Abstract
Background & objectives:
The frequency and predictors of pancreatitis in primary hyperparathyroidism (PHPT) are not well understood. The objective of the present study was to evaluate the frequency of pancreatitis in patients with PHPT and its association with clinical and biochemical parameters of the disease.
Methods:
In this retrospective study all consecutive patients with PHPT registered in the PHPT registry (www.indianphptregistry.com) from the year 2004 to 2013 were included. The clinical, biochemical and radiological parameters related to pancreatitis were evaluated in histologically proven PHPT patients.
Results:
A total of 218 patients (63 men; mean age: 40.6±14.4 yr) underwent surgery for PHPT during the study. Pancreatitis occurred in 35 [16%, 18 acute and 17 chronic pancreatitis (CP)] patients and male:female ratio was 1:0.94. Skeletal manifestations were seen less frequently in PHPT with pancreatitis as compared to that of PHPT without pancreatitis. PHPT with pancreatitis had significantly higher serum calcium (12.4±2.0 vs. 11.7±1.5 mg/dl, P<0.05) in comparison to PHPT without pancreatitis. PHPT with acute pancreatitis (AP) had higher serum calcium (P<0.05) and parathyroid hormone (PTH) (P<0.05) levels than PHPT with CP. Curative parathyroidectomy improved the symptoms associated with pancreatitis as there was no recurrence in AP group, whereas recurrence was observed only in about 10 per cent patients of the CP group.
Interpretation & conclusions:
Pancreatitis was observed in 16 per cent of PHPT patients with male predominance in the study population. No recurrence of AP was observed after curative surgery. It may be proposed that serum amylase with calcium and PTH should be measured in all patients of PHPT with pain abdomen to rule out pancreatitis.
Keywords: Bone, hypercalcaemia, pain abdomen, pancreatitis, primary hyperparathyroidism
Primary hyperparathyroidism (PHPT) is characterized by inappropriately elevated parathyroid hormone (PTH) level with hypercalcaemia. The classical PHPT was described as systemic disease involving bone, kidney, gastrointestinal tracts, nervous and cardiovascular systems1. The symptomatic form of PHPT is prevalent in developing countries such as India and China, but uncommon in developed countries2,3,4,5,6.
Pancreatitis in PHPT patients have been mostly reported in the form of case reports and case series and occasionally original research article7,8,9,10,11. However, the causal association between pancreatitis and PHPT remains controversial. Both acute and chronic forms of pancreatitis have been reported in PHPT patients8,10,12. The prevalence of pancreatitis was found to be higher in PHPT patients as compared to general inpatient population10. In PHPT, studies from the western population have shown the prevalence of pancreatitis ranging from 3.2 to 8.1 per cent with predominantly acute pancreatitis (AP) cases13,14,15. Indian studies have reported a higher prevalence of pancreatitis in PHPT ranging from 6.8 to 13 per cent with almost equal number of chronic and AP cases7,8,10,16,17,18.
The purpose of the present retrospective study was to understand the frequency and predictors of pancreatitis in PHPT and also to differentiate patients with PHPT with pancreatitis from PHPT without pancreatitis on the basis of their clinical and biochemical parameters and response after curative parathyroidectomy.
Material & Methods
Data of the patients were retrieved from PHPT registry of the department of Endocrinology, Post-graduate Institute of Medical Education and Research, Chandigarh, India. All patients with histopathologically proven PHPT, from January 2004 to December 2013 were included in the study. The clinical and biochemical details of all the PHPT patients are also maintained in this user-based Indian PHPT registry programme (www.indianphptregistry.com). The diagnosis of PHPT was based on inappropriately elevated PTH despite elevated corrected serum calcium level. Preoperatively, parathyroid tumour was localized using ultrasound of the neck and sestamibi scintigraphy. Patients with secondary or tertiary hyperparathyroidism were excluded from the study. Cure of PHPT was defined as >50 per cent reduction in PTH (between the 3rd and 7th post-operative day) with normalization of serum calcium. If immediate post-operative PTH was higher, PTH was repeated at three months; and if PTH level was normalized by then, these patients were considered as cured.
The diagnosis of AP was made on the basis of the presence of two of the following three findings: abdominal pain consistent with AP (duration <6 months), elevation in serum amylase or lipase more than three times the upper limit of normal reference range (RR) and radiological evidence of AP19.
The diagnosis of chronic pancreatitis (CP) was made on the basis of clinical and radiological investigations. A thorough diagnostic evaluation was done in patients with chronic abdominal pain (duration >6 months), and the diagnosis of CP was established if there was evidence of pancreatic calcification on abdominal X-ray and/or ultrasonography and/or abdominal computed tomography and/or there were characteristic ductal changes on magnetic resonance cholangiopancreatography and/or endoscopic retrograde cholangiopancreatography or endoscopic ultrasound showed features consistent with the diagnosis of CP20.
A detailed history specifically assessing the family history, alcohol consumption and the presence and severity of abdominal pain was recorded. The absence of alcohol consumption was confirmed by repeated interviews of the patient as well as of the family members. A diagnosis of alcohol-induced CP was established in patients with features of CP who had consumed >50 g/day of alcohol for at least five years. The patients with hereditary pancreatitis (as determined by family history), and alcohol-induced pancreatitis were excluded from the study21,22.
The clinical and biochemical parameters of PHPT patients with pancreatitis were compared with PHPT patients without pancreatitis. Further, PHPT patients with AP (PHPT-AP) were compared with CP (PHPT-CP) patients. The study protocol was approved by the ethics committee of the institute.
Biochemical and hormonal investigations: Serum calcium (RR 8.6-10.2 mg/dl), inorganic phosphate (RR, 2.7-4.5 mg/dl), albumin (RR, 3.4-4.8 mg/dl), alkaline phosphatase (RR, 40-129 IU/l), creatinine (RR, 0.6-1.3 mg/dl) and amylase (RR, 28-100 U/l) were measured using autoanalyzer (Roche Diagnostics, Modular P 800, Indianpolis, USA). Plasma intact PTH (iPTH) (RR, 15-65 pg/ml) and 25(OH) D (RR, 11.1-42.9 ng/ml) were measured by the second-generation electrochemiluminescence immunoassay (Roche ELESYS, 2010, USA). Anaemia was defined as a haemoglobin level <13 g/dl for male and <12 g/dl for female. Vitamin D deficiency was defined as 25(OH) D level <20 ng/ml.
Statistical analysis: Statistical analysis was carried out using GraphPad Prism version 5 (GraphPad Software Inc., California, USA). Data are expressed as mean±standard deviation unless specified. The Student's t test for normally distributed data otherwise the Mann-Whitney U-test was used for comparing continuous variables and Chi-square test was performed for comparison between categorical variables. Binary logistic regression analysis was performed to calculate odds ratio (OR) for the development of pancreatitis in relation to high calcium and elevated PTH levels.
Results
A total of 218 symptomatic PHPT patients were included in the study. Detailed demography, clinical, biochemical and histopathological findings of patients with PHPT are given in Table I. The major clinical manifestations of PHPT were weakness and easy fatigability, followed by anaemia, bone pain, nephrolithiasis and pain abdomen. Bone pain, anaemia and gall stones were more frequent in the female, and pancreatitis was more common in male PHPT patients. Pre-operative, serum calcium and plasma iPTH were 11.7±1.6 mg/dl and 744.4±755.4 pg/ml (3-4328 pg/ml), respectively. A total of 139 (63.8%) PHPT patients were vitamin D deficient (25(OH) D level <20 ng/ml) with mean 25(OH) D level was 26.8±31.4 ng/ml.
Table I.
Demographics, clinical, biochemical and histopathological findings of primary hyperparathyroidism patients (n=218)
| Parameters | Values |
|---|---|
| Demographics | |
| Age (mean) yr | |
| Male | 36.7±15.2 |
| Female | 41.9±13.7 |
| Sex (female:male) | 2.47:1 (female 155, male 63) |
| Clinical features (%) | |
| Weakness and fatigue | 133 (61) |
| Bone pain | 115 (52.7) |
| Fracture | 61 (28) |
| Nephrolithiasis | 80 (37.7) |
| Nephrocalcniosis | 64 (29.3) |
| Pain abdomen | 67 (30.9) |
| Anaemia | 116 (53.2) |
| Nausea and vomiting | 61 (28) |
| Gall stones/cholecystectomy | 34 (15.6) |
| Anorexia | 66 (30.3) |
| Psychiatry abnormalities | 44 (20.2) |
| Pancreatitis | 35 (16) |
| Palpable nodules | 28 (12.8) |
| Biochemistry | |
| Calcium (mg/dl) | 11.7±1.6 (8.5-16.3) |
| iPTH (pg/ml) | 744.4±755.4 (3-4348) |
| Inorganic phosphate (mg/dl) | 3.0±1.1 (1.19-10) |
| Alkaline phosphatase (IU/l) | 301.0±492.1 (53-4238) |
| 25(OH) vitamin D (ng/ml) | 26.8±31.4 (3-120) |
| Creatinine (mg/dl) | 1.3±1.5 (0.1-9.0) |
| Pathology | |
| Tumour weight (g) | 5.67±6.37 (0.1-38.4) |
| Type | Adenoma=96.3% (n=210), hyperplasia=3.2% (n=7), carcinoma=0.5% (n=1) |
iPTH, intact parathyroid hormone
Pancreatitis in primary hyperparathyroidism
Demographics: Thirty five (16%) PHPT patients had co-existing pancreatitis. Eighteen (51.4%) of these patients had AP and 17 (48.6%) had CP. There were more female patients in the PHPT patients group without pancreatitis (75.4 vs. 24.6%), the male had a higher proportion in PHPT patients with pancreatitis (51.4 vs. 48.6%) (P=0.0013). No age difference was observed between PHPT patients with and without pancreatitis, but male patients in PHPT with pancreatitis group were comparatively younger than female patients (34.8±13.8 vs. 44.6±12.7 yr, P<0.05). The incidence of pancreatitis in PHPT patients decreased despite the progressive increase in the total number of PHPT cases over 10 years. The proportion of pancreatitis cases in PHPT patients in the first five years (2004-2008) was significantly higher (20 out of 79 patients, 24.4%) than the past five years (2009-2013) (15 out of 139 patients, 11.1%) (P<0.05) (Figure). There was a single patient of parathyroid carcinoma who developed pancreatitis. None of the patients with parathyroid hyperplasia developed pancreatitis.
Figure.
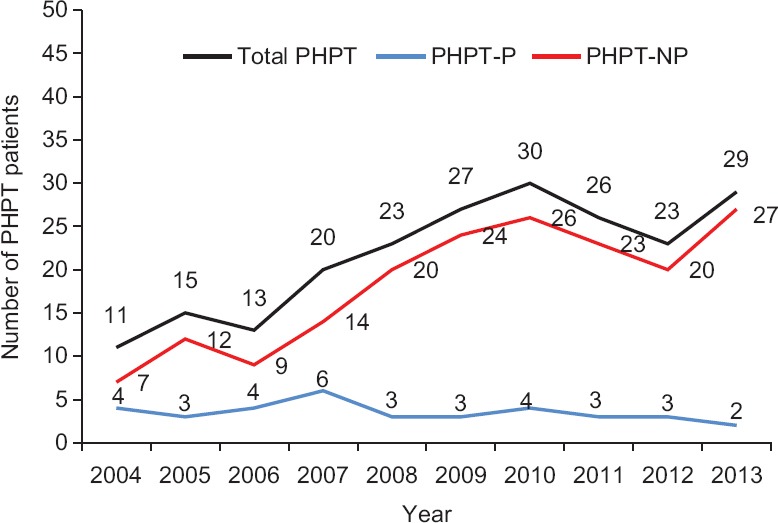
Year-wise distribution of number of total primary hyperparathyroidism patients (PHPT), primary hyperparathyroidism-with pancreatitis (PHPT-P) and with no pancreatitis (PHPT-NP).
Clinical features: Abdominal pain, nausea, vomiting and weakness and easy fatigability were the dominant clinical manifestations in PHPT patients with pancreatitis. Weight loss was observed in 14 (40%) patients with pancreatitis. Detailed comparative clinical data are given in Table II. Skeletal manifestations were less frequent in PHPT with pancreatitis group as compared to that of PHPT without pancreatitis (P<0.01). Bone pain and fracture were present in only nine (25.7%) and two (5.7%) cases of PHPT with pancreatitis, respectively. Eight patients (22.8%, 7 females and 1 male) of PHPT with pancreatitis had concurrent gall stone disease but underwent cholecystectomy before the development of pancreatitis. Nephrolithiasis and nephrocalcinosis were observed in 14 (40%) and 11 (31.4%) patients of PHPT with pancreatitis, respectively. Seven (20%) PHPT with pancreatitis patients had diabetes mellitus. There was no significant difference in the clinical manifestations between genders. The single patient with parathyroid carcinoma had almost all the symptoms of AP.
Table II.
Comparison of clinical and biochemical parameters of primary hyperparathyroidism with pancreatitis with non-pancreatitis primary hyperparathyroidism patients
| Symptoms | PHPT-NP (n=183) | PHPT-P (n=35) | P |
|---|---|---|---|
| Age (yr) | 40.6±14.5 (11-74) | 40.5±13.7 (18-64) | 0.964 |
| Sex (female:male) | 3.06:1 | 0.94:1 | 0.0013** (OR=3.25, 95% CI=1.54-6.83) |
| Bone pain, n (%) | 106 (57.9) | 9 (25.7) | 0.0005*** (OR=0.25, 95% CI=0.11-0.57) |
| Fracture, n (%) | 59 (32.2) | 2 (5.7) | 0.0014** (OR=0.13, 95% CI=0.03-0.55) |
| Weight loss, n (%) | 45 (26.2) | 14 (40) | 0.098 (OR=1.87, 95% CI=0.88-3.98) |
| Weakness and fatigue, n (%) | 112 (61.2) | 21 (63.2) | 0.893 (OR=0.95, 95% CI=0.45-1.99) |
| Anorexia, n (%) | 56 (28.4) | 10 (28.6) | 0.811 (OR=0.91, 95% CI=0.41-2.01) |
| Nausea and vomiting, n (%) | 38 (20.8) | 23 (65.7) | <0.001*** (OR=7.31, 95% CI=3.34-16.02) |
| Abdominal pain, n (%) | 35 (19.1) | 32 (91.4) | <0.001*** (OR=45.1, 95% CI=13.06-155.8) |
| Psychiatric abnormalities, n (%) | 34 (18.6) | 10 (28.6) | 0.177 (OR=1.75, 95% CI=0.77-3.99) |
| Nephrolithiasis, n (%) | 66 (36.1) | 14 (40) | 0.658 (OR=1.18, 95% CI=0.56-2.48) |
| Nephrocalcinosis, n (%) | 53 (29) | 11 (31.4) | 0.769 (OR=1.12, 95% CI=0.51-2.46) |
| Gall stone, n (%) | 26 (14) | 8 (22.8) | 0.196 (OR=1.79, 95% CI=0.73-4.36) |
| Calcium (mg/dl) | 11.7±1.5 (8.5-15.9) | 12.4±2.0 (9.6-16.3) | 0.011* |
| iPTH (pg/ml) | 795.5±786.6 (69.29-4328) | 526.1±552.8 (3-3000) | 0.061# |
| Inorganic phosphate (mg/dl) | 3.0±1.2 (1.19-10) | 3.0±1.1 (1.6-8.0) | 0.943# |
| Alkaline phosphatase (IU/l) | 364.3±536.3 (64-4238) | 237.2.0±268.2 (53-1166) | 0.267# |
| 25(OH) vitamin D (ng/ml) | 26.1±25 (3-120) | 20.3±17.8 (3-81) | 0.142# |
| Creatinine (mg/dl) | 1.4±1.2 (0.2-9.0) | 1.0±0.6 (0.1-3.8) | 0.12# |
P *<0.05, **<0.01, ***<0.001, #Mann-Whitney U-test was applied for comparison of the data. OR with 95% CI is provided for the categorical data. OR, odds ratio; CI, confidence interval; iPTH, intact parathyroid hormone; PHPT-P, primary hyperparathyroidism with pancreatitis; PHPT-NP, primary hyperparathyroidism with non-pancreatitis
Biochemical parameters: Comparative biochemistry of PHPT patients with and without pancreatitis is given in Table II. Mean serum amylase level in PHPT patients with pancreatitis was 449.4±466.8 U/l (58-2098 U/l). Serum calcium level in PHPT with pancreatitis was significantly higher than the patients having PHPT without pancreatitis (12.4±2.0 mg/dl vs. 11.7±1.5 mg/dl, P<0.011).
Comparison of primary hyperparathyroidism with acute pancreatitis versus primary hyperparathyroidism with chronic pancreatitis: Eight male and nine female patients were in PHPT-CP group, whereas there were 10 male and eight female patients in PHPT-AP group. No age difference was observed between these groups. Duration of pain abdomen in PHPT-AP patients varied from 10 days to six months with a number of episodes of pain abdomen experienced by each patient varied from 1 to 3. Three patients developed pancreatitis after hospitalization, three patients had two episodes, two patients had three episodes and 10 had minimum one episode of pancreatitis before hospitalization. Pancreatic calcification and diabetes mellitus were observed in eight (47.2%) and five (29.4%) of PHPT-CP patients, respectively. The PHPT-AP group had a significantly higher number of patients with bone problems (44.4%, P<0.01) as compared to the PHPT-CP group. Other clinical features of PHPT were comparable between both the groups.
Both plasma iPTH (731.1±680.3 vs. 280.2±133 pg/ml, P<0.05) and serum calcium level (13.13±0.47 mg/dl vs. 11.67±0.41 mg/dl, P<0.05) were significantly higher in the PHPT-AP group than the PHPT-CP group. Other biochemical parameters were comparable between the two groups (Table III).
Table III.
Primary hyperparathyroidism patients with chronic pancreatitis vs. acute pancreatitis
| Parameters | PHPT-AP (n=18), n (%) | PHPT-CP (n=17), n (%) |
|---|---|---|
| Male/female | 10/8 | 8/9 |
| Pancreatic calcification | 0 | 8 (47) |
| Diabetes mellitus | 2 (11) | 5 (29.4) |
| Pain abdomen | 15 (83.3) | 17 (100) |
| Nausea and vomiting | 13 (72.2) | 10 (58.8) |
| Weight loss | 5 (27.8) | 9 (52.9) |
| Bone disease | 8 (44.4) | 1 (5.9)*** |
| Nephrolithiasis | 6 (33.3) | 8 (47) |
| Nephrocalcinosis | 6 (33.3) | 5 (29.4) |
| Cholecystectomy | 2 (11.1) | 6 (35.3) |
| Serum calcium (mg/dl) | 13.13±2.0 | 11.67±1.69* |
| Inorganic phosphate (mg/dl) | 2.87±0.55 | 3.12±1.4# |
| iPTH (pg/ml) | 731.1±680.3 | 280.2±133*,# |
| 25(OH) vitamin D (ng/ml) | 24.7±23.1 | 16.8±12.2# |
| Creatinine (mg/dl) | 0.8±0.4 | 1.1±0.8# |
P *<0.05, ***<0.001, #Mann-Whitney U-test was applied for the analysis. iPTH, intact parathyroid hormone; PHPT-AP, primary hyperparathyroidism patients with acute pancreatitis; PHPT-CP, primary hyperparathyroidism patients with chronic pancreatitis
Effect of parathyroidectomy on pancreatitis: All the patients were cured of PHPT after parathyroidectomy. Significant improvement was observed in post-operative mean serum calcium (9.1±1.2 mg/dl, P<0.001) and iPTH levels (68.4±68.9 pg/ml, P<0.001) compared to the pre-operative levels. Post-operative mean phosphate level was 2.9±1.1 mg/dl. The mean follow up of the PHPT patients after curative parathyroidectomy was 3.2 yr (6 months - 4 yr). There was no recurrence of symptoms in the PHPT-AP group; whereas, only two patients (11.7%) PHPT-CP group had persistent symptoms of pancreatitis after curative parathyroidectomy.
Predictors of pancreatitis in primary hyperparathyroidism: Binary logistic regression analysis was performed for the outcome of pancreatitis within PHPT. By using binary logistic regression analysis, high serum calcium (OR=1.26) [1.015-1.559, 95% confidence interval (CI) P=0.036] was found to be a risk factor for pancreatitis. However, high PTH level [OR=1.004 (1.001-1.008, 95% CI) P=0.013] was found to be a risk factor for bone diseases in PHPT patients with pancreatitis. Further, comparison of the PHPT-AP group with the PHPT-CP group revealed that high PTH level [OR=1.0004, (1.0001-1.0008, 95% CI) P=0.013], not hypercalcaemia [OR=0.66, (0.35-1.24, 95% CI), P=0.19] was able to differentiate the PHPT-AP group from the PHPT-CP group.
Discussion
In this study, 16 per cent of PHPT patients had pancreatitis; of these, 51.4 per cent have AP at the time of diagnosis. The studies from the western population have shown 3.2-8.1 per cent prevalence of pancreatitis in patients with PHPT, but Indian studies have reported higher prevalence (6.8-13%)7,10,12,13. Delay in diagnosis as well as variation in the clinical presentation (symptomatic vs. asymptomatic) are the possible explanation for the two-fold higher prevalence of pancreatitis in patients with PHPT in our and other studies from India. In this study, 51.4 per cent patients of PHPT with pancreatitis were male, which was in accordance with previous studies9,12,23. In a community-based study by Khoo et al24, it has been shown that male:female ratio in PHPT with pancreatitis was similar to that of PHPT without pancreatitis patients (70% females, 30% males). No gender difference was observed between PHPT patients affected with acute and CP in this study. Gender-wise representation of AP and CP in PHPT was not reported previously. Abdominal pain was the most common (91.4%) clinical manifestation of PHPT with pancreatitis in our study and the same was reported by others as well8,10,25.
Skeletal manifestations such as bone pain and fracture were observed in less number of PHPT patients with pancreatitis; however, in PHPT patients without pancreatitis bone involvements was the major clinical manifestation. Other studies have also reported a low frequency of bone disease in PHPT with pancreatitis patients10,13. Further, skeletal problems were lower in the PHPT-CP group in our study. Serum calcium was significantly higher in the PHPT with pancreatitis group. This finding was in accordance with previous studies10,12. When we compared the calcium level between the PHPT-CP and PHPT-AP group, serum calcium was significantly higher with increased PTH level in the PHPT-AP group. Thus, more severe hypercalcaemia was associated with the acute presentation of pancreatitis. Severe hypercalcaemia would have precipitated pancreatitis in PHPT patients which brought these patients earlier to physician resulting in early case detection and less time for bone involvement. For CP group less severe disease (lower calcium and PTH as compared to PHPT-AP group) may be the possible cause for the lesser bone problem. The PTH levels of both the PHPT-AP and the PHPT-NP group were comparable and higher to the PTH level of the PHPT-CP group.
All the PHPT patients with pancreatitis underwent parathyroidectomy. After curative parathyroidectomy, none of the PHPT-AP group patients showed recurrence of pancreatitis. Only two PHPT-CP patients showed persistent symptoms of pancreatitis after three months of the follow up. Other studies have also reported a recurrence of pancreatitis after curative parathyroidectomy13,26. Carnaille et al12 also reported that none of PHPT patients with AP had a recurrence of symptoms, but six out of 22 (27.3%) subacute or CP patients had persistent symptoms of pancreatitis.
Hypercalcaemia from any source acts as a risk factor of pancreatitis. Experimental studies have shown that hypercalcaemia leads to the formation of pancreatic calculi resulting in ductal obstruction, and pancreatitis27. Hypercalcaemia can also activate trypsinogen to trypsin conversion that results into autodigestion of the pancreas and subsequent pancreatitis28. Felderbauer et al23,29 demonstrated that PHPT patients with AP had greater frequency of mutations in serine protease inhibitor kazal type 1, cystic fibrosis transmembrane conductance regulator, and chymotrypsin C genes. Hence, hypercalcaemia, as well as genetic factors may be implicated in the pathogenesis of pancreatitis in PHPT. Further studies are needed considering genomics and proteomics approach to elucidate the effect of hypercalcaemia on acute or CP in PHPT30.
In conclusion, the frequency of pancreatitis in PHPT was 16 per cent in the study population with almost equal number of acute and CP cases. There was significantly higher serum calcium in the patients having PHPT with pancreatitis as compared to those having PHPT without pancreatitis. Marked improvement in pancreatitis related symptoms was seen after curative parathyroidectomy. Hence, it is proposed that all the patients of PHPT with pain abdomen (present or past history) may undergo serum amylase with serum calcium and PTH estimation to rule out the possibility of pancreatitis.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Fraser WD. Hyperparathyroidism. Lancet. 2009;374:145–58. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 2.Shah VN, Bhadada S, Bhansali A, Behera A, Mittal BR. Changes in clinical & biochemical presentations of primary hyperparathyroidism in India over a period of 20 years. Indian J Med Res. 2014;139:694–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao L, Liu JM, He XY, Zhao HY, Sun LH, Tao B, et al. The changing clinical patterns of primary hyperparathyroidism in Chinese patients: Data from 2000 to 2010 in a single clinical center. J Clin Endocrinol Metab. 2013;98:721–8. doi: 10.1210/jc.2012-2914. [DOI] [PubMed] [Google Scholar]
- 4.Shah VN, Bhadada SK, Bhansali A, Behera A, Mittal BR, Bhavin V. Influence of age and gender on presentation of symptomatic primary hyperparathyroidism. J Postgrad Med. 2012;58:107–11. doi: 10.4103/0022-3859.97171. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg SJ, Clarke BL, Peacock M, Bandeira F, Boutroy S, Cusano NE, et al. Current issues in the presentation of asymptomatic primary hyperparathyroidism: Proceedings of the fourth international workshop. J Clin Endocrinol Metab. 2014;99:3580–94. doi: 10.1210/jc.2014-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhadada SK, Arya AK, Mukhopadhyay S, Khadgawat R, Sukumar S, Lodha S, et al. Primary hyperparathyroidism: Insights from the Indian PHPT registry. J Bone Miner Metab. 2018;36:238–45. doi: 10.1007/s00774-017-0833-8. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal A, George RK, Gupta SK, Mishra SK. Pancreatitis in patients with primary hyperparathyroidism. Indian J Gastroenterol. 2003;22:224–5. [PubMed] [Google Scholar]
- 8.Bhadada SK, Udawat HP, Bhansali A, Rana SS, Sinha SK, Bhasin DK. Chronic pancreatitis in primary hyperparathyroidism: Comparison with alcoholic and idiopathic chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:959–64. doi: 10.1111/j.1440-1746.2007.05050.x. [DOI] [PubMed] [Google Scholar]
- 9.Gupta AK, Madnani M, Mistry J, Soni H, Shah A, Patel KS, et al. Primary hyperparathyroidism with pancreatitis: Experience of management in 5 patients with review of literature. Indian J Gastroenterol. 2014;33:484–6. doi: 10.1007/s12664-014-0470-2. [DOI] [PubMed] [Google Scholar]
- 10.Jacob JJ, John M, Thomas N, Chacko A, Cherian R, Selvan B, et al. Does hyperparathyroidism cause pancreatitis? A South Indian experience and a review of published work. ANZ J Surg. 2006;76:740–4. doi: 10.1111/j.1445-2197.2006.03845.x. [DOI] [PubMed] [Google Scholar]
- 11.Lenz JI, Jacobs JM, Op de Beeck B, Huyghe IA, Pelckmans PA, Moreels TG. Acute necrotizing pancreatitis as first manifestation of primary hyperparathyroidism. World J Gastroenterol. 2010;16:2959–62. doi: 10.3748/wjg.v16.i23.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carnaille B, Oudar C, Pattou F, Combemale F, Rocha J, Proye C. Pancreatitis and primary hyperparathyroidism: Forty cases. Aust N Z J Surg. 1998;68:117–9. doi: 10.1111/j.1445-2197.1998.tb04719.x. [DOI] [PubMed] [Google Scholar]
- 13.Sitges-Serra A, Alonso M, de Lecea C, Gores PF, Sutherland DE. Pancreatitis and hyperparathyroidism. Br J Surg. 1988;75:158–60. doi: 10.1002/bjs.1800750224. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd JJ. Hyperparathyroidism presenting as pancreatitis or complicated by postoperative pancreatitis. Aust N Z J Surg. 1996;66:85–7. doi: 10.1111/j.1445-2197.1996.tb01117.x. [DOI] [PubMed] [Google Scholar]
- 15.Koppelberg T, Bartsch D, Printz H, Hasse C, Rothmund M. Pancreatitis in primary hyperparathyroidism (pHPT) is a complication of advanced pHPT. Dtsch Med Wochenschr. 1994;119:719–24. doi: 10.1055/s-2008-1058752. [DOI] [PubMed] [Google Scholar]
- 16.Shah VN, Bhadada SK, Bhansali A, Behera A, Bhattacharya A, Nahar U, et al. Effect of gender, biochemical parameters & parathyroid surgery on gastrointestinal manifestations of symptomatic primary hyperparathyroidism. Indian J Med Res. 2014;139:279–84. [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury SD, Kurien RT, Pal S, Jeyaraj V, Joseph AJ, Dutta AK, et al. Acute pancreatitis and hyperparathyroidism: A case series. Indian J Gastroenterol. 2014;33:175–7. doi: 10.1007/s12664-013-0430-2. [DOI] [PubMed] [Google Scholar]
- 18.Singh DN, Gupta SK, Kumari N, Krishnani N, Chand G, Mishra A, et al. Primary hyperparathyroidism presenting as hypercalcemic crisis: Twenty-year experience. Indian J Endocrinol Metab. 2015;19:100–5. doi: 10.4103/2230-8210.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banks PA, Freeman ML Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 20.Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184–97. doi: 10.1016/S0140-6736(10)61852-1. [DOI] [PubMed] [Google Scholar]
- 21.Chari ST, Mohan V, Jayanthi V, Snehalatha C, Malathi S, Viswanathan M, et al. Comparative study of the clinical profiles of alcoholic chronic pancreatitis and tropical chronic pancreatitis in Tamil Nadu, South India. Pancreas. 1992;7:52–8. doi: 10.1097/00006676-199201000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Gabbrielli A, Mutignani M, Pandolfi M, Perri V, Costamagna G. Endotherapy of early onset idiopathic chronic pancreatitis: Results with long-term follow-up. Gastrointest Endosc. 2002;55:488–93. doi: 10.1067/mge.2002.122651. [DOI] [PubMed] [Google Scholar]
- 23.Felderbauer P, Karakas E, Fendrich V, Bulut K, Horn T, Lebert R, et al. Pancreatitis risk in primary hyperparathyroidism: Relation to mutations in the SPINK1 trypsin inhibitor (N34S) and the cystic fibrosis gene. Am J Gastroenterol. 2008;103:368–74. doi: 10.1111/j.1572-0241.2007.01695.x. [DOI] [PubMed] [Google Scholar]
- 24.Khoo TK, Vege SS, Abu-Lebdeh HS, Ryu E, Nadeem S, Wermers RA. Acute pancreatitis in primary hyperparathyroidism: A population-based study. J Clin Endocrinol Metab. 2009;94:2115–8. doi: 10.1210/jc.2008-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padma S, Sundaram PS. Parathyroid scintigraphy, histopathology correlation in patients with tropical pancreatitis and coexisting primary hyperparathyroidism. Indian J Nucl Med. 2013;28:5–10. doi: 10.4103/0972-3919.116796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer S, Ammann R. Chronic pancreatitis and primary hyperparathyroidism. Schweiz Med Wochenschr. 1994;124:1344–8. [PubMed] [Google Scholar]
- 27.Mithöfer K, Fernández-del Castillo C, Frick TW, Lewandrowski KB, Rattner DW, Warshaw AL. Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology. 1995;109:239–46. doi: 10.1016/0016-5085(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 28.Frick TW, Fernández-del Castillo C, Bimmler D, Warshaw AL. Elevated calcium and activation of trypsinogen in rat pancreatic acini. Gut. 1997;41:339–43. doi: 10.1136/gut.41.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felderbauer P, Karakas E, Fendrich V, Lebert R, Bartsch DK, Bulut K, et al. Multifactorial genesis of pancreatitis in primary hyperparathyroidism: Evidence for “protective” (PRSS2) and “destructive” (CTRC) genetic factors. Exp Clin Endocrinol Diabetes. 2011;119:26–9. doi: 10.1055/s-0030-1255106. [DOI] [PubMed] [Google Scholar]
- 30.Varshney S, Bhadada SK, Arya AK, Sharma S, Behera A, Bhansali A, et al. Changes in parathyroid proteome in patients with primary hyperparathyroidism due to sporadic parathyroid adenomas. Clin Endocrinol (Oxf) 2014;81:614–20. doi: 10.1111/cen.12479. [DOI] [PubMed] [Google Scholar]


