Abstract
Objective:
Alcohol-related disorders (i.e., abuse and dependence) are significant problems that may result in numerous negative consequences. Although a number of studies have examined factors that predict alcohol abuse and dependence in European samples, only a few studies have examined whether genetic and environmental factors influence the pathogenesis of alcohol-related disorders among African Americans. The present study examined whether gene (internalizing symptoms polygenic risk score) by environment (parental monitoring, community disadvantage) interactions were associated with alcohol-related disorders in a sample of African American adults.
Method:
Participants (N = 640; 39.7% male) were initially recruited for an elementary school–based universal prevention trial in a mid-Atlantic city and followed into adulthood. Participants reported on their perceptions of parental monitoring in sixth grade. At 30 years of age, participants reported on their alcohol abuse and dependence, and DNA was obtained and genotyped using Affymetrix 6.0 microarrays. An internalizing symptoms polygenic risk score was created using discovery samples results from a genome-wide association study (GWAS) that involved three large population-based studies. Community disadvantage was calculated based on census data when participants were in first grade.
Results:
There was a significant interaction between the internalizing symptoms polygenic risk score and community disadvantage such that exposure to higher community disadvantage was associated with lower risk for alcohol-related disorders among participants with a higher internalizing symptoms polygenic risk score.
Conclusions:
Our findings highlight that higher genetic loading for internalizing symptoms may protect urban African Americans from alcohol-related disorders, particularly in more disadvantaged areas.
Alcohol abuse and dependence are complex disorders that have been linked to a host of negative outcomes, including unemployment, risky sexual behaviors, violence, motor vehicle accidents, and neuropsychiatric disorders (Chou et al., 2006; Cook et al., 2006; Hasin et al., 2007; Kendler et al., 2017; White et al., 2015). Lifetime prevalence rates of alcohol abuse and dependence range from 11.8% to 13.2%, with more than 30% of adults reporting experiencing an alcohol use disorder during their lifetime (Haberstick et al., 2014; Hasin et al., 2007). Given the deleterious sequelae associated with alcohol abuse and dependence, it is important to identify individual (e.g., genetic) and contextual characteristics that are associated with these disorders to inform interventions aimed at reducing the prevalence and impact of these problems.
One individual-specific feature that may underpin alcohol abuse and dependence is genetic loading for internalizing symptoms (e.g., anxiety, depressive symptoms), although there is a dearth of research in this area. Extant literature has examined a syndrome encompassed in internalizing symptom disorders, specifically depression, in relation to alcohol abuse and dependence. Small to moderate positive correlations between depression and alcohol use frequency and dependence have been observed in adult twins of European ancestry (rs ranging from .001 to .60) (Andersen et al., 2017; Kendler et al., 1993; Tambs et al., 1997; Torvik et al., 2017). Other work has shown that in samples of predominantly European descent, a genetic locus on chromosome 1 was associated with both alcohol dependence and major depressive disorder symptoms (Nurnberger et al., 2001). Paralleling this work, several genetic variants of candidate genes (e.g., 5-HTTLPR, CHRM2) have been associated with internalizing symptoms and heavy alcohol use and disorders in samples of predominantly European ancestry (Kleinjan et al., 2015; Saraceno et al., 2009).
The studies referenced above suggest that the genetic architecture of internalizing symptoms may be similar to alcohol-related disorders among individuals of European descent. However, it is unclear whether these findings apply to urban African Americans, given that these individuals may experience more frequent and severe environmental stressors (e.g., higher levels of community disadvantage) that exacerbate their risk for developing alcohol-related disorders (Wallace et al., 2017). Moreover, although most twin and candidate gene studies have revealed a relationship between internalizing symptoms and alcohol-related disorders, recent work indicates that numerous genetic variants are involved in the pathogenesis of substance use problems (Dick, 2017).
One method to examine multiple genetic influences of internalizing symptoms in relation to alcohol-related disorders is through the creation of polygenic risk scores (PRS). These scores are generated by identifying single nucleotide polymorphisms (SNPs) associated with a phenotype and may have greater predictive power compared to a single candidate gene or SNP (Musci et al., 2016). Examining whether there is an association between polygenic markers of internalizing symptoms and alcohol-related disorders may also allow researchers to ascertain the degree of genetic overlap between these conditions (Hart & Kranzler, 2015). This knowledge may help elucidate individual pathways toward alcohol abuse and dependence and have the potential to improve diagnoses, treatment, and prevention of these problems. Despite the advantages of examining polygenic influences underpinning internalizing symptoms in relation to alcohol-related disorders, no research has examined these relationships to our knowledge.
Studies examining the effects of the interplay between polygenic markers of internalizing symptoms with contextual factors is also wanting. Consistent with problem behavior theory, individual and environmental factors (e.g., parental monitoring) likely interact to influence risk for substance use problems (Jessor, 1987). Parents who monitor their children may structure their time and supervise them, which might protect them from affiliating with deviant peers and/or being exposed to illicit drugs (Dishion & McMahon, 1998); these parenting behaviors may also mitigate risk for heavy alcohol use in adolescence and, in turn, reduce the risk for alcohol-related disorders in adulthood (Guo et al., 2001; Klima et al., 2014).
There appears to be a relative dearth of work that has examined whether parental monitoring influences the development of alcohol-related disorders among youth with different genetic loading for internalizing symptoms. Available work indicates that among predominantly European American adults with higher levels of phenotypic internalizing symptoms, parental monitoring had no effect on alcohol abuse or dependence (Hill et al., 2010). However, it is unclear whether these results would apply when considering the joint contributions of an internalizing symptoms PRS and parental monitoring on the development of alcohol-related disorders among African Americans.
Neighborhood disadvantage may also play a role in the development of alcohol-related disorders. Higher levels of neighborhood disadvantage have been predictive of heavy alcohol use and dependence (Lambert et al., 2004; Winstanley et al., 2008). Individuals living in more disadvantaged neighborhoods tend to have greater exposure to crime, less community supervision of youth behavior, and fewer institutional resources (Ross & Mirowsky, 2001). In addition, in more impoverished neighborhoods, youth are more likely to be offered drugs, encounter adults using substances, and perceive drug use as normative (Galea et al., 2005; Wallace & Muroff, 2002); these environments may facilitate more frequent alcohol use in adulthood. Residing in a disadvantaged neighborhood may also result in demoralization and induce feelings of hopelessness (Ross & Mirowsky, 2001). Consistent with the stress reduction hypothesis, youth may use alcohol to reduce the stress associated with residing in a disadvantaged community (Rhodes & Jason, 1990). Among youth higher in genetic loading for internalizing problems, exposure to community disadvantage may result in heavy alcohol use in adulthood to attenuate negative affect, although there is a dearth of work in this area.
Although prior work suggests that genetic factors related to internalizing symptoms and environmental characteristics (i.e., parental monitoring, community disadvantage) may contribute to alcohol-related disorders, it is unclear based on available work whether the interaction between internalizing symptoms polygenic influences and contextual factors are associated with the development of alcohol-related disorders among African American adults. We sought to address these gaps by examining whether (a) an internalizing symptoms PRS was associated with alcohol-related disorders, and (b) parental monitoring and community disadvantage moderated the relation between the internalizing symptoms PRS and alcohol-related disorders in a sample of African Americans.
Method
Participants
The study’s analytic sample was drawn from two cohorts of participants in a randomized controlled trial of elementary school–based universal prevention interventions. The goals of the interventions were to improve academic achievement and reduce disruptive behaviors in first grade and beyond. Participants were followed periodically from first grade to young adulthood. The present study was approved by a university review board. Young adults provided written consent at approximately 20 years old, whereas parents provided written consent and youth provided assent if participants were younger than 18 years old.
In total, 2,311 individuals were available for recruitment in first grade, of which 871 completed assessments on alcohol-related disorders and provided a successfully assayed DNA sample at age 30 years. Owing to the relatively small proportion of European Americans in the sample and to further reduce the possibility of population stratification (described in more detail in the Appendix at the end of the article), we restricted the sample to only African American participants, resulting in a final sample of 640 individuals. Participant demographic information for the analytic sample is outlined in Table 1. The analytic sample (i.e., 640 participants) significantly differed (p < .05) from the whole sample (i.e., 2,311 participants) in that the analytic sample had a greater proportion of females than the whole sample.
Table 1.
Sample characteristics
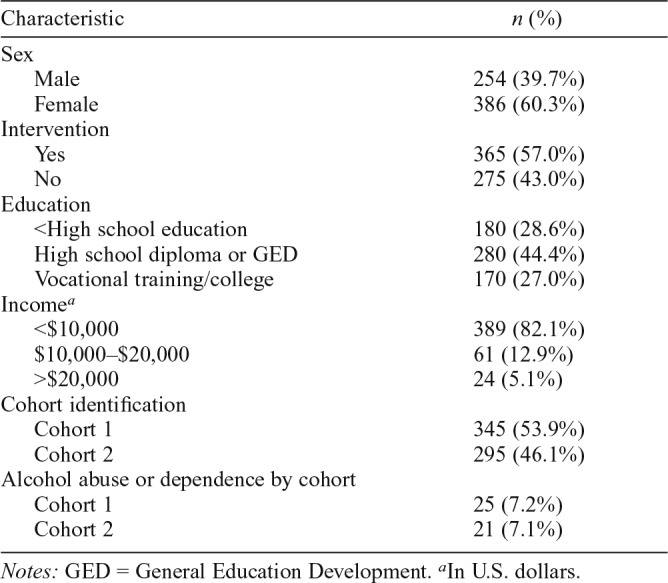
| Characteristic | n (%) |
| Sex | |
| Male | 254 (39.7%) |
| Female | 386 (60.3%) |
| Intervention | |
| Yes | 365 (57.0%) |
| No | 275 (43.0%) |
| Education | |
| <High school education | 180 (28.6%) |
| High school diploma or GED | 280 (44.4%) |
| Vocational training/college | 170 (27.0%) |
| Incomea | |
| <$10,000 | 389 (82.1%) |
| $10,000–$20,000 | 61 (12.9%) |
| >$20,000 | 24 (5.1%) |
| Cohort identification | |
| Cohort 1 | 345 (53.9%) |
| Cohort 2 | 295 (46.1%) |
| Alcohol abuse or dependence by cohort | |
| Cohort 1 | 25 (7.2%) |
| Cohort 2 | 21 (7.1%) |
Notes: GED = General Education Development.
In U.S. dollars.
Measures
Alcohol abuse and dependence.
When participants were approximately 20 years old, alcohol abuse and dependence were assessed. Although African Americans tend to display lower rates of alcohol abuse or dependence at this age relative to European Americans, this developmental period is often marked by a plethora of social and contextual changes that have been shown to exacerbate risk for the development of significant alcohol problems across racial groups (Auerbach & Collins, 2006; Delucchi et al., 2008; Smith et al., 2014). The Composite International Diagnostic Interview–University of Michigan Version (CIDI-UM; Kessler et al., 1994) was used to determine past-year alcohol-related disorders. The CIDI-UM specifies the exact wording and sequence of questions and provides a complete set of categories for classifying respondents’ replies. Past-year alcohol abuse and dependence diagnoses were derived in accord with criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994), using a computerized scoring algorithm. In terms of the CIDI-UM, Kessler et al. (1998) reported good to excellent agreement with diagnoses derived by psychiatrists using the Structured Clinical Interview for the DSM-III-R (SCID; Spitzer et al., 1992).
Parental monitoring.
The Structured Interview of Parent Management Skills and Practices–Youth Version was used to assess parental monitoring (Capaldi & Patterson, 1989). This scale assesses how often youth tell their parents when they will be back or leave a note about their whereabouts when they go out. Seven items were rated on a 5-point Likert scale (1 = all of the time to 5 = never). An average parental monitoring score was calculated, with higher scores reflecting more parental monitoring. Capaldi and Patterson (1989) report adequate internal consistency and test–retest reliability for the monitoring subscale.
Community disadvantage.
All available first-grade participants’ home addresses (n = 99.2%) were geocoded in ArcMap (Environmental Systems Research Institute, Inc., Redlands, CA). A spatial join (appends data from two map layers using geographic location) was conducted to determine the census tract for each participant. A community disadvantage score was calculated using census tract–level items from the 1990 and 2000 decennial census (U.S. Census Bureau, 2009). The items used to create the index include the percentages of (a) adults age 25 years or older with a college degree, (b) owner-occupied housing, (c) households with incomes below the federal poverty threshold, and (d) female-headed households with children. We used Ross & Mirowsky’s (2001) formula to generate the index: {[(c / 10 + d / 10) – (a / 10 + b / 10)] / 4}. The score has a possible range of -5 (low disadvantage) to +5 (high disadvantage).
Discovery sample.
The discovery results used to generate the PRS for the present study were provided by a genome-wide meta-analysis of internalizing symptoms among preschool-aged children that was conducted by the Early Genetics and Lifecourse Epidemiology Consortium (EAGLE; Benke et al., 2014). Internalizing symptoms were measured using 34 items from the Child Behavior Checklist collected across three samples (Benke et al., 2014). This study evaluated 4,596 total children across more than 2.4 million imputed SNPs and reported modest SNP-based heritabilities for internalizing symptoms.
PRS generation.
Blood or saliva samples were obtained from participants when they were about 30 years old. Using the discovery genome-wide association study (GWAS) list available from the EAGLE Consortium (Benke et al., 2014), our GWAS panel contained 741,174 (26.3%) SNPs directly genotyped from this list. After imputation, 2,554,305 (90.5%) SNPs from the discovery dataset were available in the current sample. Palindromic (A/T or C/G) SNPs were excluded, as methods for properly orienting strand from discovery to test data sets require precise knowledge of the true minor allele frequency and haplotype structure of the test sample.
This is challenging in admixed data sets. Given the high degree of inter-marker linkage disequilibrium (correlation at markers close together along a chromosome), information lost in excluding palindromic SNPs will be reflected in nearby SNPs. To account for linkage disequilibrium (LD), two rounds of LD-based results clumping were conducted in PLINK 2.0 (Chang et al., 2015) against the HapMap Phase III Release 2 Build 36 reference panel (International HapMap 3 Consortium, 2010), resulting in 219,312 selected SNPs. The 4,840 SNPs with p values below our chosen threshold of .01 were used.
Raw scores were generated in the imputed dosage dataset in PLINK 2.0 (Chang et al., 2015). Mean imputation was done for missing genotypes, and alleles were weighted by the effect sizes from the discovery GWAS. The raw PRS was regressed on the 10 ancestry principal components we identified (see the Appendix). The z-scored residuals from these regressions were the continuous ancestry-corrected scores that we used in the primary analyses.
Statistical analyses
The primary analyses were conducted using IBM SPSS Statistics for Windows, Version 25 (IBM Corp., Armonk, NY). The demographic and participant variables were coded as follows: males = 0; females = 1; no intervention = 0; intervention = 1; no abuse or dependence = 0; abuse or dependence = 1. Preliminary analyses were conducted to test for gene by environment correlations. Significant correlations between an individual’s genetic features and environmental factors may indicate that genes and environments are not independent of each other and can result in spurious interaction effects (Dick, 2011). Thus, we tested for this possibility.
We conducted logistic regressions to examine the main effects of the independent variables (participant sex, intervention status, the internalizing symptoms PRS, parental monitoring, community disadvantage) on alcohol-related disorders. Step 1 included the main effects of these variables. Step 2 included Step 1 variables and the contextual variable (parental monitoring or community disadvantage) × the internalizing symptoms PRS interaction term. All continuous predictor variables were z scored (M = 0, SD = 1).
Significant interactions and slopes were plotted using an automated spreadsheet (Dawson, 2014). This spreadsheet allowed us to visualize the relationship between the predictor (the internalizing symptoms PRS) and alcohol-related disorders at different levels of the moderator (high, average, and low parental monitoring or community disadvantage). Post hoc probing involved creating new variables at the mean and ±.5 SD from the z-scored values of the moderator (Aiken & West, 1991; Cohen & Cohen, 1983; Holmbeck, 2002). New interaction terms were created from these variables.
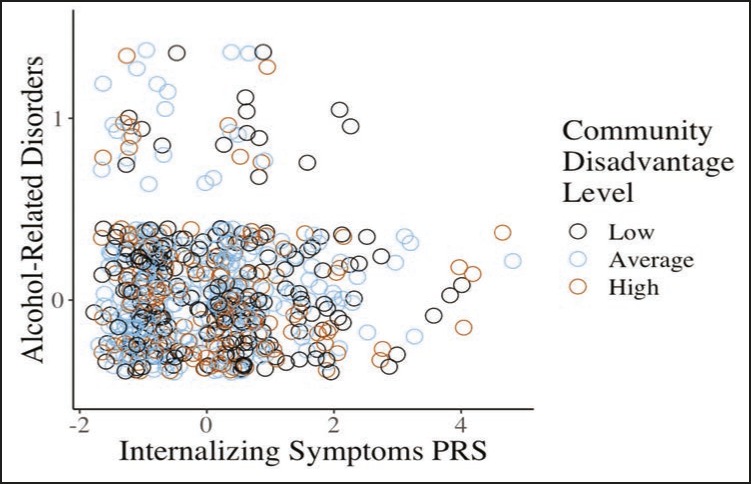
For significant interactions, the post hoc regressions involved the internalizing symptoms PRS, the contextual variable (at the mean and ±.5 SD from the mean), and the Context × Internalizing Symptoms PRS interaction. In graphing the interactions, we included the unstandardized betas (slopes) that were at the mean and ±.5 SD from the moderator. For significant interactions, we also created scatterplots of having alcohol-related disorders as a function of the internalizing symptoms PRS for participants living in low, average, and high community disadvantaged settings. These scatterplots were created using the ggplot package in R, specifically the “geom jitter” function (R Core Team, 2013; Wickham, 2016).
Results
Bivariate correlations and descriptive statistics of study variables are presented in Table 2. There was a very small, nonsignificant (a) negative correlation between the internalizing symptoms PRS and parental monitoring (r = -.06, p = .163), and (b) positive correlation between the internalizing symptoms PRS and community disadvantage (r = .003, p = .935). Given these nonsignificant associations, we were able to rule out gene–environment correlations. About 7% of participants (n = 46) reported alcohol abuse or dependence.
Table 2.
Bivariate correlations, means, standard deviations, and ns of study variables
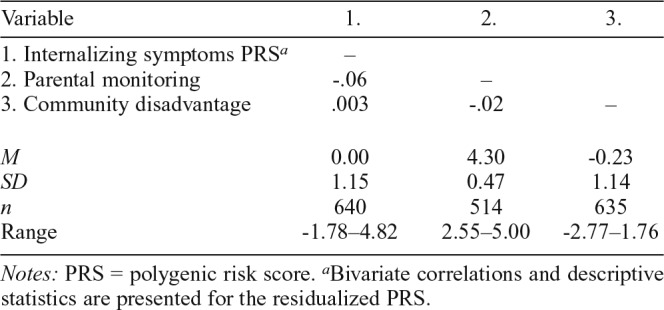
| Variable | 1. | 2. | 3. |
| 1. Internalizing symptoms PRSa | – | ||
| 2. Parental monitoring | -.06 | – | |
| 3. Community disadvantage | .003 | -.02 | – |
| M | 0.00 | 4.30 | -0.23 |
| SD | 1.15 | 0.47 | 1.14 |
| n | 640 | 514 | 635 |
| Range | -1.78–4.82 | 2.55–5.00 | -2.77–1.76 |
Notes: PRS = polygenic risk score.
Bivariate correlations and descriptive statistics are presented for the residualized PRS.
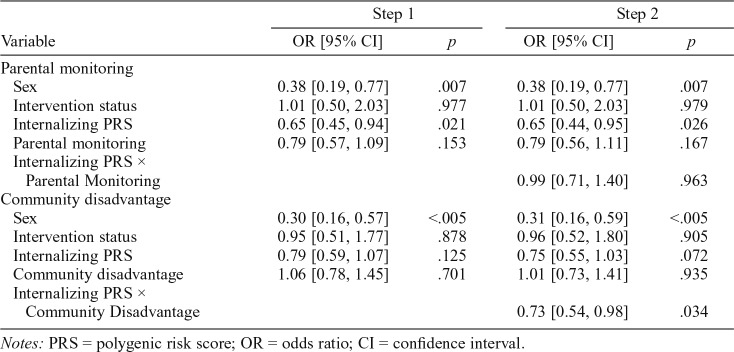
There were main effects of parental monitoring such that higher parental monitoring predicted a lower log odds of alcohol-related disorders (Table 3). After we adjusted for participant sex and intervention status, the internalizing symptoms PRS showed a trend for being significantly negatively associated with alcohol-related disorders (odds ratio = 0.78, 95% CI [0.58, 1.04], p = .095). The Internalizing Symptoms PRS × Community Disadvantage interaction was significantly associated with alcohol-related disorders; however, the Internalizing Symptoms PRS × Parental Monitoring interaction was not. Figure 1 displays a scatterplot of having alcohol-related disorders as a function of the internalizing symptoms PRS for participants living in low, average, and high community disadvantaged settings.
Table 3.
Summary of logistic regression analyses involving the internalizing symptoms PRS, parental monitoring, and community disadvantage in predicting alcohol-related disorders
| Variable | Step 1 |
Step 2 |
||
| OR [95% CI] | p | OR [95% CI] | p | |
| Parental monitoring | ||||
| Sex | 0.38 [0.19, 0.77] | .007 | 0.38 [0.19, 0.77] | .007 |
| Intervention status | 1.01 [0.50, 2.03] | .977 | 1.01 [0.50, 2.03] | .979 |
| Internalizing PRS | 0.65 [0.45, 0.94] | .021 | 0.65 [0.44, 0.95] | .026 |
| Parental monitoring | 0.79 [0.57, 1.09] | .153 | 0.79 [0.56, 1.11] | .167 |
| Internalizing PRS × Parental Monitoring | 0.99 [0.71, 1.40] | .963 | ||
| Community disadvantage | ||||
| Sex | 0.30 [0.16, 0.57] | <.005 | 0.31 [0.16, 0.59] | <.005 |
| Intervention status | 0.95 [0.51, 1.77] | .878 | 0.96 [0.52, 1.80] | .905 |
| Internalizing PRS | 0.79 [0.59, 1.07] | .125 | 0.75 [0.55, 1.03] | .072 |
| Community disadvantage | 1.06 [0.78, 1.45] | .701 | 1.01 [0.73, 1.41] | .935 |
| Internalizing PRS × Community Disadvantage | 0.73 [0.54, 0.98] | .034 | ||
Notes: PRS = polygenic risk score; OR = odds ratio; CI = confidence interval.
Figure 1.
Scatterplot of having alcohol-related disorders in the context of low (.5 SD below the mean), average (mean), and high (.5 SD above the mean) community disadvantage based on participants’ internalizing symptoms polygenic risk score (PRS). 0 = no alcohol abuse or dependence, 1 = alcohol abuse or dependence.
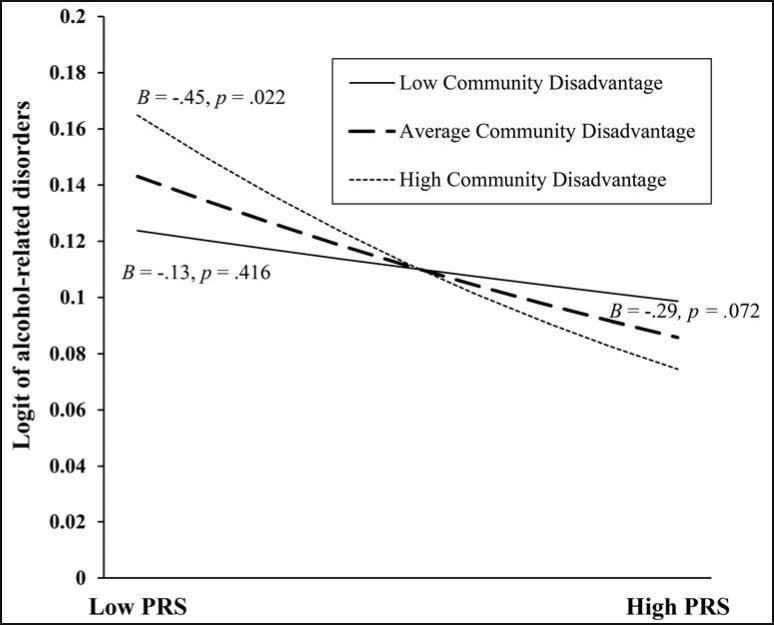
Post hoc probing of the Internalizing Symptoms PRS × Community Disadvantage interaction indicated that the slope for high community disadvantage was significant, but the slopes representing average and low community disadvantage were not (Figure 2). In the context of higher community disadvantage, participants with a higher internalizing symptoms PRS exhibited a lower log odds of alcohol-related disorders than participants with a lower internalizing symptoms PRS. However, exposure to average and low community disadvantage did not alter the likelihood of alcohol-related disorders based on participants’ internalizing symptoms PRS.
Figure 2.
Relation between the internalizing symptoms polygenic risk score (PRS) and alcohol-related disorders in the context of low (.5 SD below the mean), average (mean), and high (.5 SD above the mean) community disadvantage. Note: The y-axis was truncated to improve visibility of the slopes and corresponding p values. PRS = polygenic risk score.
Discussion
Although a number of studies have indicated a link between genetic factors (e.g., 5-HTTLPR variants) associated with internalizing symptoms and alcohol-related disorders among samples of European ancestry (e.g., Andersen et al., 2017; Saraceno et al., 2009), there is a paucity of such studies among African Americans. Moreover, no work to our knowledge has examined whether polygenic loading for internalizing symptoms confers risk or resilience for alcohol-related disorders in the context of parental monitoring and community disadvantage in an urban, African American sample. The present study sought to address these gaps by examining whether alcohol-related disorders were associated with (a) polygenic influences of internalizing symptoms and (b) the interaction of these polygenic influences with contextual factors.
In the context of higher community disadvantage, youth with lower genetic loading for internalizing symptoms had a greater likelihood of having an alcohol use disorder relative to youth with higher genetic loading for internalizing symptoms. No research to our knowledge has examined whether the interaction between community disadvantage and an internalizing symptoms PRS is associated with the development of alcohol-related disorders. However, our results are consistent with limited research linking temperamental inhibition (a potential phenotype of internalizing symptoms) and community disadvantage to alcohol use (Andreas & Watson, 2016). Findings from this study indicated that greater exposure to neighborhood problems was associated with heavier alcohol consumption among individuals lower in temperamental inhibition (Andreas & Watson, 2016).
It is possible that youth with lower genetic loading for internalizing symptoms may be less inhibited, have greater reward sensitivity, and be higher in externalizing symptoms. In disadvantaged communities where drug use may be more prevalent, youth with a lower internalizing symptoms PRS may be more likely to continue using alcohol after initial experimentation and be less fearful of the negative consequences of frequent alcohol use (Dawe et al., 2004; Galea et al., 2005; Hasking et al., 2015). Among youth with a higher internalizing symptoms PRS, residing in higher crime neighborhoods may result in these youth spending more time indoors; as such, these youth may have less of an opportunity to use alcohol and thus develop an alcohol use disorder in adulthood.
We found that the internalizing symptoms PRS was not associated with alcohol-related disorders when environmental factors were not considered. The lack of an association between the internalizing symptoms PRS and alcohol-related disorders is consistent with a number of studies indicating weak associations between genetic variants and substance use disorders (Hines et al., 2015; Musci et al., 2016). Our results suggest that genetic loading for internalizing symptoms may be unrelated to alcohol-related disorders, at least among urban African Americans in our sample, and highlight that alcohol abuse and dependence may be better explained by the interplay between environmental exposures and genetic predispositions (Hines et al., 2015). Future work should examine the relationship between internalizing symptoms genetic load and alcohol-related disorders in larger African American samples, given the scarcity of research in this area.
There are some limitations of the current study. DNA was obtained from participants at a single time point. Exposure to different parenting and neighborhood contexts may result in epigenetic changes that subsequently influence an individual’s neurodevelopment, behavior, and environmental experiences (Meaney, 2010). Future work should explore epigenetic modifications that may occur over time as a result of environmental exposures. The PRS for internalizing symptoms was derived from a GWAS that included predominantly individuals of European heritage. Recent work has demonstrated using simulated data that PRS are most accurate when inferred from GWAS performed in the same ancestry group. Indeed, some work has indicated a reduction in the variance accounted for as the genetic distance between two samples increased (Martin et al., 2017). Although some decrease in variance accounted for by PRS is expected, PRS generally maintain transferability across ethnic groups (Martin et al., 2017). Genetically informed studies conducted across ethnic groups, such as that presented here, are needed to overcome the limitation of the field regarding the lack of representation of ethnic minority populations.
Despite these limitations, the present study has several strengths. Our work helps elucidate factors involved in the pathogenesis of alcohol abuse and dependence, which may inform the prevention of alcohol-related disorders and the personalization of treatments. However, before prevention and intervention programs can integrate these findings into intervention efforts, replication of our findings is needed in large, ethnically diverse samples, as well as reductions in genotyping costs and societal acceptance of genotyping for mental health outcomes. In terms of future directions, research should consider other factors (e.g., religiosity, spirituality) that have been associated with improved alcohol abuse treatment outcomes and psychological health among African Americans (Bowen-Reid & Harrell, 2002; Krentzman et al., 2010). For example, it is possible that African Americans with a lower internalizing symptoms PRS may show reduced risk for alcohol-related disorders if they maintain a spiritual or religious orientation, something future work should explore. An additional avenue for future work is to examine phenotypes associated with the internalizing symptoms PRS—such as whether this score is associated with anxiety symptoms, depressive symptoms, and externalizing behaviors—to better elucidate behavior–gene relations.
APPENDIX
DNA and genotyping
Blood or buccal samples were obtained and DNA was extracted and genotyped using Affymetrix 6.0 microarrays (Persico et al., 1996), comprising one million SNPs across the genome. Standard quality control steps were implemented to ensure accurate genotypes were included in subsequent analyses. Subjects with >5% missing genotype data were removed. SNPs were also removed from further analysis if they had a minor allele frequency < .01, missingness > .05, or departures from Hardy–Weinberg equilibrium at p < .0001 (Anderson et al., 2010). These steps were performed using PLINK 2.0 (Chang et al., 2015). Genotypes were imputed to the 1000 Genomes Phase 3 reference panel (Abecasis et al., 2010) using IMPUTE2 (Howie et al., 2009) with pre-phasing performed in SHAPEIT (Delaneau et al., 2013). Resulting variants imputed with an INFO (quality) score < 0.8 were removed. Uncertainty adjusted dosage data, instead of called alleles, were used to generate the PGS.
When exploring genetic associations, it is important to identify and control for population stratification or genetic differences between subpopulations so that any significant associations observed are not confounded by ancestry (Li, 1969). We used principal components analysis in PLINK 2.0 (Chang et al., 2015) to create the population stratification control variables. This process uses an orthogonal transformation to reduce the multi-dimensional genome-wide SNP data into a smaller number of principal components. We used all the available measured SNPs (roughly 900,000) to generate these components. Although these were not a priori identified ancestry information markers, it has been shown that “randomly” selected SNPs perform equally as well (Pritchard & Rosenberg, 1999). We included the first ten principal components in our analyses to sufficiently account for population stratification in the sample.
Footnotes
This research was funded by National Institute on Drug Abuse grants T32DA007292 (to Jill Alexandra Rabinowitz), R01DA036525 (to Brion S. Maher), and DA11796 MH57005 (to Nicholas S. Ialongo).
References
- Abecasis G. R., Altshuler D., Auton A., Brooks L. D., Durbin R. M., Gibbs R. A., McVean G. A. the 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. doi:10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken L. S., West S. G. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Andersen A. M., Pietrzak R. H., Kranzler H. R., Ma L., Zhou H., Liu X., Kramer J., Han S. Polygenic scores for major depressive disorder and risk of alcohol dependence. JAMA Psychiatry. 2017;74:1153–1160. doi: 10.1001/jamapsychiatry.2017.2269. doi:10.1001/jamapsychiatry.2017.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. A., Pettersson F. H., Clarke G. M., Cardon L. R., Morris A. P., Zondervan K. T. Data quality control in genetic case-control association studies. Nature Protocols. 2010;5:1564–1573. doi: 10.1038/nprot.2010.116. doi:10.1038/nprot.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreas J. B., Watson M. W. Person-environment interactions and adolescent substance use: The role of sensation seeking and perceived neighborhood risk. Journal of Child & Adolescent Substance Abuse. 2016;25:438–447. doi:10.1080/1067828X.2015.1066722. [Google Scholar]
- Auerbach K. J., Collins L. M. A multidimensional developmental model of alcohol use during emerging adulthood. Journal of Studies on Alcohol. 2006;67:917–925. doi: 10.15288/jsa.2006.67.917. doi:10.15288/jsa.2006.67.917. [DOI] [PubMed] [Google Scholar]
- Benke K. S., Nivard M. G., Velders F. P., Walters R. K., Pappa I., Scheet P. A., Middeldorp C. M. A genome-wide association meta-analysis of preschool internalizing problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:667–676. e7. doi: 10.1016/j.jaac.2013.12.028. doi:10.1016/j.jaac.2013.12.028. [DOI] [PubMed] [Google Scholar]
- Bowen-Reid T. L., Harrell J. P. Racist experiences and health outcomes: An examination of spirituality as a buffer. Journal of Black Psychology. 2002;28:18–36. doi:10.1177/0095798402028001002. [Google Scholar]
- Capaldi D. M., Patterson G. R. Psychometric properties of fourteen latent constructs from the Oregon Youth Study. New York, NY: Springer-Verlag; 1989. [Google Scholar]
- Chang C. C, Chow C. C, Tellier L. C., Vattikuti S., Purcell S. M., Lee J. J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. doi:10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. P., Dawson D. A., Stinson F. S., Huang B., Pickering R. P., Zhou Y., Grant B. F. The prevalence of drinking and driving in the United States, 2001-2002: Results from the National Epidemiological Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2006;83:137–146. doi: 10.1016/j.drugalcdep.2005.11.001. doi:10.1016/j.drugalcdep.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Cohen J., Cohen P. Applied multiple regression/correlation analyses for the behavioral sciences (2nd ed.) Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- Cook R. L., Comer D. M., Wiesenfeld H. C., Chang C. -C., H., Tarter R., Lave J. R., Clark D. B. Alcohol and drug use and related disorders: An underrecognized health issue among adolescents and young adults attending sexually transmitted disease clinics. Sexually Transmitted Diseases. 2006;33:565–570. doi: 10.1097/01.olq.0000206422.40319.54. doi:10.1097/01.olq.0000206422.40319.54. [DOI] [PubMed] [Google Scholar]
- Dawe S., Gullo M. J., Loxton N. J. Reward drive and rash impulsiveness as dimensions of impulsivity: Implications for substance misuse. Addictive Behaviors. 2004;29:1389–1405. doi: 10.1016/j.addbeh.2004.06.004. doi:10.1016/j. addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Dawson J. F. Moderation in management research: What, why, when and how. Journal of Business and Psychology. 2014;29:1–19. doi:10.1007/s10869-013-9308-7. [Google Scholar]
- Delaneau O., Zagury J.-F., Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nature Methods. 2013;10:5–6. doi: 10.1038/nmeth.2307. doi:10.1038/nmeth.2307. [DOI] [PubMed] [Google Scholar]
- Delucchi K. L., Matzger H., Weisner C. Alcohol in emerging adulthood: 7-year study of problem and dependent drinkers. Addictive Behaviors. 2008;33:134–142. doi: 10.1016/j.addbeh.2007.04.027. doi:10.1016/j.addbeh.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. M. Gene-environment interaction in psychological traits and disorders. Annual Review of Clinical Psychology. 2011;7:383–409. doi: 10.1146/annurev-clinpsy-032210-104518. doi:10.1146/annurev-clinpsy-032210-104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. M. Commentary for special issue of prevention science “using genetics in prevention: Science fiction or science fact? Prevention Science. 2017;19:101–108. doi: 10.1007/s11121-017-0828-7. doi:10.1007/s11121-017-0828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion T., McMahon R. J. Parental monitoring and the prevention of child and adolescent problem behavior: A conceptual and empirical formulation. Clinical Child and Family Psychology Review. 1998;1:61–75. doi: 10.1023/a:1021800432380. [DOI] [PubMed] [Google Scholar]
- Galea S., Rudenstine S., Vlahov D. Drug use, misuse, and the urban environment. Drug and Alcohol Review. 2005;24:127–136. doi: 10.1080/09595230500102509. doi:10.1080/09595230500102509. [DOI] [PubMed] [Google Scholar]
- Guo J., Hawkins D., Hill K. G., Abbott R. D. Childhood and adolescent predictors of alcohol use and dependence in adulthood. Journal of Studies on Alcohol. 2001;62:754–762. doi: 10.15288/jsa.2001.62.754. doi:10.15288/jsa.2001.62.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick B. C., Young S. E., Zieger J. S., Lessem J. M., Hewitt J. K., Hopfer C. J. Prevalence and correlates of alcohol and cannabis use disorders in the United States: Results from the National Longitudinal Study of Adolescent Health. Drug and Alcohol Dependence. 2014;136:158–161. doi: 10.1016/j.drugalcdep.2013.11.022. doi:10.1016/j.drugalcdep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A. B., Kranzler H. R. Alcohol dependence genetics: Lessons learned from genome-wide association studies (GWAS) and post-GWAS analyses. Alcoholism: Clinical and Experimental Research. 2015;39:1312–1327. doi: 10.1111/acer.12792. doi:10.1111/acer.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D. S., Stinson F. S., Ogburn E., Grant B. F. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. doi:10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hasking P., Boyes M., Mullan B. Reward and cognition: Integrating reinforcement sensitivity theory and social cognitive theory to predict drinking behavior. Substance Use and Misuse. 2015;50:1316–1324. doi: 10.3109/10826084.2015.1005315. doi:10.3109/10826084.2015.1005315. [DOI] [PubMed] [Google Scholar]
- Hill K. G., Hawkins J. D., Bailey J. A., Catalano R. F., Abbott R. D., Shapiro V. Person-environment interaction in the prediction of alcohol abuse and alcohol dependence in adulthood. Drug and Alcohol Dependence. 2010;110:62–69. doi: 10.1016/j.drugalcdep.2010.02.005. doi:10.1016/j.drugalcdep.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines L. A., Morley K. I., Mackie C., Lynskey M. Genetic and environmental interplay in adolescent substance use disorder. Current Addiction Reports. 2015;2:122–129. doi: 10.1007/s40429-015-0049-8. doi:10.1007/s40429-015-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G. N. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Howie B. N., Donnelly P., Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genetics. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. doi:10.1371/journal. pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HapMap 3 Consortium. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. doi:10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessor R. Problem-behavior theory, psychosocial development, and adolescent problem drinking. Addiction. 1987;82:331–342. doi: 10.1111/j.1360-0443.1987.tb01490.x. doi:10.1111/j.1360-0443.1987.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Heath A. C., Neale M. C., Kessler R. C., Eaves L. J. Alcoholism and major depression in women: A twin study of the causes of comorbidity. Archives of General Psychiatry. 1993;50:690–693. doi: 10.1001/archpsyc.1993.01820210024003. doi:10.1001/archpsyc.1993.01820210024003. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Ohlsson H., Karriker-Jaffe K. J., Sundquist J., Sundquist K. Social and economic consequences of alcohol use disorder: A longitudinal cohort and co-relative analysis. Psychological Medicine. 2017;47:925–935. doi: 10.1017/S0033291716003032. doi:10.1017/S0033291716003032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R., McGonagle K., Zhao S., Nelson C. B., Hughes M., Eshleman S., Kendler K. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kessler R., Wittchen H.-U., Abelson J. M., McGonagle K., Schwarz N., Kendler K. S., Zhao S. Methodological studies of the Composite International Diagnostic Interview (CIDI) in the US National Comorbidity Study. International Journal of Methods in Psychiatric Research. 1998;7:33–55. doi: 10.1002/mpr.33. [Google Scholar]
- Kleinjan M., Rozing M., Engels R. C. M. E., Verhagen M. Co-development of early adolescent alcohol use and depressive feelings: The role of the mu-opioid receptor A118G polymorphism. Development and Psychopathology. 2015;27:915–925. doi: 10.1017/S0954579414000911. doi:10.1017/S0954579414000911. [DOI] [PubMed] [Google Scholar]
- Klima T., Skinner M. L., Haggerty K. P., Crutchfield R. D., Catalano R. F. Exploring heavy drinking patterns among Black and White young adults. Journal of Studies on Alcohol and Drugs. 2014;75:839–849. doi: 10.15288/jsad.2014.75.839. doi:10.15288/jsad.2014.75.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzman A. R., Farkas K. J., Townsend A. L. Spirituality, religiousness, and alcoholism treatment outcomes: A comparison between black and white participants. Alcoholism Treatment Quarterly. 2010;28:128–150. doi: 10.1080/07347321003648661. doi:10.1080/07347321003648661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S. F., Brown T. L., Phillips C. M., Ialongo S. N. The relationships between perceptions of neighborhood characteristics and substance use among urban African-American adolescents. American Journal of Community Psychology. 2004;34:205–218. doi: 10.1007/s10464-004-7415-3. doi:10.1007/s10464-004-7415-3. [DOI] [PubMed] [Google Scholar]
- Li C. C. Population subdivision with respect to multiple alleles. Annals of Human Genetics. 1969;33:23–29. doi: 10.1111/j.1469-1809.1969.tb01625.x. Retrieved from https://wwwncbi-nlm-nih-gov.proxy1.library.jhu.edu/pubmed/5821316. [DOI] [PubMed] [Google Scholar]
- Martin A. R., Gignoux C. R., Walters R. K., Wojcik G. L., Neale B. M., Gravel S., Kenny E. E. Human demographic history impacts genetic risk prediction across diverse populations. American Journal of Human Genetics. 2017;100:635–649. doi: 10.1016/j.ajhg.2017.03.004. doi:10.1016/j.ajhg.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M. Epigenetics and the biological definition of gene x environment interactions. Child Development. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. doi:10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Musci R. J., Masyn K. E., Benke K., Maher B., Uhl G., Ialongo N. S. The effects of the interplay of genetics and early environmental risk on the course of internalizing symptoms from late childhood through adolescence. Development and Psychopathology. 2016;28:225–237. doi: 10.1017/S0954579415000401. doi:10.1017/S0954579415000401. [DOI] [PubMed] [Google Scholar]
- Nurnberger J. I., Foroud T., Flury L., Su J., Meyer E. T., Hu K., Reich W. Evidence for a locus on chromosome 1 that influences vulnerability to alcoholism and affective disorder. American Journal of Psychiatry. 2001;158:718–724. doi: 10.1176/appi.ajp.158.5.718. doi:10.1176/appi.ajp.158.5.718. [DOI] [PubMed] [Google Scholar]
- Persico A. M., Bird G., Gabbay F. H., Uhl G. R. D2 dopamine receptor gene taqI A1 and B1 restriction fragment length polymorphisms: Enhanced frequencies in psychostimulant-preferring polysubstance abusers. Journal of Biological Psychiatry. 1996;40:776–784. doi: 10.1016/0006-3223(95)00483-1. doi:10.1016/0006-3223(95)00483-1. [DOI] [PubMed] [Google Scholar]
- Pritchard J. K., Rosenberg N. A. Use of unlinked genetic markers to detect population stratification in association studies. American Journal of Genetic Psychology. 1999;65:220–228. doi: 10.1086/302449. doi:10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. Retrieved from http://www.R-project.org. [Google Scholar]
- Rhodes J. E., Jason L. A. A social stress model of substance use. Journal of Consulting and Clinical Psychology. 1990;58:395–401. doi: 10.1037//0022-006x.58.4.395. doi:10.1037/0022-006X.58.4.395. [DOI] [PubMed] [Google Scholar]
- Ross C. E., Mirowsky J. Neighborhood disadvantage, disorder, and health. Journal of Health and Social Behavior. 2001;42:258–276. doi:10.2307/3090214. [PubMed] [Google Scholar]
- Saraceno L., Munafò M., Heron J., Craddock N., Van den Bree M. B. Genetic and non-genetic influences on the development of co-occurring alcohol problem use and internalizing symptomatology in adolescence: A review. Addiction. 2009;104:1100–1121. doi: 10.1111/j.1360-0443.2009.02571.x. doi:10.1111/j.1360-0443.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- Smith D. C., Bahar O. S., Cleeland L. R., Davis J. P. Selfperceived emerging adult status and substance use. Psychology of Addictive Behaviors. 2014;28:935–941. doi: 10.1037/a0035900. doi:10.1037/a0035900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R. L., Williams J. B. W., Gibbon M., First M. B. The structured clinical interview for DSM-III-R (SCID) Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. doi:10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Tambs K., Harris J. R., Magnus P. Genetic and environmental contributions to the correlation between alcohol consumption and symptoms of anxiety and depression: Results from a bivariate analysis of Norwegian twin data. Behavior Genetics. 1997;27:241–250. doi: 10.1023/a:1025662114352. [DOI] [PubMed] [Google Scholar]
- Torvik F. A., Rosenström T. H., Ystrom E., Tambs K., Røysamb E., Czajkowski N, Reichborn-Kjennerud T. Stability and change in etiological factors for alcohol use disorder and major depression. Journal of Abnormal Psychology. 2017;126:812–822. doi: 10.1037/abn0000280. doi:10.1037/abn0000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. American Community Survey 5-year estimates: 2005-2009. 2009. [Google Scholar]
- Wallace J. M., Muroff J. R. Preventing substance use among African-American children and youth: Race differences in risk factor exposure and vulnerability. Journal of Primary Prevention. 2002;22:235–261. doi:10.1023/A:1013617721016. [Google Scholar]
- Wallace S. A., Neilands T. B., Sanders Phillips K. Neighborhood context, psychological outlook, and risk behaviors among urban African American youth. Cultural Diversity and Ethnic Minority Psychology. 2017;23:59–69. doi: 10.1037/cdp0000108. doi:10.1037/cdp0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. Ggplot2. Elegant graphics for data analysis. New York, NY: Springer-Verlag; 2016. [Google Scholar]
- Winstanley E. L., Steinwachs D. M., Ensminger M. E., Latkin C. A., Stitzer M. L., Olsen Y. The association of self-reported neighborhood disorganization and social capital with adolescent alcohol and drug use, dependence, and access to treatment. Drug and Alcohol Dependence. 2008;92:173–182. doi: 10.1016/j.drugalcdep.2007.07.012. doi:10.1016/j.drugalcdep.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. R., Buckman J., Pardini D., Loeber R. The association of alcohol and drug use with persistence of violent offending in young adulthood. Journal of Developmental and Life Course Criminology. 2015;1:289–303. doi: 10.1007/s40865-015-0015-0. doi:10.1007/s40865-015-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]