Abstract
Objective:
Depression and cognitive impairment are often comorbid in older adults, but optimal treatment strategies remain unclear. In a two-site study, the efficacy and safety of add-on donepezil versus placebo were compared in depressed patients with cognitive impairment receiving stable antidepressant treatment.
Methods:
A randomized, double-blind, placebo-controlled trial was conducted in older adults with depression and cognitive impairment (https://clinicaltrials.gov/ct2/show/NCT01658228; NCT01658228). Patients received open-label antidepressant treatment for 16 weeks, initially with citalopram and then with venlafaxine, if needed, followed by random assignment to add-on donepezil 5–10 mg daily or placebo for another 62 weeks. Outcome measures were neuropsychological test performance (Alzheimer’s Disease Assessment Scale—Cognitive subscale [ADAS-Cog] and Selective Reminding Test [SRT] total immediate recall) and instrumental activities of daily living (Functional Activities Questionnaire).
Results:
Of 81 patients who signed informed consent, 79 patients completed the baseline evaluation. Open antidepressant treatment was associated with improvement in depression in 63.93% responders by week 16. In the randomized trial, there were no treatment group differences between donepezil and placebo on dementia conversion rates, ADAS-Cog, SRT total immediate recall, or FAQ. Neither baseline cognitive impairment severity nor apolipoprotein E e4 genotype influenced donepezil efficacy. Donepezil was associated with more adverse effects than placebo.
Conclusion:
The results do not support adjunctive off-label cholinesterase inhibitor treatment in patients with depression and cognitive impairment. The findings highlight the need to prioritize discovery of novel treatments for this highly prevalent population with comorbid illnesses.
Keywords: Mild cognitive impairment, depression, clinical trial, antidepressants, cholinesterase inhibitor
INTRODUCTION
Cognitive complaints are reported by 20%–50% of older adults, and their prevalence increases progressively with age.1,2 To varying degrees, depression affects 10%–25% of older adults.3 Cognitive impairment and depression are the most common neuropsychiatric disorders in older adults, and their co-occurrence may exceed chance.4,5 Depression is a major cause of suffering, impaired functioning, and disability, and is often comorbid with medical illnesses, with worsening of outcomes, including increased mortality.6,7 Patients presenting with comorbid depression and cognitive impairment represent a unique and understudied population. Alzheimer disease, cerebrovascular disease, and reversible cognitive impairment due to other factors may underlie this presentation.8,9
Depression in patients with cognitive impairment increases the risk of conversion to dementia.8,10,11 Treatment trials in geriatric depression have typically excluded patients with prominent memory deficits,7,12 and treatment trials in mild cognitive impairment (MCI) have usually excluded patients with major depression.13,14 There is a lack of information on the treatment of patients with comorbid depression and cognitive impairment.
In a re-analysis of a study that compared the cholinesterase inhibitor (ChEI) donepezil, vitamin E, and placebo in 769 patients with amnestic MCI and without major depression, patients with greater severity of depression were more likely to improve on donepezil compared to placebo.15 The results suggested that donepezil may improve cognition in depressed patients with amnestic MCI. In elderly patients with major depression who were not selected for having cognitive impairment, antidepressant treatment plus add-on donepezil (n = 67) or placebo (n = 63) was prescribed for 2 years.16 Donepezil treatment had no effect in cognitively intact patients with major depression, but it transiently enhanced cognition to a small degree in depressed patients with cognitive impairment. In pilot clinical trials of patients with depression and cognitive impairment, response to antidepressants was not associated with cognitive improvement.17,18 In a small pilot study, adding the ChEI donepezil to antidepressant treatment improved episodic verbal memory in some patients with comorbid depression and cognitive impairment.19
In the context of limited evidence from pilot studies suggesting possible efficacy of donepezil and an absence of systematic clinical trials of cognitive enhancers in patients with comorbid depression and cognitive impairment, we conducted a study of donepezil treatment of older adults with cognitive impairment and depression. The study design and procedures for this 18-month clinical trial have been described elsewhere.20 This randomized, double-blind study was designed to compare add-on donepezil to placebo in patients with depression and cognitive impairment who were receiving stable antidepressant treatment, with the key hypothesis being that donepezil would be superior to placebo in improving cognitive test performance. The definition of cognitive impairment required the participant to meet criteria for either early MCI (EMCI) or late MCI (LMCI; amnestic MCI) as per the Alzheimer’s Disease Neuroimaging Initiative criteria.21
The following putative moderators of cognitive improvement were examined in exploratory analyses: apolipoprotein E e4 genotype; impaired odor identification, which is known to be associated with the transition from MCI to Alzheimer disease (AD)22,23 and therefore may predict cognitive improvement on donepezil24,25; hippocampal and entorhinal cortical atrophy on magnetic resonance imaging (MRI)26–28; and hyperintensities on MRI.29,30
METHODS
Participants aged 55–95 years signed informed consent for this institutional review board–approved protocol conducted at the New York State Psychiatric Institute (NYSPI)/Columbia University Medical Center (coordinating center) and Duke University Medical Center after receiving a complete description of the study.20 Participants were recruited via referral from physicians and from newspaper advertisements. The trial was registered on clinicaltrials.gov (identifier: NCT01658228). The study was conducted from October 2012 to August 2016. Study inclusion criteria were the following: 1) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, major depression or dysthymic disorder, with 24-item Hamilton Rating Scale for Depression (HAM-D) score greater than or equal to 14;2) subjective cognitive complaints; 3) Folstein Mini-Mental State Exam (MMSE) greater than or equal to 21, with score less than or equal to 11 for delayed recall on the Wechsler Memory Scale—Revised Logical Memory II subscale or score greater than or equal to 1.5 standard deviations (SD) below norms for delayed recall on the Free and Cued Selective Reminding Test. Patients were classified as LMCI according to the Alzheimer’s Disease Neuroimaging Initiative definition, which is consistent with amnestic MCI13,31 if they scored greater than or equal to 1.5 SD below norms on the Wechsler Memory Scale—Revised Logical Memory test or the Free and Cued Selective Reminding Test conducted at screening. All other patients who met the above inclusion criteria for cognitive impairment were classified as EMCI.
Each participant was required to meet criteria for both depression and cognitive impairment. Exclusion criteria were other major pre-existing psychiatric and neurologic disorders, including dementia, acute medical illness, active suicidal ideation or suicide attempt, alcohol or substance abuse or dependence in the past 6 months, use of medication rated as the likely cause of cognitive impairment, uncontrolled hypertension, current use of effective antidepressants, current use of ChEIs or memantine, and electrocardiogram QTc interval greater than 460 msec. Patients receiving ineffective antidepressants were washed out for 1–14 days, depending on medication half-life and tolerability of washout.
Measures
The HAM-D and the Beck Depression Inventory–II were completed at each study visit. The neuropsychological battery comprised the following tests: modified Alzheimer’s Disease Assessment Scale—Cognitive subscale (ADAS-Cog; 13-item version that covers several cognitive domains), Selective Reminding Test (SRT; episodic verbal memory, 12-item 6-trial version, with three different forms used serially), Wechsler Memory Scale—Third Edition Visual Reproduction subtest (nonverbal learning and memory), Wechsler Adult Intelligence Scale—Third Edition Block Design (visuospatial skills), 15-item Boston Naming Test (confrontation naming for language), letter and animal naming (category fluency), Trail Making A (attention), Trail Making B and Stroop Color and Word Test—Interference (executive function), and Wechsler Adult Intelligence Scale—Third Edition Digit Symbol (processing speed). The two main outcome measures were ADAS-Cog total score and SRT total immediate recall score. The ADAS-Cog and SRT were administered at baseline, 16, 40, 64, and 78 weeks. The entire neuropsychological battery was administered at baseline and at 78 weeks or end of trial, if there was early termination. The results of the neuropsychological test battery were used to make the diagnosis (EMCI, LMCI, dementia with subtype of dementia) at these two time points.
At baseline, the Cumulative Illness Rating Scale for Geriatrics (medical morbidity assessment)32 and the University of Pennsylvania Smell Identification Test (UPSIT, scratch and sniff 40-item multiple-choice test) were administered. The Pfeffer Functional Activities Questionnaire (FAQ)33 was given to an informant at all visits (secondary outcome measure). Blood samples were processed by the Columbia University Human Genetics Resource Core and apolipoprotein E genotype was determined by PreventionGenetics.
MRI
Images were acquired on a Signa 3 Tesla whole body scanner (GE Medical Systems, Milwaukee, WI) using an identical model at the two sites. As described elsewhere in detail,20 sequences were three-plane localizer, three-dimensional anatomic spoiled gradient recalled acquisition in steady state aligned to the long axis of the hippocampus, and T2 fluid-attenuated inversion recovery (FLAIR). A single trained, experienced technician, who established high inter-rater reliability with expert raters (intraclass correlation coefficient [ICC] = 0.90–0.96) and showed high inter-rater reliability (ICC = 0.97–0.99),27 drew the hippocampal and entorhinal cortex regions of interest using atlas-based approaches. Patients who refused the MRI component could still participate in the clinical trial.
Hyperintensities on axial T2 FLAIR images were rated with the Fazekas modified Coffey rating scale,34 a qualitative zero to three rating of lesion severity for deep white matter hyperintensities, subcortical gray matter hyperintensities in the basal ganglia, and periventricular hyperintensities. T2 FLAIR lesion volume was measured on a parallel, semiautomated version of this scale using MRIcro.35 Study raters achieved excellent inter-rater reliability (ICC = 0.98).36
Treatment
In the first phase of 16 weeks of open-label antidepressant therapy (Phase A), citalopram was started at 10 mg and raised to 20 mg daily after 2 weeks. If there was lack of antidepressant response by 8 weeks, citalopram was switched to venlafaxine 37.5 mg daily, with the dose raised weekly over 4 weeks to reach 225 mg daily or the maximum tolerated dose. Other antidepressants were prescribed for patients with nonresponse or intolerability to citalopram and venlafaxine.
All patients who completed the first 16 weeks were eligible for the randomized phase, regardless of degree of improvement in depression with antidepressant treatment. The statistician generated the random assignment sequence, and the research pharmacist had access to the random assignment list. All raters remained blind to treatment assignment (donepezil:placebo 1:1). At 16 weeks, donepezil (or placebo) was started in randomized patients (Phase B) at 5 mg once daily and increased to 10 mg daily within 4 weeks if tolerated; the maximum tolerated dose was maintained during the trial. Adherence was monitored by pill counts. During Phase B, antidepressant treatment was continued with dose adjustment if clinically indicated.
Statistical Analyses
Means and standard deviations were computed to describe continuous variables, while frequencies and percentages were computed to describe categorical variables. White matter hyperintensity (WMH) volume measures were positively skewed even after log transformation. All WMH volume measures were converted to dichotomous variables (high-/low-volume) based on the median value for each measure. Criteria for high volumes in cm3 were deep white matter greater than0.1, periventricular hyperintensities greater than 5, sub-cortical grey hyperintensities greater than 0, and total greater than 5.4.
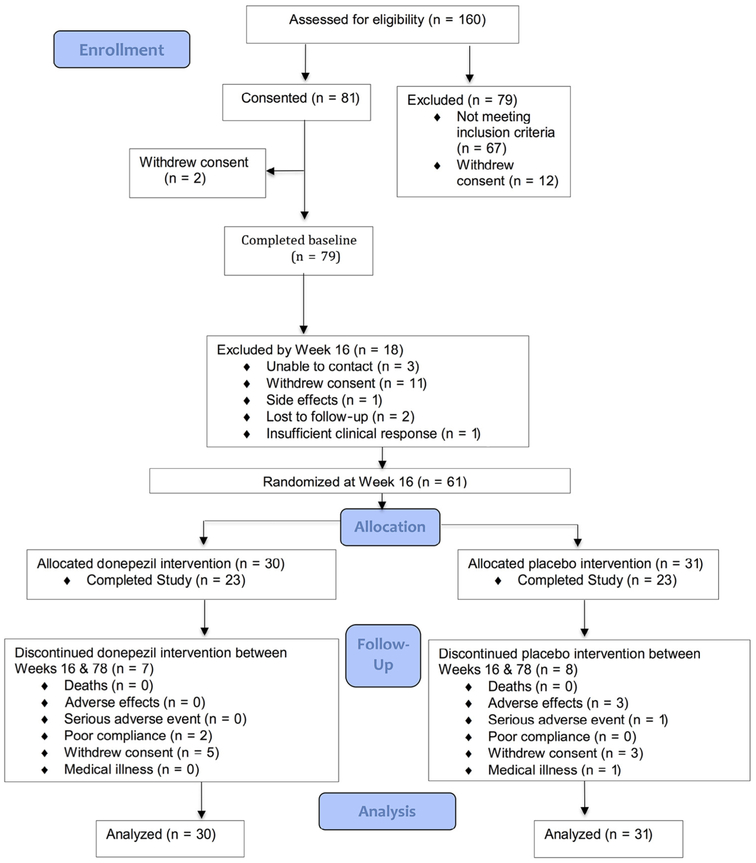
Statistical tests were performed at the 0.05 significance level. All randomized participants and all assessments were included based on the intention-to-treat principle. The targeted sample size was 80 patients, assuming 5% attrition between consecutive time points, in order to have at least 80% power to detect a moderate group difference with respect to SRT total recall. Of 160 screened patients, 81 were recruited, of whom 2 did not complete baseline procedures (Figure 1). Of the 79 participants in Phase A, 56 had MRI brain scans.
FIGURE 1.
CONSORT diagram for the donepezil treatment of older adults with cognitive impairment and depression study.
Phase A Analyses
Antidepressant response (binary variable indicating ≥50% reduction in HAM-D from baseline) over 16 weeks was modeled using logistic mixed-effects models with a random subject-specific intercept and a first-order autoregressive process within-subject covariance structure to account for repeated measures. To assess predictors of antidepressant response in exploratory analyses, separate models were fit with each of the following as the primary predictor: ApoE e4 status, baseline UPSIT score, each baseline WMH volume (high-/low-volume), hippocampal volume, and entorhinal cortex volume. All models were adjusted for baseline HAM-D score, age, gender, visit week, and site. In the models with MRI variables, intracranial volume was a covariate. Similar linear mixed-effects models were used to model the outcome of HAM-D score and to assess its association with each of these eight predictors.
Phase B Analyses
In the 61 participants randomized to donepezil or placebo at week 16, the χ2 test for independence and the independent sample t-tests were used to evaluate differences in demographic and clinical characteristics between sites. Similar analyses were conducted to evaluate differences between the donepezil and placebo groups. For the primary analysis of treatment effects, both ADAS-Cog 13 and SRT total immediate recall (at weeks 40, 64, and 78) were modeled using linear mixed-effects models with a random subject-specific intercept and a first-order autoregressive process within-subject covariance structure. The primary predictor in each model was treatment group, and each model was adjusted for the value of the outcome variable at week 16, age, site, and visit week. No evidence of treatment x visit week interaction was found, so the interaction terms were dropped from the final models. Similar models were fit separately for the EMCI and LMCI subgroups. Secondary analyses considered 1) the outcome of SRT delayed recall using similar mixed-effects models and 2) the outcome of FAQ scores (dichotomized at zero versus greater than or equal to one) using logistic mixed-effect models.
In an exploratory analysis to investigate potential moderators, similar linear mixed-effects models were considered. These models included additional terms for the potential moderator and the treatment by potential moderator interaction. The moderators assessed were ApoE e4 status, baseline UPSIT score, change in HAM-D over the first 16 weeks of the trial, each baseline WMH volume (high-/low-volume), hippocampal volume, and entorhinal cortex volume. In the models in which any of the MRI variables were considered as the potential moderator, intracranial volume was included as a covariate. To test for differential treatment effect, we tested if the coefficient for the interaction term in each model differed from zero. All mixed-effects models were fit using PROC GLIMMIX in SAS version 9.4 software (SAS Institute Inc., Cary, NC).
RESULTS
Phase A
Among the 79 participants (39 enrolled at NYSPI/Columbia and 40 at Duke), 50.63% met criteria for LMCI, mean age was 68.90 years (SD 9.00), 51.90% were male, baseline mean 24-item HAM-D was 23.0 (SD 5.1), mean UPSIT score was 29.4 (SD 7.7), and 29.11% had the ApoE e4 allele. The ethnic distribution was 73.42% white, 15.19% African American, 11.39% Hispanic.
During Phase A, 24.05% received citalopram for 16 weeks, 16.46% received citalopram for 8 weeks followed by venlafaxine for 8 weeks, 24.05% received venlafaxine for 16 weeks, and 35.44% who started on citalopram or venlafaxine switched to other antidepressants. Mean HAM-D decreased from 23.04 (SD5.10) at week 0 to 9.69 (SD 5.21) at week 16. Thirty-nine (63.93%) of the 61 subjects remaining at week 16 had shown response to open-label antidepressant treatment (>50% reduction in HAM-D scores).
Logistic mixed-effects models showed no evidence of association between either change in HAM-D score or antidepressant responder status and potential baseline moderators (ApoE e4 status, baseline UPSIT score, baseline WMH total volume (high-/low-volume), hippocampal volume, or entorhinal cortex volume), adjusting for baseline HAM-D score, age, gender, visit week, and site.
Phase B
Table 1 compares the baseline and week 16 characteristics of the sample of 61 randomized subjects. Characteristics of patients assigned to donepezil were similar to patients assigned to placebo, except that patients who received donepezil were younger; age was controlled statistically in all analyses.
TABLE 1.
Comparison of Characteristics for Participants Randomized to Donepezil or Placebo
| Variable | Variable Category |
Donepezll (n = 30) |
Placebo (n = 31) |
|
|---|---|---|---|---|
| Mean (SD) or % |
Mean (SD) or % |
Test Statistic & p Value |
||
| Site | NYSPI | 40.00% | 58.06% | |
| Age (years) | 67.10 (7.66) | 72.39 (8.90) | t(59) = −2.48 p = 0.016 | |
| Gender | Female | 53.33% | 48.39% | |
| Race | Caucasian | 70.00% | 77.42% | |
| Black/African American | 16.67% | 9.68% | ||
| Hispanic | 13.33% | 12.90% | ||
| Education (years) | 14.83 (2.95) | 15.90 (2.76) | t(59) = −1.46 p = 0.149 | |
| ApoE e4 Allele | Present | 41.38% | 29.03% | |
| HAM-D Week 0 | 22.70 (4.75) | 23.06 (6.04) | t(59) = −0.26 p = 0.794 | |
| HAM-D Week 16 | 8.73 (5.27) | 10.61 (5.07) | t(59) = −1.42 p = 0.161 | |
| HAM-D Change (week: 0–16) | −13.97 (7.01) | −12.45 (7.98) | t(59) = −0.79 p = 0.434 | |
| UPSIT (range: 0–40), Week 0 | 29.87 (8.34) | 28.61 (6.89) | t(59) = 0.64 p = 0.526 | |
| MMSE (range: 0–30), Week 0 | 27.50 (2.21) | 28.13 (1.67) | t(59) = 1.26 p = 0.216 | |
| ADAS-Cog Week 16 | 12.57 (5.52) | 13.87 (7.54) | t(59) = −0.75 p = 0.443 | |
| SRT Total Week 16 | 44.53 (11.27) | 46.58 (11.27) | t(59) = −0.71 p = 0.481 | |
| SRT Delay Week 16 | 7.40 (3.23) | 7.35 (2.82) | t(59) = 0.06 p = 0.954 | |
| Hippocampal Volume | 3.98 (0.78) | 3.80 (0.36) | t(48) = 0.52 p = 0.609 | |
| Entorhinal Cortex | 0.30 (0.07) | 0.31 (0.08) | t(48) = −0.49 p = 0.622 |
Notes: MRI at baseline was in 56 patients, of whom 50 patients were in the randomized sample. ADAS-Cog used was 13-item; HAM-D used was 24-item; SRT used was 12-item, 6-trial. Folstein MMSE range: 0–30; p values from t-test for continuous variables and χ2 test for categorical variables (degrees of freedom provided).
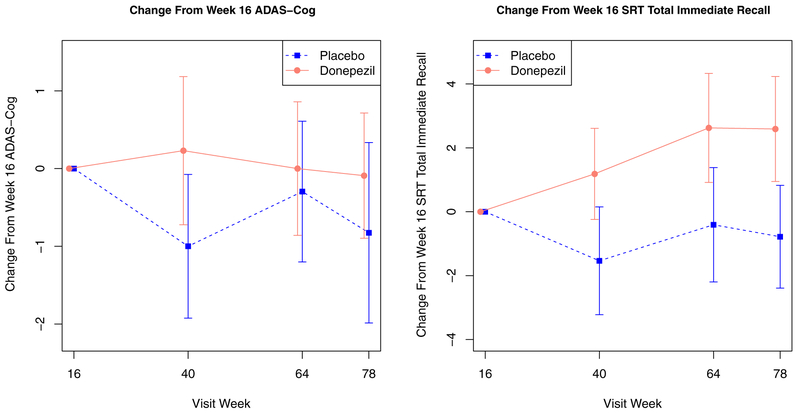
The final mean donepezil daily dose was 6.5 mg (SD4.4). Estimates derived from the linear mixed-effects models showed no evidence of a treatment effect for donepezil versus placebo on either ADAS-Cog (t(93) = 1.54, p = 0.13) or SRT total immediate recall (t(94) = 0.42, p = 0.68) (Figure 2). HAM-D scores at week 16 did not differ between patients assigned to donepezil and placebo, and the lack of treatment effect for donepezil versus placebo remained after including the HAM-D score at week 16 as a covariate. In the subsample of study completers by 78 weeks (n = 45), there was no treatment effect for donepezil versus placebo on either efficacy measure. In secondary analyses, patients assigned to donepezil and placebo did not differ in SRT delayed recall (t(94) = −0.55, p = 0.58).
FIGURE 2.
Raw mean (±1 SE) ADAS-Cog (left) and SRT total immediate recall (right) in the donepezil and placebo groups over the add-on donepezil versus placebo treatment phase. Higher ADAS-Cog score and lower SRT score indicate worse cognitive performance.
There was no difference in change in FAQ scores (dichotomized at less than or equal to one) over time for donepezil versus placebo (t(96) = 0.73, p = 0.47). Of the four patients diagnosed with dementia (all AD) during the trial, three had LMCI and one had EMCI; two were on donepezil and two were on placebo. These small numbers precluded detailed analysis of transition to dementia as an outcome.
Site by Treatment
Participants at the NYSPI/Columbia site were more racially diverse (47% non-Caucasian) and had worse cognitive scores at randomization than at Duke (7% non-Caucasian). There was no treatment by site interaction with respect to the efficacy of donepezil.
Effect of Severity of Baseline Cognitive Impairment
In subset analyses in which EMCI (n = 27) and LMCI (n = 34) classified subjects were examined separately, donepezil did not show significant differences from placebo in efficacy.
In exploratory analyses, we examined potential moderators for each of the primary outcomes. Tables 2 and 3 present model-based treatment effect estimates at different levels of the categorical and continuous moderators, respectively. Specific WMH measures were associated with the donepezil group showing greater worsening than placebo in SRT total immediate recall and ADAS-Cog (Table 2). Apolipoprotein E e4 genotype was not associated with any difference between donepezil and placebo in ADAS-Cog or SRT total immediate recall (Table 2). HAM-D scores at randomization, olfaction (UPSIT) scores, hippocampal and entorhinal cortex volumes were not associated with differences in cognitive outcomes on donepezil versus placebo (Table 3). Change in HAM-D scores during the randomized phase did not reveal any significant difference between donepezil and placebo treatment. There were no treatment group differences with respect to medical burden (Cumulative Illness Rating Scale for Geriatrics scores), number of medications taken, or anticholinergics.
TABLE 2.
Binary Moderator Analysis: Treatment Effect at Both Levels of Each Binary Moderator Compares Mean Change in Uutcome (Either ADAS-Cog or SRT Total Immediate Recall) Between Donepezil and Placebo Groups, Adjusting for Baseline Outcome Value, Age, Site, Visit Week, and Intracranicl Volume for MRI Measures
| Outcome Measure | Binary Moderator |
Treatment Effect In ApoE e4 + or High-Volume Group Mean (SE); n |
Treatment Effect In ApoE e4- or Low-Volume Group Mean (SE); n |
Test Statistic & p Value for Interaction |
|---|---|---|---|---|
| ADAS-Cog | ApoE e4+ Status | 2.53 (1.80); n = 21 | 1.19 (1.30); n = 39 | F(1,93) = 0.38 p = 0.538 |
| Deep White Matter HI | 4.24 (1.69);n = 23b | 0.74 (1.59); n = 26 | F(1,78) = 2.39 p = 0.126 | |
| Periventricular HI | 3.11 (1.69);n =24a | 1.81 (1.66); n = 25 | F(1,78) = 0.31 p = 0.576 | |
| Subcortical Grey HI | 8.00 (2.24);n = 12b | 0.94 (1.20); n = 37 | F(1,78) = 8.18 p = 0.005 | |
| Total HI | 3.49 (1.71);n = 24b | 1.68 (1.63); n = 25 | F(1,78) = 0.63 p = 0.432 | |
| SRT Total Immediate Recall | ApoE e4 Status | −0.52 (3.23); n = 21 | 1.51 (2.42); n = 39 | F(1,94) = 0.27 p = 0.603 |
| Deep White HI | −4.92 (2.95); n =23a | 6.35 (2.77);n = 26b | F(1,78) = 8.54 p = 0.005 | |
| Periventricular HI | −2.65 (3.03); n = 24 | 4.99 (2.97);n =25a | F(1,78) = 3.52 p = 0.064 | |
| Subcortical Grey HI | −3.89 (4.55); n = 12 | 2.39 (2.45); n = 37 | F(1,77) = 1.60 p = 0.210 | |
| Total HI | −2.95 (3.07); n = 24 | 5.03 (2.93);n =25a | F(1,78) = 3.86 p = 0.053 |
Notes: In the randomized sample, 50 patients had brain MRI, and assessment of HI could be conducted in 49 of these 50 patients. Tests for treatment effect within moderator subgroups are t-test contrasts estimated from the corresponding mixed-effects models. Degrees of freedom for each t-test are the same as the denominator degrees of freedom for each F statistic shown in the p value for interaction column. HI: Hyperintensity volume; SE: standard error.
p <0.10 for treatment effect within specified group.
p <0.05 for treatment effect within specified group.
TABLE 3.
Continuous Variables Moderator Analysis: Treatment Effect at 1 SD Below Mean Value of the Moderator, Mean Value of the Moderator, and 1 SD Above the Mean Value of the Moderator Compares Mean Change in Outcome (Either ADAS-Cog or SRT Total Immediate Recall) Between Donepezil and Placebo Groups, Adjusting for Baseline Outcome Value, Age, Site, Visit Week, and Intracranial Volume for MRI Measures
| Outcome Measure | Continuous Moderator |
Treatment Effect at 1 SD Below Moderator Mean Mean (SE) |
Treatment Effect at Moderator Mean Mean (SE) |
Treatment Effect at 1 SD Above Moderator Mean Mean (SE) |
Test Statistic & p Value for Interaction |
|---|---|---|---|---|---|
| ADAS-Cog | Baseline UPSIT | 0.11 (1.53) | 1.63 (1.06) | 3.15 (1.54)b | F(1,93) = 1.85 p = 0.177 |
| HAM-D Change | 2.26 (1.45) | 1.63 (1.05) | 1.01 (1.51) | F(1,93) = 0.36 p = 0.550 | |
| Hippocampal Volume | 1.43 (1.92) | 2.11 (1.17)a | 2.80 (1.85) | F(1,80) = 0.21 p = 0.646 | |
| Entorhinal Cortex Volume | 2.33 (1.64) | 2.34 (1.22)a | 2.36 (1.71) | F(1,79) = 0.00 p = 0.990 | |
| SRT Total Immediate Recall | Baseline UPSIT | 2.95 (2.82) | 0.77 (1.99) | −1.41 (2.86) | F(1,94) = 1.15 p = 0.286 |
| HAM-D Change | −1.50 (2.74) | 0.81 (2.00) | 3.12 (2.81) | F(1,94) = 1.44 p = 0.234 | |
| Hippocampal Volume | 2.61 (3.26) | 1.98 (2.07) | 1.35 (3.36) | F(1,80) = 0.06 p = 0.807 | |
| Entorhinal Cortex Volume | 0.84 (3.08) | 1.91 (2.33) | 2.98 (3.22) | F(1,80) = 0.25 p = 0.616 |
Notes: HAM-D used is 24-item; HAM-D change: 0–16 weeks in open-label phase. ADAS-Cog used is modified 13-item. Tests for treatment effect at each moderator value are t-tests for treatment effect contrasts estimated from the corresponding mixed-effects models. Degrees of freedom for each t-test are the same as the denominator degrees of freedom for each F statistic shown in the p value for interaction column. SE: standard error.
p <0.10 for treatment effect.
p <0.05 for treatment effect.
Discontinuations and Side Effects
There were 10 serious adverse events in the donepezil group and 11 serious adverse events in the placebo group, with no specific serious adverse event occurring in more than two participants per group. Nonserious adverse events that were more common with donepezil than placebo were diarrhea (40% versus 19%), headache/dizziness (23% versus 16%), fatigue/insomnia (27% versus 16%), and nightmares (13% versus 6%). Falls were more common on placebo than donepezil (16% versus 7%).
DISCUSSION
This is the first long-term randomized clinical trial of cholinesterase inhibitors in patients presenting with both depression and cognitive impairment. As expected, patients responded to antidepressant treatment in the open treatment phase.37 There were no robust biomarker predictors of antidepressant response.
Donepezil did not show superiority over placebo for the cognitive outcome measures. Few patients transitioned to dementia; this finding stands in contrast to earlier pilot studies in which there was an increased rate of dementia during follow-up.19 Differences in sample selection may partly account for the difference, though we did not observe efficacy of donepezil in the LMCI group, a group that is essentially synonymous with amnestic MCI. A study of galantamine, another ChEI, in patients with major depression who were not identified as cognitively impaired also did not find evidence of efficacy in either mood or cognition.38 In a study of donepezil in older adults with major depression, there was transient efficacy of donepezil in a subgroup that combined amnestic and nonamnestic MCI,16 but patients were selected for having major depression, and only a minority had cognitive impairment.
The lack of improvement with ChEI may be partly related to the heterogeneity in etiology. Possible etiologies include depression with reversible cognitive impairment, cerebrovascular disease leading to “vascular depression,”29,39 and Alzheimer disease. Apolipoprotein E e4 is a known risk factor, and hippocampal and entorhinal cortex atrophy is a known biomarker for MCI due to AD, but there was no interaction effect between any of these measures and donepezil treatment effect. The results indirectly support other studies showing that donepezil is ineffective in MCI due to AD.13,14 Several measures of MRI hyperintensities, which are indirect markers of cerebrovascular disease, were associated with worsening on donepezil compared to placebo. Donepezil has been reported to have some efficacy in vascular dementia, and possibly vascular cognitive impairment, but diagnostic separation from AD was not conducted systematically in those studies.40 These findings from the donepezil treatment of older adults with cognitive impairment and depression study suggest that the utility of donepezil for treating cognitive impairment in patients with significant cerebrovascular disease may need further investigation.
Moderate sample size was a limitation. The small and nonsignificant superiority of placebo over donepezil in the analyses of the main cognitive outcome measures indirectly suggests that a larger sample size is unlikely to have revealed efficacy of donepezil. The definition of depression and cognitive impairment was sufficiently broad to establish clinical generalizability, but the resultant heterogeneity may have reduced the likelihood of observing treatment effects. Participants at the two sites had similar demographics, other than race, which was expected given the population base at each site. Another limitation is the type I error rate for the analysis of the moderators, as seen in Tables 2 and 3. Out of 18 tests, only 2 are significant. This is not significantly different from what would be expected by chance using an exact test with a null hypothesis of E (“significant”) to 0.05 and assuming independence. Depression severity was very similar at the two sites. Participants at Duke showed less severe cognitive impairment, but the magnitude of the donepezil versus placebo effect did not differ by site. Dropout was above 20%, but this is not unexpected in a study of long duration.
CONCLUSIONS
The study findings are consistent with the largely negative studies of ChEI treatment of MCI in which major depression was typically excluded13,14 and suggest that donepezil cannot be recommended to treat cognitive impairment in patients with comorbid depression and cognitive impairment, including patients who meet criteria for amnestic MCI. Given the high prevalence of comorbid depression with cognitive impairment in older adults and the increased risk of dementia posed by both conditions, the findings highlight the need to prioritize discovery of novel treatments for this clinically challenging population.
Article Highlights.
A long-term randomized trial of adjunctive donepezil for comorbid depression and cognitive impairment in 79 patients on stable antidepressant treatment.
No treatment group differences between donepezil and placebo on the cognitive outcome measures of ADAS-Cog, SRT total immediate recall, and FAQ.
Study results do not support the current common practice of adjunctive off-label cholinesterase inhibitor use in patients with comorbid depression and cognitive impairment.
Acknowledgments
Davangere P. Devanand, M.D., has received advisory board fees from Acadia, Axovant, Eisai, and Genentech. He has received research support from Avanir. Pudugramam Murali Doraiswamy (PMD), M.D., has received research grants (through Duke University) from Avid, Lilly, Neuronetrix, Avanir, FORUM, and the Alzheimer’s Drug Discovery Foundation in recent years. PMD has also received speaking or advisory fees from Anthrotronix, Cognoptix, Takeda, Genomind, Targacept, NeuroCog Trials, FORUM, T3D Therapeutics, Hintsa, MindLink, Global Alzheimer’s Platform Foundation, and NeuroPro for other projects in recent years. PMD owns shares in Maxwell Health, Muses Labs, Anthrotronix, Evidation Health, Turtle Shell Technologies, and Advera Health Analytics, whose products are not discussed here. PMD is a co-inventor on patents relating to dementia biomarkers that are unlicensed. Gregory H. Pelton, M.D.; Adam Ciarleglio, Ph.D.; Howard Andrews, Ph.D.; Julia Lunsford, M.D.; John L. Beyer, M.D.; Jeffrey R. Petrella, M.D.; and Joel Sneed, Ph.D., report no competing interests. Kristina D’Antonio, M.S.W.; Jennifer Scodes, M.S.; and Michaela Ciovacco, B.A., also report no competing interests. This study is funded by the National Institute on Aging, grants R01AG040093 and R01AG041795.
References
- 1.Hanninen T, Hallikainen M, Koivisto K, et al. : A follow-up study of age-associated memory impairment: neuropsychological predictors of dementia. J Am Geriatr Soc 1995; 43:1007–1015 [DOI] [PubMed] [Google Scholar]
- 2.Jonker C, Geerlings MI, Schmand B: Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000; 15:983–991 [DOI] [PubMed] [Google Scholar]
- 3.Blazer D, Hughes DC, George LK: The epidemiology of depression in an elderly community population. Gerontologist 1987; 27:281–287 [DOI] [PubMed] [Google Scholar]
- 4.Burt DB, Zembar MJ, Niederehe G: Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull 1995; 117:285–305 [DOI] [PubMed] [Google Scholar]
- 5.Arve S, Tilvis RS, Lehtonen A, et al. : Coexistence of lowered mood and cognitive impairment of elderly people in five birth cohorts. Aging (Milano) 1999; 11:90–95 [PubMed] [Google Scholar]
- 6.Charney DS,Reynolds CF III,Lewis L,et al. :Depression and Bipolar Support Alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry 2003; 60:664–672 [DOI] [PubMed] [Google Scholar]
- 7.Alexopoulos GS: Depression in the elderly. Lancet 2005;365:1961–1970 [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Barnes LL, Mendes de Leon CF, et al. : Depressive symptoms, cognitive decline,and risk of AD in older persons. Neurology 2002; 59:364–370 [DOI] [PubMed] [Google Scholar]
- 9.Panza F, Frisardi V, Capurso C, et al. : Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry 2010; 18:98–116 [DOI] [PubMed] [Google Scholar]
- 10.Devanand DP, Sano M, Tang MX, et al. : Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry 1996; 53:175–182 [DOI] [PubMed] [Google Scholar]
- 11.Mourao RJ, Mansur G, Malloy-Diniz LF, et al. : Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: systematic review and meta-analysis. Int J Geriatr Psychiatry 2016; 31:905–911 [DOI] [PubMed] [Google Scholar]
- 12.Manning KJ, Alexopoulos GS, Banerjee S, et al. : Executive functioning complaints and escitalopram treatment response in late-life depression. Am J Geriatr Psychiatry 2015; 23:440–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen RC: Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256:183–194 [DOI] [PubMed] [Google Scholar]
- 14.Salloway S, Ferris S, Kluger A, et al. : Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 2004; 63:651–657 [DOI] [PubMed] [Google Scholar]
- 15.Lu PH, Edland SD, Teng E, et al. : Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology 2009; 72:2115–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds CF III, Butters MA, Lopez O, et al. : Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry 2011; 68:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devanand DP, Pelton GH, Marston K, et al. : Sertraline treatment of elderly patients with depression and cognitive impairment. Int J Geriatr Psychiatry 2003; 18:123–130 [DOI] [PubMed] [Google Scholar]
- 18.Nebes RD, Pollock BG, Houck PR, et al. : Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res 2003; 37:99–108 [DOI] [PubMed] [Google Scholar]
- 19.Pelton GH, Harper OL, Tabert MH, et al. : Randomized double-blind placebo-controlled donepezil augmentation in antidepressant-treated elderly patients with depression and cognitive impairment: a pilot study. Int J Geriatr Psychiatry 2008; 23:670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelton GH, Andrews H, Roose SP, et al. : Donepezil treatment of older adults with cognitive impairment and depression (DOTCODE study): clinical rationale and design. Contemp Clin Trials 2014; 37:200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu Y, Li L, Zhou TY, et al. : Alzheimer’s disease progression model based on integrated biomarkers and clinical measures. Acta Pharmacol Sin 2014; 35:1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devanand DP, Lee S, Manly J, et al. : Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 2015; 84:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts RO, Christianson TJ, Kremers WK, et al. : Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol 2016; 73:93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelton GH, Soleimani L, Roose SP, et al. : Olfactory deficits predict cognitive improvement on donepezil in patients with depression and cognitive impairment: a randomized controlled pilot study. Alzheimer Dis Assoc Disord 2016; 30:67–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devanand DP, Lentz C, Chunga RE, et al. : Change in odor identification impairment is associated with improvement with cholinesterease inhibitor treatment in mild cognitive impairment. J Alzheimers Dis 2017; 60:1525–1531 [DOI] [PubMed] [Google Scholar]
- 26.Krishnan KR, Charles HC, Doraiswamy PM, et al. : Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s disease. Am J Psychiatry 2003; 160:2003–2011 [DOI] [PubMed] [Google Scholar]
- 27.Devanand DP, Pradhaban G, Liu X, et al. : Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology 2007; 68:828–836 [DOI] [PubMed] [Google Scholar]
- 28.Chung JK, Plitman E, Nakajima S, et al. : Lifetime history of depression predicts increased amyloid-beta accumulation in patients with mild cognitive impairment. J Alzheimers Dis 2015; 45:907–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexopoulos GS, Meyers BS, Young RC, et al. : Clinically defined vascular depression. Am J Psychiatry 1997; 154:562–565 [DOI] [PubMed] [Google Scholar]
- 30.Steffens DC, Otey E, Alexopoulos GS, et al. : Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry 2006; 63:130–138 [DOI] [PubMed] [Google Scholar]
- 31.Albert MS, DeKosky ST, Dickson D, et al. : The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association work-groups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MD, Paradis CF, Houck PR, et al. : Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 1992; 41:237–248 [DOI] [PubMed] [Google Scholar]
- 33.Pfeffer RI, Kurosaki TT, Harrah CH Jr, et al. : Measurement of functional activities in older adults in the community. J Gerontol 1982; 37:323–329 [DOI] [PubMed] [Google Scholar]
- 34.Fazekas F, Chawluk JB, Alavi A, et al. : MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 1987; 149:351–356 [DOI] [PubMed] [Google Scholar]
- 35.Rorden C, Brett M: Stereotaxic display of brain lesions. Behav Neurol 2000; 12:191–200 [DOI] [PubMed] [Google Scholar]
- 36.Pimontel MA, Reinlieb ME, Johnert LC, et al. : The external validity of MRI-defined vascular depression. Int J Geriatr Psychiatry 2013; 28:1189–1196 [DOI] [PubMed] [Google Scholar]
- 37.Sheline YI, Disabato BM, Hranilovich J, et al. : Treatment course with antidepressant therapy in late-life depression. Am J Psychiatry 2012; 169:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holtzheimer PE III, Meeks TW, Kelley ME, et al. : A double blind, placebo-controlled pilot study of galantamine augmentation of antidepressant treatment in older adults with major depression. Int J Geriatr Psychiatry 2008; 23:625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnan KR, Taylor WD, McQuoid DR, et al. : Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry 2004; 55:390–397 [DOI] [PubMed] [Google Scholar]
- 40.Malouf R, Birks J: Donepezil for vascular cognitive impairment. Cochrane Database Syst Rev 2004; (1):CD004395 [DOI] [PubMed] [Google Scholar]