Abstract
Absorption of dietary lipids in the small intestine is dependent on the emulsification by bile acids (BA) and the formation of chylomicrons. Cholestyramine is a common drug used in humans—and potentially dogs—to treat BA malabsorption associated with chronic diarrhea. It is known to bind BA to form insoluble complexes, preventing their reabsorption and possibly proper emulsification and absorption of dietary fats. The objective of this study was to evaluate the effects of cholestyramine on 1) macronutrient apparent total tract digestibility (ATTD), and 2) fecal characteristics and metabolites of healthy adult dogs. We hypothesized that cholestyramine would decrease ATTD of fat and organic matter (OM), increase fecal dry matter (DM) content, and increase fecal output. Twelve healthy beagles (3.2 ± 0.8 yr; 10.4 ± 0.9 kg) were used in a randomized crossover design. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee before the study. The study included a baseline period and two 14-d experimental periods separated by a 14-d washout. All dogs were fed the same experimental diet, formulated to meet all nutrient needs recommended by AAFCO, throughout the study. Dogs were randomized into 2 groups [diet only (control) or diet + 11.4 g/d cholestyramine (8 g/d active ingredient)] in Period 1 and received the other treatment in Period 2. During the washout, all dogs were fed the diet only. Dogs were fed once daily (0800 h) to maintain BW. Total fecal output was collected during the last 4 d of each period for ATTD analysis. On day 14 of each of period, fresh fecal and blood samples were collected for metabolite analysis. Dogs fed cholestyramine had lower (P < 0.001) ATTD of DM, OM, energy, crude protein, and fat and lower (P < 0.01) fecal scores (firmer stools) than controls. Dogs fed cholestyramine had greater (P < 0.01) as-is and dry fecal output than controls. Dogs fed cholestyramine had lower (P < 0.05) fecal ammonia and phenol concentrations, but greater (P < 0.05) fecal indole, acetate, butyrate, and total short-chain fatty acid concentrations than controls. Fecal DM% and pH were greater (P < 0.01) in dogs fed cholestyramine. Our results indicate that cholestyramine, when given with a meal, is safe and well tolerated but significantly decreases nutrient digestibility and alters fecal characteristics. Future studies are required to explore the effects of cholestyramine on dogs with gastrointestinal disease.
Keywords: canine, fecal metabolites, macronutrient digestibility
INTRODUCTION
Cholestyramine is a prescription anion exchange resin that nonspecifically binds bile acids (BA) in the gastrointestinal tract, forming insoluble complexes thereby preventing their reabsorption in the ileum and promoting fecal excretion (West and Lloyd, 1975; Acalovschi and Paumgartne, 2000). Originally, cholestyramine was used to treat hypercholesterolemia as it increases BA fecal excretion, thus using endogenous cholesterol to synthesize new BA (Bergen et al., 1959; Whiteside et al., 1965). More recently, cholestyramine has been used to treat symptoms of itching during jaundice (Acalovschi and Paumgartne, 2000) and is considered the first line of treatment for BA-induced diarrhea (BAD) (West and Lloyd, 1975; Hutcheon et al., 1979; Arlow et al., 1987; Stotzer et al., 2013; Camilleri, 2015). Bile acid-induced diarrhea is most commonly observed in patients with inflammatory bowel disease (IBD) or those who have undergone ileal resection or cholecystectomy (Arlow et al., 1987; Camilleri, 2015).
Inflammatory bowel disease is a group of chronic inflammatory bowel conditions, the most common in humans being Crohn’s disease and ulcerative colitis (Vítek, 2015). However, IBD is not limited to humans. In canines, IBD is characterized as a group of chronic gastrointestinal conditions characterized by persistent/recurring gastrointestinal symptoms with an unknown etiology, and is one of the most common causes of chronic gastrointestinal disease in dogs (Simpson and Jergens, 2011; Suchodolski et al., 2012). Although cholestyramine is commonly prescribed to human patients with BAD, in canines it is primarily used to bind and excrete ingested toxins (Guentert et al., 1986). Cholestyramine may also have therapeutic potential for canine IBD; however, this has yet to be investigated.
Though cholestyramine is effective at relieving symptoms of BAD, binding BA may interfere with proper micelle formation in the small intestine and subsequently affect lipid and energy digestibility and absorption. Long-term cholestyramine supplementation has been shown to increase fecal fat content in children with hypercholesterolemia, but had no impact on circulating iron, calcium, and vitamin B12 concentrations (West and Lloyd, 1975). In contrast, in rats, cholestyramine has been shown to decrease serum iron and vitamin B12 as well as tissue nonheme iron stores (Thomas et al., 1972). In chicks, cholestyramine has been shown to impair dietary fat absorption and utilization of vitamin A and vitamin K (Whiteside et al., 1965; Furuse et al., 1997). Additionally, in humans, cholestyramine consumption has been associated with gastrointestinal symptoms including constipation and bloating (Maciejko et al., 1994). However, to our knowledge, the impact of cholestyramine on nutrient digestibility and fecal characteristics has not yet been investigated in dogs. This study, therefore, aimed to investigate the impact of short-term cholestyramine administration on macronutrient apparent total tract digestibilities (ATTD) and fecal characteristics and metabolites of healthy adult dogs. We hypothesized that cholestyramine would decrease ATTD of fat and organic matter (OM), increase fecal dry matter (DM) content, and increase fecal output.
MATERIALS AND METHODS
Animals, Diet, and Treatment
All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee before the study (protocol #16063). Twelve healthy adult [mean age = 3.17 ± 0.82 yr] female beagles [mean BW = 10.35 ± 0.88 kg; mean body condition score (BCS) = 5.25 ± 0.75] used in this study were housed individually in pens (1.22 m wide × 1.85 m long) in a temperature-controlled room (20 °C) on a 14 h light: 10 h dark cycle in the animal facility at the Veterinary Medicine Basic Sciences Building at the University of Illinois. Dogs were weighed and BCS were assessed using a 9-point scale (1: ribs, lumbar vertebrae, and pelvic bones visible from a distance, no discernible body fat; 5: ribs palpable without excess fat covering, abdominal tuck visible from above; 9: massive fat deposits across the body including neck and limbs, abdominal tuck absent, obvious abdominal distention) once a week in the morning before feeding. Dogs were provided with toys for behavioral enrichment at all times and exercised outside of their cages and socialized with each other and humans for approximately 1 h at least 3 d/wk. Dogs were evaluated and shown to be healthy before the study and after study completion. Welfare was monitored by research and animal care staff daily and trained veterinarians during the study when needed.
The ingredient and chemical composition of the experimental diet and cholestyramine are listed in Tables 1 and 2, respectively. The diet was mixed by Lortscher Animal Nutrition (Bern, KS), manufactured by AFB International (St. Charles, MO), and formulated to meet all Association of American Feed Control Officials (AAFCO, 2015) nutrient recommendations for adult dogs. All dogs were fed the same experimental diet for the duration of the study. Initial food intake was determined by calculating the maintenance energy requirement (National Research Council, 2006) and by using previous feeding records. Dogs had access to fresh water ad libitum and were fed once a day (0800 h) to maintain BW throughout the study. Food intake and refusals were measured and recorded at each meal. Cholestyramine powder (Sandoz, Holzkirchen, Upper Bavaria, Germany; 11.4 g powder, 8 g active ingredient) was mixed with water and administered via a top dressing on the food each day during experimental periods. Dogs in the control group received only diet during the experimental periods.
Table 1.
Ingredient and analyzed chemical composition of the experimental diet
| Ingredients | % as-fed |
|---|---|
| Chicken by-product meal (low-ash) | 49.00 |
| Brewer’s rice (U.S. #4) | 33.36 |
| Chicken fat | 9.00 |
| Corn, whole (U.S. #2) | 5.00 |
| Cellulose—Solka Floc 900 | 2.00 |
| Salt (sodium chloride) | 0.50 |
| Potassium chloride (50% K) | 0.45 |
| Taurine | 0.20 |
| Mineral premix1 | 0.18 |
| Vitamin premix2 | 0.18 |
| Choline chloride | 0.13 |
| Analyzed chemical composition | % |
| Dry matter (DM) | 91.1 |
| % DM basis | |
| Organic matter | 93.0 |
| Crude protein (CP) | 38.6 |
| Acid-hydrolyzed fat | 17.3 |
| Total dietary fiber | 7.2 |
| Gross energy, kcal/g DMB | 5.30 |
| Calculated metabolizable energy (ME), kcal/g DMB3 | 3.87 |
1Provided per kg diet: Mn (as MnSO4), 66.00 mg; Fe (as FeSO4), 120 mg; Cu (as CuSO4), 18.00 mg; Co (as CoSO4), 1.20 mg; Zn (as ZnSO4), 240 mg; I (as KI), 1.80 mg; Se (as Na2SeO3), 0.24 mg.
2Provided per kg diet: vitamin A, 5.28 mg; vitamin D3, 0.04 mg; vitamin E, 120.00 mg; vitamin K, 0.88 mg; thiamin, 4.40 mg; riboflavin, 5.72 mg; pantothenic acid, 22.00 mg; niacin, 39.60 mg; pyridoxine, 3.52 mg; biotin, 0.13 mg; folic acid, 0.44 mg; vitamin B12, 0.11 mg.
3ME = 8.5 kcal ME/g fat + 3.5 kcal ME/g CP + 3.5 kcal ME/g nitrogen-free extract.
Table 2.
Ingredient and analyzed chemical composition of the cholestyramine
| Active ingredients | Content, g per 11.4 g serving |
|---|---|
| Cholestyramine | 8.0 |
| Inactive ingredients1 | 3.4 |
| Analyzed chemical composition | % |
| Dry matter (DM) | 90.9 |
| % DM basis | |
| Organic matter | 99.1 |
| Crude protein (CP) | 33.4 |
| Acid-hydrolyzed fat | 0.72 |
| Total dietary fiber | N/A |
| Calculated metabolizable energy (ME, kcal/g DMB)2 | 3.50 |
1Aspartame, anhydrous citric acid, silicon dioxide, D&C Yellow No. 10, FD&C Yellow No. 6, fructose, mannitol, ammonium glycyrrhizate, orange, pectin, propylene glycolalginate, sorbitol, xanthan gum.
2ME = 8.5 kcal ME/g fat + 3.5 kcal ME/g CP + 3.5 kcal ME/g nitrogen-free extract.
Experimental Design
Dogs were randomized to an initial treatment group [control (CON) or cholestyramine (CHOL)], and a crossover design was used. The experiment consisted of a 14-d baseline period and two 14-d experimental periods separated by 14-d washout periods, for a total of five 14-d segments. Each of the 5 segments consisted of an adaption phase (day 1 to 10) and a fecal and blood collection phase (day 11 to 14).
Fecal Sample Collection
On day 11 to 14, all fecal samples were collected from the pen floor several times throughout the day. All samples were scored, weighed, and stored in Whirl-Pak bags (Nasco, Fort Atkinson, WI) at −20 °C until analysis. Samples were scored in 0.5 unit increments using the following scale: 1 = hard, dry pellets; small hard masses, 2 = hard, formed, dry stool; remains firm and soft, 3 = soft, formed, and moist stool; retains shape, 4 = soft, unformed stool; assumes shape of container, 5 = watery, liquid that can be poured. On day 14, a fresh fecal sample was collected from the pen floor of each dog within 15 min of defecation. Fecal pH was measured immediately by placing a probe directly into the sample, and then aliquots were collected. Aliquots for phenol and indole measurements were stored at −20 °C until analysis. An aliquot for ammonia, short-chain fatty acid (SCFA), and branched-chain fatty acid (BCFA) measurement was placed in 2 N HCl and stored at −20 °C until analysis. Finally, an aliquot was collected for fecal DM determination. Any remaining feces was stored at −20 °C with previous fecal samples until analysis.
Chemical Analysis of Diet, Cholestyramine, and Fecal Samples
Total fecal samples were used to analyze ATTD. Diet and fecal samples were ground using a Wiley mill (model 4, Thomas Scientific, Swedesboro, NJ) through a 2-mm screen and analyzed for DM, OM, and ash according to the Association of Official Analytical Chemists (AOAC) (AOAC, 2006). Crude protein was calculated from Leco (FP2000 and TruMac) total nitrogen values (AOAC, 2006). Total lipid content (acid-hydrolyzed fat) was determined according to the methods of the American Association of Cereal Chemists (AACC) and Budde (Budde, 1952; AACC, 1983). Total dietary fiber was determined according to Prosky et al. (1992). Gross energy was measured as kcal/g fecal sample using an oxygen bomb calorimeter (model 1261, Parr Instruments, Moline, IL). All data are reported on a dry matter basis (DMB). Each ATTD measurement was calculated as a percentage using the following equation:
Fecal Metabolite Analysis
Fecal SCFA and BCFA concentrations were determined using gas chromatography according to Erwin et al. (1961) using a gas chromatograph (Hewlett-Packard 5890A series II, Palo Alto, CA) and a glass column (180 cm × 4 mm i.d.) packed with 10% SP-1200/1% H3PO4 on 80/100+ mesh Chromosorb WAW (Supelco Inc., Bellefonte, PA). Nitrogen was used as the carrier with a flow rate of 75 mL/min. Oven, detector, and injector temperatures were 125, 175, and 180 °C, respectively. Fecal ammonia concentration was analyzed according to the method of Chaney and Marbach (1962). Fecal phenol and indole concentrations were determined using gas chromatography according to the method of Flickinger et al. (2003).
Statistical Analysis
Based on our previous feline (Deng et al., 2014; n = 12) and canine (Beloshapka et al., 2014; n = 12) experiments in this area, we estimated the need for 12 dogs per treatment to reach a significance level of 0.05 with a power of 80% for the variables of interest. We used a crossover design so that each animal received both the treatment and control provided. This design allowed the animals to serve as a self-control, reducing outcome variation as well as the number of animals needed.
All data were analyzed using SAS (version 9.4; SAS Institute, Cary, NC) using the Mixed Models procedure, testing the main effect of treatment and including the random effect of dog. Data are reported as least square means ± standard error of the means (SEM) with statistical significance set as P < 0.05 and P < 0.10 considered as trends.
RESULTS AND DISCUSSION
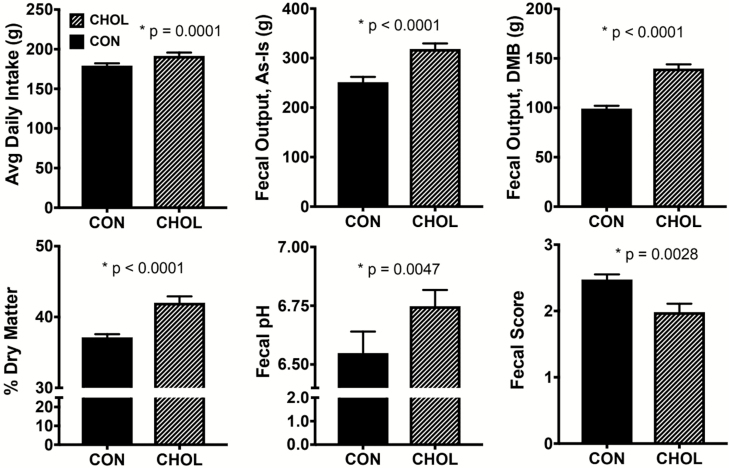
No adverse events were observed after cholestyramine supplementation during either treatment period. Average dietary intake was not different between treatment groups, but total daily intake was greater (P < 0.0001) when consumption of the 11.4 g/d cholestyramine in the CHOL group was included (Fig. 1). Dogs were fed to maintain body weight and there was no difference between groups in change in body weight from baseline. Because there was no difference in body weight change or dietary intake between groups, it does not appear that acute cholestyramine treatment caused an energy deficit. Total fecal output over the 4-d collection period was greater (P < 0.0001) in CHOL compared to CON dogs, on both an as-is and DMB. Additionally, fecal DM content (P < 0.0001) and pH (P < 0.005) were greater in CHOL dogs. Fecal scores were lower (P < 0.003) after cholestyramine treatment compared to the control (Fig. 1). Together, these data indicate that cholestyramine supplementation results in larger, drier, and more firm fecal samples. While this may suggest the development of mild constipation, the number of bowel movements per day was not different between treatment groups. Additionally, ideal fecal scores ranged from 2 to 3, so cholestyramine treatment did not result in abnormal fecal scores. In contrast, constipation is one of the most commonly reported side effects of BA sequestrants in humans (Blum and Levy, 1989; Maciejko et al., 1994).
Figure 1.
Average daily dietary intake, total fecal output, and fecal characteristics of cholestyramine-supplemented dogs. Control: solid bars, cholestyramine: patterned bars. n = 12 per group. Avg = average, DMB = dry matter basis. Fecal score scale: 1 = hard, dry pellets; small hard mass; 2 = hard formed, dry stool; remains firm and soft; 3 = soft, formed and moist stool, retains shape; 4 = soft, unformed stool; assumes shape of container; 5 = watery, liquid that can be poured.
The significant increase in fecal output, despite no change in dietary intake between groups, suggests that macronutrient ATTD was decreased after CHOL supplementation. The ATTD of DM, OM, crude protein, acid-hydrolyzed fat, and energy were lower (P < 0.001) after CHOL compared to CON (Table 3). It is commonly stated that all macronutrients should be digested at a rate of 80% or greater for dog foods (Case et al., 2011). Although cholestyramine supplementation reduced all macronutrient ATTD parameters by approximately 4 to 8 percentage units each, they did not fall below this threshold. It should be noted that the present study investigated the short-term effects of cholestyramine administration. Because CHOL is generally prescribed as a long-term medication, however, future studies investigating long-term effects on macronutrient ATTD and BW are warranted.
Table 3.
Apparent total tract macronutrient digestibility of cholestyramine-supplemented dogs
| Treatment | ||||
|---|---|---|---|---|
| CON | CHOL | |||
| --- % digestibility --- | SEM | P-value | ||
| Dry matter | 85.47 | 80.44 | 0.58 | <0.001 |
| Organic matter | 89.28 | 83.64 | 0.48 | <0.001 |
| Crude protein | 88.17 | 84.26 | 0.50 | <0.001 |
| Acid-hydrolyzed fat | 94.98 | 91.10 | 0.36 | <0.001 |
| Energy | 89.68 | 81.94 | 0.52 | <0.001 |
n = 12 per group.
Additionally, several fecal metabolites were affected by short-term cholestyramine supplementation. Fecal acetate (P < 0.001), butyrate (P = 0.004), and total SCFA (P = 0.001) were greater after CHOL compared to CON (Table 4). Production of SCFA is beneficial to the host, as they provide energy to colonocytes (particularly butyrate), stimulate synthesis of gut peptides, and initiate signal transduction pathways that promote energy utilization (Roediger, 1982; Topping and Clifton, 2001). It is unlikely that this is due to the active ingredient in cholestyramine, but rather the pectin and xanthan gum, 2 soluble, gel-forming, fermentable fibers, within the inactive ingredients in cholestyramine. Additionally, these gel-forming fibers may have bound nutrients in the small intestine, resulting in an increased fermentable substrate load in the large intestine. The inactive ingredients in the test product are likely added to improve mouthfeel because it is administered as an oral suspension. Despite a significant increase in SCFA, fecal pH was elevated (P = 0.0047) after CHOL compared to CON. It is possible that the reduction in crude protein ATTD or presence of other metabolites in complex with the cholestyramine may have contributed to the increase in fecal pH. Branched-chain fatty acids, indoles, phenols, and ammonia are nitrogenous, putrefactive compounds produced from bacterial metabolism of undigested protein in the gastrointestinal tract. Increased protein delivery to the large intestine provides more substrate for protein metabolism by bacteria (Swanson et al., 2002). Despite a decrease in crude protein ATTD, fecal ammonia concentrations decreased (P = 0.019) and neither individual nor total BCFA were affected (P > 0.05) after CHOL supplementation (Table 4). An inverse relationship was observed in the effect of CHOL on fecal indoles and phenols, with fecal indole concentrations increasing (P = 0.039) and fecal phenol concentrations decreasing (P = 0.047). Because of this inverse shift, there was no change (P > 0.05) in fecal total indole and phenol concentrations (Table 4).
Table 4.
Fecal metabolites of cholestyramine-supplemented dogs
| Treatment | ||||
|---|---|---|---|---|
| CON | CHOL | |||
| --- μmol/g DM basis --- | SEM | P-value | ||
| Total SCFA1 | 414.08 | 704.95 | 86.73 | 0.001 |
| Acetate | 229.91 | 434.48 | 52.41 | <0.001 |
| Butyrate | 62.21 | 101.29 | 14.69 | 0.004 |
| Propionate | 121.96 | 169.18 | 22.04 | 0.145 |
| Total BCFA1 | 31.60 | 34.75 | 4.23 | 0.632 |
| Isobutyrate | 11.31 | 13.36 | 1.59 | 0.479 |
| Isovalerate | 18.34 | 19.78 | 2.60 | 0.933 |
| Valerate | 1.94 | 1.60 | 0.53 | 0.250 |
| Total indoles + phenols | 4.61 | 4.66 | 0.49 | 0.945 |
| Indoles | 3.14 | 4.20 | 0.34 | 0.039 |
| Phenols | 1.47 | 0.45 | 0.31 | 0.047 |
| Ammonia | 304.54 | 230.12 | 37.50 | 0.019 |
n = 12 per group.
1Total short-chain fatty acids (SCFA) = acetate + butyrate + propionate; total branched-chain fatty acids (BCFA) = valerate + isovalerate + isobutyrate.
In summary, short-term cholestyramine supplementation decreased fecal scores and macronutrient digestibility, with a corresponding increase in fecal DM content and output in healthy dogs. Despite a decrease in crude protein ATTD, no increases in microbial protein metabolites were observed after CHOL. Fecal SCFA concentrations were increased after CHOL, likely due to the inclusion of gel-forming, fermentable fibers in the inactive ingredients that may have been directly fermented or may have increased fermentable substrate load in the large intestine via binding of small intestinal nutrients. Although macronutrient digestibility did not fall below the threshold commonly used for dog foods after cholestyramine administration, administering it long-term with lower-quality or less digestible diets should be investigated. Additionally, both short- and long-term effects of cholestryamine on these parameters should be explored in dogs with canine IBD.
Conflict of interest statement
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This work was partially supported by USDA Hatch Grant ILLU-538-937. K.S.S. and J.S.S. designed the experiment. C.A. performed the animal trials and laboratory analyses. C.A. and B.C.G. performed the statistical analyses. C.A. wrote the manuscript. The authors sincerely thank Kiley Algya, Laura Bauer, Amanda Dainton, Katelyn Detweiler, Dr. Maria de Godoy, Chelsea Iennarella, Daisy Ignatova, Lexi Meverden, Thunyaporn Phungviwatnikul, Karine Santos, and Zachary Traughber for their involvement in sample collections.
Footnotes
This work was partially supported by USDA Hatch Grant ILLU-538-937.
LITERATURE CITED
- AACC. 1983. Approved methods. 8th ed. American Association of Cereal Chemists, St Paul, MN. [Google Scholar]
- AAFCO. 2015. Official Publication. Association of American Feed Control Officials, Inc., Oxford, IN. [Google Scholar]
- Acalovschi M., and Paumgartne G., editors. 2000. Hepatobiliary diseases: cholestasis and gallstone. Kluwer Academic Publishers, BV, Dordrecht, The Netherlands: p. 80. [Google Scholar]
- AOAC. 2006. Official methods of analysis. 17th ed. Association of Official Analytical Chemists, Arlington, VA. [Google Scholar]
- Arlow F. L., Dekovich A. A., Priest R. J., and Beher W. T.. 1987. Bile acid-mediated postcholecystectomy diarrhea. Arch. Intern. Med. 147:1327–1329. doi: 10.1001/archinte.1987.00370070139021 [DOI] [PubMed] [Google Scholar]
- Beloshapka A. N., Alexander L. G., Buff P. R., and Swanson K. S.. 2014. The effects of feeding resistant starch on apparent total tract macronutrient digestibility, faecal characteristics and faecal fermentative end-products in healthy adult dogs. J. Nutr. Sci. 3:e38. doi: 10.1017/jns.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen S. S., Van Itallie T. B., Tennent D. M., and Sebrell W. H.. 1959. Effect of an anion exchange resin on serum cholesterol in man. Proc. Soc. Exp. Biol. Med. 102:676–679. doi: 10.3181/00379727-102-25358 [DOI] [PubMed] [Google Scholar]
- Blum C. B., and Levy R. I.. 1989. Current therapy for hypercholesterolemia. JAMA 261:3582–3587. doi: 10.1001/jama.1989.03420240096034 [DOI] [PubMed] [Google Scholar]
- Budde E. F. 1952. The determination of fat in baked biscuit type of dog foods. J. Assoc. Off. Agric. Chem. 35:799–805. [Google Scholar]
- Camilleri M. 2015. Bile acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver. 9:332–339. doi: 10.5009/gnl14397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case L. P., Daristotle L., Hayek M. G., and Raasch M. F.. 2011. Canine and feline nutrition: a resource for companion animal professionals. 3rd ed. Mosby Elsevier, Maryland Heights, MO: p. 180–183. [Google Scholar]
- Chaney A. L., and Marbach E. P.. 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132. [PubMed] [Google Scholar]
- Deng P., Jones J. C., and Swanson K. S.. 2014. Effects of dietary macronutrient composition on the fasted plasma metabolome of healthy adult cats. Metabolomics 10:638–650. doi: 10.1007/s11306-013-0617-7 [DOI] [Google Scholar]
- Erwin E. S., Marco G. J., and Emery E. M.. 1961. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. doi: 10.3168/jds.S0022-0302(61)89956-6 [DOI] [Google Scholar]
- Flickinger E. A., Schreijen E. M., Patil A. R., Hussein H. S., Grieshop C. M., Merchen N. R., and Fahey G. C.. 2003. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 81:2008–2018. doi: 10.2527/2003.8182008x [DOI] [PubMed] [Google Scholar]
- Furuse M., Mabayo R. T., and Okumura J.. 1997. Effects of dietary cholestyramine on the utilisation of diets containing medium or long chain triacylglycerols by chicks. Br. Poult. Sci. 38:436–438. doi: 10.1080/00071669708418016 [DOI] [PubMed] [Google Scholar]
- Guentert T. W., Schmitt M., and Defoin R.. 1986. Acceleration of the elimination of tenoxicam by cholestyramine in the dog. J. Pharmacol. Exp. Ther. 238:295–301. [PubMed] [Google Scholar]
- Hutcheon D. F., Bayless T. M., and Gadacz T. R.. 1979. Postcholecystectomy diarrhea. JAMA 241:823–824. doi: 10.1001/jama.1979.03290340041024 [DOI] [PubMed] [Google Scholar]
- Maciejko J. J., Brazg R., Shah A., Patil S., and Rubenfire M.. 1994. Psyllium for the reduction of cholestyramine-associated gastrointestinal symptoms in the treatment of primary hypercholesterolemia. Arch. Fam. Med. 3:955–960. doi: 10.1001/archfami.3.11.955 [DOI] [PubMed] [Google Scholar]
- National Research Council. 2006. Nutrient requirements of dogs and cats. National Research Council of the National Academies, Washington, DC. [Google Scholar]
- Prosky L., Asp N.-G., Schweizer T. F., DeVries J. W., and Furda I.. 1992. Determination of soluble and insoluble dietary fiber in foods and food products: collaborative study. J. AOAC 75:360–367. [PubMed] [Google Scholar]
- Roediger W. E. W. 1982. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 83:424–429. doi: 10.1016/S0016-5085(82)80339-9 [DOI] [PubMed] [Google Scholar]
- Simpson K. W., and Jergens A. E.. 2011. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet. Clin. North Am. Small Anim. Pract. 41:381–398. doi: 10.1016/j.cvsm.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Stotzer P.-O., Abrahamsson H., Bajor A., and Sadik R.. 2013. Effect of cholestyramine on gastrointestinal transit in patients with idiopathic bile acid diarrhea: a prospective, open-label study. Neuroenterology 2:1–5. doi: 10.4303/ne/235657 [DOI] [Google Scholar]
- Suchodolski J. S., Markel M. E., Garcia-Mazcorro J. F., Unterer S., Heilmann R. M., Dowd S. E., Kachroo P., Ivanov I., Minamoto Y., E. M. et al. 2012. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS One. 7:e51907. doi: 10.1371/journal.pone.0051907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson K. S., Grieshop C. M., Flickinger E. A., Bauer L. L., Healy H.-P., Dawson K. A., Merchen N. R., and Fahey G. C.. 2002. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr. 132:980–989. doi: 10.1093/jn/132.5.980 [DOI] [PubMed] [Google Scholar]
- Thomas F. B., Salsburey D., and Greenberger N. J.. 1972. Inhibition of iron absorption by cholestyramine. Demonstration of diminished iron stores following prolonged administration. Am. J. Dig. Dis. 17:263–269. doi: 10.1007/BF02232300 [DOI] [PubMed] [Google Scholar]
- Topping D. L., and Clifton P. M.. 2001. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031 [DOI] [PubMed] [Google Scholar]
- Vítek L. 2015. Bile acid malabsorption in inflammatory bowel disease. Inflamm. Bowel Dis. 21:476–483. doi: 10.1097/MIB.0000000000000193 [DOI] [PubMed] [Google Scholar]
- West R. J., and Lloyd J. K.. 1975. The effect of cholestyramine on intestinal absorption. Gut 16:93–98. doi: 10.1136/gut.16.2.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside C. H., Harkins R. W., Fluckiger H. B., and Sarett H. P.. 1965. Utilization of fat-soluble vitamins by rats and chicks fed cholestyramine, a bile acid sequestrant. Am. J. Clin. Nutr. 16:309–314. doi: 10.1093/ajcn/16.3.309 [DOI] [PubMed] [Google Scholar]