Abstract
Vitamin E (VE) is an indispensable vitamin in piglet feed formula. Among other things, it affects tissues including small intestine tissues and in particular its major unit intestinal epithelial cells. Previously, limited in vivo experiments have focused on the effect of VE on the intestine, particularly digestion and absorption. VE has been shown to inhibit proliferation of some types of cells. This experiment was conducted to test the hypothesis that VE affects intestinal functions by influencing the intestinal epithelial cell proliferation. Thirty 21-d old weaned [(Yorkshire × Landrace) × Duroc] piglets with BWs of 6.36 ± 0.55 kg were randomly divided into five VE-containing feeding formula groups. The treatments were (i) 0 IU (control), (ii) 16 IU, (iii) 32 IU, (iv) 4. 80 IU, and (v) 5. 160 IU. The treatments lasted 14 d. At the end of the experiment, all subjects were sacrificed to obtain blood and tissue samples. The results suggest that VE did not affect the growth performance. VE did tend to decrease jejunal crypt depth (linear, P = 0.056) and villus width (linear, P < 0.05). Sucrase activity significantly decreased in the adding 80 IU VE compared with the control (P < 0.05). Jejunal crypt, cell proliferation in 80 IU group significantly decreased compared with the control group (P < 0.05). This study suggests that dietary VE may affect intestinal morphology and functions by inhibiting weaned piglet jejunal epithelial cell proliferation.
Keywords: α-tocopherol, digest enzyme, histomorphology, intestine, intestinal epithelial cell proliferation, weaned piglets
INTRODUCTION
Weaning stress, in postweaning piglets, often leads to intestinal morphological changes, such as villus atrophy and crypt hyperplasia. These result from intestinal epithelial cell physiological changes that trigger reductions in digestive and absorptive capacities and decreased feed intake (Yang et al., 2013a, b, 2016a; Xiong et al., 2015). Inflammation and oxidative stress may also occur (Bomba et al., 2014; Buchet et al., 2017). In the piglet, the concentration of some essential nutrients, such as vitamin E (VE), suffering a decline after weaning (Chung et al., 1992).
VE is a common antioxidant used to alleviate oxidative stress and inflammation during pig production (NRC, 2012). In vivo and in vitro VE research has, for decades, focused the anti-oxidation effects that have been demonstrated in human blood plasma and many cell types (Burton et al., 1982; Galli et al., 2017). d-α-Tocopherol polyethylene glycol 1000 succinate, a water-soluble form of VE has been widely used as a pharmaceutical excipient and absorption enhancer. It has been demonstrated to promote intestinal epithelial cell chylomicron secretion (Fan et al., 2013). α-Tocopherol deficiencies affect intestinal transport and the passive absorption of phenolsulfonphthalein and barbital (Meshali et al., 1976). These long-known effects of VE on intestinal functions and more recent studies tending to demonstrate that VE inhibits the in vitro proliferation of various cells (Zhang et al., 2001; Kempnã et al., 2004; Olguín-Martínez et al., 2013) suggested a study of whether, how, and to what extent VE might influence intestinal epithelial cells proliferation in constituting intestinal mucosa, and majorly function as nutrient-absorption (Barker, 2014).
Little research has focused on the role of VE in intestinal absorption and digestion function, intestinal epithelial cells, or weaned piglets. It was surmised that VE may affect intestinal function by influencing intestinal epithelial cell proliferation. This study reports the effects of different dosages of α-tocopherol, the primary form of human and swine VE intake (Heinonen and Piironen, 1991; NRC, 2012), on weaned piglets growth performance. The dosages of α-tocopherol are based on a weaned piglet basal diet (NRC, 2012). Selected blood biochemical indices, morphology, digestive enzyme activities, nutrient transporters gene expressions, and jejunal epithelial cell proliferation were also measured.
MATERIALS AND METHODS
All the procedures were approved by the Animal Care and Use Committee of Hunan Normal University, Changsha City, Hunan, China.
Treatments
Thirty 21-d-old [(Yorkshire × Landrace) × Duroc] weaned piglets with a 6.36 ± 0.55kg average BW were randomly assigned to one of five possible treatments. Each treatment consisted of basal diet supplemented with either (i) 0 IU (control), (ii) 16 IU, (iii) 32 IU, (iv) 80 IU, and (v) 5. 160 IU of VE (Royal DSM NV, Shanghai, China). These levels corresponded to 0, 100, 200, 500, and 1,000% doses of VE, respectively, that the NRC (2012) recommends. The dietary-formula (Table 1) met nutrient specifications for 7–11 kg BW piglets. Piglets were individually housed and had free access to food and water throughout the duration of the study. Piglets were monitored for illness or abnormal behavior. ADG, ADFI, and ADG:ADFI (G:F) were calculated to evaluate growth performance as the previously described (Yin et al., 2001).
Table 1.
Ingredient and chemical composition of piglet diets, as fed basis
| Dietary vitamin E, IU | |||||
|---|---|---|---|---|---|
| Item | 0 | 16 | 32 | 80 | 160 |
| Ingredient, % | |||||
| Corn | 42.85 | 42.85 | 42.85 | 42.85 | 42.85 |
| Extruded corn | 20 | 20 | 20 | 20 | 20 |
| Soybean meal | 10 | 10 | 10 | 10 | 10 |
| Soy protein concentrate | 2.88 | 2.88 | 2.88 | 2.88 | 2.88 |
| Whey powder | 10 | 10 | 10 | 10 | 10 |
| Fish meal | 3 | 3 | 3 | 3 | 3 |
| Spray-dried porcine plasma | 5 | 5 | 5 | 5 | 5 |
| L-Lys | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| DL-Met | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| L-Thr | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| L-Try | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Soybean oil | 2.55 | 2.55 | 2.55 | 2.55 | 2.55 |
| Limestone | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 |
| Dicalcium phosphate | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| Choline chloride | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Antioxidants | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Citric acid | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Mineral premixaa | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin premixab | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Calculated composition | |||||
| CP, % | 18 | 18 | 18 | 18 | 18 |
| ME, kcal/kg | 3,400 | 3,400 | 3,400 | 3,400 | 3,400 |
| Ca, % | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Available P, % | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 |
| Lys,c % | 1.35 | 1.35 | 1.35 | 1.35 | 1.35 |
| Met,c % | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 |
| SAA,cd % | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 |
| Thr,c % | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 |
| Trp,c % | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
Mineral premix per kilogram of feed: 150 mg Fe (FeSO4), 100 mg Zn (ZnSO4), 30 mg Mn (MnSO4), 25 mg Cu (CuSO4), 0.5 mg I (KIO3), 0.3 mg Co (CoSO4), and 0.3 mg Se (Na2SeO3).
Vitamin premix supplied per kilogram of feed: 2,200 IU vitamin VA, 220 IU vitamin D3, 0.5 mg vitamin K3, 17.5 μg vitamin B12, 3.5 mg riboflavin, 30 mg niacin, 10 mg d-pantothenic acid, 0.05 mg biotin, 0.3 mg folic acid, 1.0 mg thiamine, 7 mg pyridoxine, and 4.0 mg ethoxyquin.
Standardized ileal digestible.
SAA = Met +Cys.
Sample Treatment and Collection
At the end of the 14-d treatment, blood samples were obtained from the jugular vein at the moment of sacrifice. Blood (10 mL each piglet) was allowed to clot for 10 hr at 4 °C in normal vacutainer. Serum was harvested after centrifugation (3,000 × g, 10 min). Serum was stored at −80 °C prior blood biochemical analysis determination. The intestines were removed aseptically. Sections, ~2 cm long of the duodenum, the middle jejunum, and the ileum were collected and preserved in a 4% neutral-buffered formalin solution. Preservation time is no longer than 7 d before treatment. The duodenum is defined as the portion of duodenal loop. The jejunum is the section between the duodenum and the ileum. Mid-jejunum mucosa collected and stored at −80 °C until being measured (Yan et al., 2018).
Blood Biochemical Analysis
The serum was thawed on ice and then centrifuged at 3,000 × rpm for 10 min under 4 °C. The listed indices were obtained from bloody serum : total protein (TP) content, alanine aminotransferase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), blood glucose (GLU), triglyceride (TG), blood cholesterol (CHOL), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL-C3), blood ammonia (NH3L), immunoglobin M (IgM), and serum oxygen (O2) using commercial kits in accordance with manufacturer instructions (Jiancheng Bioengineering Institute, Nanjing, China) and where identified using a TBA-120FR Automatic Biochemistry Radiometer (Hitachi Co., Tokyo, Japan).
Intestinal Histomorphology
Specimens of cross-sections of three intestinal segments identified above were embedded in low-melt paraffin wax and cut into 4-μm thick histological sections for hematoxylin and eosin staining. The tissue sections were measured under a microscope using a 40× combined magnification, and an image processing and analysis system (Version 1, Leica Imaging Systems Ltd., Cambridge, UK). Program Image-pro Plus 6.0 determined villus height (VH), villus width (VW), crypt depth (CD), and VH/CD ratio (VH:CD) of the small intestine. At least 20 villous with intact lamina propria of each sample were blindly selected and examined for measurement as previously described. The VH and CD mean for each section was calculated.
Digestive Enzyme Activity Assays
Jejunal mucosal tissue samples were homogenized in saline, and centrifuged (2,500 × g, 4 °C, 10 min) to produce the supernatant. The enzyme sucrase-isomaltase (SI), glucoamylase-maltase (MGAM), lactase-phlorizin hydrolase (LPH), and alkaline phosphatase (IAP) enzyme activities were analyzed using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China) and Synergy HTX Multi-Mode Microplate Reader (BioTek, VT) following the manufacturer’s instructions.
Real-Time Quantitative
Jejunal and ileal samples total RNA were isolated from liquid nitrogen frozen and ground tissues using TRIZOL regent (TaKaRa, Dalian, China) and then treated with DNase I (TaKaRa, Dalian, China) to remove trace DNA. Reverse transcription was conducted at 37 °C for 15 min and at 95 °C for 5 s. Primers used in this study were designed via Primer 5.0 for solute carrier family 1 member 1 (SLC1A1), solute carrier family 5 member 1 (SLC5A1), solute carrier family 6 member 19 (SLC6A19), solute carrier family 7 member 9 (SLC7A9), and solute carrier family 15 member 1 (SLC15A1) according to pig gene sequence (Table 2). After cDNA quantities were determined using a spectrophotometer (NanoDrop ND-1000; Thermo Fisher Scientific, DE), real-time quantitative PCR analyses were performed (ABI 7900HT Fast Real-Time PCR System; Applied Biosystems, Carlsbad, CA) using a final volume of 10 μL. After predenaturation (10 s at 94 °C), 36 cycles of amplification were conducted. Each cycle consisted of 72 °C for 35 s and 60 °C for 30 s, then, followed by a melting curve program (60–99 °C using a heating rate of 0.1 °C/s and fluorescence measurement). In each sample, the amplification of β-actin was used to normalize the expression of other genes. No difference in cycle threshold (Ct) values was observed between the control and the VE-treated piglets. Target gene mRNA expression abundance (A) was calculated as A = 2−ΔΔCt(treat−control); –ΔΔCt (treat − control) = (Ct selected gene − Ct β-actin) treat − (Ct selected gene − Ct β-actin) control. Target mRNA and β-actin mRNA were amplified at comparable efficiencies. cDNA was replaced by water for the negative controls. This method is the method described by Yang et al. (2016b, 2016c).
Table 2.
Primers used for real-time PCR analysis
| Genes | Primers | Primers sequences (5′ to 3′) | Size, bp |
|---|---|---|---|
| SLC1A1 | Forward | GGCACCGCACTCTACGAAGCA | 177 |
| Reverse | GCCCACGGCACTTAGCACGA | ||
| SLC5A1 | Forward | GTGCAGTCAGCACAAAGTGG | 127 |
| Reverse | AGGCTCCCTCCTCATTGACT | ||
| SLC6A19 | Forward | TCTGTCCACAACAACTGCGAG | 206 |
| Reverse | CAGCGAAGTTCTCCTGCGTC | ||
| SLC7A9 | Forward | GAACCCAAGACCACAAATC | 180 |
| Reverse | ACCCAGTGTCGCAAGAAT | ||
| SLC15A1 | Forward | CATCGCCATACCCTTCTG | 144 |
| Reverse | TTCCCATCCATCGTGACATT | ||
| β-actin | Forward | AGTTGAAGGTGGTCTCGTGG | 215 |
| Reverse | TGCGGGACATCAAGGAGAAG |
Immunohistochemistry
Four micrometers tissue sections were cut from paraffin-embedded samples and placed on polylysine-coated glass slides for immunohistochemistry. The samples were firstly deparaffinized in xylene twice for 10 min (one change). They were then rehydrated in a descending ethanol series, beginning with 100% ethanol, descending through 95–85% and finally to 70% ethanol, at 5 min intervals. Rehydration took 25 min altogether. The sections were then washed in PBS three times for 5 min each time. The samples were then microwave heated twice in an aqueous sodium citrate solution for 5 min each time to retrieve antigen epitopes. The sections were then thrice washed in PBS buffer for 10 min each time. Endogenous peroxidase was blocked in 3% hydrogen peroxidase for 30 min. The sections were then washed in PBS for 15 min and incubated in diluted bovine serum albumin in a moist chamber at room temperature for 30 min. They were incubated in a moist chamber with the following primary antibody: anti-Ki-67 (Abcam, Cambridge, UK, dilution 1:400; incubation 60 min). The sections were then thrice washed in PBS and treated with a secondary antibody (zsbio, Beijing, China) at room temperature to bind the primary antibody (zsbio, Beijing, China). Peroxidase activity was visualized via a brown reaction product by immersing the tissue sections in diaminobenzidine (zsbio, Beijing, China). The sections were counterstained with Mayer’s hematoxylin and coverslipped. Normal pig small intestine sections served as positive control for anti-Ki-67 and secondary antibodies. As negative control, the samples were treated as described above, except that the primary antibody was replaced with a solution of bovine serum albumin in PBS. Brown cells and total cells for at least 30 crypts of each section were counted, and the ratio of brown cells/total cells was calculated.
Statistical Analysis
Growth performance data analysis, blood biochemical analysis, and histomorphology were performed by an ANOVA with polynomial contrasts using the GLM program of SAS Institute (2000). Contrasts between treatments means, specifically results of digestive enzyme activity results, immunohistochemistry, and real-time quantitative expressions were evaluated using Student’s t-test. Data are presented as means, and P < 0.05 was considered statically significant and 0.05 < P < 0.1 was considered as tending towards significance.
RESULTS
Growth Performance and Blood Biochemical Analysis
Dietary α-tocopherol at different concentrations did not significantly affect ADG, ADFI, or G:F in weaned piglets. The 80 IU VE group had the greatest daily weight gain and greatest average daily feed intake of the five treatments (Table 3). G:F ratios were lower in the 15 and 80 IU VE groups than for control (Table 3). Blood biochemical parameters values demonstrated that adding in the 32 IU VE group, ALT was greater (linear, P = 0.039, quadratic, P = 0.092) than the other groups which had unchanged values (Table 4). A difference was observed between the 32 IU group and control with respect to IgM (linear, P = 0.032; Table 4).
Table 3.
Weaning piglets growth performance under varying vitamin E (VE) dosages1
| Dietary VE, IU | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Items | Control | 16 | 32 | 80 | 160 | Linear | Quadratic |
| ADG, g | 259.52 ± 21.0 | 247.62 ± 24.37 | 259.52 ± 18.6 | 271.43 ± 24.47 | 233.33 ± 20.76 | 1.000 | 0.662 |
| ADFI, g | 420.93 ± 23.86 | 430.7 ± 27.11 | 394.19 ± 32.51 | 459.32 ± 45.57 | 363.17 ± 22.62 | 0.553 | 0.554 |
| G:F, g/g | 0.62 ± 0.03 | 0.57 ± 0.041 | 0.67 ± 0.03 | 0.60 ± 0.04 | 0.65 ± 0.06 | 0.497 | 0.162 |
Values are expressed as mean ± SEM, n = 6.
Table 4.
Effects on blood biochemical parameters of weaning piglets fed differencing vitamin E (VE)1
| Dietary VE, IU | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Itemsa | Control | 16 | 32 | 80 | 160 | Linear | Quadratic |
| TP, IU/L | 53.15 ± 1.84 | 50.83 ± 1.41 | 52.57 ± 0.83 | 53.43 ± 1.6 | 50.02 ± 0.33 | 0.758 | 0.223 |
| ALT, IU/L | 21.68 ± 1.83 | 20.33 ± 1.25 | 28.5 ± 3.48 | 22.93 ± 1.79 | 21.24 ± 2.11 | 0.039 | 0.092 |
| AST, IU/L | 54.17 ± 10.82 | 52.5 ± 6.15 | 83 ± 22.36 | 47.67 ± 5.51 | 37.17 ± 3.48 | 0.477 | 0.545 |
| BUN, mmol/L | 3.07 ± 0.3 | 3.03 ± 0.38 | 3.52 ± 0.29 | 3.32 ± 0.3 | 3.33 ± 0.2 | 0.298 | 0.487 |
| GLU, mmol/L | 4.78 ± 0.2 | 4.45 ± 0.2 | 5.33 ± 0.3 | 4.68 ± 0.39 | 4.42 ± 0.42 | 0.227 | 0.126 |
| TG, mmol/L | 0.37 ± 0.04 | 0.45 ± 0.06 | 0.38 ± 0.04 | 0.42 ± 0.05 | 0.38 ± 0.04 | 0.855 | 0.186 |
| CHOL, mmol/L | 1.82 ± 0.12 | 2.02 ± 0.26 | 1.99 ± 0.16 | 2.03 ± 0.29 | 1.86 ± 0.1 | 0.551 | 0.647 |
| HDL, mmol/L | 0.75 ± 0.08 | 0.78 ± 0.12 | 0.91 ± 0.09 | 0.85 ± 0.12 | 0.81 ± 0.07 | 0.231 | 0.654 |
| LDL-C, mmol/L | 0.99 ± 0.06 | 1.13 ± 0.16 | 1.02 ± 0.09 | 1.11 ± 0.12 | 0.99 ± 0.07 | 0.861 | 0.407 |
| NH3L, μmol/L | 392.85 ± 15 | 381.9 ± 22.51 | 391.47 ± 13.87 | 389.83 ± 9.34 | 382.88 ± 32.31 | 0.962 | 0.683 |
| IgM, mL | 0.48 ± 0.03 | 0.46 ± 0.05 | 0.35 ± 0.03 | 0.43 ± 0.08 | 0.43 ± 0.04 | 0.032 | 0.365 |
| O2, mmol/L | 2 ± 0.29 | 1.4 ± 0.25 | 1.6 ± 0.28 | 1.4 ± 0.13 | 1.6 ± 0.16 | 0.23 | 0.168 |
Values are expressed as mean ± SEM, n = 6
TP = total protein; ALT = alanine aminotransferase; AST = aspartate transaminase; BUN = urea nitrogen; GLU = glucose; TG = triglyceride; CHOL = cholesterol; HDL = high-density lipoprotein cholesterol; LDL-C3 = low-density lipoprotein cholesterol; NH3L = blood ammonia; IgM = immunoglobin M; and O2 = serum oxygen.
Intestinal Histomorphology and Immunohistochemistry
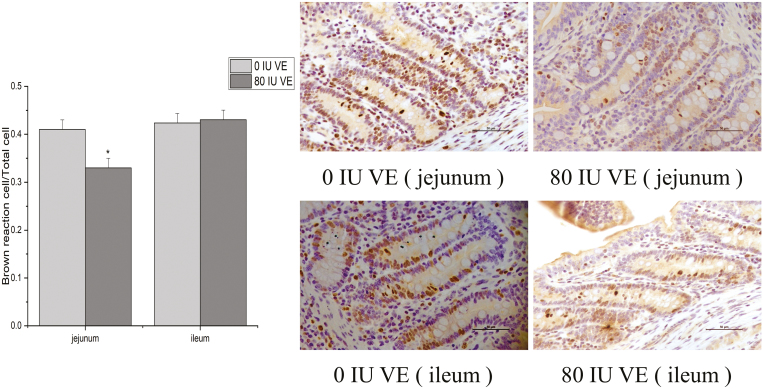
CD, the calculated mean value (linear, P = 0.056), and VW (linear, P = 0.011) revealed a significant difference between control and other groups in the jejunum (Table 5). No statistical difference was observed for the duodenum or the ileum. Mean value of VH, CD, and VW duodenal values was greater in the 80 IU group than them control (Table 5). Ileal VH and CD values were lower for the 80 IU VE group than control (Table 5). Ki-67 data for 80 IU VE and control group appear in Figure 1. Jejunal brown cells/total cells significantly decreased in the 80 IU group than in control (P < 0.05). No affect was manifested for the ileum, the number of proliferation cells and the total cells in group 80 IU VE were slightly reduced compared to control group (Figure 1).
Table 5.
Effects of dietary vitamin E (VE) on weaning piglet intestinal ileal, jejunal, and duodenal histomorphology1
| Dietary VE, IU | P-value | ||||||
|---|---|---|---|---|---|---|---|
| items | control | 16 | 32 | 80 | 160 | Linear | Quadratic |
| Duodenum | |||||||
| Villus height, μm | 301.0 ± 18.26 | 324.8 ± 35.62 | 371.19 ± 36.82 | 365.06 ± 15.51 | 305.29 ± 34.41 | 0.106 | 0.758 |
| Crypt depth, μm | 323.58 ± 16.04 | 347.1 ± 41.35 | 343.39 ± 26.08 | 372.47 ± 22.78 | 287.24 ± 23.68 | 0.613 | 0.687 |
| Villus Width, μm | 146.21 ± 8.57 | 153.02 ± 9.55 | 153.24 ± 7.22 | 166.70 ± 8.24 | 146.18 ± 5.38 | 0.536 | 0.737 |
| VH: CDa, μm:μm | 1.01 ± 0.08 | 1.09 ± 0.19 | 1.16 ± 0.12 | 1.03 ± 0.12 | 1.16 ± 0.03 | 0.412 | 0.999 |
| Jejunum | |||||||
| Villus height, μm | 332.12 ± 22.19 | 323.11 ± 11.7 | 309.06 ± 20.30 | 363.18 ± 41.52 | 294.84 ± 17.33 | 0.516 | 0.934 |
| Crypt depth, μm | 287.24 ± 12.13 | 254.42 ± 8.74 | 254.28 ± 12.28 | 235.51 ± 10.54 | 258.41 ± 13.73 | 0.056 | 0.261 |
| Villus Width, μm | 136.24 ± 2.97 | 120.62 ± 6.47 | 116.26 ± 5.39 | 118.12 ± 3.27 | 121.48 ± 6.55 | 0.011 | 0.382 |
| VH: CDa, μm:μm | 1.24 ± 0.10 | 1.28 ± 0.05 | 1.3 ± 0.12 | 1.67 ± 0.25 | 1.24 ± 0.13 | 0.775 | 0.941 |
| Ileum | |||||||
| Villus height, μm | 354.97 ± 20.5 | 353.34 ± 27.44 | 343.49 ± 17.41 | 327.79 ± 26.54 | 374.38 ± 10.0 | 0.707 | 0.876 |
| Crypt depth, μm | 232.36 ± 11.6 | 220.31 ± 13.74 | 218.51 ± 12.29 | 223.92 ± 17.0 | 215.98 ± 3.41 | 0.439 | 0.74 |
| Villus Width, μm | 125.50 ± 3.67 | 129.01 ± 4.16 | 127.04 ± 2.81 | 131.80 ± 4.13 | 133.75 ± 3.61 | 0.773 | 0.552 |
| VH: CDa, μm:μm | 1.70 ± 0.16 | 1.77 ± 0.19 | 1.71 ± 0.12 | 1.65 ± 0.23 | 1.84 ± 0.07 | 0.983 | 0.751 |
Values are expressed as mean ± SEM, n = 6.
VH: CD = villus height: crypt depth.
Figure 1.
Intestinal epithelial cell proliferation within weaning piglets jejunal and ileal crypts fed 80 and 0 IU vitamin E (VE). * indicates statistical significance (P < 0.05). Data expressed as means ± SEM; n = 6.
Digestive Enzyme Activity and Intestinal Mucosal mRNA Expression
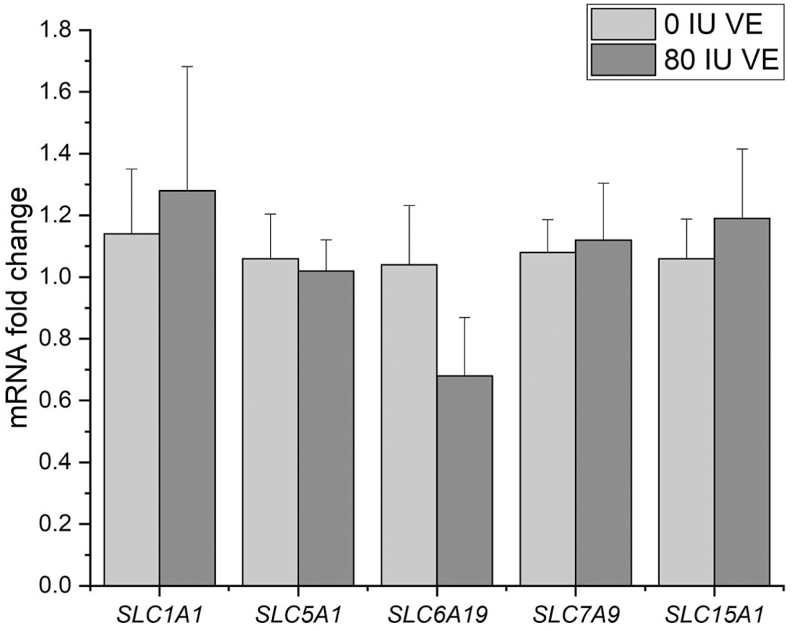
Jejunal mucosa disaccharides enzyme and IAP activities of the groups appear in Table 6. Jejunal mucosa SI activity was lower in the 80 IU VE group compared with control (P = 0.042; Table 6). It tended to decrease MGAM activity (P < 0.1) and IAP (P < 0.1) while not significantly influencing LPH activity (Table 6). SI activity fell to 4.115U/mgprot in the 80 IU group (Table 6). There were no differences in jejunal mucosal gene expression of acidic AA transporter SLC1A1, glucose transporter SLC5A1, neutral AA transporter SLC6A19, cationic and neutral AA transporter SLC7A9, and peptides transporter SLC15A1 between the 0 IU group and the 80 IU group (Figure 2). Except for gene SLC5A1 and SLC6A19, all other gene expressions were greater in the 80 IU group than in control (Figure 2).
Table 6.
Disaccharide enzyme and ALP activity in jejunal mucosa of 80 IU control groups1
| Dietary treatments | ||||
|---|---|---|---|---|
| Items | Control | 80 IU VE | P-value | |
| Sucrase–isomaltase, U/mg of protein | 18.63 ± 5.46 | 4.12 ± 1.18 | 0.042 | |
| Lactase–phlorizin hydrolase, U/mg of protein | 4.74 ± 1.32 | 3.86 ± 0.84 | 0.603 | |
| Glucoamylase–maltase, U/mg of protein | 42.15 ± 11.76 | 17.61 ± 2.92 | 0.097 | |
| Intestinal alkaline phosphatase, U/mg of protein | 119.70 ± 25.72 | 66.18 ± 5.40 | 0.097 | |
Values are expressed as mean ± SEM, n = 6
Figure 2.
Real-time quantitative expression of nutritional transporters expressed in jejunum mucosal 0 IU vitamin E (VE) and 80 IU VE groups. mRNA expression levels of SLC1A1, SLC5A1, SLC6A19, SLC7A9, and SLC15A1 were normalized using β-actin as an internal control. P-value < 0.05 indicates statistical significance. Data are expressed as means ± SEM; n = 6.
DISCUSSION
This study investigated the effects of VE as a dietary supplement on small intestine functions especially on intestinal epithelial cells. There will, inevitably, be a correspondence between diet and intestinal epithelial cell performance because intestinal lumen epithelial cells perform digestion and absorption. Diet is a prime factor regulating weaned piglets small intestine growth and maturation (Cera et al., 1988). The present study suggests that VE, in the diet, affects jejunal histomorphology by inhibiting weaned piglet jejunal intestinal epithelial cells proliferation. The effect occurs even while growth performance is not influenced. Some blood biochemical parameters and main nutrients transporters gene expression may not be affected. These findings indicate that dietary supplying with VE may affect weaned piglet small intestine absorptive and digestive functions.
Weaning stress causes several undesirable phenomena. Weaning is related to reduced feed consumption and nutrients intake (Miller et al., 1986; Yang et al., 2003b). Expecting better growth performance during pig production is reasonable. Previous studies of various VE forms (natural or synthetic), supply routes (drinking water, feed or injection), weaning ages or weight, or postweaning processing periods within nursery period when taken with our study indicates that VE appears to have no effect on growth performance in postweaning piglets (Peplowski et al., 1980; Chung et al., 1992; Ching et al., 2002; Moreira et al., 2002; Wilburn et al., 2007). However, supplying VE at the 80 IU level into feed seems to trigger a slight increase of indicators of growth performance, such as ADG, ADFI, and G:F. Studies of sows showed that significant performance improvements happened frequently in grow-finishing pigs (Niculita et al., 2007; Lu et al., 2014). One may speculate longer experiments while supplying proper amount of VE, until the end of grower I period, or using growing or grow-finishing pigs as subjects may obtain better growth performances.
The greatest amount of blood ALT and the lowest amount of IgM were detected in the 32 IU group. High concentrations of AST and ALT in the bloody serum are considered biomarkers liver and the hepatic cells damages. In this case, perhaps caused by weaning stress, and indicating that adding 32 IU does not meet the real demands of weaning piglets against weaning stress (Knudsen et al., 2016). Further studies are required to determine the changes and mechanism within weaning piglet serum and liver function. Immunoglobin M, a vertebrate antibody with the largest molecular weight, was measured by other teams interested in intestinal immune functions during weaning stress. A large number of experiments on weaning stress or other types of stress returned the same trend which is a decrease in IgM concentrations between control groups and treated groups (Blecha et al., 1981; Capolunghi et al., 2013; Ren et al., 2015). This may indicate that VE may ameliorate weaning stress.
Intestinal function is closely linked to intestinal histomorphology. Weaned piglets show a highly significant increase in CD and a dramatic reduction in VH (Hampson, 1986), suggesting the negative impact of weaning stress. Villus height increase has been generally believed to be from increased surface area capable of greater absorption of available nutrients. Deeper crypts indicate a rapid cell turnover in the villus renewal. In this study, jejunal CD significantly decreased and jejunal VH increased in the control group and the 80 IU VE group. Jejunal VW decreased.
Supplying VE at the 80 IU level ameliorates weaning stress effect. Significant decreases of CD and VW was only present in the jejunum. Immunohistochemistry Ki-67 was used to uncover possible explanations. Intestinal epithelial cell proliferation has recently been showed to have a positive connection with VH and CD in weaning piglets (Wang et al., 2018). Previous research suggests that VE dosages, within a certain range, inhibit different cell type proliferation (Zhang et al., 2001; Tappeiner et al., 2010). No researches illustrate a relationship between VE and jejunal epithelial cell production.
Small intestine epithelial cells constitute up to 90% of all cells within the crypts are an even greater proportion of cells in the villi (Chen et al., 2018). This quantity is their importance as they perform the main function of the intestine which is absorption and digestion. Small intestine epithelial cells may be biomarker to reflect intestinal function. Nutrient metabolism is changed in small intestine epithelial cells during renewal along crypt-villus axis, and the renewal process is affected by diet (Yang et al., 2016c, d; Yan et al., 2018). In the present study, dietary VE inhibits proliferation of epithelial cells in the jejunum, but not in the ileum. This suggests that jejunal and ileal cells have significantly different phenotypes and functions (Lindholm-Perry et al., 2016). Further studies are required to understand these differences. Simple location may affect performance that food carrying nutrients including VE is largely absorbed in jejunal lumen. This left less VE for ileal cells with the resulting effect.
This study suggests that disaccharides enzyme activity and IAP activity is inhibited except for LPH when 80 IU dietary VE is added. Enzyme SI and MGAM are mainly responsible for sugar source nutrients hydrolysis and digestion (Lin et al., 2012). Weaning-induced problems are caused by intestinal structure changes and decreased digestive enzymes activity (Miller, 1986). Intestinal structure correlates to digestive enzymes alteration. Enzyme SI activity reduction compared with control and treated groups conflicted with the intestinal structure. It may be associated with an unknown SI characteristic. Enzyme SI sorting is closely related to sphingolipid- and cholesterol-rich membrane rafts (Alfalah et al., 1999). Therefore, by impeding 7-ketocholesterol incorporation into sphingolipid or cholesterol-enriched domains (Royer et al., 2009), dietary VE may depress SI activity. There is a basis to speculate that changing α-tocopherol dietary concentration within a specific range may improve SI enzyme activities. There is evidence, indicating that the SI enzyme activity reduction caused by VE lessened intestinal damage (Anwar et al., 2013).
Enzyme MGAM, as an exoamylase, catalyzes maltose and maltooligosaccharides hydrolysis (Norén, 1986). Both enzymes SI and MGAM demonstrate primarily hydrolytic activity on the α-1,4 linkages. Enzyme SI hydrolytic activity is also on the α-1,6 linkages (Nichols et al., 2003). Thus, MGAM shows the same trend of enzyme activity as does SI. Enzyme LPH mean values were greater for the control group, except for the 80 IU group which showed a tiny decrease. These results are similar to a prior study showing that VE lessened intestinal damage (Anwar et al., 2013). Enzyme IAP has been regarded as an enterocyte differentiation marker and a key marker enzyme for small intestine digestion and absorption functions changes (Hodin et al., 1995). It is expressed and secreted by intestinal epithelial cells and is active in mucosal and intestinal lumen (Alpers et al., 1994; Lallès, 2010). Enzyme IAP results indicate that dietary VE may inhibit differentiation. Cell differentiation and proliferation are almost always studied together as they are phenomenon regulated by similar factors, such as crosstalk and cooperation of signaling pathways, nutrients changes and environment. Some research has shown that nonspecific tissue alkaline phosphatase knockdown impairs neural stem cell proliferation and differentiation (Kermer et al., 2010). This supports the conclusion of a relationship between this enzyme and differentiation as well as proliferation. Further research is needed to establish any connection. No statistical difference was observed between intestinal mRNA expression transporter groups, such as main sugar and AAs transporters, which suggests that sugar and AA transportation is unchanged (Chen et al., 2018).
In conclusion, this study suggests that VE may affect intestinal morphology and function by inhibiting weaned piglet intestinal epithelial cell proliferation. These findings suggest that proper supplement of VE may ameliorate weaning stress for postweaned piglets.
Conflict of interest statement. The authors declare that they have no conflict of interest.
Footnotes
This work was supported by National Key R & D Program (grant no. 2016YFD0501201), Key Programs of frontier scientific research of the Chinese Academy of Sciences (grant no. QYZDY-SSW-SMC008), National Natural Science Foundation of China (grant no. 31330075), Natural Science Foundation of Hunan Province (grant no. 2017JJ1020), and Young Elite Scientists Sponsorship Program by CAST (grant no. YESS20160086).
LITERATURE CITED
- Alfalah M., Jacob R., Preuss U., Zimmer K. P., Naim H., and Naim H. Y.. 1999. O-linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr. Biol. 9:593–596. doi: 10.1016/S0960-9822(99)80263-2 [DOI] [PubMed] [Google Scholar]
- Alpers D. H., Mahmood A., Engle M., Yamagishi F., and DeSchryver-Kecskemeti K.. 1994. The secretion of intestinal alkaline phosphatase (IAP) from the enterocyte. J. Gastroenterol. 29 Suppl. 7:63–67. [PubMed] [Google Scholar]
- Anwar M., Nanda N., Bhatia A., Akhtar R., and Mahmood S.. 2013. Effect of antioxidant supplementation on digestive enzymes in radiation induced intestinal damage in rats. Int. J. Radiat. Biol. 89:1061–1070. doi: 10.3109/09553002.2013.825062 [DOI] [PubMed] [Google Scholar]
- Barker N. 2014. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15:19–33. doi: 10.1038/nrm3721 [DOI] [PubMed] [Google Scholar]
- Blecha F., and Kelley K. W.. 1981. Effects of cold and weaning stressors on the antibody-mediated immune response of pigs. J. Anim. Sci. 53:439–447. doi:10.2527/jas1981.532439x [DOI] [PubMed] [Google Scholar]
- Bomba L., Minuti A., Moisá S. J., Trevisi E., Eufemi E., Lizier M., Chegdani F., Lucchini F., Rzepus M., Prandini A., et al. 2014. Gut response induced by weaning in piglet features marked changes in immune and inflammatory response. Funct. Integr. Genomics. 14:657–671. doi: 10.1007/s10142-014-0396-x [DOI] [PubMed] [Google Scholar]
- Buchet A., Belloc C., Leblanc-Maridor M., and Merlot E.. 2017. Effects of age and weaning conditions on blood indicators of oxidative status in pigs. PLoS ONE 12:e0178487. doi: 10.1371/journal.pone.0178487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. W., Joyce A., and Ingold K. U.. 1982. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet 2:327. doi: 10.1016/S0140-6736(82)90293-8 [DOI] [PubMed] [Google Scholar]
- Capolunghi F., Rosado M. M., Sinibaldi M., Aranburu A., and Carsetti R.. 2013. Why do we need IGM memory B cells? Immunol. Lett. 152:114–120. doi: 10.1016/j.imlet.2013.04.007 [DOI] [PubMed] [Google Scholar]
- Cera K. R., Mahan D. C., Cross R. F., Reinhart G. A., and Whitmoyer R. E.. 1988. Effect of age, weaning and postweaning diet on small intestinal growth and jejunal morphology in young swine. J. Anim. Sci. 66:574–584. doi: 10.2527/jas1988.662574x [DOI] [PubMed] [Google Scholar]
- Chen C., Yin Y., Tu Q., and Yang H.. 2018. Glucose and amino acid in enterocyte: absorption, metabolism and maturation. Front. Biosci. (Landmark Ed.). 23:1721–1739. doi: 10.2741/4669 [DOI] [PubMed] [Google Scholar]
- Ching S., Mahan D. C., Wiseman T. G., and Fastinger N. D.. 2002. Evaluating the antioxidant status of weanling pigs fed dietary vitamins A and E. J. Anim. Sci. 80:2396–2401. doi: 10.2527/2002.8092396x [DOI] [PubMed] [Google Scholar]
- Chung Y. K., Mahan D. C., and Lepine A. J.. 1992. Efficacy of dietary D-alpha-tocopherol and DL-alpha-tocopheryl acetate for weanling pigs. J. Anim. Sci. 70:2485–2492. doi: 10.2527/1992.7082485x [DOI] [PubMed] [Google Scholar]
- Fan Z., Wu J., Fang X., and Sha X.. 2013. A new function of vitamin E-TPGS in the intestinal lymphatic transport of lipophilic drugs: enhancing the secretion of chylomicrons. Int. J. Pharm. 445:141–147. doi: 10.1016/j.ijpharm.2013.01.070 [DOI] [PubMed] [Google Scholar]
- Galli F., Azzi A., Birringer M., Cook-Mills J. M., Eggersdorfer M., Frank J., Cruciani G., Lorkowski S., and Özer N. K.. 2017. Vitamin E: emerging aspects and new directions. Free Radic. Biol. Med. 102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017 [DOI] [PubMed] [Google Scholar]
- Hampson D. J. 1986. Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 40:32–40. doi: 10.1016/S0034-5288(18)30482-X [DOI] [PubMed] [Google Scholar]
- Heinonen M., and Piironen V.. 1991. The tocopherol, tocotrienol, and vitamin E content of the average Finnish diet. Int. J. Vitam. Nutr. Res. 61:27–32. [PubMed] [Google Scholar]
- Hodin R. A., Chamberlain S. M., and Meng S... 1995. Pattern of rat intestinal brush border enzyme gene expression changes with epithelial growth state. Am. J. Physiol. 269:C385–C391. doi: 10.1016/0014-5793(95)00814-P [DOI] [PubMed] [Google Scholar]
- Kermer V., Ritter M., Albuquerque B., Leib C., Stanke M., and Zimmermann H.. 2010. Knockdown of tissue nonspecific alkaline phosphatase impairs neural stem cell proliferation and differentiation. Neurosci. Lett. 485:208–211. doi: 10.1016/j.neulet.2010.09.013 [DOI] [PubMed] [Google Scholar]
- Knudsen A. R., Andersen K. J., Hamilton-Dutoit S., Nyengaard J. R., and Mortensen F. V.. 2016. Correlation between liver cell necrosis and circulating alanine aminotransferase after ischaemia/reperfusion injuries in the rat liver. Int. J. Exp. Pathol. 97:133–138. doi: 10.1111/iep.12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallès J. P. 2010. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr. Rev. 68:323–332. doi: 10.1111/j.1753-4887.2010.00292.x [DOI] [PubMed] [Google Scholar]
- Lin A. H., Lee B. H., Nichols B. L., Quezada-Calvillo R., Rose D. R., Naim H. Y., and Hamaker B. R.. 2012. Starch source influences dietary glucose generation at the mucosal α-glucosidase level. J. Biol. Chem. 287:36917–21. doi: 10.1074/jbc.M112.378331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Harper A. F., Zhao J., Estienne M. J., and Dalloul R. A.. 2014. Supplementing antioxidants to pigs fed diets high in oxidants: I. Effects on growth performance, liver function, and oxidative status. J. Anim. Sci. 92:5455–5463. doi: 10.2527/jas.2013-7109 [DOI] [PubMed] [Google Scholar]
- Meshali M. M., and Nightingale C. H.. 1976. Effect of alpha tocopherol (vitamin E) deficiency on intestinal transport of passively absorbed drugs. J. Pharm. Sci. 65:344–348. doi: 10.1002/jps.2600650307 [DOI] [PubMed] [Google Scholar]
- Miller B. G., James P. S., and Smith M. W.. 1986. Effect of weaning on the capacity of pig intestinal villi to digest and absorb nutrients. J. Agric. Sci. 107:579–589. doi: 10.1017/S0021859600069756 [DOI] [Google Scholar]
- Moreira I., and Mahan D. C.. 2002. Effect of dietary levels of vitamin E (all-rac-tocopheryl acetate) with or without added fat on weanling pig performance and tissue alpha-tocopherol concentration. J. Anim. Sci. 80:663–669. doi: 10.2527/2002.803663x [DOI] [PubMed] [Google Scholar]
- Nichols B. L., Avery S., Sen P., Swallow D. M., Hahn D., and Sterchi E.. 2003. The maltase-glucoamylase gene: common ancestry to sucrase-isomaltase with complementary starch digestion activities. Proc. Natl. Acad. Sci. USA. 100:1432–1437. doi: 10.1073/pnas.0237170100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculita P., Popa M. E., Ghidurus M., and Turtoi M.. 2007. Effect of vitamin E in swine diet on animal growth performance and meat quality parameters. Pol. J. Food Nutr. Sci. 57:125–129. [Google Scholar]
- Norén O., Sjöström H., Cowell G. M., Tranumjensen J., Hansen O. C., and Welinder K. G.. 1986. Pig intestinal microvillar maltase-glucoamylase. structure and membrane insertion. J. Biol. Chem. 261:12306–12309. doi: 10.1016/0014-5793(86)80927-9 [DOI] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. National Academic Press, Washington, DC. [Google Scholar]
- Peplowski M. A., Mahan D. C., Murray F. A., Moxon A. L., Cantor A. H., and Ekstrom K. E.. 1980. Effect of dietary and injectable vitamin e and selenium in weanling swine antigenically challenged with sheep red blood cell. J. Anim. Sci. 51:344–51. doi: 10.1080/00071668008416704 [DOI] [PubMed] [Google Scholar]
- Ren M., Zhang S. H., Zeng X. F., Liu H., and Qiao S. Y.. 2015. Branched-chain amino acids are beneficial to maintain growth performance and intestinal immune-related function in weaned piglets fed protein restricted diet. Asian-Austral. J. Anim. Sci. 28:1742–1750. doi: 10.5713/ajas.14.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer M. C., Lemaire-Ewing S., Desrumaux C., Monier S., Pais de Barros J. P., Athias A., Néel D., and Lagrost L.. 2009. 7-ketocholesterol incorporation into sphingolipid/cholesterol-enriched (lipid raft) domains is impaired by vitamin E: a specific role for alpha-tocopherol with consequences on cell death. J. Biol. Chem. 284:15826–15834. doi: 10.1074/jbc.M808641200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappeiner C., Meyenberg A., Goldblum D., Mojon D., Zingg J. M., Nesaretnam K., Kilchenmann M., and Frueh B. E.. 2010. Antifibrotic effects of tocotrienols on human tenon’s fibroblasts. Graefes Arch. Clin. Exp. Ophthalmol. 248:65–71. doi: 10.1007/s00417-009-1168-5 [DOI] [PubMed] [Google Scholar]
- Wang L., Yan S., Li J., Li Y., Ding X., Yin J., Xiong X., Yin Y. and Yang H.. 2018. Rapid Communication: the relationship of enterocyte proliferation with intestinal morphology and nutrient digestibility in weaning piglets. J. Anim. Sci. doi: 10.1093/jas/sky388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilburn E. E., Mahan D. C., Hill D. A., Shipp T. E., and Yang H.. 2008. An evaluation of natural (RRR-alpha-tocopheryl acetate) and synthetic (all-rac-alpha-tocopheryl acetate) vitamin E fortification in the diet or drinking water of weanling pigs. J. Anim. Sci. 86:584–591. doi: 10.2527/jas.2007-0377 [DOI] [PubMed] [Google Scholar]
- Xiong X., Yang H., Tan B., Yang C., Wu M., Liu G., Kim S. W., Li T., Li L., Wang J., et al. 2015. Differential expression of proteins involved in energy production along the crypt-villus axis in early-weaning pig small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 309:G229–G237. doi: 10.1152/ajpgi.00095.2015 [DOI] [PubMed] [Google Scholar]
- Yan S., Long L., Zong E., Huang P., Li J., Li Y., Ding X., Xiong X., Yin Y. and Yang H.. 2018. Dietary sulfur amino acids affect jejunal cell proliferation and functions by affecting antioxidant capacity, Wnt/β-Catenin and the mechanistic target of rapamycin signaling pathways in weaning piglets. J. Anim. Sci. 96:5124–5133. doi: 10.1093/jas/sky349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Xiong X., and Yin Y.. 2013a. Development and renewal of intestinal villi in pigs. Springer Vienna. In: Blachier F., Wu G., and Y. Yin, editor, Nutritional and physiological functions of amino acids in pigs. Springer, New York: p. 29–47. [Google Scholar]
- Yang H. S., Fu D. Z., Kong X. F., Wang W. C., Yang X. J., Nyachoti C. M., and Yin Y. L.. 2013b. Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned huanjiang mini-pig piglets. J. Anim. Sci. 91:2740–2748. doi: 10.2527/jas.2012-5795 [DOI] [PubMed] [Google Scholar]
- Yang H., Xiong X., Wang X., Li T., and Yin Y.. 2016a. Effects of weaning on intestinal upper villus epithelial cells of piglets. PLoS ONE. 11:e0150216. doi: 10.1371/journal.pone.0150216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Xiong X., Wang X., and Yin Y.. 2016b. Mammalian target of rapamycin signaling pathway changes with intestinal epithelial cells renewal along crypt-villus axis. Cell. Physiol. Biochem. 39:751–759. doi: 10.1159/000445665 [DOI] [PubMed] [Google Scholar]
- Yang H., Xiong X., and Yin Y.. 2016c. Metabolomic analysis of intestinal epithelial cell maturation along the crypt–villus axis. RSC Adv. 6:27566–27574. doi: 10.1039/c5ra27722a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang X., Xiong X., and Yin Y.. 2016d. Energy metabolism in intestinal epithelial cells during maturation along the crypt-villus axis. Sci. Rep. 6:31917. doi: 10.1038/srep31917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y. L., Baidoo S. K., Schulze H., and Simmins P. H.. 2001. Effects of supplementing diets containing hulless barley varieties having different levels of non-starch polysaccharides with β-glucanase and xylanase on the physiological status of the gastrointestinal tract and nutrient digestibility of weaned pigs. Livest. Prod. Sci. 71:97–107. doi: 10.1016/S0301-6226(01)00214-7. [DOI] [Google Scholar]
- Zhang Y., Yasumoto Y., Mei C., and Arima T.. 2001. Vitamin E inhibits proliferation of primary cultured human mesangial and endothelial cells. Nephron 89:291–296. doi: 10.1159/000046088. [DOI] [PubMed] [Google Scholar]