Abstract
Nasopharyngeal carcinoma is a rare disease in Western countries. Nevertheless, its incidence in China, Singapore, and other Eastern countries reaches 20 cases per 100,000 people. Being an extremely chemo- and radiosensitive disease, upfront treatment often consists in the association of intensity-modulated radiation therapy and concurrent cisplatin. Unfortunately, about 20% of the patients suffer from a radioresistant disease which recurs after upfront therapy. For these patients, mainly available therapeutic options consist in systemic therapy, in particular poly-chemotherapy. In those showing a single locoregional recurrence, chemotherapy is not considered to be the preferred approach and other different strategies may be employed. Re-irradiation and surgery are strategies that are always used more often, albeit related to high risk of morbidity. Immunotherapy and targeted therapy, such as heavy ions-based re-irradiations, are experimental but very intriguing options.
Keywords: nasopharyngeal carcinoma, re-irradiation, poly-chemotherapy, radioresistant, immunotherapy
Background
Nasopharyngeal carcinoma (NPC) represents a rare disease in the Western countries, such as Europe and USA, while its frequency reaches 20 cases per 100,000 people in the Eastern countries, such as China, Japan, and Singapore.1,2 From the histologic point of view, we can recognize two main types, namely squamous cell carcinoma and undifferentiated carcinoma, whose frequency often depends on the geographic area where the tumor is diagnosed. In fact, undifferentiated carcinoma, which strongly correlates with Epstein–Barr virus (EBV) infection, is very common in Eastern countries, while squamous cell carcinoma is prevalent in USA and Europe.3
Clinically, we can recognize three main disease presentation settings, namely early stage (T1–N0M0), locally advanced (from T2–N0, until T4–N3M0), and recurrent/metastatic disease, which are also differently approached from a therapeutic point of view.
Surgery is very difficult to perform due to the anatomic localization of the nasopharynx, which renders the radical interventions very hard to obtain. Fortunately, being an extremely chemo- and radiosensitive disease (in particular those related to EBV infection), early-stage and locally advanced NPC are currently managed with radiotherapy alone (in early stage disease) or combined chemo-radiotherapy (for locally advanced tumors).
On the basis of high-level evidence, intensity-modulated radiotherapy (IMRT) alone or with chemotherapy has become the primary treatment for early or locally advanced NPC, producing a 5-year survival rate of about 85%–90%.4–6 Nevertheless, about 8%–10% of these patients experience a recurrent disease and most of them develop distant metastases, while regional recurrences are less common.7–9
Early-stage and locally advanced NPC generally carry a good prognosis, but for patients with recurrent/metastatic disease, options are limited. The outcome for patients with recurrent or metastatic NPC (R/M NPC) is very poor, with a median overall survival (OS) of about 20 months.10 In this review, we will summarize the therapeutic options in patients with a diagnosis of recurrent NPC after a previous upfront therapy (radiation or chemoradiation), highlighting the importance of strategies different from the classical chemotherapy, such as re-irradiation, targeted therapy, salvage surgery, and immunotherapy.
Role of chemotherapy in recurrent NPC
Standard-of-care treatment for recurrent NPC is composed of platinum-containing multiagent chemotherapy.11 Despite numerous clinical trials, development of new systemic therapies for recurrent NPC, in the past 20 years, has been scarce. Platinum-containing doublet chemotherapy is generally regarded as the standard treatment for these patients; however, just one Phase III randomized clinical trial, conducted by Zhang et al,12 has evaluated the efficacy and toxicity of gemcitabine plus cisplatin (GP) vs the historical standard fluorouracil plus cisplatin (FP). This is the first and only randomized, Phase III, head-to-head clinical trial of first-line chemotherapy in recurrent NPC. The study enrolled 362 patients and randomly assigned them to receive gemcitabine plus cisplatin (experimental arm) or 5-fluorouracil plus cisplatin (standard arm). The results indicated that the median progression-free survival (PFS) was 7 months in the experimental arm and 5.6 months in the standard group (HR 0.55; P<0.0001), and overall response rate (ORR) was 64% in the gemcitabine arm and 42% in the 5-fluorouracil arm. Due to the statistically significant improvement in PFS, gemcitabine plus cisplatin became the standard first-line treatment for recurrent NPC.
Several Phase II trials employing platinum-based combination regimens, conducted before and after this backbone Phase III study, have reported an ORR ranging from 54% to 78% and a median time to progression of 7–11 months. In addition to gemcitabine or 5-fluorouracil in combination with platinum, taxanes (including paclitaxel and docetaxel) combined with platinum have also been widely used.13–16
Lately, Ma et al conducted a pooled meta-analysis on a total of 973 patients from 14 Phase II single-arm clinical trials, with the aim to evaluate the efficacy of commonly used first-line chemotherapy in recurrent NPC.17 As result, the authors identified four mainly employed regimens, namely 5-fluorouracil plus platinum (FP), gemcitabine plus platinum (GP), taxanes plus platinum (TP), and triplet combination regimens. Of these, triplet combination regimens demonstrated to have the best short-term efficacy with a highest ORR (0.74), followed by TP regimen with an ORR of 0.60. GP and FP regimens showed the worst results with an ORR of 0.54 and 0.52, respectively. In addition, the 6-month PFS rate of triplet combination regimens was ranked top again (0.83), followed by GP regimen (0.69), while the last was FP regimen (0.58). Finally, TP regimen showed highest 1-year OS rate of 0.79, followed by the GP regimen and triplet combination regimen, which showed similar results, with a rate of 0.71 and 0.74, respectively. FP regimen showed the worse results, with a 1-year OS rate of 0.63. The authors concluded that among the four commonly used first-line chemotherapy regimens for recurrent NPC, triplet combination regimens showed best short-term efficacy without improving prognosis; on the other hand, TP and GP regimens demonstrated the best long-term efficacy, while FP regimen was the least effective.
Notably, none of the triplet combination regimens analyzed in the above-mentioned study contained taxanes associated with platinum, not solving the question concerning the usefulness of taxanes. Lately, in a Phase II study, Wang et al treated 37 patients with recurrent NPC unsuitable for locoregional treatments, with the combination of cisplatin, paclitaxel, and 5-fluorouracil.18 The results showed that the ORR was 66.7%, and the PFS and the OS were 8.5 and 27.2 months, respectively. Toxicity was mild to moderate, being mainly constituted by grade 3 neutropenia. The conclusion was that TPF triplet chemotherapy showed a high response rate for locoregionally recurrent NPC with an acceptable toxicity profile. Unfortunately, the data regarding this type of approach are scarce, and also taking into account its toxicity, TPF is currently not recommended as first-line therapy for patients with recurrent NPC. Further Phase III trials are warranted to assess the efficacy of the TPF regimens (containing paclitaxel or docetaxel).
Actually, due to the lack of Phase III clinical trials, when the systemic therapy has been chosen for patients with recurrent NPC, the best treatment option is the combination of cisplatin and gemcitabine. Use of more than two drugs has not been shown to be superior to their doublet counterparts. Several trials reported on poly-chemotherapy for recurrent NPC which demonstrated encouraging response rates but was also associated with more treatment-related toxicities, and more importantly, they have not been compared with the standard cisplatin–fluorouracil or gemcitabine–cisplatin doublet regimen.19,20
In patients who develop disease progression after first-line chemotherapy, treatment options are scarce, because there are few clinical trials assessing the efficacy of chemotherapy as second-line therapy. Anecdotal publications consisting mainly of retrospective studies have demonstrated that the use of methotrexate, bleomycin, epidoxorubicin, and mitoxantrone produced an objective response rates between 15% and 30%.21–23 Gemcitabine and capecitabine are the drugs that have obtained the best results in terms of efficacy and activity, offering an ORR between 24% and 48% and median PFS between 4 and 14 months, while docetaxel, as a single agent, produced a response rate of 37% and a median PFS of 5 months.24–26 A number of reports have investigated the use of metronomic chemotherapy as second/third-line therapy in NPC and the most employed drug has been oral cyclophosphamide. Interestingly, disease control rate of 57.1% was observed in some reports with a median PFS of 4.47 months.27,28
The role of targeted therapy
The rationale of the targeted therapy is the identification of a disrupted intracellular pathway, linked to cell survival and/or apoptosis, which can be effectively inhibited by drugs. Like other squamous cell head and neck cancers, epidermal growth factor receptor (EGFR) is also highly expressed in NPC;29 thus, the use of EGFR inhibitors has been taken into account in several clinical trials. Erlotinib, gefitinib, and cetuximab have been tested in Phase II clinical trials in patients with recurrent/metastatic NPC without evidence of efficacy and with low activity.30–32 The EXTREME (Erbitux in first-line Treatment of REcurrent or MEtastatic head and neck cancer) Phase III study has established that the association of cetuximab with the standard PF (cisplatin-fluorouracil) significantly prolonged OS when compared with the standard PF in squamous cell carcinoma of the head and neck. Nevertheless, these results were not applicable in NPC.33
Vascular endothelial growth factor (VEGF) through interaction with its receptor VEGF-R is able to activate several downstream intracellular pathways linked directly and indirectly with neo-angiogenesis and cell survival. VEGF-R overexpression has been found in 60%–67% of patients affected with NPC and is linked to poor prognosis.34 Several oral available multikinase inhibitors have been tested in Phase II clinical trials in patients with recurrent NPC. Sorafenib is also able to inhibit the VEGF-R, affecting the downstream pathways related to its stimulation. Unfortunately, addition of sorafenib to the standard PF regimen has not provided good results in terms of efficacy, activity, and safety in clinical trials.35,36 Sunitinib and pazopanib are two very similar anti-angiogenic drugs, targeting VEGF-R (and also c-KIT in the case of pazopanib), which have been also tested in Phase II trials against recurrent NPC. Results have been scarce at the cost of a significant toxicity consisting mainly in cutaneous rash and gastrointestinal side effects.37,38 Data regarding other anti-angiogenic agents, including bevacizumab, are scarce and at present neo-angiogenesis blockade does not represent a therapeutic option in NPC.
Re-irradiation in NPC
Thanks to the ascent of the precision RT techniques, such as IMRT, local control of primary NPC has been optimized. Consequently, the pattern of relapses has been dominated by distant metastatic recurrences. However, a subset of almost 10% of patients with NPC remains affected by radioresistant disease and develop local recurrences.39 In these patients, nasopharyngectomy or re-irradiation may be the only curative options. The feasibility of surgery is, however, affected by the ability to obtain adequate margins in a confined, previously irradiated anatomic space. Starting from this assumption, when a local treatment is chosen, re-irradiation is the preferred option. The efficacy of the re-irradiation, nevertheless, may be counter-balanced by the high toxicity which could arise from the procedure, especially regarding the adjacent neural and mucosal normal structures.
The IMRT use and the consequent image guidance have strongly helped to avoid the technical limitations of conventional two- (2D) and three-dimensional (3D) RT, and they have improved the therapeutic ratio of salvage RT. In fact, re-irradiation with conventional external beam techniques has yielded largely unsatisfactory results with high rates of late complications even with transition from 2D to 3D conformal techniques.40,41
Leong et al performed a wide meta-analyses upon 12 studies in which a total of 1,768 patients with recurrent NPC were treated with re-irradiation, using IMRT, with or without concurrent chemotherapy. As a result, they discovered that the 5-year OS rate was 41%, indicating that a significant proportion of patients with local recurrence can achieve long-term survival with re-irradiation. Unfortunately, the overall grade 5 toxicity rate in this study was 33%, and this represents a very high rate of treatment-related mortality.42 Mucosal necrosis or massive hemorrhage was by far the common cause, representing at least 40% of such deaths. The second most common causes of grade 5 toxicities reported were feeding difficulties and radiation encephalopathy. The conclusion is that IMRT is the preferred option in case of re-irradiation, but it is not able to avoid severe RT-related toxicities, which account for a substantial proportion (up to 50%) of mortality.41,42 Consequently, a stratification of patients based on their risk to develop severe toxicity is strongly needed.
In the above-mentioned study, the authors identified some features related to poor prognosis in re-irradiated patients. The classification of the recurrent tumor was deemed to be the most crucial step; in fact, the 5-year local control and OS rates for recurrent T1 tumors were significantly higher than for recurrent T3 tumors (35% and 27% vs 11% and 4%, respectively). The time interval from primary radiotherapy seemed to be another crucial factor impacting on prognosis, and it was found that time to recurrence >36 months was associated favorably with local control. Finally, the total dose administered has also been shown to be a significant factor, and it was observed that cumulative doses of >100 Gy were associated with a higher rate of severe toxicities.
Lately, Han et al have tried to identify a prognostic index, based on patients, disease, and previous treatment-related features, with an aim to exclude those who will definitely not benefit from re-irradiation.43 They discovered some disease-related (age, T classification at recurrence, complications after previous RT) and treatment-related (recurrence volume and planned re-IMRT dose) variables which help to identify two distinct categories of patients, namely those at low and high risk of toxicity and recurrence after re-irradiation. The authors concluded that the re-irradiation, using IMRT, should be reserved to patients at low risk of recurrence and/or severe toxicity (low r-T stage, younger patients, low or no complications during previous RT).
Hua et al restrospectively analyzed data regarding 38 patients with diagnosis of recurrent T3/T4 NPC and treated by re-irradiation with IMRT. They discovered that the main cause of treatment failure was the under-dosage. Under-dosage in most of the treatment plans was inevitable because of the prior RT exposure and the close proximity to the critical neurologic structures. The degree of under-dosage, which was measured with GTV D95, was more severe with r-T4 disease. Additionally, authors highlighted that the main cause of death was the massive epistaxis and that despite the use of IMRT, the risk of any grade 3 complication was over 70%.44 The conclusions were that adequate tumor dose coverage is crucial for a favorable treatment outcome, but the severe consequent complications were extremely common.
On the basis of the afore-mentioned data, IMRT, at present, remains the most effective method for patients with recurrent NPC. However, re-irradiation with IMRT is associated with a considerable risk of severe complications, including mucosal necrosis, massive bleeding, cranial neuropathy, and temporal lobe necrosis. Considering the limited efficacy (especially for r-T3/4 disease) and the high probability of fatal complications, the 2-year OS rate is limited to only ~40%.45
Accelerated beams of heavy, charged particles and protons have a finite range and a distant Bragg peak. Dosimetry studies have shown that both carbon ion RT (CIRT) and proton or heavy ion (non-carbon) therapy enable the delivery of high-dose RT to the target volume, while sparing organs at risk, thereby enhancing the therapeutic ratio over IMRT in patients with NPC.46,47 CIRT and proton therapy may be considered more efficacious than IMRT, and these features are probably due to their unique DNA damage signature, which is characterized by clustered lesions which overload the DNA repair capacity of malignant cells. Hu et al,48 in a Phase II clinical trial, treated 75 patients with diagnosis of recurrent NPC with a course of 55–60 Gy of CIRT. As result, the 1-year overall survival, disease-specific survival, PFS, local recurrence-free survival, regional recurrence-free survival, and distant metastasis-free survival rates were 98.1%, 98.1%, 82.2%, 86.6%, 97.9%, and 96.2%, respectively. No patient developed acute toxicity of grade 2 during CIRT. Late severe toxicities (grade 3 or 4) were infrequent, but included mucosal necrosis (9.3%), xerostomia (1.3%), and temporal lobe necrosis (1.3%).
CIRT is an intriguing strategy for treating recurrent NPC, but it is currently in an experimental phase.
The role of surgery in recurrent NPC
Although surgery is almost never chosen as upfront therapy in NPC, its role in recurrent disease has been revalued. Surgical strategies strongly depend on the kind of recurrence, and a laterocervical lymph node metastases may be treated with elective neck dissection, which currently represents the gold standard of treatment. The management of the neck is often governed by the localization of the primitive tumor and in the case of NPC, the neck dissection regards the levels from II to V. In some selective cases, surgeons prefer to perform an extensive neck dissection, removing also other anatomical structures such as sternocleidomastoids muscle, jugular vein, and spinal accessory nerve.11 Another surgical strategy is nasopharyngectomy which, if employed in a selected group of NPC patients with limited tumor volume in the post-nasal space, might achieve a long-term control rate of over 50%.49 Nasopharyngectomy may be performed using two different surgical accesses: the trans-maxillary access, obtained between the two maxillary processes and putting up the nasal pyramid, or as an alternative the lateral trans-pharyngeal access can be employed. In both cases, it is a demolition intervention, and the post-surgical outcome may be difficult. In the literature, another less destructive intervention has been described, the endoscopic nasopharyngectomy. Liu et al critically evaluated the use of endoscopic nasopharyngectomy in the treatment of recurrent NPC. The authors observed a 2-year DFS and OS of 90.0% and 100%, respectively. They, moreover, performed a review of the literature regarding similar cases. The integration of their data with those observed in the literature showed that in a total number of 300 patients treated with the endoscopic resection of recurrent NPC, the recurrence-free survival and OS were 85.8% and 82.9%, respectively. Most of them (56.1%) were recurrent T1 lesions.50
Immunotherapy in recurrent NPC
Immunotherapy may be defined as the therapeutic strategy aimed at reinforcing the immune system and causing it to react against cancer cells. Several strategies of immunotherapy have been developed in the recent years and all of them have a common characteristic: rendering T-cytotoxic lymphocytes the ability to attack the tumor. Tumor vaccines constitute the easier strategy; however, there are only few of them available in the clinical practice. Adoptive immunotherapy consists instead in the direct administration of a population of tumor-specific antigens that can elicit T-lymphocytes, which have been firstly selected and then rearranged in order to attack the tumor.51 Direct administration of soluble cytokines has been employed for many years, providing limited results. Finally, the most used immunotherapy strategy, especially in the last years, is the modification of the tumor microenvironment (TME), which helps in eliminating the inhibitory stimuli that the TME exerts on the immune cells.
EBV induces neoplastic transformation of epithelial cells of the nasopharynx by various molecular mechanisms mostly involving activation of oncogenes and inactivation of tumor-suppressor genes. EBV infection, which is mainly responsible for NPC development, can also induce the expression of several immunogenic peptides on the plasma membrane of the infected cells. These peptides may be used as target for immunotherapy.52 A vaccination with modified vaccinia virus Ankara (MVA) recombinant vector has been experimented in clinical trials enrolling patients with advanced chemorefractory NPC. MVA-EL encodes a fusion protein derived from two EBV-associated antigens EBNA1 (EBV Nuclear Antigen 1) and LMP2 (Latent Membrane Protein 2), and it has been designed with the aim to boost T-cell immunity to these antigens. This vaccine has been demonstrated to be safe in two Phase I trials.53,54 Dendritic cells are cells belonging to the immune system, able to capture, process, and present tumor-associated antigens (TAAs) to lymphocytes, rendering them ability to react against the presented TAAs. Vaccines may also be constituted by dendritic cells pulsed with NPC-TAA. This approach has been widely employed in different Phase II clinical trials, enrolling recurrent NPC-affected patients and obtaining fairly good results.55
Adoptive immunotherapy is defined as the direct activation of effector T-cells (CD8+) stimulated in vitro and reinfused intravenously. This strategy has been employed in recurrent NPC in several Phase II and III trials, both as first and in following lines of therapy.56,57 The mediocre objective responses obtained and its association with certain amount of toxicity have risen some questions. In particular, it has been clarified that the afore-mentioned adoptive immunotherapy strategies are not able to generate lymphocytes particularly specific and able to selectively attack the tumor, due to the fact that the immunogenic potency of the employed TAAs (mostly viral proteins) is low. Thus, a number of strategies to increase the specificity of T-lymphocytes against TAA have been developed, and the most recent is the CAR (chimeric antigen receptors) technology. CAR are chimeric transmembrane receptors constituted by an antigen-specific single-chain variable fragment (against a predetermined TAA) fused with the CD3 intracellular domain (the so-called TCR, namely T-cell receptor). The aim of this strategy is to obtain a class of T-cytotoxic lymphocytes able to recognize and eliminate TAA with high selectivity. An ongoing Phase I trial is evaluating the intratumoral administration of CAR T-cells in locally advanced/recurrent metastatic head and neck squamous cell carcinomas including NPC.58
The most exciting strategy of immunotherapy is the use of the so-called checkpoint inhibitors, which are the drugs able to remove the inhibitory stimuli elicited by the TME upon the cytotoxic T-lymphocytes. TME may negatively regulate T-cells in two main steps: the “priming phase”, during which the naïve T-cells become able to react against cancer stimulated by DC, and the “effector phase”, during which the specific T-cells attack the tumor cells and destroy them. The first phase is strongly regulated by the interaction between CTLA-4 (cytotoxic t-lymphocyte associated antigen-4) and its ligands, B7.1 and CD28. The interaction between CTLA-4 (present on naïve T-cell) and B7.1 (present on dendritic cells) is able to provoke T-cell anergy. Antibodies against CTLA-4 are widely employed in clinics for the treatment of several solid tumors. Unfortunately, none of them are available for NPC.51,59–61 During the “effector phase”, the selective T-cells attack tumor cells recognizing the TAA and provoke their apoptosis, but this phenomenon may be inhibited by the interaction between PD-1 (programmed death-1), present on T-cell membrane, and programmed death-ligand 1 (PDL-1), expressed on cell surface of several tumor cells, including NPC. This last interaction provokes T-cells anerg and may be blocked using drugs able to interfere with this linkage, that is, anti PD-1 and anti-PDL-1 antibodies.62,63
As highlighted before, expression of some viral proteins, such as EBNA-1 or LMP-1 and 2, in NPC cells can elicit a virus-specific immune response in patients with NPC. LMP-1 expression and interferon-gamma activation can synergistically induce the expression of PDL-1 (PD-L1) in NPC cells. In fact, PD-L1 expression is reported to occur in 89%–95% of NPC tumors. This consideration has provided the rationale for using checkpoint inhibitors in NPC.
KEYNOTE-028 is a nonrandomized multi-cohort, Phase Ib trial of pembrolizumab in patients with PD-L1-positive advanced solid tumors. The cohort enrolling NPC cases included 27 patients who had progressed after a first-line chemotherapy containing platinum. As a result, partial response and stable disease were observed in seven and 14 patients, respectively, for an ORR of 25.9%. Drug-related adverse events that occurred in 15% of patients included rash (26%), hypothyroidism (18.5%), and fatigue (18.5%), with only one case of sepsis.64 In a similar study, Ma et al in a multinational study evaluated the antitumor activity of nivolumab in patients with NPC who had progressed after a first line of chemotherapy containing platinum. A total of 44 patients were enrolled and the ORR (the sum of complete and partial responses) was 20.5%, with a 1-year OS rate and 1-year PFS rate of 59% and 19.3%, respectively. A subgroup analysis showed that the proportion of patients who responded was higher among those with PD-L1-positive tumors (at least 1% expression) than those with PD-L1-negative tumors.65
Overall, immunotherapy has proven its effectiveness in NPC in clinical trials, but unfortunately its use remains experimental.
Conclusion
NPC is a rare disease in Western countries, while its relevance in China and Eastern countries remains notably high, representing a considerable health problem worldwide. Being an extremely chemo- and radiosensitive disease, especially tumors with undifferentiated histology, most of the patients affected by NPC are effectively cured with upfront chemo-radiotherapy, which currently includes the association of IMRT and cisplatin. Nevertheless, 10%–15% of these patients are radioresistant and experience a recurrence, which in most cases manifests with distant metastases. Systemic therapies are the gold standard in this case, but locoregional recurrences, namely localized in the nasopharynx or in laterocervical lymph nodes, should be managed differently. Based on the literature data, laterocervical metastases, if alone and isolated, are more suitable to be treated with surgery,11 while nasopharyngeal masses are managed differently. In fact, a number of clinical reports have highlighted that some recurrences could be irradiated safely and have also good probabilities to respond to re-irradiation, based on some tumor- and patient-related features. T1 and T2 recurrent NPCs, for example, should be treated with re-irradiation, because they have good probabilities to respond and the related toxicities are low and manageable.41,42 On the other hand, re-irradiation in T3 and T4 recurrent NPCs is accompanied by high toxicity and low probability of response.41–43 Surgery in the case of nasopharyngeal recurrence is difficult to perform, and there are only anecdotic data in literature regarding the nasopharyngectomy. The more radical techniques should be preferred in site of the endoscopic nasopharyngectomy, being the firsts much more radical and thus usable also for locally advanced diseases. When the re-irradiation has been chosen as therapeutic strategy, the IMRT must be preferred to conformal 3D, and the proton therapy such as that of CIRT remains the only experimental procedure.
Systemic therapies are far more used in clinical practice, and in particular two-drug chemotherapy currently represents the gold standard as first-line therapy for recurrent NPC.11 The combination of cisplatin and gemcitabine remains the gold standard, being the sole composition that has demonstrated more efficacy when compared with the old standard PF. Nowadays, three-drug regimens, albeit promising, are not employed due to their higher associated toxicity.19,20 Mono-chemotherapy remains the standard after the failure of a first-line chemotherapy, and targeted therapy has no role in recurrent NPC.
Immunotherapy is a promising strategy, having demonstrated efficacy both in first-line and in following lines of therapy, and, importantly, NPC is a tumor able to elicit a robust immune response in the host. It is probably due to its viral-induced cancerogenesis, which allows the expression of several viral antigens of cancer cells on the cell surface. LMP-1 and 2, as well as EBNA, are recognized by the immune system as non-self, provoking a considerable immune response. This feature has been taken into account, and a number of clinical trials employing active and adoptive immunotherapy have been carried out in recurrent NPC. Unfortunately, the results in terms of activity and efficacy have not been so encouraging, and hence these strategies are not included among the standards.53–55
The use of the checkpoint inhibitor is at an early stage, so more Phase II and III clinical trials are warranted to assess their efficacy in recurrent NPC.
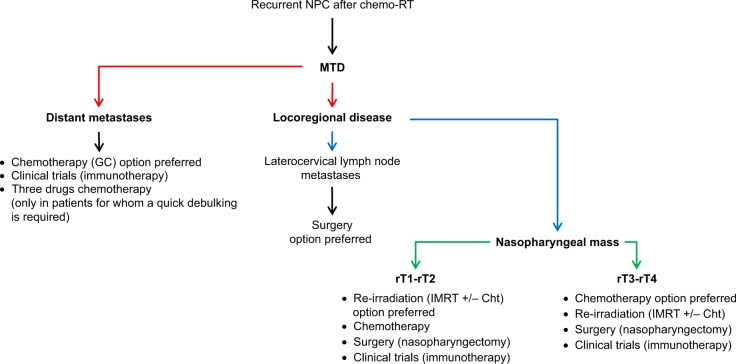
Figure 1 describes the flowchart applicable in presence of a diagnosis of recurrent NPC.
Figure 1.
Flowchart illustrating the optimal approach in patients with a diagnosis of recurrent NPC.
Abbreviations: NPC, nasopharyngeal carcinoma; chemo-RT, chemo-radiotherapy; MTD, multidisciplinary team management; GC, cisplatin-gemcitabine; IMRT, intensity-modulated radiation therapy; Cht, chemotherapy; rT1, recurrent T1; rT2, recurrent T2; rT3, recurrent T3; rT4, recurrent T4.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chan AT, Teo PM, Johnson PJ. Nasopharyngeal cancer. Cancer Treat Res. 2003;114:275–293. doi: 10.1007/0-306-48060-3_11. [DOI] [PubMed] [Google Scholar]
- 2.Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30:114–119. doi: 10.5732/cjc.010.10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caponigro F, Longo F, Ionna F, Perri F. Treatment approaches to nasopharyngeal carcinoma: a review. Anticancer Drugs. 2010;21(5):471–477. doi: 10.1097/CAD.0b013e328337160e. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–655. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Hong S, Wang Y, et al. Development and external validation of nomograms for predicting survival in nasopharyngeal carcinoma patients after definitive radiotherapy. Sci Rep. 2015;5:15638. doi: 10.1038/srep15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perri F, della Vittoria Scarpati G, Buonerba C, et al. Combined chemo-radiotherapy in locally advanced nasopharyngeal carcinomas. World J Clin Oncol. 2013;4(2):47–51. doi: 10.5306/wjco.v4.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper JS, del Rowe J, Newall J. Regional stage IV carcinoma of the nasopharynx treated by aggressive radiotherapy. Int J Radiat Oncol Biol Phys. 1983;9(11):1737–1745. doi: 10.1016/0360-3016(83)90428-5. [DOI] [PubMed] [Google Scholar]
- 8.Geara FB, Glisson BS, Sanguineti G, et al. Induction chemotherapy followed by radiotherapy versus radiotherapy alone in patients with advanced nasopharyngeal carcinoma: results of a matched cohort study. Cancer. 1997;79(7):1279–1286. doi: 10.1002/(sici)1097-0142(19970401)79:7<1279::aid-cncr2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Perri F, Bosso D, Buonerba C, Lorenzo GD, Scarpati GD. Locally advanced nasopharyngeal carcinoma: current and emerging treatment strategies. World J Clin Oncol. 2011;2(12):377–383. doi: 10.5306/wjco.v2.i12.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network NCCN Guidelines: Head and neck cancer version 2. [Accessed December 20, 2018]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- 12.Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388(10054):1883–1892. doi: 10.1016/S0140-6736(16)31388-5. [DOI] [PubMed] [Google Scholar]
- 13.Ji JH, Yun T, Kim SB, et al. A prospective multicentre phase II study of cisplatin and weekly docetaxel as first-line treatment for recurrent or metastatic nasopharyngeal cancer (KCSG HN07-01) Eur J Cancer. 2012;48(17):3198–3204. doi: 10.1016/j.ejca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Chua DT, Sham JS, Au GK. A phase II study of docetaxel and cisplatin as first-line chemotherapy in patients with metastatic nasopharyngeal carcinoma. Oral Oncol. 2005;41(6):589–595. doi: 10.1016/j.oraloncology.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Mccarthy JS, Tannock IF, Degendorfer P, Panzarella T, Furlan M, Siu LL. A phase II trial of docetaxel and cisplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. Oral Oncol. 2002;38(7):686–690. doi: 10.1016/s1368-8375(01)00134-8. [DOI] [PubMed] [Google Scholar]
- 16.Caponigro F, Longo F, Perri F, Ionna F. Docetaxel in the management of head and neck cancer. Anticancer Drugs. 2009;20(8):639–645. doi: 10.1097/cad.0b013e32832c9c51. [DOI] [PubMed] [Google Scholar]
- 17.Ma SX, Zhou T, Huang Y, et al. The efficacy of first-line chemotherapy in recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta-analysis. Ann Transl Med. 2018;6(11):201. doi: 10.21037/atm.2018.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Wang ZQ, Jiang YX, et al. A triplet chemotherapy regimen of cisplatin, fluorouracil and paclitaxel for locoregionally recurrent nasopharyngeal carcinoma cases contraindicated for re-irradiation/surgery. Expert Opin Pharmacother. 2016;17(12):1585–1590. doi: 10.1080/14656566.2016.1204293. [DOI] [PubMed] [Google Scholar]
- 19.Boussen H, Cvitkovic E, Wendling JL, et al. Chemotherapy of metastatic and/or recurrent undifferentiated nasopharyngeal carcinoma with cisplatin, bleomycin, and fluorouracil. J Clin Oncol. 1991;9(9):1675–1681. doi: 10.1200/JCO.1991.9.9.1675. [DOI] [PubMed] [Google Scholar]
- 20.Su WC, Chen TY, Kao RH, Tsao CJ. Chemotherapy with cisplatin and continuous infusion of 5-fluorouracil and bleomycin for recurrent and metastatic nasopharyngeal carcinoma in Taiwan. Oncology. 1993;50(4):205–208. doi: 10.1159/000227179. [DOI] [PubMed] [Google Scholar]
- 21.Shiu WCT, Tsao SY. Efficacy of 4-epidoxorubicin in advanced nasopharyngeal carcinoma. Clin Trials J. 1989;26:149–152. [Google Scholar]
- 22.Dugan M, Choy D, Ngai A, et al. Multicenter phase II trial of mitoxantrone in patients with advanced nasopharyngeal carcinoma in Southeast Asia: an Asian-Oceanian clinical Oncology Association group study. J Clin Oncol. 1993;11(1):70–76. doi: 10.1200/JCO.1993.11.1.70. [DOI] [PubMed] [Google Scholar]
- 23.Ma BB, Tannock IF, Pond GR, Edmonds MR, Siu LL. Chemotherapy with gemcitabine-containing regimens for locally recurrent or metastatic nasopharyngeal carcinoma. Cancer. 2002;95(12):2516–2523. doi: 10.1002/cncr.10995. [DOI] [PubMed] [Google Scholar]
- 24.Foo KF, Tan EH, Leong SS, et al. Gemcitabine in metastatic nasopharyngeal carcinoma of the undifferentiated type. Ann Oncol. 2002;13(1):150–156. doi: 10.1093/annonc/mdf002. [DOI] [PubMed] [Google Scholar]
- 25.Ciuleanu E, Irimie A, Ciuleanu TE, Popita V, Todor N, Ghilezan N. Capecitabine as salvage treatment in relapsed nasopharyngeal carcinoma: a phase II study. J Buon. 2008;13(1):37–42. [PubMed] [Google Scholar]
- 26.Ngeow J, Lim WT, Leong SS, et al. Docetaxel is effective in heavily pretreated patients with disseminated nasopharyngeal carcinoma. Ann Oncol. 2011;22(3):718–722. doi: 10.1093/annonc/mdq425. [DOI] [PubMed] [Google Scholar]
- 27.André N, Carré M, Pasquier E. Metronomics: towards personalized chemotherapy? Nat Rev Clin Oncol. 2014;11(7):413–431. doi: 10.1038/nrclinonc.2014.89. [DOI] [PubMed] [Google Scholar]
- 28.Gnoni A, Silvestris N, Licchetta A, et al. Metronomic chemotherapy from Rationale to clinical studies: a dream or reality? Crit Rev Oncol Hematol. 2015;95(1):46–61. doi: 10.1016/j.critrevonc.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Fujii M, Yamashita T, Ishiguro R, Tashiro M, Kameyama K. Significance of epidermal growth factor receptor and tumor associated tissue eosinophilia in the prognosis of patients with nasopharyngeal carcinoma. Auris Nasus Larynx. 2002;29(2):175–181. doi: 10.1016/s0385-8146(01)00135-3. [DOI] [PubMed] [Google Scholar]
- 30.Chua DT, Wei WI, Wong MP, Sham JS, Nicholls J, Au GK. Phase II study of gefitinib for the treatment of recurrent and metastatic naso-pharyngeal carcinoma. Head Neck. 2008;30(7):863–867. doi: 10.1002/hed.20792. [DOI] [PubMed] [Google Scholar]
- 31.Ma B, Hui EP, King A, et al. A phase II study of patients with metastatic or locoregionally recurrent nasopharyngeal carcinoma and evaluation of plasma Epstein-Barr virus DNA as a biomarker of efficacy. Cancer Chemother Pharmacol. 2008;62(1):59–64. doi: 10.1007/s00280-007-0575-8. [DOI] [PubMed] [Google Scholar]
- 32.You B, Le Tourneau C, Chen EX, et al. A phase II trial of erlotinib as maintenance treatment after gemcitabine plus platinum-based chemotherapy in patients with recurrent and/or metastatic nasopharyngeal carcinoma. Am J Clin Oncol. 2012;35(3):255–260. doi: 10.1097/COC.0b013e31820dbdcc. [DOI] [PubMed] [Google Scholar]
- 33.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 34.Hui EP, Chan AT, Pezzella F, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8(8):2595–2604. [PubMed] [Google Scholar]
- 35.Elser C, Siu LL, Winquist E, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25(24):3766–3773. doi: 10.1200/JCO.2006.10.2871. [DOI] [PubMed] [Google Scholar]
- 36.Xue C, Huang Y, Huang PY, et al. Phase II study of sorafenib in combination with cisplatin and 5-fluorouracil to treat recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol. 2013;24(4):1055–1061. doi: 10.1093/annonc/mds581. [DOI] [PubMed] [Google Scholar]
- 37.Lim WT, Ng QS, Ivy P, et al. A phase II study of pazopanib in Asian patients with recurrent/metastatic nasopharyngeal carcinoma. Clin Cancer Res. 2011;17(16):5481–5489. doi: 10.1158/1078-0432.CCR-10-3409. [DOI] [PubMed] [Google Scholar]
- 38.Hui EP, Ma BB, King AD, et al. Hemorrhagic complications in a phase II study of sunitinib in patients of nasopharyngeal carcinoma who has previously received high-dose radiation. Ann Oncol. 2011;22(6):1280–1287. doi: 10.1093/annonc/mdq629. [DOI] [PubMed] [Google Scholar]
- 39.Ng WT, Lee MC, Chang AT, et al. The impact of dosimetric inadequacy on treatment outcome of nasopharyngeal carcinoma with IMRT. Oral Oncol. 2014;50(5):506–512. doi: 10.1016/j.oraloncology.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Lee AW, Law SC, Foo W, et al. Retrospective analysis of patients with nasopharyngeal carcinoma treated during 1976–1985: survival after local recurrence. Int J Radiat Oncol Biol Phys. 1993;26(5):773–782. doi: 10.1016/0360-3016(93)90491-d. [DOI] [PubMed] [Google Scholar]
- 41.Chang JT, See LC, Liao CT, et al. Locally recurrent nasopharyngeal carcinoma. Radiother Oncol. 2000;54(2):135–142. doi: 10.1016/s0167-8140(99)00177-2. [DOI] [PubMed] [Google Scholar]
- 42.Leong YH, Soon YY, Lee KM, Wong LC, Tham IWK, Ho FCH. Long-term outcomes after reirradiation in nasopharyngeal carcinoma with intensity-modulated radiotherapy: a meta-analysis. Head Neck. 2018;40(3):622–631. doi: 10.1002/hed.24993. [DOI] [PubMed] [Google Scholar]
- 43.Han F, Zhao C, Huang SM, et al. Long-term outcomes and prognostic factors of re-irradiation for locally recurrent nasopharyngeal carcinoma using intensity-modulated radiotherapy. Clin Oncol. 2012;24(8):569–576. doi: 10.1016/j.clon.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Hua YJ, Han F, Lu LX, et al. Long-term treatment outcome of recurrent nasopharyngeal carcinoma treated with salvage intensity modulated radiotherapy. Eur J Cancer. 2012;48(18):3422–3428. doi: 10.1016/j.ejca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Kong L, Wang L, Shen C, et al. Salvage intensity-modulated radiation therapy (IMRT) for locally recurrent nasopharyngeal cancer after definitive IMRT: a novel scenario of the modern era. Sci Rep. 2016;6:32883. doi: 10.1038/srep32883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin R, Slater JD, Yonemoto LT, et al. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy-dose-volume histogram analysis. Radiology. 1999;213(2):489–494. doi: 10.1148/radiology.213.2.r99nv29489. [DOI] [PubMed] [Google Scholar]
- 47.Feehan PE, Castro JR, Phillips TL, et al. Recurrent locally advanced nasopharyngeal carcinoma treated with heavy charged particle irradiation. Int J Radiat Oncol Biol Phys. 1992;23(4):881–884. doi: 10.1016/0360-3016(92)90663-3. [DOI] [PubMed] [Google Scholar]
- 48.Hu J, Bao C, Gao J, et al. Salvage treatment using carbon ion radiation in patients with locoregionally recurrent nasopharyngeal carcinoma: initial results. Cancer. 2018;124(11):2427–2437. doi: 10.1002/cncr.31318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei WI, Chan JY, Ng RW, Ho WK. Surgical salvage of persistent or recurrent nasopharyngeal carcinoma with maxillary swing approach-critical appraisal after 2 decades. Head Neck. 2011;33(7):969–975. doi: 10.1002/hed.21558. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Yu H, Sun X, et al. Salvage endoscopic nasopharyngectomy for local recurrent or residual nasopharyngeal carcinoma: a 10-year experience. Int J Clin Oncol. 2017;22(5):834–842. doi: 10.1007/s10147-017-1143-9. [DOI] [PubMed] [Google Scholar]
- 51.Pisconti S, Della Vittoria Scarpati G, Facchini G, et al. The evolving landscape of immunotherapy against cancer. WCRJ. 2018;5(1):e1042. [Google Scholar]
- 52.Perri F, della Vittoria Scarpati G, Giuliano M, et al. Epstein-Barr virus infection and nasopharyngeal carcinoma: the other side of the coin. Anticancer Drugs. 2015;26(10):1017–1025. doi: 10.1097/CAD.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 53.Hui EP, Taylor GS, Jia H, et al. Phase I trial of recombinant modified vaccinia Ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res. 2013;73(6):1676–1688. doi: 10.1158/0008-5472.CAN-12-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin CL, Lo WF, Lee TH, et al. Immunization with Epstein-Barr virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. 2002;62(23):6952–6958. [PubMed] [Google Scholar]
- 55.Lin CL, Lo WF, Lee TH, et al. Immunization with Epstein-Barr virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. 2002;62(23):6952–6958. [PubMed] [Google Scholar]
- 56.He J, Tang XF, Chen QY, et al. Ex vivo expansion of tumor-infiltrating lymphocytes from nasopharyngeal carcinoma patients for adoptive immunotherapy. Chin J Cancer. 2012;31(6):287–294. doi: 10.5732/cjc.011.10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chia WK, Teo M, Wang WW, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther. 2014;22(1):132–139. doi: 10.1038/mt.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Schalkwyk MC, Papa SE, Jeannon JP, Guerrero Urbano T, Spicer JF, Maher J. Design of a phase I clinical trial to evaluate intratumoral delivery of ErbB-targeted chimeric antigen receptor T-cells in locally advanced or recurrent head and neck cancer. Hum Gene Ther Clin Dev. 2013;24(3):134–142. doi: 10.1089/humc.2013.144. [DOI] [PubMed] [Google Scholar]
- 59.Menon S, Shin S, Dy G. Advances in cancer immunotherapy in solid tumors. Cancers. 2016;8(12):106. doi: 10.3390/cancers8120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kyi C, Postow MA. Immune checkpoint inhibitor combinations in solid tumors: opportunities and challenges. Immunotherapy. 2016;8(7):821–837. doi: 10.2217/imt-2016-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist. 2015;20(7):812–822. doi: 10.1634/theoncologist.2014-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amm E, Crittenden M, Wargo J. Combination immunotherapy development in melanoma. Am Soc Clin Oncol Educ Book. 2018;38:197–207. doi: 10.1200/EDBK_201131. [DOI] [PubMed] [Google Scholar]
- 63.Eggermont AM, Maio M, Robert C. Immune checkpoint inhibitors in melanoma provide the cornerstones for curative therapies. Semin Oncol. 2015;42(3):429–435. doi: 10.1053/j.seminoncol.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed Death-Ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 2017;35(36):4050–4056. doi: 10.1200/JCO.2017.73.3675. [DOI] [PubMed] [Google Scholar]
- 65.Ma BBY, Lim WT, Goh BC, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the Mayo Clinic phase 2 Consortium (NCI-9742) J Clin Oncol. 2018;36(14):1412–1418. doi: 10.1200/JCO.2017.77.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]