Abstract
Introduction:
The development of oral sustained release dosage forms has been a longstanding goal due to the potential for ease of administration, improved pharmacokinetics, reduced dosing frequency, and improved adherence. The benefits of multi-day single dose drug delivery are evident in the success and patient adoption of injected or implanted dosage forms. However, in the space of oral medications, all current commercially-available gastric resident dosage forms, and most in development, are limited to gastric residence of less than one day.
Areas covered:
Reviews of systems to extend gastric residence reveals that one-day or more residence has been an unmet challenge. New dosage forms are in development that seek to address many of the key physiological and design challenges of long-term gastric retention beyond 24 hours and up to a week or longer. The present analysis highlights the design, material considerations, and implications of unfolding dosage form systems with ultra-long-term gastric residence.
Expert opinion:
The development of oral dosage forms providing sustained release of high potency medications over days or weeks could transform care, significantly decrease patient burden in chronic disease management and improve outcomes.
Keywords: gastric retentive dosage forms, HIV, malaria, oral ultra-long-acting, sustained release
1. Introduction
The development of extended release oral medications with continuous drug delivery from the gastrointestinal tract (GIT) has long been a goal as this could improve pharmacokinetics (PK), reduce side effects while maintaining efficacy throughout the day, enable better adherence to less-frequent dosing schedules, and potentially facilitate directly observed treatment. Most importantly, this could be achieved from a patient-friendly and non-invasive route of drug administration. However, no currently marketed oral medication achieves gastric retention beyond 24 hours [1]. The dynamic environment of the stomach, particularly its primary clearance mechanism, the strong muscle contractions, and expulsion forces associated with migrating myoelectric complex (MMC) activity, make even multi-hour retention challenging and unreliable. Over the decades, significant progress has been made to extend gastric residence of oral medications. As presented in Table 1, there are a number of systems that have been designed to prolong gastric residence [2–6]. The platforms and approaches increase gastric retention through mechanisms including density (high-density sedimentation or low-density floatation), bioadhesion or mucoadhesion, magnetic localization, and expansion (unfolding and/or swelling); these have all been shown to prolong in vivo gastric residence to varying degrees [7–10]. Density-controlled systems and mucoadhesive compositions have shown potential for multi-hour retention in preclinical models but limited clinical demonstration beyond 5 to 8 hours, with even more limited consistency and retention in the fasted state [7,10].
Table 1.
Systems for extending gastric residence of an oral dosage form.
| Gastric Residence Approach | Description |
|---|---|
| Floating | System is less dense than gastric fluid and remains buoyant in the stomach, reducing probability of drainage. Two major types of flotation-based systems are low density and gas forming systems. |
| Swellable | System swells in the stomach and is retained as long as its size is too large to permit passage through the pylorus and/or a house keeping wave is able to expel. |
| Expandable | System expands or unfolds to a geometry that can prevent passage through the pylorus. Gastric residence is achieved through a combination of size and biomechanics. |
| Mucoadhesive | System reversibly adheres to the mucosal surface of the stomach and prolongs retention until displacement or loss of adhesion, or turnover of the mucous layer |
| High Density | System is more dense than gastric contents and sinks to the bottom of the stomach and is preferentially retained in gastric folds |
| Magnetic | A magnet is placed in the abdomen next to the stomach and the system is retained in the stomach through magnetic immobilization. |
The most promising long-lasting gastric retentive dosage forms (GRDFs) to date (FDA-approved or in late-stage clinical development) are all expansible and/or swellable designs that partially resist the chemical and mechanical processes of gastric emptying. Whereas the transit time of small indigestible solids (<1–2 mm in diameter) is approximately 2 hours or less in a fasted stomach, the expansible GRDFs seek to attain, and durably sustain, a size larger than about 2 cm in at least two dimensions to resist passage through the pylorus [11]. Gastric-retentive delivery platforms must account for the influence of food and drinks, fed and fasted effects on drug solubility and absorption, the impact of human factors such as physical activity, and individual biological and medical characteristics including comorbidities and co-administered medications [7].
Design challenges and potential sources of variability that must be considered are summarized in Table 2. This article will focus on the considerations for developing ultra-long-lasting GRDFs with expandable designs. Other GRDF systems have been reviewed in detail elsewhere [2–5,7,8,10,12–14].
Table 2.
Challenges to, and Potential Sources of Variability with, Expandable GDRFs
| Challenges to Gastric Retention | Potential Sources of Variability |
|---|---|
|
Mechanical: Digestive churn and housekeeping contraction waves |
Behavioral factors:
Eating habits, diet, fluid and alcohol consumption, physical activity. |
|
Hydrodynamic: Propulsive and retropulsive jets of gastric fluid |
Population diversity:
pH, motility, and size differences; comorbidities; medications that alter gastric environment |
|
Chemical: Enzymatic, acidic, and hydration-based digestive processes |
Anatomical or physiological:
mucosal abnormalities, surgical history, emesis |
1.1. Expandable GRDFs
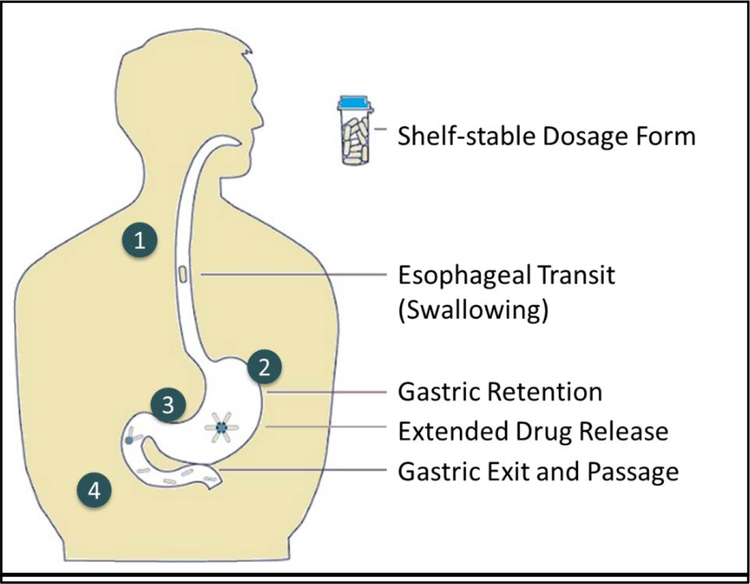
Expandable delivery platforms operate through mechanisms that increase the size of the dosage form to greater than the pylorus at its widest state; a measure which varies from 5.6–22.1mm in diameter [15], but most commonly is reported to be between 11 and 13mm [7,15,16]. Unlike the adhesive or buoyant properties of other approaches, the size and biomechanical properties of expandable GRDFs determine their ability to resist housekeeping waves, offering the potential to have limited dependence on stomach physiological states (fasted or fed state) [7]. Expandable GRDFs should satisfy the following criteria: convenient and reliable ingestion and swallowing, prompt unfolding or expansion upon entry into the stomach, durability to gastric forces, safe and non-irritating residence in the stomach for prolonged periods, release of drug at an optimal rate (largely independent of pH, fed/fasted state, and liquid content), and safe exit via the GIT upon complete drug release without adverse effects. These steps are illustrated in Figure 1.
Figure 1. Considerations for ultra-long-gastric drug delivery.
(1) Shelf-stable pill form suitable for consistent swallowing by patients, (2) pill or its contents must be reliably retained for at least the targeted drug elution period, (3) extended and steady drug release across population and behavioral factors of patients, and (4) predictable, complete, and safe exit from the stomach and intestinal tract by absorption and/or passage.
1.2. Established approaches
One expandable GRDF design has multiple products on the market and a second design is in late stage clinical trials. Depomed’s Acuform® technology is a marketed product platform that uses a swelling polymer to create an expanding pill that achieves gastric retention for 8–10 hours [17]. This system has demonstrated an ability to reduce dosing frequency to once or twice daily, depending on the application, while achieving the targeted drug plasma levels and reducing peak-to-trough fluctuations. However, polymer swelling can take up to 30 minutes and therefore the capsule must be administered with a meal. Additionally, the swollen capsule softens such that retention for longer durations has not been seen consistently.
IntecPharma’s Accordion Pill™ (AP), currently in Phase III clinical trials, consists of a multilayered film folded up within a capsule. Upon swallowing, the capsule dissolves and the film unfolds and expands up to 3.5cm in full width, with resulting gastric retention for up to about 12 hours [18]. The polymeric outer membrane is enteric, designed to remain intact during gastric retention and dissolve during intestinal passage. Compared to an oral immediate release version of the same drug taken every 4 hours, the AP formulation taken twice daily on an 8 hour interval offers a prolonged absorption phase, reduced maximum concentration (Cmax), and reduced amplitude of peak-to-trough plasma levels during the dosing period [19]. This was highlighted in the results from the Phase II clinical trials comparing the AP Carbidopa/Levodopa (CD/LD) to a commercial CD/LD formulation with an equivalent daily dose [19]. Mean residence time is substantially greater, approximately 7–8 hours, when taken with meals than when taken in the fasted state (approximately 4–5 hours) and it is therefore recommended that AP-CD/LD should be taken with meals [20]. One concern with the use of an enteric polymer to provide the outer film structure is that if the layer contributes to the control of release rate of the drug within the stomach, loss of that enteric layer could potentially lead to accelerated drug release within the intestine from an early passage of the dosage form out of the stomach.
1.3. Crossing the 24-hour boundary
Achieving beyond 12 to 24-hours of gastric retention is a hurdle that has yet to be surmounted in a clinical product. Translating in vitro designs and concepts into in vivo success and clinical development for ultra-long-acting GRDFs requires innovation beyond the leading systems described above. A fundamentally revised vision of the requirements and design possibilities is needed to cross this hurdle and achieve dosage forms with one-week or longer retention. Recent work, described below, has advanced the development of such GRDFs with demonstrations of week-long gastric retention in a large animal model.
In the following sections we highlight several approaches taken recently to the design, material selection, and production of novel long-acting expandable GRDFs that attempt to deliver therapies for a week or in some cases longer. These approaches vary from early prototypes that are open to pharmaceutically-novel materials and processes with limited scalability, as well as a design that focuses on materials and processes that are well precedented in the formulation and production of marketed pharmaceutical and biomedical products.
2. Design and material selection for ultra long-acting GRDFs
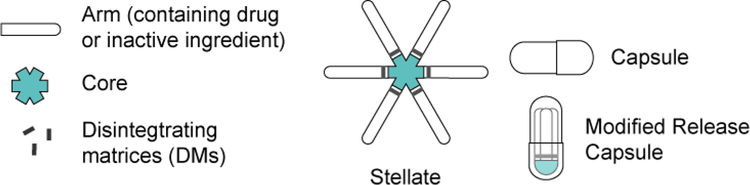
The biomechanical forces generated by the stomach are both complex and repetitive, and the chemical environment of the stomach is harsh [1]. Moreover, even before administration, the dosage form must conform to the shape of a capsule for oral administration, which can place substantial physical stress on an expandable GRDF. The added performance requirements of a multi-day gastric resident dosage form, in particular the ability to control drug release while maintaining physical properties and controlling dissolution or disintegration, suggests a need for a separation of functions. In the absence of a single material that offers all of the properties required to meet these performance criteria, a composite design is likely necessary (see Figure 2).
Figure 2.
Essential features of the modular stellate design for an ultra long-acting expandable GRDF
Pioneering work in unfolding GRDFs indicated the potential to extend gastric residence beyond 24 hours in vivo [21]. Unfolding systems such as the tetrahedron described in the 1980s had advantages over swelling systems in the use of durable materials to achieve the size and, importantly for systems that will reside in the gastric cavity over multiple meals, had an open framework to avoid interference with the passage of foods and liquids. These early unfolding systems relied on elastomeric and structural biocompatible polymers in achieving 24 hour residence in a dog model [21]. Translation of the > 24 hour residence to the clinic was unsuccessful however [22].
Recent work on the unfolding modular GRDF approach revisited previously described and new geometries [23] including rings, polygons, and stellates (i.e., star-shaped) configurations. A stellate design was preferred over alternative geometries because the stellate can fold into the shape of a standard hard capsule with less deformation and stress concentrations than other geometries: conforming a tetrahedron to a capsule shape requires multiple bending axes, and a ring or polygon must twist as well as bend to fit inside a capsule [23]. In addition, the stellate geometry offers more efficient capsule packing, filling a larger fraction of the capsule volume and thereby enabling the potential for greater drug load capacity [23]. The stellate also presents redundancies in establishing the effective size of the dosage form through multiple arms, whereas ring-based folded structures and tetrahedrons are subject to single point-of-failure losses in form, transforming with a single break into string-like shapes that lack the size in at least two dimensions required to maintain gastric retentive properties.
Controlling polymer shape and degradation are vital in creating a platform that that could be retained in the stomach for at least a week, delivering medication in a sustained manner. Previous research on the gastric retention of foldable encapsulated structures suggested certain general geometries and critical dimensions [21,22]. In practice, a dosage form must achieve more than just the performance goals of gastric residence and drug release; it must do so in a predictable and controlled fashion and must consider the entire course of dosage form passage from swallowing to excretion. Furthermore, real world environmental factors such as those listed in Table 1 must be addressed by any comprehensive modular design.
As shown in Figure 2, the stellate-based ultra-long-acting dosage form has a modular design and consists of three major components: an elastomeric core, disintegrating matrices, and drug arms. All three components are joined together to form a stellate geometry that can fold into the shape similar to a standard hard capsule.
There are several potential solutions for design and formulation of each key aspect of the stellate. Every design decision introduces constraints. The principles of the stellate elements seen in Figure 2 will be described below, including early prototypes using novel and off-the-shelf materials, and stellates that were designed for rapid translation into clinical use through accepted pharmaceutical materials and processes.
2.1. Elastomeric core
The elastomeric core provides flexibility and enables the stellate to fold into a capsule and rebound into an open configuration upon capsule disintegration. The flexibility of the core also impacts gastric retention, providing elastic bending with the motions of the stomach while allowing all other components to retain their form. During the potentially hundreds of gastric housekeeping waves that occur in a week, this shape-restoring flexibility is likely essential to prevent the stellate from being prematurely compressed in any prolonged manner to a size that would pass through the pylorus. Key properties for the elastomeric core include both elastic and viscoelastic mechanical attributes. Careful selection and balancing of these properties is necessary because they contribute to multiple performance requirements. For example, the elastic modulus of the material as well as specific features of the geometry are critical to achieving gastric residence; if too soft the form will fold too readily during gastric contraction waves and pass into the intestines; if too stiff the form could suffer from creep, i.e., permanent deformation while stored in the capsule.
The demands on an elastomeric component in a GRDF with prolonged gastric residence led to an open evaluation of both novel materials as well as a variety of biocompatible materials commonly used in medical devices. For an early prototype, a novel material was synthesized which for the first time combined an enteric functionality with robust elasticity. This pH-responsive supramolecular gel remained stable in the acid environment of the stomach but dissolved in the neutral pH environment of the small and large intestines [24]. The gel consisted of poly(acryloyl 6-aminocaproic acid) and poly(methacrylic acid-co-ethyl acrylate) held together via hydrogen bonding between the side chain carboxyl groups [24]. The entrapped water in the network likely contributed to its elastomeric properties [24].
An alternative core composition was developed comprising a polyurethane made by crosslinking poly(caprolactone) diols and triols in the presence of high molecular weight poly(caprolactone). This material offered robust adhesion and interaction with candidate materials for the controlled-release drug arms, good elasticity and some creep resistance. Based on the ratios of the three components and the cross-linking density, the elastic properties were tunable [23]. However, the curing process included a reactive ingredient that required isolation from drug substances to avoid potential covalent contamination. A further challenge of implementing this approach is that novel materials, even those with well characterized components, face increased regulatory demands.
Commercially-available materials not yet used in marketed pharmaceuticals provided additional opportunities, including thermoplastic polyurethane elastomers (TPUs) of the Elastollan® series. These polymers provided mechanical stability and good resiliency to the gastric environment [25]. As commercially-sourced materials, they offered relatively high batch-to-batch consistency and processing and manufacturing of the dosage form was simple. However, these materials displayed modest creep resistance, raising a potential risk to long-term storage stability of TPUs folded into capsules.
Liquid silicone rubber (LSR) is a commercially-available material with a long history of safe biomedical use and the potential to meet the design requirements of the elastomeric core. Cross-linked LSR has approval precedence in gastrostomy tubes and medical devices with < 30-day implantation and is used in the manufacture of skin contact parts such as eye goggles and masks as well as infant care products. The safe use and regulatory precedence of this elastomer, paired with its superior viscoelastic attributes, very low compression set, and good processability make it a strong candidate for providing the critical elastic properties required of a stellate GRDF design.
2.2. Dosage form arms
Much like the sides of polygonal dosage forms, the multiple arms of the stellate dosage form serve two purposes: maintain the size of the dosage form and hence prevent exit through the pylorus and provide controlled drug release. Importantly for multiple-day sustained delivery, release of drug should not compromise the ability to stay resident in the stomach. That is, the gastric retentive properties of the stellate should be sustained over a period of time at least as long as the time required for complete drug release and generally longer than the intended dosing interval. This approach has led to two design approaches for the drug arms; 1) two-part designs where a robust polymer provides a form in which a softer drug-containing matrix resides, and 2) a monolithic design where the drug is dispersed within a matrix of the sturdy polymer.
Two part arm designs have been described for use in the delivery of the hydrophobic antiretrovirals dolutegravir, cabotegravir, and rilpivirine [25]. In this design, each arm consisted of an open V-shaped structure that provided support to a drug-containing polymeric matrix. The matrix was formulated for controlled drug release and was backfilled into the cavity [25]. For hydrophobic drugs with low water solubility, soft formulations comprised of drug suspended or solubilized in water-soluble polymers such as poly(ethylene glycol) enabled more rapid release of drug which otherwise faced limitations of sufficient delivery, even in a sustained residence system. Water-sensitive poly(anhydrides) can also control drug release through surface-based degradation mechanisms rather than water transport mechanisms. A variety of different materials and compositions can be used for two-part arm designs, including designs compatible with scaled up manufacturing processes.
Alternatively, for many drugs, monolithic drug arms can achieve both controlled drug release while maintaining the structure needed for gastric residence. Such drug arms are composed of a biocompatible, water-insoluble polymer blended with drug and soluble excipients. The drug and excipients dissolve over time leaving behind a porous polymer matrix. In the case of a drug with limited diffusivity through the polymer itself, the drug release is primarily driven by water permeating through the pores of the solid matrix. The polymer matrix may not dissolve or degrade over the time scale of drug release. The biomechanical integrity needed for gastric retention is maintained independent of drug release such that arms depleted of drug remain intact and able to withstand gastric forces. An early embodiment of this monolithic-matrix drug arm approach has been described for the delivery of an antimalarial agent, ivermectin [23]. In this case, linear poly(caprolactone) (PCL) provided the structure and a rate-limiting dissolution barrier while polymeric surfactants (such as Pluronic® P407) and pore-forming gastric acid-soluble agents (such as Eudragit® E) tuned the release rate. Monolithic dispersions of crystalline, amorphous, or molecular drug can be manufactured by hot melt extrusion. Advantages of the monolithic design include protection of the drug from the gastric environment, as demonstrated for ivermectin [23], and control of drug release rate under varying gastric and intestinal conditions. The insolubility of PCL in water and alcohol and insensitivity to gastric pH, enzymes, and fatty dietary agents contributes to independence of the drug release from gastric environmental factors – a critical feature for clinical utility. Selection of excipients with similar insensitivity and chemical stability to these factors further provides robustness to release rate and minimizes the potential for dose dumping. Table 3
Table 3.
Common dosage forms for sustained systemic delivery at a fixed site for longer than 1 day.
| Extended Delivery System | Key Attributes |
|---|---|
| Transdermal Patches |
Pros Self-administered, needle-free Cons Visible (may not be discreet), limited drug load, low transmission rate, poor skin permeation of most drugs, skin irritation with extended application, potential to fall off, frequent administration, inconsistent adhesion |
| Injectable Depots |
Pros Exceptionally consistent drug delivery rate for current generation of formulations, good to excellent PK, continuous medication once administered, duration of a single administration can approach three or more months, compatible with direct observed therapy by clinician Cons Injection, often requires caregiver administration, patients commonly discontinue therapy, large injection volumes and/or lower doses or limitation to very potent agents, potential for burst release especially for hydrophilic drugs, dosing tails after therapeutic period, poor reversibility |
| Implantable Systems |
Pros Excellent control of delivery rate, potential for extended delivery periods out to six months or longer Cons Surgery, cost, potential limited patient access, dose limitation, poor reversibility, implant site erosion and side effects, retrieval often requires a second surgery |
| Vaginal Systems |
Pros Prolonged release, self-administration possible, discreet Cons Gender specificity, lifestyle interference, low compliance, variable systemic delivery in some applications |
2.3. Disintegrating matrices
The recent work on ultra-long GRDFs has highlighted the need for a component of the design which provides for programmed exit from the stomach and clearance through the gastrointestinal tract. One version of this design element was demonstrated in the case of GRDFs made from a durable hydrogel that is stable in the gastric environment but which dissolves upon administration of triggering molecules [26]. Specifically, these materials are made of interpenetrating networks of two polymers cross-linked by calcium ions and small molecules containing disulfide bonds [26]. These polymers can be dissolved by co-administration of generally recognized as safe (GRAS) chemicals such as ethylenediaminetetraacetic acid and glutathione and inducing passage [26].
In the case of the stellate GRDF design, programmed passage is achieved independently of the drug releasing matrix by incorporating disintegrating matrices; small segments that join the stellate arms to the elastomeric core and that gradually weaken and can break under physiological conditions, causing a gradual compromise of the ability to resist gastric contraction waves [23]. These components are responsible for controlling the timing of gastric exit of the dosage form independent of the rate or extent of drug release and can also be formulated to facilitate passage through the intestines and lower GI tract.
Compositions that change their properties in a time-dependent manner, for example by gradually softening, dissolving, or breaking down when hydrated, are good candidates for providing timed gastric exit. Disintegrating matrices composed of an alcohol-insoluble enteric polymer, which dissolves at intestinal pH but not at gastric pH, can accelerate softening and disintegration following gastric exit.
2.4. Capsule configurations
Before reaching the stomach and releasing drug, GRDFs must first be orally ingested in a capsule or related form, and the methods for encapsulation of the enfolded structure should be designed based on specific objectives pertaining to gastric retention. A capsule’s primary aim is to contain and transport the formulation to the stomach and then dissolve, therein allowing the stellate to unfold into an open configuration. Capsules therefore must be designed for stability during esophageal transit and rapid opening in the stomach; the latter of which is critical to ensuring a high percentage of dosage forms open to the form capable of achieving gastric retention. Unique to expandable GRDFs is the need for resilience to the outward-directed forces exerted by the enclosed folded form, which could impact capsule stability if not considered.
2.5. In vitro and in vivo testing
Design and development of expandable GRDFs is predicated on identification of meaningful in vitro and in vivo methods in order to establish proof-of-concept and guide iterative development. Such methods are not readily available commercially for many GRDF properties.
2.5.1. In vitro testing
As with most drug delivery systems, in vitro modeling of the performance of GRDF materials and designs can facilitate faster development, create opportunities for improved performance, support bridging studies, and can form the basis of quality control assays. Given the unique attributes of an ultra-long GRDFs, existing methods were adapted and new tests developed. In particular, composite tests that reflect properties of the complete multicomponent dosage form are important for GRDFs with modular designs. For example, composite resistance of a dosage form to folding in the stomach can be simulated by the force required to push it through a pylorus-sized funnel. Fatigue cycling to simulate repeated gastric contractions can be performed by capturing different bending modes of stellate dosage forms. Adhesion and bonding of the components to each other can be evaluated separately using flexural testing apparatuses. Drug release can be modeled using simulated gastric and intestinal fluids, evaluating the effects of different media and pH on drug release, drug stability, and the effects of hydration on material properties [27].
2.5.2. In vivo testing and animal models
In vitro studies struggle to capture the complexity of the in vivo gastric environment. As such, animal evaluation remains an essential component of the development and testing of GRDFs. Unusually for pharmaceutical products, unfolding GRDFs are often not amenable to testing in small animals due to the size of the dosage forms and importance of the gastric dimensions on their retention, thereby requiring advancement to large animals earlier in development. The principal species for evaluation of GRDFs have included dog [21,22,28] and pig [23]. Pigs share anatomic size and similar intestinal physiology with humans, while dogs are well studied from a toxicological standpoint as well as from gastric physiology, drug absorption, and other pharmacokinetic standpoints. Dogs have the additional benefit of readily swallowing capsules without need for any sedation and are more amenable to pharmacokinetic sampling than pigs.
3. Applications for an ultra long-acting oral dosage form
To date, continuous drug delivery from a dosage form to one site in the body for longer than 24 hours has only been achieved by non-oral routes of delivery (see Table 3). An ultra-long-acting oral pill may offer the potential to promote adherence in typically non-adherent populations, improve pharmacologic consistency, simplify dosing regimens, and enable directly observed therapy. The potential of achieving reduced patient burden with improved pharmacokinetics without the need for injections or surgical procedures makes the opportunity attractive, especially when considering patients wary of needle pricks or those residing in resource-limited settings. In developing countries or areas with limited infrastructure or need for inconspicuous medication practices, infrequent dosing of a discreet dosage form could present substantial advantages. Oral ultra-long acting formulations could also offer significant advantages over injectable dosage forms, including convenience, ease of dosing without discomfort, and substantial elimination of the long pharmacokinetic tails of many injectable agents; the latter of which could be a problem in infectious disease applications where subtherapeutic drug levels risk fostering drug resistance [29,30]. From a commercial perspective, ultra-long acting dosage forms can also provide opportunities for differentiation in crowded therapeutic classes as well as provide expanded market access.
To achieve these benefits, however, consistent delivery must be achieved for substantially longer than current dosage forms allow. In the case of chronic diseases, the impact of moving past once daily pills will likely only be achieved once dosage forms that can deliver for one week are available to patients. For acute, brief indications, shorter duration intervals of 2–5 days may be impactful, though adherence to acute therapies is often quite a bit higher than for chronic therapies. The design and properties of ultra-long-acting dosage forms should be tuned to the specific requirements of each indication, including not only drug release properties, but duration of retention and delivery, as well as the amount of overlap between dosing intervals.
3.1. Adherence as a barrier
For patients who take medications every 12–24 hours, longer acting therapies have the potential to improve quality of life by addressing regimen complexity and pill burden, as well as alleviate frustrations associated with daily reminders of their chronic illness. Prior evaluation has shown that as many as half of all patients with chronic illnesses do not adhere to their medications faithfully [31], with adherence decreasing significantly for every daily pill required [32]. Poor adherence to prescribed medications is a costly global issue, leading to avoidable hospitalizations with economic consequences including billions of dollars a year and a cost in human lives of hundreds of thousands of premature deaths [31]. Many patients who must take one or more pills daily experience compliance issues stemming from both biological and psychosocial barriers that prevent them from taking their medication as prescribed. These barriers include forgetfulness, having other priorities, deciding to omit a pill, a lack of information, perceived side effects, and lack of confidence in their physicians [31].
A meta-analysis of adherence rates for once-weekly versus once-daily oral dosing regimens found the odds of being adherent were 1.9-times higher in the weekly-dosing group than the daily [33]. This finding was consistent across all published studies which have evaluated the question and highlights the potential impact of long-acting delivery systems in addressing medication non-compliance.
Ultra-long-acting oral formulations can improve pharmacologic consistency even under conditions of perfect adherence, but in the real world where non-adherence is common, the pharmacologic advantage could be profound. In non-adherent patients, inter-day variability is exacerbated by missed doses, which can lead to markedly subtherapeutic levels for substantial fractions of the week. For drugs whose mechanism of action depends on consistent target engagement (true for many anti-infectious, antiviral, anti-inflammatory applications), missed daily doses can have a greater impact than the delayed doses of longer half-life or longer effective half-life sustained release drug formulations. Combination therapy is mandated for HIV antiretroviral treatment (ART) and often also for prevention in high risk populations [34–38]. Sub-optimal ART adherence is common and strongly associated with unfavorable outcomes including treatment failure and drug resistance [39,40]. The potential benefit of oral long-acting therapies has been shown in the case of HIV where modeling suggests a significant improvement in treatment success and reduction in drug resistance [25]. Depending on the indication, these improvements in pharmacology may lead to direct improvements in therapeutic efficacy in real world use.
Mass drug administration (MDA) campaigns in developing countries are another special case where achieving high coverage (through access and adherence) in an exposed population is critical for campaign effectiveness. Oral long-acting options have clear advantages in this context versus either traditional oral dosage forms or injectable long-acting formulations. Malaria MDA campaigns are an example where supplementing traditional anti-malarial agents with transmission suppressing agents such as a long-acting ivermectin that interrupt the mosquito life cycle could make a large impact [23].
3.2. Applications of ultra-long GRDFs
Oral extended release formulations of existing and novel drugs offer unique possibilities to expand and improve therapy options in many disease areas. Long gastric retention time and sustained drug delivery could provide opportunities for new approaches in treating a number of disease states where infrequent oral dosing could see rapid adoption and significant therapeutic benefit, such as HIV treatment and prophylaxis, neuropsychiatric treatments, substance use disorder medication assisted treatment, and other chronic diseases where adherence and outcomes are strongly linked.
The adaptability of the platform and its flexibility to incorporate different drugs lends itself to wide-ranging applications (see Table 4). Opportunities for oral long-acting GRDFs fall into several categories: a) improving the pharmacokinetics of existing small molecule drugs (often those with short half-lives or narrow therapeutic indices); b) long-acting oral versions of drugs where an adherence premium would be expected to improve outcomes significantly; c) combinations of multiple agents in a single long-acting form; d) point of care single dose administration regimens that achieve a full treatment course; e) sustained local delivery to the GIT to maximize local therapy or drug-on-target effects by having a proximal depot; f) continuous oral low dose administration of drugs that do not require systemic exposures (topical therapy); and g) longer-term, development of new chemical entities well suited to oral ultra-long-acting delivery, for e.g. drugs requiring a low dose.
Table 4.
Areas of Opportunity for Oral Ultra Long-acting Dosage Forms
| Existing Non-Proprietary Agents | Provide patient-centric options for superior pharmacology and adherence for existing small molecule drugs. Especially for medications with:
|
| Combination Products | Where multiple oral drugs can be combined in a long-acting formulation:
|
| Targeted or Non-systemic Delivery | Conditions where targeted GI delivery improves bioavailability or avoids systemic drug exposure:
|
| Point of Care Administration | Regimens that achieve a full treatment course in a single, long-acting dose:
|
| New Chemical Entities | Development of new therapeutic agents with low dose (high potency), where:
|
4. Conclusion
Delivering orally available drugs more efficiently, consistently, and effectively at longer dosing intervals has driven oral formulation development for decades. Extended release forms that improve delivery of drugs through longer-lasting gastric retention have seen significant advancements in the past two decades. Multiple commercial products are based on floating GRDFs that extend residence by hours, and up to 12-hour residence can be achieved with commercially available expanding GRDFs. Additionally, new oral dosage forms based on the unfolding modular drug delivery platforms described in depth within this review have demonstrated preclinical potential as ultra-long GRDFs and are entering early clinical development. However, many challenges remain to demonstrate consistency and safety, and much progress remains to be made in the development and commercialization of sustained release, long-acting medications. Clinical experience with the GRDFs in development will likely provide substantial learnings on the translation to human physiology, the specific challenges of managing inter-individual variability, and the impact of human factors on the potential clinical impact of ultra-long-acting oral medications.
5. Expert opinion
Many approved once-daily oral medications, from osmotic pumps developed by Alza to laser drilled controlled release pores to newer swellable pills, have incorporated drug delivery technologies to extend gastric residence, prolong exposure, or enhance bioavailability. As previous reviews of the field have highlighted, although great strides have been made in extended release oral dosing, innovations have struggled to push beyond once daily administration due to the strong clearance mechanisms of the GIT. There remains a need to specifically design solutions to the challenges of the gastric environment, which remains an unproven endeavor in the clinic for even the most advanced approaches. Key findings include that many approaches are limited by variability of the gastric condition between subjects and especially over time. For example, density systems are subject to patient orientation and fluid content in the stomach, adhesive systems are subject to mucosal turnover, and expansile systems may not be able to durably maintain size and shape against the forces of the stomachs migrating motor complexes.
The barrier to achieving less frequent than daily oral dosing is furthermore twofold: technical and behavioral. An evident hurdle has been the technical challenges of sustaining delivery beyond a day. In part, however, the barrier to developing long-acting oral therapies has also been due to the fact that once daily oral medication has a certain rhythm and logic for patients and has long been considered standard care. Taking medicine every day sounds simple and is an appealing treatment option for many patients, even when other routes of administration are also possible. The problematic reality that many people do not achieve this seemingly simple task has complex roots. The psychology of managing chronic, and especially preventive, therapies with daily medicine is familiar to many of us: we accept as a given that daily therapy is achievable and even desirable despite the data that even patients with motivation and incentives commonly miss doses, and even more individuals fail to persist with chronic therapies beyond a few months [41]. In some conditions, the concept of a “pill holiday” has emerged as a way to manage the underappreciated psychological challenges of taking a medicine every day, even as the pharmacokinetic implications of non-compliance have real-life health consequences. If achieved, long-acting oral medications have the potential to address these inherent psychological challenges of daily oral dosing, offering regular pill holidays. Moving to less frequent dosing carries its own challenge, however, as the next common dosing interval for most patients is not every other day but once weekly, which represents a heretofore unachievable duration for most drugs.
As the research described above indicates, design and material considerations are key in systematically addressing the barriers to gastric retention, and the evolution of new materials will likely remain a key area for advancement of long-term gastric retention in the future. Modularity is one approach which offers better control over key functional requirements by dividing the critical needs across multiple features and materials. A composite design should also offer the ability to tune properties and strategies depending on the unique requirements of each product, whether tunable duration or different physiochemical requirements of the drug. The tradeoff of modularity and multiple materials, however, is greater product complexity and manufacturing requirements. Opportunities for advancements include the development of: a) more predictive in vitro assays for in vivo performance, particularly with respect to complex properties like timing of gastric exit; b) physiologically responsive materials with predictable properties in the stomach; c) strategies for stabilizing unstable drugs; d) more low-dose oral drugs that could enable long-acting therapies for diseases where current options are limited (i.e. HIV or epilepsy); and e) evidence of translation of GRDFs into human populations with varying gastric physiologies.
New treatment approaches may be possible if the current results for ultra-long-acting GRDFs in large animals can be translated into human studies. New treatment paradigms, including potentially changing the standard of care towards directly observed therapy or point of care treatment may become more feasible for many conditions. Moments of transition in care are well characterized times of high non-adherence, and are therefore opportunities for enhancing treatment outcomes, and this is a key area where long-acting oral dosing can make an enormous difference. Whether at discharge from the hospital or through structured treatment programs such as for mental health, addiction, or dementia, reliable and effective ultra-long-acting oral therapies promise to change the way providers treat and patients experience both chronic and acute illnesses. The substantial promise of these approaches can only be achieved by continued careful engineering and clinical testing to refine and advance our understanding of how to develop ultra-long-acting oral drugs.
Article highlights.
Description of currently marketed oral dosage forms which have achieved substantially extended, but <24-hour, gastric residence
Challenges of an oral dosage form with sustained release of medication over a week or longer with reference to recent advancements
Design and material considerations of modular unfolding gastric retentive dosage forms (GRDFs)
Challenges and potential sources of variability for GRDFs
Challenges of in vitro to in vivo translation for GRDFs
Applications where once-weekly dosing have the potential to improve medication adherence and health outcomes over once daily oral dosing are presented with focus on pharmacokinetic advantages
Acknowledgments
Funding
This authors were supported in part by NIH Grants AI131416, EB024484, TR001889, and the Division of Gastroenterology, Brigham and Women’s Hospital.
Footnotes
Declaration of interest
The authors are co-inventors on multiple provisional patent applications describing various design and manufacturing aspects of gastric resident systems. D Altreuter, T Grant, C Kruger, G Traverso and A Bellinger have a financial interest in Lyndra, Inc, a biotechnology company developing gastric resident drug delivery systems. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
* of interest
** of considerable interest
- 1.Koziolek M, Grimm M, Schneider F, Jedamzik P, Sager M, Kühn J-P, et al. Navigating the human gastrointestinal tract for oral drug delivery: Uncharted waters and new frontiers. Adv Drug Deliv Rev. 2016. June 1;101:75–88. [DOI] [PubMed] [Google Scholar]
- 2.Mandal UK, Chatterjee B, Senjoti FG. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian J Pharm Sci. 2016. October 1;11(5):575–84. [Google Scholar]
- 3.Lopes CM, Bettencourt C, Rossi A, Buttini F, Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int J Pharm. 2016. August 20;510(1):144–58. [DOI] [PubMed] [Google Scholar]
- 4.Awasthi R, Kulkarni GT. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: where do we stand? Drug Deliv. 2016;23(2):378–94. [DOI] [PubMed] [Google Scholar]
- 5.Nayak AK, Maji R, Das B. Gastroretentive drug delivery systems: a review. Asian J Pharm Clin Res. 2010;(1):9. [Google Scholar]
- 6.Pathak K, Akhtar N, Singh S. Gastroretentive carrier systems in the delivery of therapeutic actives: an updated patent review. Pharm Pat Anal. 2015;4(6):453–74. [DOI] [PubMed] [Google Scholar]
- 7.Streubel A, Siepmann J, Bodmeier R. Gastroretentive drug delivery systems. Expert Opin Drug Deliv. 2006. March 28;3(2):217–33. [DOI] [PubMed] [Google Scholar]
- 8.Pawar VK, Kansal S, Garg G, Awasthi R, Singodia D, Kulkarni GT. Gastroretentive dosage forms: A review with special emphasis on floating drug delivery systems. Drug Deliv. 2011. February 1;18(2):97–110. [DOI] [PubMed] [Google Scholar]
- 9.Hwang S-J, Park H, Park K. Gastric Retentive Drug-Delivery Systems. Crit Rev Ther Drug Carr Syst. 1998;15(3):42. [PubMed] [Google Scholar]
- 10.Prinderre P, Sauzet C, Fuxen C. Advances in gastro retentive drug-delivery systems. Expert Opin Drug Deliv. 2011. September 1;8(9):1189–203. [DOI] [PubMed] [Google Scholar]
- 11.Davis SS, Hardy JG, Fara JW. Transit of pharmaceutical dosage forms through the small intestine. Gut. 1986. August 1;27(8):886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waterman KC. A critical review of gastric retentive controlled drug delivery. Pharm Dev Technol. 2007;12(1):1–10. [DOI] [PubMed] [Google Scholar]
- 13.Sathish D, Himabindu S, Kumar YS, Shayeda null, Rao YM. Floating drug delivery systems for prolonging gastric residence time: a review. Curr Drug Deliv. 2011. September;8(5):494–510. [DOI] [PubMed] [Google Scholar]
- 14.Pawar VK, Kansal S, Asthana S, Chourasia MK. Industrial perspective of gastroretentive drug delivery systems: physicochemical, biopharmaceutical, technological and regulatory consideration. Expert Opin Drug Deliv. 2012. May;9(5):551–65. [DOI] [PubMed] [Google Scholar]
- 15.Malik Z, Sankineni A, Parkman HP. Assessing pyloric sphincter pathophysiology using EndoFLIP in patients with gastroparesis. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2015. April;27(4):524–31. [DOI] [PubMed] [Google Scholar]
- 16.Ferrua M, Singh R. Modeling the Fluid Dynamics in a Human Stomach to Gain Insight of Food Digestion. J Food Sci. 2010. September;75(7):R151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Depomed. Acuform® Drug Delivery [Internet]. Enhancing therapies for optimized drug delivery. [cited 2018 May 1]. Available from: http://www.depomed.com/technology
- 18.Technology | The Accordion PillTM [Internet]. IntecPharma. [cited 2018 May 1]. Available from: http://intecpharma.com/technology/
- 19.IntecPharma. The Accordion PillTM Carbidopa/Levodopa: AP – CD/LD [Internet]. IntecPharma Pipeline. [cited 2018 May 21]. Available from: http://intecpharma.com/pipleline/ap-cdld/
- 20.Michael Gendreau R, Optimizing MD Delivery of Carbidopa/Levodopa via the Accordion PillTM: Comparative PK and Safety From 2 Randomized Crossover Studies in Healthy Volunteers. Poster Presentation presented at: American Academy of Neurology 2018 Annual Meeting (AAN 2018); 2018. April 23; Los Angeles. [Google Scholar]
- 21.Cargill R, Caldwell LJ, Engle K, Fix JA, Porter PA, Gardner CR. Controlled Gastric Emptying. 1. Effects of Physical Properties on Gastric Residence Times of Nondisintegrating Geometric Shapes in Beagle Dogs. Pharm Res. 1988;05(8):533–6.*This paper describes the use of unfolding systems that can dosed orally, and can reside in the stomach for ~1day.
- 22.Fix JA, Cargill R, Engle K. Controlled gastric emptying. III. Gastric residence time of a nondisintegrating geometric shape in human volunteers. Pharm Res. 1993. July;10(7):1087–9.*This paper was one of the first experimentations of the tetrahedron shape expandable dosage forms in humans
- 23.Bellinger AM, Jafari M, Grant TM, Zhang S, Slater HC, Wenger EA, et al. Oral, ultra-long-lasting drug delivery: Application toward malaria elimination goals. Sci Transl Med. 2016. November 16;8(365):365ra157–365ra157.**This paper shows for the first time gastric resistance of an orally deliverable system for more than a week.
- 24.Zhang S, Bellinger AM, Glettig DL, Barman R, Lee Y-AL, Zhu J, et al. A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat Mater. 2015. October;14(10):1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirtane AR, Abouzid O, Minahan D, Bensel T, Hill AL, Selinger C, et al. Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat Commun. 2018. January 9;9(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Pang Y, Zhang S, Cleveland C, Yin X, Booth L, et al. Triggerable tough hydrogels for gastric resident dosage forms. Nat Commun. 2017. July 25;8(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dressman JB, Reppas C. In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2000. October;11 Suppl 2:S73–80. [DOI] [PubMed] [Google Scholar]
- 28.Cargill R, Engle K, Gardner CR, Porter P, Sparer RV, Fix JA. Controlled gastric emptying. II. In vitro erosion and gastric residence times of an erodible device in beagle dogs. Pharm Res. 1989. June;6(6):506–9. [DOI] [PubMed] [Google Scholar]
- 29.Roberts JA, Kruger P, Paterson DL, Lipman J. Antibiotic resistance--what’s dosing got to do with it? Crit Care Med. 2008. August;36(8):2433–40.*This paper shows the use of erodible materials for extended gastric residence and safe passage from the gastrointestinal tract.
- 30.Guillemot D, Carbon C, Balkau B, Geslin P, Lecoeur H, Vauzelle-Kervroëdan F, et al. Low Dosage and Long Treatment Duration of β-Lactam: Risk Factors for Carriage of Penicillin-Resistant Streptococcus pneumoniae. JAMA. 1998. February 4;279(5):365–70. [DOI] [PubMed] [Google Scholar]
- 31.Osterberg L, Blaschke T. Adherence to Medication. N Engl J Med. 2005. August 4;353(5):487–97. [DOI] [PubMed] [Google Scholar]
- 32.Coleman CI, Limone B, Sobieraj DM, Lee S, Roberts MS, Kaur R, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm JMCP. 2012. September;18(7):527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iglay K, Cao X, Mavros P, Joshi K, Yu S, Tunceli K. Systematic Literature Review and Meta-analysis of Medication Adherence With Once-weekly Versus Once-daily Therapy. Clin Ther. 2015. August;37(8):1813–1821.e1. [DOI] [PubMed] [Google Scholar]
- 34.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016. September 1;375(9):830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dehne KL, Dallabetta G, Wilson D, Garnett GP, Laga M, Benomar E, et al. HIV Prevention 2020: a framework for delivery and a call for action. Lancet HIV. 2016;3(7):e323–332. [DOI] [PubMed] [Google Scholar]
- 36.Elion R, Coleman M. The preexposure prophylaxis revolution: from clinical trials to routine practice: implementation view from the USA. Curr Opin HIV AIDS. 2016. January;11(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet Lond Engl. 2013. November 2;382(9903):1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho DD, Pomerantz RJ, Kaplan JC. Pathogenesis of Infection with Human Immunodeficiency Virus. N Engl J Med. 1987. July 30;317(5):278–86. [DOI] [PubMed] [Google Scholar]
- 39.Streeck H, D’Souza MP, Littman DR, Crotty S. Harnessing CD4+ T cell responses in HIV vaccine development. Nat Med. 2013. February;19(2):143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cihlar T, Fordyce M. Current status and prospects of HIV treatment. Curr Opin Virol. 2016;18:50–6. [DOI] [PubMed] [Google Scholar]
- 41.Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, et al. Full Coverage for Preventive Medications after Myocardial Infarction. N Engl J Med. 2011. December 1;365(22):2088–97. [DOI] [PubMed] [Google Scholar]