Abstract
STUDY QUESTION
Do embryos with delayed blastulation have inferior reproductive potential or poorer outcomes due in part to embryo and endometrial synchrony?
SUMMARY ANSWER
Diminished outcomes in embryos with delayed blastulation undergoing fresh embryo transfer (ET) may be attributed to a loss of embryonic-endometrial synchrony.
WHAT IS KNOWN ALREADY
Embryos that blastulate slowly have lower sustained implantation rates (SIR) than those which blastulate normally on Day 5 (D5). Traditionally this has been attributed to reduced embryo quality; however, dyssynchrony with the endometrium is also a possibility and has not been fully described. This convenient cohort composed of groups that resulted from a practice wide change in laboratory protocol allows for evaluation of embryo and endometrial synchrony as it related to blastocyst expansion.
STUDY DESIGN, SIZE, DURATION
A retrospective cohort analysis was carried out from January 2009 to February 2013. Three cohorts were identified: D5 ET, D6 ET and frozen ET that comprised 822 patients, 763 patients and 718 patients, respectively. Each of these cohorts had slowly blastulating and normally blastulating embryos identified.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The study setting was academic affiliated private practice. All first fresh or cryopreserved ETs from 2010 to 2013 were studied. Non-biopsied embryos were classified into two groups on D5: slowly blastulating (Morula-Gardner 1) or normally blastulating (Gardner 2–6). Only single ETs or transfer of two embryos within the same classification group were included. Outcomes were compared between classification groups in embryos undergoing transfer on D5, D6, or after cryopreservation. This assesses the impact of transfer timing in fresh cycles as well as isolating a pure embryonic factor in frozen ET cycles. Sustained implantation was defined as heart beat detection at discharge sonogram at 8 weeks gestation. SIR was defined as the number of embryos transferred meeting criteria for sustained implantation divided by the total number of embryos transferred.
MAIN RESULTS AND THE ROLE OF CHANCE
In total, 3391 embryos were transferred to 1966 patients. On D5, SIRs were significantly lower with slowly blastulating embryos (44% versus 64% in women <35 years of age (P < 0.001) and 18% versus 56% in women ≥35 years of age (P < 0.001)). Fresh D6 ETs also had significantly lower SIRs for embryos that were slowly blastulating on D5 (52% versus 63% in <35 years of age (P < 0.05) and 32% versus 48% in ≥35 years of age (P < 0.005)) despite continued development to full blastocysts and being morphologically equivalent at the time of ET, suggesting dyssynchrony. However, when slowly blastulating embryos underwent vitrification and then ET, they had SIRs which were equivalent to their normally blastulating counterparts (57% versus 60% in <35 years of age (P = 0.5) and 37% versus 42% in ≥35 years of age (P = 0.3)). An intraclass correlation and a generalized estimating equation corrected for patient age was performed which confirmed these findings. The normalization in cryopreserved ETs indicates that dyssynchrony may be a major adverse factor limiting outcomes with late blastulating embryos in fresh cycles.
LIMITATIONS, REASONS FOR CAUTION
This is a retrospective study comprising cohorts from a convenient sample chosen due to a uniform change in embryology laboratory protocol regarding ET day, however, this was done independent of the management of embryo and endometrial synchrony. Although strict ultrasound and serum progesterone criteria were utilized to make endometrial receptivity uniform, pathologic states of pre-receptive and post-receptive endometrium cannot be ruled out.
WIDER IMPLICATIONS OF THE FINDINGS
The data surrounding embryo and endometrial synchrony should be considered in patients undergoing IVF and attention to the variations in blastulation rates can be applied to any patient undergoing extended embryo culture.
STUDY FUNDING/COMPETING INTERESTS
None.
Keywords: synchrony, blastocyst, endometrial receptivity, embryonic competence, embryo-endometrial synchrony
Introduction
ART has evolved at a rapid pace. Great strides have been made in enhancing embryo selection due in large part to the development of extended embryo culture (Reh et al., 2010). Additionally, the advent of vitrification has enabled safe cryopreservation and warming of embryos (Abdelhafez et al., 2010). These innovations have allowed for a renewed focus on embryo and endometrial synchrony: the concept that there are finite periods of time during which the embryo is able to implant and the endometrium is able to accept it (Shapiro et al., 2008; Van Voorhis and Dokras, 2008). The question now: are these singular yet interdependent events?
WHAT DOES THIS MEAN FOR PATIENTS?
Embryos that grow more slowly in the laboratory during the early steps of IVF have lower implantation rates than those that grow normally and are transferred on Day 5. This has been explained so far by an embryo which grows more slowly being of lower quality. However, timing may also be a factor: the new idea is that both embryo growth and timing with the lining of the uterus may be important.
This study was possible because of a change in laboratory protocol, which allowed researchers to analyze embryo and endometrial timing in relation to embryo growth speed—either slow or normal.
The results showed that normally developing embryos had the same high implantation rates on Day 5 and Day 6 after fresh transfer to the uterus. In contrast, the slowly developing embryos had poorer outcomes on both Days 5 and 6. However, when slowly growing embryos were frozen and then transferred they had implantation rates that were the same as their normally growing counterparts.
This suggests that the timing of embryo growth and the state of the endometrial lining (which is vital for the developing embryo) may be a major adverse factor limiting successful outcome when slowly growing embryos are used in fresh IVF cycles. These results should help to understand and improve the overall success of IVF.
Extended culture is central to answering this question. In nature, gametes meet, unite, and travel in the form of a developing embryo down the fallopian tube and ultimately enter the endometrial cavity on Day 5 or 6 (D5 or 6) (Norwitz et al., 2001). Additionally, given that the embryo begins major transcription at the two cell stage resulting in autocrine and paracrine signaling, it would seem that replacement at various time points may alter the microenvironment in which the embryo exists in the uterus (Boiso et al., 2002).
Current extended culture practices allow for good survival of blastocyst in vitro. Additionally, it has allowed for enhanced selection with morphologic assessment of embryos prior to replacement on D5 or 6 in order to select against those not destined by developmental potential to reach blastocyst stage (Reh et al., 2010), clearly a requirement for a successful pregnancy. Traditionally, embryologic grading results in better scores being assigned to more rapidly developing embryos and thus preferential transfer of these embryos (Dokras et al., 1993; Van Voorhis and Dokras, 2008). This differential developmental pace was recognized over two decades ago. But does this differential mean that those embryos that develop more slowly, despite eventually fully blastulating, are intrinsically of poorer reproductive potential than their more rapidly dividing counterparts? Perhaps this grading paradigm is measuring something different entirely. Could slowly developing embryos have adequate reproductive potential that is thwarted by embryo-endometrial dyssynchrony?
This theory is harmonious with the literature on premature progesterone rise during stimulated cycles in ART (Van Voorhis and Dokras, 2008). There is a physiologic shift in the rise of progesterone seen during IVF cycles as comparted to natural cycles (Franasiak et al., 2016). This advancement is confirmed on uterine histologic analysis (Mirkin et al., 2004). Although the precise progesterone threshold required to induce a secretory change in the endometrium is still debated (Silverberg et al., 1994; Chetkowski et al., 1997; Bosch et al., 2003; Melo et al., 2006; Venetis et al., 2007), this premature rise suggests that the threshold, whatever it is, is crossed ~16 h early in IVF. The secretory change in the endometrium then progresses along a cascade of events that culminate in a period of approximately 48 h—the ‘window of embryonic receptivity’—which now exists 16 h earlier in IVF when compared to the natural cycle. This fact may be at the core of why embryos which blastulate more quickly—thus reaching their window of embryonic ability to implant—align more often with the window of endometrial receptivity (Shapiro et al., 2001).
In order to test this theory, the present study compared sustained implantation rates (SIR) between two groups—slowly blastulating embryos and normally blastulating embryos—transferred in one of three settings: fresh on D5, fresh on D6, or in a subsequent frozen embryo cycle. It has been noted that controlled ovarian stimulation can affect endometrial receptivity (Horcajadas et al., 2008). In order to limit this effect and ensure uniformity to the extent possible, the patients included had ultrasound assessment of the endometrium and serum assessment of progesterone on the day of ovulation trigger to assess for eligibility for fresh embryo transfer (ET). Given prior literature, it could be assumed that late blastulating embryos would fare poorly compared to their normally blastulating counterparts when transferred on D5 or D6. However, if decreased SIR was due to dyssynchrony between the late expanded embryo’s window of ability to implant and the endometrium window of ability to accept the embryo, we would anticipate normalization of SIR in a frozen cycle that has been normalized to assure synchrony.
Materials and Methods
In order to assess and better characterize the impact of embryo-endometrium synchrony on sustained implantation, comparison groups were first established. This was accomplished by embryologic grading on D5 with separation into slowly blastulating and normally blastulating groups, defined below. Then three comparisons were made in order to answer specific questions.
First, SIRs were compared when these embryos were transferred in fresh cycles on D5. This was done to serve as a confirmation of prior literature and lend validity to the comparison groups. Second, SIRs were compared when these embryos were transferred in fresh cycles on D6. At this point the slowly blastulating embryos, as designated on D5, had fully blastulated and were morphologically indistinguishable from normally blastulating embryos. The comparison here was to further clarify the impact of embryo selection on the SIR. If this comparison were to show a difference, it is possible that embryo-endometrial synchrony could be playing a role, rather than embryo quality alone. Finally, SIRs were compared when embryos that had been designated slowly blastulating and normally blastulating in their prior fresh cycle on D5 were vitrified and transferred in a subsequent cryopreserved cycle. This serves to not only control for embryo quality as all were allowed to fully blastulate as with the D6 fresh transfer, but also now control for synchrony. If asynchrony was at work, vitrification and subsequent ET should normalize SIRs between the groups. Due to the fact some cases involved two embryos transferred from the same morphologic grading class, intraclass correlation was performed and, if greater than 10%, a generalized estimating equation (GEE) was planned for each analysis.
Population
The patients examined were from January 2009 to February 2013. During this time frame changes in laboratory protocol practice wide allowed for analysis of the groups. Embryo transfer timing changed from D5 to the morning of D6 as a practice standard, creating the fresh D5 and fresh D6 transfer groups. Additionally, preimplantation genetic screening for aneuploidy (PGT-A) validation was ongoing which required automatic freezing of embryos due to study parameters. Those patients in the control group who did not undergo trophectoderm biopsy and PGT-A served as the cryopreservation cycle cohort. Only embryos that had not undergone biopsy for genetic testing and PGT-A were included in these comparison groups.
Ovarian stimulation protocols did not change during this time frame and were executed for patients per practice routine. Briefly, standard regimens for controlled ovarian hyperstimulation using FSH/hMG were employed along with GnRH agonist (long down-regulation or microdose flare) or GnRH antagonist to prevent a premature LH surge. The protocol was determined by the patient’s primary physician. Monitoring of the IVF cycles was per practice routine. Oocyte maturation was induced with recombinant hCG or with GnRH agonist when two or three follicles reached or exceeded 17–18 mm diameter. Transvaginal oocyte aspiration was performed ~34–36 h later.
Embryos from only the first fresh or cryopreserved cycle were included, thus patients with recurrent implantation failure suggestive of an endometrial defect were not included. Only single ETs or transfer of two embryos within the same group, defined below, were included. As per the standard clinic protocol, patients underwent ultrasound and serum progesterone monitoring on the day of ovulation trigger. The monitoring was performed on the morning of the trigger. Patients with serum progesterone levels greater than or equal to 2.0 ng/ml were excluded from a fresh transfer. Further, patients with ultrasound evidence of progesterone effect, as evidenced by lack of a trilaminar lining, were excluded. Finally, patients were excluded if their endometrium was less than 6 mm thick at surge.
Per practice standard, patient undergoing fresh ET had luteal support provided by vaginal progesterone suppositories with serum progesterone monitoring to ensure adequate support. For patients who underwent a frozen ET, this was accomplished utilizing a programmed step-up oral estradiol regimen followed by i.m. progesterone in oil administration. The ET occurred after five completed days of progesterone supplementation. An ultrasound was performed on the day prior to embryo transfer to ensure the endometrial lining was >6 mm thick and showed appropriate progesterone effect. Monitoring of progesterone levels was accomplished utilizing hormone assessment in serum (SIEMENS Advia Centaur; Tarrytown, NY, USA).
Sustained implantation was defined as heart beat detection at discharge sonogram at 8 weeks gestation. The sustained implantation rate was defined as the number of embryos transferred meeting criteria for sustained implantation over the total number of embryos transferred.
Characterization of embryos
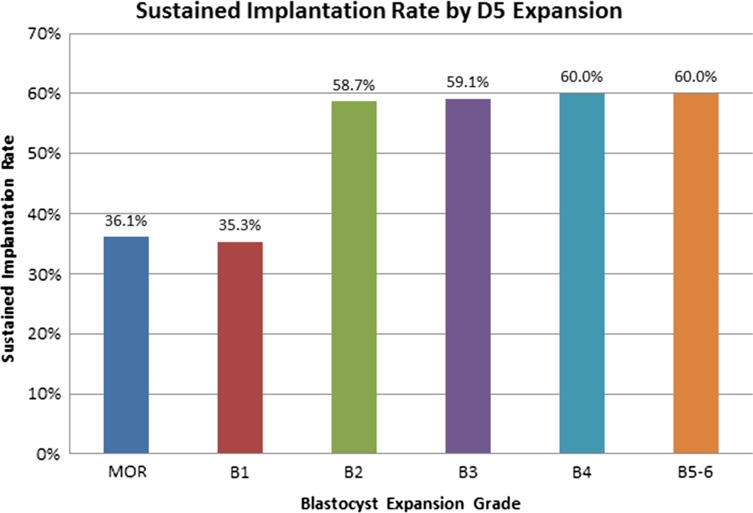
Once the inclusion and exclusion criteria were applied the embryo grading on D5 was examined. In order to determine a cut off for the slowly blastulating versus normally blastulating embryo groups, we examined SIRs across embryo grades at our institution, independent of timing of ET. The SIRs are depicted in Fig. 1. There was a clear demarcation between morula-Gardner 1 and Gardner 2–6. Thus, embryos were classified into two groups based upon D5 assessment: slowly blastulating (morula-Gardner 1) or normally blastulating (Gardner 2–6) (Gardner et al., 2000).
Figure 1.
Overall sustained implantation rates of human embryos meeting the Gardner inclusion criteria. There is a clear demarcation between morula-Gardner 1 and Gardner 2–6 (Gardner et al., 2000). Sustained implantation rate (SIR) was defined as the number of embryos transferred meeting criteria for sustained implantation divided by the total number of embryos transferred. D5: Day 5.
Statistical analysis
Once the embryos had been characterized as slowly blastulating or normally blastulating on D5, SIRs were compared for ETs on D5 fresh, D6 fresh, and ETs in cryopreservation cycles. The data was further subdivided by patient age into less than 35 years, and 35 years and older. Differences in SIRs between the morula-Gardner 1 group and the Gardner 2–6 group were compared using Chi-square tests.
Given the fact that two embryos were transferred in some cases an intraclass correlation was calculated and noted to be 26%. Given this was greater than the 10% allowed, a GEE corrected for oocyte age was utilized. In all cases, an alpha error less than 0.05 were considered significant. IBM SPSS Statistics for Windows, Version 24.0 (IBM Corp. Armonk, NY, USA) was utilized.
Ethical approval
The retrospective data review was approved by the Western internal review board #20021333 protocol #00-014.
Results
There were 3391 embryos transferred to 1966 patients that met criteria for inclusion during this time frame. Of these, a total of 1474 fresh embryos were transferred to 822 patients on D5; 763 fresh embryos transferred to 451 patients on D6; and 1154 cryopreserved embryos transferred to 718 patients. These transfers include only single ETs or double ETs where the embryos were classified in the same developmental group. The ET by patient age group and embryo group are shown in Table I. This data represents the number of embryos which achieved sustained implantation over the total number of embryos transferred in those groups. There was no difference noted by transfer order.
Table I.
Summarized data detailing the sustained implantation rates by embryo transfer day when grouped based upon Day 5 expansion.
| SIR (embryos with sustained implantation/total embryo transferred) by embryo transfer (ET) Day (Fresh D5, Fresh D6, Cryo) and D5 Expansion | |||
|---|---|---|---|
| Age group (years) | P-value | ||
| D5 ET Slow | D5 ET Normal | ||
| <35 | 45/102(44%) | 493/771(64%) | <0.001 |
| ≥35 | 16/90(18%) | 286/511(56%) | <0.001 |
| D6 ET Slow | D6 ET Normal | ||
| <35 | 52/101(52%) | 212/339(63%) | 0.05 |
| ≥35 | 37/116(32%) | 99/207(48%) | 0.005 |
| Cryo ET Slow | Cryo ET Normal | ||
| <35 | 236/416(57%) | 145/243(60%) | 0.5≥ |
| ≥35 | 140/378(37%) | 49/117(42%) | 0.3 |
Numbers represents number of embryos with sustained implantation divided by the total number of embryos transferred. Statistical analysis by chi-square.
Day 5 fresh transfer
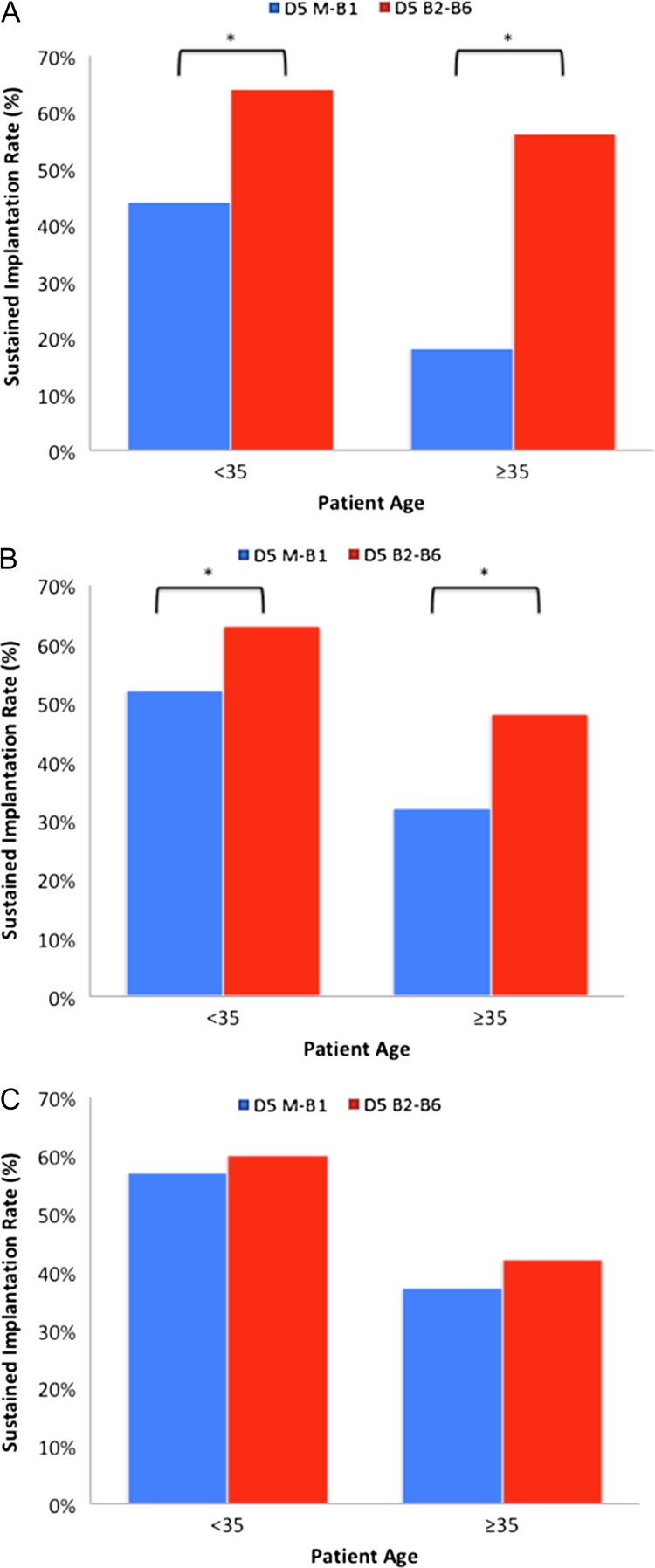
When D5 fresh transfers were compared, SIRs were lower with embryos in the slow (morula-Gardner 1) group when compared to the normal (Gardner 2–6) group in patients under age 35 years (44% and 64%, respectively, P < 0.001) as well as patients 35 years and older (18% and 56%, respectively, P < 0.001). The results of this comparison are seen in Fig. 2A. The GEE showed 0.438 [95% CI 0.328–0.584] (P < 0.001). This data alone does not address whether slow embryos arrested, were of intrinsically poorer quality, or were simply dysynchronous with the endometrium.
Figure 2.
SIRs for slowly and normally blastulating human embryos on Day 5 or Day 6 fresh embryo transfer and frozen embryo transfer. (A) SIRs of embryos transferred on Day 5 (D5) in a fresh cycle. The M-B1 represents the slowly blastulating group and the B2-B6 represents the normally blastulating group. The outcomes were significantly lower in the slowly blastulating group (P < 0.001 for both age groups, in years). (B) SIRs of embryos transferred on D6 in a fresh cycle. Although improved, the outcomes remained significantly lower in the slowly blastulating group (P < 0.05 for under 35 years and P < 0.005 for ages 35 years and older). (C) SIRs of embryos transferred in a cryopreservation cycle. The outcomes were similar (P = 0.5) for those under 35 years and (P = 0.3) for 35 years and older.
Day 6 fresh transfer
Subsequently, the slowly blastulating group and normally blastulating groups were compared when D6 fresh ETs were undertaken. In this group, embryos were now morphologically similar at time of transfer despite their D5 classifications. Furthermore, the arrested embryos in the slowly blastulating group were not available for ET. Thus, if it was an embryo arrest issue or the slowly blastulating embryos were of inferior quality, this group should have shown normalization. Interestingly, embryos classified as slowly blastulating, despite having higher implantation rates than on D5 (Fig. 2A), still had markedly lower SIRs on D6 fresh ET. Lower rates were seen in patients under age 35 years (52% and 63%, respectively, P < 0.05) as well as patients 35 years and older (32% and 48%, respectively, P < 0.005). The GEE mixed effects model showed 0.493 [95% CI 0.378–0.643] (P < 0.001). The results are summarized in Fig. 2B. Thus, despite the fact the embryos did not arrest and morphologically appeared similar, there was still impaired implantation, suggesting synchrony may play a role.
Frozen ET
Finally, SIRs between embryos designated as slowly blastulating and normally blastulating on D5 in their retrieval cycle were compared when they underwent ET in cryopreservation cycles. SIRs between the two groups were found to be equivalent in patients under 35 years (57% and 60%, respectively, P = 0.5) as well as patients aged 35 years and older (37% and 42%, respectively, P = 0.3). Figure 2C summarizes the results. Importantly, slowly blastulating embryos actually had a higher SIR after a cryopreservation cycle than when they underwent a fresh D5 transfer (P = 0.02). The GEE showed 0.931 [95% CI 0.683–1.268] (P = 0.650). The normalization in cryopreservation ETs indicates that embryo-endometrial dyssynchrony may be a major adverse factor limiting outcomes with late blastulating embryos.
Discussion
The data suggest that slowly blastulating embryos have equivalent reproductive potential to normally blastulating embryos when embryo-endometrial synchrony is accounted for. This study contributes to the paradigm shift involving focusing on the embryo and the endometrium as singular and interdependent entities when evaluating synchrony. It is no longer only about premature progesterone rises and endometrial advancement—the embryo and the endometrium ought to be considered simultaneously.
Our findings, which show decreased SIRs when D5 fresh ET is undertaken with slowly blastulating embryos, are consistent with prior literature. What was not apparent in prior data was whether these embryos are intrinsically inferior or if they were biologically competent given the correct conditions. It is known that blastulation rates differ given oocyte age. Shapiro et. al. showed that patient’s aged less than 30 years had much higher blastulation rates prior to D6 than did patient’s aged 31–34 and those aged 35–40 years (Shapiro et al.). Similarly, Forman et al. showed that patients aged 35 years and above have a significantly higher proportion of embryos which have failed to blastulate by D5 when compared to those patients under 35 years of age (Forman et al., 2013).
As for the clinical outcomes for these late blastulating embryos, similar to the shift in window seen with premature rises in progesterone in relation to the endometrium, the shift in the embryonic window confers poorer outcomes. Implantation rates of embryos which blastulate on D6 versus D5 were decreased by 15–18% (Barrenetxea et al., 2005; Shapiro et al., 2008). Initially, one might suspect that this is due to some intrinsic deficit in the embryos. However, the data presented here suggests that the decreased outcomes may be due, in large part, to timing and not to intrinsic deficits in embryonic reproductive competence. The normally blastulating embryos had an equivalent and high SIR on D5 and D6 fresh transfer. The slowly blastulating embryos had poorer outcomes on Day 5 and, although improved, persistently poorer outcomes on fresh Day 6 ET.
The fact that fresh D6 ET still showed a difference between the two groups despite being morphologically identical at the time of ET—the slowly blastulating embryos having time to fully blastulate in extended culture—and having accounted for embryo arrest in the slowly blastulating group is of interest. It suggests that simply performing morphologic assessment on D6 alone may be insufficient to actively manage embryonic dyssynchrony. Of interest is the ability to normalize SIRs between these two groupings by vitrifying embryos and transferring them in a subsequent, and presumably synchronous, cryopreservation cycle.
A retrospective analysis of embryo-endometrial synchrony is complex and comes with expected limitations. The three groups comprising D5 fresh transfer, D6 fresh transfer, and frozen ET represent a convenient cohort. The changes in laboratory practice allowing for these cohorts occurred uniformly at our center. Thus, the groups were created out of uniform practice changes that were independent of the management of embryo-endometrial synchrony. Although efforts were made to control the endometrium given practice standards surrounding ultrasound and serum assessment on the day of ovulation trigger, the possibility of endometrial pathology leading to a pre-receptive or post-receptive state which would be able to be replicated cycle to cycle cannot be entirely excluded (Ruiz-Alonso et al., 2013; Franasiak et al., 2016). Of note, only first ETs were included so as to limit the effect of repeated implantation failure patients. Further aspects of endometrial transcriptome profiling must be considered in light of the controlled ovarian stimulation in fresh ET cycles (Horcajadas et al., 2008). Finally, it is possible that some additional selective pressure was applied in the frozen ET group by the ability of the embryo to survive vitrification and warming; however, there was not a higher rate of survival in the normally blastulating embryos as compared to the slowly blastulating embryos.
The data presented here lend support to the possibility that a subset of the failures seen in IVF may be due to embryo-endometrial dyssynchrony. It is clear that attention must be paid to endometrial factors that advance the endometrial window of receptivity as well as controlling for factors related to the embryonic window during which it is capable of implantation.
Authors’ roles
The authors listed on this manuscript substantially contributed to the study conception and design, acquisition and interpretation of data, and drafting/revising the manuscript. All have given final approval of the version to be published.
Funding
None.
Conflict of interest
The authors have nothing to disclose/declare.
References
- Abdelhafez FF, Desai N, Abou-Setta AM, Falcone T, Goldfarb J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: a systematic review and meta-analysis. Reprod Biomed Online 2010;20:209–222. [DOI] [PubMed] [Google Scholar]
- Barrenetxea G, López de Larruzea A, Ganzabal T, Jiménez R, Carbonero K, Mandiola M. Blastocyst culture after repeated failure of cleavage-stage embryo transfers: a comparison of day 5 and day 6 transfers. Fertil Steril 2005;83:49–53. [DOI] [PubMed] [Google Scholar]
- Boiso I, Veiga A, Edwards RG. Fundamentals of human embryonic growth in vitro and the selection of high-quality embryos for transfer. Reprod Biomed Online 2002;5:328–350. [DOI] [PubMed] [Google Scholar]
- Bosch E, Valencia I, Escudero E, Crespo J, SimonSimón C, RemohiRemohí J, Pellicer A. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril 2003;80:1444. [DOI] [PubMed] [Google Scholar]
- Chetkowski R, Kiltz R, Salyer W. In premature luteinization, progesterone induces secretory transformation of the endometrium without impariment of embryo viability. Fertil Steril 1997;68:292–297. [DOI] [PubMed] [Google Scholar]
- Dokras A, Sargent IL, Barlow D. Human blastocyst grading an indicator of developmental potential? Hum Reprod 1993;8:2119–2127. [DOI] [PubMed] [Google Scholar]
- Forman EJ, Franasiak JM, Hong KH, Scott RT. Late expanding euploid embryos that are cryopreserved (CRYO) with subsequent synchronous transfer have high sustained implantation rates (SIR) similar to fresh normally blastulating euploid embryos. Fertil Steril 2013;100:S99. [Google Scholar]
- Franasiak JM, Ruiz-Alonso M, Scott RT, Simón C. Both slowly developing embryos and a variable pace of luteal endometrial progression may conspire to prevent normal birth in spite of a capable embryo. Fertil Steril 2016;105:861–866. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 2000;73:1155–1158. [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Minguez P, Dopazo J, Esteban FJ, Dominguez F, Giudice LC, Pellicer A, Simon C. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab 2008;93:4500–4510. [DOI] [PubMed] [Google Scholar]
- Melo MAB, Meseguer M, Garrido N, Bosch E, Pellicer A, Remohi J. The significance of premature luteinization in an oocyte-donation programme. Hum Reprod 2006;21:1503–1507. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Nikas G, Hsiu JG, Diaz J, Oehninger S. Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotropin-stimulated cycles. J Clin Endocrinol Metab 2004;89:5742–5752. [DOI] [PubMed] [Google Scholar]
- Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survivial of early pregnancy. N Engl J Med 2001;345:1400–1408. [DOI] [PubMed] [Google Scholar]
- Reh A, Fino E, Krey L, Berkeley A, Noyes N, Grifo J. Optimizing embryo selection with day 5 transfer. Fertil Steril 2010;93:609–615. [DOI] [PubMed] [Google Scholar]
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, Carrera J, Vilella F, Pellicer A, Simón C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril 2013;100:818–824. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Ross R. Contrasting patterns in in vitro fertilization pregnancy rates among fresh autologous, fresh oocyte donor, and cryopreserved cycles with the use of day 5 or day 6 blastocysts may reflect differences in embryo-endometrium synchrony. Fertil Steril 2008;89:20–26. [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Richter KS, Harris DC, Daneshmand ST. A comparison of day 5 and day 6 blastocyst transfers. Fertil Steril 2001;75:1126–1130. [DOI] [PubMed] [Google Scholar]
- Silverberg K, Martin M, Olive DL, Burns WiN, Schenken RS. Elevated serum progesterone levels on the day of human chorionic gonadotropin administration in in vitro fertilization cycles do not adversely affect embryo quality. Fertil Steril. 1994;61:508–513. doi: 10.1016/s0015-0282(16)56584-4. [DOI] [PubMed] [Google Scholar]
- Van Voorhis BJ, Dokras A. Delayed blastocyst transfer: is the window shutting? Fertil Steril 2008;89:31–32. [DOI] [PubMed] [Google Scholar]
- Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update 2007;13:343–355. [DOI] [PubMed] [Google Scholar]