Abstract
Background & objectives:
Bisphenol-A (BPA) and phthalates are utilized widely in consumer products. Due to their ubiquitous presence in the environment, a concern is expressed worldwide about their possible effect on human reproductive health. This study was conducted to compare the internal exposure of BPA and phthalates (using their metabolites as biomarkers) in plasma samples of infertile and fertile women.
Methods:
A sensitive gas chromatographic-mass spectrometric (GC-MS) method was developed to simultaneously quantify BPA and four phthalate monoester metabolites [namely mono-methyl phthalate (MMP), mono-benzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP) and mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP)] in human plasma. The method was validated using charcoal-stripped human plasma. Activated charcoal was also utilized to reduce contamination from reagents. The method was designed to account for and/or eliminate background contamination from all sources.
Results:
The limit of quantification for the method was 5 ng/ml for MMP and MBzP, while 1 ng/ml for BPA, MEHP and MEHHP, respectively. The precision and accuracy were well within the acceptable range. BPA was detectable in 77 per cent of plasma samples of infertile women and 29 per cent of fertile women. All the four phthalate metabolites were detected in plasma samples of both fertile and infertile women.
Interpretation & conclusions:
A GC-MS was developed and validated to estimate the BPA and four phthalate monoester metabolites in human plasma. It was utilised to analyse the plasma samples from fertile and infertile women. The infertile women showed significantly higher plasma concentrations of MBzP, BPA and MEHHP as compared to fertile women. The levels of MMP and MEHP were not significantly different between the two groups. Further studies need to be done to confirm these preliminary findings.
Keywords: Bisphenol-A, endometriosis, gas chromatographic-mass spectrometric, mono-2-ethylhexyl phthalate and mono-(2-ethyl-5-hydroxyhexyl) phthalate, mono-benzyl phthalate, mono-methyl phthalate, polycystic ovary syndrome
Bisphenol-A (BPA) and phthalates are high-volume production industrial chemicals. These are utilized in the manufacturing of plastics and epoxy resins that are ubiquitous in our environment and also in our daily lives. BPA is found in reusable plastic containers, food and beverage can liners, thermal receipt papers, baby bottles, detergents, soaps, cosmetics, dental sealants, etc, whereas phthalates are used in polyvinyl chloride (PVC) plastics, beauty and infant products, medical devices and the enteric coating of some medications1. These environmental chemicals are also known as endocrine-disrupting chemicals (EDCs) as these interfere with the functioning of the endocrine system. The functioning of endocrine system is important in females as the menstrual cycle and fertility are sensitive to hormone imbalances2. The ability of BPA and phthalates to cause female reproductive toxicity leading to infertility is reviewed by several groups2,3,4,5. The increasing number of epidemiological studies show a widespread exposure of these chemicals to the general population including pregnant women, children and foetuses3,6,7,8. These chemicals have been quantified in urine, blood, breast milk, amniotic fluid and cord blood8,9. The presence of these contaminants in amniotic fluid and cord blood indicates that these may pass transplacentally to the foetus9. The developing foetus is dependent on sex steroids and thyroid hormones for maturation10. These chemicals not only affect the developing embryo, but also have been shown to alter adult ovarian function by targeting steroidogenesis5. Hence, these agents have the potential to disrupt reproductive cyclicity and may also cause transgenerational effects by targeting oocyte maturation and maternal sex chromosomes5. Results from basic research and epidemiological studies indicate that additional human biomonitoring studies are required for EDCs such as BPA and phthalates that are widespread in food and living environment11.
To obtain valid biomonitoring data, accurate, precise, highly sensitive and selective multianalyte, validated analytical methods for extraction, separation and detection of the environmental chemicals are required12. However, even with the application of sophisticated and accurate mass spectrometric methods, external contamination with ubiquitous environmental chemicals such as BPA and phthalates during and after sample collection can compromise the analytical results. Therefore, it is essential to identify the possible sources of laboratory contamination and their reduction to generate valid biomonitoring data.
In the current research work, a simple, sensitive and cost-effective gas chromatographic-mass spectrometric (GC-MS) method combined with solid-phase extraction (SPE) procedure was developed to measure the concentrations of the following four phthalate monoester metabolites: mono-methyl phthalate (MMP), mono-benzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP) and mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and BPA simultaneously inhuman plasma samples. The method was validated for linearity, limit of quantification (LOQ), accuracy and precision. This validated method was utilized for the analysis of plasma samples obtained from fertile and infertile women.
Material & Methods
Study design
The study protocol was approved by the Ethics Committees of ICMR-National Institute for Research in Reproductive Health (NIRRH), King Edward Memorial Hospital, Nowrosjee Wadia Maternity Hospital, Mumbai, India. The study was registered with Clinical Trials Registry-India (CTRI, CTRI/2015/04/005664). From May to August, 2015, a total of 45 fertile and 34 infertile women were enrolled in the study from Infertility and Family Welfare Clinics of NIRRH, King Edward Memorial Hospital, Nowrosjee Wadia Maternity Hospital, Mumbai. Written informed consent was obtained from all the participants before enrolling them in the study.
Infertile women group: Inclusion criteria-Women between 20 and 40 yr of age, attending infertility outpatient department, diagnosed with infertility [endometriosis, polycystic ovary syndrome (PCOS)] where male factor is normal.
Exclusion criteria-Women on prolonged hormonal medications.
Fertile women group: Inclusion criteria-Married women between 20 and 40 yr of age, with proven fertility and no evidence of any gynaecological disorders, who achieved pregnancy naturally and delivered recently (within one year).
Exclusion criteria-Women on prolonged hormonal medications.
The fertile and infertile groups were living in urban areas and with no history of any occupational exposure to reproductive toxicants. They were non-smoker and were not consuming alcohol.
Sample collection and storage: The blood samples were withdrawn using a double-sided needle which allowed direct vein blood collection into EDTA glass vacutainers. Approximately 10 ml of peripheral venous blood was collected from each study participant. The plasma was separated and stored upright at −80°C in high-density polyethylene vials until analysis. The analysis was conducted at Guru Nanak Institute for Research and Development, Guru Nanak Khalsa College, Mumbai and Dr. P.S. Ramanathan Advanced Instrumentation Centre, Ramnarain Ruia College, Mumbai.
The analytical standards for BPA, MMP, MBzP and ammonium acetate were procured from Sigma-Aldrich (Bengaluru, India), whereas the analytical standards for MEHP, MEHHP, MiBP-d4 and BPA-d6 were procured from Toronto Research Chemicals, Canada. The derivatizing reagents N, O-bis(trimethylsilyl)trifluoroacetamide (BSTFA), trimethylchloro silane (TMCS) and ammonium acetate were purchased from Sigma-Aldrich (Bengaluru, India). LCMS-grade acetone, acetonitrile and methanol were procured from J.T. Baker, India. High-performance liquid chromatography (HPLC)-grade water was supplied by Rankem, India. Fresh frozen human plasma was purchased from Bombay Municipal Blood Centre (Shantilal Mathurdas Blood Centre, Mumbai, India) and stored at −80°C until use. B-glucuronidase (Escherichia coli K12) was supplied by Roche Biomedical, USA. Polymer DVB cartridges (30 mg, capacity - 1 ml) were purchased from Orochem, India.
Gas chromatographic-mass spectrometric instrumental conditions for BPA and phthalate estimation: GC-MS analysis was performed using Shimadzu GCMS QP-2010 Ultra system (Shimadzu, Japan) with AOC-20i injector. The system was equipped with fused silica Rtx-5 Sil MS silarylene capillary column with dimensions 30 m×0.25 mm×0.25 μm. Helium (0.82 ml/min) was used as a carrier gas. The programme used for GC oven temperature is given in Table I. The mass selective detector was operated in single-ion monitoring (SIM) mode for quantitative determinations. The selected ions for the quantification of individual anlytes are given in Table II. The injection port temperature was set at 250°C. The transfer line of the GC to the MS was set at 270°C, and the electron impact (EI) ion source of the MS was set at 230°C. The ionization of sample components was performed in the EI mode (70 eV). A solvent delay time of 5 min was used to protect the ion multiplier of the MS instrument from saturation. The mass spectrometer was calibrated every day, using perfluorotributylamineas calibration standard. The samples were injected in splitless mode. Chromatogram of blank human plasma was spiked with standard mixture of BPA and phthalate monoesters. The individual analytes were identified on the basis of their retention time and specific ions selected for quantification which are given in Table II. These ions were selected based on the mass spectra of standard compound.
Table I.
Temperature programme for column oven
| Rise in temperature per min (°C) | Temperature (°C) | Hold time (min) |
|---|---|---|
| - | 120 | 1 |
| 15 | 200 | 1 |
| 5 | 220 | 2 |
| 10 | 290 | 10 |
| Total programme time | 30.33 |
Table II.
List of selected qualifier and quantifier ions for bisphenol-A, phthalate metabolites and their internal standards
| Parent compound | Metabolite selected for analysis | Retention time (min) | Quantifier ion (m/z) | Qualifier ion (m/z) |
|---|---|---|---|---|
| BPA | Free BPA | 13.96 | 357 | 372 |
| BPA internal standard | BPA-d6 | 13.89 | 360 | 378 |
| DMP | MMP | 6.24 | 237 | 163, 238 |
| BBzP | MBzP | 13.87 | 179 | 222, 194 |
| DEHP | MEHP | 12.22 | 239 | 223, 221, 149 |
| MEHHP | 15.94 | 221 | 295, 265, 311 | |
| Phthalate Internal Standard | MiBP-d4 | 7.97 | 227 | 225, 243 |
DMP, dimethyl phthalate; DEHP, di-2-ethylhexyl phthalate; BBzP, benzyl butyl phthalate; BPA, bisphenol-A; MMP, mono-methyl phthalate; MBzP, mono-benzyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEHHP, mono-2-ethyl-5-hydroxyhexyl phthalate
Stripping of human plasma with activated charcoal: The contamination of phthalates and BPA was observed in human blank plasma (n=3) procured from a blood bank. Activated carbon (100 mg/ml) was added to the human plasma and kept overnight on shaker at room temperature (RT). The activated charcoal was separated by centrifugation and subsequent filtration with 0.45 μ syringe filter. This charcoal-stripped plasma was used to prepare calibration and quality control (QC) plasma standards.
Treatment of HPLC-grade water with activated charcoal and C-18 flash cartridges: In addition to blank plasma, contamination of phthalates and BPA was also observed in reagent blanks extracted by SPE. The HPLC-grade water utilized for the preparation of SPE mobile phases was found to be the main source of contamination. Therefore, HPLC-grade water (100 ml) was treated with 4 g of activated carbon and kept on shaker at RT for overnight. The activated carbon was separated from water by centrifugation and subsequent filtration with 0.2 μ filter. This purified water (15 ml per cartridge) was further passed through C-18 flash cartridges (2 g) pre-conditioned with 10 ml of methanol and 3 ml of activated carbon-purified water.
Contamination from experimental apparatus: Along with the above procedures, all the experiments were preferably performed using glass apparatus as BPA and phthalates can leach from the plastic ware. All the glassware were rinsed with methanol and heated at 120°C for three hour and wrapped in aluminium foil until analysis to avoid the contamination. Only polypropylene tubes (non-PVC containing) and/or glass containers were used throughout the study.
Method validation
Preparation of standard: All the initial, intermediate and mixed standard stock solutions of BPA, 4 phthalates monoester and their internal standard (MiBP-d4 and BPA-d6) were prepared in methanol. All the stock solutions were prepared in a volumetric flask and stored at 2°C-8°C until use.
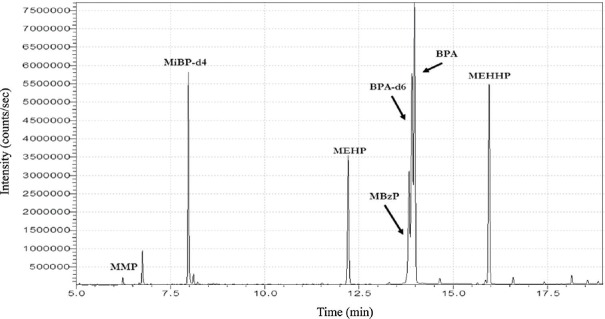
Selectivity, sensitivity and specificity: Compounds were quantified using SIM mode. Analytes appeared to be well resolved and free from interference peaks (Figure). The identity of the chromatographic peak was confirmed not only by its retention time but also by its mass spectrum. The sensitivity of the analytical method was determined on the basis of signal-to-noise ratio. The lowest concentration with signal-to-noise ratio more than 10 was considered as LOQ.
Figure.
Total ion chromatogram human plasma sample spiked with BPA and phthalate metabolites.
Linearity: The linearity of the calibration curve (CC) was evaluated from three consecutively prepared CC batches at eight levels in the concentration ranging from 5 to 200 ng/ml. The peak area ratios of analytes and the corresponding internal standard were used to calculate the correlation coefficient, intercept and slope.
Accuracy and precision: The precision and accuracy of the method was evaluated by spiking mixed stock of BPA and phthalate monoesters at 15 and 100 ng/ml plasma concentrations. Three precision and accuracy batches consisting of five replicate sample of each concentration level were injected on three consecutive days to determine the intra- and interday accuracy and precision. Precision was calculated as the relative standard deviation (RSD) for both intra- and inter day. Accuracy was determined as the degree of closeness of the determination value to the true value. Acceptable precision was defined by a RSD <15 per cent for the standards and <20 per cent at LOQ. Accuracy was acceptable between 80 and 120 per cent.
Study sample preparation and deglucuronidation: The individual plasma samples (500 μl) collected from fertile and infertile women were spiked with internal standard mixed stock solution to give a final concentration of 50 ng/ml of each internal standard. To this,100 μl of ammonium acetate buffer (pH 6.5) and 10 μl of β-glucuronidase (E. coli K12) were added. The samples were mixed and incubated overnight at 37°C to allow for deglucuronidation. Following enzymatic hydrolysis, the samples were diluted with 0.1M formic acid (500 μl) and loaded onto the SPE cartridge.
Solid-phase extraction and derivatization procedure: The cartridges were conditioned with methanol (2 ml), acetonitrile (1 ml) and 0.1 M formic acid (2 ml). The samples were diluted with 500 μl of 0.1 M formic acid and loaded onto the SPE cartridge. The cartridge was washed with purified water (1 ml) and 10 per cent methanol in water (2 ml). The samples were then eluted with acetonitrile (1 ml). The eluate was evaporated to dryness at 50°C under a stream of nitrogen in low-volume nitrogen evaporator for 10 min. In order to dissolve residue and carry out the derivatization, 100 μl of acetone and 200 μl of BSTFA with one per cent TMCS were added. Tubes were closed and heated at 60°C for one hour. After the derivatization process was completed, 1 μl of the reaction mixture was injected into the GC-MS system for analysis.
Quality control of samples: Each batch of 20 samples was analyzed with CC and two QC samples (15 and 100 ng/ml). The sample analysis was carried out by careful control of any possible contamination to achieve a low-level background contamination.
Statistical analysis: The GC solutions software, (Shimadzu, Japan) was used for data acquisition and analysis. The ratios between the peak areas in samples (calibration, QC and unknowns) and the corresponding internal standards were calculated. The concentrations of unknown samples were calculated using linearity regressed line. All validation data were expressed as mean±SD or as the RSD. The two-sided independent samples t test was used to evaluate the group differences in age, height, weight, body mass index (BMI), age at menarche and concentration of the selected EDCs between fertile and infertile women groups. Values of the compounds below the LOQ were replaced by LOQ/√2.
Results
Method development and validation: A 30.33 min GC-MS method for simultaneous determination of BPA, four phthalate metabolites and their internal standards was developed. The Figure shows extracted ion chromatograms of a pooled plasma sample spiked with 1000 ng/ml mixed stock solution of all analytes. The blank plasma samples (treated with activated charcoal) and reagent blank samples (treated with activated charcoal and C-18 Flash Cartridges) did not show any significant interfering peaks at the retention time of selected target analytes and their internal standards. This proved the specificity and selectivity of the method. The chromatographically screened interference-free activated charcoal-treated human blank plasma was further utilized to prepare CC and QC samples. The CCs were linear over the range of 5-200 ng/ml. Linearity, expressed as the correlation coefficients (R2), provided a value of 0.9912 all above for the linear range, as shown in Table III. The LOQs for BPA and phthalate metabolites are also summarized in Table III. The results for accuracy and precision are given in Table IV. At both levels, the interday accuracy ranged from 84.73 to 111.27 per cent for all the analytes, whereas the intraday accuracy was between 83.31 and 114.1 per cent. The interday precision of the method for all the analytes ranged from 3.79 to 10.91 per cent at the low-spike level and from 4.4 to 11.72 per cent at the high-spike level. While the intraday precision of the method for all the analytes ranged from 9.02 to 15.25 per cent at the low-spike level and from 3 to 8.63 per cent at the high-spike level. Overall, the method demonstrated a good accuracy and precision within the acceptance range. Table V shows the basic characteristics and reproductive history of the study participants. No significant difference in age, BMI, height and age at menarche was observed between these groups. The plasma levels of BPA and phthalate metabolites in fertile and infertile women are presented in Tables VI-VIII.
Table III.
Linear range, correlation coefficients and limit of quantification for bisphenol-A and phthalate metabolites
| Compounds | Linear range (ng/ml) | Correlation coefficients (R2) | LOQ (ng/ml) |
|---|---|---|---|
| BPA | 5-200 | 0.9966 | 1 |
| MMP | 5-200 | 0.9912 | 5 |
| MBzP | 5-200 | 0.9940 | 5 |
| MEHP | 5-200 | 0.9924 | 1 |
| MEHHP | 5-200 | 0.9956 | 1 |
LOQ, limit of quantification; Abbreviations are as given in Table II
Table IV.
Intra- and inter-day precision and accuracy (%) for bisphenol-A and phthalate metabolites
| Compounds | Interday (n=5) | Intraday (n=15) | ||
|---|---|---|---|---|
| 15 ng/ml | 100 ng/ml | 15 ng/ml | 100 ng/ml | |
| BPA | ||||
| Mean BCC | 12.71 | 107.11 | 12.5 | 104.01 |
| Precision (%) | 8.72 | 8.31 | 15.25 | 5.97 |
| Accuracy (%) | 84.73 | 84.73 | 83.31 | 104.01 |
| MMP | ||||
| Mean BCC | 12.75 | 108.75 | 14.43 | 111.00 |
| Precision (%) | 8.2 | 5.14 | 10.74 | 3.00 |
| Accuracy (%) | 85 | 108.75 | 96.21 | 111.00 |
| MBzP | ||||
| Mean BCC | 14.7 | 111.27 | 14.96 | 108.92 |
| Precision (%) | 9.8 | 11.72 | 9.4 | 4.73 |
| Accuracy (%) | 98 | 111.27 | 99.74 | 108.92 |
| MEHP | ||||
| Mean BCC | 13.67 | 104.33 | 13.34 | 109.88 |
| Precision (%) | 10.91 | 9.85 | 9.02 | 4.91 |
| Accuracy (%) | 91.11 | 104.33 | 88.94 | 109.88 |
| MEHHP | ||||
| Mean BCC | 12.87 | 106.06 | 14.93 | 114.1 |
| Precision (%) | 3.79 | 4.4 | 14.34 | 8.63 |
| Accuracy (%) | 85.81 | 106.06 | 99.52 | 114.1 |
BCC, back calculated concentration; Abbreviations are as given in Table II
Table V.
Reproductive history of fertile and infertile women
| Characteristics | Infertile women group (n=45) | Fertile women group (n=34) |
|---|---|---|
| Age (yr) | 28.4±4.4 | 26.8±3.8 |
| BMI (kg/m2) | 25.6±4.7 | 22.9±4.5 |
| Height (cm) | 152.89±6.33 | 151.3±5.9 |
| Weight (kg) | 60.01±11.23 | 52.6±11.4 |
| Age at menarche (yr) | 13.89±1.40 | 14.1±1.4 |
| Duration of infertility (yr) | 4.72±3.27 | NA |
aData expressed as mean±SD. BMI, body mass index; SD, standard deviation; NA, not available
Table VI.
Plasma levels of bisphenol-A and phthalate metabolites (ng/ml)
| Compounds | Infertile women (samples analyzed=45) | Fertile women (samples analyzed=34) | ||||
|---|---|---|---|---|---|---|
| Per cent detects | Mean±SD | 95% CI | Per cent detects | Mean±SD | 95% CI | |
| BPA | 75.6 | 4.66±3.52* | 3.60-5.72 | 29.41 | 2.64±3.99 | 1.24-4.03 |
| MMP | 35.6 | 23.79±40.21 | 11.71-35.87 | 55.88 | 22.49±31.69 | 11.43-33.55 |
| MBzP | 68.9 | 19.9±27.33** | 11.69-28.12 | 5.88 | 5.72±6.82 | 3.34-8.10 |
| MEHP | 4.4 | 1.39±3.2 | 0.43-2.35 | 8.82 | 1.01±1.25 | 0.57-1.45 |
| MEHHP | 75.6 | 6.76±5.54*** | 5.10-8.43 | 17.65 | 1.71±3.24 | 0.58-2.85 |
aData expressed as mean±SD. P*<0.05, **<0.01,***<0.001 against fertile women group. CI, confidence interval; Abbreviations are as given in Table II
Table VIII.
Plasma levels of bisphenol-A and phthalate metabolites in infertile women group (ng/ml)
| Compounds | PCOS (n=31) | Endometriosis (n=11) | PCOS + endometriosis (n=3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Range | Mean | n | Range | Mean | n | Range | Mean | |
| BPA | 30 | <LOQ-13.86 | 5.80±3.05 | 3 | <LOQ-5.31 | 4.59±1.22 | 1 | <LOQ-13.17 | 13.17 |
| MMP | 9 | <LOQ-175.63 | 71.65±63.16 | 7 | <LOQ-85.625 | 40.98±29.03 | 2 | <LOQ-35.63 | 21.88±19.45 |
| MBzP | 27 | <LOQ-123.73 | 25.71±26.69 | 4 | <LOQ-116.50 | 40.88±51.43 | 1 | <LOQ-20.11 | 20.11 |
| MEHP | 0 | - | - | 2 | <LOQ-17.75 | 15.96±2.53 | 0 | - | - |
| MEHHP | 27 | <LOQ-17.22 | 8.76±4.28 | 5 | <LOQ-14.76 | 5.43±5.53 | 2 | <LOQ-19.10 | 16.28±3.99 |
aData expressed as mean±SD. n, per cent women detected with target compounds. PCOS, polycystic ovary syndrome; Abbreviations are as given in Table II
Table VII.
Percentile of plasma levels of bisphenol-A and phthalate metabolites
| Compounds | Infertile women group (n=45) | Fertile women group (n=34) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | p25 | p50 | p75 | Maximum | p | Minimum | p25 | p50 | p75 | Maximum | |
| BPA | <LOQ | <LOQ | 5.09 | 6.15 | 13.86 | 0.0013 | <LOQ | <LOQ | <LOQ | 3.303 | 16.75 |
| MMP | <LOQ | <LOQ | <LOQ | 28.13 | 175.6 | 0.25 | <LOQ | <LOQ | 7.585 | 27.05 | 124.3 |
| MBzP | <LOQ | <LOQ | 12.09 | 20.05 | 123.7 | <0.0001 | <LOQ | <LOQ | <LOQ | <LOQ | 35.89 |
| MEHP | <LOQ | <LOQ | <LOQ | <LOQ | 17.75 | 0.27 | <LOQ | <LOQ | <LOQ | <LOQ | 6.97 |
| MEHHP | <LOQ | <LOQ | 6.5 | 10.58 | 19.1 | <0.0001 | <LOQ | <LOQ | <LOQ | <LOQ | 18.48 |
LOQ, limit of quantification; Abbreviations are as given in Table II
Bisphenol-A: BPA was detectable in 77 per cent (n=34) of plasma samples of infertile women and 29 per cent (n=45) of fertile women. The plasma BPA levels were significantly (P<0.05) higher in infertile women as compared to fertile women. The mean concentration of BPA in plasma of infertile women was 4.66 ng/ml, and the range was <LOQ to 13.86 ng/ml in fertile women. The mean plasma concentration was 2.64 ng/ml, and the range was <LOQ to 16.75 ng/ml in fertile women.
Phthalate metabolites: All the four phthalate metabolites analyzed were detected in plasma samples of both fertile and infertile women. The plasma levels of MEHHP and MBzP in infertile women were significantly higher than that of fertile women. MEHHP was detected in 76 per cent of infertile women with a mean concentration of 6.76 ng/ml. The mean concentration of MBzP was 19.9 ng/ml (range <LOQ to 123.7 ng/ml), and it was detected in 69 per cent of infertile women plasma samples.
Discussion
This pilot study was designed to estimate the exposure of BPA and phthalates in infertile and fertile women. Studies conducted in the past mainly quantified urinary excretion levels of BPA and phthalates. Although concentrations in urine samples provide valuable exposure information, the concentrations of excreted substances are dependent on water intake. Therefore, toxicokinetics are easier to interpret from measurements in blood13.
BPA and phthalates are ubiquitously present in the laboratory environment, experimental apparatus and medical and storage devices. Contamination with these chemicals was the major challenge during method development, validation and sample analysis, which could compromise the analytical results. Contamination of phthalates and BPA was found in human blank plasma purchased from blood bank and also in reagents and solvents. Especially in purchased blank plasma, a strong interfering peak of MEHP was observed. Also, trace contamination of BPA was also observed in blank plasma. MEHP is a primary metabolite of di-(2-ethylhexyl) phthalate (DEHP), which is utilized in materials such as blood storage bags and medical tubings1. Selection of relevant analyte-free matrix to prepare CC and QC samples is an essential prerequisite for method development and validation. The contaminated blank plasma contains an unknown concentration of the analyte, thus making it unsuitable for the preparation of CC and QC samples. Therefore, endogenous phthalates and BPA were removed from the human plasma by stripping with activated charcoal.
In addition to blank plasma, a low-level contamination of phthalates and BPA was also observed in reagent blanks extracted by SPE. To minimize this contamination, all the HPLC-grade solvents (methanol, acetonitrile and acetone) were replaced by solvents of higher purity, i.e. LC-MS grade. Even after replacing the solvents, contamination of target analyte was observed in the reagent blanks. The HPLC-grade water utilized for the preparation of SPE reagents was found to be the main source of contamination. Therefore, the HPLC-grade water was also treated with activated charcoal and further passed through C-18 flash cartridges for removal of trace-level contaminants. The chromatographically screened plasma was further utilized for the preparation of calibration and QC samples. Also, to avoid any contamination during sample collection, the samples were collected with double-sided needle into glass vaccutainers.
The literature for human plasma or serum BPA levels is sparse compared to that of the urinary BPA levels1. Of the 79 samples tested in this study, BPA was detectable in 44 samples (56%). The plasma BPA levels were significantly higher in infertile women compared to fertile women. The results obtained in the present study were comparable with those of previous studies which consisted of infertile women with different endocrinological problems and their controls. However, in many of these previous studies, the serum BPA levels were measured by ELISA14,15,16. The plasma BPA levels in PCOS women in our study were five times higher than the serum levels obtained in studies conducted earlier14,15,16, whereas the plasma BPA levels in endometriosis women in our study were 1.6 times higher than the serum levels of Italian women with endometriosis17.
Studies18,19,20 conducted in India showed an association of phthalates with endometriosis. In these studies, parent phthalate diesters were estimated in the blood samples of women with endometriosis and their controls. In these studies, the levels of DEHP in endometriosis patients were significantly higher than that of control. Also, the association of endometriosis with significantly higher plasma DEHP and MEHP levels was reported by Cobellis et al21. We estimated phthalate monoesters as biomarkers of phthalate exposure to overcome the limitation of phthalate contamination as these are ubiquitous laboratory contaminants.
To assess the DEHP exposure, both its primary metabolites, i.e. MEHP and secondary oxidative metabolite, i.e. MEHHP were estimated. In contrast to the previous studies13, compared to plasma MEHP levels, higher plasma levels as well as higher detection rate of MEHHP were observed in the samples analyzed. In our study, MEHP was detected in only six per cent of plasma samples, whereas MEHHP was detected in 51 per cent of samples. Also, in infertile women, the concentration of MEHHP was approximately five times higher than that of MEHP. Estimation of phthalate monoesters in blood or serum is more prone to contamination from phthalate diesters. Especially, contamination from DEHP during collection and storage is more likely possible, which is mainly utilized in medical devices. These phthalate diesters hydrolyze to their respective monoesters by serum enzymes and erroneously increase the monoester levels. The MEHHP levels would not be overestimated by external DEHP contamination as these cannot be formed by enzymatic hydrolysis. Kato et al13 reported that the estimated MEHP levels in archived serum samples included an unknown contribution from the MEHP formed from the lipase hydrolysis of DEHP contamination as no attempt was made to eliminate the lipase activity. In the present study, a double-sided needle was utilized to collect the samples which avoided the contamination during collection. Moreover, most of the sample processing was performed in glass apparatus. The undetectable levels of MEHP in many of the samples indicated that the contamination was low.
The current study had limitations of small number of sample size and only four phthalate metabolites were estimated. Future studies should be conducted with larger sample size with more number of phthalate metabolites.
In conclusion, a GC-MS method was developed for simultaneous estimation of four phthalate monoester metabolites and BPA in human plasma. The method was found to be sensitive, accurate and precise. Activated charcoal was found to be effective in the removal of BPA and phthalate contamination. Compared to fertile women, significantly higher concentrations of BPA, MBzP and MEHHP observed in infertile women suggest their possible association with infertility. These preliminary data indicate the requirement of more extensive biomonitoring studies with larger study groups.
Acknowledgment
The authors acknowledge the Director, ICMR-National Institute for Research in Reproductive Health, Mumbai, for providing the research facilities and Guru Nanak Institute for Research and Development, Guru Nanak Khalsa College, Ramnarain Ruia College, and Shimadzu Analytical India Pvt. Ltd., Mumbai, for providing analytical facilities.
Footnotes
Financial support & sponsorship: The authors acknowledge the Indian Council of Medical Research, New Delhi, India for providing financial support.
Conflicts of Interest: None.
References
- 1.Olsén L, Lampa E, Birkholz DA, Lind L, Lind PM. Circulating levels of bisphenol A (BPA) and phthalates in an elderly population in Sweden, based on the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Ecotoxicol Environ Saf. 2012;75:242–8. doi: 10.1016/j.ecoenv.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Nicolopoulou-Stamati P, Pitsos MA. The impact of endocrine disrupters on the female reproductive system. Hum Reprod Update. 2001;7:323–30. doi: 10.1093/humupd/7.3.323. [DOI] [PubMed] [Google Scholar]
- 3.Caserta D, Di Segni N, Mallozzi M, Giovanale V, Mantovani A, Marci R, et al. Bisphenol A and the female reproductive tract: An overview of recent laboratory evidence and epidemiological studies. Reprod Biol Endocrinol. 2014;12:37. doi: 10.1186/1477-7827-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huo X, Chen D, He Y, Zhu W, Zhou W, Zhang J. Bisphenol-A and female infertility: A possible role of gene-environment interactions. Int J Environ Res Public Health. 2015;12:11101–16. doi: 10.3390/ijerph120911101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uzumcu M, Zachow R. Developmental exposure to environmental endocrine disruptors: Consequences within the ovary and on female reproductive function. Reprod Toxicol. 2007;23:337–52. doi: 10.1016/j.reprotox.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Rocca C, Tait S, Guerranti C, Busani L, Ciardo F, Bergamasco B, et al. Exposure to endocrine disrupters and nuclear receptor gene expression in infertile and fertile women from different Italian areas. Int J Environ Res Public Health. 2014;11:10146–64. doi: 10.3390/ijerph111010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vagi SJ, Azziz-Baumgartner E, Sjödin A, Calafat AM, Dumesic D, Gonzalez L, et al. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: A case-control study. BMC Endocr Disord. 2014;14:86. doi: 10.1186/1472-6823-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caserta D, Maranghi L, Mantovani A, Marci R, Maranghi F, Moscarini M. Impact of endocrine disruptor chemicals in gynaecology. Hum Reprod Update. 2008;14:59–72. doi: 10.1093/humupd/dmm025. [DOI] [PubMed] [Google Scholar]
- 9.Mallozzi M, Bordi G, Garo C, Caserta D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: A review on the major concerns. Birth Defects Res C Embryo Today. 2016;108:224–42. doi: 10.1002/bdrc.21137. [DOI] [PubMed] [Google Scholar]
- 10.Fudvoye J, Bourguignon JP, Parent AS. Endocrine-disrupting chemicals and human growth and maturation: A focus on early critical windows of exposure. Vitam Horm. 2014;94:1–25. doi: 10.1016/B978-0-12-800095-3.00001-8. [DOI] [PubMed] [Google Scholar]
- 11.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calafat AM, Needham LL. What additional factors beyond state-of-the-art analytical methods are needed for optimal generation and interpretation of biomonitoring data? Environ Health Perspect. 2009;117:1481–5. doi: 10.1289/ehp.0901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato K, Silva MJ, Reidy JA, Hurtz D, 3rd, Malek NA, Needham LL, et al. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect. 2004;112:327–30. doi: 10.1289/ehp.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96:E480–4. doi: 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi T, Tsutsumi O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun. 2002;291:76–8. doi: 10.1006/bbrc.2002.6407. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J. 2004;51:165–9. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- 17.Cobellis L, Colacurci N, Trabucco E, Carpentiero C, Grumetto L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed Chromatogr. 2009;23:1186–90. doi: 10.1002/bmc.1241. [DOI] [PubMed] [Google Scholar]
- 18.Reddy BS, Rozati R, Reddy S, Kodampur S, Reddy P, Reddy R. High plasma concentrations of polychlorinated biphenyls and phthalate esters in women with endometriosis: A prospective case control study. Fertil Steril. 2006;85:775–9. doi: 10.1016/j.fertnstert.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Reddy BS, Rozati R, Reddy BV, Raman NV. Association of phthalate esters with endometriosis in Indian women. BJOG. 2006;113:515–20. doi: 10.1111/j.1471-0528.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- 20.Rozati R, Simha BG, Bendi N, Sekhar C. Evaluation of the phthalate esters in South Indian women with endometriosis. Int J Fertil Steril. 2008;1:165–70. [Google Scholar]
- 21.Cobellis L, Latini G, De Felice C, Razzi S, Paris I, Ruggieri F, et al. High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum Reprod. 2003;18:1512–5. doi: 10.1093/humrep/deg254. [DOI] [PubMed] [Google Scholar]