Abstract
Cervical carcinoma is one of the most common and dreaded diseases of women, and in India, it accounts for 16 per cent of total cervical cancer cases occurring globally. The situation is more alarming in the rural areas where the majority of women are illiterate and ignorant about the hazards of cervical cancer. Different screening strategies such as rural cancer registries and camp approach for cancer detection have been found useful in minimizing the problem of cervical cancer in the villages. Various screening techniques such as visual inspection with acetic acid, visual inspection with Lugol's iodine, visual inspection with magnification devices-magnavisualizer, Pap smear and HPV-DNA testing have been suggested and tried under low-resource settings of our country, and cervical cytology screening has been found effective in reducing incidence of the disease. In the present review, feasibility of different screening methods has been assessed to find out the most suitable mode applicable at the rural level. Single lifetime screening particularly of high-risk women along with analysis of cost-effective tumour markers such as Argyrophilic nucleolar organizer regions (AgNOR) counts to discriminate high-risk dysplasia cases appears to be an appropriate approach in fighting against cervical cancer.
Keywords: AgNOR, carcinoma cervix, rural cervical cancer screening, single lifetime cytological screening, squamous intraepithelial lesion
Introduction
Carcinoma of the uterine cervix is a major health problem faced by the Indian women, and every year, approximately 120,000 women develop this disease1. India accounts for 15.2 per cent of the total cervical cancer deaths in the world2. Although the incidence of carcinoma cervix has declined in the urban population, in the rural areas it continues to be highly prevalent3. The usual 10-20 years of natural history of progression from mild dysplasia to carcinoma cervix makes this cancer as relatively early preventable disease and provides the rationale for screening4. Despite existence of national guidelines, the screening coverage in India is appalling low. As a result, the diagnosis of carcinoma cervix is based on opportunistic screening or after the onset of the symptoms. Although the data from the 20 population-based cancer registries in India indicate a steady decline in the carcinoma cervix incidence over the last two decades, the disease still occupies number two position with a high risk of the disease5,6.
The carcinoma cervix is predicted to decrease by 2020, and there are many factors contributing to its decline. The improvement in the living standard and awareness among women through print and audio-visual media has resulted in a decline in the incidence of cervical cancer. Regular cervical cytological examination by all sexually active women can prevent the occurrence of carcinoma cervix7. In addition, awareness for genital hygiene and visiting hospital at pre-clinical stage are the contributory factors for the control of carcinoma cervix in urban settings. The situation of cancer prevalence is alarming in rural population where the majorities of women are illiterate and are ignorant about the factors that contribute to the development of cervical cancer. They are socio-economically weak and have poor hygienic conditions and many other risk factors such as early age marriage and multiple pregnancy. In addition, medical facilities, advice and awareness programmes are almost non-existent8.
In the present review, an attempt has been made to identify the different barriers which are impediment to the rural cervical cancer screening, factors influencing the awareness and ways to create awareness of the disease among rural women. Different strategies to control cervical cancer at rural level such as rural cancer registries and camp approach have been discussed, and different diagnostic methods applicable to low resource settings have been compared to find out the most suitable modality feasible for mass rural cervical cancer screening.
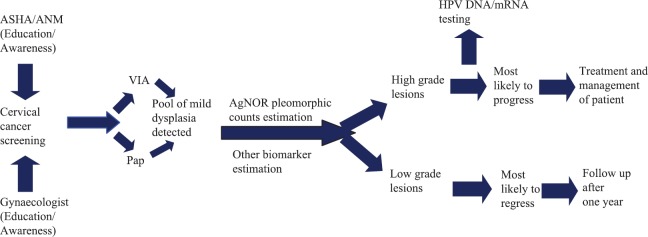
Creating awareness about the hazards of cervical cancer among rural women and apprising them about the utility of early detection tests and their availability have been the main focus for any rural screening programme. Dhamija et al9 surveyed to assess the knowledge, attitude and practice among rural women and found that younger women had better awareness and knowledge about cervical cancer and various risk factors, signs and symptoms associated with the disease. Literacy drive for education and exposure to family planning devices are found to be important in creating awareness about this disease. Early episodes of gynaecological problems and treatment lead to higher awareness, and it was felt that efforts should be made to innovate ways to reach older and illiterate women for better awareness in the community9 (Fig. 1).
Fig. 1.
Factors that influence awareness of the disease.
Source: Ref. 9.
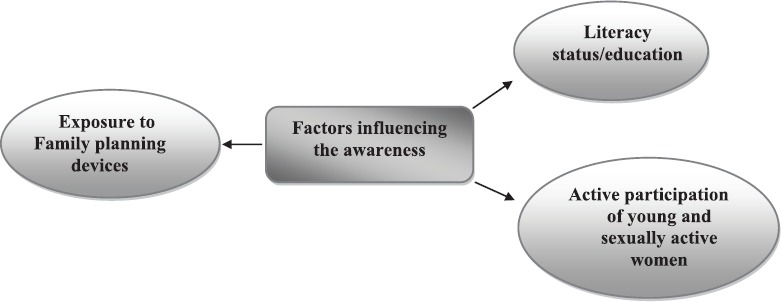
Risk factors: Tripathi et al10 have reported that health of rural women and their access to health facility is compromised due to socio-cultural, economical and environmental factors. They found that awareness about symptoms, possibility of early detection, available tests and possibility of the cure of the disease among rural women was low. They observed that main barriers faced by them were cognitive, i.e. ignorance about the disease and that the awareness scores were significantly associated with age, education and income and the number of persons with history of cancer in the family (Fig. 2).
Fig. 2.
Barriers which lead to poor rate of detection of cervical cancer at an early stage.
Source: Ref.10.
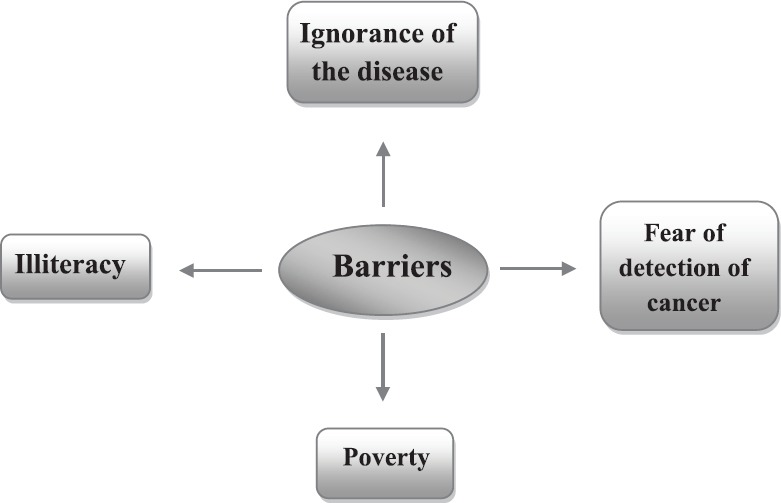
Isaac et al11 in a study in low-resource setting in rural Tamil Nadu, India, found that the uptake of screening was initially low despite access to screening programmes. However, an increase in the community awareness enhanced the number of women undergoing screening. This can be achieved through participation and necessary output and optimization of health literacy, belief and attitude of the community11 (Fig. 3). A study by Aswathy et al12 on cervical cancer screening programme in Kerala reported that appropriate knowledge on cervical cancer was a critical element in determining whether women would undergo a Pap test or not.
Fig. 3.
Variety of ways to create awareness among public.
Source: Ref. 11.
Although the three-fourth of the rural population in south India knew that carcinoma cervix could be detected early by a screening test, only 2.2 per cent had the Pap test done. This was reported by Sudhir13, who emphasized that lack of awareness of either symptoms or disease, lack of knowledge to go where for Pap test, never seen or felt that it was needed, were the factors for not undergoing screening. Hence, in the context of implementing a successful carcinoma cervix prevention programme, it becomes very essential to identify the reasons which are preventing the women for using these services13. Further psychological factors such as fear that the test would be painful, anxiety about the result and embarrassment also play major role. The International Agencies for Research on Cancer also supports the facts that the women fail to undergo screening due to many factors such as lack of resources, insufficient knowledge, difficulty in accessing the health care delivery system, culture and psychological facts, fear of pain, lack of family support and no active involvement of community14. Denny et al15 compared screening programmes for cervical cancer in many developing countries and found that competitive health needs, limited human and financial resources, poorly developed health care services, women uninformed and disempowered and widespread poverty were the main barriers for screening and country-specific solutions need to be found.
A high prevalence of important risk factors associated with carcinoma cervix such as age, age at marriage, age of first childbirth, parity, family planning practices and reproductive tract infections has been reported in rural areas of West Bengal16. It was suggested that screening and early detection efforts could be directed especially to the group at risk16. In another study preventing and treating cervical cancer and reducing the burden were to be possible by targeting resources to the areas with the high prevalence of risk factors17. In India, women do not have access to effective screening programme, and it has been estimated that without a major improvement in cytology services, it will not be possible to screen even 25 per cent of the population once in a lifetime in the near future18.
Rani et al19 surveyed regarding awareness of Pap smear and cervical cancer vaccines among women attending a tertiary hospital in the eastern part of Uttar Pradesh and found the awareness was very low in rural women and literacy and socio-economic status played important role.
Risk factors associated with of cervical cancer
Oncogenic human papillomaviruses (HPVs) are widely implicated with the development of cervical cancer20. Other risk factors such as early age of marriage, multiple sexual partners, multiple pregnancies, poor genital hygiene and lack of awareness, may be involved in modifying the risk of developing cervical cancer in women. The worldwide prevalence of HPV infection is high (9-13%) and is the most common sexually transmitted infection, with no specific treatment21.
Mostly Genital HPV infections are asymptomatic and unapparent and studies indicate that nearly all cervical cancer cases are caused by genital infection with specific high-risk HPV types. With use of the cervical smear Pap test or VIA, or application of effective HPV-DNA detection procedures, precursors of cervical cancer can be easily detected and successfully treated at an early stage. Thus, cervical cancer can be easily prevented with regular screening programmes.
Different ingredients of rural screening programme
Different rural screening strategies have been developed to detect cervical cancer in its pre-invasive phase to bring down the incidence and associated mortality due to the disease. These rural screening programmes are summarized below:
Rural cancer registries
The first rural cancer registry was set up in 1987 at Barshi, Maharashtra, to cover rural areas of Barshi, Paranda and Bhum Tahsils by adopting a methodology suitable for rural areas22. Registry activity has been accepted by the villagers, and its activity has increased cancer awareness in the population22. It has also increased the frequency of early detection of cervical cancer by more than twofold in the past 16 years (1994-2010) and significantly decreased the relative frequency of death22. The whole process has positive impact on society, awareness, stage at presentation and survival of the cancer patients.
To overcome the adverse conditions in the rural areas, the registry adopted case finding in the community itself. The registry investigators visited the villages at least twice a year to identify the cases. All those with gynaecological symptoms such as heavy bleeding and white discharge were invited to attend the cancer screening camps held by registry in each zone consisting of roughly 35 villages comprising a 35,000 population where diagnostic procedures were carried out. A total of 65 cervical cancer cases were registered in the area in the year 201022.
The following rural cancer registries have been established in the last few decades23: (i) A rural registry covering Ahmadabad rural district, Gujarat, was established in 2007; (ii) Under Tata Memorial Center, Mumbai, a rural population-based cancer registry was started in 2009 at Dervan covering entire Ratnagiri district population. A total of 700 different cases of cancer have been reported until now; (iii) During 2010, a rural population-based cancer registry was started at Sevagram, Maharashtra; and (iv) Another rural cancer registry was set up in Sindbuderg district in the Konkan area of Maharashtra in April 2011.
Camp approach
Nene et al24 correlated effectiveness of this approach in the early diagnosis and treatment of cervical cancer. In the rural area of Barshi Tehsil of Maharashtra where the camp was organized between 1982 and 1987, the acceptance of cytological screening was poor, thereby indicating that mere organization of the camp was not sufficient to motivate the women and to subject them to the Pap smear examination24. By using fully equipped cancer detection mobile van, a total of 2846 women were screened in two rounds between 1982 and 1987. The overall acceptance rate for Pap smear screening was 8.3 per cent and the rate was higher in the younger women than in the postmenopausal women. The clinical symptoms were higher in the women screened (45%) and leucorrhoea and bleeding were the most common symptoms. A high prevalence of cytological atypias was seen in this group. They were able to detect 25 cases of cervical carcinoma by cytology in the camp. Further, they found that apprehension for undergoing Pap smear was the main cause of poor acceptance of the screening which was particularly very low in the older women. Pre-camp educational drive to motivate the women is mandatory. Another study has reported usefulness of camp organized in Delhi in finding abnormal cytology in women suspected of carcinoma on clinical examination and emphasized the need for cervical cancer screening of women at regular intervals through camp approach in the country25.
Karunakaran et al26 undertook a camp based cross-sectional study in Karindalam village in Kerala to assess the Pap smear test, knowledge, attitude and practice regarding carcinoma cervix and its screening methods. Community-level women volunteers were used to mobilize women for the camp, and a face-to-face interview was held using a pre-tested questionnaire and Pap test done. The Pap smear identified 0.6 per cent of women with high intraepithelial lesions and adequate follow up was offered.
During ongoing rural cervical cancer screening programme carried out at Kakori and Malihabad blocks of Lucknow, Uttar Pradesh, Misra et al27 found that the overall acceptance rate of Pap smear screening was approximately 32 per cent of motivated women who attended the camp. This could be made possible by counselling and distribution of pamphlets. The acceptance rate was higher in younger women as they were mostly symptomatic. The response of older women was low, and this might be due to fear of detection of any abnormal pathology. This study showed that lack of education and poverty prevailing in the rural region were the main factors responsible for low acceptance rate.
Through services of village health nurses (VHNs)
In a study conducted in Tamil Nadu, village health nurses (VHNs) were trained for identifying different cervical lesions and to take satisfactory Pap smears. In a two-year period, 101 VHNs were trained and 6459 eligible women were screened. The programme was successful as 80 per cent of the Pap smears taken by the VHN were adequate28.
Alternative approaches to diagnose cervical cancer at low-resource settings and in rural areas
Several strategies have been explored to diagnose cervical cancer in its early stage in the rural settings and different studies involving different techniques to detect the disease have been undertaken. These are visual inspection of the cervix (VIA), Pap smear examination (cytology) and HPV-DNA detection.
Visual inspection
The high burden of cervical carcinoma and paucity of workforce in cytology in developing countries have led to screening by visual methods tests such as VIA and visual inspection with Lugol's iodine (VILI) and visual inspection with magnification devices-magnavisualizer (VIAM). This is to be implemented in rural areas taking into consideration the cultural background and available facilities. Cytological screening was not possible to cover even 20 per cent of the existing cases in the near future29.
The main advantage of the visual assessment is the immediate availability of the results which permits diagnosis and/or treatment to be performed in the same sitting. Pap smear test was compared with visual detection tests for screening cervical cancer for developing the optimal strategy for low-resource settings30. It was seen that Pap test had low sensitivity but high specificity, whereas the visual detection methods had high sensitivity was cheaper than Pap test. The VIAM may be more beneficial over VIA in doubtful cases30. Deodhar et al31 have also found high specificity of Pap smear than visual inspection such as VIA and VILI but less sensitive in detecting cervical intraepithelial neoplasia (CIN) and cancer cases. In another study VIA was found to detect higher proportion of CIN II and III than cytology in all age groups while cytological screening was less sensitive in women more than 40 yr of age32. In a rural community setting of north India (Dadri, Uttar Pradesh), Satyanarayana et al33 compared screening by Pap, VIA and VILI. Screened positive cases were referred for colposcopy and confirmation for histology. The sensitivity of detecting of CIN+ lesions was higher with VIA and VILI, but specificity was high with Pap. They suggested that VIA screening was a feasible primary screening method for detecting high-grade CIN and could be used at places where the Pap test was not feasible.
Manisha et al34 compared the efficacy of VIA, cytology and colposcopy in a rural hospital of Wardha and found that sensitivity of VIA in detecting cervical cancer as 95.2 per cent compared to 94 per cent with Pap and colposcopy and specificity of 44.1 per cent as against 81.4 per cent with Pap and 67.4 per cent with colposcopy. They recommended VIA a better screening test due to its high sensitivity, simple administration and low cost34.
Cytological screening
The main advantage of cytological screening is that it permits to detect the disease in its pre-invasive phase which can be easily treated. Cytological screening has been found to reduce the incidence of cervical cancer by 80 per cent35. Although cytological screening in urban areas revealed a low incidence of squamous intraepithelial lesion (SIL), the situation was alarming in rural area of Lucknow, Uttar Pradesh, where the incidence of SIL was found as high as 20 per cent27, whereas the hospital-based screening extended to over a period of 35 years showed an incidence of 4.8 per cent of SIL in urban area36. This might be due to poor hygienic conditions and associated persistent vaginal infections. In rural women, much higher (10.5%)37 SIL rate was found than that (4.5%) observed in urban setup. A higher incidence of SIL (17 and 11%)38,39 has been reported in the rural women.
Parimala et al40 conducted cervical cytology screening in rural women aged between 30 and 60 yr in Thirumazhisai health centre in Chennai, Tamil Nadu, and found high incidence of high-grade lesion (CIN3) in rural women which were susceptible to develop into cervical carcinoma. They felt that regular cytological screening was needed among the women of low socio-economic status as well as awareness to be created on cervical cancer and its complications40.
In a study conducted in a tertiary care hospital in the rural area of Himachal Pradesh, cervical cancer screening was done in symptomatic women using Pap smear and Pap smear was found to be as a sensitive and a specific method in detecting pre-cancerous lesions of cervix41. In a cervical cancer screening programme conducted in a tertiary care hospital of Tripura abnormal Pap smears were observed. Pap smear was suggested to be an elementary, economical, safe and yet highly sensitive screening test for early detection of carcinoma cervix42.
HPV-DNA detection
Nene et al43 analyzed the factors associated with participation in cervical cancer screening and treatment and evaluated the cost-effectiveness of VIA, cytological screening and HPV testing in reducing the incidence and mortality from carcinoma cervix in Maharashtra. The acceptance of screening was higher in the educated women, and it was felt that less educated women should be motivated to further increasing screening uptake43. In another study, HPV testing showed a high sensitivity and specificity than Pap and VIA, but 87.6 per cent of the total CIN and cancer cases detected were missed by the programme failures44. The use of a less invasive and more user-friendly primary screening strategy such as self-collected swab or urine for HPV-DNA testing may be required to achieve the coverage essential for effective decline in cervical cancer mortality45.
Deodhar et al46 carried out HPV study in the inflammatory and CIN lesions in rural community of Sholapur and Osmanabad district of Maharashtra. The overall prevalence of high risk HPV was 37.6 per cent with inflammatory lesions and grade I CIN, 65.3 per cent in grade II, 80.6 per cent in grade III and 86.5 per cent in cervical cancer cases. The HPV genotype data showed higher prevalence of HPV-16/18 in cancer specimens indicating that prophylactic 16/18 vaccination would have a significant impact on the prevention of carcinoma cervix in India46. In a study in the rural community setting of Uttar Pradesh all the married women aged 30-59 yr were targeted for screening by care HPV, Pap test and VIA. The sensitivity for detection of CIN was higher with HPV followed by Pap and VIA, respectively and hence care HPV testing was considered superior to VIA and Pap test for detection of high-grade CIN in rural community setting47.
Results of studies involving different techniques alone or in combination for comparing the results undertaken by the different investigators in different parts of the country are summarized in Fig. 4. Another cervical cancer screening programme carried out of a low-resource setting in West Bengal found HPV positivity in 4.75 per cent of 44,110 women screened by hybrid capture test48. The detection rate of CIN3+ by HPV test was 3.9 per cent per 1000 women. However, compliance of follow up at one year was poor. This study demonstrated feasibility of implementing HPV detection-based screening using existing primary health care infrastructure48. Bobdey et al49 compared sensitivity and specificity of different detection techniques such as VIA, VILI, cytology and HPV detection in the rural areas of Mizoram and Dibrugarh States and suggested that cervical cancer screening programme based on visual screening test should be adapted as an integral part of primary healthcare set up in resource-poor country like India. A study on Pap smear cytology and HPV-DNA testing in the women of urban and peri-urban areas of Telangana and Andhra Pradesh found HPV prevalence of 14.7 per cent50. Of these, 1.8 per cent were high-risk types and all these were associated with abnormal Pap smear. Feasibility of self-collected samples for HPV was evaluated in women of Kaniyambadi block of Vellore district in south India and high-risk HPV was found in 5.9 per cent and low-risk types in 2.7 per cent of women examined51. The women preferred and accepted self-collection as convenient and painless than taken by health workers.
Fig. 4.
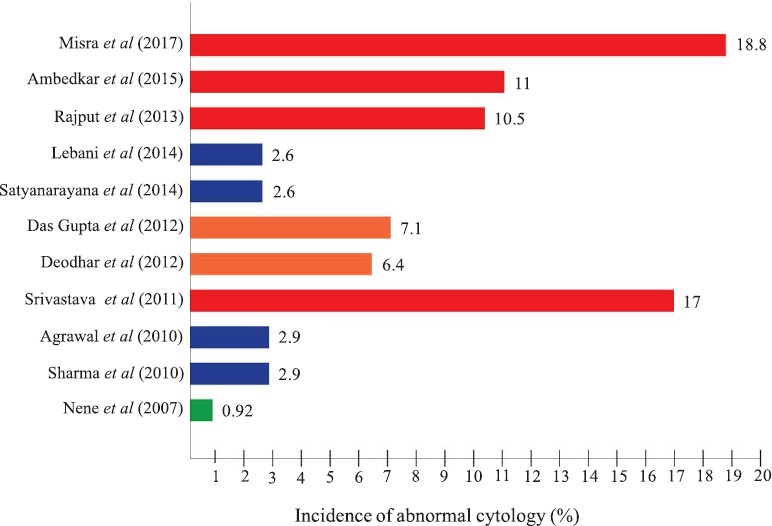
Cytological findings reported by different workers in rural screening programme.
Source: Refs 25, 27, 30-33, 37-39, 43, 47.
To make sample collection and HPV-DNA detection by PCR most simple and cost-effective, a non-invasive ‘paper smear’ method for dry collection and storage in room temperature was developed45. This method has been very commonly used now for an easy non-invasive detection of HPV. A large multi-centric study was carried out using urine sampling in remote tribal populations from three different tribal-populated States, Madhya Pradesh, Chhattisgarh and Jharkhand51. A high prevalence of high-risk HPV, particularly HPV-16, was detected in both tribal young adolescent girls and sexually active tribal women52. Furthermore, another study was carried out using urine sampling in urban and rural school girls to show a higher prevalence of HPV infection in rural adolescent girls52.
HPV vaccination
HPV vaccination can make major breakthrough in the control of carcinoma cervix in India. Sarkar et al53 in a study conducted among HPV-infected female population in West Bengal found that HPV vaccines offered protection against HPV-16/18 and some cross-protection to a few associated genotypes. These vaccines are less likely to offer protection against cervical cancer in HPV positive women and those infected with 16/18 oncogenic HPV genotypes. Chatterjee et al54 analyzed the status of HPV vaccination in the country and found that despite the efforts to introduce HPV vaccination in the National Immunization Programme and bring down the vaccine cost, challenges such as inadequate epidemiological evidence for disease prioritization, duration of vaccine use, and vaccine acceptance in implementing HPV vaccination in India appeared to be major hurdle.
Role of tumour markers
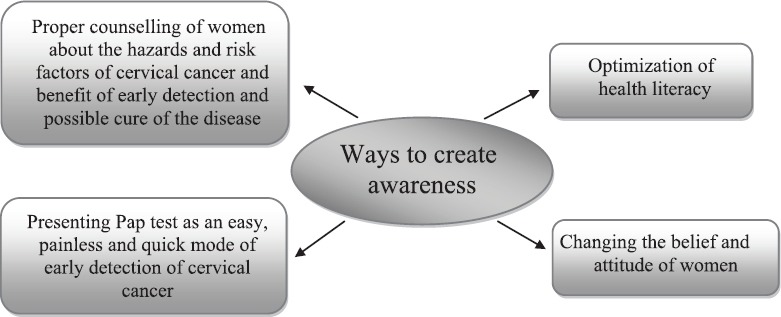
Although according to recent WHO guidelines55, all women between the age group of 21-65 yr should be screened every three years to detect any precancerous lesions occurring, but due to paucity of funds and lack of workforce in cytology, this is not feasible in developing counties like India. Consequently, various alternatives have been suggested to suit the health outlay of the country. The suggestion put forward by the WHO (1996)56 for single lifetime screening after 40 years has been to find out a large number of precancerous cases whose treatment would check the progression of the lesion to malignancy and bring down the incidence of cervical cancer in the population screened56. This would also suit the health budget of the country and could be easily introduced in the National Cancer Control Programme. Misra et al57 suggested three categories of high-risk group of women, namely higher age and parity and women with gynaecological symptoms for a single lifetime screening to extend broad coverage, and this could be beneficial to rural screening programme in our country.
It is well-established fact that 90 per cent of mild dysplasia cases regress spontaneously or after treatment and only 10 per cent of them either persist or progress to higher grade, and in all probabilities, these cases may be HPV positive35. If these 10 per cent of cases can be picked up from the pool, it will obviate the burden of follow up of all mild dysplasia cases. This could be made possible by application of tumour markers which are of low cost, easy to apply and reliable. Although high-risk HPV-DNA testing is one such reliable marker and is being used in western countries, its high cost makes it prohibitive in poor Indian setting. One inexpensive and easily affordable tumour marker which has been tried by different workers is AgNOR pleomorphic counts which showed rise with severity of cervical lesions58,59. Further, it was seen that majority of mild dysplasia cases with high AgNOR pleomorphic mean counts showed either persistence or progression to higher grade60.
Possible strategies for rural screening
Since the current concept of cervical cancer screening depends on the resources available, it may be adopted accordingly. The following alternatives have been suggested: (i) In developing countries like India where resource crunch and paucity of workforce in cytology are the major problems, a single lifetime screening especially in rural population with emphasis on the high-risk groups of women is most feasible approach to detect the disease in its pre-invasive stage. (ii) Application of AgNOR pleomorphic counts as a cheaper test to discriminate high-risk dysplasia cases whose immediate treatment will check the progression of the lesion to carcinoma. This will obviate the burden of follow up of all mild dysplasia cases which is a tedious problem and the selective follow up of high risk cases will definitely bring down the incidence of carcinoma cervix and associated mortality in the rural population. (iii) There is a need to enhance the efficacy and sensitivity of the cytological screening programme along with increasing the trained workforce in this field. Such a strategy needs to be adopted by our health managers for the National Cancer Control Programme (Fig. 5).
Fig. 5.
Strategy for control of cervical cancer most suitable for low-resource settings. ASHA, Accredited Social Health Activist; ANM, Auxiliary Nurse Midwifery; VIA, visual inspection of cervix with acetic acid.
There is also a need for educational intervention and awareness programme not only to augment HPV immunization programme but also screening for a primary prevention and control of cervical cancer in India. For a mass HPV vaccination programme in India, affordability and accessibility are of major concern. For various issues specific to the region, a cost-effective second-generation vaccine is the need of the hour. Hence, routine cytological screening should be continued to be used to detect and treat women who are infected before vaccination or with other HPV types not covered by the vaccine.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Jacob M. Information, education & communication: Corner stone for preventing cancer of the cervix. Indian J Med Res. 2012;136:182–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Bangalore: National Cancer Registry Programme; 2009. National Cancer Registry Programme. Time trends and cancer incidence rates: 1982–2005. [Google Scholar]
- 4.World Health Organization. Geneva: WHO; 2006. Comprehensive cervical cancer control: A guide to essential practice; pp. 32–49. [PubMed] [Google Scholar]
- 5.Incidence and distribution of cancer. Bangalore: 2010. Nov, National Cancer Registry Programme. Indian Council of Medical Research. Three year report of population based cancer registries: 2006-2008. [Google Scholar]
- 6.Government of India-World Health Organization Collaborative. Programme (2004–2005): Guidelines for cervical cancer screening programme. Department of Cytology & Gynaecological Pathology, Postgraduate Institute of Medical Education and Research, Chandigarh. 2006 [Google Scholar]
- 7.Takiar R, Nadayil D, Nandakumar A. Projections of number of cancer cases in India (2010-2020) by cancer groups. Asian Pac J Cancer Prev. 2010;11:1045–9. [PubMed] [Google Scholar]
- 8.Badwe RA, Dikshit R, Laversanne M, Bray F. Cancer incidence trends in India. Jpn J Clin Oncol. 2014;44:401–7. doi: 10.1093/jjco/hyu040. [DOI] [PubMed] [Google Scholar]
- 9.Dhamija S, Sehgal A, Luthra UK, Sehgal K. Factors associated with awareness and knowledge of cervical cancer in a community: Implication for health education programmes in developing countries. J R Soc Health. 1993;113:184–6. doi: 10.1177/146642409311300407. [DOI] [PubMed] [Google Scholar]
- 10.Tripathi N, Kadam YR, Dhobale RV, Gore AD. Barriers for early detection of cancer amongst Indian rural women. South Asian J Cancer. 2014;3:122–7. doi: 10.4103/2278-330X.130449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaac R, Finkel M, Olver I, Annie IK, Prashanth HR, Subhashini J, et al. Translating evidence into practice in low resource settings: Cervical cancer screening tests are only part of the solution in rural India. Asian Pac J Cancer Prev. 2012;13:4169–72. doi: 10.7314/apjcp.2012.13.8.4169. [DOI] [PubMed] [Google Scholar]
- 12.Aswathy S, Quereshi MA, Kurian B, Leelamoni K. Cervical cancer screening: Current knowledge & practice among women in a rural population of Kerala, India. Indian J Med Res. 2012;136:205–10. [PMC free article] [PubMed] [Google Scholar]
- 13.Sudhir, Krishna K. Knowledge and practice about cervical cancer screening among women in a rural population of south India. Sch J Appl Med Sci. 2014;2:689–93. [Google Scholar]
- 14.International Agency for Research on Cancer. Handbook of cancer prevention: cervical cancer screening. Vol. 10. Lyon, France: International Agency for Research on Cancer; 2003. [Google Scholar]
- 15.Denny L, Quinn M, Sankaranarayanan R. Screening for cervical cancer in developing countries. Vaccine. 2006;24(Suppl 3):S3/71–7. doi: 10.1016/j.vaccine.2006.05.121. [DOI] [PubMed] [Google Scholar]
- 16.Das Gupta A, Naskar NN, Rama R, Deb S. A community based study of the prevalence of risk factor of carcinoma cervix in married women of rural area of West Bengal. Indian J Community Med. 2012;27:36–9. [Google Scholar]
- 17.Sreedevi A, Javed R, Dinesh A. Epidemiology of cervical cancer with special focus on India. Int J Womens Health. 2015;7:405–14. doi: 10.2147/IJWH.S50001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senapathy JG, Umadevi P, Kannika PS. The present scenario of cervical cancer control and HPV epidemiology in India: An outline. Asian Pac J Cancer Prev. 2011;12:1107–15. [PubMed] [Google Scholar]
- 19.Rani A, Singh K, Thapa S. A survey of awareness of Pap smear and cervical cancer vaccine among women at tertiary care centre in Eastern Uttar Pradesh India. Int J Reprod Contracep Obstet Gynecol. 2015;4:439–41. [Google Scholar]
- 20.Das BC, Gopalkrishna V, Sharma JK, Roy M, Luthra UK. Human papillomavirus DNA in urine of women with preneoplastic and neoplastic cervical lesions. Lancet. 1992;340:1417–8. [PubMed] [Google Scholar]
- 21.Hussain S, Bharadwaj M, Nasare V, Kumari M, Sharma S, Hedau S, et al. Human papillomavirus infection among young adolescents in India: Impact of vaccination. J Med Virol. 2012;84:298–305. doi: 10.1002/jmv.22261. [DOI] [PubMed] [Google Scholar]
- 22.Jayant K, Nene BM, Badwe RA, Panse NS, Thorat RV, Khan FY. Rural cancer registry at Barshi, Maharashtra and its impact on cancer control. Natl Med J India. 2010;23:274–7. [PubMed] [Google Scholar]
- 23.Nandakumar A. ICMR-NCPR-COD desk. Cancer Registry Abstract 2011; 16 : 1-2. [accessed on December 28, 2016]. Available from: http://www.ncdirindia.org/CRAB/C_2011/CRAB_2011.pdf .
- 24.Nene BM, Jayant K, Malvi SG, Dale PS, Deshpande R. Experience in screening for cervical cancer in rural areas of Barshi Tahsil (Maharashtra) Indian J Cancer. 1994;31:34–40. [PubMed] [Google Scholar]
- 25.Sharma P, Rahi M, Lal P. A community-based cervical cancer screening program among women of Delhi using camp approach. Indian J Community Med. 2010;35:86–8. doi: 10.4103/0970-0218.62576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karunakaran U, Thekkandathil N, Divakaran B, Joseph MM, Kannankai S, Kumaran JA. Cervical cancer screening program - A camp based cross sectional study among rural women in North Kerala. Sci J Public Health. 2017;5:215–23. [Google Scholar]
- 27.Misra JS, Srivastava AN, Gupta HP. Impact of literacy status on the cervical cancer screening in rural women of India. Invest Gynecol Res Womens Health. 2017;1:IGRWH.000510. [Google Scholar]
- 28.Gajalakshmi CK, Krishnamurthi S, Ananth R, Shanta V. Cervical cancer screening in Tamilnadu, India: A feasibility study of training the village health nurse. Cancer Causes Control. 1996;7:520–4. doi: 10.1007/BF00051884. [DOI] [PubMed] [Google Scholar]
- 29.Ananth R. Downstaging of cervical cancer. J Indian Med Assoc. 2000;98:41–4. [PubMed] [Google Scholar]
- 30.Aggarwal P, Batra S, Gandhi G, Zutshi V. Comparison of Papanicolaou test with visual detection tests in screening for cervical cancer and developing the optimal strategy for low resource settings. Int J Gynecol Cancer. 2010;20:862–8. doi: 10.1111/IGC.0b013e3181e02f77. [DOI] [PubMed] [Google Scholar]
- 31.Deodhar K, Sankaranarayanan R, Jayant K, Jeronimo J, Thorat R, Hingmire S, et al. Accuracy of concurrent visual and cytology screening in detecting cervical cancer precursors in rural India. Int J Cancer. 2012;131:E954–62. doi: 10.1002/ijc.27633. [DOI] [PubMed] [Google Scholar]
- 32.Dasgupta S, Bhattacharya S. Is visual inspection with acetic acid better than cervical cytology to screen women ≥ 40 years of age for carcinoma cervix? A cross-sectional study on proportion of screen-positive women (by VIA and cervical cytology) having CIN II/III lesion on cervical biopsy: Difference between two age groups and among screening methods. Arch Gynecol Obstet. 2012;285:1731–6. doi: 10.1007/s00404-012-2228-3. [DOI] [PubMed] [Google Scholar]
- 33.Satyanarayana L, Asthana S, Bhambani S, Sodhani P, Gupta S. A comparative study of cervical cancer screening methods in a rural community setting of North India. Indian J Cancer. 2014;51:124–8. doi: 10.4103/0019-509X.138172. [DOI] [PubMed] [Google Scholar]
- 34.Manisha S, Bagde N, Shrivastava D. Visual inspection of cervix with acetic acid: An alternative to cytology and colposcopy in early screening of cervical cancer in low-resource setup. J Datta Meghe Inst Med Sci Univ. 2017;12:32–4. [Google Scholar]
- 35.Miller AB, Nazeer S, Fonn S, Brandup-Lukanow A, Rehman R, Cronje H, et al. Report on consensus conference on cervical cancer screening and management. Int J Cancer. 2000;86:440–7. doi: 10.1002/(sici)1097-0215(20000501)86:3<440::aid-ijc22>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 36.Misra JS, Srivastava S, Singh U, Srivastava AN. Risk-factors and strategies for control of carcinoma cervix in India: Hospital based cytological screening experience of 35 years. Indian J Cancer. 2009;46:155–9. doi: 10.4103/0019-509x.49155. [DOI] [PubMed] [Google Scholar]
- 37.Rajput N, Verma YS, Ahirwar G. Detection of abnormal cervical cytology by Pap smear and comparison between rural and urban women. J Evol Med Dent Sci. 2013;2:7923–30. [Google Scholar]
- 38.Srivastava M, Srivastava OP, Jaiswal SS. Pattern of cervical smear cytology in rural medical college. Pravara Med Rev. 2011;3:4–8. [Google Scholar]
- 39.Ambedkar RKV, Srinivasmurthi BC, Srinivasamurthy BC, Balamurugan M. Clinicopatological significance of Pappinocalaou smears study of post-menopausal women in a rural tertiary care centre. Clin Cancer Investig. 2015;4:147–51. [Google Scholar]
- 40.Parimala A, Sharma N, Srinivasan JK. Screening of cancer cervix: Pap smear in rural India. Int J Reprod Contracept Obstet Gynecol. 2016;5:2113–5. [Google Scholar]
- 41.Verma A, Verma S, Vashist S, Attri S, Singhal A. A study on cervical cancer screening in symptomatic women using Pap smear in a tertiary care hospital in rural area of Himachal Pradesh, India. Middle East Fertil Soc J. 2017;22:39–42. [Google Scholar]
- 42.Khasnabish S, Chakraborty R, Chakraborty D, Hati GC. Study of cervical pap smear study and its utility in cancer screening – An experience in a tertiary care hospital of Tripura, North Eastern state of India. J Evid Based Med Healthc. 2017;4:2936–9. [Google Scholar]
- 43.Nene B, Jayant K, Arrossi S, Shastri S, Budukh A, Hingmire S, et al. Determinants of womens participation in cervical cancer screening trial, Maharashtra, India. Bull World Health Organ. 2007;85:264–72. doi: 10.2471/BLT.06.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gravitt PE, Paul P, Katki HA, Vendantham H, Ramakrishna G, Sudula M, et al. Effectiveness of VIA, pap, and HPV DNA testing in a cervical cancer screening program in a peri-urban community in Andhra Pradesh, India. PLoS One. 2010;5:e13711. doi: 10.1371/journal.pone.0013711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kailash U, Hedau S, Gopalkrishna V, Katiyar S, Das BC. A simple ‘paper smear’ method for dry collection, transport and storage of cervical cytological specimens for rapid screening of HPV infection by PCR. J Med Microbiol. 2002;51:606–10. doi: 10.1099/0022-1317-51-7-606. [DOI] [PubMed] [Google Scholar]
- 46.Deodhar K, Gheit T, Vaccarella S, Romao CC, Tenet V, Nene BM, et al. Prevalence of human papillomavirus types in cervical lesions from women in rural Western India. J Med Virol. 2012;84:1054–60. doi: 10.1002/jmv.23310. [DOI] [PubMed] [Google Scholar]
- 47.Labani S, Asthana S, Sodhani P, Gupta S, Bhambhani S, Pooja B, et al. CareHPV cervical cancer screening demonstration in a rural population of North India. Eur J Obstet Gynecol Reprod Biol. 2014;176:75–9. doi: 10.1016/j.ejogrb.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Mittal S, Mandal R, Banerjee D, Das P, Ghosh I, Panda C, et al. HPV detection-based cervical cancer screening program in low-resource setting: Lessons learnt from a community-based demonstration project in India. Cancer Causes Control. 2016;27:351–8. doi: 10.1007/s10552-015-0708-z. [DOI] [PubMed] [Google Scholar]
- 49.Bobdey S, Sathwara J, Jain A, Balasubramaniam G. Burden of cervical cancer and role of screening in India. Indian J Med Paediatr Oncol. 2016;37:278–85. doi: 10.4103/0971-5851.195751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thumoju S, Mohiuddin MK, Sirumalla C, Rani VU, Salma M, Ahuja YR. Cervical cancer screening program in Hyderabad and surrounding peri-urban areas, South India: Prevalence of high risk HPV subtypes. Am J Cancer Biol. 2016;4:6–15. [Google Scholar]
- 51.Peedicayil A, Abraham P, Prasad L, Jeyaseelan S, Abraham S, Kurian P, et al. Community prevalence of human pappiloma virus by self collected samples in South India. Indian J Gynecol Oncol. 2016;14:16. [Google Scholar]
- 52.Sharma K, Kathait A, Jain A, Kujur K, Raghuwanshi S, Bharti AC, et al. Higher prevalence of human papillomavirus infection in adolescent and young adult girls belonging to different Indian tribes with varied socio-sexual lifestyle. PLoS One. 2015;10:e0125693. doi: 10.1371/journal.pone.0125693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarkar K, Pal R, Bal B, Saha B, Bhattacharya S, Sengupta S, et al. Oncogenic HPV among HIV infected female population in West Bengal, India. BMC Infect Dis. 2011;11:72. doi: 10.1186/1471-2334-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chatterjee S, Chattopadhyay A, Samanta L, Panigrahi P. HPV and cervical cancer epidemiology - Current status of HPV vaccination in India. Asian Pac J Cancer Prev. 2016;17:3663–73. [PubMed] [Google Scholar]
- 55.World Health Organization. WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. [accessed on December 28, 2016]. Available from: https://apps.who.int/iris/bitstream/handle/10665/94830/9789241548694_eng.pdf;jsessionid=3386CA30BC67F30495F9114CC9E0C653?sequence=1 . [PubMed]
- 56.World Health Organization. Geneva: WHO; 1996. [accessed on December 28, 2016]. The World health report: 1996: fighting disease, fostering development/report of the Director- General. Available from: http://www.who.int/iris/handle/10665/36848 . [Google Scholar]
- 57.Misra JS, Srivastava AN, Das V. Single life time cytological screening in high risk women as an economical and feasible approach to control cervical cancer in developing countries like India. Asian Pac J Cancer Prev. 2015;16:859–62. doi: 10.7314/apjcp.2015.16.3.859. [DOI] [PubMed] [Google Scholar]
- 58.Misra JS, Das V, Srivastava AN, Singh U, Singh M. AgNOR counts in cervical smears under normal and other cytopathologic conditions. Anal Quant Cytol Histol. 2005;27:337–40. [PubMed] [Google Scholar]
- 59.Srivastava A, Srivastava S, Bansal C, Misra J. Diagnostic importance of AgNOR pleomorphism in cervical carcinogenesis. Ecancermedicalscience. 2013;7:287. doi: 10.3332/ecancer.2013.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Misra JS, Chhavi, Singh M, Srivastava AN, Das V. Assessment of potential of AgNOR counts as tumor marker in cervical carcinogenesis. Diagn Cytopathol. 2008;36:194–5. doi: 10.1002/dc.20718. [DOI] [PubMed] [Google Scholar]