Abstract
STUDY QUESTION
Does an individualized serum anti-Müllerian hormone (AMH) based FSH dosing algorithm used in a GnRH antagonist protocol increase the proportion of patients with an intended number of oocytes (5–14) retrieved compared with a standard regimen?
SUMMARY ANSWER
The AMH-based individualized algorithm did not increase the proportion of patients with an intended oocyte retrieval.
WHAT IS KNOWN ALREADY
Individualizing treatment for ovarian stimulation by serum AMH or antral follicle count can theoretically improve the ratio between benefits and risks. Current data suggest that there may be a reduced risk of ovarian hyperstimulation syndrome (OHSS), but without improved pregnancy or live birth rates. Only two randomized controlled trials (RCTs) have examined the potential of AMH-based algorithms to optimize the FSH dosing in ovarian stimulation.
STUDY DESIGN, SIZE, DURATION
A dual-center open-label investigator-driven RCT was conducted between January 2013 and November 2016. Eligibility was assessed in 269 women and 221 were randomized 2:1 between individualized and standard dosing groups. Women with pretreatment serum AMH > 24 pmol/L had 100 IU/day of recombinant FSH (rFSH); AMH 12–24 pmol/L had 150 IU/day of rFSH, and AMH < 12 pmol/L had maximal stimulation with corifollitropin 100 or 150 mg depending on bodyweight ±60 kg. The standard group had 150 IU/day of rFSH irrespective of pretreatment AMH. All patients followed the GnRH-antagonist protocol.
The sample size calculation assumed that individualized dosing by AMH would reduce the proportion of unintended oocyte yield (outside the 5–14 range) by 50%, from 35 to 17.5%. In a 2:1 randomization this required 216 patients: 144 in the individualized and 72 patients in the standard group (80% power, 5% significance).
PARTICIPANTS/MATERIALS, SETTING, METHODS
All women had a presumed ovulatory normal menstrual cycle, were aged 25–38 years, weighed < 75 kg, had pretreatment AMH 4–40 pmol/L, did their first IVF or ICSI cycle and had two ovaries accessible to oocyte retrieval. Recruitment was conducted from both participating sites. Women were excluded if diagnosed with anovulatory polycystic ovary syndrome, endometriosis grade III/IV, hydrosalpings on ultrasound, recurrent miscarriages (≥3), FSH > 12 IU/L or major medical disorders.
MAIN RESULTS AND THE ROLE OF CHANCE
After randomization 149 women were allocated to the individualized group and 72 to the standard group. The primary outcome of women with an intended (5–14) number of oocytes retrieved was similar in the individualized (n = 105) versus the standard (n = 55) rFSH treatment group (72% [95% CI 64–79%] versus 78% [95% CI 67–86%], respectively, P = 0.68, between group standardized mean difference (SMD) −6%, 95% CI: −19–8%). In the high AMH stratum of the individualized group, significantly more women (n = 13) had an unintended low number of oocytes (<5) retrieved (38% [95% CI: 23–55%]) compared with the standard group (6% [95% CI 0.3–24%], P = 0.029, between group SMD 32%, 95% CI: 9–56%). Conversely, in the low pretreatment AMH stratum, individualized dosing using corifollitropin reduced the proportion of unintended low responders to 24% (95% CI: 12–40%) compared with 47% (95% CI: 26–69%) in the standard group, P = 0.10, between group SMD −23% (95% CI: −54–8%). OHSS was diagnosed in four women (two in each study arm), and all cases were mild. Daily luteal phase questionnaire reporting showed similar wellbeing in terms of abdominal distention, abdominal pain, dyspnea and occurrence of bleeding between groups. The cumulative live birth rate per started cycle was similar (32 and 35%) comparing the individualized with the standard group.
LIMITATIONS, REASONS FOR CAUTION
This study was powered for showing differences only in the distribution of oocyte retrieval when comparing individualized and standard groups, therefore additional results should be viewed with caution. In addition, there was a change of AMH assay halfway through the study period and the possibility that corifollitropin being introduced to a subgroup of the intervention has introduced confounding cannot be ruled out.
WIDER IMPLICATIONS OF FINDINGS
In the expected high responder AMH stratum, 100 IU/day is an insufficient rFSH dose in a high proportion of patients. Further research might explore the 125 IU/day dose for the high AMH segment.
STUDY FUNDING/COMPETING INTEREST(S)
None for the submitted work. ICMJE declared personal interests for two of the authors.
TRIAL REGISTRATION NUMBER
EUDRACT registration number: 2012-004969-40.
TRIAL REGISTRATION DATE
27 November 2012.
DATE OF FIRST PATIENT’S ENROLLMENT
10 January 2013.
Keywords: individualized FSH dosing, anti-Müllerian hormone, ovarian stimulation, FSH, randomized controlled trial, ovarian response, oocyte retrieval
Introduction
Individualized FSH dosing using antral follicle count (AFC) or anti-Müllerian hormone (AMH) as ovarian reserve tests (ORT) has been introduced to optimize the ratio between benefits and risks of ovarian stimulation for IVF. Theoretically, individualized dosing could provide the targeted number of oocytes, which in turn could increase the pregnancy rate and lower the risk of ovarian hyperstimulation syndrome (OHSS). Earlier randomized controlled studies (RCTs) of individualized dosing targeted the retrieval of 9–11 oocytes, ranging from 5 to 14 or 8 to 14 (Popovic-Todorovic et al., 2003; Olivennes et al., 2015; Allegra et al., 2017; Nyboe Andersen et al., 2017). The recently published large Dutch optimization of cost effectiveness through Individualized FSH Stimulation dosages for IVF Treatment: A randomized trial (OPTIMIST trials) (Oudshoorn et al., 2017; van Tilborg et al., 2017a,b) used AFC as the ORT and found that individualization did not increase pregnancy rates, but may reduce the risk of OHSS (Oudshoorn et al., 2017).
WHAT DOES THIS MEAN FOR PATIENTS?
Women who seek assisted reproduction treatment for IVF typically undergo hormonal ovarian stimulation with follicle stimulating hormone (FSH) to develop a reasonable number of mature follicles containing healthy eggs. Retrieval of 5–14 egg cells from a single cycle balances the chance of a live birth versus the risk of ovarian hyperstimulation syndrome (OHSS).
Hormonal stimulation algorithms using ovarian reserve tests (ORT) have been introduced to increase the proportion of women who develop an appropriate number of eggs. Using the anti-Müllerian hormone (AMH) level in serum before treatment as an ORT, this study divided participants into expected low (AMH < 12 pmol/L), medium (AMH 12–24 pmol/L) and high (AMH > 24 pmol/L) response groups. The participants were randomized to receive either low, normal or high FSH doses for ovarian stimulation based on their AMH. The standard group all had 150 IU/day of FSH irrespective of their AMH level.
The AMH-based stimulation did not increase the proportion of women who reached the intended target of eggs. Conversely, in the expected high response group a statistically significant proportion of women developed unintended few egg cells, because the low dose of 100 IU of FSH/day was insufficient.
Patients should be informed that dosing with 150 IU/day of FSH is recommended, but patients with high ORTs, such as AMH, could be dosed with around 125–137 IU/day to reduce their risk of OHSS.
Apart from the recent Evidence-based Stimulation Trial With Human rFSH in Europe and Rest of World (ESTHER) trial (Nyboe Andersen et al., 2017), earlier RCTs on individualized dosing have mainly used the long GnRH agonist protocol. There is a need for studies examining individualized dosing in the short GnRH antagonist protocol that is used more frequently today due to a more positive patient experience as well as a better balance between risk in terms of OHSS (Toftager et al., 2016) and benefit in terms of cumulative live birth rates (Toftager et al., 2017).
Currently, the best biomarkers for response prediction seem to be AMH and AFC. Both predict low and excessive responses to ovarian stimulation (Broer et al., 2009), but AFC may have higher inter-observed variability between clinics in multicenter trials and may therefore be a less appropriate parameter to use (Nelson et al., 2015a). Additionally, the clinical use of AMH-based dosing models (La Marca and Sunkara, 2014) has been tested, firstly in observational studies (Nelson et al., 2009; Yates et al., 2011) and recently in RCTs (Allegra et al., 2017; Nyboe Andersen et al., 2017). This development has been facilitated by the new fully automated assay for AMH measurements, providing faster and more reproducible results than the earlier ELISA methods (Nelson et al., 2015b).
Based on the currently available evidence (Nyboe Andersen et al., 2017; Oudshoorn et al., 2017; van Tilborg et al., 2017a,b; Lensen et al., 2018), any advantage of individualized dosing seems to improve the safety of treatment. Optimizing this, we especially need to improve the ‘down-dosing’ of FSH in expected hyper-responders without losing their potential to have sufficient oocyte yields. The objective of the study was to increase the proportion of patients with the intended retrieval of 5–14 oocytes using an AMH-based dosing algorithm.
Materials and Methods
Participants
This was a dual-center, RCT comparing patients treated in an individualized AMH-based FSH dosing algorithm with patients treated with a standard FSH dose of 150 IU/day.
Infertile patients were included prior to their first IVF or ICSI cycle from the Fertility clinics at Rigshospitalet and North Zealand Hospital, during the period January 2013 to November 2016. North Zealand Hospital functioned as a satellite IVF clinic. All oocyte retrievals and single blastocyst transfers were performed centrally at Rigshospitalet. In total 221 women were randomized.
The main inclusion criteria were: indication for IVF or ICSI; first treatment at the clinic; age ≥25 and ≤38 years; serum AMH 4–40 pmol/L; normal menstrual cycles of 24–35 days interval presumed to be ovulatory; bodyweight <75 kg; two ovaries accessible to oocyte retrieval; uterus with presumed normal function based on sonography; and willingness and ability to sign the informed consent.
The main exclusion criteria were anovulatory polycystic ovary syndrome, endometriosis grade III/IV, hydrosalpinx on ultrasound, recurrent miscarriages (≥3), FSH >12 IE/L and major medical disorders. There were no restrictions in terms of BMI.
Screening was carried out on Days 2–5 of the menstrual cycle within 3 months prior to start of stimulation. Screening involved transvaginal ovarian sonography with measurement of ovarian volume and counting of antral follicles below 10 mm diameter.
Treatment
Participants were randomized at Day 3 of a spontaneous menstrual bleeding. All patients fulfilling the criteria for participation were randomized in a 2:1 distribution between the individualized and standardly dosed groups. Ovarian stimulation with FSH was started on Day 3 in an antagonist cycle.
Individualized group
Patients were FSH-dosed as follows: High AMH (>24 pmol/L) had minimal dosing of 100 IU/day (rFSH; Puregon®, Organon, The Netherlands). Normal AMH (12–24 pmol/L) had standard dosing of 150 IU/day (rFSH; Puregon®). Low AMH (<12 pmol/L) had maximal dosing using corifollitropin (Elonva® 100 or 150 μg according to bodyweight below or above 60 kg, Elonva®; Merck Sharp & Dohme B.V., Kenilworth, NJ, USA).
Standard group
All patients had rFSH (Puregon® 150 IU/day) irrespective of AMH levels.
For all patients in both arms a fixed dose regiment was used throughout the stimulation period. In two patients (one in each group) the clinician decided to decrease the FSH dose at Day 8 due to risk of OHSS.
From Day 6 the GnRH antagonist ganirelix (Orgalutran®, Merck Sharp & Dohme B.V., USA) 0.25 mg/day was administered s.c. once daily. Patients stimulated with corifollitropin started GnRH antagonist on Day 5. Criterion for triggering of ovulation with hCG (Ovitrelle® 250 μg, Merck Serono Europe Limited, London, UK) was three follicles ≥17 mm. Triggering could be done on that day or one day after. If less than three mature follicles were present and the clinician had no expectation that more mature follicles could be achieved, triggering and oocyte retrieval versus IUI was discussed. Patients treated with corifollitropin (Elonva®) were given rFSH 200 IU/day in those cases that continued stimulation beyond Day 8. Patients were examined with sonography and baseline blood sampling of FSH, LH, estradiol, progesterone and AMH.
All had luteal support using vaginal progesterone (Lutinus® 100 mg three times a day for 14 days, Ferring Pharmaceuticals, Saint-Prex, Switzerland), starting 2 days after oocyte retrieval and continued until the day of hCG-testing. Vaginal progesterone was withdrawn in all patients on the day of hCG-testing, 14 days after blastocyst transfer.
A single blastocyst was transferred at Day 5 after retrieval. If a blastocyst was not available a compacted embryo could be transferred. Outcome of pregnancy was recorded for all fresh cycle transfers and additionally from all frozen embryo transfers (FET) of cryopreserved blastocysts transferred within 1 year after final patient enrollment.
From Day 5 after oocyte retrieval and onwards, patients daily assessed their general wellbeing in terms of abdominal distention, abdominal pain and dyspnea ranging on a scale of none, mild, moderate or severe. Additionally, they answered if bleeding occurred until the day of the hCG-testing.
Primary end point
The primary end point was the proportion of patients in the individualized group versus the standard group who had an intended (5–14) versus an unintended (<5 or ≥15) number of oocytes retrieved.
Unintended responses included patients who had oocyte retrieval canceled due to insufficient response, excessive number of follicles, or had canceled their blastocyst transfer due to freezing of all blastocysts in order to avoid OHSS. No member of the trial initiators performed the oocyte retrievals.
Secondary end points
Additionally, the trial examined the effect of individualization on the rates of excessive response (>15 oocytes), OHSS occurrence, pregnancy, ongoing pregnancy (>7 weeks’ gestation) and live birth as well as the subjective wellbeing of patients in the luteal phase of treatment.
Randomization
Randomization was performed by a computer-generated randomization list and the classical sealed envelope system, where the next numbered envelope was taken for each new randomized patient. Randomization was arranged using a variable block-size with either nine or six in the block, and a 2:1 distribution of randomization between the individualized and standardly dosed patients. The practical procedure was undertaken by a person that was independent of the doctor responsible for the treatment. Participants were randomized at Day 3 of the cycle when the ovarian stimulation was started.
AMH analysis
Serum AMH was measured using the Immunotech RIA AMH/MIS kit (catalog number A16507, ELISA, Enzyme ImmunoAssay, Beckman Coulter, France, with intra- and inter-assay coefficients of variation ≤12.3 and ≤14.2%, respectively) from study start to 1 June 2015. Hereafter, the fully automated Elecsys AMH Assay (Elecsys, intra- and inter-assay coefficients of variation ≤1.8 and ≤4.4%, respectively, Roche, Switzerland) was used. The Immunotech assay was used in 154 patients (57%) and the Elecsys in 115 patients (43%). In our clinic, a previous analysis of more than 400 samples with both assays found a conversion factor of 0.8. Therefore, Elecsys-derived values were 0.8 of the value obtained with Immunotech. As a result, the cut-off levels between low, intermediate and high AMH in the first 154 patients were 15 and 30 pmol/L whereas the cut-offs were 12 and 24 pmol/L in the 115 patients included after the change of analysis. All AMH values in the present article are expressed as with the Elecsys assay.
Sample size and statistical analyses
We used data from our own earlier trial (Popovic-Todorovic et al., 2003) where patients in the comparator arm were given a dose of 150 IU of rFSH (Puregon®) and where 65% of the patients had an intended response (5–14 oocytes), whereas 35% had unintended response. Calculation of the sample size assumed that individualized dosing based on AMH would reduce unintended responses by 50%, from 35 to 17.5%. In our earlier study we observed a reduction from 35 to 25%, mainly due to no reduction in excessive responders following rFSH doses around 125 IU/day. In the present study, we decided to lower the dose of rFSH to 100 IU/day in patients with a predicted high response. We assumed it would be realistic to reduce the proportion of unintended responses to 17.5%. This would, when using a 2:1 randomization, require 216 patients, 144 in the individualized and 72 patients in the standard arm (80% power, 5% significance).
The Chi2-test, Student’s t-test and Mann–Whitney U test were used for different comparisons, as appropriate. Statistically significant differences were set at P < 0.05. Data analysis was based on an intention-to-treat (ITT) analysis. In terms of oocyte yield and embryo development, data are related to the number who had oocyte retrieval (numbers specified in Table II). Parameters of success (pregnancy, ongoing, live birth) and risk (OHSS) were reported in the ITT population. Complete case analyses were used in all calculations as data including the primary outcome mostly considered counts, proportions and means. To test whether the scarce missing data had any effect on the results, multiple imputation (Donders et al., 2006) was carried out using the mice statistical package in R (Rstudio Software, version 1.0.136). The pmm method was applied for continuous and the polyreg for categorical variables. After imputation, no statistically significant alterations to the data were seen, hence results are reported as a complete case analysis.
Table II.
Clinical outcome by individualized AMH-based or standard dosing.
| Type of dosing | ||||
|---|---|---|---|---|
| Individualized | Standard | |||
| N = 146a | N = 71a | P | SMD 95% CI | |
| Follicles aspiratedb | 8.6 ± 3.5 | 9.0 ± 4.3 | 0.23e | −0.4 (−0.8 to 1.6) |
| Oocytes retrieved per pretreatment AMH (pmol/L) | ||||
| Low (AMH 4–11.99) | 7.3 ± 3.6 | 5.1 ± 3.3 | 0.04e | 2.2 (0.2 to 4.1) |
| Normal (AMH 12–23.99) | 8.1 ± 3.5 | 8.5 ± 3.3 | 0.58e | −0.4 (−1 to 1.8) |
| High (AMH 24–40) | 6.3 ± 3.5 | 10.6 ± 3.6 | 0.00e | −4.3 (−6.4 to −2.1) |
| Total | 7.5 ± 3.6 | 8.1 ± 3.9 | 0.25e | −0.6 (−0.5 to 1.7) |
| Oocytes retrieved | 0.68f | |||
| <5—unintended low | 34 (23.3) | 13 (18.3) | 0.51g | 5% (−7 to 17) |
| 5–14—intended | 105 (71.9) | 55 (77.5) | 0.48g | −6% (−19 to 8) |
| ≥15—unintended high | 7 (4.8) | 3 (4.2) | 0.98g | 0.6% (−6 to 9) |
| Duration of stimulation (d) | 8.8 ± 1.4 | 8.2 ± 1.3 | 0.00e | 0.6 (0.2 to 1.0) |
| Total FSH dose (IU)c | 1.177 ± 262 | 1.239 ± 166 | 0.09e | −62 (−23 to 111) |
| Fertilization | ||||
| Fertilized oocytes | 3.9 ± 2.5 | 3.7 ± 2.5 | 0.78e | 0.2 (−0.9 to 0.5) |
| Rate of fertilization | 54 ± 27 | 46 ± 28 | 0.04g | 8% (16 to 0.01) |
| Embryo development | ||||
| Embryos Day 2 | 4.1 ± 2.6 | 4.0 ± 2.7 | 0.82e | 0.1 (−0.9 to 0.6) |
| Blastocysts Day 5 | 1.9 ± 1.6 | 1.9 ± 1.9 | 0.57e | 0 (−0.5 to 0.5) |
| Blastocyst transfer | 122 (83.6) | 56 (78.9) | 0.40f | 4.7% (−8 to 17) |
| No transfer | 24 (16.4) | 15 (21.1) | ||
| OHSS any typed | 2/149 (1.3) | 2/72 (2.8) | 0.73g | 1.5% (−7 to 4) |
| Positive hCGd | 57/149 (38.3) | 32/72 (44.4) | 0.34g | −6.1% (−21 to 9) |
| Ongoing pregnancy (week 7)d | 44/149 (29.5) | 21/72 (29.2) | 0.79g | 0.3% (−13 to 14) |
| Live birthd | ||||
| Per fresh cycle | 41/149 (27.5) | 21/72 (29.2) | 0.79g | −1.7% (−15 to 12) |
| Per FET | 6/22 (27.3) | 4/7 (57.1) | 0.16g | −30% (−80 to 20) |
| Cumulative | 47/149 (31.5) | 25/72 (34.7) | 0.64g | −3.2% (−17 to 11) |
Values are mean ± SD and count (column %).
OHSS, ovarian hyperstimulation syndrome; FET, frozen embryo transfer; SMD, standardized mean difference.
aAnalyses for women who had oocyte retrieval. unless otherwise specified.
bMissing data on follicles aspirated (n = 8).
cFSH dose comparing medium and high pretreatment AMH-strata.
dIn the intention-to-treat population.
et-test.
fChi2-test.
gMann–Whitney U test
Prior to first patient enrollment, the study was enrolled in the EUDRACT online registry (2012-004969-40). The Scientific Ethical Committee of the Copenhagen Region and the Danish Data Protection Agency approved the study in December 2012 (H-3-2012-149 and 30-0895, respectively). The study was monitored by the Good Clinical Practice Unit (GCP) of the Danish Capital Region. All participants provided a written informed consent to participation.
Results
Eligibility was assessed in 269 women and 221 were eventually randomized to either the individualized (N = 149) or the standard (N = 72) group. Each group was evenly distributed by their pretreatment AMH levels between the low, medium and high strata (individualized group: 22.1, 55.4, 23.5% and standard group: 27.8, 47.2, 25.0%, respectively).
Table I shows baseline pretreatment characteristics between the individualized and the standard group including the pretreatment AMH, FSH, AFC and AMH/AFC values in the three AMH strata.
Table I.
Baseline characteristics by individualized AMH-based or standard dosing.
| Type of dosing | ||
|---|---|---|
| Individualized | Standard | |
| N = 149 | N = 72 | |
| Age (y), mean ± SD | 32.2 ± 3.3 | 31.6 ± 3.2 |
| Age groups, N (%) | 29 (19.5) | 21 (29.2) |
| <30 | 74 (49.7) | 37 (51.4) |
| 30–34 | ||
| 35–38 | 46 (30.9) | 14 (19.4) |
| BMI (kg/m2), mean ± SD | 22.2 ± 4.1 | 22.0 ± 2.6 |
| Duration of infertility (months), mean ± SD | 27.3 ± 13.0 | 27.1 ± 10.3 |
| Main cause of infertility, N (%) | ||
| Male | 75 (50.3) | 45 (62.5) |
| Tubal | 14 (9.4) | 4 (5.6) |
| Combined factors | 4 (2.7) | 1 (1.4) |
| Unexplained | 56 (37.6) | 22 (30.6) |
| Primary infertility, N (%) | 103 (69.1) | 54 (75.0) |
| Cycle length (d), mean ± SD | 28.2 ± 1.8 | 28.1 ± 1.9 |
| Ovarian volume (mL), mean ± SD | 6.1 ± 2.6 | 6.2 ± 2.8 |
| AFC, mean ± SD | 20.3 ± 7.3 | 19.3 ± 7.5 |
| AMH (pmol/L)*, median (IQR) | 16.8 (12.0–23.0) | 15.2 (10.6–23.5) |
| FSH (IU/L), median (IQR) | 7.5 (6.2–8.9) | 7.2 (6.1–8.4) |
| Treatment groups by AMH, N (%) | ||
| AMH 4–11.9 | 33 (22.1) | 20 (27.8) |
| AMH 12–23.9 | 81 (55.4) | 34 (47.2) |
| AMH 24–40 | 35 (23.5) | 18 (25.0) |
| Pretreatment stimulation characteristics by AMH stratum | ||
| AMH 4–11.9 pmol/L | ||
| AMH, median (IQR) | 9.6 (6.6–10.7) | 8.8 (6.6–9.6) |
| FSH, median (IQR) | 7.8 (6.5–9.2) | 7.2 (6.8–8.6) |
| AFC, mean ± SD | 14.3 ± 4.8 | 12.9 ± 3.9 |
| AMH/AFC | 0.64 ± 0.18 | 0.68 ± 0.27 |
| AMH 12–23.9 pmol/L | ||
| AMH, median (IQR) | 16.8 (13.6–20.0) | 15.6 (13.0–20.0) |
| FSH, median (IQR) | 7.3 (6.2–9.0) | 7.2 (6.2–8.8) |
| AFC, mean ± SD | 19.7 ± 5.4 | 19.7 ± 6.0 |
| AMH/AFC | 0.93 ± 0.33 | 0.87 ± 0.20 |
| AMH 24–40 pmol/L | ||
| AMH, median (IQR) | 33 (28–35) | 27 (24–29) |
| FSH, median (IQR) | 7.3 (6.6–8.0) | 6.0 (5.6–7.9) |
| AFC, mean ± SD | 27.5 ± 7.3 | 25.8 ± 7.5 |
| AMH/AFC | 1.22 ± 0.06 | 1.17 ± 0.4 |
AMH, anti-Müllerian hormone; AFC, antral follicle count. No statistical differences between groups using Student’s t-test, Mann–Whitney U test or Chi2-test as appropriate. *All serum AMH values in the present article are expressed as with the Elecsys assay.
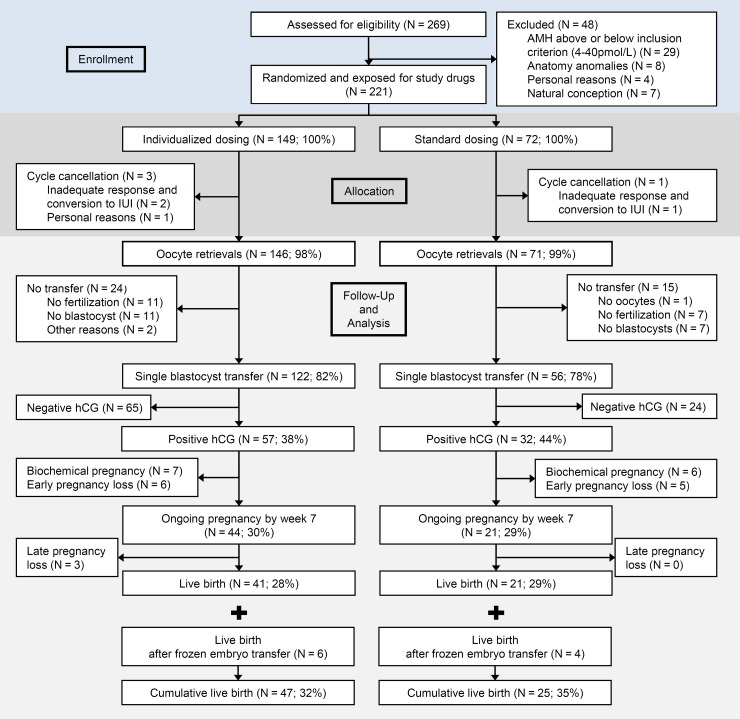
Figure 1 shows the flow of patients through the trial. A similar proportion of started cycles had oocyte retrieval (individualized group 146/149 (98%); standard group 71/72 (99%)). Oocyte retrieval cancellation due to inadequate response was seen in two cases in the individualized and one case in the standard group. Considering the patients who had oocytes retrieved, a similar proportion of the two groups had blastocyst transfer (122/146, 82%; versus 56/71 (79%) in the individualized and standard group, respectively).
Figure 1.
Patient flow and outcome during the study of AMH-based individualized FSH dosing in a GnRH antagonist protocol, from assessment of eligibility to cumulative live birth rate. AMH, anti-Müllerian hormone.
Table II reports all primary and secondary outcomes including between-group standardized mean differences and their 95% CI. Table II shows the mean number of oocytes retrieved per pretreatment AMH strata. In the individualized group the low AMH stratum had more oocytes retrieved compared with the standardly dosed (7.3 ± 3.6 versus 5.1 ± 3.3, P = 0.04, respectively). Conversely, in the high AMH stratum fewer oocytes were retrieved in the individualized group (6.3 ± 3.5 versus 10.6 ± 3.6, P < 0.01, respectively). Also shown in Table II, the intended outcome of 5–14 oocytes was near equally achieved in the individualized versus the standard group (72% [95% CI: 64–79%] and 78% [95% CI: 67–86%], respectively). In the individualized group 23% (95% CI: 17–31%) of patients had a low response (<5 oocytes) and 4.8% (95% CI 2–9%) had a high response (≥15 oocytes) versus 18% (95% CI: 11–29%) and 4.2% (95% CI: 1–11%) in the standard group, respectively. None of these differences were statistically significant.
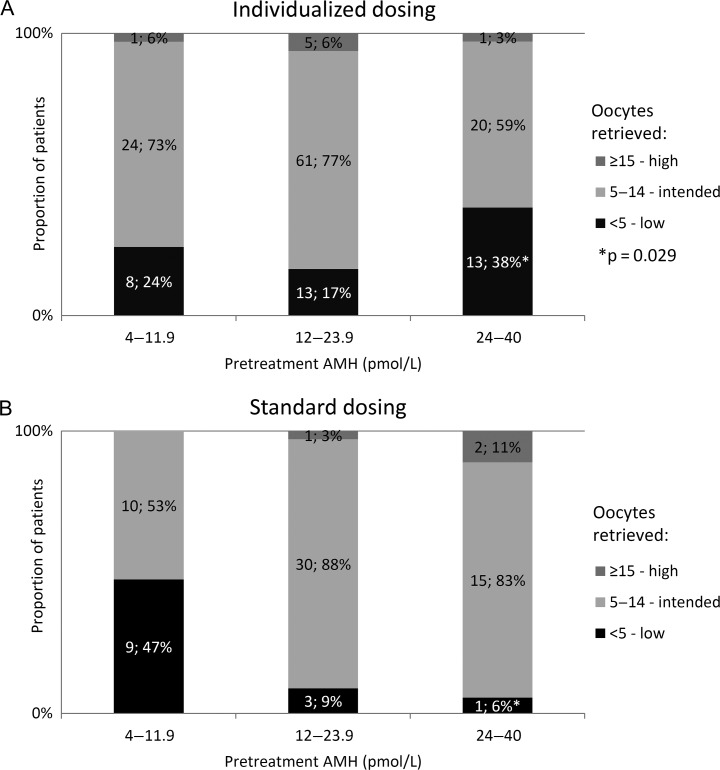
Figure 2 shows the relative distribution of oocytes retrieved per pretreatment AMH strata in the two study groups. For patients in the high pretreatment AMH stratum exposed to individualized dosing of 100 IU/day, 38% (95% CI: 23–55%) had an unintended low response (<5 oocytes) compared to only 6% (95% CI: 0.3–24%) of standard group patients within this AMH stratum who had 150 IU/day (P = 0.029). Scrutinizing the individualized group, the 13 patients in the high AMH stratum who had less than five oocytes compared with the 20 high AMH patients who had the intended response (5–14 oocytes) there were no statistically significant differences in terms of mean age (31.9 ± 3.8 versus 32.6 ± 3.0 years), weight (61.8 ± 8.1 versus 61.6 ± 7.2 kg) or BMI (21.6 ± 2.3 versus 21.7 ± 1.8 kg/m2) or median AMH levels within the stratum (33.0 [inter-quartile range (IQR) 29.6–35.2] pmol/L versus 29.8 [IQR 26.9–34.0] pmol/L), pretreatment FSH levels (7.5 [IQR 6.3–9.3] versus 7.2 [IQR 6.8–7.7] U/L) or the AMH/AFC ratio (1.1 ± 0.3 versus 1.4 ± 0.4, P = 0.074).
Figure 2.
Proportion of patients with a low, intended or high response in terms of oocytes retrieved according to pretreatment AMH. (A) Individualized AMH-based dosing and (B) standard dosing. Difference in distribution within each AMH stratum was evaluated by a two proportions z-test.
In terms of an unintended high response within this stratum, there was no statistically significant difference. Conversely, among patients in the low pretreatment AMH stratum treated with individualized dosing using corifollitropin, the proportion of unintended low responders was reduced to 24% (95% CI: 12–40%) compared with 47% (95% CI: 26–69%) in the standard group. This difference was not significant (P = 0.10).
Table II also shows that the number of aspirated follicles on the day of oocyte retrieval was evenly distributed between groups and the fertilization rate was marginally improved in the individualized group (P = 0.04). The number of embryos available as well as the number and grading of blastocysts were similar between groups (grading data not shown).
In two patients, both in the individualized group, all blastocysts were frozen. One of these had early onset OHSS, the other had symptoms of infection at the day of transfer. Both patients had a subsequent transfer of a thawed blastocyst. Excessive responses comprised 4.8 versus 4.2%, comparing the individualized with the standard group, respectively. GnRH agonist triggering was not used. In the individualized group two of 149 patients (1.3%) had OHSS. One patient from the medium AMH-stratum had severe early onset OHSS, cryopreservation of all blastocysts and required 2 days of hospitalization, and one patient in the low AMH-stratum had late onset moderate OHSS without hospitalization. In the standard group, 2 of 72 (2.8%) patients developed OHSS. Both originated from the high AMH stratum and had late onset moderate OHSS during early pregnancy without hospitalization.
All clinical outcomes were similar. The live birth rate per single blastocyst transfer was 34% (95% CI: 26–42%) and 38% (95% CI: 26–51%) and as seen in Fig. 1 the live birth rate per started cycle was 28% (95% CI: 21–35%) versus 29% (95% CI: 20–40%) in the individualized versus the standard group, respectively. Similar proportions of the randomized patients had blastocysts available for cryopreservation (21% in the individualized and 20% in the standard group). As shown in Fig. 1 and Table II, 22 of 30 patients with cryopreserved blastocysts in the individualized group had a FET cycle and 6 (27%) had live births. In the standard group, 7 of 14 patients had a FET cycle and 4 (57%) had live births. All results were statistically non-significant. Overall, the cumulative live birth rate per started cycle was similar (32 and 35%) comparing the individualized with the standard group.
Missing data were scarce regarding these main objective data as only five women did not have an ovarian volume estimation and data on the number of follicles at day of aspiration were missing in eight cases. Performing multiple imputation on these values did not change the findings in Tables I and II.
Table III shows subjective discomforts during the luteal phase with no differences between the individualized and standard group regarding abdominal distention, abdominal pain, dyspnea and bleeding pattern. Complete data were collected from 78% of participants and are shown. Missing data were mostly caused by lack of blastocyst transfers and thus subsequent poor compliance of the patients. To test for the effect of this large proportion of missing data, both multiple imputation and classifying the missing values as an individual level within the categories was done. Neither method showed statistically significant changes to the results.
Table III.
Patient experiences during the luteal phase according to type of dosing and pretreatment AMH.
| Type of dosing and pretreatment AMH (pmol/L) | ||||||
|---|---|---|---|---|---|---|
| AMH 4–11.9 n = 43 |
AMH 12–23.9 n = 89 |
AMH 24–40 n = 40 |
||||
| AMH-based | Standard | AMH-based | Standard | AMH-based | Standard | |
| Abdominal distension, P = 0.13 | ||||||
| None | 2 (7.1) | 2 (13.3) | 5 (8.3) | 5 (17.2) | 0 (0.0) | 0 (0.0) |
| Mild | 17 (60.7) | 5 (33.3) | 29 (48.3) | 13 (44.8) | 11 (40.7) | 4 (30.8) |
| Moderate | 5 (17.9) | 8 (53.3) | 19 (31.7) | 10 (34.5) | 10 (37.0) | 6 (46.2) |
| Severe | 4 (14.3) | 0 (.0) | 7 (11.7) | 1 (3.4) | 6 (22.2) | 3 (23.1) |
| Abdominal pain, P = 0.90 | ||||||
| None | 4 (14.3) | 2 (13.3) | 7 (11.7) | 2 (6.9) | 3 (11.1) | 0 (0.0) |
| Mild | 11 (39.3) | 5 (33.3) | 28 (46.7) | 15 (51.7) | 16 (59.3) | 7 (53.8) |
| Moderate | 11 (39.3) | 5 (33.3) | 19 (31.7) | 8 (27.6) | 5 (18.5) | 5 (38.5) |
| Severe | 2 (7.1) | 3 (20.0) | 6 (10.0) | 4 (13.8) | 3 (11.1) | 1 (7.7) |
| Dyspnoea, P = 0.21 | ||||||
| None | 17 (60.7) | 13 (86.7) | 41 (68.3) | 20 (69.0) | 11 (40.7) | 9 (69.2) |
| Mild | 10 (35.7) | 2 (13.3) | 16 (26.7) | 7 (24.1) | 11 (40.7) | 2 (15.4) |
| Moderate | 1 (3.6) | 0 (0.0) | 2 (3.3) | 2 (6.9) | 5 (18.5) | 2 (15.4) |
| Severe | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Grading of bleeding after transfer, P = 0.79 | ||||||
| Menstruation | 15 (83.3) | 9 (81.8) | 30 (73.2) | 13 (68.0) | 12 (75.0) | 7 (77.8) |
| Spotting | 3 (16.7) | 2 (18.2) | 11 (26.8) | 6 (32.0) | 4 (25.0) | 2 (22.2) |
Values are count and column proportions (%). No statistical differences between groups using Chi2-test.
Questionnaires were returned by 172 of 221 patients (22% missing data).
Discussion
Individualized AMH-based FSH dosing in the antagonist protocol using the selected algorithm did not increase the proportion of patients with an intended oocyte retrieval (5–14).
Comparing the intervention group exposed to individualized dosing with the standardly dosed group, patients in the low AMH segment had significantly more (7.3 ± 3.6 versus 5.1 ± 3.3) oocytes retrieved, whereas the patients in the high AMH segment had significantly less (6.3 ± 3.5 versus 10.6 ± 3.6). However, despite these apparently favorable changes in terms of benefits to risk ratio, the overall proportions of patients who responded within the predefined intended target were similar, with 72% in the individualized group and 78% in the standard group. Likewise, unintended responses defined as either too few (<5) or too many (≥15) oocytes were equal. Among patients with a high AMH-based ovarian reserve, the reduction in rFSH dose to 100 IU/day in the individualized group displayed unwanted consequences as 38% of these patients had less than five oocytes. Therefore, if AMH is used alone for individualized rFSH dosing 100 IU/day is insufficient, also in patients presumed to be able to have a high response based on their AMH.
The 100 IU/day was based on our own earlier experience with an RCT on rFSH dosing (Popovic-Todorovic et al., 2003) where around 125 IU/day was found not to lower the number of patients with excessive responses. We assume some of this apparent discrepancy may be related to the prior use of the long GnRH agonist protocol, whereas the GnRH antagonist protocol was applied in the present study. The recently published large Dutch multicenter ‘OPTIMIST’ study predominantly used GnRH agonist protocols and administered 100 IU/day of rFSH to expected high responders (AFC > 15). The authors also reported a large number of poor responses (29%) and a 3-fold increase in cycle cancellation due to insufficient follicular growth (Oudshoorn et al., 2017).
The insufficiency of 100 IU/day of rFSH is likely caused by not reaching the threshold for most follicles. In the early rFSH dose finding studies after introduction of Puregon® on the market, ‘standard IVF patients’ exposed to 100 IU/day had insufficient follicle development and cancellation of the cycle in as many as 24% of cases (Out et al., 1999). These findings, our data and the recent studies on individualized dosing show that 100 IU/day is insufficient in a proportion of ‘standard IVF patients’, as well as in predicted hyper-responding patients, irrespective of whether the prediction is based on AFC or AMH and irrespective of protocol used (Out et al., 1999; Oudshoorn et al., 2017).
Another factor influencing dosage is bodyweight, recently combined with AMH in the dosing algorithm developed for the new follitropin delta (Rekovelle®). In the large ESTHER-1 multicenter study (1326 patients), patients with AMH above 15 pmol/L were dosed per kilogram (Nyboe Andersen et al., 2017) in order to allow better ‘down-dosing’ with rFSH. Indeed, this dosing model increased the proportion of patients within the targeted number of oocytes (8–14).
To explore the insufficiency of 100 IU/day of rFSH, we compared patients within the high AMH stratum who had <5 or 5–14 oocytes retrieved in the present study. They did not differ in terms of age, bodyweight, BMI, FSH or AMH levels or AMH/AFC ratio. In a large American study on anovulatory infertility (Mumford et al., 2016) a high AMH/AFC ratio predicted unresponsiveness to ovulation induction and suggested that high intrafollicular AMH levels could diminish FSH sensitivity. In the present study, we found the same trend among our ovulatory patients in the high AMH stratum, as the ratio was increased (1.4 ± 0.4) in the group who had <5 oocytes retrieved compared with the group who had 5–14 (1.1 ± 0.3) (P = 0.07). However, our small sample size limits conclusions regarding this hypothesis of high intrafollicular AMH levels as a cause of relative insensitivity to FSH stimulation and since this outcome was not a predefined end point, the result is merely hypothesis developing.
This investigator-driven dual center study was not powered for such rare events as excessive responses (>15 oocytes), OHSS or OHSS related preventative actions such as cancellation of the oocyte retrieval, agonist triggering or freeze all. The data showed no difference by choice of protocol. The OPTIMIST trials reported a statistically significant increase (P = 0.002) in cycle cancellation due to excessive responses comparing the daily dose of 150IU with 100IU, albeit at the expense of a cancellation rate of 21% due to insufficient growth (Oudshoorn et al., 2017). The benefits were an overall reduction of OHSS (all types). The ESTHER-1 trial using the antagonist protocol showed a reduction in excessive responses by individualization and less patients with OHSS and OHSS preventive measures (Nyboe Andersen et al., 2017). Accordingly, the recent meta-analysis (Lensen et al., 2018) concluded that the benefit of individual dosing models could be a reduction in OHSS incidence.
We assessed the patient-reported subjective wellbeing during the luteal phase in order to evaluate whether individualized dosing could decrease the discomforts by reducing the number of hyper responses. Unfortunately, 22% of ITT participants were lost to follow-up despite being invited to return their questionnaires. Non-respondents predominantly did not have a blastocyst transfer and therefore lost the incentive to return. Within the patients who had a blastocyst transfer the data were robust, in line with the randomization and we saw no improvement by individualization. However, as the proportion of missing data was substantial caution in the interpretation of these results is warranted.
Although not statistically powered to analyze pregnancy rates, the study found similar singleton ongoing pregnancy rates per transfer (44/122 = 36% versus 21/56 = 38% comparing individualized with standard group, respectively) and live birth per started cycle (28 versus 29%), suggesting neither benefit nor harm of the individualized dosing regimen, irrespective of which parameter was used. Additionally, the cumulative live birth rates were similar at 32 and 35% after completion of FET cycles within 1 year of study completion, comparing individualized with standard group, respectively.
A major limitation of this study was the change of AMH assay halfway through the study period. Supplementary Figure S1 illustrates the measured median and inter-quartile range pr. AMH stratum in each assay and Supplementary Fig. S2 the dispersion of values with stratum medians. All Immunotech values were converted to corresponding Elecsys values using the 0.8 factor. Following this conversion, no statistical differences were observed between the two assays. This change was unexpected at the time of study design and introduce an obvious methodological limitation. However, following the considerations of the Supplementary figures, we feel confident that both assays gave sufficiently comparable measures of AMH values for these to be pooled.
The expected extreme cases of ovarian stimulation (AMH < 4 and > 40 pmol/L, FSH > 12 IU/L) and the patients with a bodyweight > 75 kg were excluded. This selection bias limits the generalizability of our results but were chosen in this 2012-designed study for ethical reasons to counteract extreme responses in the standard arm in which all patients had 150 IU/day irrespective of their AMH (Arce et al., 2014). The recently advanced understanding of this area (Nyboe Andersen et al., 2017; Oudshoorn et al., 2017; Lensen et al., 2018) suggests a different setup and it would have been interesting to see the 100 IU/day effect on the patients with AMH > 40 pmol/L.
Likewise, predefining cut-off levels for AMH subgroups is debatable. The present study based the cut-off levels on previous work using the Immunotech assay, suggesting a pragmatic and clinically applicable model with regard to prediction of both oocytes and live birth rate (Nelson et al., 2007). Our cut-off for more aggressive stimulation with corifollitropin (Elonva®) was based on earlier uncontrolled studies using AMH to determine protocol and FSH doses (Nelson et al., 2009; Yates et al., 2011). In our study, the predefined cut-offs corresponded to quartiles of the population as ~25% had an AMH < 12 pmol/L, 50% between 12 and 24 pmol/L, and 25% > 24 pmol/L. Similar cut-offs were used by Anckaert et al. (2012) relating the responses and OHSS risks to these AMH quartiles. As a clinically applicable model we therefore find merit in this division. Recent trials on individualization chose to dose according to the AMH range (Allegra et al., 2017; Nyboe Andersen et al., 2017) and this offers a novel way to examine the concept.
In our center, substantial experience with corifollitropin (Elonva®) stimulation is available. Previously, we presented the analysis of 599 stimulations cycles with no OHSS cases, when the drug was selectively given to patients with AMH in the lower AMH segment (Nielsen et al., 2016). Instead of using a high daily dose of rFSH for the low AMH stratum in the individualized group we chose to use corifollitropin due to ease of administration, a lower cost for the patient and current evidence suggesting an oocyte yield similar to 300 IU/daily of rFSH (Boostanfar et al., 2016). Nonetheless, whether corifollitropin being introduced to a subgroup of the intervention has introduced confounding cannot be ruled out. Also, of the 34 women treated with corifollitropin, 25 needed additional daily FSH doses of 200IU beyond Day 8 of stimulation (6 patients had 1 day, 11 had 2 days, 5 had 3 days and 3 had 4 days). It can be argued that an addition of daily 300IU would have better mimicked the corifollitropin dosage. However, considering empirical experience of slight dosage changes beyond Day 8 of stimulation, we believe this would be of a minor importance for the oocyte yield.
To our knowledge, this RCT is only the second of its kind to investigate individualized dosing in a mandatory antagonist protocol with single blastocyst transfers. As such, it helps to dispel doubts of the concept. The trial was not blinded but none of the authors participated in the oocyte retrievals that provided the primary end point. Additionally, this trial set out to evaluate a concept without any predetermined preference within the investigators and so we would consider any unblinding biases to the results to be negligible.
The clinical implication of administering 100 IU/day of rFSH in predicted hyper-responders is a large proportion of seemingly under-dosed patients. As we are presently not able to predict who will respond appropriately, this group could be dosed in the range 125–137.5 IU/day in future trials that also could include a more specified hyper response group for testing the low dosage regimen. However, the meta-analysis by Lensen et al. (2018) indicates that this is unlikely to increase pregnancy rates and should be powered for a safety outcome. Dosing with <150 IU/day also in the antagonist protocol should otherwise be avoided in accordance with a recently stated recommendation by the Editor in Chief of ‘Human Reproduction’, Hans Evers (Evers, 2017). Optimizing the safety aspect, hyper-responders may also benefit from both agonist triggering and a freeze-all approach. This concept currently await the results of ongoing European RCTs (Stormlund et al., 2017).
Likewise, based on the present understanding it seems clear that more aggressive FSH dosing in patients with a low or limited ovarian reserve may squeeze out one or two more oocytes (Oudshorn et al. 2018; Nyboe Andersen et al., 2018) but this will also not increase pregnancy rates. Herein we agree with the 2015 Broekmans argument that one more oocyte will probably not produce any benefit for the live birth rate (Broekmans, 2015).
The main findings in the present study are rather consistent with recently published multicenter trials. Individualized FSH dosing induce minor changes in oocyte numbers in patients with low and high AMH, but these changes improve neither pregnancy nor live birth rates.
Supplementary Material
Authors’ roles
J.F.P. contributed to the acquisition and analysis of the data and drafted the article. E.C.L., L.F.A. and A.N.A. made substantial contribution to the concept and design of the study as well as the acquisition of the data. A.N.A. furthermore contributed to the analysis and interpretation of data. K.T., A.E., D.N., L.H. contributed to acquisition of the data. All authors made contributions to drafting and revising the article critically for important intellectual content. All authors approved the final version of the article to be published.
Funding
This research article did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. All trial funding was attained by the budgets of the institutions participating and hence completed without any involvement from external sponsors.
Conflict of interest
Outside the submitted work Lars Franch Andersen reports grants and personal fees from Ferring, Merck Serono, MSD and Gedeon-Richter. Outside the submitted work Anders Nyboe Andersen reports grants and personal fees from Ferring, MSD and Gedeon-Richter and personal fees and honoraria from Merck Serono, MSD, Novo-Nordisk and Abbott. All other authors declare no conflicts of interest.
References
- Allegra A, Marino A, Volpes A, Coffaro F, Scaglione P, Gullo S, La Marca A. A randomized controlled trial investigating the use of a predictive nomogram for the selection of the FSH starting dose in IVF/ICSI cycles. Reprod Biomed Online 2017;34:429–438. [DOI] [PubMed] [Google Scholar]
- Anckaert E, Smitz J, Schiettecatte J, Klein BM, Arce J-C. The value of anti-Mullerian hormone measurement in the long GnRH agonist protocol: association with ovarian response and gonadotrophin-dose adjustments. Hum Reprod 2012;27:1829–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce J-C, Klein BM, La Marca A. The rate of high ovarian response in women identified at risk by a high serum AMH level is influenced by the type of gonadotropin. Gynecol Endocrinol 2014;30:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boostanfar R, Gates D, Guan Y, Gordon K, McCrary Sisk C, Stegmann BJ. Efficacy and safety of frozen-thawed embryo transfer in women aged 35 to 42 years from the PURSUE randomized clinical trial. Fertil Steril 2016;106:300–305.e5. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ. The sub-optimal response to controlled ovarian stimulation: manageable or inevitable? Hum Reprod 2015;30:2009–2010. [DOI] [PubMed] [Google Scholar]
- Broer SL, Mol BWJ, Hendriks D, Broekmans FJM. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril 2009;91:705–714. [DOI] [PubMed] [Google Scholar]
- Donders ART, van der Heijden GJMG, Stijnen T, Moons KGM. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 2006;59:1087–1091. [DOI] [PubMed] [Google Scholar]
- Evers J. Editors Highlights 2017. https://www.eshre.eu/Publications/Journals/Human-Reproduction/Editor-highlight/Previous-highlights_2017.aspx.
- La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update 2014;20:124–140. [DOI] [PubMed] [Google Scholar]
- Lensen SF, Wilkinson J, Leijdekkers JA, La Marca A, Mol BWJ, Marjoribanks J, Torrance H, Broekmans FJ. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI). Cochrane database Syst Rev 2018;2:CD012693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford SL, Legro RS, Diamond MP, Coutifaris C, Steiner AZ, Schlaff WD, Alvero R, Christman GM, Casson PR, Huang H et al. . Baseline AMH level associated with ovulation following ovulation induction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2016;101:3288–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Klein BM, Arce JC. Comparison of antimüllerian hormone levels and antral follicle count as predictor of ovarian response to controlled ovarian stimulation in good-prognosis patients at individual fertility clinics in two multicenter trials. Fertil Steril 2015. a;103:923–930.e1. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Pastuszek E, Kloss G, Malinowska I, Liss J, Lukaszuk A, Plociennik L, Lukaszuk K. Two new automated, compared with two enzyme-linked immunosorbent, antimüllerian hormone assays. Fertil Steril 2015. b;104:1016–1021.e6. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod 2007;22:2414–2421. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Yates RW, Lyall H, Jamieson M, Traynor I, Gaudoin M, Mitchell P, Ambrose P, Fleming R. Anti-Müllerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod 2009;24:867–875. [DOI] [PubMed] [Google Scholar]
- Nielsen AP, Korsholm A-S, Lemmen JG, Sylvest R, Sopa N, Nyboe Andersen A. Selective use of corifollitropin for controlled ovarian stimulation for IVF in patients with low anti-Müllerian hormone. Gynecol Endocrinol 2016;32:625–628. [DOI] [PubMed] [Google Scholar]
- Nyboe Andersen A, Nelson SM, Fauser BCJM, García-Velasco JA, Klein BM, Arce J-C, Tournaye H, ESTHER-1 study group H, De Sutter P, Decleer W, Petracco A et al. . Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril 2017;107:387–396.e4. [DOI] [PubMed] [Google Scholar]
- Olivennes F, Trew G, Borini A, Broekmans F, Arriagada P, Warne DW, Howles CM. Randomized, controlled, open-label, non-inferiority study of the CONSORT algorithm for individualized dosing of follitropin alfa. Reprod Biomed Online 2015;30:248–257. [DOI] [PubMed] [Google Scholar]
- Oudshoorn SC, van Tilborg TC, Eijkemans MJC, Oosterhuis GJE, Friederich J, van Hooff MHA, van Santbrink EJP, Brinkhuis EA, Smeenk JMJ, Kwee J et al. . Individualized versus standard FSH dosing in women starting IVF/ICSI: an RCT. Part 2: The predicted hyper responder. Hum Reprod 2017;32:2506–2514. [DOI] [PubMed] [Google Scholar]
- Out HJ, Lindenberg S, Mikkelsen AL, Eldar-Geva T, Healy DL, Leader A, Rodriguez-Escudero FJ, Garcia-Velasco JA, Pellicer A. A prospective, randomized, double-blind clinical trial to study the efficacy and efficiency of a fixed dose of recombinant follicle stimulating hormone (Puregon) in women undergoing ovarian stimulation. Hum Reprod 1999;14:622–627. [DOI] [PubMed] [Google Scholar]
- Popovic-Todorovic B, Loft A, Bredkjaeer HE, Bangsbøll S, Nielsen IK, Andersen AN. A prospective randomized clinical trial comparing an individual dose of recombinant FSH based on predictive factors versus a ‘standard’ dose of 150 IU/day in ‘standard’ patients undergoing IVF/ICSI treatment. Hum Reprod 2003;18:2275–2282. [DOI] [PubMed] [Google Scholar]
- Stormlund S, Løssl K, Zedeler A, Bogstad J, Prætorius L, Nielsen HS, Bungum M, Skouby SO, Mikkelsen AL, Andersen AN et al. . Comparison of a ‘freeze-all’ strategy including GnRH agonist trigger versus a ‘fresh transfer’ strategy including hCG trigger in assisted reproductive technology (ART): a study protocol for a randomised controlled trial. BMJ Open 2017;7:e016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toftager M, Bogstad J, Bryndorf T, Løssl K, Roskær J, Holland T, Prætorius L, Zedeler A, Nilas L, Pinborg A. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod 2016;31:1253–1264. [DOI] [PubMed] [Google Scholar]
- Toftager M, Bogstad J, Løssl K, Prætorius L, Zedeler A, Bryndorf T, Nilas L, Pinborg A. Cumulative live birth rates after one ART cycle including all subsequent frozen-thaw cycles in 1050 women: secondary outcome of an RCT comparing GnRH-antagonist and GnRH-agonist protocols. Hum Reprod 2017;32:556–567. [DOI] [PubMed] [Google Scholar]
- van Tilborg TC, Oudshoorn SC, Eijkemans MJC, Mochtar MH, van Golde RJT, Hoek A, Kuchenbecker WKH, Fleischer K, de Bruin JP, Groen H et al. . Individualized FSH dosing based on ovarian reserve testing in women starting IVF/ICSI: a multicentre trial and cost-effectiveness analysis. Hum Reprod 2017. a;32:2485–2495. [DOI] [PubMed] [Google Scholar]
- van Tilborg TC, Torrance HL, Oudshoorn SC, Eijkemans MJC, Koks CAM, Verhoeve HR, Nap AW, Scheffer GJ, Manger AP, Schoot BC et al. . Individualized versus standard FSH dosing in women starting IVF/ICSI: an RCT. Part 1: The predicted poor responder. Hum Reprod 2017. b;32:2496–2505. [DOI] [PubMed] [Google Scholar]
- Yates AP, Rustamov O, Roberts SA, Lim HYN, Pemberton PW, Smith A, Nardo LG. Anti-Mullerian hormone-tailored stimulation protocols improve outcomes whilst reducing adverse effects and costs of IVF. Hum Reprod 2011;26:2353–2362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.