Abstract
STUDY QUESTION
How did the field of stem cell research develop in the years following the derivation of the first human embryonic stem cell (hESC) line?
SUMMARY ANSWER
Supported by the increasing number of clinical trials to date, significant technological advances in the past two decades have brought us ever closer to clinical therapies derived from pluripotent cells.
WHAT IS KNOWN ALREADY
Since their discovery 20 years ago, the use of human pluripotent stem cells has progressed tremendously from bench to bedside. Here, we provide a concise review of the main keystones of this journey and focus on ongoing clinical trials, while indicating the most relevant future research directions.
STUDY DESIGN, SIZE, DURATION
This is a historical narrative, including relevant publications in the field of pluripotent stem cells (PSC) derivation and differentiation, recounted both through scholarly research of published evidence and interviews of six pioneers who participated in some of the most relevant discoveries in the field.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The authors all contributed by researching the literature and agreed upon body of works. Portions of the interviews of the field pioneers have been integrated into the review and have also been included in full for advanced reader interest.
MAIN RESULTS AND THE ROLE OF CHANCE
The stem cell field is ever expanding. We find that in the 20 years since the derivation of the first hESC lines, several relevant developments have shaped the pluripotent cell field, from the discovery of different states of pluripotency, the derivation of induced PSC, the refinement of differentiation protocols with several clinical trials underway, as well as the recent development of organoids. The challenge for the years to come will be to validate and refine PSCs for clinical use, from the production of highly defined cell populations in clinical grade conditions to the possibility of creating replacement organoids for functional, if not anatomical, function restoration.
LIMITATIONS, REASONS FOR CAUTION
This is a non-systematic review of current literature. Some references may have escaped the experts’ analysis due to the exceedingly diverse nature of the field. As the field of regenerative medicine is rapidly advancing, some of the most recent developments may have not been captured entirely.
WIDER IMPLICATIONS OF THE FINDINGS
The multi-disciplinary nature and tremendous potential of the stem cell field has important implications for basic as well as translational research. Recounting these activities will serve to provide an in-depth overview of the field, fostering a further understanding of human stem cell and developmental biology. The comprehensive overview of clinical trials and expert opinions included in this narrative may serve as a valuable scientific resource, supporting future efforts in translational approaches.
STUDY FUNDING/COMPETING INTEREST(S)
ESHRE provided funding for the authors’ on-site meeting and discussion during the preparation of this manuscript. S.M.C.S.L. is funded by the European Research Council Consolidator (ERC-CoG-725722-OVOGROWTH). M.P. is supported by the Special Research Fund, Bijzonder Onderzoeksfonds (BOF01D08114). M.G. is supported by the Methusalem grant of Vrije Universiteit Brussel, in the name of Prof. Karen Sermon and by Innovation by Science and Technology in Flanders (IWT, Project Number: 150042). A.V. and B.A. are supported by the Plataforma de Proteomica, Genotipado y Líneas Celulares (PT1770019/0015) (PRB3), Instituto de Salud Carlos III. Research grant to B.H. by the Research Foundation—Flanders (FWO) (FWO.KAN.2016.0005.01 and FWO.Project G051516N). There are no conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
Not applicable.
ESHRE Pages are not externally peer reviewed. This article has been approved by the Executive Committee of ESHRE.
Keywords: pluripotency, human embryonic stem cell (hESC), human-induced pluripotent stem cells (hiPSC), regenerative medicine, differentiation, clinical trials
Introduction
In 1998, a report published in the scientific journal Science marked the beginning of the modern era of regenerative medicine (Thomson et al., 1998). For the first time, scientists were able to derive stem cells from a human embryo and show that these cells could be maintained in the so-called pluripotent state.
Pluripotency is a unique characteristic of stem cells; a pluripotent cell can divide indefinitely into daughter cells, while at the same time retaining the capacity to differentiate into any cell type of the human body when submitted to the appropriate stimuli. Human embryonic stem cells (hESCs) are pluripotent, and their derivation sparked new possibilities, from the production of ‘spare parts’ to treating a plethora of degenerative conditions, the study of early embryonic development, to revolutionizing drug screening and development and broadening the spectrum of human toxicology research.
WHAT DOES THIS MEAN FOR PATIENTS?
We reviewed the history of human pluripotent stem cell (hPSC) derivation, in the context of the scientific and technical environment at the time, giving a historical account of the development of the field of stem cell research with a special emphasis on regenerative medicine and clinical applications. Furthermore, we organized interviews with several stem cell scientists at the forefront of basic and clinical research to give the reader a qualitative account of the field, as well as a perspective on the future developments at the cutting edge of research.
This 20th anniversary of the derivation of human embryonic stem cell (hESC) lines offers an ideal opportunity to look back over the past 20 years in this field, as well as to look forward to what the future may hold. With this review, we hope to inspire young investigators today to continue working on their research in this fascinating topic.
In the 20 years since, some of these promises have been fulfilled, some roadblocks have appeared and some new players have entered the stage of pluripotency. The aim of this review is to tell the story of this amazing journey and to reveal its most salient moments through the voice of some of the pioneers in this exciting field.
Materials and Methods
The literature search for the preparation of this non-systematic review was carried out in PubMed including articles written in English between 1998 and 2018. Moreover, suggestions from the pioneers interviewed on seminal contributions were included in the paper. Further, reports in the media were scanned to include up to date information for topics not always reported in the scientific literature. Finally, the database ClinicalTrials.gov and other Clinical Trials Registries were scanned for relevant ongoing trials.
The derivation of the first hESC line
In the mid-90s of the last century, one of the most active centers of stem cell research, at that time restricted to mouse work in most countries, was the Wisconsin National Primate Research Center in the USA. It was here that scientists had derived in 1994 the first primate ESC line from rhesus macaque embryos. As customary at the time, the line was cultured over a feeder layer of mouse fibroblasts and the scientists were able to show the cells ability to continually grow in culture for more than 1 year. Moreover, once released from stem cell culture condition and injected into immunodeficient mice these cells could differentiate spontaneously into derivatives of the three germ layers: endoderm, ectoderm and mesoderm, a test of pluripotency still used to this day (Thomson et al., 1995).
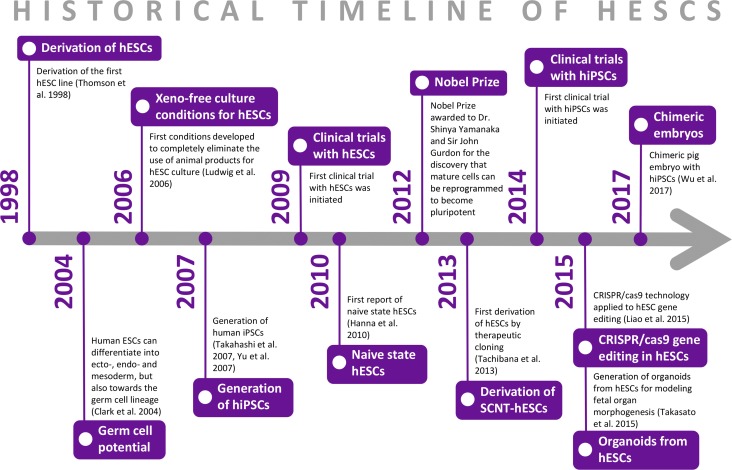
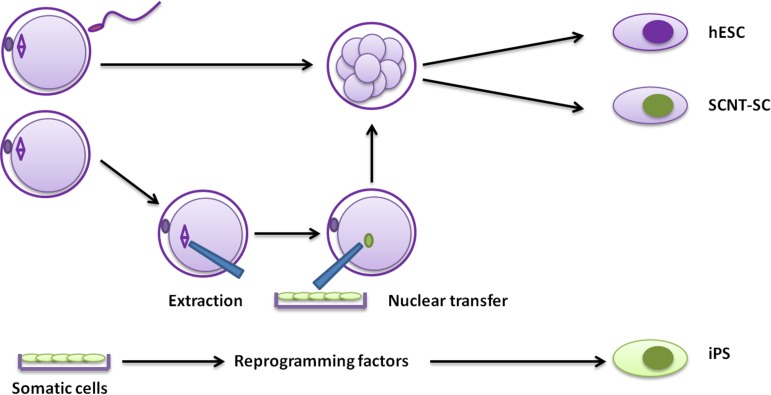
Without any doubts, their experience with rhesus ESCs accelerated their learning curve in primate stem cell handling, and the same team of scientists led by Dr J. A Thomson was able to report, 4 years later, the derivation of the first hESC line (Thomson et al., 1998) (Fig. 1). The researchers used 36 embryos from patients who underwent IVF, who donated their embryos after completing their treatment. From those embryos, 14 inner cell masses (ICMs) were isolated, and five hESC lines were derived (Fig. 2). It is interesting to note that one of those lines, H9, has been used in clinical trials several years following its derivation.
Figure 1.
Historical timeline of human embryonic stem cells. hESCs: human embryonic stem cells, hiPSC: human-induced pluripotent stem cells, CRISPR/Cas 9: clustered regularly interspaced short palindromic repeat/ CRISPR associated 9.
Figure 2.
Types of human pluripotent stem cells. Three pathways to the generation of pluripotent cells are described: hESC derivation from preimplantation-stage embryos (hESC; top), derivation after somatic cell nuclear transfer (SCNT-SC; middle) and derivation through reprogramming of somatic cells (iPS; bottom). SCNT-SC: somatic cell nuclear transfer-stem cells; iPS: induced pluripotent stem cells.
Around the same time, hESC derivation was also attempted at the National University Hospital in Singapore. In 1994, Bongso et al. cultured whole blastocysts on human tubal ampullary epithelium and succeeded in obtaining cells that retained stem cell-like morphology (Bongso et al., 1994). Although these cultures differentiated after several passages, this was the first report of the successful isolation of human ICM cells and their continued culture in vitro. A few years later, in 2000, Reubinoff et al., independently reported the establishment of hESC lines with similar properties to those of Thomson et al. (1995), obtaining ICM clusters from human blastocysts using immunosurgery and culturing them on mouse embryonic fibroblasts. Their report further validated the potential of hESCs by demonstrating that they could be directed toward the neuronal lineage, through isolation and culture of neuronal progenitor cells from differentiating hESCs. Dr A. Trounson, a pioneer in the field, offers his testimonial: ‘When Martin Pera joined me from Oxford he thought we had human ESCs, so I sent Ben Reubinoff (our PhD student) to Singapore to make them again. He brought back some of the colonies, which converted to hESCs and we set about characterizing them, using markers Martin had for setting up the teratoma assays, just before we received the Thomson paper for review.’ (Supplementary data).
This research certainly paved the way for the large number of pluripotent stem cell lines produced to date and generated considerable optimism regarding stem cell biology. Notably, the scientific and medical potential of hESCs could not have been realized without the progress made in the field of assisted reproduction at the time, and particularly the use of surplus IVF embryos, donated by patients for research purposes.
The news and promise of hESCs sparked the imagination of scientists and the general public alike, and the race to repeat the results was fierce, compiled by the fact that very few researchers had at the time seen primate stem cells. As Dr M. Stojkovic, whose team derived the first hESC line in the UK, recalls: ‘I remember very well the following picture: in the front of our microscope was Thomson’s paper and images of hESC colonies and Majlinda Lako and myself starting our open-air work: cutting the colony, passaging the cells… both of us had never seen or worked with hESCs before.’ (Supplementary data).
The hope that stem cells could provide potential therapies in regenerative medicine prompted further research toward the directed differentiation of hESCs toward specialized cell types (Trounson, 2006). Derivation reports showed that suboptimal culture conditions led to spontaneous differentiation of hESCs (Reubinoff et al., 2000, Thomson et al., 1998). Subsequently, Schuldiner et al. demonstrated that the differentiation of hESCs in vitro could be controlled by using several factors to enrich for specific embryonic germ layers (Schuldiner et al., 2000). This work provided a valuable framework for the directed derivation of a number of specialized cell types from hESCs, including mature neurons (Reubinoff et al., 2001, Zhang et al., 2001), cardiomyocytes (Kehat et al., 2001, Mummery et al., 2003) and insulin-producing cells (Assady et al., 2001). These early reports were fundamental for further in vitro manipulations of pluripotent stem cells (PSCs), setting the stage for future clinical applications.
Induced PSCs
In 2006, the PSC field was revolutionized again by the generation of induced PSC (iPSC), a technology pioneered by Dr S. Yamanaka’s lab in Kyoto, Japan. Yamanaka’s team demonstrated that forced expression of four transcription factors (Pou5f1, pou class 5 homeobox 1; Sox2, SRY-Box 2; Klf4, kruppel like factor 4 and c-Myc, c-myc) could reprogram adult mouse cells into a pluripotent state remarkably similar to that of ESCs (Fig. 2) (Takahashi and Yamanaka, 2006). Less than 1 year later, the same technology was used for the derivation of human iPSCs (hiPSCs) (Takahashi et al., 2007; Yu et al., 2007), providing an alternative source of human pluripotent stem cells (hPSCs) without the need to use human embryos, thus alleviating some of the ethical concerns associated with hESCs (Fig. 1). The profound impact of iPSC technology on the study of cell biology, and especially nuclear reprogramming, was recognized when Dr S. Yamanaka, along with Dr J. Gurdon, received the Nobel Prize for Physiology or Medicine in 2012 (Fig. 1). Interestingly, when asked about the name iPSC, S. Yamanaka explained: ‘In naming iPS cells, I used a small letter for “i” after the model of “iPod” hoping that the name would be easy to be remembered.' (Supplementary data).
The initial methods for iPSC derivation used retroviral or lentiviral vectors to deliver the four reprogramming factors (Takahashi et al., 2007; Yu et al., 2007), which resulted in the integration of the foreign DNA into the host cell genome, thus carrying the risk of insertional mutagenesis. To overcome this issue, various non-integrating methods for human iPSC generation have been developed, including episomal DNA plasmids (Okita et al., 2011a), Sendai virus (Fusaki et al., 2009), adenovirus (Stadtfeld et al., 2008), synthesized modified mRNAs (Warren et al., 2010) and proteins (Kim et al., 2009). Several cell sources and combination of fewer, or different, factors have also been successfully used for reprogramming, making it safer and more efficient in the process (reviewed in (Takahashi and Yamanaka, 2016)).
One of the main advantages of human iPSCs is their potential to model disease in vitro. In contrast to hESCs, which can also carry a genetic disease but are derived from embryos after PGD, the genotype of iPSCs can be directly linked to the disease phenotype in the patient/cell donor. iPSCs can be derived from patients carrying a disease-causing mutation and differentiated into disease-relevant cell types, offering an unlimited source of cells for studying genotype–phenotype relationships. Already in 2009, the derivation of patient-specific iPSCs from a child with spinal muscular atrophy showed disease-related deficits in the motor neurons generated in vitro (Ebert et al., 2009). Since then, an increasing number of disease models with iPSCs have been generated, especially for monogenic diseases, such as Rett syndrome (Marchetto et al., 2010) and type 2 long QT syndrome (Itzhaki et al., 2011), but also for genetically complex or sporadic diseases, such as Alzheimer’s disease (Israel et al., 2012) and Parkinson’s disease (Nguyen et al., 2011). hiPSCs can successfully recapitulate disease pathogenesis in vitro and are now increasingly used for validating and screening new therapeutic compounds (reviewed in (Suh, 2017)). In addition, iPSCs hold great promise for developing personalized treatments. Patient-specific iPSCs may be used for predicting the patient’s response to specific treatment strategies, as was indicated in a recent study that showed concordant results for pharmacological response between iPSCs and a patient with type 3 long QT syndrome (Malan et al., 2016). Furthermore, iPSCs may be used in regenerative medicine either as an autologous cell source or as a HLA-matched allogeneic cell source for transplantation, minimizing the risk of rejection and the use of long-term immunosuppression. In 2014, the first clinical study using hiPSC-derived cells was initiated (Fig. 1).
Somatic cell nuclear transfer derived stem cells
Somatic cell nuclear transfer (SCNT), colloquially known as cloning, is the process of transferring the nuclear DNA of a donor somatic cell into an enucleated oocyte, followed by embryo development (Fig. 2) (Wilmut et al., 2002). When the SCNT embryo is transferred to a surrogate recipient with the aim to achieve a live birth, the process is defined as reproductive cloning. The first success in mammals was achieved with the birth of Dolly the sheep in 1996 (Wilmut et al., 1997), cloned from a differentiated mammary epithelial cell. This successful attempt proved that it is possible to revert the differentiated status of the somatic nucleus to totipotency (reprogramming) (Wilmut et al., 2002). However, when pluripotent SCNT stem cells are harvested from the reconstructed SCNT embryo, the process is called therapeutic cloning, aiming at deriving pluripotent stem cells for future cell therapy and research purposes (Fig. 2). The advantage of therapeutic cloning over ESCs is that SCNT stem cells, like iPSCs, are genetically identical to the somatic cell they are derived from, thereby overcoming immune rejection, inherently valuable for future clinical applications. Somatic cell nuclear transfer was first attempted in amphibians due to the comparatively large size of the eggs, enabling easier micromanipulation coupled with the possibility of using considerable numbers of eggs and embryos. Tadpoles developed following transfer of nuclei from early cleavage stage embryos to enucleated eggs (Briggs and King, 1952). Subsequently, the group of Dr J. Gurdon (Gurdon et al., 1958) transplanted the nucleus of a tadpole intestinal cell into an enucleated frog egg, succeeding in the creation of viable tadpoles that were genetically identical to the one from which the intestinal cell was obtained. This was the first experiment to show that differentiated cells could be set back to an embryonic state.
Since Dolly, several attempts have been made to generate SCNT-ESCs in several mammalian species, due to their potential benefits in biomedical applications such as allo-transplantation and personalized drug selection (Matoba and Zhang, 2018). These attempts have further enabled optimization of the SCNT process, including cell cycle synchronization between donor cells and recipient oocytes, erasure of epigenetic marks by using donor cells of varying ages and from different tissues, as well as the addition of small molecules and the modification of culture conditions (Akagi et al., 2011). The first primate SCNT-ESCs were derived in the rhesus macaque from adult skin fibroblasts, partly owing to the non-invasive removal of the spindle-chromosome complex by polarized microscopy (Byrne et al., 2007). Although very successful in all species tested, pseudoblastocyst development following human SCNT was not achieved, with most SCNT embryos arresting at the stage of embryonic genome activation (Heindryckx et al., 2007). The first successfully reconstructed human SCNT pseudoblastocysts were reported by French et al. (2008), however the derivation of SCNT-ESC lines was not attempted. The key to success was minor SCNT technological adjustments and the use of in vivo matured oocytes from young donors. Subsequently, Noggle and collaborators adjusted the conventional SCNT approach by transferring the somatic nucleus into a non-enucleated recipient oocyte. The reconstructed embryos developed well, and several SCNT-ESC lines were derived, albeit triploid (Noggle et al., 2011).
The group of Dr S. Mitalipov (Tachibana et al., 2013) was the first to succeed in the production of SCNT-hESCs lines (Fig. 1), later reproduced by a handful of groups (Chung et al., 2015; Wolf et al., 2017). As S. Mitalipov highlights himself: ‘We demonstrated that cytoplasmic factors present in mature human oocytes are capable of converting the transplanted nuclear genomes from somatic cells (skin fibroblasts) to become “oocyte-like”. We then used such skin-derived oocytes to develop into blastocysts and ESCs.’ (Supplementary data).
The biggest hurdle for human SCNT applications remains the scarcity of human oocytes. A highly debated research question is whether the SCNT-ESC represent a better reprogramming method compared to iPSC (Matoba and Zhang, 2018). An issue of SCNT-ESCs and iPSCs is their propensity to retain a so-called somatic epigenetic memory, i.e. a partial epigenetic state, characteristic of the somatic cell used to derive them, which may lead to biases or limitations in their fate choice following differentiation into cells of a particular lineage, as shown in the mouse. In-depth analysis of mouse SCNT-PSCs has shown that they are molecularly closer to and functionally indistinguishable from mESCs derived from IVF-fertilized embryos, as compared to both mouse and hiPSCs (Ma et al., 2014; Mishra et al., 2018).
Toward clinical-grade hPSCs: development of fully defined culture conditions
Currently, thousands of hPSC lines have been derived and are available for research purposes (https://hpscreg.eu/; https://cells.ebisc.org; http://www.hipsci.org/). However, most of these lines are not suitable for clinical use as they have been derived and maintained in complex and poorly defined culture systems containing several xenogeneic components. Conversely, clinical-grade hPSC need to be generated and maintained in fully defined, xeno-free culture conditions, in compliance with current good manufacturing practices (GMPs).
The importance of clinical-grade cells has been recognized for several years now, as expressed in the words of pioneer Dr O. Hovatta: ‘[…] we saw that the hESC lines derived using mouse cells as feeder cells, and using bovine serum and other ingredients with animal origin in the culture medium, were quite suboptimal in quality thinking of infection risks and functional quality in clinical work. Hence, we decided to develop better derivation systems.’ (Supplementary data).
In fact, as mentioned by Dr O. Hovatta, the first methods described for the derivation of hESC entailed the use of mouse feeder cell layers and medium containing fetal bovine serum (FBS), two factors that may contain animal pathogens and immunogens. FBS can be replaced by Knock-Out Serum Replacement (SR), which contains several amino acids, vitamins, antioxidants and trace elements, but also proteins including lipid-rich albumin which seems to play an important role in hPSC self-renewal (Garcia-Gonzalo and Izpisua Belmonte, 2008). Although more defined than FBS, SR is still xenogeneic and therefore maintains the risk for pathogenic contamination of hPSCs, which may be transmitted to patients upon transplantation (Martin et al., 2005). The use of human cells, mainly foreskin fibroblast cells, as feeder layers was the first important step toward xeno-free hPSC cultures (Richards et al., 2002; Amit et al., 2003; Hovatta et al., 2003). However, if used for clinical-grade hPSC derivation and culture, the human feeder cells must also be produced under current GMP conditions (Prathalingam et al., 2012).
The first feeder-free hESC derivation was reported in 2005, using extracellular matrix extracted from mouse embryonic fibroblasts (Klimanskaya et al., 2005). They applied a serum-free medium with high concentrations of basic fibroblast growth factor (bFGF) to support hESC growth in the absence of fibroblasts. One year later, Ludwig and colleagues composed the first defined hESC derivation and culture medium, termed TeSR1. The formulation of the medium was created by testing the effect on hESC marker expression after the systematic addition of growth factors. One of the major advantages of this approach was that bFGF and transforming growth factor β 1 (TGFβ1), important factors for hESC maintenance, were identified. These factors are significantly different than those that were known for mouse ESCs (mESC) (Ludwig et al., 2006). Ludwig and colleagues also generated an artificial human extracellular matrix with a combination of human collagen IV, laminin, fibronectin and vitronectin to support hESC derivation and long-term culture. These conditions, for the first time, completely eliminated the use of animal products (Ludwig et al., 2006) (Fig. 1). Further progress in the study of the interactions between different media components led to the formulation of a completely chemically-defined albumin-free medium, composed of eight factors: Essential 8 medium (E8). E8 is composed of insulin, selenium, transferrin, l-ascorbic acid, bFGF and TGFβ (or Nodal) in DMEM/F12 with pH adjusted with NaHCO3 (Chen et al., 2011).
The first surface coatings used to substitute feeder cells were often protein mixtures obtained from cell cultures from which the exact composition can vary significantly from lot to lot (Klimanskaya et al., 2005; Ludwig et al., 2006). Matrigel, for example, is an extracellular protein mixture secreted by Engelbreth–Holm–Swarm mouse sarcoma cells, consisting of structural proteins such as laminin or collagen, growth factors (like TGFβ) and other proteins in small amounts (Hughes et al., 2010). During their search for more defined coatings, several groups identified extracellular matrix proteins with primary roles in supporting hPSC self-renewal and pluripotency. The recombinant forms of these proteins were then used for derivation and maintenance of hPSC lines. Known examples are vitronectin, laminin and fibronectin (Braam et al., 2008; Rodin et al., 2010). In 2014, Rodin et al. produced a specific subtype of laminin, LN-521, which allowed single-cell passaging of hPSC (Rodin et al., 2014), until then only possible with the use of Rho-associated coiled-coil containing protein kinase (ROCK) pathway inhibitors (Watanabe et al., 2007). Moreover, the combination of LN-521 with E-cadherin highly improved the efficiency of clonal hPSC culture and allowed hESC derivation from single blastomeres (Rodin et al., 2014).
As an alternative to recombinant proteins, synthetic polymers such as amino-propylmethacrylamide (APMAAm), poly(methyl vinyl ether-alt-maleic anhydride) (PMVE-alt-MA) and poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl) ammonium hydroxide] (PMEDSAH) have been used, resulting in a fully defined surface coating for hPSC culture (Shao et al., 2015). Finally, physical methods have also been used for surface optimization. Successful hPSC cultures have been described on both oxygen plasma-etched and UV ozone radiation modified tissue culture polystyrene (PE-TCP) (Mahlstedt et al., 2010; Saha et al., 2011).
One of the most crucial points of improvement for the future will be the upscaling of hPSC cultures through 3D systems. Large-scale hPSC production will be essential for their successful application in regenerative medicine. The number of cells required for effective cell therapy treatment varies according to the therapeutic goal but is expected to range between millions and billions of cells (Serra et al., 2012). While the 2D culture systems remain time-consuming and labor-intensive, 3D automated culture systems, e.g. with stirred-tank bioreactors, may provide a solution for the mass production of high quality hPSC with minimal labor and costs (Steiner et al., 2010; Fluri et al., 2012; Shafa et al., 2012). To avoid hydrodynamic stress and agglomeration of growing spherical aggregates, researchers have attempted to encapsulate hPSCs in hydrogel scaffolds (Li et al., 2018). With this last approach, systems can be set up that are simple, scalable, highly efficient, defined and GMP-compatible.
An important aspect to keep in mind is that culture conditions may affect the genetic and epigenetic stability of the hPSC cells. An elegant study by Jacobs et al. showed that the decreased pH of the culture medium, resulting from high-density culture, has a direct influence on DNA damage and genomic instability of the cells (Jacobs et al., 2016). Highly recurrent chromosomal abnormalities over culture passages have been reported by various labs worldwide, reflecting the progressive adaptation of hPSCs to culture conditions and the culture advantage conferred to the cells by these genetic changes (Spits et al., 2008; International Stem Cell et al., 2011; Nguyen et al., 2013). An example is the duplication of the long arm of chromosome 20. This abnormality has been reported in ~20% of hPSC lines worldwide, and culture takeover of the mutant cells is driven by overexpression of the B-cell lymphoma 2 like 1 (BCL2L1) gene, which leads to resistance to apoptosis upon dissociation (Avery et al., 2013; Nguyen et al., 2014). Similarly, also at the epigenetic level, the loss of DNA methylation and specific histone modifications has been linked to suboptimal culture conditions (Nazor et al., 2012; Geens et al., 2016; Geens and Chuva De Sousa Lopes, 2017).
States of pluripotency: a model of early embryonic development
In the past two decades, insights into early embryo development have broadened our perception of pluripotency. As such, pluripotency is no longer viewed as a fixed state but rather a highly dynamic, malleable signaling network (Wu and Izpisua Belmonte, 2015; Weinberger et al., 2016; Smith, 2017). Unraveling the complete potency spectrum and its transitions will remain central to our understanding of lineage commitment.
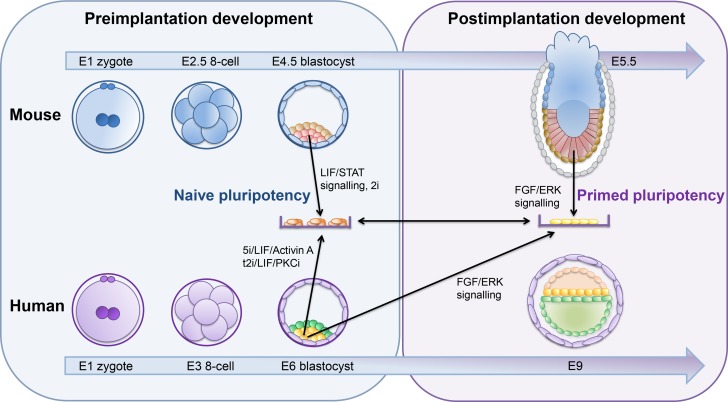
mESCs are one of the earliest and better characterized models of pluripotency (Xue et al., 2011). Derived from the ICM of mouse blastocysts, mESCs demonstrated characteristic features of pluripotency, including long-term self-renewal, ability to differentiate toward all germ layers, high single-cell clonogenicity and efficient contribution to chimeras (Evans and Kaufman, 1981; Martin, 1981) (Fig. 3). hESCs derived from human preimplantation embryos, however, were markedly different from mESCs (Thomson et al., 1998); hESCs had an epithelial morphology, could not be propagated efficiently as single cells and had different growth requirements (Thomson et al., 1998). It soon became evident that hESCs rely on different signaling pathways to maintain pluripotency (Vallier et al., 2005; Nichols and Smith, 2009) (Fig. 3).
Figure 3.
States of pluripotency: primed vs naive. A comparison between potency states of cells in the developing embryo from mice (top) and human (bottom) and the in vitro manipulation necessary to maintain/convert each stage to the other. LIF/STAT, leukemia inhibitory factor/signal transducer and activator of transcription 3; PKCi, protein kinase C inhibitor; E, day of embryonic development; FGF, fibroblast growth factor; ERK, extracellular regulated kinase.
Some years later, mouse epiblast stem cells (mEpiSCs) were isolated from post-implantation embryos and were found to share many similarities with hESCs (Brons et al., 2007; Tesar et al., 2007). Their transcriptome was similar to that of the post-implantation epiblast (Brons et al., 2007; Tesar et al., 2007), indicating that hESCs were more representative of later stages of embryo development. Subsequently, two states of pluripotency were proposed: naive and primed (Nichols and Smith, 2009) (Fig. 3). Accordingly, mESCs exist in a naive state, which constitutes the functional in vitro equivalent of the preimplantation epiblast, while hESCs are in a primed state. The naive or ground state of pluripotency is characterized by a seemingly unbiased differentiation potential, low variability in pluripotency linked gene expression, global DNA hypo-methylation and two active X-chromosomes in female cells (Nichols and Smith, 2009; Hackett and Surani, 2014; Davidson et al., 2015; Van der Jeught et al., 2015; Weinberger et al., 2016) (Fig. 3). Conversely, cells in the primed state display distinct pluripotency associated gene patterns, DNA hyper-methylation, X-chromosome inactivation and inefficiency in forming chimeras; this state corresponds to the transition of naive epiblast cells toward a more committed state in vivo (Nichols and Smith, 2009; Hackett and Surani, 2014) (Fig. 3).
The notion that pluripotency exists in at least two distinct forms prompted further research toward the identification of in vitro culture conditions that stabilize the naive state in humans. Naive-like hESCs were first obtained by transgene-mediated reprogramming of primed hESCs (Hanna et al., 2010) (Fig. 1). However, the resulting naive hESCs required continued expression of integrated transgenes for long-term self-renewal. To address this limitation, several groups attempted to modify the cell culture conditions in order to induce naive characteristics in hESCs (Xu et al., 2010; Gu et al., 2012). Soon after, optimized chemically defined conditions were established, with the naive human stem cell medium (NHSM) allowing rapid conversion of primed hESCs to the naive state (Gafni et al., 2013). The resulting hESCs retained molecular and functional properties similar to naive mESCs. In a more systematic approach, Theunissen et al. identified a combination of five kinase inhibitors that generated hESCs expressing genes associated with human preimplantation development and the ground state of pluripotency (Theunissen et al., 2014). However, several hESC lines presented with an abnormal karyotype, leading to the notion that naive hPSCs may be more prone to genomic instability in culture. Takashima et al. reported that short-term overexpression of NANOG and kruppel like factor 2 (KLF2) were sufficient to reset the human pluripotency network (Takashima et al., 2014). To date, several other conversion protocols have been established (Chan et al., 2013; Valamehr et al., 2014; Ware et al., 2014; Duggal et al., 2015; Carter et al., 2016). Derivation of naive hESCs directly from the blastocyst ICM has also been achieved, albeit generally at low efficiency (Gafni et al., 2013; Theunissen et al., 2014; Ware et al., 2014) and frequently resulting in an abnormal karyotype (Theunissen et al., 2014; Guo et al., 2016).
The generation of naive hESCs allowed for their molecular signature to be described in detail. However, the methods used to generate naive hESCs vary considerably, restricting the primed state at different molecular levels. In the embryo, the transition to lineage commitment is highly efficient (Smith, 2017), yet during in vitro culture, altered conditions may modify this progression, contributing to inconsistent and inefficient directed differentiation (Warrier et al., 2017). Notably, current naive hESCs do not readily differentiate and must first undergo a ‘priming’ step (Takashima et al., 2014; Irie et al., 2015; Smith, 2017).
The possibility to obtain different types of stem cells illustrates that pluripotency is highly determined by the synergistic interplay between embryo developmental stage and the micro-environment. Considering future therapeutic applications, unraveling the full potency spectrum of hESCs may enhance efficiency and control over directed differentiation. Exploring cellular plasticity during embryogenesis and recapitulating this potential in vitro will undoubtedly have profound effects on both the reproductive and stem cell fields.
Differentiation of PSCs
To date, hPSCs have been differentiated to many cell types, either by directed differentiation (to a specific cell type of interest, for example cardiomyocytes, dopaminergic neurons or pancreatic beta-cells) or by undirected differentiation (for example in embryoid bodies).
For some purposes, the generation of a single-cell type may be desirable (e.g. to model heart disease, Parkinson’s disease, diabetes). However, the initial population of PSCs is usually rather heterogeneous, hence differentiation protocols need constant optimization to increase their efficiency. A limitation of in vitro differentiation is that the final maturation of differentiated cells in culture is often partial, corresponding to a ‘fetal’ phenotype instead of a more ‘adult’ (or mature) one. Scientists are still attempting to identify factors that may be used to mature the cells further. In this sense, it is important to note that initiatives to help determine the exact ‘maturation level’ of a differentiated cell are emerging. One such initiative is KeyGenes, a platform that includes transcriptomics data of human fetal and adult organs and compares the transcriptional profile of differentiated cells to in vivo organs at different developmental stages (Roost et al., 2015). For example, this platform was used to demonstrate the fidelity of differentiation of kidney-organoids to the kidney, providing fast and reliable quantification of the differentiation protocol used and revealing that after 16 days of differentiation the organoids resembled first trimester kidneys (Takasato et al., 2015). Another initiative that will surely contribute to increase our understanding of specific cell types and signals produced at every stage of maturation to help improve the quality and efficiency of differentiation protocols is the Human Cell Atlas project (Rozenblatt-Rosen et al., 2017) that will generate single-cell transcriptomics data from human organs.
Organoids and chimeras
More recently, there has been a significant effort to generate human organoids (or mini-organs) by taking advantage of patient-specific PSCs for drug testing and disease modeling. Organoids are typically free-floating hollow spheres made of cells, with self-organizing (and self-patterning) properties that recapitulate some aspects of development and organ morphogenesis. Typically, they have epithelial or epithelial and mesenchymal architectural compartments, with the apical part of the epithelial compartment on the inside bordering a fluid-filled cavity. Organoids are usually maintained long-term in defined culture medium on Matrigel-scaffolds. However, Matrigel is an undefined product, making organoids unsuitable for regenerative medicine applications. To date, hPSCs have been used to generate organoids from the digestive system (liver, small intestine, stomach), urogenital tract (kidney), neural system (brain, pituitary gland, inner ear, retina), exo/endocrine glands (mammary glands, thyroid) and respiratory system (lung) (Huch and Koo, 2015; Kretzschmar and Clevers, 2016; McCauley and Wells, 2017) (Fig. 1). One of the advantages of organoids is the potential for scaling up at an industrial level for translational applications. With the current screening technologies, organoid systems could be exploited to model patient-specific drug response to tumorigenesis (Arai et al., 2015; Crespo et al., 2017; Qu et al., 2017) or infectious diseases (Zika virus) (Qian et al., 2016).
In addition to organoids, efforts are also underway to generate fully grown organs in interspecies-chimeras (organism composed of a mix of at least two genetically distinct cells), including human–animal chimeras, broadening the application of patient-specific PSCs. Initial ground-breaking work from Kobayashi and colleagues in 2010 showed that wild-type rat PSCs could integrate in mice blastocysts mutants for PDX1 (pancreatic and duodenal homeobox 1) (that develop to birth but die shortly afterward, as they lack the pancreas) and resulted in viable PDX1-/- mouse progeny with a fully functional pancreas made of rat cells (Kobayashi et al., 2010). This successful application was taken a step further and the technology to generate pig blastocyst mutants for PDX1 was subsequently developed (Matsunari et al., 2013). Next steps include investigating whether human PSCs have the potential to integrate into mutant pig blastocysts, such that a human pancreas can be developed in the pig. This concept known as ‘organ farming’ may have important implications to resolve the shortage of organs available for transplantation (heart, kidney, pancreas), as it would be possible to generate patient-specific human organs in pigs. However, recent work introducing rat and human PSCs in porcine and bovine blastocysts has demonstrated limited integration and development of chimeric pig embryos until mid-gestation (Wu et al., 2017) (Fig. 1).
In vitro gamete derivation
Producing gametes in vitro by directed differentiation of hPSCs represent a complex goal, which may be used in the future to provide for an unlimited source of in vitro-produced gametes for toxicology and pharmacology studies, but possibly also for granting certain infertile couples the possibility to have genetic children.
Germ cell development is a highly orchestrated process, controlled by a unique set of genetic and epigenetic regulators, taking into account important variables like primordial germ cell (PGC) state, meiosis and gonadal somatic cells with important differences between mouse and human models (Eguizabal et al., 2016). Thus far, researchers have been successful in deriving in vitro germ cells from mouse PSCs, however many obstacles remain to be overcome for the robust generation of mature gametes in humans (Yamashiro et al., 2018).
In vitro differentiation of hPSCs into male germ cells
An important first step to achieve functional gametes from PSCs is to reach the PGC state. Putative PGC-like cells have already been derived in vitro from hPSCs (Fig. 1) (Clark et al., 2004) by several culture approaches such as embryoid body formation (Bucay et al., 2009), monolayer differentiation (Tilgner et al., 2008, 2010) or co-culture with fetal gonads (Park et al., 2009). Also the PGC state can be reached from different pluripotency states (primed and naive) (Mitsunaga et al., 2017; Sasaki et al., 2015).
The second step for male germ cell differentiation is reaching the post-meiotic stage of spermatozoa. Unfortunately, differentiation past the PGC stage has proven challenging in human. One of the strategies employed includes supplementing the differentiation medium with growth factors such as bone morphogenetic protein (BMP)-4 (Tilgner et al., 2008), bFGF, retinoid acid and R115866 (Eguizabal et al., 2011) and hormones such as insulin and testosterone (Easley et al., 2012). Several groups have attempted overexpression of specific male germ cell genes, such as deleted in azoospermia (DAZ), deleted in azoospermia like (DAZL) and BOULE (Kee et al., 2009; Panula et al., 2011) or VASA (Tilgner et al., 2008, 2010; Medrano et al., 2012) to guide differentiation toward spermatozoa.
Recently, hiPSCs from azoospermic and normospermic males were directly transplanted into mouse testis, partially colonizing the testicular niche and showing signs of early spermatogenesis (Ramathal et al., 2014), raising the question of whether an initial in vitro differentiation step is needed at all. In a mouse model, it has been demonstrated that transplanting in vitro-produced PGC-like cells from PSCs into mouse testis resulted in functional sperm, capable of producing healthy and fertile offspring (Hayashi et al., 2011; Nakaki et al., 2013), representing the most important proof of functional male germ cells from PSCs.
So far, the need of a natural testicular niche to obtain mature functional spermatozoa is still present, and in vitro production of spermatozoa is currently not possible in humans (Zhou et al., 2016).
In vitro differentiation of hPSCs into female germ cells
In 2003, Dr H. Schöler´s group demonstrated for the first time that mESCs were able to form follicle-like structures comprising oocyte-like cells, however their functionality was not proven (Hubner et al., 2003). Several years later, in 2012, Hayashi et al. used female mPSCs and induced them into PGC-like cells, which were then aggregated with fetal ovarian somatic cells and transplanted under the ovarian bursa. PGC-derived immature oocytes were obtained and were subsequently matured and fertilized in vitro and the resulting embryos were transplanted into foster mothers (Hayashi et al., 2012).
Later, Hikabe et al. reconstituted the complete process of oogenesis in vitro from mESCs and iPSCs (derived from fetal and adult tail tip fibroblasts) to generate oocyte-like cells, however with lower success rates in comparison to Dr K. Hayashi's earlier results (Hikabe et al., 2016).
Recently, Jung et al. (Jung et al., 2017) showed that DAZL and BOULE can modulate hESC entry into meiosis, and that growth differentiation factor 9 (GDF9) and BMP15 can induce folliculogenesis in differentiated hESCs. The follicle-like cells derived resembled in vivo primordial follicle, as proven by transplantation experiments.
At present, robust in vitro production of functional human oocytes from pluripotent cells remains a distant prospect.
Genetic modifications in hPSCs
Over the past decades, several gene editing strategies have been employed in hESCs and hiPSCs, each with benefits and limitations.
The first efforts to manipulate the hESC genome involved untargeted transgenic approaches, used to monitor cellular differentiation (Eiges et al., 2001). Bacterial plasmids or viral vectors were used to randomly integrate reporter genes, using cell-specific promoters to drive expression (Eiges et al., 2001; Gerrard et al., 2005). Similarly, a number of fluorescent reporter lines were generated to monitor differentiation toward specific lineages, allowing for the identification of a variety of hESC derivatives (Lavon et al., 2004; Singh Roy et al., 2005; Huber et al., 2007) However, untargeted transgenic technology was soon replaced by targeted approaches for gene editing in order to achieve specificity.
Homologous recombination (HR)-based gene editing allowed integration of a nucleotide sequence into a specific site within the hESC genome (Mansour et al., 1988; Meyn, 1993; Giudice and Trounson, 2008). HR-mediated methods utilize the innate DNA repair machinery of the cell to alter or replace a specific nucleotide sequence by a homologous one (Leavitt and Hamlett, 2011; Brookhouser et al., 2017). Gene targeting by HR in mESCs (Smithies et al., 1985; Thomas and Capecchi, 1987) proved paramount for characterizing gene function and investigating human disease. However, classical methods of HR in mESCs did not prove straightforward in hESCs. The first report demonstrating HR-based gene editing in hESCs targeted the X-linked, hypoxanthine-guanine phosphoribosyl transferase (HPRT) gene and pluripotency marker POU5F1 (Zwaka and Thomson, 2003), providing key parameters for further efforts in the field, at the time. Notably, HR-based gene targeting was also applied to correct gene mutations in hESCs and model disease (Urbach et al., 2004; Ruby and Zheng, 2009). Although these reports demonstrated the viability of HR-based gene editing they also underscored the technical challenges involved. Poor single-cell survival resulted in considerable cell death and low transfection efficiencies in hESCs (Zwaka and Thomson, 2003; Urbach et al., 2004; Irion et al., 2007; Di Domenico et al., 2008; Ruby and Zheng, 2009; Brookhouser et al., 2017). Moreover, as recombination activity is heavily dependent on cell type and cell cycle, applications of this method were relatively limited (Eid and Mahfouz, 2016; Chandrasekaran et al., 2017).
Other approaches aimed at promoting HR-based targeting in hESCs proved more successful. In the past decades, enzymatically induced DNA double-strand breaks (DSBs) significantly increased the efficiency of HR-mediated gene editing (Donoho et al., 1998). Genomic DSBs are generated by engineered sequence specific nucleases and repaired either by non-homologous end-joining (NHEJ) or homology-directed repair (HDR). The synthetic nucleases used for editing DNA in hESCs include zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeat (CRISPR)-associated Cas nucleases (Chandrasekaran et al., 2017). There have been several reports demonstrating the efficacy and value of all three approaches for editing hPSC genomes.
ZFN applications range from the introduction of reporter genes to monitor pluripotency and differentiation (Hockemeyer et al., 2009), to the correction of disease-causing mutations in hiPSCs, including sickle cell anemia (Sebastiano et al., 2011), α-thalassemia (Chang and Bouhassira, 2012) and several neurodegenerative diseases (Fong et al., 2013; Kiskinis et al., 2014). TALENs emerged as an alternative to ZFNs, although similar in architecture, the binding affinity and assembly of functional TALENs is inherently more successful (Li et al., 2014). TALEN-mediated approaches have been employed by several groups for the generation of hPSC reporter lines (Luo et al., 2014), biallelic gene knockout and to generate various diseases models using hiPSCs (Soldner et al., 2011; Ding et al., 2013).
Unlike ZFNs and TALENs, limited by their high cost and poorer specificity, Cas nucleases rapidly became the preferred enzymes for genome editing due to their higher efficiency and versatility (Plaza Reyes and Lanner, 2017). The most commonly applied Cas9 enzyme is guided by a short single guide RNA (gRNA) molecule, which can be easily engineered. CRISPR/Cas9 gene editing undoubtedly revolutionized human stem cell research, providing vast opportunities for genetic manipulation, further exemplified by the plethora of basic and translational applications to date. To explore underlying mechanisms of gene regulation, Liao et al., for instance, describe the targeted disruption of active DNA methyltransferases (DNMTs) in hESCs, unraveling the role of these enzymes (Liao et al., 2015) (Fig. 1). Furthermore, gene knock-in by HDR-mediated CRISPR/Cas has been applied for the generation of numerous reporter lines (Balboa et al., 2017) and serves as a powerful therapeutic strategy for the correction of specific mutant genes, as well as modeling human disease by generating mutant hPSCs. The HDR-based approach may also be useful in cases where generating patient-specific hiPSCs is unfeasible, creating opportunities for studying a wide range of genetic pathologies (Zhang et al., 2017). The CRISPR/Cas9 system has been applied for the study of genetic diseases including Duchenne muscular dystrophy (Young et al., 2016), Huntington’s disease (Shin et al., 2016), β-thalassemia (Xie et al., 2014) and sickle cell anemia (Hanna et al., 2007).
Along with its success, several limitations remain to be overcome prior to clinical applications of gene editing in hPSCs. Off target effects, resulting from the random integration of nucleotides, still persist, while exploring novel safe delivery strategies is also necessary (Zhang et al., 2017). Moreover, the safety and efficacy of the edited cells require evaluation prior to implementation in a clinical setting. Elucidating the full extent of off target effects and improving editing efficiencies in hPSCs will require constant innovation in both gene editing and stem cell research. Nevertheless, the complementary nature of these two fields has certainly allowed remarkable progress. Manipulating the hPSC genome to unravel gene function and underlying processes of human development will continue to enhance stem cell technologies. Concurrently, disease modeling and therapeutic approaches will further foster the ultimate vision of clinical applications through personalized regenerative medicine.
Clinical trials with hPSC
Cell therapies with hPSC are emerging as a possible solution to degenerative diseases. As Dr P. Andrews points out: ‘[…] it is remarkable that 20 years on from Jamie Thomson’s landmark paper, clinical trials of pluripotent stem cell derivatives are in hand or on the near horizon for a range of medical conditions […]’ (Supplementary data). hPSCs are in clinical trials for a range of conditions, including macular degeneration, spinal cord injury, type I diabetes, heart disease and Parkinson’s disease (Trounson, and De Witt, 2016). About 30 clinical trials are currently ongoing with hESC-derived cells and the first patients are being treated with hiPSC-derived cells (Table I).
Table I.
Clinical trials with cells derived from human embryonic stem cell and human-induced pluripotent stem cells.
| No. | Disease | Cell origin | Device | Derived cells | Sponsor | Country | Phase | Start date | Final date | No. of pat. | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT01217008 | SCI | hESC | GRNOPC1 | Oligodendrocytes | Asterias Biotherapeutics | USA | I | Oct-10 | Jul-13 | 5 | Completed |
| NCT01344993 | AMD | hESC | MA09-hRPE | Retinal pigmented epith. | Astellas Inst. Regen.Med. | USA | I/II | Apr-11 | Aug-15 | 13 | Completed |
| NCT01345006 | SMD | hESC | MA09-hRPE | Retinal pigmented epith. | Astellas Inst. Regen.Med. | USA | I/II | Apr-11 | Aug-15 | 13 | Completed |
| NCT01469832 | SMD | hESC | MA09-hRPE | Retinal pigmented epith. | Astellas Inst. Regen.Med. | UK | I/II | Nov-11 | Sep-15 | 12 | Completed |
| NCT01625559 | SMD | hESC | MA09-hRPE | Retinal pigmented epith. | CHABiotech CO., Ltd | Korea | I | Sep-12 | Jun-15 | 3 | Unknown |
| NCT01674829 | AMD | hESC | MA09-hRPE | Retinal pigmented epith. | CHABiotech CO., Ltd | Korea | I/IIa | Sep-12 | Apr-16 | 12 | Unknown |
| NCT01691261 | AMD | hESC | PF-05206388 | Retinal pigmented epith. | Pfizer | UK | I | Jun-15 | Nov-16 | 10 | Ongoing |
| NCT02057900 | IHD | hESC | CD15+ Isl-1+ progen. | Assistance Pub. Hôp. Paris | France | I | Jun-13 | Jun-18 | 6 | Recruiting | |
| NCT02239354 | T1DM | hESC | VC-01™ | β Cell progenitors | ViaCyte | USA | I/II | Sep-14 | Jan-21 | 40 | Ongoing |
| NCT02286089 | AMD | hESC | OpRegen | Retinal pigmented epith. | Cell Cure Neurosciences | Israel | I/II | Mar-15 | Sep-19 | 15 | Recruiting |
| NCT02302157 | SCI | hESC | AST-OPC1 | Oligodendrocits progenit. | Asterias Biotherapeutic. | USA | I/II | Mar-15 | Dic-18 | 35 | Recruiting |
| NCT02445612 | SMD | hESC | MA09-hRPE | Retinal pigmented epith. | Astellas Inst. Regen.Med. | USA | I/II | Jul-12 | Dic-29 | 13 | Ongoing |
| NCT02452723 | PD | phESC | ISC-hpNSC | Neural stem cells | Cyto Therapeutics Pty Lim. | Australia | I | Mar-16 | Mar-19 | 12 | Recruiting |
| NCT02463344 | AMD | hESC | MA09-hRPE | Retinal pigmented epith. | Astellas Inst. Regen.Med. | USA | I/II | Jul-12 | Dic-29 | 11 | Ongoing |
| NCT02464956 | AMD | hiPS autol | Retinal pigmented epith. | Moorfields Eye Hosp. NHS | UK | I | Jul-15 | Apr-16 | 10 | Unknown | |
| NCT02590692 | AMD | hESC | CPCB-RPE1 | Retinal pigmented epith. | Regenerative Patch Tech. | USA | I/II | Oct-15 | Sep-22 | 20 | Recruiting |
| NCT02749734 | AMD; SMD | hESC | ESC-RPE | Retinal pigmented epith. | Southwest Hospital, China | China | I | May-15 | Dec-17 | 15 | Recruiting |
| NCT02755428 | AMD | hESC | MA09-hRPE | Retinal pigmented epith. | Chinese Academy Sc. | China | I | Apr-17 | Dec-20 | 10 | Recruting |
| NCT02903576 | AMD; SMD | hESC | Retinal pigmented epith. | Federal Univ. São Paulo | Brazil | I/II | Aug-15 | Jun-19 | 18 | Recruiting | |
| NCT02923375 | GVHD | hiPSC allog | CYP-001 | MSC | Cynata Therapeutics | Australia | I | Mar-17 | Sep-19 | 16 | Recruiting |
| NCT02941991 | SMD | hESC | MA09-hRPE | Retinal pigmented epith. | Astellas Inst. Regen.Med. | UK | I/II | Jun-13 | Dec-19 | 11 | Ongoing |
| NCT03046407 | AMD | hESC | ESC-RPE | Retinal pigmented epith. | Chinese Academy Sc. | China | I | Mar-17 | Dec-20 | 10 | Recruiting |
| NCT03119636 | PD | hESC | Neural precursors | Chinese Academy Sc. | China | I/II | May-17 | Dec-20 | 50 | Recruiting | |
| NCT03162926 | T1DM | hESC | VC-02 | β Cell progenitors | ViaCyte | USA | I | Jul-17 | Jun-18 | 15 | Recruiting |
| NCT03163511 | T1DM | hESC | VC-02 | β Cell progenitors | ViaCyte | USA | I/II | Jul-17 | Dec-20 | 55 | Recruiting |
| ChiCTR-OCB-15005968 | SOSD | hESC | Corneal epithelium | Eye Institute Xiamen Univ. | China | I/II | Oct-15 | Dec-18 | 20 | Recruiting | |
| ChiCTR-OCB-15007054 | AMD | hESC | ESC-RPE | Retinal pigmented epith. | Chinese Academy Sc. | China | I | Jun-16 | Jun-17 | 10 | Recruiting |
| ChiCTR-OCB-15007055 | RPD | hESC | ESC-RPE | Retinal pigmented epith. | Chinese Academy Sc. | China | I | Sep-15 | Dec-17 | 10 | Recruiting |
| AMD | hiPSC allog | Retinal pigmented epith. | RIKEN Center for Dev.Biol | Japan | I | Feb-17 | 5 | Recruiting | |||
| UMIN000011929 | AMD | hiPS autol | Retinal pigmented epith. | RIKEN Center for Dev.Biol | Japan | I | Sep-14 | Sep-15 | 1 | Suspended | |
| PD | hiPSC allog | Dopamine-secreting nerve | Center for iPS Cel Res. | Japan | I | 2018 |
hESC, human embryonic stem cell; hiPSC, human-induced pluripotent stem cell; MSC, mesenchymal stem cell; allog, allogenic; epith, ephitelial; progen, progenitors; pat, patient; autol, autologous.
Most of the information regarding the results of clinical trials with PSCs in this section have been obtained from sponsor company press releases or press articles. Whenever the results arise from a scientific publication, the reference is provided.
The first clinical trial with cells from hPSCs was launched in 2010 for patients with spinal cord injury (Fig. 1). Geron corp. initiated a Phase I study to evaluate the safety of the use of oligodendrocyte precursors derived from hESCs (GRNOPC1), in patients with recent spinal cord injury. One year later, the company canceled the trial for economic reasons, reporting that no significant side effects were observed in any of the five patients treated. In 2014, Asterias Biotherapeutics reinitiated the assay (by renaming the product as AST-OPC1), initiating a Phase I/II trial in which it intended to treat 35 patients with different cell doses. Preliminary data reported by the company at the ISCCR meeting in June 2017 showed cavitation, improved myelin coating, neovascularization and the production of neuronal growth stimulating factors, in addition to the absence of relevant side effects and good tolerance for the immunosuppressive treatment.
In 2011, advanced cell technologies (ACTs) initiated a Phase I trial for the treatment of age-related macular degeneration (AMD) and Stargardt’s disease by transplantation of retinal pigmented epithelium (RPE) cells derived from hESC (MA09-hRPE). ACT has published preliminary (Schwartz et al., 2012) and medium term results (Schwartz et al., 2015) without side effects and improved vision in 17 out of 18 treated patients. Currently, ACT (which was renamed Ocata therapeutics and later Astellas Institut for Regenerative Medicine) has four other clinical trials underway for the treatment of macular degeneration in the USA and UK. In addition, MA09-hRPE is also being used in two Phase I/II trials in South Korea with similar positive results (Song et al., 2015). Dr Coffey´s group have reported primary and secondary outcomes from the first two patients in a Phase I clinical trial sponsored by Pfizer in the UK (da Cruz et al., 2018). One year post-treatment, the best corrected visual acuity (BCVA) improved from 10 to 39 and from 8 to 29 letters in Patients 1 and 2, respectively. Reading speed improved from 1,7 to 82,8 and from 0 to 47,8 words/min.
In an article published recently in Ophtalmology (Mehat et al., 2018), the authors did not find any evidence of uncontrolled proliferation or inflammatory responses after the transplantation. Borderline improvements in BCVA in four participants either were unsustained or were matched by a similar improvement in the untreated contralateral eye. Microperimetry demonstrated no evidence of benefit at 12 months in the 12 participants.
Other clinical trials for the treatment of macular degeneration by hESC-derived RPE have been registered such as those carried out by Pfizer (UK), Regenerative Patch Technologies (USA), Cell Cure Neurosciences (Israel), Federal University of São Paulo (Brazil), Chinese Academic of Science (China), Southwest Hospital, Chongqing (China), all registered at Clinical Trials.gov (https://clinicaltrials.gov). In the Chinese Clinical Trial Register (www.chictr.org), there are two clinical trials for macular degeneration from The Chinese Academy of Sciences and one trial from The Eye Institute of Xiamen University with corneal epithelium derived from hESCs for severe ocular surface disease.
In 2013, the first Phase I clinical trial for the treatment of six patients with cardiac ischemia with cardiac progenitors derived from hESCs was started in the Assistance Publique Hôpitaux in Paris. The preliminary results of the first treated patient were published by Menasche et al. (2015) and reported an improvement of cardiac function and absence of important side effects.
In 2014, a Phase I/II study for the treatment of 65 patients with type 1 diabetes was initiated by Viacyte. A device (VC-01) consisting of a semipermeable membrane that encapsulates the β cell progenitor cells had been designed. This device, implanted subcutaneously, allows the entry of oxygen and nutrients, as well as the release of insulin and other hormones, while protecting the cells from the autoimmune reaction that causes type 1 diabetes. The company announced in August 2017 two more trials with a modified device (VC-02) that allows direct vascularization into itself.
In 2017, the Australian company Cyto Therapeutics Pty Limited initiated a study in 12 patients with Parkinson’s disease with neuronal progenitors from parthenogenetic hESCs (ISC-hpNSC). After 6 months of treatment, a reduction of the off period was observed in the treated patients, as well as an improvement in motor and cognitive abilities. Another clinical trial for Parkinsons’s disease had been announced in June 2017 by The Chinese Academy of Sciences consisting of the transplantation of neuronal precursors derived from hESCs (50 patients; Phase I).
In 2014, the Riken Institute in Japan treated a patient with cells obtained from hiPSCs for the first time (Fig. 1). These were RPEs derived from autologous hiPSCs to avoid immune rejection. The patient did not present any side effects after the treatment. The trial was suspended when attempting to treat a second patient as mutations were detected in the generated hiPSCs (Mandai et al., 2017). Due to these findings and to the fact that the generation of patient-specific iPSCs is time-consuming and expensive, Riken opted for the use of tested and safe allogenic hiPSCs. A new clinical trial has been initiated involving the generation of a hiPSC bank from peripheral blood samples with the most frequent homozygous HLA haplotypes that could match a sufficient proportion of the general population (Okita et al., 2011b). Riken announced the treatment of the first patient with RPE derived from allogeneic hiPSCs in February 2017.
The Moorfields Eye Hospital NHS Foundation Trust (UK) reported in May 2015 that the first AMD patient was treated with hiPSC-derived RPE. To date, no results have been published so far.
Cynata Therapeutics (Australia) started a clinical trial in May 2017 with mesenchymal cells from allogeneic hiPSC (CYP-001) for the treatment of graft versus host disease (GVHD). Improvement in the severity of the GVHD in the first eight patients was announced in January 2018. The Center for iPS Cell Research in Japan reported in February 2017 that a trial for the treatment of Parkinson’s with allogeneic hiPSCs will soon be conducted.
Clinical trials registered in Clinical Trials.gov and other registries are shown in Table I.
Misconduct in pluripotent cell research
Stimulus-triggered acquisition of pluripotency
In January 2014, two Nature papers seemed to revolutionize stem cell derivation by showing that somatic cells could be ‘reprogrammed’ to PSCs simply by subjecting them to certain types of stress, e.g. submersion in weak acid, physical trauma or bacterial toxins. However, within weeks of the paper’s publication errors were reported and scientist trying to replicate this stimulus-triggered acquisition of pluripotency (also known as STAP) method all failed. Lead author Dr H. Obokata was accused of misconduct and in August 2014 the papers were retracted.
Human SCNT embryos
Dr W. Hwang and his former colleagues at Seoul National University, South Korea claimed in 2004 and 2005 to have produced human SCNT embryos and the successful derivation of ESCs. This purported breakthrough was published in Science, however both papers were soon retracted based on suspicions of fraud. The ball started rolling when it was found that two female scientists in the lab donated eggs for this research, a violation of international ethics guidelines. The scientific claims were further examined and the final report from the investigation committee of Seoul National University concluded that the authors of the two papers published in Science engaged in research misconduct, with the papers containing fabricated data. Once considered a national hero in South Korea, receiving huge financial support from the government, Dr W. Hwang became an outcast.
Conclusion
The first 20 years following the derivation of hESCs have proven rich in developments. The multi-disciplinary nature of the field with tremendous potential for a vast range of diagnostic and therapeutic applications has been generating immense excitement in medical research. From the derivation of iPSCs to the discovery of different states of pluripotency, and the refinement of differentiation protocols and gene editing technologies. At present, several clinical trials are underway and preliminary results are promising. In the years to come, pluripotent cells will come of age, and regenerative therapies in several fields of medicine will profit from the production of highly defined cell populations in clinical grade conditions. Although the road to the seamless application of pluripotent human cells in the clinic is still treacherous, exceptional advancement in just 20 years provides a positive outlook on the future of this discipline. To conclude with the words of Dr P. Andrews: ‘Predicting the future is always dangerous and the only certain thing is that we will use stem cell biology in the future in ways that we do not imagine today.’
Supplementary Material
Acknowledgements
The authors wish to thank Shinya Yamanaka, Alan Trounson, Outi Hovatta, Peter W Andrews, Miodrag Stojkovic and Shoukhrat Mitalipov for their contribution, and Sarai Brazal for assistance.
Authors’ roles
C.E., B.A., S.M.C.S.L., M.G., B.H., S.P., M.P., R.V. and A.V. concieved and designed the study, performed literature search and manuscript writing. All authors revised and approved the final manuscript.
Funding
ESHRE provided funding for the authors’ meeting on-site and discussion during the preparation of this manuscript. S.M.C.S.L. is funded by the European Research Council Consolidator (ERC-CoG-725722-OVOGROWTH). M.P. is supported by the Special Research Fund, Bijzonder Onderzoeksfonds (BOF01D08114). M.G. is supported by the Methusalem grant of Vrije Universiteit Brussel, in the name of Prof. Karen Sermon and by Innovation by Science and Technology in Flanders (IWT, Project Number: 150042). A.V. and B.A. are supported by the Plataforma de Proteomica, Genotipado y Líneas Celulares (PT1770019/0015) (PRB3), Instituto de Salud Carlos III. Research grant to BH by the Research Foundation–Flanders (FWO) (FWO.KAN.2016.0005.01 and FWO.Project G051516N) and Innovation by Science and Technology in Flanders (IWT, Project Number: 150042).
Conflict of interest
None declared.
References
- Akagi S, Matsukawa K, Mizutani E, Fukunari K, Kaneda M, Watanabe S, Takahashi S. Treatment with a histone deacetylase inhibitor after nuclear transfer improves the preimplantation development of cloned bovine embryos. J Reprod Dev 2011;57:120–126. [DOI] [PubMed] [Google Scholar]
- Amit M, Margulets V, Segev H, Shariki K, Laevsky I, Coleman R, Itskovitz-Eldor J. Human feeder layers for human embryonic stem cells. Biol Reprod 2003;68:2150–2156. [DOI] [PubMed] [Google Scholar]
- Arai S, Miyauchi M, Kurokawa M. Modeling of hematologic malignancies by iPS technology. Exp Hematol 2015;43:654–660. [DOI] [PubMed] [Google Scholar]
- Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes 2001;50:1691–1697. [DOI] [PubMed] [Google Scholar]
- Avery S, Hirst AJ, Baker D, Lim CY, Alagaratnam S, Skotheim RI, Lothe RA, Pera MF, Colman A, Robson P et al. BCL-XL mediates the strong selective advantage of a 20q11.21 amplification commonly found in human embryonic stem cell cultures. Stem Cell Reports 2013;1:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa D, Weltner J, Novik Y, Eurola S, Wartiovaara K, Otonkoski T. Generation of a SOX2 reporter human induced pluripotent stem cell line using CRISPR/SaCas9. Stem Cell Res 2017;22:16–19. [DOI] [PubMed] [Google Scholar]
- Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, Lebrin F, Kats P, Hochstenbach R, Passier R et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells 2008;26:2257–2265. [DOI] [PubMed] [Google Scholar]
- Briggs R, King TJ. Transplantation of living nuclei from blastula cells into enucleated frogs’ eggs. Proc Natl Acad Sci USA 1952;38:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongso A, Fong CY, Ng SC, Ratnam S. Isolation and culture of inner cell mass cells from human blastocysts. Hum Reprod 1994;9:2110–2117. [DOI] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007;448:191–195. [DOI] [PubMed] [Google Scholar]
- Brookhouser N, Raman S, Potts C, Brafman D. May I cut in? Gene editing approaches in human induced pluripotent stem cells. Cells 2017;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucay N, Yebra M, Cirulli V, Afrikanova I, Kaido T, Hayek A, Montgomery AM. A novel approach for the derivation of putative primordial germ cells and sertoli cells from human embryonic stem cells. Stem Cells 2009;27:68–77. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 2007;450:497–502. [DOI] [PubMed] [Google Scholar]
- Carter MG, Smagghe BJ, Stewart AK, Rapley JA, Lynch E, Bernier KJ, Keating KW, Hatziioannou VM, Hartman EJ, Bamdad CC. A primitive growth factor, NME7AB, is sufficient to induce stable naive state human pluripotency; reprogramming in this novel growth factor confers superior differentiation. Stem Cells 2016;34:847–859. [DOI] [PubMed] [Google Scholar]
- Chan YS, Goke J, Ng JH, Lu X, Gonzales KA, Tan CP, Tng WQ, Hong ZZ, Lim YS, Ng HH. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell 2013;13:663–675. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Bouhassira EE. Zinc-finger nuclease-mediated correction of alpha-thalassemia in iPS cells. Blood 2012;120:3906–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods 2011;8:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YG, Matoba S, Liu Y, Eum JH, Lu F, Jiang W, Lee JE, Sepilian V, Cha KY, Lee DR et al. Histone demethylase expression enhances human somatic cell nuclear transfer efficiency and promotes derivation of pluripotent stem cells. Cell Stem Cell 2015;17:758–766. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran AP, Song M, Ramakrishna S. Genome editing: a robust technology for human stem cells. Cell Mol Life Sci 2017;74:3335–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AT, Bodnar MS, Fox M, Rodriquez RT, Abeyta MJ, Firpo MT, Pera RA. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum Mol Genet 2004;13:727–739. [DOI] [PubMed] [Google Scholar]
- Crespo M, Vilar E, Tsai SY, Chang K, Amin S, Srinivasan T, Zhang T, Pipalia NH, Chen HJ, Witherspoon M et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med 2017;23:878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol 2018;36:328–337. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Callaway ES, Kim E, Weeks BR, Fan YY, Allred CD, Chapkin RS. Targeted deletion of p53 in Lgr5-expressing intestinal stem cells promotes colon tumorigenesis in a preclinical model of colitis-associated cancer. Cancer Res 2015;75:5392–5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Lee YK, Schaefer EA, Peters DT, Veres A, Kim K, Kuperwasser N, Motola DL, Meissner TB, Hendriks WT et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell 2013;12:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico AI, Christodoulou I, Pells SC, McWhir J, Thomson AJ. Sequential genetic modification of the hprt locus in human ESCs combining gene targeting and recombinase-mediated cassette exchange. Cloning Stem Cells 2008;10:217–230. [DOI] [PubMed] [Google Scholar]
- Donoho G, Jasin M, Berg P. Analysis of gene targeting and intrachromosomal homologous recombination stimulated by genomic double-strand breaks in mouse embryonic stem cells. Mol Cell Biol 1998;18:4070–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal G, Warrier S, Ghimire S, Broekaert D, Van der Jeught M, Lierman S, Deroo T, Peelman L, Van Soom A, Cornelissen R et al. Alternative routes to induce naive pluripotency in human embryonic stem cells. Stem Cells 2015;33:2686–2698. [DOI] [PubMed] [Google Scholar]
- Easley CAt, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE et al. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep 2012;2:440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF Jr., Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 2009;457:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguizabal C, Herrera L, De Onate L, Montserrat N, Hajkova P, Izpisua Belmonte JC. Characterization of the epigenetic changes during human gonadal primordial germ cells reprogramming. Stem Cells 2016;34:2418–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguizabal C, Montserrat N, Vassena R, Barragan M, Garreta E, Garcia-Quevedo L, Vidal F, Giorgetti A, Veiga A, Izpisua Belmonte JC. Complete meiosis from human induced pluripotent stem cells. Stem Cells 2011;29:1186–1195. [DOI] [PubMed] [Google Scholar]
- Eid A, Mahfouz MM. Genome editing: the road of CRISPR/Cas9 from bench to clinic. Exp Mol Med 2016;48:e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiges R, Schuldiner M, Drukker M, Yanuka O, Itskovitz-Eldor J, Benvenisty N. Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Curr Biol 2001;11:514–518. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292:154–156. [DOI] [PubMed] [Google Scholar]
- Fluri DA, Tonge PD, Song H, Baptista RP, Shakiba N, Shukla S, Clarke G, Nagy A, Zandstra PW. Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nat Methods 2012;9:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H, Wang C, Knoferle J, Walker D, Balestra ME, Tong LM, Leung L, Ring KL, Seeley WW, Karydas A et al. Genetic correction of tauopathy phenotypes in neurons derived from human induced pluripotent stem cells. Stem Cell Reports 2013;1:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AJ, Adams CA, Anderson LS, Kitchen JR, Hughes MR, Wood SH. Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts. Stem Cells 2008;26:485–493. [DOI] [PubMed] [Google Scholar]
- Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci 2009;85:348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013;504:282–286. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Izpisua Belmonte JC. Albumin-associated lipids regulate human embryonic stem cell self-renewal. PLoS One 2008;3:e1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geens M, Chuva De Sousa Lopes SM. X chromosome inactivation in human pluripotent stem cells as a model for human development: back to the drawing board? Hum Reprod Update 2017;23:520–532. [DOI] [PubMed] [Google Scholar]
- Geens M, Seriola A, Barbe L, Santalo J, Veiga A, Dee K, Van Haute L, Sermon K, Spits C. Female human pluripotent stem cells rapidly lose X chromosome inactivation marks and progress to a skewed methylation pattern during culture. Mol Hum Reprod 2016;22:285–298. [DOI] [PubMed] [Google Scholar]
- Gerrard L, Zhao D, Clark AJ, Cui W. Stably transfected human embryonic stem cell clones express OCT4-specific green fluorescent protein and maintain self-renewal and pluripotency. Stem Cells 2005;23:124–133. [DOI] [PubMed] [Google Scholar]
- Giudice A, Trounson A. Genetic modification of human embryonic stem cells for derivation of target cells. Cell Stem Cell 2008;2:422–433. [DOI] [PubMed] [Google Scholar]
- Gu Q, Hao J, Zhao XY, Li W, Liu L, Wang L, Liu ZH, Zhou Q. Rapid conversion of human ESCs into mouse ESC-like pluripotent state by optimizing culture conditions. Protein Cell 2012;3:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, von Meyenn F, Santos F, Chen Y, Reik W, Bertone P, Smith A, Nichols J. Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem Cell Reports 2016;6:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB, Elsdale TR, Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature 1958;182:64–65. [DOI] [PubMed] [Google Scholar]
- Hackett JA, Surani MA. Regulatory principles of pluripotency: from the ground state up. Cell Stem Cell 2014;15:416–430. [DOI] [PubMed] [Google Scholar]
- Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci USA 2010;107:9222–9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 2007;318:1920–1923. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011;146:519–532. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 2012;338:971–975. [DOI] [PubMed] [Google Scholar]
- Heindryckx B, De Sutter P, Gerris J, Dhont M, Van der Elst J. Embryo development after successful somatic cell nuclear transfer to in vitro matured human germinal vesicle oocytes. Hum Reprod 2007;22:1982–1990. [DOI] [PubMed] [Google Scholar]
- Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, Shimamoto S, Imamura T, Nakashima K, Saitou M et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016;539:299–303. [DOI] [PubMed] [Google Scholar]
- Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC, Katibah GE, Amora R, Boydston EA, Zeitler B et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol 2009;27:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovatta O, Mikkola M, Gertow K, Stromberg AM, Inzunza J, Hreinsson J, Rozell B, Blennow E, Andang M, Ahrlund-Richter L. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum Reprod 2003;18:1404–1409. [DOI] [PubMed] [Google Scholar]
- Huber I, Itzhaki I, Caspi O, Arbel G, Tzukerman M, Gepstein A, Habib M, Yankelson L, Kehat I, Gepstein L. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J 2007;21:2551–2563. [DOI] [PubMed] [Google Scholar]
- Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF 3rd, Boiani M, Scholer HR. Derivation of oocytes from mouse embryonic stem cells. Science 2003;300:1251–1256. [DOI] [PubMed] [Google Scholar]
- Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development 2015;142:3113–3125. [DOI] [PubMed] [Google Scholar]
- Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 2010;10:1886–1890. [DOI] [PubMed] [Google Scholar]
- International Stem Cell I, Amps K, Andrews PW, Anyfantis G, Armstrong L, Avery S, Baharvand H, Baker J, Baker D, Munoz MB, Beil S et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol 2011;29:1132–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie N, Weinberger L, Tang WW, Kobayashi T, Viukov S, Manor YS, Dietmann S, Hanna JH, Surani MA. SOX17 is a critical specifier of human primordial germ cell fate. Cell 2015;160:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irion S, Luche H, Gadue P, Fehling HJ, Kennedy M, Keller G. Identification and targeting of the ROSA26 locus in human embryonic stem cells. Nat Biotechnol 2007;25:1477–1482. [DOI] [PubMed] [Google Scholar]
- Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 2012;482:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 2011;471:225–229. [DOI] [PubMed] [Google Scholar]
- Jacobs K, Zambelli F, Mertzanidou A, Smolders I, Geens M, Nguyen HT, Barbe L, Sermon K, Spits C. Higher-density culture in human embryonic stem cells results in DNA damage and genome instability. Stem Cell Reports 2016;6:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Xiong J, Ye M, Qin X, Li L, Cheng S, Luo M, Peng J, Dong J, Tang F et al. In vitro differentiation of human embryonic stem cells into ovarian follicle-like cells. Nat Commun 2017;12:15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 2009;462:222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 2001;108:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009;4:472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskinis E, Sandoe J, Williams LA, Boulting GL, Moccia R, Wainger BJ, Han S, Peng T, Thams S, Mikkilineni S et al. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell 2014;14:781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R. Human embryonic stem cells derived without feeder cells. Lancet 2005;365:1636–1641. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, Sato H, Lee YS, Usui J, Knisely AS et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 2010;142:787–799. [DOI] [PubMed] [Google Scholar]
- Kretzschmar K, Clevers H. Organoids: modeling development and the stem cell niche in a dish. Dev Cell 2016;38:590–600. [DOI] [PubMed] [Google Scholar]
- Lavon N, Yanuka O, Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation 2004;72:230–238. [DOI] [PubMed] [Google Scholar]
- Leavitt AD, Hamlett I. Homologous recombination in human embryonic stem cells: a tool for advancing cell therapy and understanding and treating human disease. Clin Transl Sci 2011;4:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Lin H, Du Q, Liu K, Wang O, Evans C, Christian H, Zhang C, Lei Y. Scalable and physiologically relevant microenvironments for human pluripotent stem cell expansion and differentiation. Biofabrication 2018;10:025006. [DOI] [PubMed] [Google Scholar]
- Li M, Suzuki K, Kim NY, Liu GH, Izpisua Belmonte JC. A cut above the rest: targeted genome editing technologies in human pluripotent stem cells. J Biol Chem 2014;289:4594–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Karnik R, Gu H, Ziller MJ, Clement K, Tsankov AM, Akopian V, Gifford CA, Donaghey J, Galonska C et al. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat Genet 2015;47:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 2006;24:185–187. [DOI] [PubMed] [Google Scholar]
- Luo Y, Rao M, Zou J. Generation of GFP reporter human induced pluripotent stem cells using AAVS1 safe harbor transcription activator-like effector nuclease. Curr Protoc Stem Cell Biol 2014;29:5A 7 1–18A 7 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Morey R, O’Neil RC, He Y, Daughtry B, Schultz MD, Hariharan M, Nery JR, Castanon R, Sabatini K et al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature 2014;511:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlstedt MM, Anderson D, Sharp JS, McGilvray R, Munoz MD, Buttery LD, Alexander MR, Rose FR, Denning C. Maintenance of pluripotency in human embryonic stem cells cultured on a synthetic substrate in conditioned medium. Biotechnol Bioeng 2010;105:130–140. [DOI] [PubMed] [Google Scholar]
- Malan D, Zhang M, Stallmeyer B, Muller J, Fleischmann BK, Schulze-Bahr E, Sasse P, Greber B. Human iPS cell model of type 3 long QT syndrome recapitulates drug-based phenotype correction. Basic Res Cardiol 2016;111:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med 2017;376:1038–1046. [DOI] [PubMed] [Google Scholar]
- Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 1988;336:348–352. [DOI] [PubMed] [Google Scholar]
- Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 2010;143:527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 1981;78:7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med 2005;11:228–232. [DOI] [PubMed] [Google Scholar]
- Matoba S, Zhang Y. Somatic cell nuclear transfer reprogramming: mechanisms and applications. Cell Stem Cell 2018;23:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunari H, Nagashima H, Watanabe M, Umeyama K, Nakano K, Nagaya M, Kobayashi T, Yamaguchi T, Sumazaki R, Herzenberg LA et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci USA 2013;110:4557–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley HA, Wells JM. Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development 2017;144:958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano JV, Ramathal C, Nguyen HN, Simon C, Reijo Pera RA. Divergent RNA-binding proteins, DAZL and VASA, induce meiotic progression in human germ cells derived in vitro. Stem Cells 2012;30:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehat MS, Sundaram V, Ripamonti C, Robson AG, Smith AJ, Borooah S, Robinson M, Rosenthal AN, Innes W, Weleber RG et al. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells in macular degeneration. Ophthalmology 2018;125:1765–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J 2015;36:2011–2017. [DOI] [PubMed] [Google Scholar]
- Meyn MS. High spontaneous intrachromosomal recombination rates in ataxia-telangiectasia. Science 1993;260:1327–1330. [DOI] [PubMed] [Google Scholar]
- Mishra S, Kacin E, Stamatiadis P, Franck S, Van der Jeught M, Mertes H, Pennings G, De Sutter P, Sermon K, Heindryckx B et al. The role of the reprogramming method and pluripotency state in gamete differentiation from patient-specific human pluripotent stem cells. Mol Hum Reprod 2018;24:173–184. [DOI] [PubMed] [Google Scholar]
- Mitsunaga S, Odajima J, Yawata S, Shioda K, Owa C, Isselbacher KJ, Hanna JH, Shioda T. Relevance of iPSC-derived human PGC-like cells at the surface of embryoid bodies to prechemotaxis migrating PGCs. Proc Natl Acad Sci USA 2017;114:9913–9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 2003;107:2733–2740. [DOI] [PubMed] [Google Scholar]
- Nazor KL, Altun G, Lynch C, Tran H, Harness JV, Slavin I, Garitaonandia I, Muller FJ, Wang YC, Boscolo FS et al. Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell 2012;10:620–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schule B, Dolmetsch RE, Langston W et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 2011;8:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HT, Geens M, Mertzanidou A, Jacobs K, Heirman C, Breckpot K, Spits C. Gain of 20q11.21 in human embryonic stem cells improves cell survival by increased expression of Bcl-xL. Mol Hum Reprod 2014;20:168–177. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Geens M, Spits C. Genetic and epigenetic instability in human pluripotent stem cells. Hum Reprod Update 2013;19:187–205. [DOI] [PubMed] [Google Scholar]
- Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell 2009;4:487–492. [DOI] [PubMed] [Google Scholar]
- Noggle S, Fung HL, Gore A, Martinez H, Satriani KC, Prosser R, Oum K, Paull D, Druckenmiller S, Freeby M et al. Human oocytes reprogram somatic cells to a pluripotent state. Nature 2011;478:70–75. [DOI] [PubMed] [Google Scholar]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K et al. A more efficient method to generate integration-free human iPS cells. Nat Methods 2011. a;8:409–412. [DOI] [PubMed] [Google Scholar]
- Okita K, Nagata N, Yamanaka S. Immunogenicity of induced pluripotent stem cells. Circ Res 2011. b;109:720–721. [DOI] [PubMed] [Google Scholar]
- Panula S, Medrano JV, Kee K, Bergstrom R, Nguyen HN, Byers B, Wilson KD, Wu JC, Simon C, Hovatta O et al. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet 2011;20:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Galic Z, Conway AE, Lindgren A, van Handel BJ, Magnusson M, Richter L, Teitell MA, Mikkola HK, Lowry WE et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells 2009;27:783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prathalingam N, Ferguson L, Young L, Lietz G, Oldershaw R, Healy L, Craig A, Lister H, Binaykia R, Sheth R et al. Production and validation of a good manufacturing practice grade human fibroblast line for supporting human embryonic stem cell derivation and culture. Stem Cell Res Ther 2012;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 2016;165:1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Han B, Gao B, Bose S, Gong Y, Wawrowsky K, Giuliano AE, Sareen D, Cui X. Differentiation of human induced pluripotent stem cells to mammary-like organoids. Stem Cell Reports 2017;8:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramathal C, Durruthy-Durruthy J, Sukhwani M, Arakaki JE, Turek PJ, Orwig KE, Reijo Pera RA. Fate of iPSCs derived from azoospermic and fertile men following xenotransplantation to murine seminiferous tubules. Cell Rep 2014;7:1284–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 2000;18:399–404. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol 2001;19:1134–1140. [DOI] [PubMed] [Google Scholar]
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol 2002;20:933–936. [DOI] [PubMed] [Google Scholar]
- Rodin S, Antonsson L, Niaudet C, Simonson OE, Salmela E, Hansson EM, Domogatskaya A, Xiao Z, Damdimopoulou P, Sheikhi M et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun 2014;5:3195. [DOI] [PubMed] [Google Scholar]
- Rodin S, Domogatskaya A, Strom S, Hansson EM, Chien KR, Inzunza J, Hovatta O, Tryggvason K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol 2010;28:611–615. [DOI] [PubMed] [Google Scholar]
- Roost MS, van Iperen L, Ariyurek Y, Buermans HP, Arindrarto W, Devalla HD, Passier R, Mummery CL, Carlotti F, de Koning EJ et al. KeyGenes, a tool to probe tissue differentiation using a human fetal transcriptional atlas. Stem Cell Reports 2015;4:1112–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Stubbington MJT, Regev A, Teichmann SA. The Human Cell Atlas: from vision to reality. Nature 2017;550:451–453. [DOI] [PubMed] [Google Scholar]
- Ruby KM, Zheng B. Gene targeting in a HUES line of human embryonic stem cells via electroporation. Stem Cells 2009;27:1496–1506. [DOI] [PubMed] [Google Scholar]
- Saha K, Mei Y, Reisterer CM, Pyzocha NK, Yang J, Muffat J, Davies MC, Alexander MR, Langer R, Anderson DG et al. Surface-engineered substrates for improved human pluripotent stem cell culture under fully defined conditions. Proc Natl Acad Sci USA 2011;108:18714–18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Yokobayashi S, Nakamura T, Okamoto I, Yabuta Y, Kurimoto K, Ohta H, Moritoki Y, Iwatani C, Tsuchiya H et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 2015;17:178–194. [DOI] [PubMed] [Google Scholar]
- Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 2000;97:11307–11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 2012;379:713–720. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 2015;385:509–516. [DOI] [PubMed] [Google Scholar]
- Sebastiano V, Maeder ML, Angstman JF, Haddad B, Khayter C, Yeo DT, Goodwin MJ, Hawkins JS, Ramirez CL, Batista LF et al. In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells 2011;29:1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Brito C, Correia C, Alves PM. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol 2012;30:350–359. [DOI] [PubMed] [Google Scholar]
- Shafa M, Day B, Yamashita A, Meng G, Liu S, Krawetz R, Rancourt DE. Derivation of iPSCs in stirred suspension bioreactors. Nat Methods 2012;9:465–466. [DOI] [PubMed] [Google Scholar]
- Shao Y, Sang J, Fu J. On human pluripotent stem cell control: the rise of 3D bioengineering and mechanobiology. Biomaterials 2015;52:26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Roy N, Nakano T, Xuing L, Kang J, Nedergaard M, Goldman SA. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp Neurol 2005;196:224–234. [DOI] [PubMed] [Google Scholar]
- Shin JW, Kim KH, Chao MJ, Atwal RS, Gillis T, MacDonald ME, Gusella JF, Lee JM. Permanent inactivation of Huntington’s disease mutation by personalized allele-specific CRISPR/Cas9. Hum Mol Genet 2016;25:4566–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Formative pluripotency: the executive phase in a developmental continuum. Development 2017;144:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS. Insertion of DNA sequences into the human chromosomal β-globin locus by homologous recombination. Nature 1985;317:230–234. [DOI] [PubMed] [Google Scholar]
- Soldner F, Laganiere J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe LI, Myers RH, Lindquist S et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 2011;146:318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, Shim SH, Del Priore LV, Lanza R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports 2015;4:860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits C, Mateizel I, Geens M, Mertzanidou A, Staessen C, Vandeskelde Y, Van der Elst J, Liebaers I, Sermon K. Recurrent chromosomal abnormalities in human embryonic stem cells. Nat Biotechnol 2008;26:1361–1363. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science 2008;322:945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D, Khaner H, Cohen M, Even-Ram S, Gil Y, Itsykson P, Turetsky T, Idelson M, Aizenman E, Ram R et al. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat Biotechnol 2010;28:361–364. [DOI] [PubMed] [Google Scholar]
- Suh W. A new era of disease modeling and drug discovery using induced pluripotent stem cells. Arch Pharm Res 2017;40:1–12. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 2013;153:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat Rev Mol Cell Biol 2016;17:183–193. [DOI] [PubMed] [Google Scholar]
- Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015;526:564–568. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D, Santos F, Clarke J, Mansfield W et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 2014;158:1254–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007;448:196–199. [DOI] [PubMed] [Google Scholar]
- Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 2014;15:524–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 1987;51:503–512. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Fairley CK, Ugoni AM, Forbes AB, Purcell PM, Desmond PV, Smallwood RA, McNeil JJ. Risk factors for the development of amoxycillin-clavulanic acid associated jaundice. Med J Aust 1995;162:638–640. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–1147. [DOI] [PubMed] [Google Scholar]
- Tilgner K, Atkinson SP, Golebiewska A, Stojkovic M, Lako M, Armstrong L. Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells 2008;26:3075–3085. [DOI] [PubMed] [Google Scholar]
- Tilgner K, Atkinson SP, Yung S, Golebiewska A, Stojkovic M, Moreno R, Lako M, Armstrong L. Expression of GFP under the control of the RNA helicase VASA permits fluorescence-activated cell sorting isolation of human primordial germ cells. Stem Cells 2010;28:84–92. [DOI] [PubMed] [Google Scholar]
- Trounson A. The production and directed differentiation of human embryonic stem cells. Endocr Rev 2006;27:208–219. [DOI] [PubMed] [Google Scholar]
- Trounson A, De Witt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol 2016;17:194–200. [DOI] [PubMed] [Google Scholar]
- Urbach A, Schuldiner M, Benvenisty N. Modeling for Lesch-Nyhan disease by gene targeting in human embryonic stem cells. Stem Cells 2004;22:635–641. [DOI] [PubMed] [Google Scholar]
- Valamehr B, Robinson M, Abujarour R, Rezner B, Vranceanu F, Le T, Medcalf A, Lee TT, Fitch M, Robbins D et al. Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Reports 2014;2:366–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L, Alexander M, Pedersen RA. Activin/nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 2005;118:4495–4509. [DOI] [PubMed] [Google Scholar]
- Van der Jeught M, O’Leary T, Duggal G, De Sutter P, Chuva de Sousa Lopes S, Heindryckx B. The post-inner cell mass intermediate: implications for stem cell biology and assisted reproductive technology. Hum Reprod Update 2015;21:616–626. [DOI] [PubMed] [Google Scholar]
- Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S et al. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci USA 2014;111:4484–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010;7:618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier S, Van der Jeught M, Duggal G, Tilleman L, Sutherland E, Taelman J, Popovic M, Lierman S, Chuva De Sousa Lopes S, Van Soom A et al. Direct comparison of distinct naive pluripotent states in human embryonic stem cells. Nat Commun 2017;8:15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 2007;25:681–686. [DOI] [PubMed] [Google Scholar]
- Weinberger L, Ayyash M, Novershtern N, Hanna JH. Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat Rev Mol Cell Biol 2016;17:155–169. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Beaujean N, de Sousa PA, Dinnyes A, King TJ, Paterson LA, Wells DN, Young LE. Somatic cell nuclear transfer. Nature 2002;419:583–586. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature 1997;385:810–813. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Morey R, Kang E, Ma H, Hayama T, Laurent LC, Mitalipov S. Concise Review: embryonic stem cells derived by somatic cell nuclear transfer: a horse in the race? Stem Cells 2017;35:26–34. [DOI] [PubMed] [Google Scholar]
- Wu J, Izpisua Belmonte JC. Dynamic pluripotent stem cell states and their applications. Cell Stem Cell 2015;17:509–525. [DOI] [PubMed] [Google Scholar]
- Wu J, Platero-Luengo A, Sakurai M, Sugawara A, Gil MA, Yamauchi T, Suzuki K, Bogliotti YS, Cuello C, Morales Valencia M et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell 2017;168:473–486 e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Ye L, Chang JC, Beyer AI, Wang J, Muench MO, Kan YW. Seamless gene correction of beta-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res 2014;24:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhu X, Hahm HS, Wei W, Hao E, Hayek A, Ding S. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci USA 2010;107:8129–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue K, Ng JH, Ng HH. Mapping the networks for pluripotency. Philos Trans R Soc Lond B Biol Sci 2011;366:2238–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro C, Sasaki K, Yabuta Y, Kojima Y, Nakamura T, Okamoto I, Yokobayashi S, Murase Y, Ishikura Y, Shirane K et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science 2018;362:356–360. [DOI] [PubMed] [Google Scholar]
- Young CS, Hicks MR, Ermolova NV, Nakano H, Jan M, Younesi S, Karumbayaram S, Kumagai-Cresse C, Wang D, Zack JA et al. A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell 2016;18:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–1920. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 2001;19:1129–1133. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang Y, Gao F, Han S, Cheah KS, Tse HF, Lian Q. CRISPR/Cas9 genome-editing system in human stem cells: current status and future prospects. Mol Ther Nucleic Acids 2017;9:230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wang M, Yuan Y, Wang X, Fu R, Wan H, Xie M, Liu M, Guo X, Zheng Y et al. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell 2016;18:330–340. [DOI] [PubMed] [Google Scholar]
- Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol 2003;21:319–321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.