Abstract
The research study aimed to study the effect of short term exposure to light basically red, blue and white on the autonomic tone of essential hypertensive individuals. The objective was to find out the baseline cardiac autonomic function along with the effect of these lights on the cardiac autonomic function among them. Till date few if any study have been conducted upon the individuals with certain disorder as common as essential hypertension. This was a cross sectional observational study conducted in the institute itself that included 77 newly diagnosed hypertensive subjects who willingly participated in the study. After written informed consent, brief history taking with the help of self-made questionnaire and clinical examination, they were randomized to different intervention groups (IG) namely IG I (red) IG II (blue) and IG III (white). HRV analysis of the last 5-6 minutes of both the baseline and color exposure was finally analyzed using MS Excel version 13 and Graph Pad Prism version 7.05. Different HRV parameters have been found to be affected differently on different color exposures. Red has shown to have an impact, mainly on the sympathetic system whereas white showed a dominant vagal component thus acting as a parasympathetic regulator. On one hand, where no conclusive result was found on blue light exposure, white light showed the most prominent results affecting various time and frequency components of HRV like SDRR, TP, LF etc. The present study, both, contradicts as well as supports various other works done on the similar area of interest. One reason of such high variation in different results is because HRV is itself a very dynamic function affected by even a slight change in both the internal and external environment of the subject. As artificial lights of various colors are part and parcel of the aesthetics and designing of most of the work environment all over world, it is very pertinent to study its impact upon human health status. The outcome of the study may play a decisive role in the diagnostics and therapeutics of essential hypertension in days to come. Furthermore, on the basis of the present findings, a future study could be undertaken with bigger data base addressing the limitations of the present study to find some conclusive evidence in the area highlighted.
Keywords: Autonomic function, colored light, Drug naïve essential hypertension, HRV
Introduction
Every third urban and fourth rural citizen of India is hypertensive and only 25 to 42% of them are actually aware of it.[1] The condition is more or less similar in even in most of the developed countries of the world. According to a published report, the load of some 20 million office visits of Canadian family to the family physicians in Canada annually.[2,3] Moreover, pharmacological management, which itself has financial constraints, simple non-pharmacological measures including the life style measures could be an effective mean to decrease the disease burden over the society.
Essential hypertension, also known as primary hypertension is the chronic elevation of blood pressure (BP) and has its origin from an unknown cause, found in 95% of the hypertensive patients; also it has been found to have a strong genetic pre-disposition (around 50%). It has been found that red color increases excitement and BP, whereas blue color decreases excitement and BP, thus signifying their possible role in either elevating or alleviating hypertension.[4] We already know that stressed environment such as workplace has been implicated in the pathogenesis of hypertension, indicating the effect of stress on essential hypertension. There are studies signifying the need and effect of anti-stress programs being implicated at the workplace to alleviate hypertension among the inmates.[5] These individuals are more likely to suffer from myocardial infarcts and atherosclerosis and are predisposed to heart failures and cerebral hemorrhage. Therefore, a slight modulation of working environment based upon the scientific evidences may possibly decrease the incidences of heart failure, chronic kidney disease, strokes, etc.
Despite some piece meal studies scarcity prevails regarding the concrete evidence of effect of short-term exposure to different color light on various psycho-physiological parameters. More so, little if any evidence of the same is available on the subjects of common clinical conditions such as essential hypertension, which has pandemic prevalence. Presently, essential hypertension is by and large non-curable and can only be treated.[6]
Color of light is a frequently used tool to modulate the ambiance of working environment. With advent of sophisticated organic light-emitting diodes (OLED) of various colors, the endeavor became very handy and cheap aesthetic component at most of the workplaces. Such lightings are known to have some impact on our mind and physical states, especially in various diurnal conditions such as during work, study, rest, and sleep.[7] Different light components have apparently different effect upon the various physiological parameters.[8,9] The effect of color on mood, anxiety, and depression, etc., has been effectively studied in the past.[9,10] Thus, few wavelength of light may be involved in modulating blood pressure, bringing role of environmental factors into play.[11] Not only the wavelengths but also the color temperature plays a significant role in effecting various physiological parameters.[12] A non-visual response produced by light, mediated through the retino-hypothalamic tract has been explored by the scientists.[4,13] It also causes shift in the circadian rhythm (through melatonin and its mechanism), which cannot be neglected either.[14,15] A study done on the school children concluded that colors such as blue and pink in the dental set-up could enhance a positive attitude, whereas black and red could develop a negative outlook in their mind.[16] In addition, chromotherapy- applying a scientific basis for the treatment of various diseases, uses the visible part of the electromagnetic spectrum of radiations,[17] thus, opening new avenues of research.
Present study aims to find out the effect of short-term exposure of red, blue, and white colored light on the autonomic reactivity of normal and hypertensive individuals.
Materials and Methods
Methods
Following all the inclusion and exclusion criteria, a total of 96 subjects with essential hypertension (having no known cause of hypertension) have been recruited for the study in the anticipation of possible redundancy and dropouts. Inclusion criteria included age group from 18 to 60 years and subject having newly diagnosed essential hypertension cases with no known etiological factors present/diagnosed. Prior ethical clearance has been taken from the Institutional Ethical Committee of AIIMS Patna vide letter no. IEC/AIIMS/Pat/220/2018. The subjects with any of the following conditions had been excluded from the study-
Subjects taking any drug/medication/nutritional supplements which is known to affect cardiac autonomic functions
Subjects with associated acute/chronic illnesses
Smokers, alcoholic, and drug edict individuals
Females of premenopausal age group and pregnancy condition
Subjects with known sleep disturbances or disorders of sleep.
After written informed consent, brief history taking, and clinical examination, the subjects were randomized to intervention group I; intervention group II; and intervention group III keeping 32 subjects in each group. A standard environment has been maintained in the laboratory including maintaining the standard temperature, light, and noise level for the baseline recording of optimum heart rate variability (HRV) parameters. After baseline recording, the subjects of group I were exposed to red light, group II with blue light, and group III with white light, maintaining a uniform light intensity for all the groups. One subject in group I and nine subjects, each in group II and III have left the study because of subjective discomfort or apprehensions. A total of 31 subjects in group I (Red; 620– 720 nm), 23 in group II (Blue; 492-455 nm), and 23 in group III (White) have successfully completed the study, and their data have been used for further analysis. Figure 1 shows the baseline (Dark) followed by interventional (colored light) exposure and HRV recording. Last 5 min. HRV recording has been used for analysis purpose.
Figure 1.
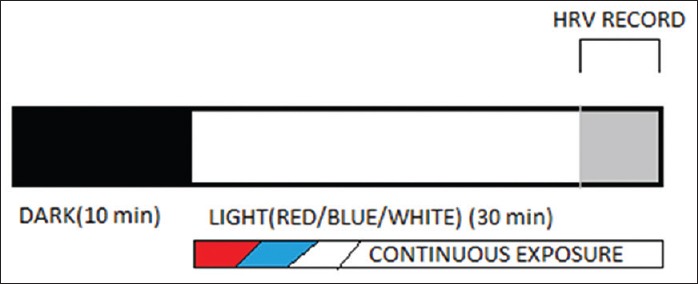
Diagramatic representation of Methodology; subjects were exposed to basal (Dark) condition for 10 min. followed by light exposure for 30 min. followed by. Last 5 min. of the record from both phase has been used for HRV analysis
The cardiac autonomic function testing tool, i.e. HRV using Power lab instrument version 7 (AD Instruments ltd, Australia.) have been used to test the sympathovagal balance of each individual. The individuals were first exposed for 10 min in the dimly lit peaceful environment. This followed a session of 30 min continuous exposure of red, blue, or white using fluorescent OLED light strips of same intensity and illuminance. These strips were fitted in the autonomic laboratory of the department taking proper care that all the three strips were at almost same distance from the subject, so as to rule out the distance factor. The intervention Group I, II, and III were namely red, blue, and white, respectively. During whole event, continuous 3 lead Electro cardiogram (ECG) have been recorded using the specific equipment mentioned above. The last 5–6 min of both the baseline recording and intervention phase recording have been used for the HRV analysis purpose. Data analysis was done using Microsoft Excel version 13 and Graph Pad Prism version 7.05. The HRV parameters used for analysis include Standard deviation of NN intervals (SDNN), Root Mean square of Standard Deviations (of NN intervals) (RMSSD) number of pairs of successive NN intervals greater than 50 ms (pNN50), total power (TP), High frequency (HF), Low Frequency (LF) Very High Freqency(VLF), HF: LF, SD1, and SD2 (Standard Deviations 1 &2).
A brief descriptions of the HRV parameters studied in the present study are as follows:[16]
Time domain variables
SDNN: Standard deviation of NN intervals. It casts the overall parasympathetic and sympathetic influences on HRV
rMSSD: It is the square root of the mean squared differences of successive NN intervals (rMSSD). It is one of the slow changing components of HRV
pNN50: The number of pairs of successive NN intervals greater than 50 ms. NN50 and the proportion calculated from the ratio between NN50 and the total number of NN intervals is pNN5O. It directly correlated with the variability if the heart and its adaptive capacity
TP: Total Power. It determines about the variance of NN intervals over the temporal segment.
Frequency domain variables
For short duration recording (2–5 min), the peaks that is identified are
Very low frequency (VLF): < 0.04 Hz. Not much role in short-term exposure to light as it is also a slow changing frequency component of HRV
Low frequency (LF): 0.04–0.15 Hz. Corresponds mainly to sympathetic along with some contribution from parasympathetic nervous system
High frequency (HF): 0.15–0.4 Hz. Modulated by the parasympathetic nervous system
(LF/HF): It is an index of the sympathovagal balance
SD1: This standard deviation is used as a marker of vagal influence
SD2: This standard deviation is used as are presentation of delayed R-R interval changes basically correlating to the sympathetic activity.
Observation and Result
The study group consisted of 50 males and 27 female subjects who could complete the study successfully. The age distribution of the subject included 8 subjects having age less than 30 years, 39 subjects with age between 30 and 50 years, and 30 subjects with age more than 50 years. Despite a difference in the number of male and female subjects participating in the study, no significant differences have been found between the mean values of various HRV parameters studied (P > 0.05) except that of pRR50 [ref. Table 1] in the baseline recordings. While, despite of wider age range of the study population, no significant differences have been observed in the baseline HRV parameters studied among subjects of different age groups [ref. Table 2].
Table 1.
Baseline recording of different HRV parametres; gender distribution
| Parameters | Males (n=50) | Females (n=27) | P | Significance |
|---|---|---|---|---|
| SDRR | 40.52±26.51 | 44.26±30.32 | 0.5761 | All are non-significant except Prr50. |
| RMSSSD | 38.70±39.19 | 40.14±38.8 | 0.8775 | |
| pRR50 | 0.09±0.16 | 0.10±0.187 | 0.0403 | |
| TP | 1510.91±1793.42 | 2244.47±3448.94 | 0.2221 | |
| VLF | 543.48±590.05 | 571.06±764.07 | 0.8607 | |
| LF | 374.11±452.75 | 533.70±768.02 | 0.2543 | |
| HF | 541.01±991.49 | 990.11±1912.85 | 0.1778 | |
| LF/HF | 1.70±2.15 | 28.40±27.48 | 0.3843 | |
| SD1 | 27.40±27.74 | 54.25±35.48 | 0.8793 | |
| SD2 | 48.76±28.19 | 53.53±51.94 | 0.4592 |
Table 2.
Baseline recording of different HRV parametres; age wise distribution
| Parameters | <30 (yrs) (n=8) | 30-50 (years) (n=39) | >50 (years) (n=30) | P | Significance |
|---|---|---|---|---|---|
| SDRR | 37.01±14.51 | 41.20±20.68 | 44.02±37.83 | 0.8057 | Non-significant |
| RMSSSD | 26.76±10.64 | 37.95±31.15 | 45.02±50.98 | 0.3713 | |
| Prr50 | 0.03±0.04 | 0.10±0.16 | 0.10±0.2 | 0.8231 | |
| TP | 1362.66±977.52 | 1521.12±1331.81 | 2220.7±3735.7 | 0.4672 | |
| VLF | 752.82±631.12 | 523.29±497.83 | 539.25±833.20 | 0.6600 | |
| LF | 413.48±297.66 | 387.63±351.51 | 493.2±849.33 | 0.7612 | |
| HF | 194.34±148.89 | 550.64±792.09 | 1041.5±2034.5 | 0.1964 | |
| LF/HF | 2.67±1.87 | 1.57±2.14 | 1.25±1.14 | 0.1515 | |
| SD1 | 16.80±7.54 | 26.86±22.06 | 32.00±36.08 | 0.3771 | |
| SD2 | 49.06±20.56 | 50.23±22.88 | 51.76±41.74 | 0.9682 |
The mean systolic BP of the study subjects were 150.49 ± 19.9 mm Hg, whereas the diastolic BP was found to be 94.57 ± 14.52 mm Hg [ref. Table 3].
Table 3.
Distribution of age, BMI, PR, SBP, and DBP among study population
| Determinants | Mean |
|---|---|
| Age (Years) | 48.04±14.26 |
| BMI (kg/m2) | 25.69±4.17 |
| PR (bpm) | 86.14±13.10 |
| SBP (mm of hg) | 150.49±19.9 |
| DBP (mm of Hg) | 94.57±14.52 |
BMI: Body mass index; bpm: Beats per minute; PR: Pulse Rate, SBP and DBP: Systolic and diastolic BP, respectively
The subjects were randomly assigned to intervention groups I (Red), II (Blue), and III (white) based upon the color of light exposed to them after the baseline recording. The baseline parameters observed among the individual group subjects are summarized in the Table 4.
Table 4.
Overall baseline HRV parameters of the study population (n=77)
| Determinants | Mean | Standard deviation | Standard error |
|---|---|---|---|
| SDRR | 40.21 | 22.66 | 2.583 |
| RMSSSD | 38.81 | 36.31 | 4.137 |
| Prr50 | 1.766 | 2.244 | 0.2558 |
| TP | 1254 | 1001 | 114.1 |
| VLF | 498.3 | 468.6 | 53.4 |
| LF | 442.2 | 575.7 | 65.61 |
| HF | 504.9 | 675.6 | 76.99 |
| LF/HF | 1.457 | 1.793 | 0.204 |
| SD1 | 29.19 | 26.47 | 3.017 |
| SD2 | 50.68 | 30.61 | 3.489 |
The HRV parameters after exposure to different color lights to the different intervention group individuals has been summarized in Table 5.
Table 5.
Pre-exposure (baseline) HRV parameters and values after exposure to individual color lights in the different intervention group subjects
| Parameters | Red (Mean±SD) (Intervention group I) | Blue (Mean±SD) (Intervention group II) | White (Mean±SD) (Intervention group III) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | Before | After | P | |
| SDRR | 36.56±16.54 | 39.45±18.22 | 0.1359 | 37.21±19.31 | 36.55±16.2 | 0.4469 | 48.11±30.57 | 61.95±64.1 | 0.0697 |
| RMSSD | 33.29±21.66 | 33.3±22.82 | 0.4982 | 33.94±24.96 | 62.13±17.56 | 0.3885 | 51.12±55.36 | 68.71±99.03 | 0.1109 |
| Prr50 ⌂ | 1.836±2.565 | 2.807±3.184 | 0.0305 | 1.607±1.969 | 2.498±3.303 | 0.0520 | 1.829±2.125 | 2.335±2.376 | 0.0817 |
| TP ⌂▲ | 1192±878.5 | 1555±1190 | 0.0316 | 1136±883.3 | 1302±745.6 | 0.1835 | 1455±1252 | 2103±1786 | 0.0031 |
| VLF ▲ | 462.3±422.1 | 546.4±384.7 | 0.1315 | 511.3±510 | 595.5±488.8 | 0.2067 | 533.7±502.2 | 1035±1299 | 0.0318 |
| LF | 375.4±365.8 | 476.5±340.6 | 0.0987 | 324.7±325.7 | 488.6±531.4 | 0.0915 | 649.9±889.2 | 899.5±1171 | 0.1753 |
| HF▲ | 466.4±442.6 | 586.4±673.2 | 0.1064 | 313.8±363.8 | 593.1±1050 | 0.1268 | 747.9±1037 | 1144±1483 | 0.0325 |
| LF/HF⌂ | 0.9568±0.6986 | 1.413±1.053 | 0.0206 | 1.263±0.7942 | 1.935±2.393 | 0.0850 | 2.325±2.929 | 2.147±2.414 | 0.3249 |
| SD1 | 27.23±19.4 | 27.95±25.85 | 0.4346 | 23.84±17.83 | 24.99±14.59 | 0.3665 | 37.17±38.57 | 45.43±57.16 | 0.1384 |
| SD2 | 48.74±31.6 | 52.19±27.42 | 0.2945 | 46.04±22.83 | 46.07±21.72 | 0.4976 | 58.51±35.54 | 65.46±48.08 | 0.1688 |
▲: Significant in intervention group III; ⌂: Significant in intervention group I
The HRV parameters before exposure (baseline) and after exposure to different light to the respective intervention group has been analyzed statistically by applying paired t test for comparison of means using MS Excel version 13 and statistical software GraphPad Prism version 7.05. Following observations have been made in the statistical outcome of the data.
H F to LF ratio Shows Sympathetic dominance after exposure to red light [Graph 1]
White light exposure tends to increase parasympathetic dominance [Graph 2]
The Red light exposures Tends to increase variability depicted by increase in p RR50 [Graph 3] and Total power [Graph 4] though white light shows highest variability as depicted through SDRR [Graph 5] and RMSSD’ [Graph 6] analysis.
LF has shown maximum increase in white light exposure [Graph 7] but similar type of response also observed in H F parameters too after exposure to white light [Graphs 8 and 9]
White light shows significant parasympathetic activation in Comparison to blue and red light exposures.
Certain HRV parameters such as PRR50 (P value = 0.0305), TP (P value = 0.0316), and LF/HF ratio (P value = 0.0206) have shown significant difference compared to baseline values when the participants were subjected to red light exposure [see Graphs 3, 4, 9 and 10]
SDRR, VLF, and HF values do not show any significant difference statistically when compared to the baseline values (P value >0.1) [see Graphs 2, 5 and 8]
Few parameters including LF have shown some significance when compared to baseline value (0.05 < P value > 0.1) [see Graphs 1 and 7]
Even SD1, SD2, and RMSSD also do not show any significant change compared to baseline [see Graph 6]
Exposure to blue light in the hypertensive individuals (intervention group II) apparently did not bring any statistically significant effect on most HRV parameters when compared to the baseline values, but certain parameters such as PRR50 (P value = 0.0520), LF (P value = 0.0915), and LF/HF ratio (P value = 0.0850) have shown P value closer to the significance level (P < 0.1)
White light exposure apparently caused significant change in the parameters including TP (P value = 0.0031), VLF (P value = 0.0318), and HF (P value = 0.0325). SDRR (P value = 0.067 and Prr50 (P value = 0.0817), though not significant in strict sense when compared to the baseline values, were very close to the significance level of P = 0.05. There is no significant difference found in SD1, SD2, RMSSD, and LF.
Graph 1.
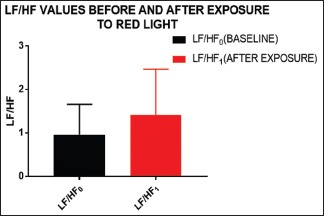
Increased LF/HF directly correlates with the increase in LF or decrement in HF, both stating its sympathetic dominance
Graph 2.
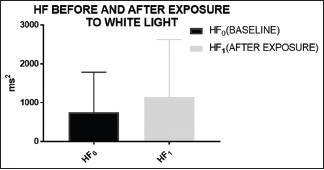
It can be seen clearly that after exposure to white light, HF component of the HRV (frequency domain parameter) has increased. Exposure to white light thus leads to parasympathetic activation
Graph 3.
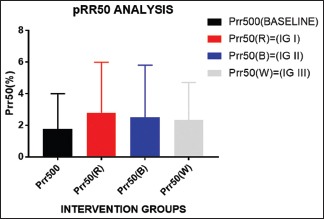
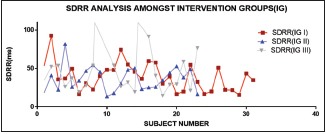
pRR50 is the direct measure of variability. Higher the value of pRR50, higher is the variability. Significantly, after ANOVA analysis, it has been found that red has got the highest variability
Graph 4.
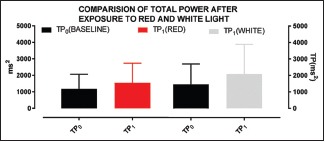
Total power has been found significant in both red and white because its fluctuation is linked with that of variation in HF, LF, or both. From the analysis of above graphs, it can be deciphered that TP found significant in red is owing to LF and in white owing to HF. To an extent, it shows vagal dominance in white and sympatho-dominance in red
Graph 5.
Maximum peak is observed in white light showing increased variability followed by red and blue in both the graphs, i.e. SDNN and that of rMSSD. Increase in variability in these parameters (time domain HRV component) is co-related with improved life expectancy and better prognosis. Although RMSSD it is not of much signifance, it is a slow changing time domain parameter
Graph 6.
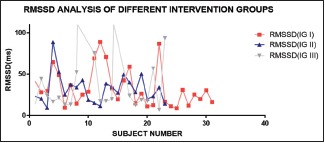
RMSSD analysis of different intervention groups, the white group shows apperently higher values in comparison to others
Graph 7.
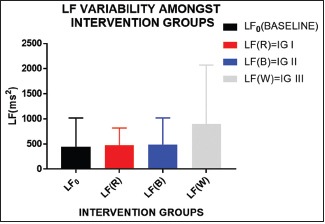
LF has maximally increased in white light exposure and negligible effect on red and blue light; however, its effect is determined in relation with other parameter, as it is affected by both sympathetic and parasympathetic activity
Graph 8.
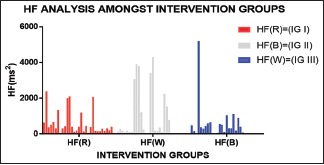
The following graph depicts both the mean and standard deviation of HF
Graph 9.
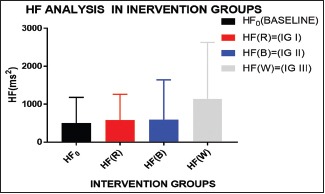
Almost similar kind of graph as LF is obtained in HF, but the increment in both HF and LF depicts the parasympathetic dominance in white light exposure (IG III) and almost similar/not so significant effect in red and blue exposure (IG I and II)
Graph 10.
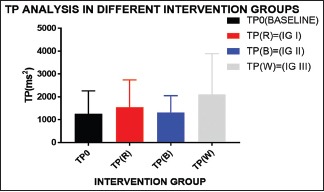
Maximum increase in the total power is seen in IG III depicting parasympathetic activation, majorly in white light exposure
To ascertain if an individual color of light have got significantly higher impact upon the HRV parameters in hypertensive individuals of the study population, repeated measure one- way analysis of variance (ANOVA) has been done. The outcome of the ANOVA can be presented as detailed in the table below [ref. Table 6].
Table 6.
ANOVA analysis for comparing the means of intervention groups I, II, and III
| Determinants | Red (L); n=31 | Blue (L); n=23 | White (L); n=23 | P |
|---|---|---|---|---|
| SDRR☆☆☆☆☆ | 39.45±18.22 | 36.55±16.2 | 61.95±64.1 | 0.0168 |
| RMSSD | 33.3±22.82 | 32.13±17.56 | 68.71±99.03 | 0.0251 |
| Prr50 | 2.807±3.184 | 2.498±3.303 | 2.335±2.376 | 0.2622 |
| TP☆☆☆ | 1555±1190 | 1302±745.6 | 2103±1786 | 0.0197 |
| VLF | 546.4±384.7 | 595.5±488.8 | 1035±1299 | 0.0770 |
| LF☆☆☆ | 476.5±340.6 | 488.6±531.4 | 899.5±1171 | 0.0341 |
| HF | 586.4±673.2 | 593.1±1050 | 1144±1483 | 0.0470 |
| LF/HF | 1.413±1.053 | 1.935±2.393 | 2.147±2.414 | 0.3374 |
| SD1 | 27.95±25.85 | 24.99±14.59 | 45.43±57.16 | 0.1090 |
| SD2 | 52.19±27.42 | 46.07±21.72 | 65.46±48.08 | 0.1869 |
Significant difference was found in SDRR, RMSSD, TP, LF, and HF when overall analysis of different colors was done using ANOVA. All the parameters mentioned above have shown significantly higher value in white group than all other groups
Discussion
HRV is one of the recent electrophysiological tools to understand the complex mechanism of autonomic homeostatic adaptation in challenging environment including physical and psychological challenges. It is closely associated with the autonomic nervous regulatory mechanisms which in turn regulate most organ systems of human body including cardiovascular, gastrointestinal, genito-urinary systems, etc., Variations in the HRV parameters are non-linear and are brought about by both physiological and pathological conditions. The overall regulation depends upon complex oscillatory mechanisms involving overall sympathovagal balance of the individual.[15,16,17]
Exposure to lights of different color (wavelengths) and intensity is known to affect the HRV by means of various mechanisms including the modulation of sympathovagal balance, affecting the renin-angiotensin system, affecting endocrine milieu, acting through anterior and posterior hypothalamic stimulations, etc.[8] Various studies have been done in bits and pieces to identify and quantify the effect of various color lights upon the different HRV parameters without any conclusive finding rather contradictory findings at times.[15,16]
In the present study, both time and frequency domain analysis of HRV parameters have been done to find out if red, blue, and white color lights have got any effect on the HRV as well as the sympathovagal balance of a hypertensive individual or not. The result of the study shows that both white and red color exposure to the study population caused a significant change in certain time and frequency domain parameters.
White light exposure has been documented to increase the TP, VLF, and HF component of HRV significantly. Moreover, SDRR and PRR50 values also registered an increase in the values compared to the baseline values closer to the significance level. The increase in the time domain parameters, i.e. SDRR and PRR50 indicates an increase in the variability of the heart rate with the exposure to white Light Emitting Diod (LED) light. This probably delignates some stimulatory effect of white light over HRV. The frequency domain parameters TP, VLF, and HF are mainly concerned with the sympathovagal balance of an individual. A rise in the HF parameters after exposure to white light indicates an increase in the vagal component in the subjects. For short-term HRV studies, VLF have little significance compared to the HF component;[16] its rise may possibly be the result of early sympathetic activation owing to the illuminance effect of white light exposure immediately following the dark (baseline) period.
Red light exposure to the study population has shown a significant rise in the PRR50, TP, and LF: HF ratio while modest increase in the LF component. This signifies a rise in the HRV along with some sympathetic dominance as LF/HF is reported to correlate well with sympathetic dominance. This finding is in agreement with many previous studies in which red light exposure had been linked with suppression of parasympathetic system in individual with anxiety disorders and certain other conditions.[6] Red light is known to stimulate sympathetic system by stimulation of certain areas of the posterior hypothalamus.[8]
The individuals exposed with blue light did not show any significant change in any of the HRV parameters; though PRR50, LF, and LF: HF ratio have shown definite increase compared to the baseline value closer to the significance level (P < 0.1). The previous studies in context to blue light exposure are contradictory. Some of the studies have documented suppression of vagal balance by blue light,[18,19,20] whereas others have documented a suppression of autonomic arousal and alertness.[19,21] One study on hypertensive individuals has documented a suppression of cardiac vagal modulation by blue light while no effect upon the healthy volunteers.[9] Present study has some indications, though not statistically significant, that exposure to blue light intend to increase HRV by increasing the PRR50 values and shifts the sympathoval balance toward sympathetic dominance in hypertensive individuals. The effect may possibly be an indicator of blunted baroreceptor activities that is well documented in the hypertensive individuals. Furthermore, exposure to blue light is documented to have pronounced effect upon the emotional state of an individual, which in turn could modulate the overall effect upon the HRV parameters, even in hypertensive individuals.[20,22]
The results of repeated measure ANOVA have shown white light to be the most significant factor affecting the HRV parameters in comparison to red and blue light. The HRV parameters SDRR, RMSSD, TP, LF, and HF have shown most significant change in white light condition compared to other colored light exposures. SDRR and RMSSD are associated with increased variability, whereas TP, LF, and HF powers indicate an overall parasympathetic dominance. Although LF has got both sympathetic and parasympathetic component, HF and LF together signifies parasympathetic effect rather than sympathetic dominance.[23] White light being closest to the natural day light condition is reported to have some health benefits in earlier studies too.[11] The present study may be considered consistent with such findings.
Conclusion
HRV is an important tool for assessing the sympathovagal balance in health and disease. Exposures to different colored lights are known to affect the various HRV parameters by multitude of poorly known mechanisms. In present study, effect of red, blue, and white lights have been studied on the different HRV parameters of essential hypertension subjects. White light has shown most promising effect on certain time domain and frequency domain parameters including SDRR, RMSSD, TP, LF, and HF values when compared with baseline as well as with other colored lights indicating positive effect on the variability as well as vagal balance with some sympathetic component. Red light has shown to affect variability as well as sympathetic activation to some extent. Blue light did not show and significant effect on HRV parameters though lower frequency components have shown increasing trend in comparison to the baseline values. A detailed study with bigger database may be proposed on the basis of the results of the study to gather some conclusive findings in the area.
The observations of the study and possible future extension of the study may throw some light over the simple yet effective tools to take care of essential hypertension and may add values to the non-pharmacologic prescriptions of the practitioners of family medicine at a larger perspective.
Limitations
Despite all precautions, owing to individual specific factors known to affect autonomic functions including state of mind, stress level, optimal sleep, etc., are difficult to standardize, hence, a chance of inter-individual variation in the baseline data is inevitable
Because of time constraints, sufficient sample size could not be taken, which could possibly, have affected the study
Intensity and luminescence of light have not been studied, which could possibly influence the autonomic parameters
A long-term exposure and long-term HRV study could be more effective especially to study the lower frequency components, which could not be undertaken because of resource limitations.
Financial support and sponsorship
ICMR STS project (ID: 2018-01885).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledge the contribution of Faculty of Medicine for contributing in the study by helping in the patient selection, Dr. Alok Ranjan, the faculty of bio-statistics who helped in designing the research project and analysis of the data, laboratory staffs of the Department of Physiology for their unconditional support, and above all the subjects who have shown their willingness for the study in the best interest of the society.
References
- 1.Anchala R, Kannuri NK, Pant H, Khan H, Franco OH, Di Angelantonio E, et al. Hypertension in India: A systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014;32:1170–7. doi: 10.1097/HJH.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell N, Kwong MM Canadian Hypertension Education Program. 2010 Canadian Hypertension Education Program recommendations: An annual update. Can Fam Physician. 2010;56:649–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Pimlott N. Hypertension and the family physician. Can Fam Physician. 2009;55:682. [PMC free article] [PubMed] [Google Scholar]
- 4.Daneault V, Hebert M, Albouy G, Doyon J, Dumont M, Carrier J, et al. Aging reduces the stimulating effect of blue light on cognitive brain functions. Sleep. 2014;37:85–96. doi: 10.5665/sleep.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuda E, Ogasawara H, Yoshida Y, Hayano J. Suppression of vagal cardiac modulation by blue light in healthy subjects. J Physiol Anthropol. 2016;35:24. doi: 10.1186/s40101-016-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi CJ, Kim KS, Kim CM, Kim SH, Choi WS. Reactivity of heart rate variability after exposure to coloured lights in healthy adults with symptoms of anxiety and depression. Int J Psychophysiol. 2011;79:83–8. doi: 10.1016/j.ijpsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Kurt S, Osueke KK. The effects of color on the moods of college students. SAGE Open. 2014 doi: 10.1177/2158244014525423. [Google Scholar]
- 8.Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 9.Dai Q, Uchiyama Y, Lee S, Shimomura Y, Katsuura T. Effect of quantity and intensity of pulsed light on human non-visual physiological responses. J Physiol Anthropol. 2017;36:22. doi: 10.1186/s40101-017-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skene DJ, Arendt J. Human circadian rhythms: Physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem. 2006;43:344–53. doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- 11.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 12.Annamary K, Prathima GS, Sajeev R, Kayalvizhi G, Ramesh V, Ezhumalai G. Colour preference to emotions in relation to the anxiety level among school children in Puducherry-A cross-sectional study. J Clin Diagn Res. 2016;10:ZC26–30. doi: 10.7860/JCDR/2016/18506.8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedrosa P, Mendes R, Cabral R, Martins LMDRS, Baptista PV, et al. Combination of chemotherapy and Au-nanoparticle photothermy in the visible light to tackle doxorubicin resistance in cancer cells. Sci Rep. 2018;8 doi: 10.1038/s41598-018-29870-0. doi: 10.1038/s41598-018-29870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eng JY, Moy FM, Bulgiba A. Impact of a Workplace Health Promotion Program on Employees’ Blood Pressure in a Public University. PLoS One. 2016;11:e0148307. doi: 10.1371/journal.pone.0148307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Low PA, Tomalia VA, Park KJ. Autonomic function tests: Some clinical applications. J Clin Neurol. 2013;9:1–8. doi: 10.3988/jcn.2013.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao LC, Liu YW, Tzeng NS, Kuo TB, Huang SY, Chang CC, et al. Linking an anxiety-related personality trait to cardiac autonomic regulation in well-defined healthy adults: Harm avoidance and resting heart rate variability. Psychiatry Investig. 2016;13:397–405. doi: 10.4306/pi.2016.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litscher D, Wang L, Gaischek I, Litscher G. The influence of new colored light stimulation methods on heart rate variability, temperature, and well-being: Results of a pilot study in humans. Evid Based Complement Alternat Med 2013. 2013 doi: 10.1155/2013/674183. doi: 10.1155/2013/674183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuda E, Ogasawara Hiroki, Yoshida Y, Hayano J. Exposure to blue light during lunch break: Effects on autonomic arousal and behavioral alertness. J Physiol Anthropol. 2017;36:30. doi: 10.1186/s40101-017-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhat V, Shetty P, Shashikiran HC, Sujatha KJ, Amruth P. Immediate effect of blue coloured light on autonomic variables on primary hypertension. European J Biomed Pharm Sci. 2018;5:405–8. [Google Scholar]
- 21.Schäfer A, Kratky K. The effect of coloured illumination on heart rate variability. Forschende Komplementärmedizin. 2006;13:167–73. doi: 10.1159/000092644. [DOI] [PubMed] [Google Scholar]
- 22.Samina T, Yusuf Azemi and Mohsin Raza. A critical analysis of Chromotherapy and its scientific evolution. Evid Based Complement Alternat Med. 2005;2:481–8. doi: 10.1093/ecam/neh137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huikuri HV, Tkikallio M, Airaksinen J, Mitrani R, Castellanos A, Myerburg RJ. Measurement of heart rate variability: A clinical tool or a research toy.? J Am Coll Cardiol. 1999;34:1878–83. doi: 10.1016/s0735-1097(99)00468-4. [DOI] [PubMed] [Google Scholar]


