Abstract
Although the impact of carbohydrases on performance and nutrient utilization has been well studied, their effects on immune status and intestinal microbiota are less known in pigs. This study aimed to evaluate the impact of xylanase (X) and a carbohydrase enzyme blend (EB; cellulase, ß-glucanase, and xylanase) on the immune profile of the intestine and peripheral system as well as intestinal microbes and microbial metabolites of weaned pigs fed higher fiber diets. Pigs (n = 460; 6.43 ± 0.06 kg BW; F25 × 6.0 Genetiporc) were blocked by initial BW. Pens (n = 48; 12 per treatment; 9 or 10 pigs per pen) were randomly assigned to 1 of 4 dietary treatments, including a higher fiber control diet (CON) and the CON supplemented with 0.01% X, 0.01% EB, or both enzymes (X + EB), arranged in a 2 × 2 factorial. The diets were based on corn, soybean meal, corn distillers dried grains with solubles, and wheat middlings. After 7-d adaptation to the environment, pigs were fed experimental diets ad libitum for 28 d. Blood samples were collected from the same pig within each pen on days 0, 7, 14, and 28. Intestinal tissues and digesta were collected on day 28. Bacteria 16S rRNA gene copy numbers were quantified using qPCR. The mRNA levels of colonic IL-17, occludin (OCLN), and claudin 3 (CLDN3) were greater in pigs fed diets with X + EB, but not X or EB, compared with those fed CON (P < 0.05). The EB in the diet reduced plasma IL-8 over the 28-d trial compared with diets without EB (P < 0.05). There was an X × EB interaction on plasma tumor necrosis factor α and IL-1ß (P < 0.05); their levels were decreased when X and EB were added together, but not individually, compared with CON. The EB decreased cecal propionate, butyrate, and total volatile fatty acids (P < 0.05). Pigs fed X had lower ileal Lactobacillus and greater ileal and cecal Enterobacteriaceae compared with those fed unsupplemented diets (P < 0.05). The EB decreased Lactobacillus (P < 0.05) and tended to decrease (P = 0.065) Enterobacteriaceae in the colon compared with diets without EB. In conclusion, the addition of X and EB together decreased systemic markers of immune activation, potentially diverting energy and nutrients towards growth. The EB reduced colonic Lactobacillus and cecal total volatile fatty acids, probably due to improved prececal fiber and starch degradation and thus reduced substrate availability in the large intestine. These data corroborated previously observed enhanced growth in pigs fed EB-supplemented diets.
Keywords: dietary enzymes, inflammation markers, microbiota, volatile fatty acids, swine
INTRODUCTION
Weaned pigs are not efficient at utilizing dietary fiber due to their limited physical gut capacity (Li and Patience, 2016) and fermentation capacity (Knudsen et al., 2012). Thus, nonstarch polysaccharides (NSP) degrading carbohydrases are currently supplemented to improve nutrient digestibility and growth performance of pigs (Adeola and Cowieson, 2011; Tsai et al., 2017). Additionally, carbohydrases (e.g., xylanase) degrade dietary NSP and liberate low molecular weight oligosaccharides (Lærke et al., 2015; Pedersen et al., 2015) and/or monosaccharides (Gill et al., 2000). The released oligosaccharides, especially arabinoxylan-oligosaccharides (AXOS), can then exert prebiotic effects by selectively modulating intestinal microbiota and altering host immune status through interaction with microbes or direct stimulation of epithelial cells and monocytes (Courtin et al., 2008; Chen et al., 2012; Mendis et al., 2016). Therefore, it is reasonable to hypothesize that carbohydrase supplementation in higher fiber diets (especially those abundant in arabinoxylan) may regulate intestinal microbiota and modulate immune status, either directly or indirectly, in pigs through fiber degradation and production of AXOS in the intestine.
It was previously found that a carbohydrase blend (EB; cellulase, ß-glucanase, and xylanase) enhanced growth rate of nursery pigs fed higher fiber diets, which may be partly due to improved barrier integrity and decreased inflammation markers of the small intestine (Li et al., 2018). This study is a further analysis of samples collected from the previous experiment to evaluate how the addition of xylanase (X) and/or EB affects the colonic and peripheral inflammatory cytokines as well as microbial populations in those pigs. It was hypothesized that supplementation with X and/or EB would down-regulate colonic and peripheral inflammatory status and beneficially alter the intestinal microbial population.
MATERIALS AND METHODS
All procedures in this experiment adhered to guidelines for the ethical and humane use of animals for research and were approved by the Institutional Animal Care and Use Committee at Iowa State University (#9-15-8097-S).
Animals and Experimental Design
Pigs used in this experiment and experimental design were previously described in Li et al. (2018). Briefly, 460 weaned pigs (6.43 ± 0.06 kg BW; 6.0 × F25 Genetiporc; PIC Inc., Hendersonville, TN) were blocked by initial BW and 48 pens (n = 12 per treatment) were randomly assigned to 1 of 4 dietary treatments, with 9 or10 pigs per pen. The 4 diets included a higher fiber control diet (CON) plus the CON supplemented with either X, EB, or X + EB, arranged in a 2 × 2 factorial: X (0 or 0.01%) and EB (0 or 0.01%). The inclusion rate of both enzymes was based on manufacturer’s recommendations (Huvepharma, Peachtree City, GA). The X activity was 15,000 EPU xylanase/g and 1 g of EB contained 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase. Diets were based on corn, soybean meal, corn distillers dried grains with solubles (DDGS), and wheat middlings. At weaning, all pigs had a 7-d period of acclimation to the environment and were fed a common commercial starter diet that did not contain any antibiotics or zinc or copper at levels above their nutritional requirement. The experimental diets were then fed for 28 d in 2 phases, with days 0 to 14 as Phase 1 and days 15 to 28 as Phase 2 (Table 1). Phase 1 diets contained 5% corn DDGS and 5% wheat middlings, and phase 2 diets contained 10% corn DDGS and 10% wheat middlings.
Table 1.
Ingredients and chemical composition of the basal diet (as-fed basis)
| Item | Phase 1 | Phase 2 |
| Ingredients, % | ||
| Corn | 48.11 | 49.80 |
| Reduced-oil corn DDGS | 5.00 | 10.00 |
| Wheat middlings | 5.00 | 10.00 |
| Milk whey powder | 7.50 | – |
| Menhaden select fish meal | 5.80 | 2.00 |
| Hamlet protein, HP 3001 | 8.00 | 3.00 |
| Soybean meal, 47.7 | 17.50 | 22.00 |
| Soybean oil | 0.60 | 0.60 |
| Limestone | 0.58 | 1.05 |
| L-Lys HCl | 0.42 | 0.48 |
| DL-Met | 0.14 | 0.08 |
| L-Thr | 0.09 | 0.11 |
| L-Trp | 0.02 | 0.02 |
| Phytase2 | 0.0125 | 0.0125 |
| Vitamin premix3 | 0.25 | 0.25 |
| Trace mineral premix4 | 0.15 | 0.15 |
| Tiamulin5 | 0.18 | – |
| Chlortetracycline6 | 0.40 | – |
| Carbadox7 | – | 0.20 |
| Salt | 0.25 | 0.25 |
| Calculated nutrient levels, % | ||
| ME, Mcal/kg | 3.35 | 3.29 |
| NE, Mcal/kg | 2.43 | 2.37 |
| CP | 23.86 | 22.49 |
| NDF | 9.58 | 13.33 |
| ADF | 3.73 | 4.99 |
| SID8 Lys | 1.45 | 1.31 |
Phase 1 = days 0–14; Phase 2 = days 14–28; 0.01% xylanase or a carbohydrase enzyme blend or both were mixed with premixes and then added to the basal diets
1Enzymatically treated soybean meal, Hamlet Protein Inc., Findlay, OH.
2OptiPhos 4000 G, Huvepharma Inc., Peachtree City, GA; assumed to release 0.15% standardized total tract digestible P in the diet based on manufacture’s recommendation.
3Provided 6,614 IU vitamin A, 827 IU vitamin D, 26 IU vitamin E, 2.6 mg vitamin K, 29.8 mg niacin, 16.5 mg pantothenic acid, 5.0 mg riboflavin, and 0.023 mg vitamin B12 per kg of diet.
4Provided 165 mg Zn (zinc sulfate), 165 mg Fe (iron sulfate), 39 mg Mn (manganese sulfate), 17 mg Cu (copper sulfate), 0.3 mg I (calcium iodate), and 0.3 mg Se (sodium selenite) per kg of diet.
5Denagard 10 (tiamulin, 22 g per kg), Elanco Animal Health, Greenfield, IN.
6Aureomycin 50 (chlortetracycline, 110 g per kg), Zoetis Inc., Kalamazoo, MI.
7Mecadox 2.5 (carbadox, 5.5 g per kg), Phibro Animal Health Corp., Ridgefield Park, NJ.
8SID = standardized ileal digestible.
Sample Collection
On day 0, 1 pig per pen, closest to pen average BW, was selected from which to collect repeated blood samples after being weighed and ear tagged again for identification. On days 7, 14, and 28, venous blood was collected from the same pigs by jugular venipuncture into 10-mL vacuum containers with sodium heparin (Becton Dickinson, Franklin Lakes, NJ) and placed on ice after collection. Harvested blood was centrifuged at 2,000 × g for 10 min at 4 °C, and the resulting plasma was aliquoted into 1.5-mL microcentrifuge tubes and stored at −80 °C for later analysis of cytokines, as markers of systemic inflammatory status.
On day 28, the same pig used for blood collection from each pen was euthanized by captive bolt stunning followed by exsanguination. Post-euthanasia, the abdomen was opened and the entire gastrointestinal tract was removed. Mid-colon tissues were collected, rinsed with ice-cold phosphate-buffered saline (PBS), snap-frozen in liquid nitrogen, and kept at −80 °C for later RNA extraction. Digesta samples (3 to 5 g) from distal ileum, cecum, and mid-colon were collected, snap-frozen in liquid nitrogen, and kept at −80 °C pending DNA extraction.
The pH of the digesta from the cecum and mid-colon was measured by directly inserting the probe of a portable pH meter (Oakton Instruments, Vernon Hills, IL) into the content. Digesta samples (approximately 20 mL) were then collected into 50-mL tubes and immediately stored at −20 °C pending volatile fatty acids (VFA) analysis.
Analytical Methods
The procedures for RNA isolation and real-time quantitative PCR (qPCR) to determine gene transcript abundance in colonic tissue were previously described in Li et al. (2018). Briefly, total RNA was isolated from homogenized colonic tissues (50 to 100 mg) using Trizol (1 mL; Invitrogen, Carlsbad, CA). After RNA quantification, 1 mg of isolated RNA was used for cDNA synthesis using the QuantiTect Reverse Transcription Kit (Qiagen GmbH, Hilden, Germany). The qPCR was performed in 20-µL reactions using iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA). Each reaction included 10 µL of SYBR Green Supermix, 1 µL of each forward and reverse primer, 2 µL of cDNA, and 6 µL of nuclease-free water. A no-reverse transcriptase negative control, a nuclease-free water control, and a pooled cDNA reference sample were included in each plate. Each sample was assayed in triplicate. The cycling conditions included 5-min initial denaturation at 95 °C followed by 40 PCR cycles (95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s) and a dissociation curve to verify the amplification of a single PCR product. Analyses of amplification plots were performed with the iQ5 Optical System Software version 2.0 (Bio-Rad Laboratories, Inc.) to obtain cycle threshold (Ct) values for each reaction. The mRNA abundance for each sample was normalized to a housekeeping gene (ribosomal protein—L19) and the pooled sample, and fold change was calculated using the 2−ΔΔCTmethod (Livak and Schmittgen, 2001). Plasma samples were analyzed in duplicate for interferon (IFN)-α, IFN-γ, tumor necrosis factor alpha (TNFα), interleukin (IL)-1β, IL-4, IL-6, IL-8, and IL-10 using a multiplex ELISA following the manufacturer’s recommendations (Aushon Biosystems, Billerica, MA).
The concentration of VFA was determined using Gas Chromatography (3800 Varian GC, Agilent Technologies, Santa Clara, CA). Digesta samples (1 g) were thawed and suspended in 2.5 mL of distilled water in a screw-capped tube. After being vortexed, 1 mL of the mixture was transferred into a 1.5-mL centrifuge tube and mixed with 0.2 mL of metaphosphoric acid and 0.1 mL of isocaproic acid as an internal standard (48.3 mM; Sigma-Aldrich, Saint Louis, MO). The tubes were then centrifuged at 15,000 × g at 4 °C for 20 min. Aliquots of the supernatant (1 mL) of the standard and digesta samples were transferred to 1.5-mL GC vials and 100 µL duplicates were injected into the GC for analysis. A standard curve was generated using 5 concentrations of acetate, propionate, butyrate, valerate, isobutyrate, and isovalerate (Sigma-Aldrich, Saint Louis, MO). A flame ionization detector was used with an oven temperature of 60 to 200 °C. The Nukol capillary column (15 m × 0.25 mm × 0.25 μm; Sigma-Aldrich, Bellefonte, PA) was operated with highly purified He, as the carrier gas, at 1 mL/min. Concentrations of acetate, propionate, butyrate, valerate, isobutyrate, and isovalerate were calculated using the ratio of the peak area of each compound to the internal standard and standard curve regression. Molar proportions of VFA (%) were calculated as the individual VFA/total VFA concentration × 100.
Microbial Quantification Using qPCR
Total genomic DNA was extracted from intestinal digesta (250 mg) using DNeasy PowerLyzer PowerSoil kit (Qiagen, Germantown, MD) according to manufacturer’s instructions. Genomic DNA concentration and purity were measured using a ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE). All samples had 260:280 nm ratios above 1.7. Extracted DNA from the ileal, cecal, and colonic digesta was adjusted to 5, 10, and 20 ng/µL, respectively.
The primers used to amplify the bacterial 16S rRNA gene are shown in Table 2. Lactobacillus and Bifidobacterium were selected to indicate abundance of beneficial bacteria, and Enterobacteriaceae was selected as an indicator of potential opportunistic pathogens. The PCR amplicon size for the target 16S rRNA genes was checked by running 1% gel electrophoresis. Real-time qPCR was performed in 20-µL reactions using iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). Each reaction was run in duplicate, including 10 µL of SYBR Green Supermix, 1 µL of each forward and reverse primer, 2 µL of DNA, and 6 µL of nuclease-free water. Duplicates of the no template negative control were included in each PCR run. All qPCR procedures were optimized and included: one cycle of predenaturation at 95 °C for 10 min; 40 cycles of denaturation at 95 °C for 15 s, at optimal annealing temperature for 30 s and extension at 72 °C for 60 s; one cycle of 95 °C for 1 min; and one cycle of 55 °C for 1 min. Data were collected at the extension step. Fluorescence of SYBR Green was quantified with the iQ5 Real Time PCR Detection System, and amplification plots were analyzed using the iQ5 Optical System Software version 2.0 (Bio-Rad Laboratories, Hercules, CA) to obtain the Ct values for each reaction. To determine the specificity of the amplification, melting curve analysis was performed by slow heating with an increment of 0.1 °C/s from 55 to 95 °C.
Table 2.
Sequences of primers for quantification of total bacteria and target bacterial groups
| Bacteria | Primer sequence, 5′–3′ | Annealing temperature (°C) | Product size (bp) |
| Total bacteria | |||
| Forward | CCTACGGGAGGCAGCAG | 61 | 189 |
| Reverse | ATTACCGCGGCTGCTGG | ||
| Lactobacillus spp. | |||
| Forward | AGCAGTAGGGAATCTTCCA | 62 | 341 |
| Reverse | CACCGCTACACATGGAG | ||
| Bifidobacterium spp. | |||
| Forward | TCGCGTCYGGTGTGAAAG | 60 | 243 |
| Reverse | CCACATCCAGCRTCCAC | ||
| Enterobacteriaceae family | |||
| Forward | CATTGACGTTACCCGCAGAAGAAGC | 65 | 195 |
| Reverse | CTCTACGAGACTCAAGCTTGC |
Primer sequences were from Metzler-Zebeli et al. (2013).
To quantify the 16S rRNA gene copy numbers of total bacteria and target bacteria, standard curves were generated using a series of 10-fold dilutions of purified PCR products or bacterial DNA extracted from pure cultures of specific bacteria. For total bacteria, PCR products from the amplifying microbial 16S rRNA gene using the universal primer set 27F-1492R (27F: AGAGTTTGATYMTGGCTCAG; 1492R: TACG GYTACCTTGTTACGACT) from digesta genomic DNA were purified with a PureLink PCR purification kit (Invitrogen Life Technologies, Carlsbad, CA) and used as standard DNA. Concentrations of standard DNA samples were quantified using Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Hanover Park, IL). The copy number of standard DNA molecules was calculated using the following equation: DNA (molecules/µL) = [6.02 × 1023 (molecules/mol) × DNA concentration (g/µL)]/[DNA length (bp) × 660 (g/mol/bp)]. The standard DNAs were then serially diluted 10-fold (1 × 107 to 1 × 101 copies/μL) and qPCR reactions were run to build specific standard curves. Each standard curve was constructed by linear regression of the plotted points, and Ct values were plotted against the logarithm of template copy numbers.
The gene copy numbers of total bacteria and target bacterial groups in intestinal digesta DNAs were determined by relating the Ct values to respective standard curves. The final copy numbers of total bacteria and target bacterial groups per gram of wet digesta were calculated using the equation (QM × C × DV)/(S × V), according to Metzler-Zebeli et al. (2013), where QM is the quantitative mean of the copy number, C is the DNA concentration of each sample, DV is the dilution volume of extracted DNA, S is the DNA amount (ng) in each reaction, and V is the sample weight (g) used for DNA extraction. The amplification efficiency was confirmed to be between 80% and 110% for all reactions.
Statistical Analysis
Data were analyzed as a 2 × 2 factorial in a randomized complete block design using PROC GLIMMIX of SAS 9.4 (SAS Institute Inc., Cary, NC). The 2 factors were X (0 or 0.01%) and EB (0 or 0.01%). The UNIVARIATE procedure was used to check normality and equal variance of residuals, and to identify statistical outliers (>3 standard deviations from the mean). Xylanase, EB, and their interaction were fixed effects; block was considered a random effect. The individual pig from each pen from which samples were collected was the experimental unit.
Blood cytokine data were log-transformed before analysis, using repeated measurements with collection day as the repeated effect and a variance structure of auto-regressive 1 was applied. Because baseline (day 0) IFN-α was different and IL-8 tended to be different among treatments, their concentrations were used as a covariate in the model. If there was a treatment by day interaction, means of the main effect on each day were separated using the least square means statement and the slice = day option. The interactions between X and EB within each day were calculated using the lsmestimate statement. Bacterial 16S rRNA gene copies of total and specific bacteria data were log10-transformed for analysis. Least square means of treatments were reported. Differences were considered significant if P ≤ 0.05 and a tendency if 0.05 < P ≤ 0.10.
RESULTS
Colonic Gene mRNA Abundance
Xylanase did not affect the mRNA abundance of IL1B; however, EB tended to decrease its abundance (P = 0.054; Table 3). A significant X × EB interaction (P < 0.05) was observed for colonic IL-6 mRNA, with EB increasing IL-6 mRNA in the presence of X, but not in the absence of X. The addition of X tended to decrease (P < 0.10) IL-10 mRNA in the colon of pigs receiving diets with EB, but not in diets without EB. The mRNA abundance of IL-17 tended to be greater in pigs fed diets with X, and IL-22 tended to be decreased by EB (P < 0.10). A significant X × EB interaction was observed for colonic IL-17, occludin (OCLN), and claudin-3 (CLDN3) mRNA (P < 0.05); their mRNA abundance was increased in pigs fed diets supplemented with X + EB, but not X or EB alone, compared with those fed unsupplemented CON diets (P < 0.05). Pigs consuming diets with X tended to have greater mRNA abundance of OCLN, and EB tended to increase mRNA abundance of CLDN3 and zonula occludens-1 (ZO-1; P < 0.10) compared with diets without enzyme supplementation.
Table 3.
Effect of xylanase and a carbohydrase enzyme blend on the fold change of mRNA abundance of cytokines and tight junction protein genes in the colon
| Gene name1 | Treatment2 | SEM | P | |||||
| Control | X | EB | X + EB | X | EB | X × EB | ||
| IL-1B | 3.58 | 2.91 | 2.35 | 0.87 | 0.89 | 0.245 | 0.054 | 0.491 |
| IL-6 | 1.33ab | 0.75b | 0.84ab | 1.36a | 0.22 | 0.911 | 0.370 | 0.015 |
| IL-10 | 1.65ab | 1.92ab | 2.04a | 0.83b | 0.48 | 0.199 | 0.874 | 0.055 |
| IL-17 | 1.33b | 1.42ab | 1.13b | 1.74a | 0.15 | 0.072 | 0.668 | 0.015 |
| IL-22 | 2.03 | 1.94 | 0.89 | 1.67 | 0.35 | 0.297 | 0.100 | 0.170 |
| OCLN | 1.08b | 1.20b | 0.68b | 1.53a | 0.21 | 0.052 | 0.305 | 0.014 |
| CLDN3 | 1.63b | 1.22b | 1.17b | 2.40a | 0.27 | 0.309 | 0.059 | 0.013 |
| ZO-1 | 0.41 | 0.45 | 0.44 | 0.67 | 0.11 | 0.148 | 0.075 | 0.212 |
n = 12 per treatment.
1 IL: interleukin; OCLN: occludin; CLDN3: claudin 3; ZO-1: zonula occludens-1.
2X = xylanase, EB = enzyme blend, X + EB = combination of xylanase and enzyme blend. Xylanase activity was expected to be 15,000 EPU/g; one gram of EB was expected to contain 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase; the inclusion rate of both X and EB was 0.01% according to manufacturer’s recommendation.
Plasma Cytokines
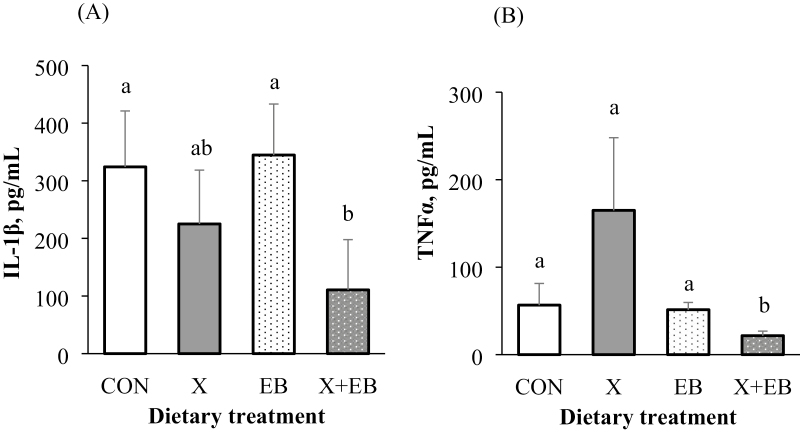
More than half of the plasma samples were below the detection limit for IL-4, IL-6, IL-10, and IFNγ; thus, no statistical analysis was performed (data not shown). No X × EB × day interaction was observed for any blood cytokines. Because there was no X × day or EB × day interaction for IL-1ß and TNFα, the overall interaction effect of X × EB on IL-1ß and TNFα was shown. Plasma levels of IL-1ß and TNFα were significantly decreased only when X and EB were added together, but not individually, compared with CON (P < 0.05; Figure 1). The EB increased plasma IFNα on day 14 compared with diets without EB (P < 0.05); however, EB had no impact on IFNα on day 7 or over the 28-d period (Table 4). Neither X nor X × EB interaction affected plasma levels of IFNα or IL-8. Pigs fed diets with EB had lower concentrations of IL-8 on day 7 and over the 28-d period in the plasma compared with those receiving diets without EB (P < 0.05).
Figure 1.
Effect of xylanase (X) and a carbohydrase enzyme blend (EB) on concentration of plasma cytokines in weaned pigs, pg/mL. (A): Interleukin (IL)-1β; (B) Tumor necrosis factor alpha (TNFα). X + EB: diets supplemented with both X and EB; a,bMeans without a common superscript differ (P < 0.05); Data were average least square means across 4 collection days (days 0, 7, 14, and 28) and n = 48 per treatment.
Table 4.
Effect of xylanase and a carbohydrase blend on plasma cytokine concentrations
| Item1 | Treatment2 | SEM | P 3,4 | |||||
| Control | X | EB | X + EB | X | EB | X × EB | ||
| IFNα, pg/mL | 0.409 | 0.341 | 0.274 | |||||
| day 0 | 199.4 | 143.8 | 86.6 | 30.0 | 27.8 | 0.356 | 0.065 | 0.973 |
| day 7 | 32.9 | 35.8 | 1.5 | 2.2 | 11.8 | 0.600 | 0.348 | 0.521 |
| day 14 | 0.3 | 1.2 | 2.4 | 1.3 | 0.5 | 0.696 | 0.001 | 0.162 |
| day 28 | 1.4 | 5.5 | 4.2 | 5.3 | 1.4 | 0.147 | 0.069 | 0.729 |
| IL-8, pg/mL | 0.543 | 0.046 | 0.727 | |||||
| day 0 | 86.4 | 14.0 | 31.9 | 10.7 | 17.6 | 0.008 | 0.239 | 0.997 |
| day 7 | 19.2 | 19.3 | 8.7 | 7.3 | 3.5 | 0.846 | 0.042 | 0.344 |
| day 14 | 4.5 | 7.4 | 3.7 | 4.1 | 0.8 | 0.529 | 0.328 | 0.787 |
| day 28 | 10.7 | 10.9 | 9.5 | 13.7 | 1.9 | 0.489 | 0.899 | 0.600 |
n = 12 per treatment for individual collection day data and 48 for 4 collection days data; data were analyzed as repeated measurements with day as the repeated effect; P (day) < 0.01 and P (X × EB × day) > 0.10
1IFNα = interferon alpha; IL = interleukin.
2X = xylanase; EB = enzyme blend; X + EB = combination of xylanase and enzyme blend. Xylanase activity was expected to be 15,000 EPU/g; one gram of EB was expected to contain 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase; the inclusion rate of both X and EB was 0.01% according to manufacturer’s recommendation.
3Baseline IFNα concentration on day 0 was different among treatments; thus, it was used as a covariate in data analysis.
4Baseline IL-8 concentration on day 0 tended to be different among treatments; thus, it was used as a covariate in data analysis.
Volatile Fatty Acids and pH
In the cecum, there was no X × EB interaction or main effect of X on any VFA or on pH (Table 5). The EB decreased the concentration of propionate, butyrate, valerate, and total VFA (P < 0.05), but did not affect acetate concentration or pH in the cecum. The molar proportion (%) of cecal acetate was higher and butyrate was lower in EB-supplemented diets than those without EB (P < 0.05).
Table 5.
Effect of xylanase and a carbohydrase enzyme blend on volatile fatty acids and pH of digesta in the cecum
| Item | Treatment1 | SEM | P | |||||
| Control | X | EB | X + EB | X | EB | X × EB | ||
| VFA2 concentration, µmol/g | ||||||||
| Acetate | 84.80 | 84.25 | 77.39 | 81.45 | 2.80 | 0.595 | 0.128 | 0.485 |
| Propionate | 39.53 | 38.15 | 32.53 | 34.26 | 1.05 | 0.907 | 0.001 | 0.300 |
| Butyrate | 16.50 | 17.54 | 12.74 | 13.11 | 0.84 | 0.556 | 0.002 | 0.780 |
| Valerate | 1.95 | 2.30 | 1.30 | 1.40 | 0.19 | 0.408 | 0.008 | 0.663 |
| Isobutyrate | 0.28 | 0.23 | 0.21 | 0.23 | 0.02 | 0.568 | 0.168 | 0.114 |
| Isovalerate | 0.30 | 0.26 | 0.27 | 0.28 | 0.02 | 0.717 | 0.865 | 0.514 |
| Total | 143.22 | 142.66 | 124.33 | 135.04 | 3.47 | 0.314 | 0.012 | 0.265 |
| pH | 5.69 | 5.64 | 5.72 | 5.74 | 0.04 | 0.758 | 0.282 | 0.568 |
| VFA molar proportion, %3 | ||||||||
| Acetate | 59.36 | 59.01 | 62.13 | 62.03 | 0.80 | 0.808 | 0.005 | 0.896 |
| Propionate | 27.71 | 26.81 | 26.13 | 26.42 | 0.50 | 0.634 | 0.132 | 0.355 |
| Butyrate | 11.31 | 12.24 | 10.35 | 10.09 | 0.49 | 0.628 | 0.030 | 0.391 |
| Valerate | 1.32 | 1.64 | 1.08 | 1.13 | 0.15 | 0.349 | 0.062 | 0.480 |
| Isobutyrate | 0.19 | 0.16 | 0.17 | 0.19 | 0.02 | 0.789 | 0.968 | 0.199 |
| Isovalerate | 0.21 | 0.19 | 0.22 | 0.23 | 0.02 | 0.831 | 0.350 | 0.657 |
n = 12 per treatment.
1X = xylanase; EB = enzyme blend; X + EB = combination of X and EB. Xylanase activity was expected to be 15,000 EPU/g; one gram of EB was expected to contain 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase; the inclusion rate of both X and EB was 0.01% according to manufacturer’s recommendation.
2VFA = volatile fatty acids.
3Calculated as the individual VFA concentration/total VFA concentration × 100%.
In the colon, the main effect of X did not affect the concentrations of any VFA (Table 6) except for isobutyrate and isovalerate, which were increased compared with diets without X (P < 0.05). Butyrate concentration tended to be lower in colon of pigs fed the EB-supplemented diet compared with pigs fed diets without EB (P < 0.10). There was a significant X × EB interaction for colonic propionate (P < 0.05); EB decreased its concentration when X was not present, but not when X was supplemented. A tendency for X × EB interaction was also observed for colonic total VFA (P < 0.10) such that EB decreased its concentration when added individually, but not together with X, compared with diets without EB. Xylanase tended to increase colonic pH when EB was not present (P < 0.10), but not when EB was present.
Table 6.
Effect of xylanase and a carbohydrase enzyme blend on volatile fatty acids and pH of digesta in the colon
| Item | Treatment1 | SEM | P | |||||
| Control | X | EB | X + EB | X | EB | X × EB | ||
| VFA2 concentration, µmol/g | ||||||||
| Acetate | 93.60 | 86.44 | 85.16 | 88.48 | 3.95 | 0.640 | 0.437 | 0.207 |
| Propionate | 44.85a | 36.64b | 37.03b | 38.13b | 2.10 | 0.126 | 0.172 | 0.048 |
| Butyrate | 23.52 | 19.85 | 18.70 | 19.88 | 1.42 | 0.358 | 0.082 | 0.078 |
| Valerate | 3.48 | 3.19 | 2.61 | 3.05 | 0.28 | 0.853 | 0.203 | 0.352 |
| Isobutyrate | 0.83 | 1.18 | 0.89 | 1.15 | 0.10 | 0.038 | 0.904 | 0.745 |
| Isovalerate | 1.11 | 1.61 | 1.18 | 1.59 | 0.15 | 0.039 | 0.917 | 0.828 |
| Total | 167.40 | 148.91 | 145.57 | 152.27 | 7.16 | 0.395 | 0.186 | 0.075 |
| pH | 5.82 | 6.14 | 5.96 | 5.94 | 0.07 | 0.081 | 0.670 | 0.053 |
| VFA molar proportion, %3 | ||||||||
| Acetate | 56.13 | 57.89 | 58.69 | 58.26 | 0.62 | 0.419 | 0.079 | 0.187 |
| Propionate | 26.78 | 24.58 | 25.17 | 24.79 | 0.45 | 0.049 | 0.275 | 0.158 |
| Butyrate | 13.90 | 13.26 | 12.72 | 12.99 | 0.48 | 0.762 | 0.233 | 0.451 |
| Valerate | 2.01 | 2.21 | 1.80 | 2.00 | 0.15 | 0.337 | 0.314 | 0.997 |
| Isobutyrate | 0.50 | 0.86 | 0.69 | 0.82 | 0.09 | 0.075 | 0.583 | 0.377 |
| Isovalerate | 0.68 | 1.20 | 0.93 | 1.14 | 0.14 | 0.072 | 0.616 | 0.434 |
n = 12 per treatment.
1X = xylanase; EB = enzyme blend; X + EB = combination of X and EB. Xylanase activity was expected to be 15,000 EPU/g; one gram of EB was expected to contain 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase; the inclusion rate of both X and EB was 0.01% according to manufacturer’s recommendation.
2VFA = volatile fatty acids.
3Calculated as the individual VFA concentration/total VFA concentration × 100%
Microbial Populations in the Intestinal Digesta
The supplementation of X and EB, individually or together, did not alter 16S rRNA gene copies of total bacteria or Bifidobacterium spp. in all 3 intestinal segments (Table 7). Unexpectedly, X decreased Lactobacillus spp. in the ileum and increased the Enterobacteriaceae family in both ileum and cecum (P < 0.05). Similarly, EB tended to reduce the gene copies of Lactobacillus spp. in both ileum and cecum (P < 0.10) and significantly decreased Lactobacillus spp. gene copies in the colon compared with diets without EB (P < 0.01). The gene copies of the Enterobacteriaceae family tended to be lower in the colon of pigs fed EB-supplemented diets compared with those who received diets without EB (P < 0.10). No X × EB interaction was detected for Enterobacteriaceae in all 3 intestinal sections.
Table 7.
Effect of xylanase and a carbohydrase enzyme blend on intestinal bacterial populations, log10 16S rRNA gene copies/g wet digesta
| Item | Treatment1 | SEM | P | |||||
| Control | X | EB | X + EB | X | EB | X × EB | ||
| Ileum | ||||||||
| Total | 9.64 | 9.70 | 9.56 | 9.68 | 0.10 | 0.482 | 0.687 | 0.840 |
| Lactobacillus spp. | 8.66 | 8.41 | 8.46 | 7.86 | 0.15 | 0.045 | 0.074 | 0.391 |
| Bifidobaterium spp. | 8.05 | 7.58 | 7.43 | 7.63 | 0.26 | 0.710 | 0.440 | 0.366 |
| Enterobacteriaceae family | 4.43 | 5.34 | 4.64 | 5.01 | 0.20 | 0.012 | 0.813 | 0.266 |
| Cecum | ||||||||
| Total | 10.17 | 10.21 | 10.16 | 10.29 | 0.04 | 0.148 | 0.581 | 0.462 |
| Lactobacillus spp. | 8.90 | 8.89 | 8.71 | 8.61 | 0.08 | 0.644 | 0.061 | 0.712 |
| Bifidobaterium spp. | 8.30 | 7.90 | 7.86 | 8.03 | 0.20 | 0.683 | 0.575 | 0.302 |
| Enterobacteriaceae family | 6.01 | 6.52 | 6.13 | 6.50 | 0.15 | 0.046 | 0.818 | 0.761 |
| Colon | ||||||||
| Total | 9.97 | 10.09 | 9.98 | 9.96 | 0.05 | 0.323 | 0.256 | 0.199 |
| Lactobacillus spp. | 9.08 | 9.18 | 8.97 | 8.77 | 0.06 | 0.601 | 0.005 | 0.085 |
| Bifidobaterium spp. | 7.57 | 7.59 | 7.61 | 7.63 | 0.15 | 0.475 | 0.318 | 0.408 |
| Enterobacteriaceae family | 6.15 | 6.61 | 6.04 | 5.99 | 0.13 | 0.290 | 0.065 | 0.191 |
n = 12 per treatment.
1X = xylanase; EB = enzyme blend; X + EB = combination of X and EB. Xylanase activity was expected to be 15,000 EPU/g; one gram of EB was expected to contain 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase; the inclusion rate of both X and EB was 0.01% according to manufacturer’s recommendation.
The relative abundance (%) of Lactobacillus spp. was decreased (P < 0.05) and Bifidobacterium spp. tended to be decreased (P < 0.10) in both ileum and cecum by the main effect of X (Table 8). The X also increased the relative abundance of Enterobacteriaceae in the ileum (P < 0.05). Pigs fed diets with EB tended to reduce the relative abundance of ileal Lactobacillus spp. (P < 0.10) and significantly reduced cecal and colonic relative abundance of Lactobacillus spp. compared with those receiving unsupplemented diets (P < 0.05). The main effect of EB and X × EB interaction had no impact on relative abundance of Bifidobacterium spp. and Enterobacteriaceae.
Table 8.
Effect of xylanase and a carbohydrase enzyme blend on relative abundance of intestinal bacteria
| Item | Treatment1 | SEM | P | |||||
| Control | X | EB | X + EB | X | EB | X × EB | ||
| Ileum, % | ||||||||
| Lactobacillus spp. | 13.25 | 10.57 | 10.75 | 3.39 | 2.48 | 0.007 | 0.075 | 0.289 |
| Bifidobaterium spp. | 4.76 | 2.62 | 3.93 | 1.64 | 0.92 | 0.098 | 0.489 | 0.954 |
| Enterobacteriaceae family | 0.001 | 0.018 | 0.003 | 0.031 | 0.01 | 0.033 | 0.939 | 0.225 |
| Cecum, % | ||||||||
| Lactobacillus spp. | 6.32 | 6.08 | 4.59 | 2.43 | 0.71 | 0.046 | 0.001 | 0.076 |
| Bifidobaterium spp. | 2.04 | 1.24 | 1.92 | 1.07 | 0.34 | 0.059 | 0.712 | 0.874 |
| Enterobacteriaceae family | 0.03 | 0.04 | 0.02 | 0.05 | 0.01 | 0.272 | 0.758 | 0.877 |
| Colon, % | ||||||||
| Lactobacillus spp. | 14.98 | 14.71 | 11.76 | 8.05 | 1.44 | 0.246 | 0.032 | 0.446 |
| Bifidobaterium spp. | 0.61 | 0.42 | 0.76 | 0.56 | 0.11 | 0.199 | 0.341 | 0.959 |
| Enterobacteriaceae family | 0.05 | 0.09 | 0.04 | 0.03 | 0.02 | 0.466 | 0.117 | 0.465 |
n = 12 per treatment.
1X = xylanase; EB = enzyme blend; X + EB = combination of X and EB. Xylanase activity was expected to be 15,000 EPU/g; one gram of EB was expected to contain 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase; the inclusion rate of both X and EB was 0.01% according to manufacturer’s recommendation.
DISCUSSION
This study was a further analysis of samples collected during experiments conducted in previous work (Li et al., 2018). It was found that EB supplementation improved small intestinal barrier function and reduced markers of immune activation in the ileum, which concurred with an improved growth rate compared with diets without EB; yet, feed intake and digestibility of energy and nutrients were not improved by EB, except for ADF (Li et al., 2018). The current study was to examine the effects of EB on colonic and peripheral inflammatory status as well as microbial populations in the same pigs. Such work will provide further evidence for the mode of action of EB in improving the growth of weaned pigs fed higher fiber diets.
The current results that EB tended to increase CLDN3 and ZO-1 mRNA and decrease IL-22 and IL-1B mRNA abundance in the colon agreed with previously reported findings in the ileum (Li et al., 2018). This may indicate that EB supplementation improves intestinal barrier function and reduces markers of inflammation not only in the small intestine but also the large intestine. Elevated levels of proinflammatory cytokines, such as IL-1β and TNFα, increase gut permeability through modulating tight junction proteins (Capaldo and Nusrat, 2009; Chen et al., 2013). Conversely, impaired barrier integrity in pigs caused by heat stress increases the translocation of lipopolysaccharide (Gabler et al., 2018), which is a potent immune stimulator to induce inflammation (Huntley et al., 2018). Thus, the reduced plasma IL-8 and colonic IL-1B mRNA in EB seemed consistent with previously observed improvements in small intestinal barrier integrity (Li et al., 2018) and the current upregulated tight junction proteins mRNA. The downregulation of IL-1B mRNA in the colon by EB may also be associated with decreased butyrate concentration. This is supported by Milo et al. (2002), who reported that VFA (acetate, propionate, and butyrate) supplementation increased ileal IL-1β and IL-6 protein abundance in pigs. Furthermore, IL-17 has been shown to stimulate the development of tight junctions in human intestinal epithelial cells, which was correlated with up-regulation of claudin-1 and claudin-2 mRNA levels (Kinugasa et al., 2000). These data partially support our findings that the greater mRNA abundance of colonic OCLN and CLDN3 in diets with X + EB coincided with elevated colonic IL-17 mRNA.
Neither X nor EB had an impact on plasma IFNα over the 28-d period, which may be explained by the fact that IFNα proteins are often associated with viral infection (Doyle, 2016). Interlukin-1ß, TNFα, and IL-8 are important inflammatory response mediators, levels of which are normally increased during an immune challenge to orchestrate host immune response (Huntley et al., 2018). However, increased amounts of proinflammatory cytokines generally have a negative influence on the growth and well-being of the animal (Elsasser et al., 1995; Huntley et al., 2018). Therefore, the lower plasma IL-8 in pigs fed EB and reduced plasma TNFα and IL-1ß in pigs fed diets with X + EB may suggest reduced systemic inflammation by carbohydrase supplementation. The observation that plasma TNFα and IL-1ß were decreased only by X + EB in comparison to CON indicates an additive impact of the 2 enzymes. Xylanase contained high xylanase activity and EB contained high cellulase and ß-glucanase activity, but low xylanase activity. The combination of X and EB may be more effective in breaking down the NSP to release low molecular weight oligosaccharides, which then can reduce inflammation (Chen et al., 2012; Mendis et al., 2016). The decreased markers of inflammation by EB (plasma IL-8 and colonic IL1B) and X + EB (plasma TNFα and IL-1ß), as well as increased indicators for improved intestinal barrier integrity, may contribute to the previously observed improvement in ADG in EB-supplemented diets (Li et al., 2018).
Exogenous enzymes may regulate intestinal microbiota through modulating undigested substrates (e.g., starch and protein) and in situ release of soluble and more easily fermentable oligosaccharides in the intestine (Bedford and Cowieson, 2012; Kiarie et al., 2013). Previous research showed that AXOS derived from arabinoxylan degradation promoted the growth of some Lactobacilli and Bifidobacteria in chickens (Courtin et al., 2008) and humans (Kontula et al., 2000; Sanchez et al., 2009). Although there is a scarcity of information about xylanase-derived AXOS on intestinal microbiota in pigs, the current finding that X decreased Lactobacillus spp. tended to decrease Bifidobacterium spp. and increased Enterobacteriaceae family in the intestine is rather unexpected, and the reasons for those findings are unclear.
The 16S rRNA gene copies and relative abundance (%) of Lactobacillus spp. (amylolytic bacteria) tended to be decreased in the ileum and were significantly decreased in the colon of pigs fed EB-supplemented diets compared with unsupplemented diets. These data appear to be due to the accelerated removal of starch by EB (Bedford and Cowieson, 2012; Zhang et al., 2018). In accordance with the current experiment, Smith et al. (2010) showed that xylanase and ß-glucanase supplementation decreased Lactobacilli populations in the ileum compared with treatment without enzymes. Similarly, Vahjen et al. (2007) reported decreased Lactobacillus spp. in the stomach by adding either a multienzyme or a monoenzyme to a wheat-based diet in nursery pigs. The authors suspected that the inhibition of bacterial growth might be protective against generating more hydrogen peroxide by Lactobacilli, potentially leading to peroxidation and subsequently impaired growth performance. In contrast, Kiarie et al. (2007) and Zhang et al. (2014) reported that the addition of a blend of carbohydrases increased the Lactobacilli count in the ileum and feces in pigs.
Because EB improved growth rate and intestinal barrier integrity (Li et al., 2018), it seems that the decrease in Lactobacillus spp. by EB supplementation may not indicate disturbed intestinal microbial homeostasis. Xu et al. (2016) speculated that the decrease in Lactobacillus on day 8 postweaning in the stomach and ileum of piglets supplemented with butyrate may not negatively affect gut health, as microbial diversity was increased and Lactobacillus was still the most predominant genus. Furthermore, pigs fed EB-supplemented diets tended to have reduced colonic Enterobacteriaceae, indicating less abundance of pathogenic bacteria, which agreed with Zhang et al. (2014) and Zhang et al. (2018). Collectively, the decreased Lactobacillus spp. abundance in the small and large intestine by X or EB may suggest reduced substrate availability, supported by Van der Meulen et al. (2001), who showed xylanase and cellulase activitity in the stomach and small intestine. Lower Lactobacillus abundance was reported in IL-22-deficient mice, with no detectable pathology or abnormal colon architecture (Zenewicz et al., 2013). Therefore, it is also possible that the decreased Lactobacillus abundance observed in EB is associated with an interaction with the host immune system (e.g., lower ileal IL-22 mRNA reported in Li et al., 2018). The discrepancies in microbiota across experiments are probably associated with differences in initial gut microbial status and age of animals, type of carbohydrases used, dietary nutrient levels, and fibrous substrate concentration and characteristics [reviewed by Kiarie et al. (2013) and Mendis et al. (2016)]. Further studies are warranted to investigate the composition and function of microbial communities more broadly, in the context of carbohydrases as feed additives, to elucidate associated functions regarding intestinal health and immunity via molecular microbiology techniques, such as 16S rRNA sequencing, metagenomics, and metatranscriptomics.
As with reduction in Lactobacillus abundance, the lower total VFA in the cecum of pigs fed EB-supplemented diets compared with unsupplemented diets may reflect reduced substrate availability. It appears that the readily fermentable fiber was degraded by EB and the released degradation products were fermented by microbes in the small intestine, resulting in less degradable substrate entering the large intestine for microbial fermentation (Zeng et al., 2018). This theory is further confirmed by Clarke et al. (2018), who reported decreased total VFA in the colon accompanied with an improved ileal digestibility of gross energy in pigs receiving xylanase and β-glucanase supplemented diets. Energy absorbed as monosaccharides (e.g., glucose) in the small intestine will be used by the pig with greater efficiency than as VFA produced by fermentation in the large intestine (Patience, 2017). Therefore, carbohydrases may improve energy utilization by shifting fiber degradation from the distal to proximal intestine. This agrees with the improved growth performance in EB-supplemented diets (Li et al., 2018). The X increased isobutyrate and isovalerate in the colon, suggesting increased fermentation of proteinaceous substrates, in agreement with Clarke et al. (2018). This is most likely due to increased degradation of easily fermentable fiber in X-supplemented diets before the colon, thus reducing substrate availability in the colon. The reduced available substrates, in turn, cause decreased fiber-utilizing bacteria and nitrogen utilization for protein synthesis by those bacteria, which consequently leads to increased proteinaceous substrates for fermentation (Clarke et al., 2018).
In conclusion, EB decreased IL-8 and the combination of X and EB decreased TNFα and IL-1ß in the plasma, possibly suggesting decreased systemic immune activation. The mRNA abundance of colonic tight junction protein genes was significantly increased. The reduced plasma proinflammatory cytokines and improved colonic tight junction protein mRNA levels by the combination of X + EB indicate additive effects of the 2 enzymes. Lower relative abundance of Lactobacillus spp. was observed in pigs fed X-supplemented diets compared with those fed diets without X. Supplementation of EB decreased the relative abundance of Lactobacillus spp. in the cecum and colon as well as total VFA in the cecum compared with diets without EB. These data may indicate improved fiber and starch degradation in the small intestine and hence decreased fermentable substrate availability for microbes in the large intestine. These data corroborated previously observed enhanced growth in pigs fed EB-supplemented diets. Further studies are warranted to investigate broader microbial population changes in response to dietary carbohydrase addition and mechanisms whereby carbohydrases alter immune response. In situ production of fiber degradation products in both small and large intestines should be considered to provide insight into the association between microbial activity and the amount as well as the type of specific NSP hydrolysis products.
Footnotes
We would like to thank Huvepharma Inc. for financial support of this research. Appreciation is also expressed to Ajinomoto Heartland, DSM Nutritional Products, and Hamlet Protein for in-kind support.
LITERATURE CITED
- Adeola O., and Cowieson A. J.. 2011. Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 89:3189–3218. doi: 10.2527/jas.2010-3715 [DOI] [PubMed] [Google Scholar]
- Bedford M., and Cowieson A.. 2012. Exogenous enzymes and their effects on intestinal microbiology. Anim. Feed Sci. Technol. 173: 76–85. doi: 10.1016/j.anifeedsci.2011.12.018 [DOI] [Google Scholar]
- Capaldo C. T., and Nusrat A.. 2009. Cytokine regulation of tight junctions. Biochim. Biophys. Acta 1788:864–871. doi: 10.1016/j.bbamem.2008.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. H., Chen Y. K., Chang H. C., and Lin S. Y.. 2012. Immunomodulatory effects of xylooligosaccharides. Food Sci. Technol. Res. 18: 195–199. doi: 10.3136/fstr.18.195 [DOI] [Google Scholar]
- Chen H., Mao X., He J., Yu B., Huang Z., Yu J., Zheng P., and Chen D.. 2013. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 110:1837–1848. doi: 10.1017/S0007114513001293 [DOI] [PubMed] [Google Scholar]
- Clarke L., Sweeney T., Curley E., Gath V., Duffy S., Vigors S., Rajauria G., and O’Doherty J.. 2018. Effect of β-glucanase and β-xylanase enzyme supplemented barley diets on nutrient digestibility, growth performance and expression of intestinal nutrient transporter genes in finisher pigs. Anim. Feed Sci. Technol. 238: 98–110. doi: 10.1016/j.anifeedsci.2018.02.006 [DOI] [Google Scholar]
- Courtin C. M., Swennen K., Broekaert W. F., Swennen Q., Buyse J., Decuypere E., Michiels C. W., De Ketelaere B., and Delcour J. A.. 2008. Effects of dietary inclusion of xylooligo‐saccharides, arabinoxylooligosaccha‐rides and soluble arabinoxylan on the microbial composition of caecal contents of chickens. J. Sci. Food Agr. 88: 2517–2522. doi: 10.1002/jsfa.3373 [DOI] [Google Scholar]
- Doyle T. 2016. The evolving role of interferons in viral eradication strategies. J. Virus Erad. 2:121–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsasser T. H., Steele N. C., and Fayer R.. 1995. Cytokines, stress, and growth modulation. In: Myers M., Murthaugh M., editors, Cytokines in animal health and disease. NY: Marcel Dekker; p. 261–290. [Google Scholar]
- Gabler N. K., Koltes D., Schaumberger S., Murugesan G. R., and Reisinger N.. 2018. Diurnal heat stress reduces pig intestinal integrity and increases endotoxin translocation. Transl. Anim. Sci. 2: 1–10. doi: 10.1093/tas/txx003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill B., Mellange J., and Rooke J.. 2000. Growth performance and apparent nutrient digestibility in weaned piglets offered wheat-, barley-or sugar-beet pulp-based diets supplemented with food enzymes. Anim. Sci. 70: 107–118. doi: 10.1017/S135772980005164X [DOI] [Google Scholar]
- Huntley N. F., Nyachoti C. M., and Patience J. F.. 2018. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotechnol. 9:47. doi: 10.1186/s40104-018-0264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie E., Nyachoti C. M., Slominski B. A., and Blank G.. 2007. Growth performance, gastrointestinal microbial activity, and nutrient digestibility in early-weaned pigs fed diets containing flaxseed and carbohydrase enzyme. J. Anim. Sci. 85:2982–2993. doi: 10.2527/jas.2006-481 [DOI] [PubMed] [Google Scholar]
- Kiarie E., Romero L. F., and Nyachoti C. M.. 2013. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 26:71–88. doi: 10.1017/S0954422413000048 [DOI] [PubMed] [Google Scholar]
- Kinugasa T., Sakaguchi T., Gu X., and Reinecker H. C.. 2000. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 118:1001–1011. doi: 10.1016/S0016-5085(00)70351-9 [DOI] [PubMed] [Google Scholar]
- Knudsen K. E. B., Hedemann M. S., and Lærke H. N.. 2012. The role of carbohydrates in intestinal health of pigs. Anim. Feed Sci. Technol. 173: 41–53. doi: 10.1016/j.anifeedsci.2011.12.020 [DOI] [Google Scholar]
- Kontula P., Suihko M.-L., Suortti T., Tenkanen M., Mattila-Sandholm T., and Von Wright A.. 2000. The isolation of lactic acid bacteria from human colonic biopsies after enrichment on lactose derivatives and rye arabinoxylo-oligosaccharides. Food Microbiol. 17: 13–22. doi: 10.1006/fmic.1999.0268 [DOI] [Google Scholar]
- Lærke H. N., Arent S., Dalsgaard S., and Bach Knudsen K. E.. 2015. Effect of xylanases on ileal viscosity, intestinal fiber modification, and apparent ileal fiber and nutrient digestibility of rye and wheat in growing pigs. J. Anim. Sci. 93:4323–4335. doi:10.2527/jas.2015-9096 [DOI] [PubMed] [Google Scholar]
- Li Q., Gabler N. K., Loving C. L., Gould S. A., and Patience J. F.. 2018. A dietary carbohydrase blend improved intestinal barrier function and growth rate in weaned pigs fed higher fiber diets. J. Anim. Sci. 96:5233–5243. doi: 10.1093/jas/sky383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., and Patience J. F.. 2016. Factors involved in the regulation of feed and energy intake of pigs. Anim. Feed Sci. Technol. 233:22–33. doi:10.1016/j.anifeedsci.2016.01.001 [Google Scholar]
- Livak K. J., and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Mendis M., Leclerc E., and Simsek S.. 2016. Arabinoxylans, gut microbiota and immunity. Carbohydr. Polym. 139:159–166. doi: 10.1016/j.carbpol.2015.11.068 [DOI] [PubMed] [Google Scholar]
- Metzler-Zebeli B. U., Schmitz-Esser S., Klevenhusen F., Podstatzky-Lichtenstein L., Wagner M., and Zebeli Q.. 2013. Grain-rich diets differently alter ruminal and colonic abundance of microbial populations and lipopolysaccharide in goats. Anaerobe 20:65–73. doi: 10.1016/j.anaerobe.2013.02.005 [DOI] [PubMed] [Google Scholar]
- van der Meulen J., Inborr J., and Bakker J. G.. 2001. Effects of cell wall degrading enzymes on carbohydrate fractions and metabolites in stomach and ileum of pigs fed wheat bran based diets. Arch. Tierernahr. 54:101–115. doi: 10.1080/17450390109381970 [DOI] [PubMed] [Google Scholar]
- Milo L. A., Reardon K. A., and Tappenden K. A.. 2002. Effects of short-chain fatty acid-supplemented total parenteral nutrition on intestinal pro-inflammatory cytokine abundance. Dig. Dis. Sci. 47:2049–2055. doi:10.1023/a:1019676929875 [DOI] [PubMed] [Google Scholar]
- Patience J. F. 2017. Meeting energy requirements in pig nutrition. In: Wiseman J., editor, Achieving sustainable production of pig meat. Cambridge, UK: Burleigh Dodds Science Publ; p. 127–143. doi:10.19103/AS,2017.0013.07 [Google Scholar]
- Pedersen M. B., Yu S., Arent S., Dalsgaard S., Bach Knudsen K. E., and Lærke H. N.. 2015. Xylanase increased the ileal digestibility of nonstarch polysaccharides and concentration of low molecular weight nondigestible carbohydrates in pigs fed high levels of wheat distillers dried grains with solubles. J. Anim. Sci. 93:2885–2893. doi: 10.2527/jas.2014-8829 [DOI] [PubMed] [Google Scholar]
- Sanchez J. I., Marzorati M., Grootaert C., Baran M., Van Craeyveld V., Courtin C. M., Broekaert W. F., Delcour J. A., Verstraete W., and Van de Wiele T.. 2009. Arabinoxylan-oligosaccharides (AXOS) affect the protein/carbohydrate fermentation balance and microbial population dynamics of the simulator of human intestinal microbial ecosystem. Microb. Biotechnol. 2:101–113. doi: 10.1111/j.1751-7915.2008.00064.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Reilly P., Sweeney T., Pierce K., Gahan D., Callan J., and O’Doherty J.. 2010. The effect of cereal type and exogenous enzyme supplementation on intestinal microbiota and nutrient digestibility in finisher pigs. Livest. Sci. 133: 148–150. doi: 10.1016/j.livsci.2010.06.049 [DOI] [Google Scholar]
- Tiwari U. P., Chen H., Kim S. W., and Jha R.. 2018. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro models. Anim. Feed Sci. Technol. 245:77–90. doi:10.1016/j.anifeedsci.2018.07.002 [Google Scholar]
- Tsai T., Dove C., Cline P., Owusu-Asiedu A., Walsh M., and Azain M.. 2017. The effect of adding xylanase or β-glucanase to diets with corn distillers dried grains with solubles (CDDGS) on growth performance and nutrient digestibility in nursery pigs. Livest. Sci. 197: 46–52. doi: 10.1016/j.livsci.2017.01.008 [DOI] [Google Scholar]
- Vahjen W., Osswald T., Schäfer K., and Simon O.. 2007. Comparison of a xylanase and a complex of non starch polysaccharide-degrading enzymes with regard to performance and bacterial metabolism in weaned piglets. Arch. Anim. Nutr. 61:90–102. doi: 10.1080/17450390701203881 [DOI] [PubMed] [Google Scholar]
- Xu J., Chen X., Yu S., Su Y., and Zhu W.. 2016. Effects of early intervention with sodium butyrate on gut microbiota and the expression of inflammatory cytokines in neonatal piglets. PloS One. 11: e0162461. doi: 10.1371/journal.pone.0162461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz L. A., Yin X., Wang G., Elinav E., Hao L., Zhao L., and Flavell R. A.. 2013. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol. 190:5306–5312. doi: 10.4049/jimmunol.1300016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Li Q., Tian Q., Xu Y., and Piao X.. 2018. The combination of carbohydrases and phytase to improve nutritional value and non-starch polysaccharides degradation for growing pigs fed diets with or without wheat bran. Anim. Feed Sci. Technol. 235: 138–146. doi: 10.1016/j.anifeedsci.2017.11.009 [DOI] [Google Scholar]
- Zhang Y., Liu Q., Zhang W., Zhang Z., Wang W., and Zhuang S.. 2018. Gastrointestinal microbial diversity and short-chain fatty acid production in pigs fed different fibrous diets with or without cell wall-degrading enzyme supplementation. Livest. Sci. 207: 105–116. doi: 10.1016/J.ENG.2017.03.010 [DOI] [Google Scholar]
- Zhang G., Yang Z., Wang Y., Yang W., and Zhou H.. 2014. Effects of dietary supplementation of multi-enzyme on growth performance, nutrient digestibility, small intestinal digestive enzyme activities, and large intestinal selected microbiota in weanling pigs. J. Anim. Sci. 92: 2063–2069. doi: 10.2527/jas.2013-6672 [DOI] [PubMed] [Google Scholar]