Abstract
Expanding hepatitis C virus (HCV) treatment coverage is challenged by limited testing and diagnosis. This study assessed the risk of exposure, for the Middle East and North Africa, by population, yields of testing, and program efficiency of testing strategies. A standardized and systematically assembled database of 2,542 HCV antibody prevalence studies on 49 million individuals was analyzed. Random effects meta‐analyses were conducted to estimate pooled measures for risk of exposure, risk ratio (RR) of exposure, and yields of testing. Program expansion path curves were calculated to assess program efficiency. Countries clustered into two patterns: generalized versus concentrated epidemics. In generalized epidemics (Egypt and Pakistan) relative to general populations, RR of exposure was 6.8 for people who inject drugs (PWID), 6.7 for populations with liver conditions, and 5.0 for populations with high‐risk health care exposures. In concentrated epidemics (remaining countries), corresponding RRs were 97.2, 45.1, and 22.2, respectively. In generalized epidemics, the number of tests needed to identify a chronic infection was 2.5 for PWID, 2.4 for populations with liver conditions, 2.7 for populations with high‐risk health care exposures, and 14.2 for general populations. In concentrated epidemics, corresponding numbers were 2.8, 8.6, 5.1, and 222.2, respectively. Program expansion path curves demonstrated major gains in program efficiency by targeting specific populations. Risk of exposure varies immensely by population and shows a distinctive hierarchy, particularly in concentrated epidemics. Testing strategies can be much more efficient through population prioritization by risk of exposure. General population testing is not programmatically efficient in concentrated epidemics.
Abbreviations
- CI
confidence interval
- GHSS
Global Health Sector Strategy on Viral Hepatitis, 2016‐2021
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- LMICs
low‐ and middle‐income countries
- MENA
Middle East and North Africa
- PWID
people who inject drugs
- RR
relative risk
- UAE
United Arab Emirates
- WHO
World Health Organization
Viral hepatitis is the seventh leading cause of mortality globally.1 A third of this mortality is attributed to hepatitis C virus (HCV), a major cause of acute viral hepatitis and liver fibrosis, cirrhosis, and cancer.1, 2, 3, 4 In the Middle East and North Africa (MENA), HCV accounts for two thirds of viral hepatitis mortality and disability‐adjusted life years.1, 4 Although exposure to HCV infection has been estimated at 1%‐3% in most countries,2, 5 disproportionate infection levels are found in the MENA countries.2, 3, 5, 6 In this region, antibody prevalence has reached high levels in populous countries, particularly in Egypt and Pakistan, at 10.0%7, 8, 9, 10 and 4.8%,11, 12, 13 respectively.
It has been recently estimated that 71 million people are chronically infected with HCV worldwide, including 15 million in the MENA region, which is the most affected.1, 4 Most of those infected, however, are unaware of their infection; infection is mostly asymptomatic until advanced stages of disease.14 With the breakthroughs in treatment, namely the development of highly efficacious oral direct‐acting antivirals (DAAs),15, 16, 17 and the rapid drop in treatment regimen costs from more than $80,000 to only $100 in some resource‐limited countries, such as Egypt18, 19 and Pakistan,20, 21 availability and accessibility of effective treatment have been rendered surmountable challenges. Nonetheless, diagnosis of those infected has become a global challenge to address this disease burden and to control transmission.
In the context of this historical opportunity to cure HCV infections, achieve large reductions in disease burden, and even eliminate this blood‐borne virus as a public health concern, the World Health Organization (WHO) formulated the Global Health Sector Strategy on Viral Hepatitis, 2016‐2021 (GHSS)22 and established service coverage targets to eliminate HCV as a public health threat by 2030.22, 23 The strategy specifically calls for scaling up testing and treatment to achieve by 2030 target coverage levels of 90% for HCV diagnosis, 80% for HCV treatment, as well as 80% reduction in HCV incidence.4, 22, 23
These ambitious targets are challenged by the logistics of establishing programs that can achieve such coverage targets, more so in low‐ and middle‐income countries (LMICs). The cost for testing, for example, remains a challenge despite the amendment of the screening algorithm from a three‐ to two‐step approach.24, 25 The latter consists of a single antibody detection test while discontinuing further confirmatory testing, followed by RNA testing to detect chronic infection.24, 25
With the identification of those infected becoming probably the leading challenge for HCV response in the MENA region and elsewhere, we aimed to address who should be tested for HCV so that test and treat programs could be scaled up efficiently and effectively, targeting those most likely to be carriers. Against a dearth of studies on HCV testing in LMICs,26, 27 we addressed specific questions for the MENA region: (1) Which populations are at higher risk of having been exposed and therefore should be tested? (2) What are the yields of a testing program targeting different populations? (3) How can testing programs be efficiently expanded? The yield of a testing program is defined here as the number of individuals needed to be tested to identify one chronically infected individual.28
We addressed these questions through an in‐depth quantitative assessment of a standardized and systematically assembled database of 2,542 HCV antibody prevalence studies on more than 49 million individuals across 24 countries, which to our knowledge is the largest HCV epidemiology database worldwide. Our overarching aim was to provide the scientific evidence necessary to inform policy and strategic prioritization and resource allocation of programming across MENA countries, the majority of which fall under the umbrella of LMICs. The criticality of this analysis arises from the fact that ill‐informed and inefficient testing could have a serious and crippling budgetary impact and may diminish the political enthusiasm to scale up testing and treatment.26
Participants and Methods
Data Sources
The source of data was the MENA HCV Epidemiology Synthesis Project database, a comprehensive database of HCV epidemiologic measures.29 The database includes 2,542 unique antibody prevalence studies from MENA countries based on testing 49,021,211 individuals, among whom 461,965 were antibody positive.
The database was populated through a series of systematic literature reviews covering every MENA country: Afghanistan,30 Algeria,31 Bahrain,32 Djibouti,33 Egypt,9, 10 Iran,34 Iraq,35 Jordan,35 Kuwait,32 Lebanon,35 Libya,31 Mauritania,31 Morocco,31 Oman,32 Pakistan,13 Palestine,35 Qatar,32 Saudi Arabia,32 Somalia,33 Sudan,33 Syria,35 Tunisia,31 the United Arab Emirates (UAE),32 and Yemen.33 These reviews were informed by the Cochrane Collaboration Handbook guidelines,36 and the findings were reported using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.37
Data sources for these reviews included international scientific databases (PubMed and Embase), regional‐ and country‐level scientific databases (WHO Index Medicus for the Eastern Mediterranean Region, Iraqi Academic Scientific Journals’ database, and Iran’s Scientific Information Database, among others), relevant country‐level (Ministries of Health and Civil Society) and international organizations’ reports and routine data, the MENA human immunodeficiency virus (HIV)/acquired immune deficiency syndrome Epidemiology Synthesis Project database,38, 39 and abstract archives of nonindexed international scientific conferences.
Search criteria were broad, combining medical index terms (MeSH/Emtree) exploded to cover all subheadings and free text terms for HCV and relevant country names. No language restrictions were used. Data from non‐English articles were extracted by native speakers. Records were considered for analysis if published after 1989, the year when HCV was first characterized.40, 41
The database also incorporates recent updates to these systematic reviews as well as unpublished country‐level routine data and thus is the most complete database for HCV infection in the MENA region. All HCV prevalence measures were subject to a detailed quality assessment as described9, 10, 13, 30, 31, 32, 33, 34, 35, 42 and as informed by the Cochrane Collaboration Handbook guidelines.36
Epidemiologic Classification
Prevalence studies were classified into six epidemiologic population categories based on an established population classification for the perceived risk of being exposed to HCV infection6, 9, 10, 13, 30, 31, 32, 33, 34, 35, 42, 43, 44, 45 and as informed by existing literature.3, 5, 46 Based on this classification, prisoners were considered a population at intermediate risk because they can be exposed to HCV through practices such as tattooing, piercing, or anal sex. Importantly, a proportion of prisoners also could be people who inject drugs (PWID), with this proportion varying immensely from one setting to another.42, 47 Patients with HIV were classified based on the status of the HIV epidemic in each country. Specifically, they were considered at high risk whenever the epidemic was dominated by iatrogenic transmission (injecting drug networks, such as in Iran, Libya, and Pakistan)47 and at intermediate risk whenever the epidemic was dominated by sexual transmission.48, 49
Definitions and examples illustrating each of these population categories are included in Fig. 1, with further details found in previous studies.6, 9, 10, 13, 30, 31, 32, 33, 34, 35, 43, 44, 45 The same definitions were applied to all studies in all countries. All studies among PWID were for current PWID, not ex‐PWID who are no longer injecting. Population‐ and country‐mixed samples were excluded from further analysis. Consequently, the analyses included a total of 2,521 prevalence studies on 48,931,779 individuals, among whom 459,598 individuals were antibody positive (Table 1).
Figure 1.

Population epidemiologic classification based on the perceived risk of having been exposed to HCV.
Table 1.
Summary of HCV Prevalence Studies Across the Mena Countries, That Were Included in Analysis, Stratified by Population Epidemiologic Category
| Population | Number of HCV Prevalence Measures | Number of Individuals Tested for HCV | Number of HCV Antibody‐Positive Individuals |
|---|---|---|---|
| People who inject drugs | 108 | 44,094 | 17,004 |
| Populations with high‐risk health care exposures | 440 | 123,249 | 45,034 |
| Populations at intermediate risk | 340 | 290,721 | 31,204 |
| General populations | 1,182 | 48,245,780 | 299,489 |
| Populations with liver conditions | 245 | 131,343 | 51,069 |
| Special clinical populations | 206 | 96,592 | 15,798 |
| All populations | 2,521 | 48,931,779 | 459,598 |
The HCV epidemic in each country was classified as either generalized or concentrated. In the absence of a working definition in the literature and in light of a broad understanding of HCV epidemiology,6, 9, 10, 13, 30, 31, 32, 33, 34, 35, 43, 44, 45, 50, 51, 52, 53, 54, 55 we defined a generalized epidemic as a pooled mean HCV antibody prevalence in the general population being ≥3% and a concentrated epidemic as a pooled mean prevalence being <3%.
Quantitative Analysis
Risk of Having Been Exposed to HCV Infection
We assumed that the risk (likelihood) of having been exposed to HCV in a population is equal to the mean HCV antibody prevalence in that population. Estimate of the risk of having been exposed to HCV for each epidemiologic population was calculated by pooling prevalence measures belonging to that population category (Fig. 1). Pooling was conducted using DerSimonian–Laird random effects models with inverse variance weighting.56 These meta‐analyses were performed at the country, epidemic (generalized/concentrated), and regional levels. The 95% confidence intervals (CIs) were further reported. Meta‐analyses were conducted in R version 3.4.157 using the meta package.58
Risk Ratios of Having Been Exposed to HCV Infection
For each country, we used the combined sample of all prevalence studies for each epidemiologic population to calculate the risk ratio (RR) of having been exposed to HCV infection in that specific population relative to every other population. Specifically, we divided the pooled proportion of HCV antibody‐positive individuals for each country for a specific epidemiologic population by that of every other population.
To estimate the pooled mean RR (at the regional level) of having been exposed to HCV for any two epidemiologic populations as well as in the subsets of countries with generalized versus concentrated epidemics, we pooled the country‐specific RRs using DerSimonian–Laird random effects meta‐analyses with inverse variance weighting.56 The 95% CIs were further reported.
Meta‐analyses were conducted in Stata/SE version 1459 using the metan package.
Yields of HCV Testing Programs
Country‐specific, epidemic‐specific, and regional estimates for the yields (number of individuals needed to be tested to identify one chronically infected individual) of potential testing programs targeting the different epidemiologic populations were derived using the inverse of the pooled mean risk of having been exposed to HCV.28 In this derivation, a spontaneous clearance rate of 25% was assumed based on estimates from prospective cohort studies.60, 61 Of note, a recent modeling study suggested that the HCV spontaneous clearance rate may have been underestimated in prospective cohort studies.62
Program Efficiency and Testing Program Expansion Path Curves
Program expansion path curves were also calculated for the yields in countries with generalized versus concentrated epidemics. A program expansion path curve is a metric of program efficiency that delineates, in a graphical representation, how a program should efficiently be expanded.63, 64 This metric as applied here describes the growth in the number of individuals needed to be tested to identify one chronically infected individual as the testing program is expanded one population at a time.
The hierarchy of population expansion was based on the hierarchy of the yield; that is, the expansion path curve was generated by first targeting the population with the lowest number of individuals needed to be tested to identify a case, then adding the population with the second lowest number, and so forth, up to the population with the highest number of individuals needed to be tested to identify a case.
Sensitivity Analyses
Estimates for the risk of having been exposed to HCV were pooled by applying weights factoring the population size of each country as derived from the United Nations World Population Prospects database.65 The impact of this weighting strategy on the RRs of having been exposed to HCV infection and on the yields of a testing program were further assessed.
Sensitivity analysis comparing estimates based on population‐based surveys to those using all measures was further conducted.
Results
The HCV prevalence measures included in analysis stratified by population epidemiologic category are summarized in Table 1.
Risk Of Having Been Exposed
Results of the pooled analyses for the risk of having been exposed to HCV are presented in Table 2. For PWID, the risk ranged between 25.0% in Lebanon and 94.2% in Libya, with a median of 52.6%. For countries with a generalized epidemic, it was 54.2%, and for those with a concentrated epidemic, it was 48.1%. For the MENA region as a whole, it was 49.1%.
Table 2.
Pooled Estimates for the Risk of Having Been Exposed to HCV Across Mena Countries, Stratified by Population Epidemiologic Category
| Country | Mean Risk of Having Been Exposed to HCV | |||||
|---|---|---|---|---|---|---|
| People Who Inject Drugs % (95% CI) | Populations With High‐Risk Health Care Exposures % (95% CI) | Populations at Intermediate Risk % (95% CI) | General Populations % (95% CI) | Populations With Liver Conditions % (95% CI) | Special Clinical Populations % (95% CI) | |
| Afghanistan | 32.87 (25.17‐41.07) | NA* | 2.34 (1.29‐3.67) | 0.74 (0.57‐0.93) | NA* | NA* |
| Algeria | NA* | 17.86 (11.25‐25.55) | 4.96 (0.00‐17.31) | 0.13 (0.03‐0.29) | NA* | 69.7† (65.05‐74.09) |
| Bahrain | NA* | 55.74 (26.35‐83.12) | NA* | NA* | NA* | 8.19 (0.00‐29.70) |
| Djibouti | NA* | NA* | NA* | 0.28 (0.21‐0.37) | 0† (0.00‐0.06) | NA* |
| Egypt | 63.00† (52.76‐72.44) | 55.44 (49.12‐61.67) | 13.99 (10.09‐18.38) | 11.83 (11.14‐12.53) | 58.81 (51.46‐65.97) | 38.67 (32.22‐45.33) |
| Iran | 52.17 (46.88‐57.43) | 24.80 (20.39‐29.48) | 6.17 (3.44‐9.58) | 0.29 (0.21‐0.37) | 7.52 (4.34‐11.43) | 2.65 (1.82‐3.61) |
| Iraq | NA* | 19.75 (15.11‐24.82) | 1.26 (0.68‐1.98) | 0.18 (0.13‐0.25) | 6.97 (3.86‐10.84) | 2.77 (1.79‐3.92) |
| Jordan | NA* | 33.17 (25.27‐41.57) | 0.66† (0.02‐3.61) | 0.15 (0.07‐0.25) | NA* | 40.56† (32.43‐49.08) |
| Kuwait | NA* | 18.74 (5.73‐36.88) | 5.34 (0.52‐14.33) | 1.37 (0.05‐4.05) | 5.88 (0.00‐23.25) | 60.60† (42.14‐77.09) |
| Lebanon | 25.03 (4.40‐54.51) | 7.39 (4.16‐11.38) | 2.16 (0.26‐5.36) | 0.15 (0.06‐0.25) | 19.57† (12.03‐29.15) | 0.00 (0.00‐0.76) |
| Libya | 94.20† (91.46‐96.72) | 27.26 (18.85‐36.57) | 5.38 (1.74‐10.73) | 1.59 (1.44‐1.76) | NA* | 37.79 (24.84‐51.69) |
| Mauritania | NA* | NA* | NA* | 1.10† (0.31‐2.91) | NA* | NA* |
| Morocco | 52.97 (33.11‐72.35) | 38.34 (18.69‐60.15) | 3.88 (2.32‐5.79) | 0.65 (0.47‐0.86) | 37.94 (3.70‐81.56) | 9.02 (3.87‐15.87) |
| Oman | 48.05† (43.64‐52.47) | 33.71 (20.83‐47.93) | NA* | 0.75 (0.60‐0.92) | NA* | 8.80 (0.92‐22.66) |
| Pakistan | 53.63 (36.21‐70.62) | 32.84 (25.34‐40.78) | 12.94 (10.85‐15.18) | 6.15 (5.68‐6.65) | 55.10 (48.19‐61.91) | 24.56 (12.58‐38.91) |
| Palestine | 41.58 (36.24‐47.02) | 10.34 (5.64‐16.17) | NA* | 0.24 (0.18‐0.30) | NA* | NA* |
| Qatar | NA* | 44.61† (35.90‐53.58) | NA* | 1.90 (1.09‐2.93) | 33.08 (18.48‐49.47) | 9.00† (4.19‐16.40) |
| Saudi Arabia | 58.67 (18.17‐93.10) | 46.74 (43.12‐50.37) | 8.95 (4.01‐15.49) | 0.75 (0.68‐0.82) | 27.23 (20.96‐33.97) | 11.16 (7.86‐14.92) |
| Somalia | NA* | NA* | 1.71 (0.10‐4.56) | 1.07 (0.32‐2.15) | 20.18 (4.52‐42.62) | NA* |
| Sudan | NA* | 18.42 (8.42‐31.07) | 0.70 (0.29‐1.24) | 1.36 (0.63‐2.31) | 7.32 (1.26‐17.0) | 5.21 (0‐17.08) |
| Syria | 39.62 (7.03‐78.46) | 43.53 (26.34‐61.56) | 2.98 (1.77‐4.45) | 0.41 (0.36‐0.47) | 1.04† (0.13‐3.69) | 48.00† (27.80‐68.69) |
| Tunisia | 85.61† (78.44‐91.11) | 25.05 (20.24‐30.17) | 6.30 (1.31‐14.38) | 0.60 (0.44‐0.79) | 22.42 (10.02‐37.78) | 10.69 (0.28‐30.97) |
| United Arab Emirates | NA* | 24.00† (19.35‐30.09) | 15.74 (3.58‐33.94) | 2.02 (1.25‐2.96) | NA* | NA* |
| Yemen | NA* | 45.03 (33.10‐57.25) | 1.63 (0.39‐3.61) | 1.61 (1.12‐2.20) | 25.09 (15.61‐35.90) | 10.32 (6.22‐15.29) |
| Pooled estimates using all measures | ||||||
| Countries with a generalized epidemic‡ | 54.24 (37.67‐70.34) | 48.97 (43.68‐54.27) | 13.33 (11.62‐15.14) | 9.42 (8.96‐9.88) | 56.72 (51.45‐61.91) | 34.05 (27.10‐41.35) |
| Countries with a concentrated epidemic§ | 48.14 (42.88‐53.43) | 26.38 (23.69‐29.15) | 4.14 (3.17‐5.22) | 0.60 (0.55‐0.64) | 15.55 (12.38‐18.98) | 7.36 (5.65‐9.26) |
| All countries | 49.06 (43.88‐54.25) | 30.10 (27.60‐32.67) | 6.59 (5.67‐7.57) | 2.44 (2.33‐2.56) | 34.75 (30.88‐38.73) | 15.63 (12.09‐19.51) |
| Sensitivity analysis‐Pooled estimates with population size weighting | ||||||
| Countries with a generalized epidemic‡ | 53.83 (37.27‐69.97) | 49.22 (44.09‐54.36) | 13.34 (11.74‐15.02) | 10.07 (9.66‐10.49) | 56.88 (51.66‐62.03) | 33.87 (26.70‐41.44) |
| Countries with a concentrated epidemic§ | 48.21 (43.44‐52.99) | 26.19 (23.35‐29.14) | 4.06 (2.99‐5.28) | 0.73 (0.69‐0.76) | 15.50 (12.45‐18.80) | 7.03 (5.87‐8.28) |
| All countries | 49.04 (43.79‐54.31) | 30.11 (27.51‐32.77) | 6.58 (5.79‐7.41) | 2.89 (2.74‐3.03) | 34.79 (31.05‐38.63) | 15.40 (11.64‐19.56) |
NA indicates that no estimates were available because no prevalence studies were identified.
Point estimate for HCV prevalence based on a single study.
Classification based on the pooled mean HCV antibody prevalence in the general population being ≥3% (includes Egypt and Pakistan).
Classification based on the pooled mean HCV antibody prevalence in the general population being <3% (includes all MENA countries other than Egypt and Pakistan).
For populations with high‐risk health care exposures, the risk ranged between 7.4% in Lebanon and 55.7% in Bahrain, with a median of 30.1%. The risk was 49.0% for countries with a generalized epidemic, 26.4% for countries with a concentrated epidemic, and 30.1% for all the MENA countries.
For populations at intermediate risk, the risk was lower, ranging between 0.7% in Jordan and 15.7% in the UAE, with a median of 4.4%. The risk was 13.3% for countries with a generalized epidemic, 4.1% for countries with a concentrated epidemic, and 6.6% for all the MENA countries.
For general populations, the risk ranged between 0.1% in Algeria and 11.8% in Egypt, with a median of 0.8%. The risk was 9.4% for countries with a generalized epidemic, only 0.6% for countries with a concentrated epidemic, and 2.4% for all the MENA countries.
For populations with liver conditions, the risk ranged between 0% in Djibouti and 58.8% in Egypt, with a median of 20.2%. The risk was 56.7% for countries with a generalized epidemic, 15.6% for countries with a concentrated epidemic, and 34.8% for all the MENA countries.
For special clinical populations, the risk ranged between 0% in Lebanon and 69.7% in Algeria, with a median of 10.5%. The risk was 34.1% for countries with a generalized epidemic, 7.4% for countries with a concentrated epidemic, and 15.6% for all the MENA countries.
Risk estimates for specific subpopulations by epidemic type and for all the MENA region are presented in Supporting Table S1.
RRs Of Having Been Exposed
The pooled mean RRs of having been exposed to HCV infection are presented in Supporting Table S2 for any two combinations of the population epidemiologic categories. These pooled mean RRs are also shown in Fig. 2 relative to general populations, for countries with a generalized epidemic (Fig. 2A), for countries with a concentrated epidemic (Fig. 2B), and for the MENA region as a whole (Fig. 2C).
Figure 2.
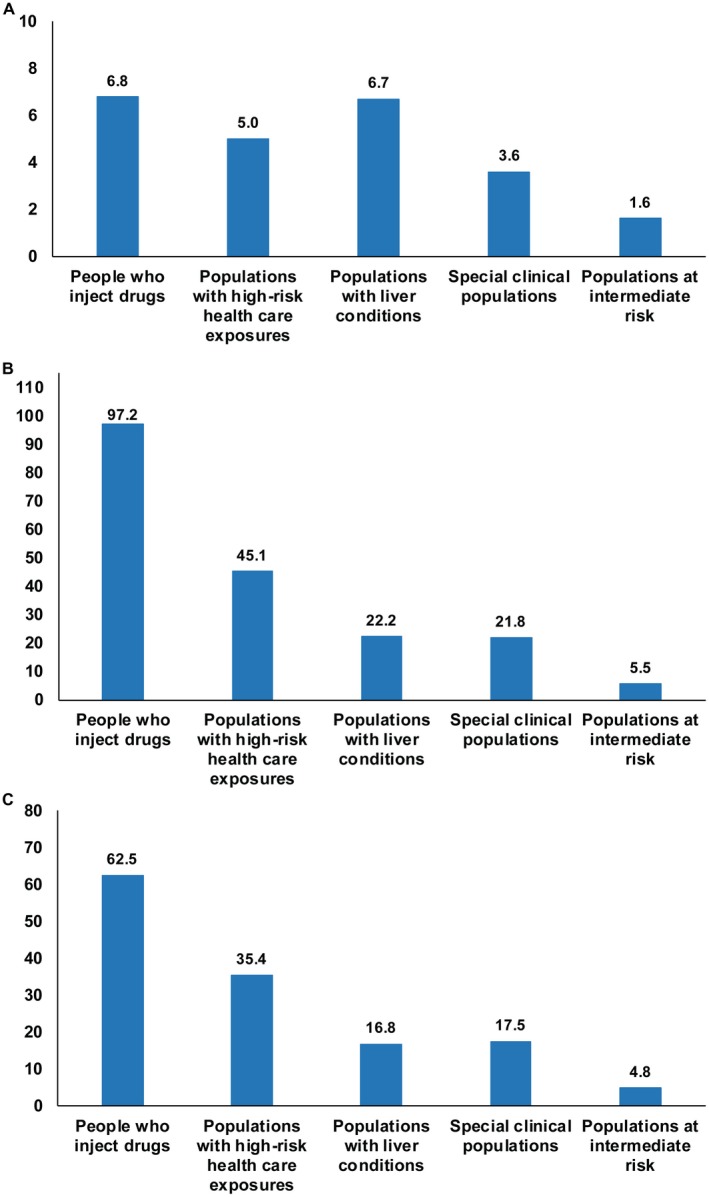
Hierarchy of risk of exposure to the infection in MENA countries. (A) Pooled RR of having been exposed to HCV, relative to general populations, for the different population epidemiologic categories for countries with a generalized epidemic (Egypt and Pakistan). (B) Pooled RR relative to general populations but for countries with a concentrated epidemic. (C) Pooled RR relative to general populations for all countries.
Relative to general populations, for countries with a generalized epidemic, the pooled mean RR ranged between 1.6 for populations at intermediate risk and 6.8 for PWID. For countries with a concentrated epidemic, the pooled mean RR ranged between 5.5 for populations at intermediate risk and 97.2 for PWID. For the MENA region as a whole, the pooled mean RR ranged between 4.8 for populations at intermediate risk and 62.5 for PWID.
Yields Of A Testing Program
The yields in a testing program, i.e., the number of individuals needed to be tested to identify one chronically infected individual, are presented in Table 3 for each population epidemiologic category for each country. For PWID, the number needed to be tested to identify one case ranged from 1.4 in Libya to 5.3 in Lebanon, with a median of 2.5. For countries with a generalized epidemic, it was 2.5, and for those with a concentrated epidemic, it was 2.8. For the MENA region as a whole, it was 2.7.
Table 3.
Yields of a Testing Program Targeting Populations at Different Risks of Being Exposed to HCV in Mena Countries
| Country | Number of Individuals Needed to Be Tested to Identify One HCV Chronically Infected Individual (95% CI) | |||||
|---|---|---|---|---|---|---|
| People Who Inject Drugs | Populations With High‐Risk Health Care Exposures | Populations at Intermediate Risk | General Populations | Populations With Liver Conditions | Special Clinical Populations | |
| Afghanistan | 4.1 (3.2‐5.3) | NA* | 57.0 (36.3‐103.4) | 180.2 (143.4‐233.9) | NA* | NA* |
| Algeria | NA* | 7.5 (5.2‐11.9) | 26.9 (7.7‐ ‐‐†) | 1025.6 (459.8‐4444.4) | NA* | 1.9 (1.8‐2.0) |
| Bahrain | NA* | 2.4 (1.6‐5.1) | NA* | NA* | NA* | 16.3 (4.5‐ ‐‐†) |
| Djibouti | NA* | NA* | NA* | 476.2 (360.4‐634.9) | NA† | NA* |
| Egypt | 2.1 (1.8‐2.5) | 2.4 (2.2‐2.7) | 9.5 (7.3‐13.2) | 11.3 (10.6‐12.0) | 2.3 (2.0‐2.6) | 3.4 (2.9‐4.1) |
| Iran | 2.6 (2.3‐2.8) | 5.4 (4.5‐6.5) | 21.6 (13.9‐38.8) | 459.8 (360.4‐634.9) | 17.7 (11.7‐30.7) | 50.3 (36.9‐73.3) |
| Iraq | NA* | 6.8 (5.4‐8.8) | 105.8 (67.3‐196.1) | 740.7 (533.3‐1,025.6) | 19.1 (12.3‐34.5) | 48.1 (34.0‐74.5) |
| Jordan | NA* | 4.0 (3.2‐5.3) | 202 (36.9‐6,666.7) | 888.9 (533.3‐1,904.8) | NA* | 3.3 (2.7‐4.1) |
| Kuwait | NA* | 7.1 (3.6‐23.3) | 25.0 (9.3‐256.4) | 97.3 (32.9‐2,666.7) | 22.7 (5.7‐ ‐‐†) | 2.2 (1.7‐3.2) |
| Lebanon | 5.3 (2.4‐30.3) | 18.0 (11.7‐32.1) | 61.7 (24.9‐512.8) | 888.9 (533.3‐2,222.2) | 6.8 (4.6‐11.1) | NA† |
| Libya | 1.4 (1.4‐1.5) | 4.9 (3.6‐7.1) | 24.8 (12.4‐76.6) | 83.9 (75.8‐92.6) | NA* | 3.5 (2.6‐5.4) |
| Mauritania | NA* | NA* | NA* | 121.2 (45.8‐430.1) | NA* | NA* |
| Morocco | 2.5 (1.8‐4.0) | 3.5 (2.2‐7.1) | 34.4 (23.0‐57.5) | 205.1 (155.0‐283.7) | 3.5 (1.6‐36.0) | 14.8 (8.4‐34.5) |
| Oman | 2.8 (2.5‐3.1) | 4.0 (2.8‐6.4) | NA* | 177.8 (144.9‐222.2) | NA* | 15.2 (5.9‐144.9) |
| Pakistan | 2.5 (1.9‐3.7) | 4.1 (3.3‐5.3) | 10.3 (8.8‐12.3) | 21.7 (20.1‐23.5) | 2.4 (2.2‐2.8) | 5.4 (3.4‐10.6) |
| Palestine | 3.2 (2.8‐3.7) | 12.9 (8.2‐23.6) | NA* | 555.6 (444.4‐740.7) | NA* | NA* |
| Qatar | NA* | 3.0 (2.5‐3.7) | NA* | 70.2 (45.5‐122.3) | 4.0 (2.7‐7.2) | 14.8 (8.1‐31.8) |
| Saudi Arabia | 2.3 (1.4‐7.3) | 2.9 (2.6‐3.1) | 14.9 (8.6‐33.3) | 177.8 (162.6‐196.1) | 4.9 (3.9‐6.4) | 11.9 (8.9‐17.0) |
| Somalia | NA* | NA* | 78.0 (29.2‐1,333.3) | 124.6 (62.0‐416.7) | 6.6 (3.1‐29.5) | NA* |
| Sudan | NA* | 7.2 (4.3‐15.8) | 190.5 (107.5‐459.8) | 98.0 (57.7‐211.6) | 18.2 (7.8‐105.8) | 25.6 (7.8‐ ‐‐†) |
| Syria | 3.4 (1.7‐19.0) | 3.1 (2.2‐5.1) | 44.7 (30.0‐75.3) | 325.2 (283.7‐370.4) | 128.2 (36.1‐1,025.6) | 2.8 (1.9‐4.8) |
| Tunisia | 1.6 (1.5‐1.7) | 5.3 (4.4‐6.6) | 21.2 (9.3‐101.8) | 222.2 (168.8‐303.0) | 5.9 (3.5‐13.3) | 12.5 (4.3‐476.2) |
| United Arab Emirates | NA* | 5.6 (4.4‐6.9) | 8.5 (3.9‐37.2) | 66.0 (45.0‐106.7) | NA* | NA* |
| Yemen | NA* | 3.0 (2.3‐4.0) | 81.8 (36.9‐341.9) | 82.8 (60.6‐119.0) | 5.3 (3.7‐8.5) | 12.9 (8.7‐21.4) |
| Yields of a testing programs based on pooling all measures | ||||||
| Countries with a generalized epidemic‡ | 2.5 (1.9‐3.5) | 2.7 (2.5‐3.1) | 10.0 (8.8‐11.5) | 14.2 (13.5‐14.9) | 2.4 (2.2‐2.6) | 3.9 (3.2‐4.9) |
| Countries with a concentrated epidemic§ | 2.8 (2.5‐3.1) | 5.1 (4.6‐5.6) | 32.2 (25.5‐42.1) | 222.2 (208.3‐242.4) | 8.6 (7.0‐10.8) | 18.1 (14.4‐23.6) |
| All countries | 2.7 (2.5‐3.0) | 4.4 (4.1‐4.8) | 20.2 (17.6‐23.5) | 54.6 (52.1‐57.2) | 3.8 (3.4‐4.3) | 8.5 (6.8‐11.0) |
| Sensitivity analysis‐Yields of a testing program based on pooling all measures with population size weighting | ||||||
| Countries with a generalized epidemic‡ | 2.5 (1.9‐3.6) | 2.7 (2.5‐3.0) | 10.0 (8.9‐11.4) | 13.2 (12.7‐13.8) | 2.3 (2.1‐2.6) | 3.9 (3.2‐5.0) |
| Countries with a concentrated epidemic§ | 2.8 (2.5‐3.1) | 5.1 (4.6‐5.7) | 32.8 (25.3‐44.6) | 182.6 (175.4‐193.2) | 8.6 (7.1‐10.7) | 19.0 (16.1‐22.7) |
| All countries | 2.7 (2.5‐3.0) | 4.4 (4.1‐4.8) | 20.3 (18.0‐23.0) | 46.1 (44.0‐48.7) | 3.8 (3.5‐4.3) | 8.7 (6.8‐11.5) |
The yield in a testing program is defined as the number of individuals needed to be tested to identify one HCV chronically infected individual.28
NA indicates that no estimates were available because no prevalence studies were identified.
Estimate could not be calculated as either HCV prevalence or the limit for its CI is 0 or too close to 0, indicating the need for an infinite number of tests.
Classification based on the pooled mean HCV antibody prevalence in the general population being ≥3% (includes Egypt and Pakistan).
Classification based on the pooled mean HCV antibody prevalence in the general population being <3% (includes all MENA countries other than Egypt and Pakistan).
For populations with high‐risk health care exposures, the number needed to be tested ranged from 2.4 in Bahrain and Egypt to 18.0 in Lebanon, with a median of 4.5. It was 2.7 for countries with a generalized epidemic, 5.1 for countries with a concentrated epidemic, and 4.4 for all the MENA countries.
For populations at intermediate risk, the number that needed to be tested ranged from 8.5 in the UAE to 202.0 in Jordan, with a median of 30.6. It was 10.0 for countries with a generalized epidemic, 32.2 for countries with a concentrated epidemic, and 20.2 for all the MENA countries.
For general populations, the number needed to be tested was higher, ranging from 11.3 in Egypt to 1,025.6 in Algeria, with a median of 177.8. It was 14.2 for countries with a generalized epidemic, 222.2 for countries with a concentrated epidemic, and 54.6 for all the MENA countries.
For populations with liver conditions, the number needed to be tested ranged from 2.3 in Egypt to 128.2 in Syria, with a median of 6.3. It was 2.4 for countries with a generalized epidemic, 8.6 for countries with a concentrated epidemic, and 3.8 for all the MENA countries.
For special clinical populations, the number needed to be tested ranged from 1.9 in Algeria to 50.3 in Iran, with a median of 12.5. It was 3.9 for countries with a generalized epidemic, 18.1 for countries with a concentrated epidemic, and 8.5 for all the MENA countries.
Yields of a testing program for specific subpopulations by epidemic type and for the MENA region are presented in Supporting Table S1.
Program Efficiency And Testing Program Expansion Path Curves
The efficiency of the testing program is illustrated in Fig. 3. For countries with a generalized epidemic (Fig. 3A), the highest program efficiency was achieved by targeting populations with liver conditions, where only two individuals needed to be tested to identify one chronically infected individual. The expansion path curve was also largely flat by expanding the program to include PWID, populations with high‐risk health care exposures, and special clinical populations; only two to four tests would be needed to identify one chronic infection. However, the curve edged upward with expansion of the program to include populations at intermediate risk and general populations, indicating diminishing returns, although still only 10 to 15 tests would be needed to identify one chronic infection.
Figure 3.
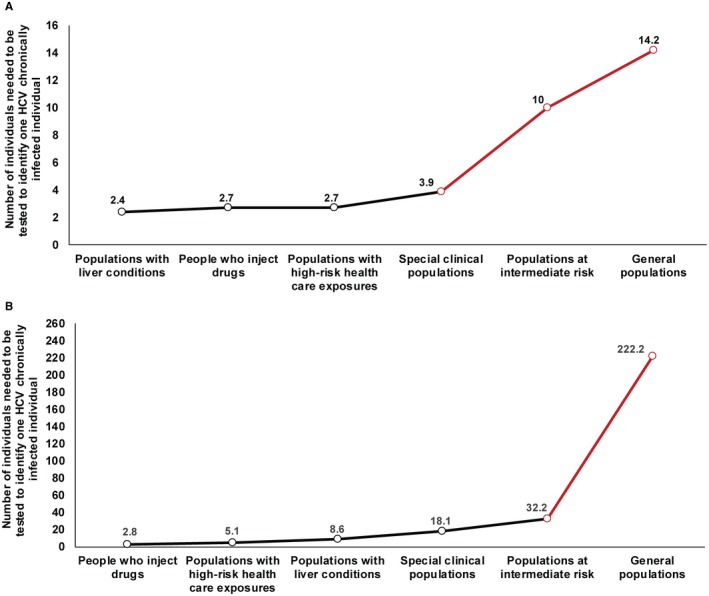
Testing program expansion path curve in MENA countries. (A) Testing program expansion path curve for the yield, i.e., for the number of tests necessary to identify one HCV chronically infected individual for countries with a generalized epidemic (Egypt and Pakistan). (B) Testing program expansion path curve but for countries with a concentrated epidemic.
For countries with a concentrated epidemic (Fig. 3B), the highest program efficiency was achieved by targeting PWID, where only three individuals needed to be tested to identify one chronically infected individual. As the program was expanded to include populations with high‐risk health care exposures and populations with liver conditions, five and nine tests, respectively, would be needed to identify a chronic infection. As the program was expanded to more populations, the curve began a rapid climb upward, highlighting the diminishing returns of testing. Most notably, expansion of the program to general populations implied exponentially more tests (>200) to identify a single chronic infection.
Sensitivity Analyses
Our estimates for the risk of having been exposed to HCV (Table 2), RRs of having been exposed to HCV infection (Supporting Table S3), and yields of a testing program (Table 3) were robust to data weighting using country population size.
Only Egypt7, 66 and Pakistan11, 12 had HCV serologic measures based on nationally representative population‐based surveys. Pooled estimates for the risk of having been exposed to HCV using these data were 8.0% (95% CI, 6.1‐9.8) for Egypt and 4.9% (95% CI, 4.7‐5.1) for Pakistan. The corresponding numbers needed to be tested were 16.9 (95% CI, 13.6‐21.7) for Egypt and 27.3 (95% CI, 26.3‐28.5) for Pakistan. These estimates are similar to those generated using the totality of the evidence (Tables 2 and 3).
Discussion
Based on analyses of a massive and systematic database of prevalence measures, we estimated the risk of exposure to HCV in the MENA region, quantified the yields of testing, and furnished a roadmap for efficiently rolling out testing programs, all for the purpose of expanding treatment coverage. The results demonstrated that major gains can be attained through a targeted approach that factors in epidemic type and is tailored to each country, thereby optimizing testing and treatment within budgetary constraints. Such a targeted approach has an additional indirect benefit of effectively curtailing transmission, as populations with a higher risk of exposure are also the same populations that are more likely to pass the infection to uninfected persons. Although the analyses are specific to MENA countries, the results are probably generalizable to other LMICs and regions with similar epidemic patterns.
These findings inform development of testing programs and policies at the country and regional levels, thereby attending to the first and fourth of the WHO’s GHSS strategic directions.22, 23 These call, respectively, for characterizing populations at increased risk of exposure and for ensuring efficiency, cost‐effectiveness, and sustainability of programs.4, 22, 23 With the constrained funding for HCV programming,67, 68, 69 the financial implications of poorly informed testing strategies and inefficient testing campaigns may cripple the political will to scale up test‐and‐treat programs, thus thwarting progress toward achieving the GHSS service coverage targets.22, 23, 70 The presented results indicate a real opportunity for MENA countries to establish and/or optimize efficient test‐and‐treat programs. Such programs would not only tackle existing infection and disease burden but also lead to a gradual decline in incidence, thus meeting GHSS targets and simultaneously achieving the 2030 elimination goal.22, 23, 70
Epidemiologically and for testing strategies, MENA countries clustered into two main patterns: generalized epidemics where HCV prevalence in the general population is ≥3% (Egypt and Pakistan) and concentrated epidemics where prevalence is <3% (remaining countries). In countries with generalized epidemics (Table 2), the risk of having been exposed was high and similar for PWID, populations with liver conditions, and populations with high‐risk health care exposures; in this last group, one in every two individuals is infected. This risk was also substantial, although less than the former groups, for special clinical populations, populations at intermediate risk, and even general populations, signifying the terminology of generalized epidemics.
Meanwhile, in countries with concentrated epidemics, there was a clear hierarchy in the risk of exposure (Table 2). PWID had the highest risk, followed by populations with high‐risk health care exposures, populations with liver conditions, and special clinical populations. The relative role of PWID was thus higher here than in generalized epidemics (Table 2; Fig. 2A,B). The risk for populations at intermediate risk and for general populations was very low (Table 2). Overall, risk estimates across countries were consistent and comparable with those of recent global reviews and estimations for the general population5 (Supporting Table S4) and PWID71 (although with some differences) for few countries with small epidemics and less data.
Remarkably, for both generalized and concentrated epidemics, the risk of exposure in clinical populations, including populations with high‐risk health care exposures and special clinical populations, was substantial if not very high, indicating a critical role for health care in transmission. Although this important role for health care may not be well appreciated,26, 72 it demonstrates that any successful test‐and‐treat strategy (broadly in LMICs) needs to consider this reality of transmission in health care settings in HCV programming.
Targeted facility‐based testing may be gradually introduced to identify clinical populations most at risk (such as dialysis or transfusion patients). Testing can later be extended to other clinical populations (such as hospital inpatients) as resources become available. Of note is that populations with liver conditions also had a high risk of exposure. Despite the variability in risk by epidemic type, this testifies to the role of HCV in liver disease in the MENA region.
A wide reaching general population testing policy in generalized epidemics is supported by the results (Table 3; Fig. 3A) because the number of tests needed to identify a chronic infection is low (<20). Such policies may include door‐to‐door community‐based testing,73 routine testing in health services, and targeted testing for populations with high risk of exposure. However, in resource‐constrained settings, a stepwise prioritization approach with targeted testing can still achieve higher program efficiency with the constraints in program resources.
Testing prioritization should be made with the aim of achieving the quickest public health gains in terms of achieving the elimination goal and thus should take into consideration testing efficiency based on the infection burden in specific population groups, the size of those population groups, and their contribution to the pool of infections within the country. For example, based on our findings, testing efficiency is highest if it targets, respectively, populations with liver conditions, PWID, and populations with high‐risk health care exposures, where only two to four tests would be needed to identify a chronic infection, followed by special clinical populations, populations at intermediate risk, and eventually general populations. However, realities on the ground, such as the size of the population of concern and its contribution to the overall pool of infection, may change the prioritization order. Of note, challenges like ensuring adequate resources and necessary laboratory infrastructure to perform testing and linkage to treatment at the district level should also be considered, even in the context of generalized epidemics.
In concentrated epidemics, a targeted testing strategy is critical for program efficiency (Table 3; Fig. 3B). Testing is most efficient by targeting PWID, followed by populations with high‐risk health care exposures, populations with liver conditions, and lastly, special clinical populations. Given the large number of tests needed to identify a single chronic infection, it will be difficult to justify commitment of large resources for testing populations at intermediate risk and general populations. For instance, >200 tests would be needed to identify a single case in general populations. This being said, modeling studies could be used to assess the full impact of testing strategies on cost before a detailed policy is established.
The provided strategies for testing are based on an epidemiologic perspective. However, other considerations can affect implementation of testing programs. Testing prioritization policies should follow treatment policies and take into consideration the availability of treatment for HCV. Testing strategies also need to factor in the ease of identifying populations at different risks of exposure as some populations may be difficult to identify and target. The size of each target population also needs to be considered. The size of the target population is an important factor; for instance, the size of PWID in a specific country may be too small relative to the overall size of the infected population, thereby affecting consideration, optimization, and logistics of resource allocation.
Population accessibility is yet one more important factor. Accessing current PWID or ex‐PWID may prove difficult.47 Risk of reinfection is another factor, such as for PWID and populations with high‐risk health care exposures. With the recent increasing availability of generic DAA medicines in MENA countries at a cost below $100, such as in Egypt18, 19 and Pakistan,20, 21 which are the two most affected countries, effort should be paid to prioritize and target testing and treatment based on the most efficient public health considerations, with a gradual scale‐up to attain universal coverage of all population groups and elimination of HCV as an epidemic of public health concern. Cost‐effectiveness is another consideration, and this varies from one country to another based on actual costs of testing and treatment and the country‐specific cost‐effectiveness threshold as set based on the per‐capita gross domestic product.74, 75
Our analyses are limited by the variability in quantity and quality of available data. For some countries, prevalence measures in some of the epidemiologic populations were not available or not sufficient to warrant estimations. On occasion, estimates were based on a small number of studies that may not have been representative of the considered population. We provided an analysis to inform testing with a focus on developing a regional strategy, but this may not be sufficient to devise a country‐specific optimal strategy. The latter may need to incorporate additional data and contextual factors and be conducted as in‐depth country‐specific analyses that include all subpopulations beyond the broad populations considered here. In the present study, however, the focus was on analyses at the regional level and by epidemic type.
The effect of age, although important, also could not be assessed as age‐stratified prevalence measures were not available for most countries. When available, these varied in how they were reported; different studies used different age group definitions, rendering a pooled analysis difficult. Another challenge was the variability in the affected age cohorts in one country to another given epidemic history, say in Egypt versus Pakistan.11, 13, 50 Inclusion of age, thus, is best addressed through in‐depth country‐specific analyses, with the development of sensitive and specific risk scores being an additional aim.
The inverse variance weighting method was used when estimating pooled HCV prevalence. However, sensitivity analysis adding country population size for weighting data had no impact on our estimates for the pooled prevalence or related measures (yields of a testing program and RRs). Another method for weighting data, such as by time, could have also been considered. Yet, epidemic time‐trend data were too limited for most countries to inform weighting that factors the epidemic’s temporal evolution. Overall, data were most complete only for Egypt and Pakistan, the two countries with generalized yet strikingly different epidemics.50, 54
Our study has key strengths. The analyzed database is, to our knowledge, the largest and most comprehensive ever assembled of HCV prevalence measures. The database was built systematically and through standardized protocols and included all available prevalence measures in all populations in MENA countries. Such scope of epidemiologic evidence allowed extensive and stratified analyses that could not have been possible without such a database.
In conclusion, population risk of exposure depends on whether the type of epidemic is generalized or concentrated. Risk of exposure varies immensely by population and shows a distinctive hierarchy in concentrated epidemics, but a less distinctive hierarchy in generalized epidemics. These findings indicate different test‐and‐treat strategies by epidemic type. In all countries, however, testing strategy should be targeted with prioritization determined by risk of exposure. Populations with liver conditions, clinical populations, and PWID should be at the core of every testing strategy. These findings inform the development of testing guidelines at the country and regional levels, thereby attending to the GHSS strategic directions to ensure program efficiency and sustainability of HCV programs.
Supporting information
Acknowledgment
We are grateful for the infrastructure support provided by the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine‐Qatar.
SEE EDITORIAL ON PAGE 321
This work was made possible by the Qatar National Research Fund (NPRP 9‐040‐3‐008 to L.J.A.).
The statements made herein are solely the responsibility of the authors. The authors are also grateful for infrastructure support provided by the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine‐Qatar.
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016;388:1081‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology 2013;57:1333‐1342. [DOI] [PubMed] [Google Scholar]
- 3. Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect 2011;17:107‐115. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . The Global Hepatitis Report. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 5. Gower E, Estes C, Blach S, Razavi‐Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61(Suppl.):S45‐S57. [DOI] [PubMed] [Google Scholar]
- 6. Chemaitelly H, Mahmud S, Chaabna K, Kouyoumjian SP, Mumtaz GR, Abu‐Raddad LJ. The Epidemiology of Hepatitis C Virus in the World Health Organization Eastern Mediterranean Region: Implications for Strategic Action. Geneva, Switzerland: World Health Organization. In press. [Google Scholar]
- 7. Ministry of Health and Population Egypt, El‐Zanaty and Associates Egypt, and ICF International . Egypt Health Issues Survey 2015. Cairo, Egypt, and Rockville, MD: Ministry of Health and Population and ICF International; 2015. [Google Scholar]
- 8. El‐Zanaty F, Way A. Egypt Demographic and Health Survey 2008. Cairo, Egypt: Ministry of Health, El‐Zanaty and Associates/Egypt, and Macro International; 2009. [Google Scholar]
- 9. Mohamoud YA, Mumtaz GR, Riome S, Miller D, Abu‐Raddad LJ. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis 2013;13:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kouyoumjian SP, Chemaitelly H, Abu‐Raddad LJ. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta‐analyses, and meta‐regressions. Sci Rep 2018;8:1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qureshi H, Bile KM, Jooma R, Alam SE, Afridi HU. Prevalence of hepatitis B and C viral infections in Pakistan: findings of a national survey appealing for effective prevention and control measures. East Mediterr Health J 2010;16(Suppl):S15‐S23. [PubMed] [Google Scholar]
- 12. Umar M, Bushra HT, Ahmad M, Data A, Ahmad M, Khurram M, et al. Hepatitis C in Pakistan: a review of available data. Hepat Mon 2010;10:205‐214. [PMC free article] [PubMed] [Google Scholar]
- 13. Al Kanaani Z, Mahmud S, Kouyoumjian SP, Abu‐Raddad LJ. The epidemiology of hepatitis C virus in Pakistan: systematic review and meta‐analyses. R Soc Open Sci 2018;5:180257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 2013;10:553‐562. [DOI] [PubMed] [Google Scholar]
- 15. Advances in the treatment of hepatitis C virus infection from EASL 2015: The 50th Annual meeting of the European Association for the Study of the Liver, April 22–26, 2015, Vienna, Austria . Gastroenterol Hepatol (N Y) 2015;11(Suppl. 3):1‐23. [PMC free article] [PubMed] [Google Scholar]
- 16. Chen C. Gilead’s new hepatitis C drug approved by FDA, priced at $74,760. https://www.bloomberg.com/news/articles/2016-06-28/gilead-wins-fda-approval-of-hepatitis-c-drug-for-all-genotypes. Published June 28, 2016. Accessed October 2, 2017.
- 17. Advances in the treatment of hepatitis C virus infection from the 2016 EASL meeting: The Annual Meeting of the European Association for the Study of the Liver, April 13–17, 2016, Barcelona, Spain . Gastroenterol Hepatol (N Y) 2016;12(Suppl. 2):1‐22. [PMC free article] [PubMed] [Google Scholar]
- 18. Kim DD, Hutton DW, Raouf AA, Salama M, Hablas A, Seifeldin IA, et al. Cost‐effectiveness model for hepatitis C screening and treatment: implications for Egypt and other countries with high prevalence. Glob Public Health 2015;10:296‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El‐Akel W, El‐Sayed MH, El Kassas M, El‐Serafy M, Khairy M, Elsaeed K, et al. National treatment programme of hepatitis C in Egypt: hepatitis C virus model of care. J Viral Hepat 2017;24:262‐267. [DOI] [PubMed] [Google Scholar]
- 20. Price of Hepatitis‐C drug fixed at Rs5,868 . https://www.thenews.com.pk/print/97674-Price-of-Hepatitis-C-drug-fixed-at-Rs5868. Published February 12, 2016. Accessed January 3, 2018.
- 21. National Hepatitis Strategic Framework launched . http://nation.com.pk/09-Oct-2017/national-hepatitis-strategic-framework-launched. Published October 9, 2017. Accessed January 3, 2018.
- 22. World Health Organization . Global Health Sector Strategy on Viral Hepatitis, 2016–2021. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 23. World Health Organization . Combating Hepatitis B and C to Reach Elimination by 2030. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 24. Vijayan T, Klausner JD. Hepatitis C: challenges and opportunities in the laboratory diagnosis of infection. MLO Med Lab Obs 2016;48(16):18. [PubMed] [Google Scholar]
- 25. World Health Organization . Guidelines on Hepatitis B and C Testing. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 26. Hutin YJ, Bulterys M, Hirnschall GO. How far are we from viral hepatitis elimination service coverage targets? J Int AIDS Soc 2018;21(Suppl. 2):e25050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zuure F, Davidovich U, Kok G, Depla AC, Hoebe C, van den Hoek A, et al. Evaluation of a risk assessment questionnaire to assist hepatitis C screening in the general population. Euro Surveill 2010;15:19539. [PubMed] [Google Scholar]
- 28. Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ 1998;317:307‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chemaitelly H, Mahmud S, Chaabna K, Kouyoumjian SP, Mumtaz GR, Abu‐Raddad LJ. The epidemiology of hepatitis C virus in the World Health Organization Eastern Mediterranean Region: Implications for strategic action. World Health Organization Report. In press. [Google Scholar]
- 30. Chemaitelly H, Mahmud S, Rahmani AM, Abu‐Raddad LJ. The epidemiology of hepatitis C virus in Afghanistan: systematic review and meta‐analysis. Int J Infect Dis 2015;40:54‐63. [DOI] [PubMed] [Google Scholar]
- 31. Fadlalla FA, Mohamoud YA, Mumtaz GR, Abu‐Raddad LJ. The epidemiology of hepatitis C virus in the Maghreb region: systematic review and meta‐analyses. PLoS One 2015;10:e0121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohamoud YA, Riome S, Abu‐Raddad LJ. Epidemiology of hepatitis C virus in the Arabian Gulf countries: systematic review and meta‐analysis of prevalence. Int J Infect Dis 2016;46:116‐125. [DOI] [PubMed] [Google Scholar]
- 33. Chaabna K, Kouyoumjian SP, Abu‐Raddad LJ. Hepatitis C virus epidemiology in Djibouti, Somalia, Sudan, and Yemen: systematic review and meta‐analysis. PLoS One 2016;11:e0149966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mahmud S, Akbarzadeh V, Abu‐Raddad LJ. The epidemiology of hepatitis C virus in Iran: systematic review and meta‐analyses. Sci Rep 2018;8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chemaitelly H, Chaabna K, Abu‐Raddad LJ. The epidemiology of hepatitis C virus in the Fertile Crescent: systematic review and meta‐analysis. PLoS One 2015;10:e0135281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, 1st ed Hoboken, NJ: Wiley‐Blackwell; 2008. [Google Scholar]
- 37. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abu‐Raddad LJ, Akala FA, Semini I, Riedner G, Wilson D, Tawil O. Characterizing the HIV/AIDS Epidemic in the Middle East and North Africa: Time for Strategic Action. Washington, DC: World Bank; 2010. [Google Scholar]
- 39. Abu‐Raddad LJ, Hilmi N, Mumtaz G, Benkirane M, Akala FA, Riedner G, et al. Epidemiology of HIV infection in the Middle East and North Africa. AIDS 2010;24(Suppl. 2):S5–S23. [DOI] [PubMed] [Google Scholar]
- 40. Choo Q‐L, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood‐borne non‐A, non‐B viral hepatitis genome. Science 1989;244:359‐362. [DOI] [PubMed] [Google Scholar]
- 41. Kuo G, Choo Q, Alter H, Gitnick G, Redeker A, Purcell R, et al. An assay for circulating antibodies to a major etiologic virus of human non‐A, non‐B hepatitis. Science 1989;244:362‐364. [DOI] [PubMed] [Google Scholar]
- 42. Heijnen M, Mumtaz GR, Abu‐Raddad LJ. Status of HIV and hepatitis C virus infections among prisoners in the Middle East and North Africa: review and synthesis. J Int AIDS Soc 2016;19:20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harfouche M, Chemaitelly H, Kouyoumjian SP, Mahmud S, Chaabna K, Al‐Kanaani Z, et al. Hepatitis C virus viremic rate in the Middle East and North Africa: systematic synthesis, meta‐analyses, and meta‐regressions. PLoS One 2017;12:e0187177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mahmud S, Al‐Kanaani Z, Chemaitelly H, Chaabna K, Kouyoumjian SP, Abu‐Raddad LJ. Hepatitis C virus genotypes in the Middle East and North Africa: distribution, diversity, and patterns. J Med Virol 2018;90:131‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mahmud S, Kouyoumjian SP, Al Kanaani Z, Chemaitelly H, Abu‐Raddad LJ. Individual‐level key associations and modes of exposure for hepatitis C virus infection in the Middle East and North Africa: a systematic synthesis. Ann Epidemiol 2018;28:452‐461. [DOI] [PubMed] [Google Scholar]
- 46. Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011;378:571‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mumtaz GR, Weiss HA, Thomas SL, Riome S, Setayesh H, Riedner G, et al. HIV among people who inject drugs in the Middle East and North Africa: systematic review and data synthesis. PLoS Medicine 2014;11:e1001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chemaitelly H, Weiss HA, Thomas SL, Calvert C, Harfouche M, Abu‐Raddad LJ. HIV epidemiology among female sex workers and their clients in the Middle East and North Africa: systematic review, meta‐analyses, and meta‐regressions. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 49. Mumtaz G, Hilmi N, McFarland W, Kaplan RL, Akala FA, Semini I, et al. Are HIV epidemics among men who have sex with men emerging in the Middle East and North Africa?: A systematic review and data synthesis. PLoS Medicine 2010;8:e1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ayoub HH, Al Kanaani Z, Abu‐Raddad LJ. Characterizing the temporal evolution of the hepatitis C virus epidemic in Pakistan. J Viral Hepat 2018;25:670‐679. [DOI] [PubMed] [Google Scholar]
- 51. Benova L, Awad SF, Miller FD, Abu‐Raddad LJ. Estimation of hepatitis C virus infections resulting from vertical transmission in Egypt. Hepatology 2015;61:834‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cuadros DF, Branscum AJ, Miller FD, Abu‐Raddad LJ. Spatial epidemiology of hepatitis C virus infection in Egypt: analyses and implications. Hepatology 2014;60:1150‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Benova L, Mohamoud YA, Calvert C, Abu‐Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta‐analysis. Clin Infect Dis 2014;59:765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ayoub HH, Abu‐Raddad LJ. Impact of treatment on hepatitis C virus transmission and incidence in Egypt: a case for treatment as prevention. J Viral Hepat 2017;24:486‐495. [DOI] [PubMed] [Google Scholar]
- 55. Harfouche M, Chemaitelly H, Mahmud S, Chaabna K, Kouyoumjian SP, Al Kanaani Z, et al. Epidemiology of hepatitis C virus among hemodialysis patients in the Middle East and North Africa: systematic syntheses, meta‐analyses, and meta‐regressions. Epidemiol Infect 2017;145:3243‐3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. Chichester, United Kingdom: John Wiley & Sons; 2009. [Google Scholar]
- 57. R Core Team . R: A Language and Environment for Statistical Computing . Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 58. Schwarzer G. meta: an R package for meta‐analysis. R. News 2007;7:40‐45. [Google Scholar]
- 59. StataCorp . Stata Statistical Software for Large Datasets: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 60. Micallef J, Kaldor J, Dore G. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat 2006;13:34‐41. [DOI] [PubMed] [Google Scholar]
- 61. Grebely J, Page K, Sacks‐Davis R, Loeff MS, Rice TM, Bruneau J, et al. InC3 Study Group. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014;59:109‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ayoub HH, Chemaitelly H, Omori R, Abu‐Raddad LJ. Hepatitis C virus infection spontaneous clearance: has it been underestimated? Int J Infect Dis 2018;75:60‐66. [DOI] [PubMed] [Google Scholar]
- 63. Hogan DR, Baltussen R, Hayashi C, Lauer JA, Salomon JA. Cost effectiveness analysis of strategies to combat HIV/AIDS in developing countries. BMJ 2005;331:1431‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Awad SF, Sgaier SK, Tambatamba BC, Mohamoud YA, Lau FK, Reed JB, et al. Investigating voluntary medical male circumcision program efficiency gains through subpopulation prioritization: insights from application to Zambia. PLoS One 2015;10:e0145729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. United Nations . World population Prospects: 2017 Revision. https://population.un.org/wpp/2018. Published June 21, 2017. Accessed March 8, 2018.
- 66. El‐Zanaty F, Way A. Egypt Demographic and Health Survey 2008. Cairo, Egypt: Ministry of Health, El‐Zanaty and Associates, and Macro International; 2009:431. [Google Scholar]
- 67. The Global Fund . Global Fund support for co‐infections and co‐morbidities, thirty‐third board meeting, March 31‐April 1. Geneva, Switzerland; 2015. [Google Scholar]
- 68. Centers for Disease Control and Prevention . HIV & HCV excerpts from HHS/CDC 2017 budget. http://www.natap.org/2016/HCV/021016_05.htm. Accessed May 14, 2018.
- 69. Qureshi H, Hirnschall G, Wiktor S. The Funding of Viral Hepatitis: The Big Question. Glascow, United Kingdom: World Hepatitis Summit; September 2‐4, 2015. [Google Scholar]
- 70. World Health Organization . Guidelines for the Screening, Care, and Treatment of Persons with Chronic Hepatitis C Infection. Geneva, Switzerland; 2016. [PubMed] [Google Scholar]
- 71. Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017;5:e1192‐e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morgan JR, Servidone M, Easterbrook P, Linas BP. Economic evaluation of HCV testing approaches in low and middle income countries. BMC Infect Dis 2017;17(Suppl. 1):697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shiha G, Metwally AM, Soliman R, Elbasiony M, Mikhail NNH, Easterbrook P. An educate, test, and treat programme towards elimination of hepatitis C infection in Egypt: a community‐based demonstration project. Lancet Gastroenterol Hepatol 2018;3:778‐789. [DOI] [PubMed] [Google Scholar]
- 74. WHO Commission on Macroeconomics and Health and World Health Organization . Macroeconomics and health: investing in health for economic development: executive summary/report of the Commission on Macroeconomics and Health. Geneva, Switzerland; 2001. [Google Scholar]
- 75. World Health Organization . Cost‐effectiveness and strategic planning (WHO‐CHOICE). www.who.int/choice/cost-effectiveness/en/. Published 2014. Accessed May 23, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


