Abstract
Background:
Asthma and chronic obstructive lung disease (COPD) together constitute a huge burden on healthcare all around the world. Treatment of these patients is largely dependent on the way the inhalation devices are used. This study aimed at measuring the impact of rectification of inhalation technique on quality of life and severity of obstruction in patients with asthma and COPD.
Materials and Methods:
A total of 45 asthma (partly controlled) and 38 patients with COPD (categories B and C) were enrolled after applying optimal exclusion criteria. These patients underwent Asthma Control Test (ACT)/COPD Assessment Test (CAT) questionnaire as a measure of quality of life and forced expiratory volume in one second (FEV1) as a measure of severity of obstruction at 0 and 4 weeks, respectively.
Results:
In asthmatics, the mean FEV1 improved from 2.0 to 2.15 L after 4 weeks of technique correction (P < 0.001). In addition, the mean ACT scores improved from 18.0 to 20.75 (P < 0.001). In patients with COPD, the mean FEV1 improved slightly from 1.54 to 1.56 L after 4 weeks of technique correction (P = 0.28). In addition, the mean CAT scores improved from 21.86 to 19.83 (P < 0.001).
Conclusion:
Demonstration of correct inhalation technique should be an indispensible part of the treatment prescription of patients with obstructive airway disease. This simple and important task can be undertaken at the level of primary care physicians in a community-based setting to improve patient compliance.
Keywords: Asthma, chronic obstructive lung disease, inhalation technique, quality of life
Introduction
Asthma and chronic obstructive lung disease (COPD) present a major public health burden worldwide.[1,2] Both environmental exposure and genetic predispositions play a role in their pathogenesis. Although significant advances have been made in therapeutics of these diseases in the past few years, yet their incidence is consistently on the rise.[1,2] The inhaled route of medication has emerged as the most preferred one because of its direct topical action and minimal systemic side effects.[3] But the efficacy of inhaled treatment is largely dependent on the way these are used. Misuse of the inhaler prevents the pharmacological agent from reaching the drug to its target, that is, the lungs.[4] Several types of portable inhaler devices are being used nowadays. Broadly, they are metered dose inhalers (MDI), dry powder inhalers (DPI), or breath actuated inhalers. The amount of drug getting deposited in the lungs depends on three main factors: the drug formulation (fine particle dose of <5 μm reaches the lungs), the technical characteristics of the device, and the ability of the patient to handle and use the device properly.[5] With the MDIs, the drug is propelled out by a propeller and a slow and steady inhalation is required; it is better done with a spacer. Whereas in a DPI the drug is broken into <5 μm initially and then followed by a forceful inhalation by the patient.[6] Breath actuated inhalers are somewhat in between the two devices. The patient generates a minimal inhalation flow rate which opens the one-way valve and allows the drug to be carried into the airways. Here, the patient himself can assess whether he has generated the minimal inspiratory flow rate or not.[7] Since all available devices require some patient skill, adequate training should always be imparted to patients regarding proper technique and handling of the device. This study aims to evaluate the impact of rectification of inhalation technique in patients with asthma and COPD. The impact was measured in terms of (i) severity of obstruction measured by forced expiratory volume in one second (FEV1) and (ii) quality of life measured by Asthma Control Test (ACT) and COPD Assessment Test (CAT) questionnaire for patients with asthma and COPD, respectively.
Materials and Methods
This was a quasi-experimental study carried out over a period of 1 year at All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India. Prior institutional ethical clearance was obtained and informed patient's consent was also taken from each patient before enrolment. Patients already diagnosed as asthma or COPD from outside our institution were initially enrolled. They were reconfirmed of their diagnosis and classified on the basis of severity after taking appropriate history and spirometry. Airway obstruction was classified as per American Thoracic Society/European Respiratory Society guidelines.[8] Children below 14 years of age were excluded from the study.
As per Global Initiative Against Asthma guidelines, asthmatics were segregated into well-controlled, partly controlled, and uncontrolled subgroups. The well-controlled group did not need any intervention and uncontrolled ones required immediate stepping up of treatment. Hence, they were excluded. The ones with partly controlled (74 in number) were further shortlisted. The inhalation technique was cross checked as per the guidelines laid down by National Asthma Council Australia.[9] Of 74, 49 were found to have a faulty technique. These 49 patients filled the ACT questionnaire at 0 weeks first and were sent home after appropriate demonstration of correct inhalation technique. Two of them were lost to follow-up and another two had exacerbation during these 4 weeks and hence were excluded. The remaining 45 had their spirometry and ACT questionnaire repeated after 4 weeks of follow-up and then compared with initial results [Figure 1].
Figure 1.
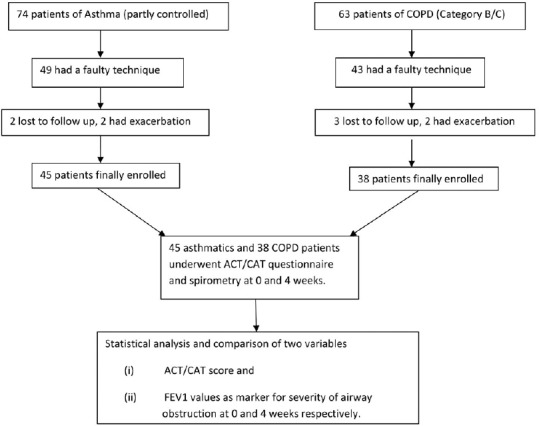
Flowchart depicting the steps involved in enrolment of patients with asthma and COPD
Similarly, patients with COPD were also segregated into categories A, B, C, and D in order of increasing severity as per Global Initiative Against Obstructive Lung Disease guidelines. Groups A and D were excluded as the former required no change in intervention and the latter needed immediate hospitalization/stepping up of treatment. Of 62 patients with COPD in category B/C, 43 were found to have a faulty technique as per National Asthma Council Australia guidelines. These 43 patients filled the CAT questionnaire at 0 weeks and were asked to follow-up after 4 weeks. Three patients were lost to follow-up and two had exacerbations during the 4 weeks. Final follow-up analysis in terms of repeat CAT questionnaire and spirometry at 4 weeks was done in 38 patients [Figure 1].
Statistical analysis
All analysis was performed using R software. Numerical variables were summarized by calculating mean and standard deviation and categorical variables as frequency and percentage. Paired t-test or Wilcoxon signed rank test was used appropriately after checking assumption of normal distribution (by Shaprio–Wilk test) for testing difference in FEV1, ACT, and CAT from baseline in patients with asthma and COPD separately. Box and Whisker plots were used for depicting difference. A P value less than 0.05 was considered statistically significant.
Results
MDIs were used by 24 of 45 asthmatics (53%) and 12 of 38 (31%) patients with COPD. Stepwise assessment of each patient was carried out. The most common error in both the subgroup of patients was that the MDIs were not being used along with a spacer (86% patients). The second most common error was not taking slow and steady breaths as required (58% patients). Other errors, for example, not shaking the MDI or removing the spacer in between, were also seen in some patients [Table 1]. Similarly, DPIs were used by 19 of 45 (40%) asthmatics and 26 of 38 (69%) patients with COPD. The most common error in DPIs was not taking a deep and forceful inspiration (60% patients) followed by not holding the breath for an adequate time (73% patients). Other errors were inappropriate sealing of the device between lips and teeth, incomplete exhalation to start with, and so on [Table 2]. Two patients with asthma used a breath actuated inhaler both of whom were generating a suboptimal inspiratory flow rate.
Table 1.
Stepwise assessment of patients using MDI
| Steps assessed in MDI use | Patients with asthma using MDI (n=24) | Patients with COPD using MDI (n=12) | ||
|---|---|---|---|---|
| Correct usage | Incorrect usage | Correct usage | Incorrect usage | |
| 1. Shake the inhaler well | 18 | 6 | 9 | 3 |
| 2. Fit the inhaler into spacer | 3 | 21 (did not use spacer) | 2 | 10 (did not use spacer) |
| 3. Seal the mouthpiece between the lips | 18 | 6 | 6 | 6 |
| 4. Breathe out gently | 19 | 5 | 8 | 4 |
| 5. Press the MDI only once | 17 | 6 | 9 | 3 |
| 6. Take slow and steady breaths 4-5 times | 8 | 14 | 5 | 7 |
| 7. Do not remove the spacer in between | 15 | 9 | 8 | 4 |
| 8. Remove the spacer | 24 | 0 | 12 | 0 |
MDI: Metered dose inhaler
Table 2.
Stepwise assessment of patients using DPI
| Steps assessed in DPI use | Patients with asthma using DPI (n=19) | Patients with COPD using DPI (n=26) | ||
|---|---|---|---|---|
| Correct usage | Incorrect usage | Correct usage | Incorrect usage | |
| 1. Place the capsule in the chamber | 18 | 1 | 24 | 2 |
| 2. Break the capsule by rotation or pressing depending on the type of device | 15 | 4 | 22 | 4 |
| 3. Hold the chamber upright correctly | 17 | 2 | 23 | 3 |
| 4. Seal the mouthpiece into the lips and teeth | 14 | 5 | 21 | 5 |
| 5. Breathe out gently | 13 | 6 | 19 | 7 |
| 6. Take a deep forceful inspiration | 7 | 12 | 11 | 15 |
| 7. Hold the breath for 8-10 s | 8 | 11 | 6 | 22 |
| 8. Remove the device from the mouth | 19 | 0 | 26 | 0 |
DPI: Dry powder inhaler
In asthmatics, the mean FEV1 improved from 2.0 to 2.15 L after 4 weeks of technique correction only [mean difference 0.15, 95% confidence interval (CI): 0.11–0.20] with a P value <0.001. In addition, the mean ACT scores improved from 18.0 to 20.75 (mean difference 3.0, 95% CI: 2.5–3.5) with a P value <0.001. In patients with COPD, the mean FEV1 improved slightly from 1.54 to 1.56 L after 4 weeks of technique correction (mean difference 0.01, 95% CI: −0.01 to 0.03) with a P value 0.280. In addition, the mean CAT scores improved from 21.86 to 19.83 (mean difference −2.99, 95% CI: −4.0 to −2.0) with a P value <0.001 [Table 3]. Figures 2 and 3 depict the differences in ACT/CAT scores and FEV1 values, respectively, at 0 and 4 weeks in patients with asthma and COPD in Box and Whisker plots.
Table 3.
Distribution of difference in FEV1 and ACT/CAT among patients with asthma and COPD
| Variable | Mean (SD) | Median (IQR) | Mean difference with 95% CI | P |
|---|---|---|---|---|
| Asthma | ||||
| FEV1 | ||||
| Baseline | 2.00 (0.38) | 1.98 (0.52) | 0.15 (0.11 to 0.20) | <0.001 |
| 4 Weeks | 2.15 (0.40) | 2.22 (0.61) | ||
| ACT | ||||
| Baseline | 18.00 (2.01) | 18 (2.25) | 3.0 (2.5 to 3.5)# | <0.001 |
| 4 Weeks | 20.75 (1.74) | 21 (2.0) | ||
| COPD | ||||
| FEV1 | ||||
| Baseline | 1.54 (0.35) | 1.57 (0.51) | 0.01 (0.01 to 0.03)# | 0.280 |
| 4 Weeks | 1.56 (0.38) | 1.59 (0.51) | ||
| CAT | ||||
| Baseline | 21.86 (4.05) | 22 (4.75) | -2.99 (-4.0 to -2.0) | <0.001 |
| 4 Weeks | 19.23 (3.87) | 19 (4.5) | ||
SD: Standard deviation; IQR: Interquartile range; CI: Confidence interval; FEV1: Forced expiratory volume in one second; CAT: COPD Assessment Test; COPD: Chronic obstructive lung disease
Figure 2.
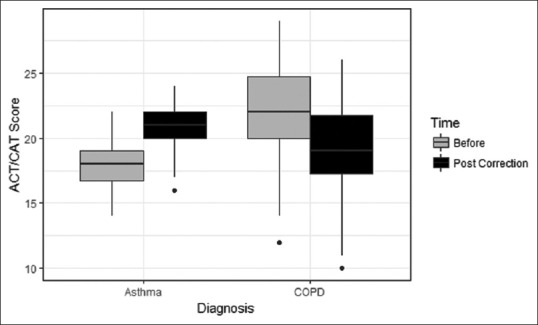
Box and Whisker plot depicting the difference achieved in ACT and CAT scores in patients with asthma and COPD at 0 and 4 weeks, respectively
Figure 3.
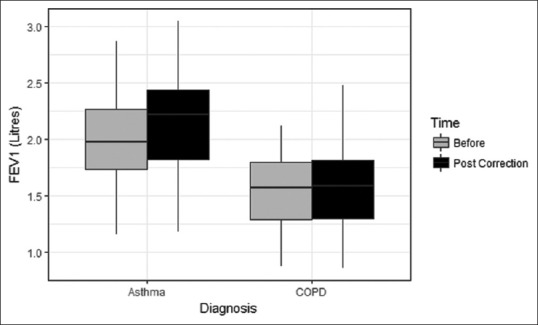
Box and Whisker plot depicting the difference achieved in FEV1 in patients with asthma and COPD at 0 and 4 weeks, respectively
Discussion
The results from the study are really alarming. About 66% of partly controlled asthmatics and 69% of patients with category C/D COPD had poor inhalation technique. Other similar studies have reported up to 50% prevalence of poor inhaler technique.[9,10]
Aerosol mechanics
The mechanics involved in drug intake through these devices need to be thoroughly understood by every physician involved in prescription of these inhaled drugs. Deposition of aerosols in the lungs is controlled by three main laws of aerosol kinetics.[11] (a) Inertial impaction: applicable for particles >8 μm in diameter which get deposited in the upper respiratory tract when the airstream is fast and changes direction. (b) Gravitational sedimentation: it is a time-dependent process whereby particles 1–8 μm in size settle down in smaller airways in the presence of a large cross-sectional area and low flows. (c) Brownian diffusion: particles <1 μm in diameter remain suspended in the air and are expired out. All these principles become clinically relevant when the patients are advised appropriate instructions/steps [Table 1]. When an MDI is used without a spacer, only around 10% of the drug reaches the small airways. The rest is deposited above the larynx because of inertial impaction. Use of a spacer not only increases this deposition to more than 50% by counteracting the inertial impaction but also solves the problem of hand–mouth co-ordination.[11] Instructing the patient to hold a breath while using a MDI or a DPI makes use of the principle of gravitational sedimentation for maximal drug delivery. The principle of Brownian diffusion is not clinically relevant in drug delivery as the smallest particles (<1 μm) are expired as such.
Device selection and demonstration
At the initial encounter, appropriate device selection individualized for each patient is of utmost importance. It is well proven that inhalation required through MDIs is slow with a lower flow rate (30 L/min), whereas inhalation needed through DPIs is fast with a higher flow rate (30–90 L/min).[12] The fact that 26 of 38 patients with COPD having a mean age of 58.13 years were prescribed a DPI in our study clearly shows lack of subject knowledge among physicians at this first step. In MDI usage, approximately 90% of patients with asthma and COPD were not using a spacer along. It is well documented that using a spacer along overcomes the problem of inertial impaction and increases the drug deposition from 10% (without spacer) to 50% (with spacer).[13] The next most common faulty step was not taking slow and deep breaths as required. In DPI usage, the most common faulty steps were lack of forceful deep inspiration and breath holding. This not only prevents the drug to reach its target in lower airways but also deprives the patient of the beneficial effect of gravitational sedimentation.[11] Mere correction of inhalation technique significantly improved the quality of life in terms of ACT and CAT scores in patients with asthma and COPD in this study. In addition, severity of obstruction (FEV1) was statistically improved in patients with asthma. These results clearly conveyed that demonstration of correct technique is an inevitable and integral part of inhaler prescription.
Patient education and the role of primary care physicians
Despite the fact that educating the patients about inhalation technique is an integral part of treatment plan of obstructive airway disease, this study clearly reflects that this component is missing grossly at primary healthcare settings.[14,15] Patient characteristics, namely, age, gender, level of education, and concomitant emotional problems, have been variably associated with incorrect inhalation technique in previous studies.[16] The fact that simple correction of inhalation technique significantly improves the quality of life in both patients with patients asthma and COPD implies that this practical component of treatment prescription needs far more emphasis than what is currently given. Primary care and family physicians can play a vital role in increasing the aderence to inhalation therapy in patients with patients asthma and COPD.[17] Various modalities in routine practice can positively influence patient outcomes in obstructive airway disease.
Training of healthcare providers themselves should be done before they are asked to educate the patients about the devices and their benefits.[18,19] A simple one to two session training programmes to family physicians can markedly improve the patient compliance by virtue of changing the physician attitude toward inhaler teaching and its proper implementation.[20] Considering the role of general practitioners and primary care physicians in healthcare setting of any country, large-scale randomized control trials are underway to compare the patient outcomes based on the educational intervention of general practitioners.[21] Better control of the diseases at initial visits in primary health centers can not only be useful for better disease control in the patients but also reduce the burden of emergency admissions at tertiary healthcare centers
Giving optimal time to the patients so as to check their techniques completely and solve any queries of the patients, if they have. The techniques should be rechecked at frequent visits even if they have been demonstrated at the initial visit. Patients do tend to forget the techniques and introduce new errors in due course of time[22,23]
Group instructions with the help of multimedia devices can have a better impact on the patients’ understanding of the disease and its management.[24]
Conclusion
We conclude that an overwhelming proportion of patients with patients asthma and COPD unaware about the correct inhalation technique. Demonstration of correct inhalation technique and repeated cross checking at follow-up visits is an equally important part of treatment prescription which should be encouraged by educating the patients by the use of multimedia devices or group sessions. The role of primary care and family physicians can be crucial as their intervention at the primary health care setting can be instrumental in decreasing the morbidity of these diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Global initiative for asthma report. Global strategy for the diagnosis and prevention. Global initiative against asthma (updated 2017) [Last accessed on 2018 Jun 02]. Available from: http://www.ginasthma.org .
- 2.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–82. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 3.Papi A, Haughney J, Virchow JC, Roche N, Palkonen S, Price D. Inhaler devices for asthma: A call for action in a neglected field. Eur Respir J. 2011;37:982–5. doi: 10.1183/09031936.00150910. [DOI] [PubMed] [Google Scholar]
- 4.Giraud V, Roche N. Misuse of corticosteroid metered dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:256–61. doi: 10.1183/09031936.02.00218402. [DOI] [PubMed] [Google Scholar]
- 5.Roche N, Chrystyn H, Lavorini F, Agusti A, Virchow JC, Dekhuijzen R et al. Effectiveness of inhaler devices in adult asthma and COPD. EMJ Respir. 2013;1:64–71. [Google Scholar]
- 6.Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta 2 agonist particle size. Am J Respir Crit Care Med. 2005;172:1497–504. doi: 10.1164/rccm.200410-1414OC. [DOI] [PubMed] [Google Scholar]
- 7.Newman SP, Weisz AW, Talaee N, Clarke SW. Improvement of drug delivery with a breath actuated pressurised aerosol for patients with poor inhaler technique. Thorax. 1991;46:712–6. doi: 10.1136/thx.46.10.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 9.Bryant L, Bang C, Chew C, Baik SH, Wiseman D. Adequacy of inhaler technique used by people with asthma or chronic obstructive pulmonary disease. J Prim Health Care. 2013;5:191–8. [PubMed] [Google Scholar]
- 10.Melani A, Bonavia M, Cilenti V, Cinti C, Lodi M, Martucci P, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–8. doi: 10.1016/j.rmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Stuart BO. Deposition of inhaled aerosols. Arch Intern Med. 1973;131:60–73. [PubMed] [Google Scholar]
- 12.Ammari WG, Chrystyn H. Optimizing the inhalation flow and technique through metered dose inhalers of asthmatic adults and children attending a community pharmacy. J Asthma. 2013;50:505–13. doi: 10.3109/02770903.2013.783064. [DOI] [PubMed] [Google Scholar]
- 13.Newman SP. Aerosols and the Lung. In: Clarke SW, Pavia D, editors. Therapeutic aerosols. Butterworth: England; 1984. pp. 197–224. [Google Scholar]
- 14.Prabhakaran L, Lim G, Abisheganaden J, Chee CBE, Choo YM. Impact of an asthma education programme on patient's knowledge, inhaler technique and compliance to treatment. Singapore Med J. 2006;47:225–31. [PubMed] [Google Scholar]
- 15.Janson S, McGrath K, Covington J, Cheng S, Boushey H. Individualized asthma self management improves medication adherence and markers of asthma control. J Allergy Clin Immunol. 2009;123:840–6. doi: 10.1016/j.jaci.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rootmensen G N, Keimpema A R J, Jansen H M, Haan R J. Predictors of incorrect inhalation technique in patients with asthma or COPD: A study using a validated videotaped scoring method. J Aerosol Med and Pulm Drug Deliv. 2010;5:323–8. doi: 10.1089/jamp.2009.0785. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan A, Price D. Matching inhaler devices with patients: The role of primary care physician. Can Respir J. 2018;23:9. doi: 10.1155/2018/9473051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melani AS. Inhalatory therapy training: A priority challenge for the physician. Acta Biomed. 2007;78:233–45. [PubMed] [Google Scholar]
- 19.Chapman KR, Voshaar T, Virchow JC. Inhaler choice in primary practice. Eur Resp Rev. 2005;14:117–22. [Google Scholar]
- 20.Leung JM, Bhutani M, Leigh R, Pelletier D, Good C, Sin DD. Empowering family physicians to impart proper inhaler teaching to patients with chronic obstructive pulmonary disease and asthma. Can Respir J. 2015;22:266–70. doi: 10.1155/2015/731357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez JL, Alarcon RLV, Leiva VA, Becerra ML, Fernandez FL, Fonseca PB. Efficacy of an educational intervention in primary health care in inhalation techniques: Study protocol for a pragmatic cluster randomized controltrial. Trials. 2016;17:144. doi: 10.1186/s13063-016-1269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochrane MG, Bala MV, Downs KE, Masukopf J, Ben-Joseph RH. Inhaled corticosteroids for asthma therapy: Patient compliance, devices and inhalation technique. Chest. 2000;117:542–50. doi: 10.1378/chest.117.2.542. [DOI] [PubMed] [Google Scholar]
- 23.Kamps AW, Brand PL, Roorda RJ. Determinants of correct inhalation technique in children attending a hospital based asthma clinic. Acta Paediatr. 2002;91:159–63. doi: 10.1080/080352502317285144. [DOI] [PubMed] [Google Scholar]
- 24.Vander Palen J, Klein JJ, Kerkhoff AH, van Herwaarden CL. Evaluation of the effectiveness of four different inhalers in patients with chronic obstructive pulmonary disease. Thorax. 1995;50:1183–7. doi: 10.1136/thx.50.11.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]


