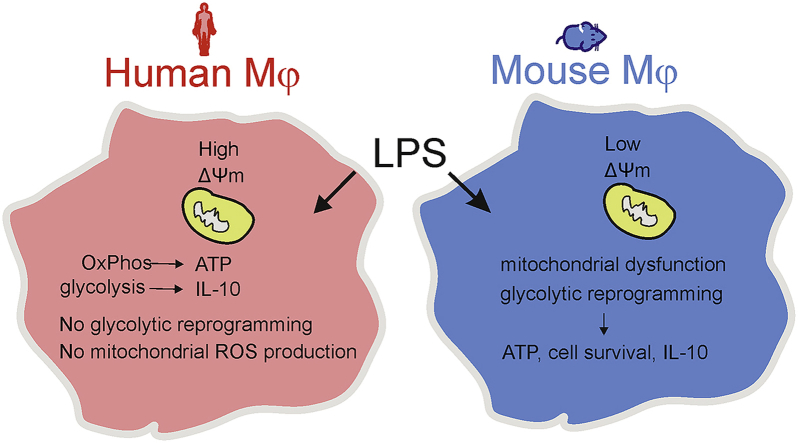
Abstract
Macrophages adopt different phenotypes in response to microenvironmental changes, which can be principally classified into inflammatory and anti-inflammatory states. Inflammatory activation of macrophages has been linked with metabolic reprogramming from oxidative phosphorylation to aerobic glycolysis. In contrast to mouse macrophages, little information is available on the link between metabolism and inflammation in human macrophages. In the current report it is demonstrated that lipopolysaccharide (LPS)-activated human peripheral blood monocyte-derived macrophages (hMDMs) fail to undergo metabolic reprogramming towards glycolysis, but rely on oxidative phosphorylation for the generation of ATP. By contrast, activation by LPS led to an increased extracellular acidification rate (glycolysis) and decreased oxygen consumption rate (oxidative phosphorylation) in mouse bone marrow-derived macrophages (mBMDMs). Mitochondrial bioenergetics after LPS stimulation in human macrophages was unchanged, but was markedly impaired in mouse macrophages. Furthermore, treatment with 2-deoxyglucose, an inhibitor of glycolysis, led to cell death in mouse, but not in human macrophages. Finally, glycolysis appeared to be critical for LPS-mediated induction of the anti-inflammatory cytokine interleukin-10 in both human and mouse macrophages. In summary, these findings indicate that LPS-induced immunometabolism in human macrophages is different to that observed in mouse macrophages.
Keywords: Bioenergetics, Inflammation, Macrophage, Metabolic reprogramming, Mitochondrial function
Graphical abstract
Highlights
-
•
Human inflammatory macrophages rely on oxidative phosphorylation rather than glycolysis for ATP production.
-
•
Mouse but not human macrophages display bioenergetic dysfunction upon inflammatory activation.
-
•
Glycolysis is dispensable for the survival of human inflammatory macrophages.
1. Introduction
Metabolic reprogramming refers to changes in bioenergetic pathways in activated immune cells. Some of the key metabolic cascades that are modulated include glycolysis, oxidative phosphorylation, the pentose phosphate pathway, fatty acid oxidation and amino acid metabolism [1,2]. In particular, aerobic glycolysis has been shown to be induced in a variety of activated immune cells such as NK cells, dendritic cells, B cells and effector T cells [1], which in turn modulate their functional characteristics. Notably, the paradigm that metabolic changes may contribute to the functionality of immune cells may open new options for therapeutic interventions in inflammatory diseases [2,3].
Metabolic reprogramming from oxidative phosphorylation to glycolysis is also a hallmark of activated inflammatory (M1) macrophages. In contrast, anti-inflammatory (M2) macrophages have been shown to rely primarily on oxidative phosphorylation (OxPhos) [4,5]. In inflammatory macrophages a break in the TCA cycle with accumulation of citrate and succinate has been reported, which might account for the pro-inflammatory phenotype characterized by increased reactive oxygen species (ROS), nitric oxide (NO) and prostaglandin production [4,6]. Most of the experimental findings on metabolic reprogramming in macrophages have been obtained from studies in mouse macrophages, but as recently highlighted by Van den Bossche and colleagues, very little information is available on metabolic reprogramming in human macrophages [5]. Of note, considerable divergences have been reported in the immunological responses of human and mouse macrophage to TLR4 signaling [7,8]. Hence, the major goal of the current study was to determine metabolic reprogramming in human macrophages in response to the prototypical inflammatory stimulus LPS. Upon LPS activation, human peripheral blood monocyte-derived macrophages (hMDMs) do not exhibit the switch to glycolysis and bioenergetic dysfunction that have previously been established for mouse macrophages. Moreover, activated hMDMs rely on OxPhos rather than glycolysis for ATP production unlike mouse macrophages. Finally, studies using 2-deoxyglucose (2-DG), an inhibitor of glycolysis, indicate that the glycolytic pathway is directly involved in cell survival of mouse, but not human macrophages.
2. Results and discussion
2.1. Bioenergetic dysfunction and metabolic reprogramming to aerobic glycolysis is not a feature of human inflammatory macrophages
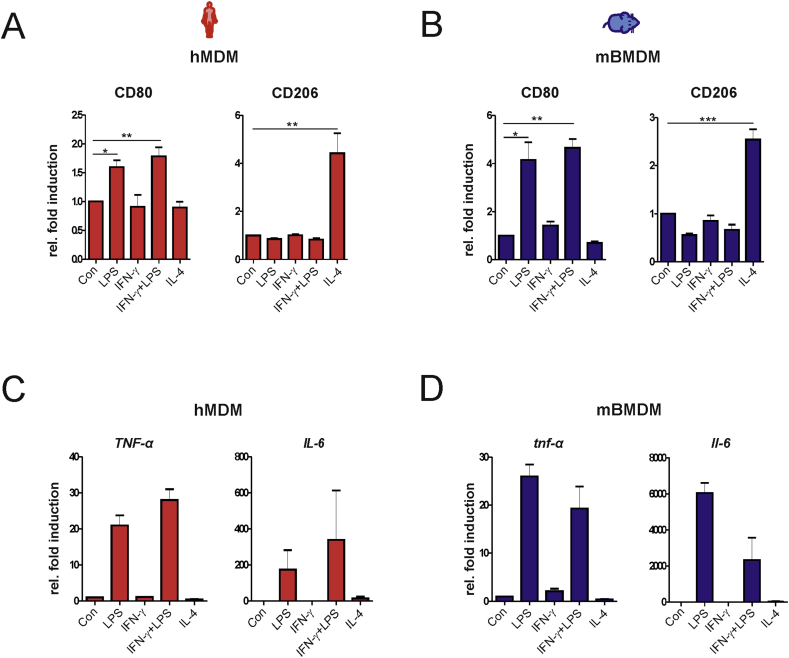
To assess the principal comparability of primary human and mouse macrophages in inflammatory conditions, hMDMs and mBMDMs were characterized for their ability to polarize into either: 1) the inflammatory M1 state after exposure to LPS or IFN-γ+LPS or 2) the anti-inflammatory M2 state after stimulation with IL-4. Treatment with LPS and IFN-γ+LPS, but not IL-4, induced M1 polarization in both hMDMs and mBMDMs as indicated by increased surface expression of CD80 (Figs. S1A and B). In contrast, only treatment with IL-4 increased the expression of CD206 associated with M2 polarization of both human and mouse macrophages (Figs. S1A and B). Further, mRNA expression of the pro-inflammatory genes TNF-α and IL-6 was only induced by treatment with LPS and IFN-γ+LPS, but not by IL-4 (Figs. S1C and D). These findings indicate that both human and mouse primary macrophages similarly undergo M1 or M2 polarization in our experimental setting. Moreover, stimulation with LPS alone is sufficient to induce the inflammatory phenotype.
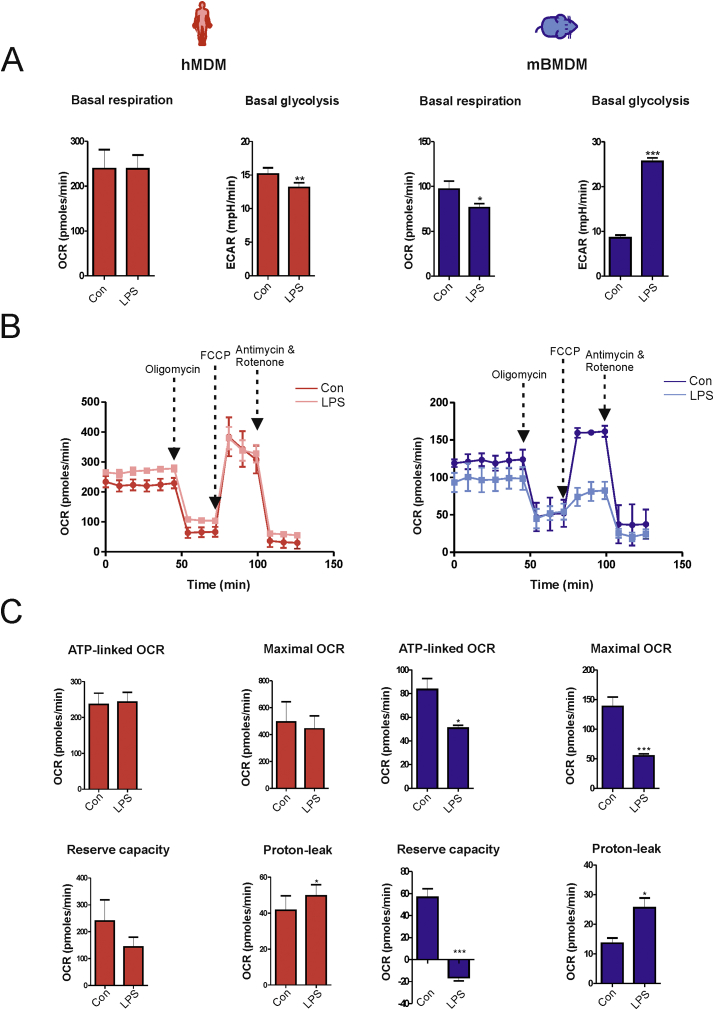
Accumulating evidence indicates that metabolic reprogramming of immune cells is an important determinant of their function [9]. To investigate the metabolic alterations in activated human macrophages, the bioenergetic profile of unstimulated and LPS-activated macrophages was assessed with the Seahorse XF-analyzer. Oxygen consumption rate (OCR), an index of OxPhos in LPS-activated hMDMs (16 h), was comparable to that of unstimulated cells, whereas the extracellular acidification rate (ECAR), an index of glycolysis, was mildly but significantly reduced (Fig. 1A). This finding was unexpected because inflammatory activation of mouse macrophages has been shown to cause reduced OxPhos and increased glycolysis [10,11]. Accordingly, LPS activation (16 h) in mBMDMs caused a marked reduction in OCR and a concomitant increase in ECAR (Fig. 1A). Stimulation with LPS minimally altered the Mito Stress profile in hMDMs (Fig. 1B). Although a tendency towards a decrease in reserve capacity and an increase in proton leak was observed, ATP-linked OCR and maximal OCR were not significantly affected after LPS activation in hMDMs (Fig. 1C). In contrast, stimulation with LPS in mBMDMs led to impaired mitochondrial bioenergetics (Fig. 1B) with a significant reduction in respiratory parameters such as ATP-linked OCR, reserve capacity and maximal OCR (Fig. 1B and C). These data indicate that LPS stimulation does not affect mitochondrial bioenergetics in human macrophages.
Fig. 1.
LPS induces glycolysis and mitochondrial dysfunction in mouse but not in human macrophages. Untreated (Con) or LPS-activated (16 h) mouse (mBMDM) and human (hMDM) macrophages were subjected to a Mito Stress test using a Seahorse XF-analyzer. (A) Basal oxygen consumption rate (OCR), an index of oxidative phosphorylation, and extracellular acidification rate (ECAR), an index of glycolysis, are shown. (B) A representative Seahorse plot of the Mito Stress test assessed by recording OCR after injection of oligomycin (1 μg/ml), carbonyl cyanide-4-(trifluoromethoxy), phenylhydrazone (FCCP, 0.7 μM), and rotenone (1 μM) plus antimycin (1 μM) from three independent experiments is shown. (C) The values of the indicated respiratory parameters were calculated from the Mito Stress test and are shown as mean ± SEM of three independent experiments. Statistical analysis was performed using Student's t-test (Con Vs LPS); *p < 0.05, **p < 0.01, ***p < 0.001.
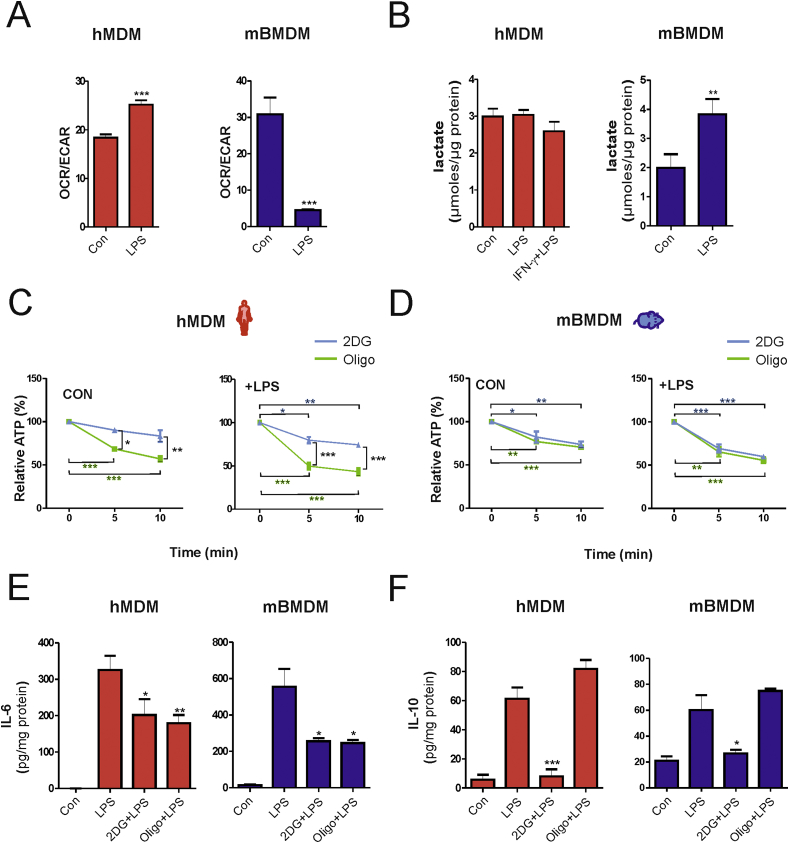
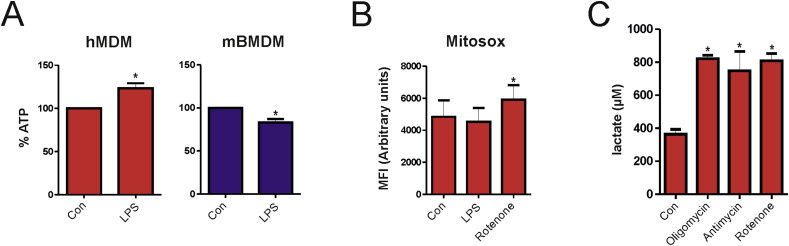
Different responses to LPS in human and mouse macrophages were also observed for the OCR/ECAR ratio, an important indicator of cellular metabolism. While activation of hMDMs with LPS resulted in an increase of OCR/ECAR ratio, treatment of mBMDMs with LPS significantly decreased this parameter (Fig. 2A). Accordingly, levels of the end product of glycolysis, lactate, remained unchanged in culture media of LPS-stimulated hMDMs, but were increased in LPS-treated mBMDMs (Fig. 2B). These data suggest that glycolysis is preferentially adopted by mouse but not human macrophages upon activation with LPS. A possible explanation for this differential metabolic reprogramming might be that upon attenuation of OxPhos, glycolysis is required to compensate for the reduced capacity of mitochondrial ATP production in mouse macrophages. To test this hypothesis we determined the role of OxPhos and glycolysis for ATP production in human and mouse macrophages. A time-dependent decrease of ATP levels was significantly higher in hMDMs treated with the OxPhos inhibitor oligomycin (4 μM) in comparison to those treated with the glycolysis inhibitor 2-deoxyglucose (2DG) (5 mM). This effect was observed in both unstimulated and LPS-activated cells (Fig. 2C), indicating that OxPhos is the predominant source of ATP production. By contrast, ATP levels were reduced to a similar extent by both 2DG and oligomycin in unstimulated and LPS-activated mBMDMs (Fig. 2D) suggesting that these cells substantially rely on both glycolysis and OxPhos for energy generation. Of note, total ATP levels were significantly reduced after LPS activation in mBMDMs, but increased in LPS-activated hMDMs (Fig. S2A), which might be due to the fact that glycolysis is less efficient in comparison to OxPhos for generation of ATP. To substantiate the role of OxPhos for ATP production in hMDMs, we also analyzed mitochondrial ROS production because mitochondrial function has been shown to shift from energy metabolism to ROS production in inflammatory mouse macrophages [10]. However, our analysis in human macrophages revealed that LPS-activation did not significantly alter mitochondrial ROS production, whereas treatment with the complex I inhibitor rotenone increased ROS levels (Fig. S2B). Additionally, the ability of human macrophages to induce glycolysis was tested. Upon treatment with mitochondrial respiratory complex inhibitors, hMDMs exhibited substantial induction of glycolysis as shown by increased lactate levels (Fig. S2C), indicating that human macrophages can switch to glycolysis when responding to stimuli different from LPS. Collectively, these findings indicate that, in contrast to mouse macrophages, human macrophages do not exhibit bioenergetic dysfunction upon stimulation with LPS.
Fig. 2.
Activated mouse and human macrophages display differential dependence on metabolic pathways for energy production but not for cytokine secretion. (A) Mean values of OCR/ECAR ratio in untreated (Con) or LPS-activated (16 h) human (hMDM) and mouse (mBMDM) macrophages from three independent experiments. (B) Lactate production measured in the culture supernatants and normalized to the total protein of cell lysates. (C–D) Intracellular ATP levels were determined before and after addition of 2-deoxyglucose (2DG, 5 mM) or oligomycin (oligo, 4 μM) for the indicated time points. (E–F) Human (hMDM) or mouse (mBMDM) macrophages were stimulated with LPS alone or in combination with 2DG (5 mM) or oligo (2 μM) for 8 h (mBMDM) and 16 h (hMDM), respectively. Culture supernatants were collected for ELISA to detect (E) IL-6 and (F) IL-10 and the values obtained were normalized to the total protein content of the respective total cell lysates. Values represent mean ± SEM of three independent experiments. Statistical analysis was performed using either a Student's t-test (Con vs. LPS) for OCR/ECAR and lactate (2DG vs. Oligo) or One-way ANOVA with Tukey's post-hoc analysis for ATP (0 min vs 5/10 min) and ELISA (LPS vs. LPS+2DG/oligo). *p < 0.05, **p < 0.01,***p < 0.001*.
In T cells, early activation of glycolysis is involved in the regulation of cytokine synthesis [12]. Hence, we also evaluated whether metabolic differences between human and mouse macrophages contribute differentially to the inflammatory cytokine response. To this end, the pro-inflammatory cytokine IL-6 and the anti-inflammatory cytokine IL-10 were determined in supernatants from hMDMs and mBMDMs stimulated with LPS alone or LPS in the presence of either 2DG or oligomycin. As shown in Fig. 2E, 2DG or oligomycin blocked LPS-induced secretion of IL-6 in both cell types. Interestingly, treatment with 2DG, but not with oligomycin, abrogated the LPS-induced secretion of IL-10 in both hMDMs and mBMDMs (Fig. 2F). These findings indicate that the differential species-specific metabolic reprogramming by LPS does not seem to affect IL-6 and IL-10 production and that glycolysis but not OxPhos is necessary for the production of the anti-inflammatory cytokine IL-10 in human and mouse macrophages. IL-10 is an important anti-inflammatory cytokine, which regulates the function of inflammatory immune cells. IL-10 has been shown to work in an autocrine fashion to regulate NO production in mouse macrophages [13]. Moreover, IL-10 production enables glycolysis to be kept in check and also promotes mitophagy of dysfunctional mitochondria [14]. The dependence of macrophages on glycolysis for IL-10 production as observed in this study suggests that a potential translational approach based on inhibition of glycolysis in human macrophages might be unfavorable during inflammatory conditions. This notion is further supported by recent findings showing that 2DG blocked the anti-inflammatory polarization of macrophages [15].
2.2. Glycolysis is dispensable for the maintenance of mitochondrial membrane potential (ΔΨm) and cell survival in human macrophages
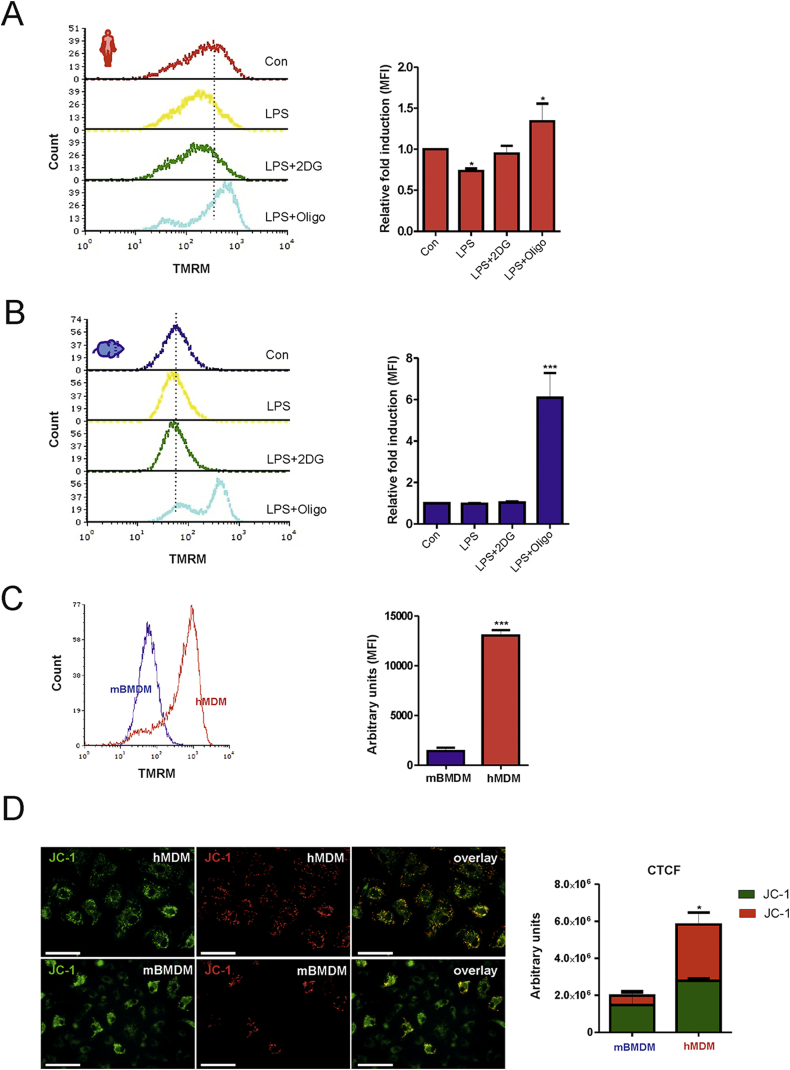
It has been reported for astrocytes [16] and dendritic cells [17] that mitochondrial dysfunction leads to the use of glycolysis-derived ATP to maintain ΔΨm, in which case Fo/F1 ATP synthase works in the reverse direction to hydrolyze ATP. To evaluate whether glycolysis-derived ATP may affect ΔΨm in our experimental setting, LPS-activated hMDMs were treated with 2DG to inhibit glycolysis or oligomycin to block Fo/F1 ATP synthase 30 min prior to tetramethylrhodamine methyl ester perchlorate (TMRM) staining. We found that 2DG did not affect ΔΨm, whereas oligomycin caused hyperpolarization of ΔΨm indicating that the Fo/F1 synthase was working in the forward direction (Fig. 3A). Interestingly, treatment with 2DG in LPS-activated mBMDMs also did not have a significant impact on ΔΨm, while oligomycin hyperpolarized ΔΨm (Fig. 3B). These findings rule out the use of glycolysis-derived ATP to sustain ΔΨm in LPS-activated primary macrophages.
Fig. 3.
Glycolysis-derived ATP is not used to sustain ΔΨm in mouse and human macrophages. (A) Human (hMDM) or (B) mouse (mBMDM) macrophages were stimulated with LPS for 16 h followed by treatment with oligomycin (2 μM) and 2-DG (5 mM) for an additional 30 min. Cells were subjected to TMRM staining and analyzed by flow cytometry. A representative histogram plot is shown. The mean fluorescent intensity (MFI) from three independent experiment ± SEM is shown as fold induction relative to untreated (Con) cells. (C) Untreated mouse (mBMDM) and human (hMDM) macrophages were subjected to staining with TMRM for 30 min and analyzed by flow cytometry. A representative histogram and the MFI from three independent experiments ± SEM are shown. (D) Untreatred human (hMDM) and mouse (mBMDM) macrophages were subjected to staining with the cationic dye JC-1 for 30 min. A representative image from three independent experiments is shown. Green color JC-1 monomers are indicative of low ΔΨm and red color polymers are indicative of high ΔΨm. Bars (200 μm). The corrected total cell fluorescence (CTCF) is shown as a bar graph. Statistical analysis was performed using Student's t-test (mBMDM vs. hMDM) or One-way ANOVA with Tukey's post-hoc analysis (Con vs. LPS/LPS+2DG/LPS+oligo); *p < 0.05, **p < 0.01,***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
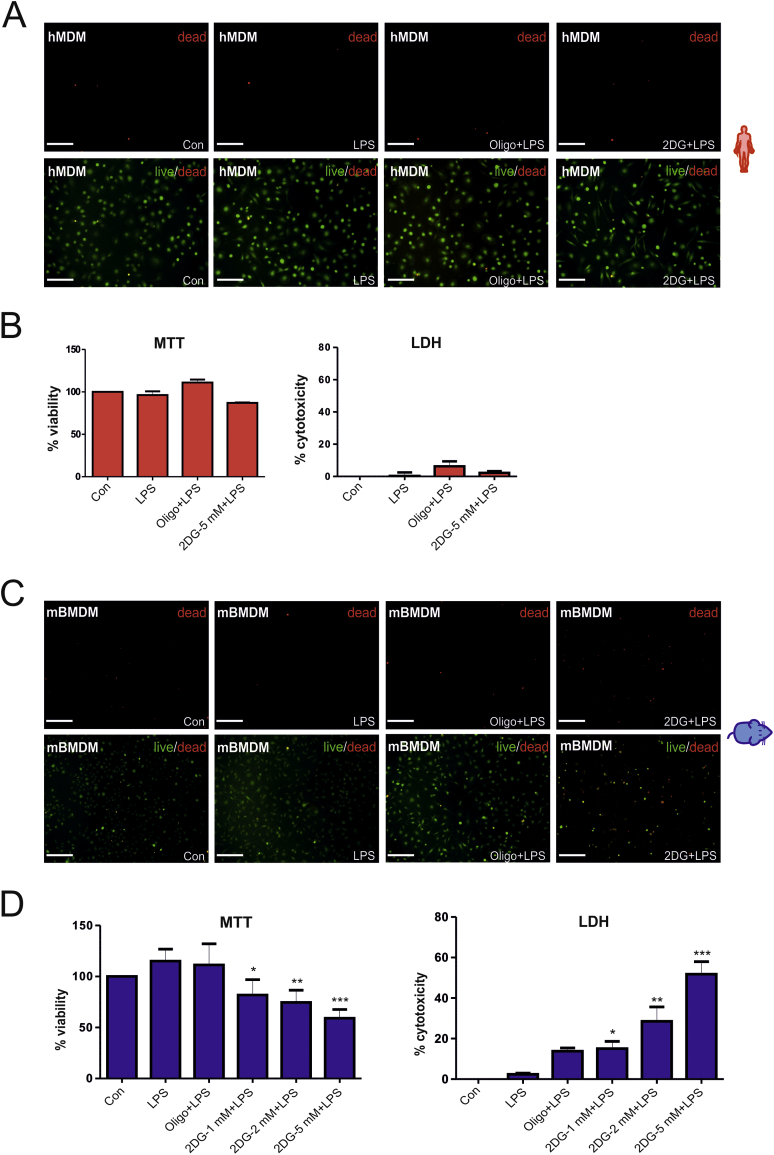
Notably, hMDMs exhibited a higher ΔΨm than mBMDMs (Fig. 3C), which was also confirmed by staining with a second cationic dye JC-1 that revealed red fluorescence for hMDMs (indicative of a high ΔΨm) and green fluorescence for mBMDMs (indicative of a low ΔΨm) (Fig. 3D). These observations led us to speculate that differences in the basal ΔΨm might reflect the cellular resistance to stress-induced cell death, a phenomenon that has previously been reported in hybridoma and colonic tumor cell populations [18,19]. Hence, we tested whether induction of glycolysis in activated mouse macrophages is necessary to maintain cell survival. As expected, neither LPS and 2DG (5 mM) nor LPS and oligomycin (2 μM) treatment affected viability of hMDMs after 24 h as determined by live/dead staining, MTT and LDH assays (Fig. 4A, -B). By contrast, a marked reduction in cell viability was noted in mBMDMs stimulated with LPS in the presence of 2DG (5 mM) (Fig. 4C). The effect of 2DG on cell viability of mBMDMs was dose-dependent (Fig. 4C and D) and oligomycin decreased cell viability only to a minor extent (Fig. 4C and D). Of note, treatment with 2DG alone decreased cell viability in unstimulated mBMDMs (not shown). In summary, glycolysis appears to be essential for cell survival in activated mouse macrophages, but not in human macrophages.
Fig. 4.
Inhibiting glycolysis induces cell death in activated mouse but not human macrophages. Macrophages were stimulated with LPS alone or in the presence of different concentration of 2-deoxyglucose (2DG) or oligomycin (oligo, 2 μM) for 24 h and subjected to the indicated analysis. (A) Live/dead staining of human macrophages (hMDM). Representative fluorescence microscopy images of three independent experiments. Bars (200 μm). (B) MTT assay and (B) LDH assay in hMDM. (C) Live/dead staining of mouse macrophages (mBMDM). Representative fluorescence microscopy images of three independent experiments. Bars (200 μm) (D) MTT assay and LDH assay in mBMDM. Values represent the mean ± SEM of three independent experiments. Statistical analysis was performed using One-way ANOVA with Tukey's post-hoc analysis; (LPS Vs LPS+2DG/LPS+oligo) *p < 0.05, **p < 0.01,***p < 0.001.
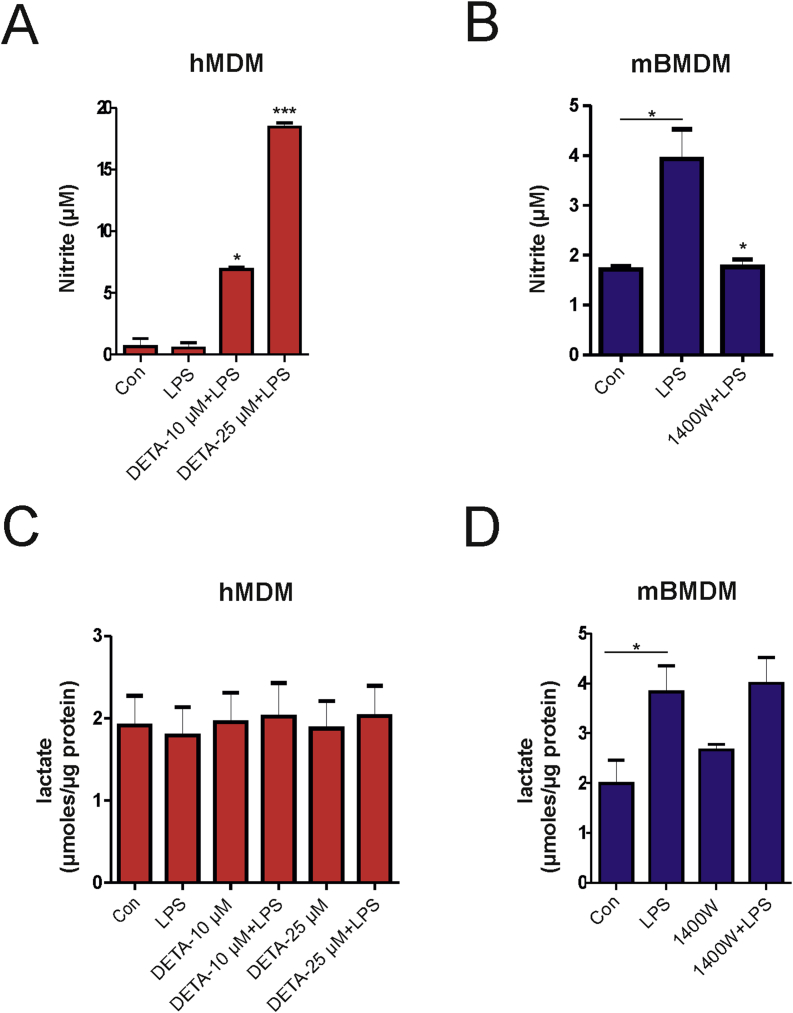
As it is established that mouse but not human macrophages generate significant amounts of nitric oxide (NO) upon LPS stimulation in vitro [18] (Figs. S3A and B), we explored a role for nitric oxide (NO) in induction of glycolysis. Treatment of hMDMs with a NO donor (DETA NONOate) in the presence of LPS increased nitrite levels in the supernatant (Fig. S3B), but did not affect glycolysis as lactate production was unchanged (Fig. S3D). Similarly, treatment with the iNOS inhibitor 1400W inhibited LPS-dependent production of NO in mouse macrophages (Fig. S3A), but failed to block the production of lactate indicating that NO does not affect glycolysis (Fig. S3C) and confirmed observations from a previous study [11]. In summary NO does not regulate glycolysis in LPS-activated macrophages.
3. Conclusion
Although the human and mouse immune system exhibit extensive similarities, which makes it a primary tool of choice for research, several evolutionary divergences have been demonstrated [20]. The current study identifies a difference in metabolic reprogramming in response to the TLR4 ligand LPS between human and mouse macrophages indicating that inflammatory activation of human macrophages is not driven by glycolytic reprogramming. The differences in ΔΨm and resistance to mitochondrial dysfunction that was observed in this study might also be of evolutionary significance and has to be explored further. While we acknowledge the limitation of this study that might arise from the use of hMDMs (which is the most widely used model of human macrophages) in comparison to BMDMs from mouse, our findings are in accordance with a previous observation that human lung macrophages displayed no metabolic alterations upon LPS stimulation in vitro [21]. The increased glycolysis characteristic of mouse macrophages with mitochondrial dysfunction appears to play a role in cell survival as previously shown for astrocytes [22]. It should be noted that metabolic reprogramming is not restricted to glycolysis and changes in other metabolic pathways such as fatty acid oxidation could occur. In conclusion, the findings from this study suggest the need to validate data obtained on immunometabolism from rodent macrophages in human macrophages to confirm their relevance in translational medicine.
Author contributions
VV, FG, RF, RM and SI designed the experiments. VV, PP, RF, LB and HF conducted experiments. VV, PP, FG, RF, RM and SI wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Acknowledgement
We wish to thank Anette Sarti and Sylvie Manin for their excellent technical assistance. The authors also like to thank Dr. Christos Chinopoulos for the experimental advices to perform mitochondrial membrane potential analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101147.
Contributor Information
Roberta Foresti, Email: roberta.foresti@inserm.fr.
Stephan Immenschuh, Email: immenschuh.stephan@mh-hannover.de.
Materials and methods
Reagents
Antibodies for flow cytometry CD80 and CD206 and ELISA kits for mouse and human IL-6 and IL-10 were purchased from Biolegend (San Diego, CA, USA). Recombinant cytokines M-CSF, IL-4 and IFN-γ was purchased from PeproTech Inc, USA. Lipopolysaccharide serotype 0111:B4 was from Invivogen (San Diego, CA, USA). Human AB serum was purchased from c.c. pro. GmbH (Thuringia, Germany). DMEM, RPMI-1640 and all the other reagents unless specified were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Mice
Wild type C57BL/6J 6-12 weeks old mice (Charles river, Sulzfeld, Germany) were kept on a normal laboratory diet and were housed in cages under standardized environmental conditions (12-h light/dark cycle, 23 ± 1 °C and 55 ± 1% relative humidity). All experiments were approved by the Committee for Animal Welfare.
Cell isolation and culture
Human peripheral mononuclear blood cells were isolated from healthy donors by density centrifugation using lymphosep (c.c. pro GmbH, Thuringia, Germany) and differentiated in RPMI 1640 medium containing 5% AB-serum, 100 U/ml penicillin, and 10 mg/ml streptomycin and 25 ng/ml recombinant human macrophage-colony stimulating factor (M-CSF). Mouse bone marrow cells were isolated from the tibia and femur of C57BL6/J and differentiated in DMEM high glucose media containing 10% fetal bovine serum (FBS) (Merck), 100 U/ml penicillin, 10 mg/ml streptomycin and 25 ng/ml recombinant mouse M-CSF. Cells were harvested on the 7th day of differentiation and plated in a 12-well plate for experiments. All treatments were done in 1% serum containing 12.5 ng/ml M-CSF and LPS was used at a concentration of 1 μg/ml.
Analysis of mRNA expression
RNA isolation was performed using an RNeasy mini kit (Qiagen GmbH, Hilden, Germany). High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA) was used for cDNA synthesis. Primers for quantification of mRNA levels of TNF-α, IL-6 and GAPDH were from Applied Biosystems. Amplification was performed with TaqMan Gene Expression Master Mix on a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). Thermal cycling was performed at 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. GAPDH was used as a control for normalization of cDNA values. The ΔΔCT method was used to semi quantify mRNA levels.
Extracellular flux assay
Real time bioenergetic profile of mBMDMs and hMDMs were obtained by measuring oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) using a Seahorse XF extracellular flux analyzer (Seahorse Bioscience, Inc, North Billerica, MA, USA). Briefly, mBMDMs or hMDMs were seeded at a density of 50,000 cells per well. After overnight culture, cells were left untreated or treated with LPS for 16 h. Cells were then washed and the medium was replaced with FCS- and bicarbonate-free DMEM medium supplemented with 4.5 g/L d-glucose and 2 mM glutamine. Following incubation in an incubator without CO2 at 37 °C for 60 min, basal OCR and ECAR were recorded for 30 min. Mito Stress assay was performed by sequential addition of 1 μg/ml oligomycin (inhibitor of ATP synthesis), 0.7 μM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, uncoupling agent) and 1 μM rotenone/antimycin A (inhibitors of complex I and complex III of the respiratory chain, respectively). Parameters such as ATP-linked OCR, maximal OCR, reserve capacity and proton leak were calculated from Mito Stress assays of three independent experiments as previously described [23,24].
Flow cytometry
At the end of the experiment, cells were detached with accutase (Capricorn Scientific GmbH, Germany), collected in FACS tube and washed once with PBS. Cells were stained with antibodies for mouse and human CD80 and CD206 for 20 min followed by two wash steps and resuspended in 300 μL volume PBS. Cells were analyzed using a FACS Canto II flow cytometer and FACSDiva Software (BD Biosciences, San Jose, CA, USA). The mean fluorescence intensity from three independent experiments were calculated and shown.
Mitochondrial membrane potential
Mitochondrial membrane potential was assessed either by JC-1 or TMRM (Cayman Chemical, MI, USA). Cells were stained with JC-1 (1 μm) and TMRM (20 nM) for 30 min at 37 °C followed by fluorescence microscopy or FACS analysis. A positive control (FCCP 20 μM) was included but not shown. For JC-1 staining, the microscopic images taken with Olympus IX81 was quantified using Image J. Briefly, the corrected total cell fluorescence of approximately 100 cells was calculated and represented as a bar graph.
Mitosox assay
To determine mitochondrial ROS production, cells were stained with 5 μm of Mitosox (Thermo Fisher Scientific, Inc. Waltham, MA, USA) for 10 min at the end of the experiment followed by FACS analysis.
MTT assay
Cells were washed with PBS and incubated with 0.5 mg/ml MTT in RPMI or DMEM medium for 2 h. The resulting formazan crystals were dissolved in DMSO and absorbance was measured at 570 nm using a spectrophotometer. The percentage of viable cells was calculated by the following formula: A570 of treated cells/A570 of non-treated cells x 100.
ATP assay
ATP levels were determined using a ATP assay kit (Cayman Chemical, MI, USA) according to the manufacturer's protocol. The ATP levels determined after addition of 2DG or oligomycin are expressed as percentage in relation to the levels determined before addition which was taken as 100%.
Other biochemical assays
Measurements of lactate (Cayman Chemical, MI, USA), cytokines (Biolegend, San Diego, CA, USA), nitrite (Promega, Madison, Wisconsin, USA) and LDH (Sigma-Aldrich, St. Louis, MO, USA) were performed using the respective assay kits according to the manufacturers' instruction.
Live or dead cell assay
A live or dead cell assay (Abcam, Cambridge, MA, USA) was performed according to the manufacturers' instruction and analyzed by fluorescent microscopy using the Olympus IX81 microscope (Olympus). The pictures obtained were processed using image J software.
Statistical analyses
All statistical data analysis was performed using One-way ANOVA with Post Tukey's test or Student's t-test as indicated in the figure legends using GraphPad Prism Version 5 (GraphPad Prism Software Inc.).
Funding resources
This work was supported by funding from a PHC Procope/DAAD Exchange Program between France and Germany, INSERM and University Paris Est Créteil, the Deutsche Forschungsgemeinschaft (IM 20/4-1 to SI) and the European Union and the State of Niedersachsen (EFRE ZW6-85007634 to SI). The funding bodies had no role in the preparation and submission of the manuscript.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
M1 and M2 polarization of human and mouse macrophages. hMDMs and mBMDMs were stimulated with IFN-γ (10 ng/ml), IL-4 (20 ng/ml) or LPS for 24 h. In cell cultures treated with IFN-γ+LPS, LPS was added after 6 h of pulsing with IFN‑γ. (A‑B) The expression of M1 marker CD80 and M2 marker CD206 was analyzed by flow cytometry and the fold induction of mean fluorescence intensity relative to unstimulated control macrophages is shown (n = 3). (C-D) TNF-α, and IL6 expression was analyzed by quantitative real-time PCR. GAPDH was used as the house-keeping gene and the fold induction relative to control wells is shown (n=3). The values shown represent mean ± SEM of three independent experiments; One-way ANOVA with Tukey’s post-hoc analysis; *p < 0.05, **p < 0.01, ***p < 0.001.
figs2.
Oxidative phosphorylation is the primary source of ATP generation in hMDMs. (A) Total ATP levels were determined in control and LPS-activated hMDMs and mBMDMs. Values in relation to control, which is taken as 100% from three independent experiments, are shown. (B) hMDMs were treated with LPS for 16 h followed by staining with Mitosox for 30 min. Treatment with the complex I inhibitor rotenone (2 μM) for 30 min was used as a positive control. The mean fluorescence intensity from three independent experiments is shown as arbritrary units. (C) hMDMs were left untreated or treated with oligomycin (2 μM), antimycin A (5 μM) and rotenone (1 μM) as indicated for 8 h and the supernatant was used for lactate measurements. Values from two independent experiments are shown (A) Student’s t test (B-C) One-way ANOVA with Tukey’s post-hoc analysis; *p < 0.05.
figs3.
Nitric oxide (NO) does not contribute to the induction of glycolysis. (A) Human (hMDM) or (B) mouse (mBMDM) were treated for 16 h with the indicated compounds and nitrite levels were determined in culture supernatants. (C-D) Human (hMDM) or mouse (mBMDM) were treated for 16 h with the indicated agents and the lactate levels were determined in the culture supernatants. Values obtained were normalized to total protein from total cell lysates. Values shown represent mean ± SEM of three independent experiments. Statistical analysis was done using One-way ANOVA with Tukey's post-hoc analysis; *p < 0.05, **p < 0.01, ***p < 0.001.
References
- 1.O'Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarrinpar A., Bensinger S.J. The therapeutic potential of T cell metabolism. Am. J. Transplant. 2017;17:1705–1712. doi: 10.1111/ajt.14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornberg M.D., Bhargava P., Kim P.M., Putluri V., Snowman A.M., Putluri N., Calabresi P.A., Snyder S.H. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science. 2018;360:449–453. doi: 10.1126/science.aan4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Neill L.A. A metabolic roadblock in inflammatory macrophages. Cell Rep. 2016;17:625–626. doi: 10.1016/j.celrep.2016.09.085. [DOI] [PubMed] [Google Scholar]
- 5.Van den Bossche J., O'Neill L.A., Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406. doi: 10.1016/j.it.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Kelly B., O'Neill L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorresteijn M.J., Paine A., Zilian E., Fenten M.G., Frenzel E., Janciauskiene S., Figueiredo C., Eiz-Vesper B., Blasczyk R., Dekker D., Pennings B., Scharstuhl A., Smits P., Larmann J., Theilmeier G., van der Hoeven J.G., Wagener F.A., Pickkers P., Immenschuh S. Cell-type-specific downregulation of heme oxygenase-1 by lipopolysaccharide via Bach 1 in primary human mononuclear cells. Free Radic. Biol. Med. 2015;78:224–232. doi: 10.1016/j.freeradbiomed.2014.10.579. [DOI] [PubMed] [Google Scholar]
- 8.Schroder K., Irvine K.M., Taylor M.S., Bokil N.J., Le Cao K.A., Masterman K.A., Labzin L.I., Semple C.A., Kapetanovic R., Fairbairn L., Akalin A., Faulkner G.J., Baillie J.K., Gongora M., Daub C.O., Kawaji H., McLachlan G.J., Goldman N., Grimmond S.M., Carninci P., Suzuki H., Hayashizaki Y., Lenhard B., Hume D.A., Sweet M.J. Conservation and divergence in Toll-like receptor 4-regulated gene expression in primary human versus mouse macrophages. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E944–E953. doi: 10.1073/pnas.1110156109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce E.L., Pearce E.J. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills E.L., Kelly B., Logan A., Costa A.S.H., Varma M., Bryant C.E., Tourlomousis P., Dabritz J.H.M., Gottlieb E., Latorre I., Corr S.C., McManus G., Ryan D., Jacobs H.T., Szibor M., Xavier R.J., Braun T., Frezza C., Murphy M.P., O'Neill L.A. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470 e413. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Bossche J., Baardman J., Otto N.A., van der Velden S., Neele A.E., van den Berg S.M., Luque-Martin R., Chen H.J., Boshuizen M.C., Ahmed M., Hoeksema M.A., de Vos A.F., de Winther M.P. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17:684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Menk A.V., Scharping N.E., Moreci R.S., Zeng X., Guy C., Salvatore S., Bae H., Xie J., Young H.A., Wendell S.G., Delgoffe G.M. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 2018;22:1509–1521. doi: 10.1016/j.celrep.2018.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baseler W.A., Davies L.C., Quigley L., Ridnour L.A., Weiss J.M., Hussain S.P., Wink D.A., McVicar D.W. Autocrine IL-10 functions as a rheostat for M1 macrophage glycolytic commitment by tuning nitric oxide production. Redox Biol. 2016;10:12–23. doi: 10.1016/j.redox.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ip W.K.E., Hoshi N., Shouval D.S., Snapper S., Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–519. doi: 10.1126/science.aal3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Q., Chu Z., Zhu L., Yang T., Wang P., Liu F., Huang Y., Zhang F., Zhang X., Ding W., Zhao Y. 2-Deoxy-d-Glucose treatment decreases anti-inflammatory M2 macrophage polarization in mice with tumor and allergic airway inflammation. Front. Immunol. 2017;8:637. doi: 10.3389/fimmu.2017.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida A., Almeida J., Bolanos J.P., Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15294–15299. doi: 10.1073/pnas.261560998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everts B., Amiel E., van der Windt G.J., Freitas T.C., Chott R., Yarasheski K.E., Pearce E.L., Pearce E.J. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Follstad B.D., Wang D.I., Stephanopoulos G. Mitochondrial membrane potential differentiates cells resistant to apoptosis in hybridoma cultures. Eur. J. Biochem. 2000;267:6534–6540. doi: 10.1046/j.1432-1327.2000.01743.x. [DOI] [PubMed] [Google Scholar]
- 19.Houston M.A., Augenlicht L.H., Heerdt B.G. Stable differences in intrinsic mitochondrial membrane potential of tumor cell subpopulations reflect phenotypic heterogeneity. Int. J. Cell. Biol. 2011;2011 doi: 10.1155/2011/978583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mestas J., Hughes C.C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 21.Lavrich K.S., Speen A.M., Ghio A.J., Bromberg P.A., Samet J.M., Alexis N.E. Macrophages from the upper and lower human respiratory tract are metabolically distinct. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;315(5):L752–L764. doi: 10.1152/ajplung.00208.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Supplie L.M., Duking T., Campbell G., Diaz F., Moraes C.T., Gotz M., Hamprecht B., Boretius S., Mahad D., Nave K.A. Respiration-deficient astrocytes survive as glycolytic cells in vivo. J. Neurosci. 2017;37:4231–4242. doi: 10.1523/JNEUROSCI.0756-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dranka B.P., Benavides G.A., Diers A.R., Giordano S., Zelickson B.R., Reily C., Zou L., Chatham J.C., Hill B.G., Zhang J., Landar A., Darley-Usmar V.M. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic. Biol. Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill B.G., Benavides G.A., Lancaster J.R., Jr., Ballinger S., Dell'Italia L., Jianhua Z., Darley-Usmar V.M. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]