Abstract
Although the effect of levocarnitine (L‐carnitine) on hyperammonemia has been reported in patients with liver cirrhosis (LC), its effect on sarcopenia remains to be elucidated. We assessed the effects of L‐carnitine on sarcopenia in patients with LC. We retrospectively evaluated 52 patients with LC who were treated with L‐carnitine for more than 3 months between February 2013 and June 2017. Computed tomography was used to measure the cross‐sectional area of the skeletal muscles at the level of the third lumbar vertebra. The relative change in skeletal muscle index (SMI) per year (ΔSMI/year) was computed in each patient. We evaluated the relationship between ΔSMI/year and various parameters, such as age, sex, liver functional reserve, and dose of L‐carnitine. The median ΔSMI/year for all patients was −0.22%. The ΔSMI/year values in Child‐Pugh classes A, B, and C were not significantly different among the three groups. There was no significant relationship between ΔSMI/year and sex, age, body mass index, and sarcopenia. Multivariate analysis showed that only a high dose of L‐carnitine (odds ratio [OR], 4.812; 95% confidence interval [CI], 1.233‐18.784; P = 0.024) was associated with increased muscle mass. The L‐carnitine high‐dose group included a significantly larger number of patients with increased muscle mass compared with the low‐dose group (OR, 3.568; 95% CI, 1.138‐11.185; P = 0.027). Administration of L‐carnitine led to a significant and gradual reduction in serum ammonia levels. Conclusion: L‐carnitine seems to suppress the progression of sarcopenia dose dependently, and this was noted to be associated with the improvement of hyperammonemia in patients with LC.
Abbreviations
- BCAA
branched‐chain amino acid
- BMI
body mass index
- CI
confidence interval
- CT
computed tomography
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- L3
level of the third lumbar vertebra
- LC
liver cirrhosis
- L‐carnitine
levocarnitine
- OR
odds ratio
- SMA
skeletal muscle area
- SMI
skeletal muscle index
Sarcopenia (loss of skeletal muscle mass and strength) is common in patients with liver cirrhosis (LC), with an estimated prevalence of 40% to 70%. Sarcopenia exacerbates survival, quality of life (QOL), and outcome after liver transplant in patients with LC.1, 2, 3
Levocarnitine (L‐carnitine) transports long‐chain fatty acids from the cytosol into the mitochondrial matrix for subsequent β‐oxidation. L‐carnitine is also required for the outflow of acyl‐coenzyme A (CoA) from the mitochondrion. Moreover, L‐carnitine regulates the pyruvate dehydrogenase complex, which is an important metabolic enzyme known to regulate the pool of free CoA.4 Lack of L‐carnitine is associated with not only impaired fatty acid metabolism but also carbohydrate utilization disorder and insulin sensitivity.5 Previous studies reported that L‐carnitine supplementation has a positive impact on oxidative stress, inflammation, fatigue, QOL, nutritional status, and sarcopenia in the elderly6 and also in patients with chronic diseases, such as cancer, chronic kidney disease, and human immunodeficiency virus infection, as well as those with chronic hepatitis C infection.7 However, the effect of L‐carnitine on sarcopenia has not been examined in patients with LC. We conducted the present study to assess the clinical effects of L‐carnitine on sarcopenia and serum ammonia level in these patients.
Patients and Methods
Patients
We reviewed 138 patients with LC who received L‐carnitine (Otsuka Pharmaceutical, Tokyo, Japan) in our department during the period February 2013 to June 2017. The reason for the administration of L‐carnitine in these patients was hepatic encephalopathy, hyperammonemia, muscle cramps, or a combination of these conditions. Among these patients, 75 patients had been on L‐carnitine for more than 3 months. Within that group, 52 patients underwent computed tomography (CT) before and during L‐carnitine supplementation. We enrolled these 52 patients in this retrospective study. The diagnosis of LC was confirmed by liver biopsy and laboratory and clinical data. The dose of L‐carnitine used in these patients ranged from 300 to 3,000 mg/day, and the dose was selected by the attending physician. We evaluated the effects of L‐carnitine on serum ammonia level and muscle mass in patients with LC. The study protocol was approved by the Human Ethics Review Committee of Hiroshima University, and a signed consent form was obtained from each patient. The study also complied with the Treaty of Helsinki.
Evaluation of Skeletal Muscle Mass and Diagnosis of Sarcopenia
CT imaging was used to measure the cross‐sectional area of skeletal muscles at the level of the third lumbar vertebra (L3). Images were analyzed with sliceOmatic V4.3 software (TomoVision, Montreal, Canada), which allows specific tissue demarcation using previously reported Hounsfield unit (HU) thresholds. For analysis, the skeletal muscle was identified and then quantified by an HU threshold ranging from −29 to 150.8 The skeletal muscle area (SMA) (cm2) was then divided by the height squared to yield the skeletal muscle index (SMI) (cm2/m2). The time‐course change in SMI per year (ΔSMI/year [%]) was then computed as follows: ΔSMI/year (%) = (SMI at final CT scan – SMI at initial CT scan) / SMI at initial CT scan × 100 / interval between CT scans (years). The Japanese Society of Hepatology set the criteria for evaluation of sarcopenia in patients with liver disease in 2016.9 Based on these criteria, loss of muscle mass is diagnosed with a CT‐based L3 SMI of ≤42 cm2/m2 in male patients and ≤38 cm2/m2 in female patients. We used the above cut‐off values to define muscle mass reduction and diagnosis of sarcopenia in the present study.
CT examination at our hospital is routinely conducted in patients with LC for screening and follow‐up of hepatocellular carcinoma (HCC) at least once within 6 months. Patients with LC with serum albumin of ≤3.5 g/dL or with a branched‐chain amino acid (BCAA) to tyrosine ratio of ≤4 are treated with BCAA. For oral BCAA supplementation, patients receive three packets of Livact (EA Pharma, Tokyo, Japan) per day or one to three packets of Aminoleban EN (Otsuka Pharmaceutical, Tokyo, Japan) per day according to nutritional status. For this study, we analyzed the effects of various factors on the ΔSMI/year, such as sex, liver functional reserve, and dose of L‐carnitine.
Statistical Analysis
Continuous variables are presented as median (range). The paired or nonpaired Student t test was used to assess the significance of differences when comparing continuous data. Pearson’s chi‐square test was used for categorical data. To identify factors that contributed to the change in muscle mass, multivariate analysis was performed through stepwise forward selection (P < 0.15) logistic regression using all variables estimated in the univariate analysis. P < 0.05 was considered to reflect statistical significance. All statistical analyses were performed using SPSS for Windows, version 20.0 (SPSS, Inc., Chicago, IL).
Results
Patient Characteristics and Laboratory Data
Characteristics of the study patients (32 men and 20 women) are shown in Table 1. The median age was 72 years (range, 44‐85 years). The median body mass index (BMI) and L3 SMI were 22.5 kg/m2 (range, 17.1‐38.6 kg/m2) and 42.1 cm2/m2 (range, 32.2‐72.3 cm2/m2), respectively. The etiology of liver disease was hepatitis B virus (HBV) infection (n = 8), hepatitis C virus (HCV) infection (n = 23), alcohol related (n = 11), and other etiologies (n = 10), including primary biliary cholangitis, autoimmune hepatitis, and idiopathic causes. The Child‐Pugh class was classified as A, B, and C in 14, 29, and 9 patients, respectively. Among the 52 study patients, 28 (54%) patients had HCC at study entry. Sarcopenia was confirmed in 19 (36.5%) of the 52 patients. Among the study patients, 48 (92%) were treated with BCAAs at the commencement of L‐carnitine. The median daily dose and duration of treatment with L‐carnitine were 1,274 mg/day (range, 300‐3,000 mg/day) and 348 days (range, 93‐744 days), respectively. No adverse events associated with L‐carnitine administration were recorded in any patient. Body weight (P = 0.004), BMI (P = 0.001), and SMI (P < 0.001) were significantly lower in the sarcopenia group than in the non‐sarcopenia group. The dose of L‐carnitine (P = 0.017), leukocyte count (P = 0.008), and platelet count (P = 0.006) were significantly higher in the sarcopenia group than in the non‐sarcopenia group.
Table 1.
Clinical and Laboratory Findings of Study Patients
| Total | Sarcopenia Group | Non‐Sarcopenia Group | P Value* | |
|---|---|---|---|---|
| (n = 52) | (n = 19) | (n = 33) | ||
| Age (years) | 72 (44‐85) | 74 (44‐84) | 65 (44‐85) | 0.248 |
| Sex (male/female) | 32/20 | 10/9 | 22/11 | 0.316 |
| Height (cm) | 159.3 (135.5‐174.8) | 157.0 (143.8‐174.8) | 160.4 (135.5‐174.0) | 0.767 |
| Body weight (kg) | 57.2 (36‐109.8) | 49.9 (37.9‐72.5) | 62.4 (36‐109.8) | 0.004 |
| BMI (kg/m2) | 22.5 (17.1‐38.6) | 20.9 (17.1‐24.7) | 23.5 (17.6‐38.6) | 0.001 |
| SMI (cm2/m2) | 42.1 (32.2‐72.3) | 35.7 (32.2‐39.3) | 48.6 (38.2‐72.3) | <0.001 |
| Etiology of cirrhosis (HBV/HCV/alcohol/other) | 8/23/11/10 | 1/10/4/4 | 7/13/7/6 | |
| Child‐Pugh (A/B/C) | 14/29/9 | 7/8/4 | 7/21/5 | |
| Presence of HCC (yes/no) | 28/24 | 9/10 | 19/14 | 0.477 |
| Patients treated with BCAAs (n [%]) | 48 (92) | 19 (100) | 29 (88) | 0.151 |
| Dose of L‐carnitine (mg/day) | 1,274 (300‐3,000) | 1,596 (409‐3,000) | 1,083 (300‐3,000) | 0.017 |
| Duration of L‐carnitine treatment (days) | 348 (93‐744) | 298 (104‐744) | 361 (93‐742) | 0.464 |
| Leukocyte count (/μL ) | 3,920 (1,640‐10,270) | 4,650 (2,290‐10,270) | 3,450 (1,640‐6,690) | 0.008 |
| Hemoglobin (g/dL) | 10.6 (6.9‐16.2) | 10.6 (8.3‐13.8) | 10.6 (6.9‐16.2) | 0.334 |
| Platelet count (/μL) | 96,000 (30,000‐284,000) | 142,000 (41,000‐284,000) | 81,000 (30,000‐262,000) | 0.006 |
| Total bilirubin (mg/dL) | 1.1 (0.5‐16.5) | 1.0 (0.5‐5.1) | 1.2 (0.5‐16.5) | 0.417 |
| AST (IU/L) | 37 (19‐219) | 46 (20‐219) | 36 (19‐87) | 0.155 |
| ALT (IU/L) | 25 (11‐175) | 27 (14‐175) | 25 (11‐67) | 0.331 |
| Albumin (g/dL) | 3.4 (2.2‐4.4) | 3.2 (2.2‐4.4) | 3.4 (2.2‐4.4) | 0.925 |
| Ammonia (μg/dL) | 68 (13‐220) | 59 (13‐220) | 80 (20‐209) | 0.28 |
| BUN (mg/dL) | 19 (6.6‐52.8) | 21.1 (11.4‐52.8) | 17 (6.6‐38.1) | 0.095 |
| Creatinine (mg/dL) | 0.81 (0.54‐6.66) | 0.81 (0.62‐6.66) | 0.80 (0.54‐2.28) | 0.268 |
| Fasting blood glucose (mg/dL) | 110 (71‐300) | 109 (72‐284) | 110 (71‐300) | 0.879 |
| HbA1c (%) | 5.6 (3.9‐8.8) | 5.6 (4.1‐8.8) | 5.7 (3.9‐7.2) | 0.684 |
| Total cholesterol (mg/dL) | 140 (78‐257) | 138 (78‐211) | 152 (93‐257) | 0.106 |
| Triglyceride (mg/dL) | 78 (18‐277) | 56 (18‐277) | 83 (44‐157) | 0.988 |
Values are presented as number of patients and median (range).
Comparison of clinical characteristics by BCAA supplementation. Statistical analysis was performed using the chi‐square test or nonpaired Student t test. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; HbA1c, hemoglobin A1c.
Rate of Change in Muscle Mass
The median ΔSMI/year for all patients was −0.22% (range, −37.4%‐41.2%), meaning that sarcopenia worsened during the study period in some patients but improved in others. The median ΔSMI/year for Child‐Pugh class A, B, and C was 5.2%, −1.7%, and 1.2%, respectively (P not significant). The median ΔSMI/year for the male patients and female patients was −1.94% and 2.73%, respectively (P = 0.213). The median ΔSMI/year for patients aged <72 and ≥72 years was −1.11% and 1.53%, respectively (P = 0.155). The median ΔSMI/year was −4.77% and 1.96% for those with a BMI of <22.5 and ≥22.5 kg/m2, respectively (P = 0.595). The median ΔSMI/year for patients with and without sarcopenia at the commencement of L‐carnitine administration was 1.24% and −1.74%, respectively (P = 0.334).
Factors Associated with Increased Muscle Mass
We defined patients whose ΔSMI/year was ≥0 as those with an increase in muscle mass following treatment with L‐carnitine. Of the 52 patients, 26 (50%) showed increased muscle mass. We performed Pearson's chi‐square test and multivariate logistic regression analysis to identify those factors that contributed to this observed increase (Table 2). Univariate analysis showed a close relationship between increased muscle mass and a high dose of L‐carnitine (median dose of L‐carnitine ≥1,274 mg/day) (Table 2). Multivariate analysis identified a high dose of L‐carnitine (odds ratio [OR], 4.812; 95% confidence interval [CI], 1.233‐18.784; P = 0.024) to be an independent and significant contributor to the observed increase in muscle mass (Table 2). Therefore, we examined muscle mass change by dose of L‐carnitine. We defined patients with L‐carnitine dose above the median (≥1,274 mg/day) as the high‐dose group (n = 26) and those with lower doses as the low‐dose group (n = 26). In these patients, we defined muscle mass increase as ΔSMI/year ≥0. Muscle mass increased only in 9 patients (35%) in the low‐dose group. On the other hand, muscle mass increased in 17 patients (65%) in the high‐dose group. The high‐dose group included a significantly larger number of patients with increased muscle mass than the low‐dose group (P = 0.027). This finding suggests that treatment with high‐dose L‐carnitine increases muscle mass in patients with LC.
Table 2.
Results of Multivariate Regression Analysis to Identify Factors That Influenced the Increase In Muscle Mass Induced By Treatment With L‐Carnitine
| Variables | n | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | (95% CI) | P Value | AOR | (95% CI) | P Value | ||
| Sex | |||||||
| Male | 32 | 1 | Reference | ‐ | |||
| Female | 20 | 1.929 | (0.620‐6.000) | 0.254 | ‐ | ||
| Age | |||||||
| under 65 years | 20 | 1 | Reference | ‐ | |||
| 66‐75 years | 16 | 1.286 | (0.343‐4.816) | 0.709 | ‐ | ||
| over 76 years | 16 | 0.778 | (0.203‐2.913) | 0.709 | ‐ | ||
| BMI | |||||||
| <18.5 kg/m2 | 5 | 0.706 | (0.105‐4.758) | 0.549 | ‐ | ||
| 18.5‐24.9 kg/m2 | 35 | 1 | Reference | ‐ | |||
| ≥25 kg/m2 | 12 | 1.482 | (0.394‐5.579) | 0.559 | ‐ | ||
| Etiology of cirrhosis | |||||||
| HBV | 8 | 1 | Reference | ‐ | |||
| HCV | 23 | 2.593 | (0.494‐13.612) | 0.232 | ‐ | ||
| Alcohol | 11 | 1.389 | (0.216‐8.916) | 0.551 | ‐ | ||
| Others | 10 | 1.111 | (0.164‐7.506) | 0.648 | ‐ | ||
| Ascites | |||||||
| Absence | 30 | 1 | Reference | ‐ | |||
| Presence | 22 | 1 | (0.333‐3.005) | 1.000 | ‐ | ||
| Child‐Pugh class | |||||||
| A | 14 | 1 | Reference | ‐ | |||
| B | 29 | 0.609 | (0.168‐2.207) | 0.449 | ‐ | ||
| C | 9 | 0.938 | (0.173‐5.070) | 0.637 | ‐ | ||
| HCC | |||||||
| Absence | 24 | 1 | Reference | ‐ | |||
| Presence | 28 | 1 | (0.336‐2.976) | 1.000 | ‐ | ||
| Sarcopenia | |||||||
| Absence | 33 | 1 | Reference | ‐ | |||
| Presence | 19 | 1.181 | (0.381‐3.655) | 0.773 | ‐ | ||
| Dose of L‐carnitine | |||||||
| <1,274 mg/day* | 26 | 1 | Reference | 1 | Reference | ||
| ≥1,274 mg/day | 26 | 3.568 | (1.138‐11.185) | 0.027 | 4.812 | (1.233‐18.784) | 0.024 |
| Duration of L‐carnitine treatment | |||||||
| <348 days* | 26 | 1 | Reference | ‐ | |||
| ≥348 days | 26 | 1.86 | (0.619‐5.588) | 0.267 | ‐ | ||
| Platelet count | |||||||
| <100,000/μL | 27 | 1 | Reference | ‐ | |||
| ≥100,000/μL | 25 | 1.591 | (0.532‐4.757) | 0.405 | ‐ | ||
| Total bilirubin | |||||||
| <1.1 mg/dL | 26 | 1 | Reference | ‐ | |||
| ≥1.1 mg/dL | 26 | 0.735 | (0.247‐2.186) | 0.579 | ‐ | ||
| Albumin | |||||||
| <3.0 g/dL | 14 | 1 | Reference | ‐ | |||
| 3.0‐3.4 g/dL | 18 | 2.25 | (0.536‐9.450) | 0.265 | ‐ | ||
| ≥3.5 g/dL | 20 | 2.2 | (0.540‐8.957) | 0.268 | ‐ | ||
| Ammonia† | |||||||
| <80 μg/dL | 27 | 1 | Reference | 1 | Reference | ||
| ≥80 μg/dL | 21 | 2.763 | (0.851‐8.965) | 0.087 | 3.133 | (0.786‐12.488) | 0.106 |
| HbA1c‡ | |||||||
| <6.3 % | 36 | 1 | Reference | ‐ | |||
| ≥6.3 % | 11 | 0.931 | (0.240‐3.612) | 0.918 | ‐ | ||
| a‐fetoprotein† | |||||||
| <100 ng/mL | 35 | 1 | Reference | ‐ | |||
| ≥100 ng/mL | 13 | 1.694 | (0.462‐6.211) | 0.424 | ‐ | ||
*median; †data of 4 patients were missing; ‡data of 5 patients were missing.
Abbreviations: AOR, adjusted odds ratio; HbA1c, hemoglobin A1c.
Effects of L‐Carnitine on Serum Ammonia Levels
Treatment with L‐carnitine was associated with a steady reduction in serum ammonia level (Fig. 1A). Serum ammonia levels at 3 and 12 months after the commencement of L‐carnitine therapy were significantly lower than that at baseline (before administration) (P = 0.009 and P = 0.002, respectively). There was no significant difference in serum ammonia levels between the low‐dose group and the high‐dose group before administration of L‐carnitine (P = 0.138). Serum ammonia levels after administration of L‐carnitine in the low‐dose group tended to decrease, but the difference was not statistically significant (Fig. 1B). In the high‐dose group, however, serum ammonia level at 12 months after administration of L‐carnitine was significantly lower than the baseline value (P = 0.013) (Fig. 1C).
Figure 1.
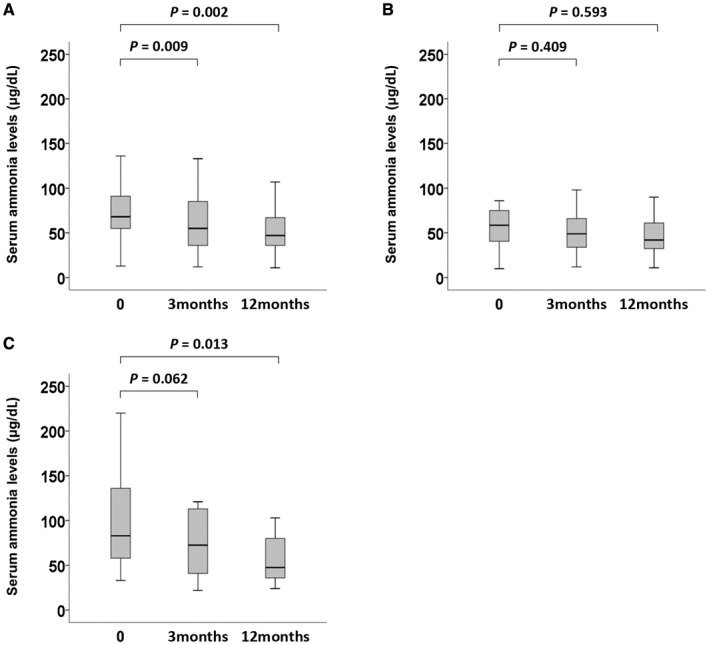
Effects of L‐carnitine on serum ammonia levels in (A) overall patients (n = 52), (B) the L‐carnitine low‐dose group (n = 26), and (C) the L‐carnitine high‐dose group (n = 26). Box and whisker plots show changes in serum ammonia level at study entry and 3 and 12 months following the start of treatment with L‐carnitine. Data were analyzed by the paired Student t test. In these plots, lines within the boxes represent median values; the upper and lower lines of the boxes represent the 25th and 75th percentiles, respectively.
Discussion
The reported median value of ΔSMA/year in patients with LC is −2.2%.10 The terms ΔSMA/year and ΔSMI/year have exactly the same meaning because SMI is computed by dividing SMA by the square of the height. The median ΔSMI/year in patients with LC in our study was −0.22%, which was relatively higher than the above reported value.
Our results suggest that administration of L‐carnitine reduces muscle mass loss in patients with LC. The multivariate analysis conducted in this study also showed that the observed increase in muscle mass correlated significantly with the use of high‐dose L‐carnitine. Significantly more patients with LC treated with high‐dose L‐carnitine showed improvement in muscle mass compared with those treated with low‐dose L‐carnitine. This result indicated the usefulness of L‐carnitine in the therapeutic management of sarcopenia in patients with LC.
Sarcopenia develops due to either a reduction in protein synthesis, an increase in proteolysis, or both. The mechanism of sarcopenia in patients with LC is multifactorial and can include hyperammonemia,11 low levels of BCAAs (including leucine), low testosterone,12 low growth hormone, and high levels of inflammatory cytokines. Hyperammonemia in skeletal muscle induces transcriptional up‐regulation of myostatin13 and increases autophagy,14 both of which contribute to sarcopenia in patients with LC.
L‐carnitine is reported to have a protective effect against hepatic encephalopathy in mice and rats.15 Various other reports have described the usefulness of L‐carnitine in reducing serum ammonia concentration in clinical studies.16, 17, 18 Moreover, it is reported that ammonia‐lowering therapy improved skeletal muscle phenotype, function, and molecular perturbations of hyperammonemia in an in vitro and in vivo model. 19 Our results also showed that administration of L‐carnitine significantly reduced serum ammonia levels. Taking these facts together, it seems possible to halt the progression of sarcopenia by improving hyperammonemia with administration of L‐carnitine. Our results showed no significant relation between ΔSMI/year and hepatic functional reserve, sex, age, BMI, and presence of sarcopenia. These results may be due to the small number of subjects in this study. BCAAs are considered to be a favorable source of protein synthesis. On the other hand, besides the urea cycle of liver, ammonia can be detoxified in the skeletal muscles by the process for glutamine synthesis using BCAAs. Therefore, BCAAs are thought to suppress the progression of sarcopenia through protein synthesis and ammonia reduction. However, intake of BCAAs was not extracted as a significant factor contributing to muscle mass increase in univariate analysis (data not shown). This is presumably because 92% of the subjects in this study were taking BCAAs. Nonetheless, there is no consensus on the appropriate dose of L‐carnitine for patients with LC. In this study, the median dose of L‐carnitine (≥1,274 mg/day) was chosen as the cut‐off value. Our results showed a significant decrease in ammonia levels and a significant increase in muscle mass in the L‐carnitine high‐dose group. These results suggest that the dose used in Japan (1,500‐3,000 mg/day) is adequate and clinically beneficial.
Our study has several limitations. First, the study did not include a control group. In patients with cirrhosis, hyperammonemia and hepatic encephalopathy affect prognosis, so basically we treat them adequately. Therefore, there are a few patients with hyperammonemia who do not take L‐carnitine in our institution. A prospective study in a controlled manner is needed in the future. Second, this is a retrospective study with a small number of subjects. Moreover, the dose of L‐carnitine was left to the discretion of the attending physician rather than set at a particular dose. Further studies of larger sample numbers are needed to examine the effect of L‐carnitine dose on the outcome of treatment in patients with LC.
In conclusion, we have demonstrated in the present study that administration of L‐carnitine seems to suppress the progression of sarcopenia dose dependently. This was noted to be associated with improvement of hyperammonemia in patients with LC. Further studies are necessary to validate the efficacy and safety of this treatment and to determine the optimal dose and duration of treatment with L‐carnitine.
Potential conflict of interest: Nothing to report.
References
- 1. Montano‐Loza AJ, Meza‐Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166‐173. [DOI] [PubMed] [Google Scholar]
- 2. Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, Ito T, et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant 2013;13:1549‐1556. [DOI] [PubMed] [Google Scholar]
- 3. Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015;31:193‐199. [DOI] [PubMed] [Google Scholar]
- 4. Bremer J. Carnitine–metabolism and functions. Physiol Rev 1983;63:1420‐1480. [DOI] [PubMed] [Google Scholar]
- 5. Ringseis R, Keller J, Eder K. Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency. Eur J Nutr 2012;51:1‐18. [DOI] [PubMed] [Google Scholar]
- 6. Malaguarnera M, Cammalleri L, Gargante MP, Vacante M, Colonna V, Motta M, et al. L‐Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. Am J Clin Nutr 2007;86:1738‐1744. [DOI] [PubMed] [Google Scholar]
- 7. Malaguarnera M, Vacante M, Bertino G, Neri S, Malaguarnera M, Gargante MP, et al. The supplementation of acetyl‐L‐carnitine decreases fatigue and increases quality of life in patients with hepatitis C treated with pegylated interferon‐α 2b plus ribavirin. J Interferon Cytokine Res 2011;31:653‐659. [DOI] [PubMed] [Google Scholar]
- 8. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1998;85:115‐122. [DOI] [PubMed] [Google Scholar]
- 9. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016;46:951‐963. [DOI] [PubMed] [Google Scholar]
- 10. Hanai T, Shiraki M, Ohnishi S, Miyazaki T, Ideta T, Kochi T, et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res 2016;46:743‐751. [DOI] [PubMed] [Google Scholar]
- 11. Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle 2012;3:225‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol 2016;65:906‐913. [DOI] [PubMed] [Google Scholar]
- 13. Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF‐κB‐mediated mechanism. Proc Natl Acad Sci U S A 2013;110:18162–18167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, et al. Hyperammonemia‐mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab 2012;303:983‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horiuchi M, Kobayashi K, Tomomura M, Kuwajima M, Imamura Y, Koizumi,, et al. Carnitine administration to juvenile visceral steatosis mice corrects the suppressed expression of urea cycle enzymes by normalizing their transcription. J Biol Chem 1992;267:5032‐5035. [PubMed] [Google Scholar]
- 16. Malaguarnera M, Vacante M, Motta M, Giordano M, Malaguarnera G, Bella R, et al. Acetyl‐L‐carnitine improves cognitive functions in severe hepatic encephalopathy: a randomized and controlled clinical trial. Metab Brain Dis 2011;26:281‐289. [DOI] [PubMed] [Google Scholar]
- 17. Iwasa M, Sugimoto R, Ishihara T, Sekoguchi‐Fujikawa N, Yoshikawa K, Mifuji‐Moroka R, et al. Usefulness of levocarnitine and/or branched‐chain amino acids during invasive treatment for hepatocellular carcinoma. J Nutr Sci Vitaminol (Tokyo) 2015;61:433‐440. [DOI] [PubMed] [Google Scholar]
- 18. Shiraki M, Shimizu M, Moriwaki H, Okita K, Koike K. Carnitine dynamics and their effects on hyperammonemia in cirrhotic Japanese patients. Hepatol Res 2017;47:321‐327. [DOI] [PubMed] [Google Scholar]
- 19. Kumar A, Davuluri G, Silva RNE, Engelen MPKJ, Ten Have GAM, Prayson R, et al. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology 2017;65:2045‐2058. [DOI] [PMC free article] [PubMed] [Google Scholar]


