Abstract
STUDY QUESTIONS
The primary objective is to investigate if continuous use of oral contraceptives is non-inferior compared to long-term pituitary desensitization with a GnRH agonist prior to IVF/ICSI in patients with moderate to severe endometriosis with regard to treatment efficacy. Secondary objectives concern treatment safety and cost-effectiveness.
WHAT IS KNOWN ALREADY
Long-term pituitary desensitization with a GnRH agonist for 3–6 months prior to IVF/ICSI improves clinical pregnancy rates in women suffering from endometriosis. However, discussion about this treatment strategy exists because of its uncomfortable side effects. Alternatively, IVF/ICSI pre-treatment with continuously administered oral contraceptives may offer fewer side-effects and lower (in)direct costs, as well as encouraging IVF outcomes in women with endometriosis. To date, these two different IVF/ICSI pre-treatment strategies in women with endometriosis have not been directly compared.
STUDY DESIGN, SIZE, DURATION
An open-label, parallel two-arm randomized controlled multicenter trial is planned, including patients with moderate to severe endometriosis. To demonstrate an absolute difference of 13% (delta of 10% with non-inferiority margin of 3%) with a power of 80% 137 patients per group are sufficient. Taking into account a withdrawal of patients of 10% and a cancelation rate of embryo transfer after ovarian pick up of 10% (for instance due to fertilization failure), the sample size calculation is rounded off to 165 patients per group; 330 patients in total will be included. After informed consent, eligible patients will be randomly allocated to the intervention or reference group by using web based block randomization stratified per centre. Study inclusion is expected to be complete in 3–5 years.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The research population consists of patients with moderate to severe endometriosis (ASRM III/IV) who are scheduled for their first, second or third IVF/ICSI treatment attempt. Women aged over 41 years, younger than 18 years, with a known contraindication for the use of oral contraceptives and/or GnRH agonists or with severe male factor infertility will be excluded from participation. After informed consent patients are allocated to the intervention group (one-phase oral contraceptive continuously during three subsequent months) or the reference group (three Leuprorelin 3.75 mg i.m./s.c. depot injections during three subsequent months). Tibolon 2.5 mg can be given daily as add-back therapy in the reference group. After 3 months of pre-treatment the IVF/ICSI stimulation phase will be started. The primary outcome is live birth rate after fresh embryo transfer. Secondary outcomes are cumulative live birth rate after one IVF/ICSI treatment cycle (including fresh and frozen embryo transfers up to 15 months after randomization), ongoing pregnancy rate and time to pregnancy. In addition, treatment outcome parameters, adverse events, side-effects during the first 3 months, complications, recurrence of endometriosis (complaints), quality of life, patient preferences, safety and costs effectiveness will be reported. Measurements will be performed at baseline and at 3, 6, 9, 12 and 15 months after randomization.
STUDY FUNDING/COMPETING INTEREST(s)
All authors have no conflict of interest related to this manuscript. The department of reproductive medicine of the Amsterdam UMC location VUmc has received several research and educational grants from Guerbet, Merck and Ferring not related to the submitted work.
TRIAL REGISTRATION NUMBER
The trial is registered as the COPIE trial (Continuous use of Oral contraceptives as an alternative for long-term Pituitary desensitization with a GnRH agonist prior to IVF/ICSI in Endometriosis patients) in the Dutch Trial Register (Ref. No. NTR6357, http://www.trialregister.nl).
TRIAL REGISTRATION DATE
16 March 2017.
DATE OF FIRST PATIENT’S ENROLMENT
Enrollment is planned for November 2018.
Keywords: endometriosis, assisted reproduction, IVF/ICSI outcome, pregnancy, cost effectiveness, infertility, GnRH agonist/antagonist
Introduction
Endometriosis is associated with subfertility in up to 50% of patients (Meuleman et al., 2009). In patients with severe endometriosis, subfertility is presumed to be based on a distortion of the pelvic anatomy, a hostile peritoneal environment, a decreased oocyte quality, a diminished ovarian reserve (due to the presence of endometrioma or prior ovarian surgery) and/or an impaired implantation due to an altered endometrial receptivity (Giudice, 2010; de Ziegler et al., 2010a).
What this means for patients.
Women with moderate to severe endometriosis receiving IVF or ICSI are nowadays advised to use a GnRH agonist during 3 months before IVF or ICSI in order to increase pregnancy rates. A GnRH-agonist treatment could be accompanied with unpleasant side effect such as hot flushes and vaginal dryness. In this study, we would like to investigate if pre-treatment with an oral contraceptive is as effective in pursuing pregnancy after IVF or ICSI with lower side effects and less costs.
Especially in cases of tubal dysfunction, in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) are stated as appropriate treatments for subfertility in endometriosis patients by the European Society of Human Reproduction and Embryology (ESHRE) guideline (Dunselman et al., 2014). However, inferior success rates are especially described in patients with moderate to severe endometriosis (American Society for Reproductive Medicine (ASRM) Stage III and IV) and in patients with endometrioma(s) (Barnhart et al., 2002; Coccia et al., 2011; Opoien et al., 2012; Johnson and Hummelshoj, 2013).
To improve pregnancy rates after IVF/ICSI, it is recommended to precede IVF/ICSI by long-term pituitary desensitization with a GnRH agonist for 3–6 months, since this is shown to improve clinical pregnancy rates (CPR) (Dale et al., 1990; Dicker et al., 1990, 1992; Nakamura et al., 1992; Chedid et al., 1995; Rickes et al., 2002; Surrey et al., 2002; Sallam et al., 2006; Ma et al., 2008). However, the mechanism responsible for this increase in clinical pregnancy rate is not yet clarified and more research is needed.
Furthermore, as both the Cochrane and ESHRE recommendations are based upon only three small randomized studies (N = 165) (Dicker et al., 1992; Rickes et al., 2002; Surrey et al., 2002), executed in a different IVF/ICSI treatment era in which more aggressive stimulation was used and multiple embryos were transferred, debate about this treatment strategy exists. Additionally, different study-populations with varying degrees of endometriosis (ASRM Stage II–IV) were included in these trials (Dicker et al., 1992; Rickes et al., 2002; Surrey et al., 2002), which could have influenced the results.
It is also postulated that the use of a long-term pituitary desensitization regime may lower ovarian response to ovarian stimulation, especially in poor responders (Griesinger et al., 2008). In addition, uncomfortable side effects, such as vasomotor instability, are often reported by patients. These side effects might result in a restrained attitude in prescribing this treatment strategy for a longer period of time, although add-back therapy is available to diminish these vasomotor side-effects (Hornstein et al., 1998; Zupi et al., 2004). On the other hand, patients who used long-term GnRH agonists prior to IVF reported to be motivated to use this treatment strategy again, despite side-effects, in a next IVF/ICSI attempt (van der Houwen et al., 2014a).
Alternatively, the effect of continued use of oral contraceptives (OCs) for 6–8 weeks prior to IVF/ICSI has been investigated (de Ziegler et al., 2010b). Non-randomized data show that this treatment is favourable in patients with severe endometriosis undergoing IVF/ICSI, as clinical pregnancy rates were higher compared to endometriosis patients treated without OCs and comparable to that of control patients without endometriosis (de Ziegler et al., 2010b). However, a randomized comparison between continuous use of OCs and long-term pituitary desensitization with a GnRH agonist prior to IVF/ICSI in patients with endometriosis has not yet been made.
The daily uncomfortable vasomotor side-effects that are associated with a GnRH agonist treatment without add-back therapy, are negatively associated with work-ability and increase the risk of sickness absence (Simoens et al., 2011, 2012; Geukes et al., 2012; de Graaff et al., 2013). It is currently unknown if this can be positively influenced by using add-back therapy.
Although the tolerability of OC is higher compared to GnRH agonists (with or without add-back therapy) (Berlanda et al., 2016), its use is known to be often associated with migraine, weight gain, depression and with a higher risk on venous and arterial thromboembolism (Hee et al., 2013, Beyer-Westendorf, et al., 2018). By using OC continuously, a reduction in monthly blood loss is expected combined with a decrease in dysmenorrhea compared to cyclic use of OCs. However, this seems to be related with a higher risk on spotting (Hee et al., 2013).
Due to potentially fewer side effects, improvement of societal productivity can be expected in women receiving continuous OC treatment instead of GnRH agonist treatment. Moreover, since GnRH agonists are over 40 times more expensive than OCs, a reduction in directs costs of medication used in IVF/ICSI can be achieved by prolonged use of OCs prior to IVF/ICSI treatment.
Therefore, this study is conducted to investigate if continuous use of OC is non-inferior to long-term pituitary desensitization with a GnRH agonist (as standard care) combined with add-back therapy (if necessary) prior to IVF/ICSI treatment, with regard to treatment efficacy, safety and cost-effectiveness.
Outcomes
The primary outcome is live birth rate after fresh embryo transfer (ET). Secondary outcomes are listed in Table I. Measurements will be performed at baseline (t0), three (t1), six (t2), nine (t3), twelve (t4) and fifteen (t5) months after randomization.
Table I.
Secondary outcomes.
| Clinical |
|
| Treatment |
|
| Endometriosis |
|
| Cost-effectiveness and BIA |
|
| Factors to be taken into account |
|
IVF = in vitro fertilization; ICSI = intracytoplasmic sperm injection; EHP-30 = Endometriosis Health Profile 30; ET= embryo transfer; hCG = human chorionic gonadotrophin; TVS = transvaginal sonography; VAS = Visual Analogue Scale; BIA = budget impact analysis; iMCQ = iMTA Medical Consumption Questionnaire; iPCQ = iMTA Productivity Cost Questionnaire; iMTA = Institute for Medical Technology Assessment; MUSA = Morphological Uterus Sonographic Assessment (van den Bosch et al., 2015); OC = oral contraceptive.
Materials and Methods
The study will have a parallel two-arm randomized controlled non-inferiority design and will be open-label. Recruitment of patients will be performed in two tertiary care centres in the Netherlands. The research population will consist of women with endometriosis ASRM Stage III or IV who are scheduled for their first, second or third IVF/ICSI treatment. The diagnosis of endometriosis has to be surgically confirmed or likely to be present based on transvaginal sonography (TVS) or magnetic resonance imaging (MRI) findings, including the presence of uni- or bilateral ovarian endometrioma and deep endometriosis. For diagnosing endometrial cysts and/or deep endometriosis diagnostic classification systems for imaging modalities like sonography and/or MRI do not exists, although guidelines help clinicians in describing sonographic findings in a structural way (Guerriero et al., 2016). Clinics involved in this study will use the systematic approach for sonography as presented by the IDEA group (Guerriero et al., 2016). Sonography and MRI are both shown to be accurate enough to diagnose endometrial cysts in the ovaries and deep endometriosis in the lower bowel and other pelvic structures (Nisenblat et al., 2016). Also women with surgically treated (i.e. remediated) moderate to severe endometriosis will be included. Excluded from participation will be women aged under 18 and over 41 years, women with a known contraindication for the use of OCs (history of venous thromboembolism (VTE), hepatic adenoma(s), positive family history for VTE and/or known thrombophilic abnormalities (Middeldorp, 2011)) or for the use of GnRH agonists (severe side-effects and/or allergic reaction to GnRH agonists), pregnant women, women with a malignancy or severe male factor infertility (i.e. azoospermia). Non-Dutch speaking women will be excluded. Women can only participate once in the study.
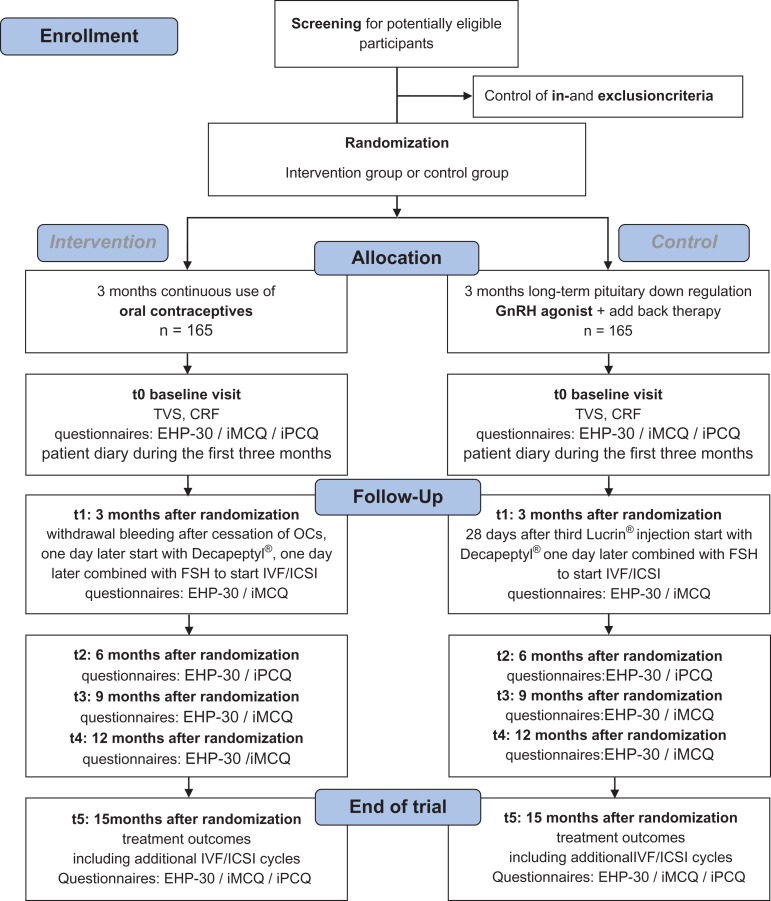
The study schedule is presented in the Consort Flow Diagram (Fig. 1). Before study entry, women will be screened for eligibility. Women who comply with all selection criteria will be informed about the trial during a visit at the outpatient clinic or IVF centre. Information will be handed out by a gynaecologist, resident, fertility physician or research nurse offering a reflection time of minimally 7 days. Informed consent must be signed before any study-related procedures can be carried out. After informed consent is obtained, women will be randomly assigned to the intervention group (one-phase oral contraceptive continuously administered during three subsequent months) or the reference group (three GnRH agonist depot injections during three subsequent months combined with add-back therapy if necessary). Randomization will be performed via web based randomly permuted blocks with variable, randomly chosen sizes (4–6–8 patients) stratified by participating centre. Drop outs after randomization will not be replaced.
Figure 1.
Consort Flow Diagram and study schedule. CRF = case record form; EHP-30 = Endometriosis Health Profile 30; GnRH = gonadotropin releasing hormone; ICSI = intracytoplasmic sperm injection; iMCQ = iMTA Medical Consumption Questionnaire; iMTA = institute for Medical Technology Assessment; iPCQ = iMTA Productivity Cost Questionnaire; IVF = in vitro fertilization; n = number; t = time point; TVS = transvaginal sonography.
After randomization, at baseline (t0) a TVS will be performed, to determine the presence of endometrioma and signs of deep endometriosis. Baseline characteristics and medical history will be listed in the case report file (CRF). Participants will be asked to complete different questionnaires at baseline (t0), three (t1), six (t2), nine (t3), twelve (t4) and fifteen (t5) months after randomization. These validated questionnaires measure quality of life of women with endometriosis (Endometriosis Health Profile 30 (EHP-30) (Jones et al., 2004)), direct and indirect medical costs (the Medical consumption Questionnaire (iMCQ) (Bouwmans, 2013a) and the Productivity Cost Questionnaire (iPCQ) (Bouwmans, 2013b)). During the first 3 months of treatment medical consultation, side-effects and absence of work will also be recorded in a patients’ diary.
All women in the intervention group will receive one-phase oral contraceptives (sub-50 OCs) continuously administered during three subsequent months (i.e. 3 × 28 days). Preferably ethinylestradiol/levonorgestrel 30/150 μg is prescribed. Since a lot of patients already use specific types of OCs, other one-phase sub-50 OCs will be accepted as well. Women will be instructed to contact the IVF department on the first day of bleeding after stopping the OC (after 3 months) treatment. On day two of the withdrawal bleeding women will visit the IVF centre and Triptorelin ‘Decapeptyl’ s.c. will be started for suppression of the luteinizing hormone (LH) peak. One day later ovarian stimulation will be started with subcutaneously injected gonadotrophins (follicle stimulation hormone (FSH)) in an individually determined dosage.
All women in the reference group will receive three Leuprorelin ‘Lucrin’ 3.75 mg i.m. or s.c. depot injections during three subsequent months. During these months Tibolon ‘Livial’ 2.5 mg tablets can be given daily as add-back therapy. Women will have an appointment at the IVF department exactly 28 days after the last Leuprorelin injection. According to protocol, daily administration of Triptorelin ‘Decapeptyl’ s.c. will then be started. One day later ovarian stimulation will be started with subcutaneously injected gonadotrophins (FSH) in an individually determined dosage.
When the ovarian stimulation cycle is started women in both groups will be monitored routinely by standard (local) protocols, depending on follicle growth monitored by TVS. FSH dose adjustments during the stimulation cycle are allowed. Monitoring will be continued until the criteria for human chorionic gonadotrophin (hCG) injection are met (three or more follicles of 17 mm), to achieve final oocyte maturation. Oocyte retrieval will be carried out 34–37 h after hCG injection. Embryos will be cultured and incubated. Embryos will be morphologically assessed by combining the number of blastomeres and the percentage of fragmentation. The embryo with the highest number of blastocysts and the least fragmentation rate will be transferred 48–80 h after oocyte retrieval. The number of morphologic top-quality embryos (TQE) will be assessed and elective single embryo transfer (eSET) will be performed in the first IVF/ICSI cycle and ≤2 embryos will be selected for transfer to the uterus in the second or third cycle of IVF/ICSI. Remaining embryos of good quality will be cryopreserved according to local protocols. Luteal support and pregnancy testing will be accomplished by local protocols.
All additional IVF/ICSI treatment cycles (including subsequent pregnancies) up to 15 months after randomization (t5) will be registered in both intervention and control groups.
Sample size
We performed a retrospective study (van der Houwen et al., 2014b) regarding the efficacy and safety of IVF/ICSI in patients with moderate to severe endometriosis, showing ongoing pregnancy rates of 20% after fresh embryo transfer. de Ziegler et al. (2010b) reported clinical pregnancy rates of almost 40% in patients with moderate to severe endometriosis with or without the presence of an endometrioma during IVF with OCs as a pre-treatment. Taking into account a miscarriage rate of 20% (Matalliotakis et al., 2007), an ongoing pregnancy rate of 30% can be expected after continuous use of OCs. Therefore, assuming that the ongoing pregnancy rate in patients treated with long-term pituitary desensitization with a GnRH agonist prior to IVF/ICSI is 20% and in patients treated with continuous use of OCs prior to IVF/ICSI is 30%, 137 patients per group are sufficient to demonstrate an absolute difference of 13% (i.e. a non-inferiority margin of 3%) with an 80% power that the live birth rate in the continuous use of OCs group is not inferior to the live birth rate in the long-term pituitary desensitization with a GnRH agonists group. The non-inferiority margin of 3% is arbitrary chosen. Justification can be arranged by performing a discrete choice experiment taking into account efficacy, safety and burden of treatment. For instance, an earlier published trial concerning patients’ preference regarding GnRH agonist or antagonists in IVF/ICSI showed that with a trade-off of 2.0% increase in pregnancy rate patients would switch from antagonists to GnRH agonists (van den Wijngaard et al., 2014). Therefore, taking into account more benefits of oral contraceptives over GnRH agonists, a non-inferiority margin of 3% appears to be justified. The significance level is set at α 0.05, one sided. To take into account loss to follow-up due to withdrawal of patients of 10% and a cancelation rate of embryo transfer after ovarian pick up of 10% (for instance due to fertilization failure) the sample size is rounded off to 165 patients per group. In total, 330 patients need to be included in this study.
Statistical analysis
SPSS will be used for statistical analysis. Continuous data will be presented as means and standard deviations (normally distributed data) or medians and ranges (non-parametrical data). In case of dichotomous or categorical data numbers with percentages will be used.
The primary analysis (live birth rate after fresh embryo transfer) will be performed according to the intention to treat (ITT) principle including all randomized patients. The effectiveness of the interventional treatment versus the reference treatment will be expressed as a rate for live birth with corresponding 95% confidence interval. We will consider the intervention inferior when the absolute difference in success rate exceeds 13% compared to the expected success rate of 30% – this is comparable to a relative difference of 0.17/0.30 is 0.57. If the left border of the 95% CI of the RR does not exceed the pre-defined threshold of 0.57 for inferiority we will consider the intervention to be non-inferior to the reference treatment. A secondary analysis for effectiveness will be done on the per-protocol (PP) population. Non-inferiority of the intervention is stated if non-inferiority is shown in both the ITT and PP population.
All secondary outcomes will be compared in both the ITT and PP analysis. The live birth rates in both groups will be compared using Kaplan–Meier analysis. Dichotomous and categorical data will be analysed using either Fisher Exact or chi-square as appropriate. For continuous outcomes, the confidence interval will be based on (the Welch version of) the t-test if the observations are normally distributed. If continuous outcomes are non-normally distributed, the Mann–Whitney-U test will be employed. A probability (P) of less than 0.05 will be considered statistically significant. Because of the non-inferiority design of this study, for a better interpretation of the findings, confidence intervals around point estimates will also be provided.
An economic evaluation will be performed alongside the randomized controlled trial from a societal and a health care perspective according to Dutch guidelines with a time horizon of 15 months after randomization (i.e. 12 months after start of hormonal stimulation for IVF/ICSI treatment) (Zorginstituut Nederland, 2016).
Cost categories that will be included are: (1) healthcare costs (primary and secondary care, complementary care and home care); (2) lost productivity costs (absenteeism from paid and unpaid work, and presenteeism) and (3) patient costs (informal care and other care services paid for by patients themselves). Valuation will be done according to Dutch costing guidelines (Hakkaart-van Roijen et al., 2016). For the valuation of health care utilization, lost productivity and informal care Dutch standard costs will be used. Medication use will be valued using prices of the Royal Dutch Society for Pharmacy. Patient and family costs other than informal care will be valued using self-reported prices. For the valuation of absenteeism from paid work, the friction cost approach will be used. To document absence from paid work the iPCQ (Bouwmans, 2013b) will be used at t0, t2 and t5. Besides, absence of work will be evaluated with a patient diary during the first 3 months of treatment.
The economic evaluation will be analysed by the ITT principle. Missing cost and effect data will be imputed using multiple imputation according to the MICE algorithm (van Buuren et al., 1999). Rubin’s rules will be used to pool the results from the different multiply imputed datasets. Multiple regression analyses will be performed to estimate cost and effect differences between OC pre-treatment and the reference treatment of long-term pituitary desensitization with a GnRH agonist, and adjusted for confounders. Incremental cost-effectiveness ratios (ICERs) will be calculated by dividing the difference in the mean total costs by the difference in mean effect between the two groups. Bias-corrected and accelerated bootstrapping with 5000 replications will be used to estimate 95% confidence intervals around the cost differences and statistical uncertainty surrounding the ICERs. Cost-effectiveness acceptability curves will also be estimated for a range of different ceiling ratios, thereby showing decision uncertainty (Fenwick et al., 2004).
In sensitivity analyses we will evaluate the effect of 50% higher or lower endometriosis-related complaints and/or side effects requiring surgery.
A budget impact analysis (BIA) is planned to be performed. The time horizon for the BIA will be 15 months after randomization up to the birth of a child. For our final BIA we will evaluate (I) a scenario where OCs will be implemented in all cases, (II) a scenario where OCs will be implemented in 70% of the cases, and (III) sensitivity analyses on productivity loss.
Data management and monitoring
The datasets generated or analysed during the current study will be available from the corresponding author on reasonable request. An interim analysis for efficacy will not be performed. All Serious Adverse Events (SAEs) and Suspected Unexpected Serious Adverse Reactions (SUSARs) will be reported to the Medical Ethics Committee (METc). All Adverse Events (AEs) will be recorded and followed until they have abated, or until a stable situation has been reached. Depending on the event, follow up may require additional tests or medical procedures as indicated, and/or referral to the general physician or a medical specialist. An annual safety report will be send to the accredited METc and the competent authority. A Data Safety Monitoring Board (DSMB) will not be installed since all medication used in this study is registered for the given indication and used in clinical practice for years.
Ethical approval
This study is approved by the National Central Committee on Research involving Human Subjects (CCMO – NL 59874.026.16) and by the METc of the VU University Medical Center (Ref. No. 2016.570). The trial is registered as the COPIE trial (Continuous use of Oral contraceptives as an alternative for long-term Pituitary desensitization with a GnRH agonist prior to IVF/ICSI in Endometriosis patients) in the Dutch Trial Register (Ref. No. NTR6357, http://www.trialregister.nl). From all participants written informed consent will be obtained before they will be enroled in the study.
Discussion
In women with moderate to severe endometriosis, IVF/ICSI treatment is frequently applied to overcome endometriosis-associated infertility (Surrey, 2015). This randomized controlled trial is the first to investigate the non-inferiority and cost-effectiveness of continuous use of OCs compared to long-term pituitary desensitization with a GnRH agonist prior to IVF/ICSI treatment in women with endometriosis ASRM Stage III/IV. An IVF/ICSI treatment strategy with continuous use of OCs holds promise to be more patient friendly as well as cost-effective compared with long-term pituitary desensitization with a GnRH agonist. To our knowledge there are currently no other (registered) ongoing trials that evaluate both treatment regimens.
The COPIE trial is an adequately powered trial. Since a new treatment regime (OCs) will be compared with the currently used standard treatment (GnRH agonist) a non-inferiority design is appropriate (Piaggio et al., 2012). In case of a superiority design, a larger sample size (i.e. 650 women in total) would be needed, but this would not necessarily lead to different conclusions. If OC prior to IVF/ICSI is non-inferior, the current standard can be replaced and less women have to experience the discomforts that are related to the use of a GnRH agonist.
Due to the different application forms of GnRH agonist (subcutaneously or intramuscularly administered) and OCs (orally administered) the study will be open-labelled. This may influence the outcomes reported by the patient, like quality of life and the experienced side-effects of both treatment regimens, but we do not expect the primary outcome to be influenced.
The risk of bias is reduced by random allocation of patients to the intervention or control group. Block-randomization, stratified for each participating centre, will be web based through which chance of allocation bias will be reduced.
To our best knowledge, it is unknown if add-back therapy has an effect on pregnancy rates after IVF/ICSI. Since the duration of pituitary desensitization is limited to 3 months, it is not obligatory to start with add-back therapy to reduce long-term side effects on bone mineral density. However, in clinical daily practice, add-back therapy can be necessary in patients receiving GnRH analogues, because of severe side effects (e.g. vasomotor complaints). Though, the hypothesis postulated by Barbieri (1992) supports the idea that endometriosis is effectively reduced in terms of endometrial growth and pain reduction by taking into account a threshold for serum estradiol of 30–50 pgram/ml. By using Tibolone 2.5 mg daily, as usually applied in add-back therapy, it is not expected that this threshold will be exceeded (Verheul et al., 2007).
For this trial we use validated questionnaires in the Dutch language. The EHP-30 is a validated and frequently used instrument to measure quality of life in women diagnosed with endometriosis (Jones et al., 2004; van de Burgt et al., 2011). The iMCQ (Bouwmans, 2013a) and iPCQ (Bouwmans, 2013b) are validated questionnaires to measure medical consumption, absence from (paid) work and loss of productivity due to health problems and are both suitable to be used in chronic conditions like endometriosis.
The study is expected to guide future management of women with severe endometriosis undergoing IVF/ICSI, either (when continuous use of OCs is the preferred strategy) improving quality of life and treatment compliance or (when continuous use of OCs is inferior to GnRH agonists) preventing the use of strategy that reduces pregnancy rates. Women and clinicians can use the results in shared-decision making, increasing the chance of pregnancy and with the potential to reduce healthcare expenditures. To improve counselling, a discrete choice experiment is planned to investigate patient preferences. We will evaluate the indirect costs of patients’ loss of societal productivity, due to endometriosis-related symptoms or medication associated side effects. The trial will be supported and promoted by the Dutch Endometriosis Foundation, the patient association for women with endometriosis in The Netherlands. By the intense collaboration with the Dutch Endometriosis Foundation and the NVOG (Dutch Society of Obstetrics & Gynaecology) Working Group on Endometriosis, the results of this trial should stimulate a prompt incorporation in guideline and daily practice. In 2015 this study was recognized by the NVOG as a first priority knowledge gap in reproductive medicine and is listed in the group of studies which are supported by the NVOG (Oepkes and Oudijk, 2015).
Authors’ roles
All authors were involved in the design of this study and made substantial contributions to this manuscript. All authors critically revised and approved the final version of the manuscript.
Conflict of interest
All authors have no conflict of interest related to this manuscript. The department of reproductive medicine of the Amsterdam UMC location VUmc has received several research and educational grants from Guerbet, Merck and Ferring not related to the submitted work.
References
- Barbieri RL. Homone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol 1992;166:740–745. [DOI] [PubMed] [Google Scholar]
- Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril 2002;77:1148–1155. [DOI] [PubMed] [Google Scholar]
- Berlanda N, Somigliana E, Viganò P, Vercellini P. Safety of medical treatments for endometriosis. Expert Opin Drug Saf 2016;15:21–30. [DOI] [PubMed] [Google Scholar]
- Beyer-Westendorf J, Bauersachs R, Hach-Wunderle V, Zotz RB, Rott H. Sex hormones and venous thromboembolism – from contraception to hormone replacement therapy. Vasa 2018;47:441–450. [DOI] [PubMed] [Google Scholar]
- Bouwmans C. Manual iMTA Medical Consumption Questionnaire (iMCQ). 2013. a. Available from: www.imta.nl. (26 June 2018, date last accessed).
- Bouwmans C. Manural iMTA Productivity Cost Questionnaire (iPCQ). 2013. b. Available from: www.imta.nl (26 June 2018, date last accessed). [DOI] [PubMed]
- Chedid S, Camus M, Smitz J, Van Steirteghem AC, Devroey P. Comparison among different ovarian stimulation regimens for assisted procreation procedures in patients with endometriosis. Hum Reprod 1995;10:2406–2411. [DOI] [PubMed] [Google Scholar]
- Coccia ME, Rizzello F, Mariani G, Bulletti C, Palagiano A, Scarselli G. Impact of endometriosis on in vitro fertilization and embryo transfer cycles in young women: a stage-dependent interference. Acta Obstet Gynecol Scand 2011;90:1232–1238. [DOI] [PubMed] [Google Scholar]
- Dale PO, Tanbo T, Abyholm T. Endometriosis-associated infertility treated by long-term gonadotropin-releasing hormone agonist administration and assisted fertilization. J In Vitro Fert Embryo Transf 1990;7:180–181. [DOI] [PubMed] [Google Scholar]
- de Graaff AA, D’Hooghe TM, Dunselman GA, Dirksen CD, Hummelshoj L, Simoens S. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod 2013;28:2677–2685. [DOI] [PubMed] [Google Scholar]
- de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet 2010. a;376:730–738. [DOI] [PubMed] [Google Scholar]
- de Ziegler D, Gayet V, Aubriot FX, Fauque P, Streuli I, Wolf JP, de Mouzon J, Chapron C. Use of oral contraceptives in women with endometriosis before assisted reproduction treatment improves outcomes. Fertil Steril 2010. b;94:2796–2799. [DOI] [PubMed] [Google Scholar]
- Dicker D, Goldman GA, Ashkenazi J, Feldberg D, Voliovitz I, Goldman JA. The value of pre-treatment with gonadotrophin releasing hormone (GnRH) analogue in IVF-ET therapy of severe endometriosis. Hum Reprod 1990;5:418–420. [DOI] [PubMed] [Google Scholar]
- Dicker D, Goldman JA, Levy T, Feldberg D, Ashkenazi J. The impact of long-term gonadotropin-releasing hormone analogue treatment on preclinical abortions in patients with severe endometriosis undergoing in vitro fertilization-embryo transfer. Fertil Steril 1992;57:597–600. [DOI] [PubMed] [Google Scholar]
- Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap A et al. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014;29:400–412. [DOI] [PubMed] [Google Scholar]
- Fenwick E, O’Brien BJ, Briggs A. Cost‐effectiveness acceptability curves–facts, fallacies and frequently asked questions. Health Econ 2004;13:405–415. [DOI] [PubMed] [Google Scholar]
- Geukes M, van Aalst MP, Nauta MC, Oosterhof H. The impact of menopausal symptoms on work ability. Menopause 2012;19:278–282. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010;362:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesinger G, Venetis CA, Marx T, Diedrich K, Tarlatzis BC, Kolibianakis EM. Oral contraceptive pill pretreatment in ovarian stimulation with GnRH antagonists for IVF: a systematic review and meta-analysis. Fertil Steril 2008;90:1055–1063. [DOI] [PubMed] [Google Scholar]
- Guerriero S, Condous G, van den Bosch T, Valentin L, Leone FP, Van Schoubroeck D, Exacoustos C, Installé AJ, Martins WP, Abrao MS et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol 2016;48:318–332. [DOI] [PubMed] [Google Scholar]
- Hakkaart-Van Roijen L, van der Linden N, Bouwmans C, Kanters T, Tan SS Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Diemen 2016. Available from: https://www.zorginstituutnederland.nl/over-ons/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg (7 August 2017, date last accessed).
- Hee L, Kettner LO, Vejtorp M. Continuous use of oral contraceptives: an overview of effects and side-effects. Acta Obstet Gynecol 2013;92:125–136. [DOI] [PubMed] [Google Scholar]
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol 1998;91:16–24. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Hummelshoj L. Consensus on current management of endometriosis. Hum Reprod 2013;28:1552–1568. [DOI] [PubMed] [Google Scholar]
- Jones G, Jenkinson C, Kennedy S. Evaluating the responsiveness of the Endometriosis Health Profile Questionnaire: the EHP-30. Qual Life Res 2004;13:705–713. [DOI] [PubMed] [Google Scholar]
- Ma C, Qiao J, Liu P, Chen G. Ovarian suppression treatment prior to in-vitro fertilization and embryo transfer in Chinese women with stage III or IV endometriosis. Int J Gynaecol Obstet 2008;100:167–170. [DOI] [PubMed] [Google Scholar]
- Matalliotakis IM, Cakmak H, Mahutte N, Fragouli Y, Arici A, Sakkas D. Women with advanced-stage endometriosis and previous surgery respond less well to gonadotropin stimulation, but have similar IVF implantation and delivery rates compared with women with tubal factor infertility. Fertil Steril 2007;88:1568–1572. [DOI] [PubMed] [Google Scholar]
- Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D’Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril 2009;92:68–74. [DOI] [PubMed] [Google Scholar]
- Middeldorp S. Is thrombophilia testing useful? Hematol Am Soc Hematol Educ Program 2011;2011:150–155. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Oosawa M, Kondou I, Inagaki S, Shibata H, Narita O, Suganuma N, Tomoda Y. Menotropin stimulation after prolonged gonadotropin releasing hormone agonist pretreatment for in vitro fertilization in patients with endometriosis. J Assist Reprod Genet 1992;9:113–117. [DOI] [PubMed] [Google Scholar]
- Nisenblat V, Prentice L, Bossuyt PM, Farquhar C, Hull ML, Johnson N. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev 2016;7:CD012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oepkes D, Oudijk MA. Van Kennishiaten naar Wetenschaps-agenda; ambitie Koepel Wetenschap. Nederlands Tijdschrift voor Obstetrie & Gynaecologie 2015;128:386–388. [Google Scholar]
- Opoien HK, Fedorcsak P, Omland AK, Abyholm T, Bjercke S, Ertzeid G, Oldereid N, Mellembakken JR, Tanbo T. In vitro fertilization is a successful treatment in endometriosis-associated infertility. Fertil Steril 2012;97:912–918. [DOI] [PubMed] [Google Scholar]
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594–2604. [DOI] [PubMed] [Google Scholar]
- Rickes D, Nickel I, Kropf S, Kleinstein J. Increased pregnancy rates after ultralong postoperative therapy with gonadotropin-releasing hormone analogs in patients with endometriosis. Fertil Steril 2002;78:757–762. [DOI] [PubMed] [Google Scholar]
- Sallam HN, Garcia-Velasco JA, Dias S, Arici A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev 2006;1:CD004635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27:1292–1299. [DOI] [PubMed] [Google Scholar]
- Simoens S, Hummelshoj L, Dunselman G, Brandes I, Dirksen C, D’Hooghe T. Endometriosis cost assessment (the EndoCost study): a cost-of-illness study protocol. Gynecol Obstet Invest 2011;71:170–176. [DOI] [PubMed] [Google Scholar]
- Surrey ES. Endometriosis-related infertility: the role of the assisted reproductive technologies. Biomed Res Int 2015;2015:482959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surrey ES, Silverberg KM, Surrey MW, Schoolcraft WB. Effect of prolonged gonadotropin-releasing hormone agonist therapy on the outcome of in vitro fertilization-embryo transfer in patients with endometriosis. Fertil Steril 2002;78:699–704. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681–694. [DOI] [PubMed] [Google Scholar]
- van de Burgt TJ, Hendriks JC, Kluivers KB. Quality of life in endometriosis: evaluation of the Dutch-version Endometriosis Health Profile-30 (EHP-30). Fertil Steril 2011;95:1863–1865. [DOI] [PubMed] [Google Scholar]
- van den Bosch T, Dueholm M, Leone FP, Valentin L, Rasmussen CK, Votino A, van Schoubroeck D, Landolfo C, Installé AJ, Guerriero S et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol 2015;46:284–298. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard L, van Wly M, Dancet EA, van Mello NM, Koks CA, van der Veen F, Mol BW, Mochtar MH. Patients’ preferences for gonadotrophin-releasing hormone analogs in in vitro fertilization. Gynecol Obstet Invest 2014;78:16–21. [DOI] [PubMed] [Google Scholar]
- van der Houwen LE, Mijatovic V, Leemhuis E, Schats R, Heymans MW, Lambalk CB, Hompes PG. Efficacy and safety of IVF/ICSI in patients with severe endometriosis after long-term pituitary down-regulation. Reprod Biomed Online 2014. b;28:39–46. [DOI] [PubMed] [Google Scholar]
- van der Houwen LE, Schreurs AM, Schats R, Lambalk CB, Hompes PG, Mijatovic V. Patient satisfaction concerning assisted reproductive technology treatments in moderate to severe endometriosis. Gynecol Endocrinol 2014. a;30:798–803. [DOI] [PubMed] [Google Scholar]
- Verheul HA, Blok LJ, Burger CW, Hanifi-Moghaddam P, Kloosterboer HJ. Levels of tibolone and estradiol and their nonsulfated and sulfated metabolites in serum, myometrium, and vagina of postmenopausal women following treatment for 21 days with tibolone, estradiol, or estradiol plus medroxyprogesterone acetate. Reprod Sci 2007;14:160–168. [DOI] [PubMed] [Google Scholar]
- Zorginstituut Nederland Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg. Diemen 2016. Available from https://www.zorginstituutnederland.nl/over-ons/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg (7 August 2017, date last accessed).
- Zupi E, Marconi D, Sbracia M, Zullo F, de Vivo B, Exacustos C, Sorrenti G. Add-back therapy in the treatment of endometriosis-associated pain. Fertil Steril 2004;82:1303–1308. [DOI] [PubMed] [Google Scholar]