. K. C.; Lee H.-Y.; Chu K.-C.; Li S.-T.; Lin Y.-C.; Ren C.-T.; Cheng T.-J. R.; Hsu T.-L.; Yu A. L.; Wu C.-Y.; Wong C.-H. Carbohydrate-based vaccines with a glycolipid adjuvant for breast cancer. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (7), 2517–2522. 10.1073/pnas.1222649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinefield H. R. Overview of the development and current use of CRM197 conjugate vaccines for pediatric use. Vaccine 2010, 28 (27), 4335–4339. 10.1016/j.vaccine.2010.04.072. [DOI] [PubMed] [Google Scholar]
- Calarese D. A.; Lee H.-K.; Huang C.-Y.; Best M. D.; Astronomo R. D.; Stanfield R. L.; Katinger H.; Burton D. R.; Wong C.-H.; Wilson I. A. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc. Natl. Acad. Sci. U. S. A. 2005, 102 (38), 13372–13377. 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murin C. D.; Julien J.-P.; Sok D.; Stanfield R. L.; Khayat R.; Cupo A.; Moore J. P.; Burton D. R.; Wilson I. A.; Ward A. B. Structure of 2G12 Fab2 in Complex with Soluble and Fully Glycosylated HIV-1 Env by Negative-Stain Single-Particle Electron Microscopy. J. Virol. 2014, 88 (17), 10177–10188. 10.1128/JVI.01229-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A.-J.; Vasiljevic S.; Pritchard L. K.; Harvey D. J.; Andev R. S.; Krumm S. A.; Struwe W. B.; Cupo A.; Kumar A.; Zitzmann N.; Seabright G. E.; Kramer H. B.; Spencer D. I. R.; Royle L.; Lee J. H.; Klasse P. J.; Burton D. R.; Wilson I. A.; Ward A. B.; Sanders R. W.; Moore J. P.; Doores K. J.; Crispin M. Composition and Antigenic Effects of Individual Glycan Sites of a Trimeric HIV-1 Envelope Glycoprotein. Cell Rep. 2016, 14 (11), 2695–2706. 10.1016/j.celrep.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. W.; Derking R.; Cupo A.; Julien J. P.; Yasmeen A.; de Val N.; Kim H. J.; Blattner C.; de la Pena A. T.; Korzun J.; Golabek M.; de los Reyes K.; Ketas T. J.; van Gils M. J.; King C. R.; Wilson I. A.; Ward A. B.; Klasse P. J.; Moore J. P. A Next-Generation Cleaved, Soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, Expresses Multiple Epitopes for Broadly Neutralizing but Not Non-Neutralizing Antibodies. PLoS Pathog. 2013, 9 (9), e1003618 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K. O.; Nicely N. I.; Wiehe K.; Bonsignori M.; Meyerhoff R. R.; Parks R.; Walkowicz W. E.; Aussedat B.; Wu N. R.; Cai F.; Vohra Y.; Park P. K.; Eaton A.; Go E. P.; Sutherland L. L.; Scearce R. M.; Barouch D. H.; Zhang R.; Von Holle T.; Overman R. G.; Anasti K.; Sanders R. W.; Moody M. A.; Kepler T. B.; Korber B.; Desaire H.; Santra S.; Letvin N. L.; Nabel G. J.; Montefiori D. C.; Tomaras G. D.; Liao H.-X.; Alam S. M.; Danishefsky S. J.; Haynes B. F. Vaccine Elicitation of High Mannose-Dependent Neutralizing Antibodies against the V3-Glycan Broadly Neutralizing Epitope in Nonhuman Primates. Cell Rep. 2017, 18 (9), 2175–2188. 10.1016/j.celrep.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks E. T.; Grimley S. L.; Cully M.; Osawa K.; Dekkers G.; Saunders K.; Rämisch S.; Menis S.; Schief W. R.; Doria-Rose N.; Haynes B.; Murrell B.; Cale E. M.; Pegu A.; Mascola J. R.; Vidarsson G.; Binley J. M. Glycoengineering HIV-1 Env creates ‘supercharged’ and ‘hybrid’ glycans to increase neutralizing antibody potency, breadth and saturation. PLoS Pathog. 2018, 14 (5), e1007024 10.1371/journal.ppat.1007024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J. H.; Song H. J.; Wang Y. D.; Stamatos N. M.; Wang L. X. Toward a carbohydrate-based HIV-1 vaccine: Synthesis and immunological studies of oligomannose-containing glycoconjugates. Bioconjugate Chem. 2006, 17 (2), 493–500. 10.1021/bc0502816. [DOI] [PubMed] [Google Scholar]
- Liu C.-C.; Zhai C.; Zheng X.-J.; Ye X.-S. Altering the Specificity of the Antibody Response to HIV gp120 with a Glycoconjugate Antigen. ACS Chem. Biol. 2016, 11 (6), 1702–1709. 10.1021/acschembio.6b00224. [DOI] [PubMed] [Google Scholar]
- Doores K. J.; Fulton Z.; Hong V.; Patel M. K.; Scanlan C. N.; Wormald M. R.; Finn M. G.; Burton D. R.; Wilson I. A.; Davis B. G. A nonself sugar mimic of the HIV glycan shield shows enhanced antigenicity. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (40), 17107–17112. 10.1073/pnas.1002717107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astronomo R. D.; Lee H. K.; Scanlan C. N.; Pantophlet R.; Huang C. Y.; Wilson I. A.; Blixt O.; Dwek R. A.; Wong C. H.; Burton D. R. A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J. Virol. 2008, 82 (13), 6359–6368. 10.1128/JVI.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astronomo R. D.; Kaltgrad E.; Udit A. K.; Wang S. K.; Doores K. J.; Huang C. Y.; Pantophlet R.; Paulson J. C.; Wong C. H.; Finn M. G.; Burton D. R. Defining Criteria for Oligomannose Immunogens for HIV Using Icosahedral Virus Capsid Scaffolds. Chem. Biol. 2010, 17 (4), 357–370. 10.1016/j.chembiol.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal-Gamse C.; Luallen R. J.; Liu B.; Fu H.; Lee F.-H.; Geng Y.; Doms R. W. Yeast-Elicited Cross-Reactive Antibodies to HIV Env Glycans Efficiently Neutralize Virions Expressing Exclusively High-Mannose N-Linked Glycans. J. Virol. 2011, 85 (1), 470–480. 10.1128/JVI.01349-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantophlet R.; Trattnig N.; Murrell S.; Lu N.; Chau D.; Rempel C.; Wilson I. A.; Kosma P. Bacterially derived synthetic mimetics of mammalian oligomannose prime antibody responses that neutralize HIV infectivity. Nat. Commun. 2017, 8 (1), 1601–1613. 10.1038/s41467-017-01640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Fu H.; Luallen R. J.; Liu B. F.; Lee F. H.; Doms R. W.; Geng Y. Antibodies elicited by yeast glycoproteins recognize HIV-1 virions and potently neutralize virions with high mannose N-glycans. Vaccine 2015, 33 (39), 5140–5147. 10.1016/j.vaccine.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M.; Le K. M.; Doores K. J.; Fulton Z.; Stanfield R. L.; Wilson I. A.; Burton D. R. Very few substitutions in a germ line antibody are required to initiate significant domain exchange. J. Virol. 2010, 84 (20), 10700–10707. 10.1128/JVI.01111-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doores K. J.; Fulton Z.; Huber M.; Wilson I. A.; Burton D. R. Antibody 2G12 Recognizes Di-Mannose Equivalently in Domain- and Nondomain-Exchanged Forms but Only Binds the HIV-1 Glycan Shield if Domain Exchanged. J. Virol. 2010, 84 (20), 10690–10699. 10.1128/JVI.01110-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.; Brown D.; Reed C.; Chung S.; Lutman J.; Stefanich E.; Wong A.; Stephan J.-P.; Bayer R. Production, characterization and pharmacokinetic properties of antibodies with N-linked Mannose-5 glycans. mAbs 2012, 4 (4), 475–487. 10.4161/mabs.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Liu Y. D.; Flynn G. C. The effect of Fc glycan forms on human IgG2 antibody clearance in humans. Glycobiology 2009, 19 (3), 240–249. 10.1093/glycob/cwn120. [DOI] [PubMed] [Google Scholar]
- Porwoll S.; Fuchs H.; Tauber R. Characterization of a soluble class I α-mannosidase in human serum. FEBS Lett. 1999, 449 (2–3), 175–178. 10.1016/S0014-5793(99)00422-6. [DOI] [PubMed] [Google Scholar]
- Higel F.; Seidl A.; Demelbauer U.; Viertlboeck-Schudy M.; Koppenburg V.; Kronthaler U.; Sörgel F.; Frieß W. N-glycan PK Profiling Using a High Sensitivity nanoLCMS Work-Flow with Heavy Stable Isotope Labeled Internal Standard and Application to a Preclinical Study of an IgG1 Biopharmaceutical. Pharm. Res. 2015, 32 (11), 3649–3659. 10.1007/s11095-015-1724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva N. S.; Klein U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 2015, 15 (3), 137–148. 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauthner M.; Havenar-Daughton C.; Sok D.; Nkolola J. P.; Bastidas R.; Boopathy A. V.; Carnathan D. G.; Chandrashekar A.; Cirelli K. M.; Cottrell C. A.; Eroshkin A. M.; Guenaga J.; Kaushik K.; Kulp D. W.; Liu J.; McCoy L. E.; Oom A. L.; Ozorowski G.; Post K. W.; Sharma S. K.; Steichen J. M.; de Taeye S. W.; Tokatlian T.; Torrents de la Peña A.; Butera S. T.; LaBranche C. C.; Montefiori D. C.; Silvestri G.; Wilson I. A.; Irvine D. J.; Sanders R. W.; Schief W. R.; Ward A. B.; Wyatt R. T.; Barouch D. H.; Crotty S.; Burton D. R. Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 2017, 46 (6), 1073–1088. 10.1016/j.immuni.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam H. H.; Melo M. B.; Kang M.; Pelet J. M.; Ruda V. M.; Foley M. H.; Hu J. K.; Kumari S.; Crampton J.; Baldeon A. D.; Sanders R. W.; Moore J. P.; Crotty S.; Langer R.; Anderson D. G.; Chakraborty A. K.; Irvine D. J. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (43), E6639–E6648. 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H.; Henrick K.; Nakamura H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. 10.1038/nsb1203-980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.