INTRODUCTION:
In 2016, over 64,000 people died of a drug overdose and half of those involved an opioid.1 Despite national efforts to curb opioid prescribing, prescription rates remain high. The decision to write an opioid prescription is complex. At times, providers may be presented with the dilemma of maintaining patient satisfaction while limiting the prescription of an expected opioid medication. Many healthcare institutions utilize patient satisfaction surveys, and some offer financial incentives to their providers based on the results. Here, we hypothesized that members of the CAFP who reported being incentivized based on formal patient satisfaction surveys were more likely to report an impact of such surveys on their opioid prescribing practices than physicians who reported not to be incentivized.
METHODS:
This study was approved by the Institutional Review Board (protocol #17–1118). We developed the survey to assess the self-perceived impact of patient satisfaction surveys on the opioid prescribing practices of family physicians. We collected data on provider and practice demographics, use of patient satisfaction surveys and incentives, and provider attitudes toward opioid prescribing for different pain categories.
The survey was accessible by a unique internet link, which was emailed to all 1404 members of the CAFP. The primary outcome was perceived impact of patient satisfaction surveys on opioid prescribing. Comparisons were made using Pearson’s chi-square tests with 2-sided asymptotic significance. For statistical comparison responses were dichotomized into “slightly to very impactful” versus “not applicable” or “not at all”.
RESULTS:
The survey response rate was 10.4% (146 responses). Clinical indications for which responders prescribe opioids included acute pain (93%), cancer pain (85%), and chronic non-malignant pain (72%). Of the 146 responders, 27% (n=39) reported using patient satisfaction surveys with incentives, 45% (n=66) reported using patient satisfaction surveys without incentives, and 28% (n=41) reported not using patient satisfaction surveys or were unsure. Thirty-six % (n=14) of physicians incentivized by patient satisfaction reported at least slight impact on their opioid prescribing compared to 12% (n=8) of physicians using surveys but not reporting financial incentives (p=0.004) (Figure 1).
Figure 1.
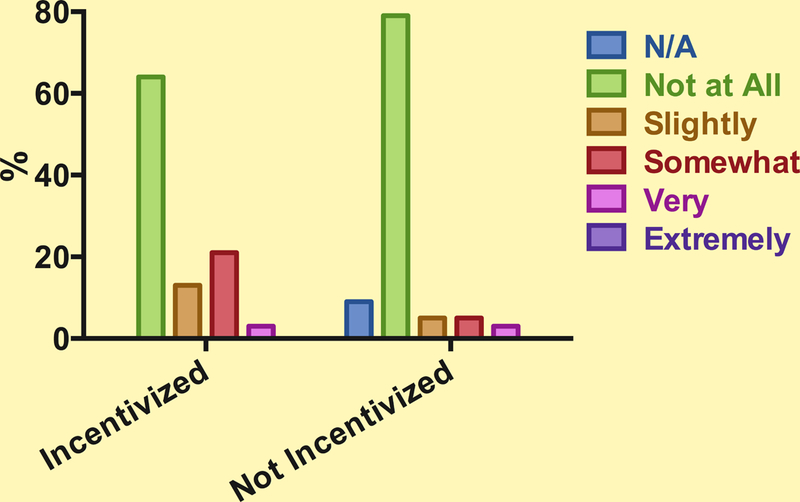
Providers’ perception of the impact patient satisfaction surveys have on their decision to prescribe opioids.
CONCLUSIONS:
Although most family physicians reported no impact of patient satisfaction surveys on their decision to prescribe opioids, those reporting financial incentives for survey results were more likely to report such an impact. A low response rate, lack of directionality of reported impact, and physician self-reporting as opposed to assessment of actual opioid prescription data limit the generalizability of these preliminary results. Further research is needed to correlate actual opioid prescribing practices with patient-satisfaction based incentives. Meanwhile, our findings are consistent with reports of potentially adverse effects from efforts to improve patient satisfaction. In a study assessing the relationship between patient satisfaction and outcomes, higher patient satisfaction was associated with increased health care costs and mortality.2 There is insufficient evidence to support the concept that patient satisfaction surveys alone reflect the true quality of care.3 Further, institutional pressure to achieve high scores on such surveys might create an inadvertent pressure to prescribe more opioids.
Family physicians may be less willing to prescribe opioids for chronic non-malignant pain compared to acute pain or cancer pain. This has been demonstrated by other studies over the past two decades and is readily apparent in the medical community.4 In 2016, the CDC published guidelines for prescribing opioids for chronic pain, which may better guide appropriate prescribing.5 Further study of the potential dilemma of balancing patient satisfaction with responsible opioid prescribing is suggested to effectively address the current opioid epidemic in the United States.
Supplementary Material
Acknowledgments
FUNDING: This work was supported by the National Institutes of Health (NIH), award number K23DA040923 to Karsten Bartels and award number K24DA032555 to Christian Hopfer. In addition, this work was supported by the institutional NIH award number UL1TR001082–05. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The NIH had no involvement in study design, collection, analysis, interpretation of data, writing of the report, or the decision to submit the article for publication.
Footnotes
PRIOR PRESENTATION: Jacqueline A. Carrico presented preliminary data of this work in abstract form in the medical student category to the IARS/AUA meeting April 28 – May 1, 2018 in Chicago, Illinois, U.S.A.
CONFLICT OF INTEREST: The authors report no conflicts of interest.
REFERENCES:
- 1.Mattson CLS, Lyna; Scholl, Lawrence; Rudd, Rose A.; Seth, Puja; Xu, Likang; Wilson, Nana Otoo; Paulozzi, Leonard J. Annual surveillance report of drug-related risks and outcomes -- United States, 20172017 August 31, 2017.
- 2.Fenton JJ, Jerant AF, Bertakis KD, Franks P. The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med 2012;172:405–11. [DOI] [PubMed] [Google Scholar]
- 3.Scott A, Sivey P, Ait Ouakrim D, et al. The effect of financial incentives on the quality of health care provided by primary care physicians. The Cochrane database of systematic reviews 2011:Cd008451. [DOI] [PubMed] [Google Scholar]
- 4.Jamison RN, Sheehan KA, Scanlan E, Matthews M, Ross EL. Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag 2014;10:375–82. [DOI] [PubMed] [Google Scholar]
- 5.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA 2016;315:1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


