Abstract
Recent studies of perfluoroalkylated substances (PFASs) have focused on the toxicity of long chain PFASs, such as PFOS or PFOA, which have been demonstrated to cause an array of developmental and behavioral effects. However, less is known about low molecular weight PFASs and alternatives. This study examined the morphometric and behavioral effects in zebrafish following developmental exposures of C6 PFASs: perfluorohexanoic acid, PFHxA, perfluorohexane sulfonate, PFHxS, and 6:2 fluorotelomer alcohol, 6:2 FTOH. Embryos were exposed to 0.02 to 20 μM concentrations of these compounds from the high stage (~3 hours post fertilization, hpf) until 120 hpf. Morphometric and gene expression endpoints were examined at 120 hpf. Genes selected for analysis were previously shown to be altered in zebrafish developmentally exposed to PFOS and PFOA. Additionally, exposed larvae were transferred to clean water and reared until 14 days post fertilization, dpf, when behavioral assays were completed and morphometric endpoints examined. While PFHxA was found to be the most acutely toxic at 120 hpf, few morphometric effects were observed. Gene expression was the most sensitive endpoint with significant increased tgfb1a, bdnf, and ap1s1 expression observed with PFHxA exposure. PFHxS exposure produced morphometric effects in the larvae, specifically increased length and yolk sac area at 2 and 20 μM. This phenotype persisted to the 14 dpf time point, where these larvae additionally displayed decreased distance traveled and crosses through the center of the arena of the behavioral assay. Exposure to 6:2 FTOH caused no morphometric effects at 120 hpf, and this compound was the least acutely toxic. However, expression of both tgfb1a and bdnf were increased by greater than 2 fold change at this time point. Effects also persisted to 14 dpf where a significant increase in distance traveled and velocity were observed in the behavioral assay. This study demonstrates effects on behavioral, morphometric and gene expression endpoints with developmental PFHxA, PFHxS, and 6:2 FTOH exposures in zebrafish.
Keywords: PFHxA, PFHxS, 6:2 FTOH, PFAS, zebrafish
1. Introduction
Perfluoroalkylated substances, PFASs, are a class of chemicals that continue to raise environmental and health concerns throughout the world. Their extensive use and structural variations in commercial products for manufacturing, their persistence in the environment, and the lack of toxicological data pose a serious data gap. Long chain PFASs in particular perfluorooctane sulfonate, PFOS, and perfluorooctanoic acid, PFOA, have been demonstrated to cause toxicity in a variety of model organisms, including hepatic, developmental, reproductive, and behavioral effects (Lau 2012; Li et al. 2017; Negri et al. 2017; Post et al. 2012; Tsuda 2016).
Due to safety concerns, companies have voluntarily switched to other compounds, including low molecular weight alternatives (Lehmler et al. 2007; Wang et al. 2013). The present study focuses on the low molecular weight PFASs, perfluorohexanoic acid, PFHxA, perfluorohexane sulfonate, PFHxS, and 6:2 fluorotelomer alcohol, 6:2 FTOH (Table 1). The fluorotelomer alcohol was selected to serve as a chain length control, as it has been shown to be readily metabolized and excreted, and because of its continued industrial use (Martin et al. 2005). In general, these compounds have shorter serum half-lives than the long chain PFASs and are therefore deemed safer for the environment (Conderet al. 2008; Lau 2012). These compounds have been detected in ground and surface water supplies across the world (Ahrens 2011; Pan et al. 2018). Additionally, PFHxS was detected in low concentrations in a variety of species; however, PFHxA levels are often below the limit of detection in environmental tissue samples (Kannan et al. 2005; Sedlak et al. 2017). Detection of these compounds in humans has been reported (Lewis et al. 2015; Olsen et al. 2017; Perez et al. 2013; Siebenaler et al. 2017), but due to increasing concerns of the bioaccumulation and toxicity associated with PFHxS, serum levels of this compound have plateaued (Land et al. 2018). PFHxA was reported in fish fillet collected from the Mississippi in 9-15% of samples at concentrations ranging from 0.12-0.18ng/g, depending on species, and PFHxS in 7-55% of samples ranging from 0.032 – 0.069 ng/g (Newsted et al. 2017).
Table 1.
Chemical structures of perfluorohexanoic acid (PFHxA), perfluorohexane sulfonate (PFHxS), and 6:2 fluorotelomer alcohol (6:2 FTOH).
| STRUCTURE | CAS NUMBER | MOLECULAR WEIGHT | |
|---|---|---|---|
| PFHxA |  |
307-24-4 | 315.05 |
| PFHxS |  |
3871-99-6 | 438.20 |
|
6:2 FTOH |
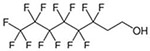 |
647-42-7 | 364.1 |
Both PFOS and PFOA have been demonstrated to act as developmental toxicants eliciting a wide array of effects, including reduced growth and offspring survivability in rodent models (Negri et al. 2017; Tsuda 2016). PFOS and PFOA exposures have also been reported to cause abnormal liver, behavioral, and reproductive functions. Fewer studies exist examining the toxicity of low molecular weight PFASs compared to the long chain PFASs; however, there has been some overlap in observed effects following exposure to the low molecular weight PFAS alternatives. Both PFHxA and PFHxS exposures caused liver hypertrophy in adult rats (Bijland et al. 2011; Butenhoff et al. 2009; Chang et al. 2018; Chengelis et al. 2009; Klaunig et al. 2014). PFHxS may also be associated with nervous system toxicity. A single exposure at postnatal day 10 in mice was shown to cause behavioral effects at 2 months of age (Viberg et al. 2013). However, other studies of PFHxS, up to 250 mg/kg, in rodents have reported no effects on behavioral outcomes (Butenhoff et al. 2009; Chang et al. 2018). In addition, behavioral effects have not been observed in rodents with PFHxA or 6:2 FTOH exposures (Chengelis et al. 2009; Klaunig et al. 2014; O'Connor et al. 2014; Serex et al. 2014).
Studies in zebrafish, including those from our lab, have demonstrated that developmental exposures to long chain PFASs, PFOS and PFOA, impact a variety of endpoints, including developmental abnormalities, changes in yolk sac size, delayed hatching, and alterations in the development of the swim bladder, liver, and muscle fibers (Chen et al. 2014; Cheng et al. 2016; Huang et al. 2010; Jantzen et al. 2016a; Zheng et al. 2011). In addition, both PFOS and PFOA have been demonstrated to alter behavioral endpoints in larval zebrafish (Hagenaars et al. 2014; Jantzen et al. 2016a; Ulhaq et al. 2013). In order to organize a similar array of data presented in this study, results will be discussed in terms of adverse outcomes pathways (AOPs). Specifically, this study will focus on growth and behavioral AOPs in terms of endpoints at the individual level as well as identifying possible molecular effects through targeted gene expression analysis.
Candidate genes for gene expression analysis were selected based on previous findings from larval and adult developmental and chronic PFAS exposures (Jantzen et al. 2016a; Jantzen et al. 2016b; Jantzen et al. 2017). These include the organic anion transporters slco2b1 and slco1d1, which are transporters and/or inhibitors of PFASs (Popovic et al. 2014; Popovic et al. 2010; Zhao et al. 2017). The remaining genes, bdnf, tgfb1a, and ap1s1, are related to central nervous, muscle, and lateral line development, respectively. Gene expression has been coupled with behavioral and morphometric analysis to determine the toxicity profiles of developmental exposures to PFHxA, PFHxS, and 6:2 FTOH in the zebrafish.
2. Materials and methods
2.1. Zebrafish Husbandry:
Zebrafish, strain AB (Zebrafish International Resource Center, Eugene, OR) were housed in Aquatic Habitat (Apopka, FL) recirculating systems. Sand and activated carbon filtered municipal water was used as source water. Water quality was monitored monthly to ensure water was maintained at <0.05 ppm nitrite, <0.2 ppm ammonia, DO, and 7.2-7.7 pH. The temperature was monitored twice daily to ensure that tank water was held between 26 and 28 °C. Fish were maintained on a 14:10 hour light: dark cycle, and fed twice daily a diet of artemia in the mornings and aquatox/tetramin flake mix in the evenings. All experiments were conducted following Rutgers University Animal Care and Facilities Committee guidelines under the zebrafish husbandry and embryonic exposure protocol (08-025).
2.2. Experimental Setup
Stock solutions of PFHxA (perfluorohexanoic acid, Oakwood Chemical, Estill, SC), 6:2 FTOH (1H,1H,2H,2H-perfluorooctan-1-ol, Sigma Aldrich, St. Louis MO), and PFHxS (trideafluorohexane-1-sulfonic acid potassium salt, Sigma Aldrich, St. Louis MO) were prepared at concentrations of 2000 μM in egg water, which is 60 μg/mL stock salts in distilled water. Table 1 contains the chemical structure of these compounds. Embryos were exposed to static, non-renewed, nominal concentrations of each compound. Figure 1 outlines the exposure paradigm, modified from OECD 212 (OECD 1998). For each treatment, healthy embryos were selected at approximately 3 hpf (hours post fertilization) where staging ranged from the 1000 cell to high stage (Kimmel et al. 1995). Exposures were continued to 120 hpf, or 5 days post fertilization (dpf), when hatched larvae were in the sac fry stage. For behavioral studies, larvae were transferred into treatment-free water and reared until analysis was completed on larvae at 14 dpf. All experiments had >85% control survival.
Figure 1.
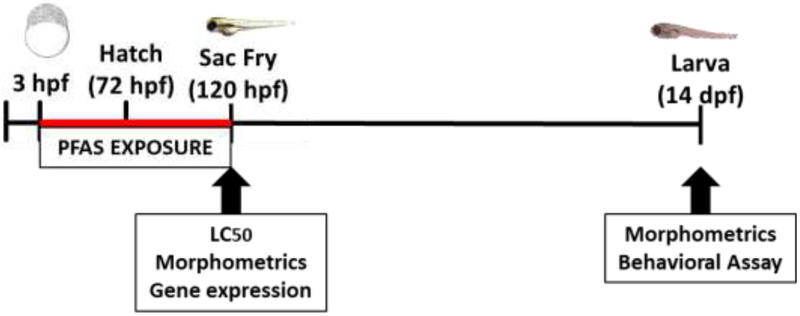
Exposure paradigm of all experiments. Waterborne PFAS exposures began at 3 hours post fertilization (hpf) and continued until 120 hpf. Endpoint examined in the sac fry larvae include LC50 determination, morphometrics, and gene expression. Larvae at 14 days post fertilization (dpf) were reared in treatment-free water until behavioral endpoints were assessed.
2.3. LC50Determination
Embryos at 3 hpf were placed into individual glass vials containing 1 mL of nominal concentrations of PFHxA, PFHxS, and 6:2 FTOH ranging from 0-1000 μM. Fifteen embryos were selected per treatment group, and embryos were monitored daily with total mortality established at 5 dpf. Experimental replicates were completed with all doses of one compound, and each experiment was independently replicated 3 times. Determination of LC50 values was completed using log probability paper and confidence intervals as outlined in the following (Litchfield and Wilcoxon 1948). Confidence intervals (95%) were determined as the doses corresponding to the 16 and 84% effect level based on the best-fit line.
2.4. Morphometric Analysis
Doses of 0, 0.02, 0.2, and 2 μM PFAS were selected to remain below the LC50 determined in the present study, which ranged from 290-830 μM (Figure 2) and align with doses of long chain PFASs tested in the zebrafish model (Jantzen et al. 2016a). A high dose (20 μM) study was carried out after the initial low dose study. For both studies fifteen embryos were individually reared in glass vials and exposed to 1 mL of treated or control egg water. Static, non-renewed exposures were continued until 120 hpf, at which point egg water was removed and replaced with 10% buffered formalin to fix the larvae for further analysis. The fixed larvae were stained following an acid-free alcian blue- alizarin red protocol, which allows for visualization of the cartilage and yolk membranes of the larvae (Walker and Kimmel 2007).
Figure 2.
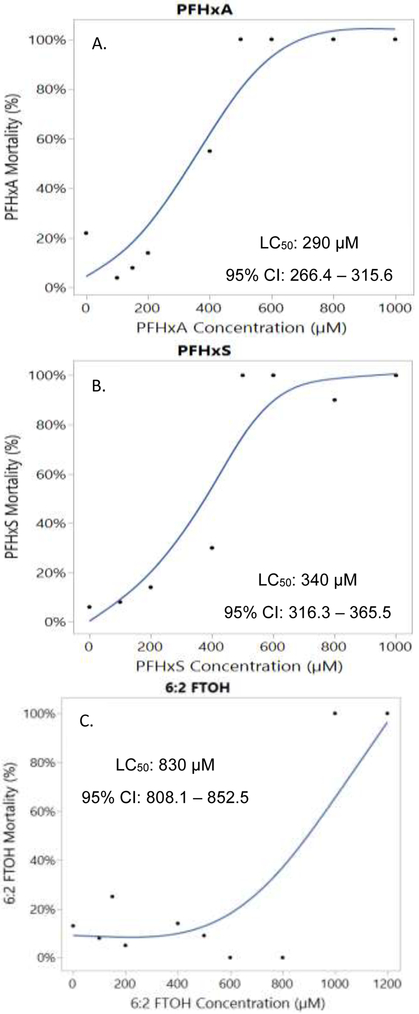
Dose response mortality curves for (A) PFHxA, (B) PFHxS, and (C) 6:2 FTOH at 5 dpf. Indicated on the figures are the calculated LC50 and 95% confidence intervals (CI).
Photographs of each sample were collected using an Olympus SZ-PT dissecting microscope mounted with a Scion digital camera (CFW-1310C). Measurements were taken using Photoshop CS2 for the following endpoints: total body length, yolk sac area, and pericardial sac area. Measurements in pixels were normalized to ruler length in Photoshop. Three independent experiments were completed which each consisted of 15 animals per dose. Mean and standard error were calculated for each endpoint in each treatment group.
2.5. Gene Expression Analysis
Twenty-five embryos were group housed in 20 mL glass vials and exposed to 8 mL of treated 0, 0.2, 2, or 20 μM PFAS in egg water. Each experiment contained 4-5 vials per treatment. At 24 hpf, dead embryos were removed from the vials to prevent fungal growth. Static, non-renewed exposures were continued until 120 hpf, at which point pooled larvae were snap frozen in liquid nitrogen. RNA was isolated following Sigma’s RNAzol protocol. Purity was assessed using a NanoDrop 1000 Spectrophotometer (ThermoScientific), RNA with A260/280 below 1.7 were excluded from analysis. RNA concentrations were diluted to 200 ng/μL in RNase-free water. RNA was converted into cDNA using the High Capacity Reverse Transcription Kit (Applied Biosystems, ThermoFisher). Gene expression was quantified via qPCR using iQTM SYBR® Green Supermix (Biorad, Hercules CA) in a Biorad iCylcer iQ machine. The qPCR protocol used was 35 cycles of 95°C for 15 seconds and 60°C for 60 seconds. The primers used in this study are outlined in Table 2. Standard curves were used to generate transcript numbers, which were normalized to beta actin, actb, as a housekeeping gene. It was determined that actb was not altered by PFAS treatment, and these results were verified using 28s. Outliers were determined as values exceeding 2 standard deviations from the mean. All experiments were independently replicated three times, and the transcript numbers were combined to determine the mean and standard error (SEM) for each treatment.
Table 2.
Gene name, function, and primers utilized in this study.
| GENE | ACCESSION NUMBER |
FORWARD PRIMER |
REVERSE PRIMER |
FUNCTION | |
|---|---|---|---|---|---|
| actb | beta-actin | NM_131031.2 | CGAGCAGGAGATGGGAACC | CAACGGAAACGCTCATTGC | Housekeeping gene |
| slco2b1 | Solute carrier organic anion transporter family member 2b1 | NM_001037678.2 | TTGCCCTGCCTCACTTCATT | AGGCTGGAGTTGAGTCTGGT | Organic anion transporter |
| slco1d1 | Solute carrier organic anion transporter family member 1d1 | NM_001348086.1 | GCCGCATTTCTTCCAAGGAC | TGTAAGGCACGGCAGAACAT | Organic anion transporter |
| tgfb1a | Transforming growth factor beta 1a | NM_182873.1 | CCAGCAGAGCACGGATAAGT | TCATATCTGCCAGACCAGCG | Muscle development |
| ap1s1 | Adaptor related protein complex 1 sigma 1 subunit | NM_200309.2 | CCGTCGAAATGATGCGCTTT | GTACTTATCCAGCACCACCTG | Vesicle formation |
| bdnf | Brain-derived neurotrophic factor | NM_001308648.1 | AGGTCCCCGTGACTAATGGT | CGCTTGTCTATTCCTCGGCA | Neuron development |
2.6. Behavioral Analysis
Twenty-five embryos were selected and exposed 3-120 hpf to 8 mL of 0, 0.2, 2, or 20 μM PFHxA, PFHxS or 6:2 FTOH. Three to four vials were established per treatment per experiment. At 120 hpf, larvae were removed from the vials into beakers containing treatment-free system water. Larvae were reared in an incubator held between 26-27°C with 14:10 hour light: dark cycle. At 120 hpf, a feeding regiment was established, twice daily Ziegler Larval AP50 (Aquatic Habitats). At 14 dpf, the larvae were assessed for behavioral endpoints and then measured for total length. The larvae were individually transferred into 1.5 mL system water per well in a clear-bottomed 24 well plate. The treatments were randomized throughout the plate.
Plates were placed in the behavior room 1 hour prior to recording with all lights on to allow for the larvae to acclimate to the novel surroundings (Metz et al. 2006). After 1 hour, the lights were turned off and recording under infrared filter began using Noldus Ethovision (Leesburg, VA) tracking software. All experimentation was restricted to 1-4 pm, when variability due to larval circadian rhythm is minimized (MacPhail et al. 2009). Four cameras were set to record 4 plates for a 30 minute period. Two recordings were completed each afternoon. Therefore, each experimental setup was recorded on 8 plates and videos. The experimental replicate was independently repeated.
Videos were analyzed through Ethovision XT Software. The parameters analyzed were total distance traveled, cross frequency (the number of crosses through the center of the arena), and mean velocity over the 30 minute assay. Each plate was considered a plate replicate with 7-8 plates in total analyzed per compound. These were collapsed from at least 2 experimental replicates and at least 4 clutches of embryos. Outliers were defined as points outside of 3 standard deviations of the mean for all control data for each endpoint. Mean values were then determined per compound concentration per plate replicate. Statistical analysis was completed on these values. Values were separated into 10 minute time bins to differentiate initial startle response from delayed responses. Total distance, number of crosses, and mean velocity are reported for each time bin, 0-10, 10-20, or 20-30 minutes, and cumulative across the assay 30 minute assay.
2.7. Statistical Analyses
Statistical analysis was completed using SigmaPlot™ 11.0 software. One-way analysis of variance (ANOVA) were completed to compare across concentrations for each compound, followed by either a Bonferroni post hoc test. High dose morphometrics were analyzed via Mann-Whitney rank sum T test. Significance was set at p<0.05.
3. Results
3.1. Lethality and morphometric effects of low molecular weight PFAS alternatives
Within Figure 2 are summarized the LC50 values for PFHxA, PFHxS, and 6:2 FTOH at 5 dpf. PFHxA was determined to be the most toxic to the developing zebrafish with an LC50 of 290 μM. The LC50 values for PFHxS and 6:2 FTOH were 340 and 830 μM, respectively. It was observed during this study that there was a delayed onset of death with exposure to PFHxS, which occurred post-hatch between 4 and 5 dpf.
Morphometric analysis was carried out on 5 dpf sac fry larvae following low dose PFAS exposures (0, 0.02, 0.2, and 2 μM), and the results are summarized in Table 3A. Following exposure to 0.2 μM PFHxA, sac fry larvae had significantly decreased total body length and yolk sac area compared to controls. However, these effects were not dose-dependent and not observed at other doses examined. PFHxS exposure at 2 μM caused a significant increase in total body length. There was a significant decrease in total body length of larvae exposed to 0.02 μM 6:2 FTOH, but this same effect was not observed at higher concentrations. In addition, PFHxS and 6:2 FTOH did not have any significant effect on yolk sac area. Pericardial sac area was measured, and no edema or significant increases in pericardial area were observed (data not shown).
Table 3.
Measurements of total body length (mm) and yolk sac area (mm2) at 5 dpf following exposure to PFHxA, PFHxS and 6:2 FTOH. (A) Values indicate mean and standard deviation of measurements. An asterisk (*) indicates p<0.05, one-way ANOVA on ranks, N=36-40.(B) Morphometric measurement at 5 dpf following exposure to 20 μM PFHxA, PFHxS and 6:2 FTOH. Values indicate mean and standard deviation of measurements. An asterisk (*) indicates p<0.05, Mann Whitney Rank Sum test, N=38-45.
| A. | ||||
|---|---|---|---|---|
| 5 DPF MORPHOMETRIC MEASUREMENTS: LOW DOSE PFAS EXPOSURES | ||||
| TOTAL BODY LENGTH (mm) | ||||
| 0 μM | 0.02 μM | 0.2 μM | 2 μM | |
| PFHxA | 4.15 ± 0.13 | 4.23 ± 0.15 | 3.96 ± 0.18* | 4.21 ± 0.17 |
| PFHxS | 4.08 ± 0.15 | 4.02 ± 0.13 | 4.04 ± 0.12 | 4.24 ± 0.14* |
| 6:2 FTOH | 4.44 ± 0.16 | 4.34 ± 0.17* | 4.39 ± 0.15 | 4.48 ± 0.15 |
| YOLK SAC AREA (mm2) | ||||
| 0 μM | 0.02 μM | 0.2 μM | 2 μM | |
| PFHxA | 0.294 ± 0.032 | 0.300 ± 0.042 | 0.242 ± 0.054* | 0.303 ± 0.038 |
| PFHxS | 0.299 ± 0.028 | 0.286 ± 0.054 | 0.304 ± 0.041 | 0.285 ± 0.035 |
| 6:2 FTOH | 0.306 ± 0.055 | 0.298 ± 0.045 | 0.291 ± 0.036 | 0.302 ± 0.036 |
| B. | ||||
|---|---|---|---|---|
| 5 DPF MORPHOMETRIC MEASUREMENTS: 20 μM PFAS EXPOSURES | ||||
| Control | PFHxA | PFHxS | 6:2 FTOH | |
| Total Body Length (mm) | 3.97 ± 0.18 | 4.00 ± 0.24 | 4.11 ± 0.32* | 3.98 ± 0.178 |
| Yolk Sac Area (mm2) | 0.293 ± 0.043 | 0.287 ± 0.044 | 0.323 ± 0.051* | 0.303 ± 0.041 |
High dose exposures of 20 μM were also completed to determine if higher concentrations of the low molecular weight alternatives were necessary to elicit morphological effects because these compounds have been reported to be less toxic than their long chain PFAS counterparts (Wang 2015). Significant morphometric effects were observed following exposure to 20 μM PFHxS (Table 3B), where larvae were significantly larger and had significantly increased yolk sac areas compared to controls. No significant effects in total length or yolk sac area were observed following exposure to 20 μM PFHxA or 6:2 FTOH. Pericardial area was also not significantly increased with any of the experimental compounds (data not shown).
3.2. Changes in gene expression with PFAS exposures at 5 dpf
Gene expression was completed after a 5 day developmental exposure of 0, 0.2, 2, and 20 μM PFHxA, PFHxS, and 6:2 FTOH. Alterations in expression of these genes have been reported in developing zebrafish following exposure to PFOS and PFOA (Jantzen et al. 2016a). Slight changes in expression of slco transporters, slco2b1 and slco1d1, were observed in 5 dpf sac fry larvae (Figure 3). There was a small, but significant decrease (0.67 fold) in expression of slco2b1 with exposure to 2 μM PFHxA; however, at 20 μM PFHxA this trend was no longer observed (Figure 3A). Expression of slco2b1 was significantly increased (1.92 fold) in the 2 μM 6:2 FTOH treatment group. Fold change gene expression of slco1d1 did not vary significantly from controls at all concentrations of PFHxA and PFHxS (Figure 3B). There was a small (1.45 fold) significant increase in expression following exposure of 20 μM 6:2 FTOH. Overall, dose-dependent changes in slco2b1 and slco1d1 gene expression were not observed with exposures to these compounds.
Figure 3.
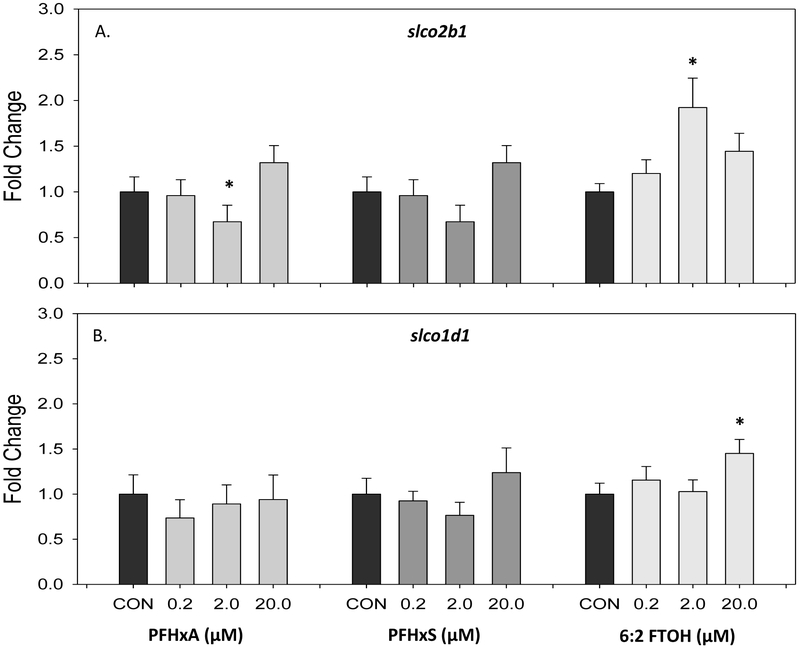
Gene expression of slco transporters at 5 dpf following exposure to 0, 0.2, 2, and 20 μM PFHxA, PFHxS, or 6:2 FTOH. Gene expression represented as mean and standard error (SEM) fold change of (A) slco2b1 (B) slco1d1. An asterisk (*) indicates p<0.05, one-way ANOVA, Bonferroni post hoc, N=8-14.
The other genes examined, tgfb1a, bdnf, and ap1s1, were more sensitive to low molecular weight PFAS exposures (Figure 4). Transforming growth factor beta 1a (tgfb1a) expression was significantly increased, between 2.04 and 2.57 fold, at all 3 doses of PFHxA examined (Figure 4A). Similarly, there was a significant dose-dependent increase in tgfb1a expression, at all doses of 6:2 FTOH, and a 2.11 fold induction in transcript level at 20 μM. This was not observed with exposure to PFHxS, where a trend of decreased tgfb1a was observed. Expression of brain-derived neurotrophic factor (bdnf) increased with developmental exposures to all low molecular weight PFAS alternatives (Figures 4B). Significant increases were observed at 20 μM PFHxA (2.69 fold) and 20 μM 6:2 FTOH (2.5 fold). Adaptor related protein complex (ap1s1) expression followed a dose-dependent increase with PFHxA exposure (Figure 4C). Expression was significantly increased from controls at 2 and 20 μM PFHxA (3.63 and 13.2 fold, respectively).
Figure 4.
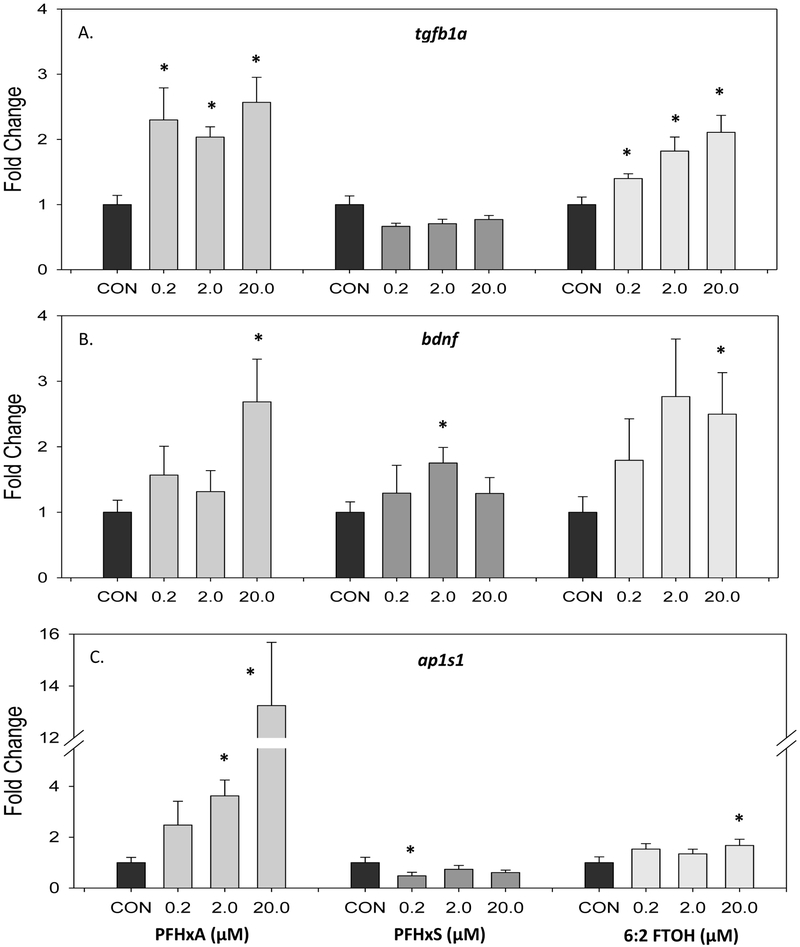
Gene expression of tgfb1a, bdnf, and ap1s1 at 5 dpf following exposure to 0, 0.2, 2, and 20 μM PFHxA, PFHxS, or 6:2 FTOH. Gene expression represented as mean and standard error (SEM) fold change of (A) tgfb1a, transforming growth factor (B) bdnf, brain-derived neurotrophic factor (C) ap1s1, adaptor related protein complex. An asterisk (*) indicates p<0.05, one-way ANOVA, Bonferroni post hoc, N=8-14.
3.3. Changes in larval morphometrics and locomotor behavior with PFAS exposures at 14 dpf
Morphometric analysis and a behavioral assay were completed at 14 dpf following a 5 day developmental exposure to 0, 0.2, 2, and 20 μM PFHxA, PFHxS, or 6:2 FTOH. Only exposure to PFHxS yielded effects on larval growth, where a significant increase in total body length occurred in the 0.2 and 2 μM treatment groups (Table 4). This same response was observed in the 5 dpf larvae exposed to PFHxS.
Table 4.
Measurements of total body length (mean ± standard deviation, in mm) at 14 dpf following developmental exposure to PFHxA, PFHxS and 6:2 FTOH. An asterisk (*) indicates p<0.05, one-way ANOVA, N=35-40.
| 14 DPF TOTAL BODY LENGTH (mm) | ||||
|---|---|---|---|---|
| Compound | 0 μM | 0.2 μM | 2 μM | 20 μM |
| PFHxA | 4.60 ± 0.18 | 4.60 ± 0.27 | 4.54 ± 0.20 | 4.66 ± 0.22 |
| PFHxS | 4.74 ± 0.25 | 4.91 ± 0.26* | 4.91 ± 0.20* | 4.85 ± 0.27 |
| 6:2 FTOH | 4.76 ± 0.24 | 4.81 ± 0.23 | 4.85 ± 0.20 | 4.74 ± 0.26 |
The 30 minute behavioral assay was divided into 10 minute time bins to differentiate between initiate startle response from the change in light stimulus and the following effects on acclimation. Figure 5 shows the mean distance traveled within each time bin as well as cumulative over the entire assay. PFHxA exposure did not affect distance traveled (Figure 5A). Following 20 μM PFHxS exposure, there was a significant decrease in total distance traveled during the behavioral assay, cumulatively, as well as in the 0-10 and 10-20 minute time bins (Figure 5B). Exposure to 6:2 FTOH increased distance traveled, where a significant increase in distance was observed at 2 μM 6:2 FTOH in the 0-10 minute time bin and cumulatively (Figure 5C).
Figure 5.
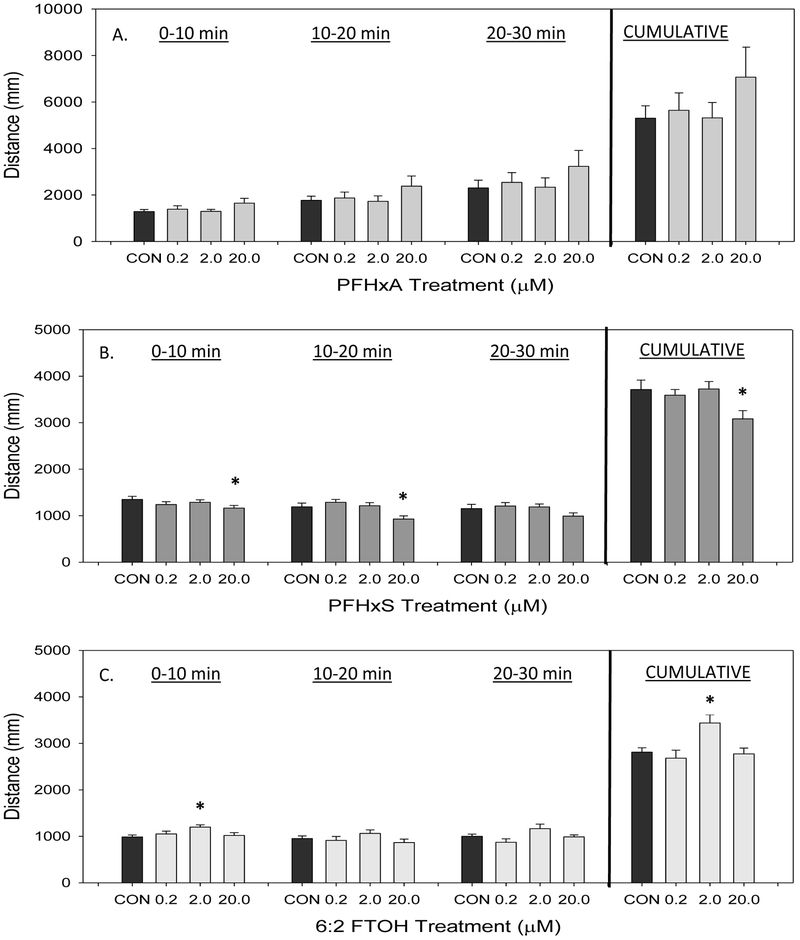
Distance traveled (mm) by developmentally PFHxA-, PFHxS-, or 6:2 FTOH-exposed larvae at 14 dpf. Data collected over a 30 minute period are divided into 10 minute time bins. Cumulative distance over the 30 minute assay is shown in the last column set. Values are reported as mean and SEM for each time bin with (A) PFHxA treatment (B) PFHxS treatment (C) 6:2 FTOH treatment. An asterisk (*) indicates p<0.05, one-way ANOVA, N=7-8.
Cross frequency of the larvae throughout the assay is shown in Figure 6. Exposure to PFHxA did not impact cross frequency (Figure 6A). Exposure to 20 μM PFHxS caused a significant decrease in cumulative larval crossing over the course of the assay (Figure 6B). There were no observed changes in cross frequency with exposure to 6:2 FTOH at any dose (Figure 6C).
Figure 6.
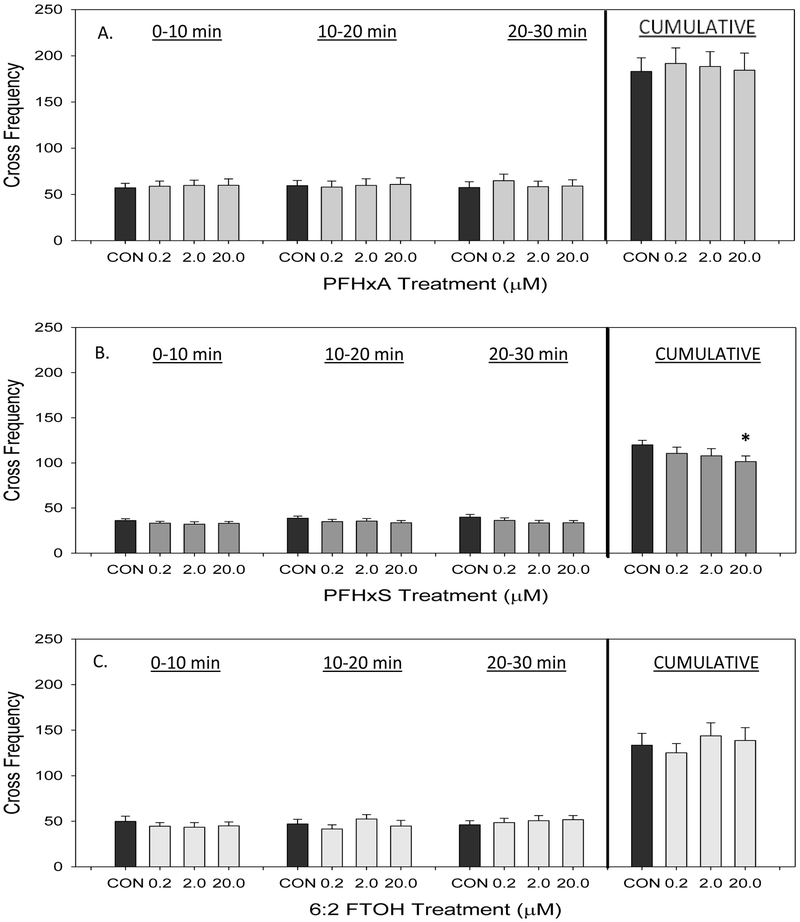
Cross frequency, or number of times the larvae swam through the center of the arena, of developmentally PFHxA-, PFHxS-, or 6:2 FTOH-exposed larvae at 14 dpf. Data collected over a 30 minute period are divided into 10 minute time bins. Cumulative distance over the 30 minute assay is shown in the last column set. Values are reported as mean and SEM for each time bin with (A) PFHxA treatment (B) PFHxS treatment (C) 6:2 FTOH treatment. An asterisk (*) indicates p<0.05, one-way ANOVA, N=7-8.
Velocity was also recorded in the locomotion assay, and these values are represented as the mean for each 10 minute time bin (Figure 7). PFHxA exposure caused no significant effects on larval velocity (Figure 7A). Similarly, no significant changes in velocity were observed with exposure to PFHxS (Figure 7B). Exposure to 20 μM 6:2 FTOH caused a significant increase in mean velocity compared to controls in the 10-20 and 20-30 minute time bins (Figure 7C). In this assay, velocity typically decreases over time, which is evident in the controls (Emran et al. 2008; Kristofco et al. 2016; MacPhail et al. 2009). However, this decrease in velocity over time is absent with exposure to 20 μM 6:2 FTOH.
Figure 7.
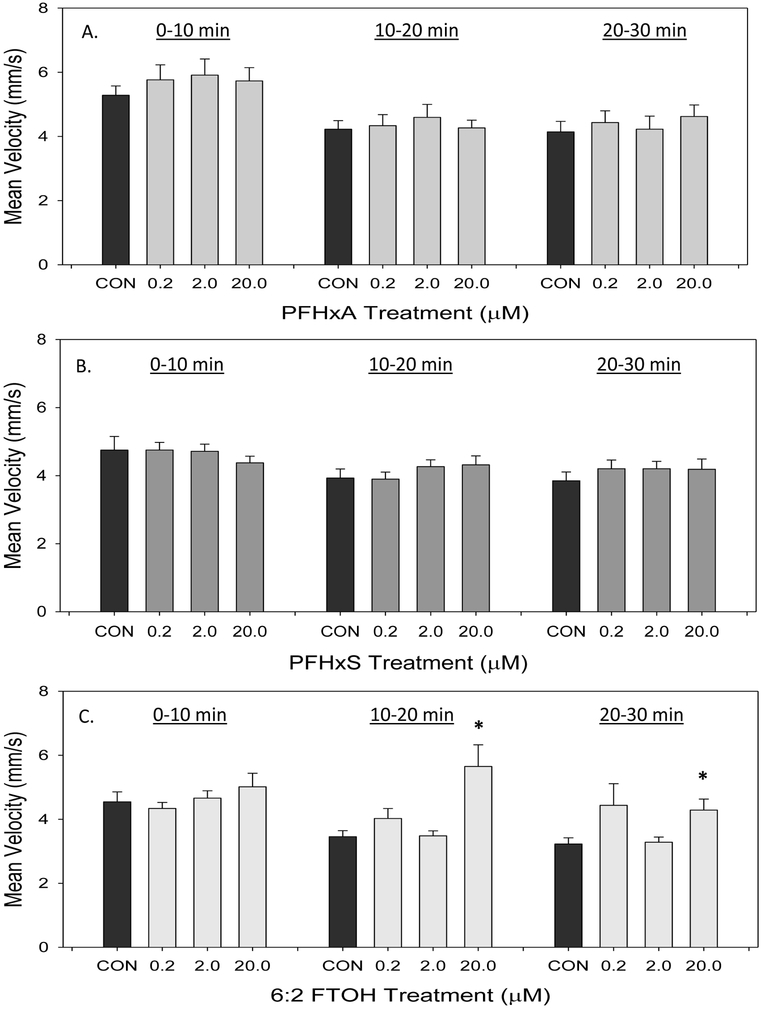
Mean velocity of developmentally PFHxA-, PFHxS-, or 6:2 FTOH-exposed larvae at 14 dpf. Data collected over a 30 minute period are divided into 10 minute time bins. Values are reported as non-cumulative mean and SEM for each time bin with (A) PFHxA treatment (B) PFHxS treatment (C) 6:2 FTOH treatment. An asterisk (*) indicates p<0.05, one-way ANOVA, N=7-8.
4. Discussion
4.1. General Conclusions
Overall, growth and behavioral endpoints measured at the individual level of these AOPs do not appear to be the most sensitive measures of PFHxA, PFHxS, and 6:2 FTOH toxicities in the developing zebrafish. PFHxS was the only compound to produce consistent morphometric effects at 5 and 14 dpf (Tables 3 and 4). In terms of the behavioral endpoints examined, the highest concentration of 20 μM PFHxS and 6:2 FTOH were the only doses at which behavioral effects were observed. Changes in gene expression, however, were observed at the lower doses examined. Significant changes in bdnf, tgfb1a, and ap1s1 transcript levels were observed with 0.2 μM concentrations of PFHxA, PFHxS, and 6:2 FTOH (Figure 4). It is possible that these compounds may be eliciting effects at low doses on the biochemical level.
While the behavioral and growth-related effects of PFHxS and 6:2 FTOH exposures were observed only at the highest concentrations, these findings do highlight that early developmental exposures can have lasting impacts. The developmentally-exposed larvae in this study received a 9 day depuration period prior to the behavioral assay, which could mean that the targeted tissues or biochemistry were permanently altered. It is also possible that internal doses of these compounds were still present at the 14 dpf time point. In adult female zebrafish, plasma half-life of PFHxA ranged between 3.2-9.6 days, and the half-life for PFHxS was measured between 6.4-14.7 days (Chen et al. 2016). In contrast, 6:2 FTOH is reported to be rapidly conjugated and excreted in cell culture (Martin et al. 2005), but its half-life has yet to be reported in zebrafish.
This study examines expression levels of some genes of interest, slco2b1, slco1d1, bdnf, tgfb1a, and ap1s1, selected based on previous data from our lab showing changes in expression of these genes with similar developmental exposure to long chain PFASs (Jantzen et al. 2016a; Jantzen et al. 2016b; Jantzen et al. 2017). Here, we observed changes in regulation of these genes as well with exposure to the low molecular weight PFAS alternatives. This suggests that there might be shared molecular targets for some PFASs. In addition, bdnf, tgb1a, and ap1s1 exist in interrelated pathways, so changes in expression of these genes may be linked to one another. These changes in expression can also provide possible explanations for the effects on growth and behavior observed at higher doses.
There were few changes in expression of the transporters, slco2b1 and slco1d1, with exposure to these compounds (Figure 3). These transporters are responsible for the transfer of endogenous ligands, such as bile acids, steroid conjugates thyroxine, bilirubin, and prostaglandins, across membranes throughout the body, but expression for these transporters is high in the liver (Klaassen and Aleksunes 2010; Popovic et al. 2010). In zebrafish, slco2b1, has high homology to human slco2b1, and both PFOS and PFHxS are substrates for human slco2b1 (Popovic et al. 2010; Zhao et al. 2017). Slco1d1 is unique to fish; however, it has been demonstrated that PFOS is a substrate and PFOA is an inhibitor of transporter function (Popovic et al. 2014). While interaction with long chain PFASs is reported in the literature, fewer studies have examined these low molecular weight alternatives (Cheng and Klaassen 2008; Kudo et al. 2002; Popovic et al. 2014; Weaver et al. 2010; Yang et al. 2010). In the present study, small changes in fold change of gene expression were observed. This is in opposition to the gene expression of 5 dpf larvae exposed to long chain PFASs. Developmental exposure to 2 μM PFOA caused a 2 fold induction of slco2b1 and PFOS caused an 8 fold induction (Jantzen et al. 2016a). Effects on the transcription level of both transporters persisted into adulthood where a significant down-regulation with exposure to PFOS (slco2b1 and slco1d1) and PFOA (slco1d1) (Jantzen et al. 2016b). A contributing factor to the lack of response observed with the low molecular weight PFASs may be that RNA was collected from whole body larvae. This disregards the tissue-specific expression pattern of these transporters (Popovic et al. 2010). Additionally, there is overlap of substrates, which might offset any effects to a specific transporter (Klaassen and Aleksunes 2010). While the protein and functional status of these transporters were not studied, these compounds appear to not target transcript expression of slco2b1 or slco1d1 in this developmental model.
4.2. Toxicity profile for PFHxA exposure in zebrafish
Effects at the individual level of the growth and behavioral AOPs were not observed in the developing larvae exposed to PFHxA. At the 0.2 μM PFHxA dose, there was a significant reduction in the overall length and yolk sac size of the 5 dpf larvae; however, this observation recovered at the higher doses (Table 3). Due to the nonmonotonic response for these endpoints, it may be that the doses examined in this study are below the LOAEL (lowest observable adverse effect level) in zebrafish. In Xenopus laevis, the lowest concentration of PFHxA that produced a reduction in body length was 1000 μM (Kim et al. 2015). Similarly, chronic and subchronic studies in rats of exposures up to 200 mg/kg/day had no effects on growth (Chengelis et al. 2009; Klaunig et al. 2014).
PFHxA exposure caused no changes in 14 dpf larval zebrafish behavior (Figures 5A, 6A, and 7A). Exposure levels may have been below of the LOAEL in this model. These findings correlated with the literature as no changes in either a locomotor assay or functional observational battery have been observed in rats exposed to PFHxA (Chengelis et al. 2009; Klaunig et al. 2014). Additionally, total length was not altered in these larvae at 14 dpf (Table 4).
The endpoints most impacted by PFHxA exposure were gene expression (Figure 4). All doses examined caused a significant induction in tgfb1a transcript expression, which is involved in the formation of muscle bundles in zebrafish (Kim and Ingham 2009). There was also a dose-dependent increase ap1s1 expression with PFHxA exposure and a 13-fold induction of expression at 20 μM PFHxA (Figure 4C). This adaptor-related protein is responsible for vesicle formation and protein movement at a subcellular level, and AP1S1 has been found to be especially critical in development of the spinal cord, transporting acetylcholine (Montpetit et al. 2008; Nakatsu et al. 2014). In addition, the expression of ap1s1 is related to that of bdnf, with bdnf able to promote expression of ap-1 and stimulate its activity (Gaiddon et al. 1996; Huynh and Heinrich 2001). Ap-1 may also be involved in a positive feedback loop with bdnf (Tuvikene et al. 2016), which would provide a possible explanation for the increased bdnf expression observed in the present study at 20 μM PFHxA (Figure 4B). Additionally, it has been reported in the literature that PFHxA accumulates in human brain tissue (Perez et al. 2013). However, these changes in gene expression do not translate to effects on growth or behavior with PFHxA exposure in the developing larvae in this study.
One goal of this study was to compare the toxicity profile of PFHxA to PFOA in the developing zebrafish, which has been demonstrated in our lab following a similar exposure paradigm (Jantzen et al. 2016a). There is evidence in the literature to suggest that PFHxA is less toxic than its long chain counterpart due to its shorter half-life (Wang et al. 2015). However, we anticipated similar responses in the behavioral and growth AOPs due to the shared carboxylic acid moiety. Developmental exposure to 2 μM PFOA caused a decreased total body length and yolk sac edema at 5 dpf (Jantzen et al. 2016a). Additionally, 2 μM PFOA caused a significant increase in distance traveled over a similar 30 minute assay at 14 dpf. However, no growth or behavioral effects were observed at these time points with PFHxA exposures. Gene expression results also did not correlate; the direction and level of change was often not consistent.
4.3. Toxicity profile for PFHxS exposure in zebrafish
Both growth and behavioral AOPs were impacted with PFHxS exposure. In the present study, there was an observed delay, post hatch, in mortality with PFHxS exposure that was not observed with PFHxA or 6:2 FTOH. To our knowledge this endpoint has not been reported in in vivo studies with PFHxS. This may suggest that events later in development are targeted by PFHxS exposure and warrant further study. There was also a significant increase in total length observed at 2 and 20 μM PFHxS, and a significant increase in yolk sac area in the 20 μM treatment group (Table 3). The increase in length persisted at 14 dpf (Table 4). These findings correspond to the literature where increased weight was observed in male mice exposed for 42 days to 0.3 and 1 mg/kg/day PFHxS (Chang et al. 2018). However, similar studies in rats have reported no effect on body weight (Butenhoff et al. 2009). In the zebrafish larvae, PFHxS exposure may be stimulating a pathway resulting in this observed increased growth.
Following 20 μM PFHxS exposure, there was a decrease in both total distance across time bins and cumulative number of crosses in the locomotor assay (Figure 5B and 6B). This reflects a decrease in activity and exploratory behavior in the larval fish, as demonstrated by the decrease in number of crosses through the center of the well. Increased time spent in the outer portion of the arena is indicative of thigmotaxis, a wall-hugging behavior associated with avoidance behavior and an anxiety-like phenotype (Champagne et al. 2010). A typical stressed phenotype, although, is also associated with increased swim distance and velocity, which were not observe in this study (Champagne et al. 2010). Similar effects were observed following a single exposure to 1.4-21 μmol/kg PFHxS at postnatal day 10 of age, mice at 2 months of age displayed decreased activity compared to controls during the first 20 minutes of the locomotor assay (Viberg et al. 2013).
These behavioral findings emphasis that early developmental exposures to PFHxS may have lasting behavioral effects on exposed populations. It is unknown what this impact will have on fish in wild populations. The decrease in distance and exploratory could be harmful if it means that a fish will not be in search of prey and mates as it reach sexual maturity, and stress responses have been demonstrated to increase lethality of certain anthropogenic compounds (Champagne et al. 2010; Relyea and Mills 2001). However, reduced stress responses are beneficial and evolutionarily adaptive for fish populations in areas of high predation (Brown et al. 2005). It is important to note that decreased behavioral response in behavioral assays has been associated with the presence of lesions in larval zebrafish, but there was an increase rather than decrease in growth (Padilla et al. 2011). It is possible that the increased size in larvae at 5 and 14 dpf is related to the decreased activity observed at 14 dpf if energy homeostasis was altered so early in development.
PFHxS and PFOS produced different phenotypes in the developing zebrafish. Following a 5 day exposure to 2 μM PFOS, the sac fry larvae were significantly smaller in length and yolk sac area compared to controls (Jantzen et al. 2016a). The opposite effects were observed in the present study, where PFHxS caused a significant increase in length, at 2 and 20 μM, and a significant increase in yolk sac area at 20 μM. By 14 dpf, the increase in length was persistent with PFHXS exposure, but the PFOS-exposed larvae were not significantly different from controls. Additionally, the behavior profile differed. Larval zebrafish developmentally exposed to 2μM PFOS displayed a hyperactive phenotype with increased distance traveled, cross frequency, and mean velocity over a similar 30 minute assay (Jantzen et al. 2016a).
4.4. Toxicity profile for 6:2 FTOH exposure in zebrafish
In terms of LC50 values, 6:2 FTOH was the least toxic of the 3 compounds examined in 5 dpf zebrafish (Figure 2). This was also reflected in the morphological data, where a significant decrease in total body length observed only at the 0.02 μM concentration (Table 3). However, adverse effects have been reported at low dose 6:2 FTOH exposures in the literature. Exposure as low as 0.82 μM impacted the gonadal somatic index in adult zebrafish and 10 μM affected the hepatosomatic index in medaka (Ishibashi et al. 2008; Liu et al. 2009). It is possible that either these morphological endpoints or early stages of zebrafish development are less sensitive to 6:2 FTOH exposures.
Behavioral endpoints were altered with exposure to 6:2 FTOH, particularly at the higher doses of 2 and 20 μM (Figures 5 and 7). Total distance traveled during the assay was significantly increased with exposure to 2 μM 6:2 FTOH in the first time bin and when examined over the entire assay (Figure 5C). This compound was the only one to impact the mean velocity of the larvae, where a significant increase in mean velocity compared to controls was observed in the 10-20 and 20-30 minute time bins (Figure 7C). There is an anticipated startle response at the start of the assay as lights are turned off stimulating motion. The initial startle response occurs, but not an acclimation to the change in stimulus in the 6:2 FTOH-exposed larvae. This may suggest either a decreased ability to habituate to the dark stimulus or an increase in basal speeds. Together, the increased swim speed and distance traveled support an elevated anxiety phenotype (Champagne et al. 2010).
Gene expression changes were observed at 5 dpf following low dose exposures to 6:2 FTOH. There was a significant dose-dependent increase in tgfb1a transcript expression with 6:2 FTOH exposure (Figure 4A). Tgfb1a is necessary for proper muscle and lateral line development, both of which can be related to the behavioral effects observed (Kim and Ingham 2009; Xing et al. 2015). Similarly, an increase in gene expression of ap1s1 and bdnf were observed with exposure to 20 μM 6:2 FTOH. Bdnf is also involved in lateral line development (Germana et al. 2010). Additionally, both bdnf and ap1s1 are also related to central nervous system development and function (De Felice et al. 2014; Montpetit et al. 2008). The changes in gene expression with 6:2 FTOH observed at 5 dpf might be related to the behavioral changes observed at 14 dpf. These changes in gene expression may reflect impacts at the biochemical level that translate to the individual larval effects in the behavioral AOP.
4.5. Conclusions
The present study is the first demonstrating the LC50 values for PFHxA, PFHxS, and 6:2 FTOH in the developing zebrafish at 5 dpf. The LC50 values were comparable for PFHxA and PFHxS; however, 6:2 FTOH was less acutely toxic. Following 0.2-20 μM exposures to these low molecular weight PFAS alternatives, few morphological effects were observed in 5 dpf larvae. Behavioral effects were observed following a 5 day developmental exposure to PFHxS and 6:2 FTOH in 14 dpf larvae. Exposure to 20 μM PFHxS caused a decrease in activity, and 2 μM 6:2 FTOH caused an increase in swim distance and velocity in the behavioral assays. Gene expression of candidate molecular targets (bdnf, ap1s1, and tgfb1a) were examined in 5 dpf larvae and unique patterns of response were observed for each compound which may be related to the growth and behavioral effects observed at the individual level of these AOPs. Additionally, gene expression was altered at the lowest concentrations examined, proving to be the most sensitive endpoint in this study. This study demonstrates developmental effects of PFHxA, PFHxS, and 6:2 FTOH in zebrafish.
Highlights.
PFHxA was the most acutely toxic, yet caused no behavioral or morphometric effects.
PFHxS impacted morphometric and behavioral endpoints at high doses.
Exposure to 6:2 FTOH impacted gene expression and behavioral endpoints.
Changes in behavior and gene expression represent subtle alterations.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [T32-ES007148 & P30 ES005022] and New Jersey Agricultural Experiment Station, NJAES-Rutgers [NJ01201].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahrens L (2011) Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate. Journal of environmental monitoring : JEM 13(1):20–31 doi: 10.1039/c0em00373e [DOI] [PubMed] [Google Scholar]
- Bijland S, Rensen PC, Pieterman EJ, et al. (2011) Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-Leiden CETP mice. Toxicological sciences : an official journal of the Society of Toxicology 123(1):290–303 doi: 10.1093/toxsci/kfr142 [DOI] [PubMed] [Google Scholar]
- Brown C, Gardner C, Braithwaite VA (2005) Differential stress responses in fish from areas of high- and low-predation pressure. J Comp Physiol B 175(5):305–12 doi: 10.1007/s00360-005-0486-0 [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Chang SC, Ehresman DJ, York RG (2009) Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reproductive toxicology 27(3-4):331–41 doi: 10.1016/j.reprotox.2009.01.004 [DOI] [PubMed] [Google Scholar]
- Champagne DL, Hoefnagels CC, de Kloet RE, Richardson MK (2010) Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav Brain Res 214(2):332–42 doi: 10.1016/j.bbr.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Chang S, Butenhoff JL, Parker GA, et al. (2018) Reproductive and Developmental Toxicity of Potassium Perfluorohexanesulfonate in CD-1 Mice. Reproductive toxicology doi: 10.1016/j.reprotox.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Chen F, Gong Z, Kelly BC (2016) Bioavailability and bioconcentration potential of perfluoroalkyl-phosphinic and -phosphonic acids in zebrafish (Danio rerio): Comparison to perfluorocarboxylates and perfluorosulfonates. The Science of the total environment 568:33–41 doi: 10.1016/j.scitotenv.2016.05.215 [DOI] [PubMed] [Google Scholar]
- Chen J, Tanguay RL, Tal TL, et al. (2014) Early life perfluorooctanesulphonic acid (PFOS) exposure impairs zebrafish organogenesis. Aquatic toxicology 150:124–32 doi: 10.1016/j.aquatox.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Lv S, Nie S, et al. (2016) Chronic perfluorooctane sulfonate (PFOS) exposure induces hepatic steatosis in zebrafish. Aquatic toxicology 176:45–52 doi: 10.1016/j.aquatox.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Cheng X, Klaassen CD (2008) Critical role of PPAR-alpha in perfluorooctanoic acid- and perfluorodecanoic acid-induced downregulation of Oatp uptake transporters in mouse livers. Toxicological sciences : an official journal of the Society of Toxicology 106(1):37–45 doi: 10.1093/toxsci/kfn161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chengelis CP, Kirkpatrick JB, Radovsky A, Shinohara M (2009) A 90-day repeated dose oral (gavage) toxicity study of perfluorohexanoic acid (PFHxA) in rats (with functional observational battery and motor activity determinations). Reproductive toxicology 27(3-4):342–51 doi: 10.1016/j.reprotox.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Conder JM, Hoke RA, De Wolf W, Russell MH, Buck RC (2008) Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environmental science & technology 42(4):995–1003 [DOI] [PubMed] [Google Scholar]
- De Felice E, Porreca I, Alleva E, et al. (2014) Localization of BDNF expression in the developing brain of zebrafish. J Anat 224(5):564–74 doi: 10.1111/joa.12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emran F, Rihel J, Dowling JE (2008) A behavioral assay to measure responsiveness of zebrafish to changes in light intensities. J Vis Exp(20) doi: 10.3791/923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiddon C, Loeffler JP, Larmet Y (1996) Brain-derived neurotrophic factor stimulates AP-1 and cyclic AMP-responsive element dependent transcriptional activity in central nervous system neurons. Journal of neurochemistry 66(6):2279–86 [DOI] [PubMed] [Google Scholar]
- Germana A, Laura R, Montalbano G, et al. (2010) Expression of brain-derived neurotrophic factor and TrkB in the lateral line system of zebrafish during development. Cell Mol Neurobiol 30(5):787–93 doi: 10.1007/s10571-010-9506-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars A, Stinckens E, Vergauwen L, Bervoets L, Knapen D (2014) PFOS affects posterior swim bladder chamber inflation and swimming performance of zebrafish larvae. Aquatic toxicology 157:225–35 doi: 10.1016/j.aquatox.2014.10.017 [DOI] [PubMed] [Google Scholar]
- Huang H, Huang C, Wang L, et al. (2010) Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquatic toxicology 98(2):139–47 doi: 10.1016/j.aquatox.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh G, Heinrich G (2001) Brain-derived neurotrophic factor gene organization and transcription in the zebrafish embryo. Int J Dev Neurosci 19(7):663–73 [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Yamauchi R, Matsuoka M, et al. (2008) Fluorotelomer alcohols induce hepatic vitellogenin through activation of the estrogen receptor in male medaka (Oryzias latipes). Chemosphere 71 (10):1853–9 doi: 10.1016/j.chemosphere.2008.01.065 [DOI] [PubMed] [Google Scholar]
- Jantzen CE, Annunziato KA, Bugel SM, Cooper KR (2016a) PFOS, PFNA, and PFOA sub-lethal exposure to embryonic zebrafish have different toxicity profiles in terms of morphometrics, behavior and gene expression. Aquatic toxicology 175:160–70 doi: 10.1016/j.aquatox.2016.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen CE, Annunziato KM, Cooper KR (2016b) Behavioral, morphometric, and gene expression effects in adult zebrafish (Danio rerio) embryonically exposed to PFOA, PFOS, and PFNA. Aquatic toxicology 180:123–130 doi: 10.1016/j.aquatox.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen CE, Toor F, Annunziato KA, Cooper KR (2017) Effects of chronic perfluorooctanoic acid (PFOA) at low concentration on morphometrics, gene expression, and fecundity in zebrafish (Danio rerio). Reproductive toxicology 69:34–42 doi: 10.1016/j.reprotox.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Tao L, Sinclair E, Pastva SD, Jude DJ, Giesy JP (2005) Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Arch Environ Contam Toxicol 48(4):559–66 doi: 10.1007/s00244-004-0133-x [DOI] [PubMed] [Google Scholar]
- Kim HR, Ingham PW (2009) The extracellular matrix protein TGFBI promotes myofibril bundling and muscle fibre growth in the zebrafish embryo. Dev Dyn 238(1):56–65 doi: 10.1002/dvdy.21812 [DOI] [PubMed] [Google Scholar]
- Kim M, Park MS, Son J, et al. (2015) Perfluoroheptanoic acid affects amphibian embryogenesis by inducing the phosphorylation of ERK and JNK. Int J Mol Med 36(6):1693–700 doi: 10.3892/ijmm.2015.2370 [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203(3):253–310 doi: 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM (2010) Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacological reviews 62(1):1–96 doi: 10.1124/pr.109.002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Shinohara M, Iwai H, et al. (2014) Evaluation of the Chronic Toxicity and Carcinogenicity of Perfluorohexanoic Acid (PFHxA) in Sprague-Dawley Rats. Toxicologic pathology doi: 10.1177/0192623314530532 [DOI] [PubMed] [Google Scholar]
- Kristofco LA, Cruz LC, Haddad SP, Behra ML, Chambliss CK, Brooks BW (2016) Age matters: Developmental stage of Danio rerio larvae influences photomotor response thresholds to diazinion or diphenhydramine. Aquatic toxicology 170:344–54 doi: 10.1016/j.aquatox.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y, Kawashima Y (2002) Sex hormone-regulated renal transport of perfluorooctanoic acid. Chemico-biological interactions 139(3):301–16 [DOI] [PubMed] [Google Scholar]
- Land M, De Wit CA, Bignert A, et al. (2018) What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review. Environ Evidence 7(4) doi: 10.1186/s13750-017-0114-y [DOI] [Google Scholar]
- Lau C (2012) Perfluorinated compounds. EXS 101:47–86 doi: 10.1007/978-3-7643-8340-4_3 [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Rama Rao VV, Nauduri D, Vargo JD, Parkin S (2007) Synthesis and Structure of Environmentally Relevant Perfluorinated Sulfonamides. Journal of fluorine chemistry 128(6):595–607 doi: 10.1016/j.jfluchem.2007.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RC, Johns LE, Meeker JD (2015) Serum Biomarkers of Exposure to Perfluoroalkyl Substances in Relation to Serum Testosterone and Measures of Thyroid Function among Adults and Adolescents from NHANES 2011-2012. Int J Environ Res Public Health 12(6):6098–114 doi: 10.3390/ijerph120606098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Gao P, Xiang P, Zhang X, Cui X, Ma LQ (2017) Molecular mechanisms of PFOA-induced toxicity in animals and humans: Implications for health risks. Environment international 99:43–54 doi: 10.1016/j.envint.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Litchfield JT Jr., Wilcoxon F (1948) A simplified method of evaluating dose-effect experiments. Fed Proc 7(1 Pt 1):240. [PubMed] [Google Scholar]
- Liu C, Yu L, Deng J, Lam PK, Wu RS, Zhou B (2009) Waterborne exposure to fluorotelomer alcohol 6:2 FTOH alters plasma sex hormone and gene transcription in the hypothalamic-pituitary-gonadal (HPG) axis of zebrafish. Aquatic toxicology 93(2-3):131–7 doi: 10.1016/j.aquatox.2009.04.005 [DOI] [PubMed] [Google Scholar]
- MacPhail RC, Brooks J, Hunter DL, Padnos B, Irons TD, Padilla S (2009) Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 30(1):52–8 doi: 10.1016/j.neuro.2008.09.011 [DOI] [PubMed] [Google Scholar]
- Martin JW, Mabury SA, O'Brien PJ (2005) Metabolic products and pathways of fluorotelomer alcohols in isolated rat hepatocytes. Chemico-biological interactions 155(3):165–80 doi: 10.1016/j.cbi.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Metz JR, Huising MO, Leon K, Verburg-van Kemenade BM, Flik G (2006) Central and peripheral interleukin-1beta and interleukin-1 receptor I expression and their role in the acute stress response of common carp, Cyprinus carpio L. J Endocrinol 191(1):25–35 doi: 10.1677/joe.1.06640 [DOI] [PubMed] [Google Scholar]
- Montpetit A, Cote S, Brustein E, et al. (2008) Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord. PLoS Genet 4(12):e1000296 doi: 10.1371/journal.pgen.1000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu F, Hase K, Ohno H (2014) The Role of the Clathrin Adaptor AP-1: Polarized Sorting and Beyond. Membranes (Basel) 4(4):747–63 doi: 10.3390/membranes4040747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri E, Metruccio F, Guercio V, et al. (2017) Exposure to PFOA and PFOS and fetal growth: a critical merging of toxicological and epidemiological data. Critical reviews in toxicology 47(6):482–508 doi: 10.1080/10408444.2016.1271972 [DOI] [PubMed] [Google Scholar]
- Newsted JL, Holem R, Hohenstein G, et al. (2017) Spatial and temporal trends of poly- and perfluoroalkyl substances in fish fillets and water collected from pool 2 of the Upper Mississippi River. Environmental toxicology and chemistry / SETAC 36(11):3138–3147 doi: 10.1002/etc.3891 [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Munley SM, Serex TL, Buck RC (2014) Evaluation of the reproductive and developmental toxicity of 6:2 fluorotelomer alcohol in rats. Toxicology 317:6–16 doi: 10.1016/j.tox.2014.01.002 [DOI] [PubMed] [Google Scholar]
- OECD (1998) Test No. 212: Fish, Short-term Toxicity Test on Embryo and Sac-Fry Stages, [Google Scholar]
- Olsen GW, Mair DC, Lange CC, et al. (2017) Per- and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000-2015. Environmental research 157:87–95 doi: 10.1016/j.envres.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Padilla S, Hunter DL, Padnos B, Frady S, MacPhail RC (2011) Assessing locomotor activity in larval zebrafish: Influence of extrinsic and intrinsic variables. Neurotoxicol Teratol 33(6):624–30 doi: 10.1016/j.ntt.2011.08.005 [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhang H, Cui Q, et al. (2018) Worldwide Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids in Surface Water. Environmental science & technology doi: 10.1021/acs.est.8b00829 [DOI] [PubMed] [Google Scholar]
- Perez F, Nadal M, Navarro-Ortega A, et al. (2013) Accumulation of perfluoroalkyl substances in human tissues. Environment international 59:354–62 doi: 10.1016/j.envint.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Popovic M, Zaja R, Fent K, Smital T (2014) Interaction of environmental contaminants with zebrafish organic anion transporting polypeptide, Oatp1d1 (Slco1d1). Toxicol Appl Pharmacol 280(1):149–58 doi: 10.1016/j.taap.2014.07.015 [DOI] [PubMed] [Google Scholar]
- Popovic M, Zaja R, Smital T (2010) Organic anion transporting polypeptides (OATP) in zebrafish (Danio rerio): Phylogenetic analysis and tissue distribution. Comparative biochemistry and physiology Part A, Molecular & integrative physiology 155(3):327–35 doi: 10.1016/j.cbpa.2009.11.011 [DOI] [PubMed] [Google Scholar]
- Post GB, Cohn PD, Cooper KR (2012) Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environmental research 116:93–117 doi: 10.1016/j.envres.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Relyea RA, Mills N (2001) Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor). Proceedings of the National Academy of Sciences of the United States of America 98(5):2491–6 doi: 10.1073/pnas.031076198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak MD, Benskin JP, Wong A, Grace R, Greig DJ (2017) Per- and polyfluoroalkyl substances (PFASs) in San Francisco Bay wildlife: Temporal trends, exposure pathways, and notable presence of precursor compounds. Chemosphere 185:1217–1226 doi: 10.1016/j.chemosphere.2017.04.096 [DOI] [PubMed] [Google Scholar]
- Serex T, Anand S, Munley S, et al. (2014) Toxicological evaluation of 6:2 fluorotelomer alcohol. Toxicology 319:1–9 doi: 10.1016/j.tox.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Siebenaler R, Cameron R, Butt CM, Hoffman K, Higgins CP, Stapleton HM (2017) Serum perfluoroalkyl acids (PFAAs) and associations with behavioral attributes. Chemosphere 184:687–693 doi: 10.1016/j.chemosphere.2017.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda S (2016) Differential toxicity between perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). J Toxicol Sci 41(Special):SP27–SP36 doi: 10.2131/jts.41.SP27 [DOI] [PubMed] [Google Scholar]
- Tuvikene J, Pruunsild P, Orav E, Esvald EE, Timmusk T (2016) AP-1 Transcription Factors Mediate BDNF-Positive Feedback Loop in Cortical Neurons. J Neurosci 36(4):1290–305 doi: 10.1523/JNEUROSCI.3360-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulhaq M, Orn S, Carlsson G, Morrison DA, Norrgren L (2013) Locomotor behavior in zebrafish (Danio rerio) larvae exposed to perfluoroalkyl acids. Aquatic toxicology 144-145:332–40 doi: 10.1016/j.aquatox.2013.10.021 [DOI] [PubMed] [Google Scholar]
- Viberg H, Lee I, Eriksson P (2013) Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology 304:185–91 doi: 10.1016/j.tox.2012.12.013 [DOI] [PubMed] [Google Scholar]
- Walker MB, Kimmel CB (2007) A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech Histochem 82(1):23–8 doi: 10.1080/10520290701333558 [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbuehler K (2015) Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions. Environment international 75:172–9 doi: 10.1016/j.envint.2014.11.013 [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbuhler K (2013) Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environment international 60:242–8 [DOI] [PubMed] [Google Scholar]
- Weaver YM, Ehresman DJ, Butenhoff JL, Hagenbuch B (2010) Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicological sciences : an official journal of the Society of Toxicology 113(2):305–14 doi: 10.1093/toxsci/kfp275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Gong B, Xue Y, et al. (2015) TGFbeta1a regulates zebrafish posterior lateral line formation via Smad5 mediated pathway. J Mol Cell Biol 7(1):48–61 doi: 10.1093/jmcb/mjv004 [DOI] [PubMed] [Google Scholar]
- Yang CH, Glover KP, Han X (2010) Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicological sciences : an official journal of the Society of Toxicology 117(2):294–302 doi: 10.1093/toxsci/kfq219 [DOI] [PubMed] [Google Scholar]
- Zhao W, Zitzow JD, Weaver Y, et al. (2017) Organic Anion Transporting Polypeptides Contribute to the Disposition of Perfluoroalkyl Acids in Humans and Rats. Toxicological sciences : an official journal of the Society of Toxicology 156(1):84–95 doi: 10.1093/toxsci/kfw236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XM, Liu HL, Shi W, Wei S, Giesy JP, Yu HX (2011) Effects of perfluorinated compounds on development of zebrafish embryos. Environmental science and pollution research international 19(7):2498–505 doi: 10.1007/s11356-012-0977-y [DOI] [PubMed] [Google Scholar]


