Abstract
Background
Resolution of inflammation is an active and dynamic process after surgery. Maresin 1 (MaR1) is one of a growing number of specialised pro-resolving lipids biosynthesised by macrophages that regulates acute inflammation. We investigated the effects of MaR1 on postoperative neuroinflammation, macrophage activity, and cognitive function in mice.
Methods
Adult male C57BL/6 (n=111) and Ccr2RFP/+Cx3cr1GFP/+ (n=54) mice were treated with MaR1 before undergoing anaesthesia and orthopaedic surgery. Systemic inflammatory changes, bone healing, neuroinflammation, and cognition were assessed at different time points. MaR1 protective effects were also evaluated using bone marrow derived macrophage cultures.
Results
MaR1 exerted potent systemic anti-inflammatory effects without impairing fracture healing. Prophylaxis with MaR1 prevented surgery-induced glial activation and opening of the blood–brain barrier. In Ccr2RFP/+Cx3cr1GFP/+ mice, fewer infiltrating macrophages were detected in the hippocampus after surgery with MaR1 prophylaxis, which resulted in improved memory function. MaR1 treatment also reduced expression of pro-inflammatory cell surface markers and cytokines by in vitro cultured macrophages. MaR1 was detectable in the cerebrospinal fluid of older adults before and after surgery.
Conclusions
MaR1 exerts distinct anti-inflammatory and pro-resolving effects through regulation of macrophage infiltration, NF-κB signalling, and cytokine release after surgery. Future studies on the use of pro-resolving lipid mediators may inform novel approaches to treat neuroinflammation and postoperative neurocognitive disorders.
Keywords: neurocognitive disorders, neuroinflammation, glia, macrophages, omega 3 fatty acids, postoperative complications, pro-resolving lipid mediators
Editor's key points.
-
•
Maresin 1 is a specialised pro-resolving lipid biosynthesised by macrophages that regulates acute inflammation.
-
•
The effects of maresin 1 on postoperative neuroinflammation, macrophage activity, and cognitive function were investigated in mouse models of orthopaedic surgery with general anaesthesia.
-
•
Maresin 1 prevented surgery-induced glial activation, opening of the blood–brain barrier and infiltration of macrophages into the hippocampus, and improved postoperative memory.
-
•
Pro-resolving lipid mediators such as maresin 1 provide novel targets to treat postoperative neuroinflammation and neurocognitive dysfunction.
Acute inflammation and activation of the innate immune response are fundamental processes after tissue trauma or infection.1 Resolution of inflammation is an active and highly controlled process orchestrated by several molecules including omega-3-derived specialised pro-resolving mediators (SPMs).2 SPMs regulate the magnitude and duration of inflammation, limiting neutrophil infiltration and promoting efferocytosis.3 Failed resolution is a key component of several inflammatory conditions and is of considerable interest in translational biomedical research.4 Maresins (macrophage mediators in resolving inflammation) are a genus of resolution agonists biosynthesised from docosahexaenoic acid (DHA) by macrophages that display anti-inflammatory and pro-resolving actions.5 Maresin 1 (MaR1; 7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid) is a family of structurally distinct autacoids, which, together with lipoxins, resolvins, and protectins, stimulate general resolution signalling.6, 7 Importantly, SPMs (including MaR1) boost self-limiting inflammatory processes and enhance bacterial clearance without being immunosuppressive.3 MaR1 was also shown to enhance microbial killing in clinical periodontitis8 and to regulate human macrophages during infectious inflammation.9
Macrophages are pivotal in response to cellular damage or invading pathogens. However, dysregulated macrophage activity, especially caused by non-resolving inflammation, can be maladaptive and pathological in several conditions ranging from atherosclerosis to neurodegeneration.10 Peripheral systemic factors, circulating monocytes, macrophages, neutrophils, and the ensuing pro-inflammatory milieu can exert multiple effects on the central nervous system (CNS), contributing to changes in neuronal function, synaptic plasticity, and glial homeostasis.11 Peripheral surgery and remote organ damage may also contribute to macrophage infiltration in the CNS, neuroinflammation, cognitive dysfunction, and overall brain injury.12, 13, 14 Perioperative neurocognitive disorders (PNDs) are a common postoperative complication in elderly patients, who represent an expanding segment of our population.15 The mechanisms underlying PNDs remain elusive and, despite a marked incidence and burden on global health, no effective therapeutics are currently available.
Systemic inflammation and pro-inflammatory cytokines released after surgery have been associated with changes in neuronal function and are highlighted as critical pathogenic mechanisms underlying cognitive decline in preclinical models.16, 17, 18 Because SPMs provide both anti-inflammatory and pro-resolving actions without being immunosuppressive,19 we tested the hypothesis that targeting macrophage trafficking into the brain using synthetic MaR1 will reduce neuroinflammation and improve cognitive outcome in a murine model of orthopaedic surgery. We also provide preliminary evidence that MaR1 levels can be measured in human cerebrospinal fluid (CSF) before and after surgery, raising the possibility that MaR1 levels could potentially play a role in resolving postoperative neuroinflammation in the human CNS.
Methods
Mice
All experiments were performed in accordance with protocols approved by the local ethics committee for animal research in Sweden (Stockholm North Animal Ethics Board) and conformed to the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines. Inbred C57BL/6 mice 10–14 weeks old were purchased from Charles River (Sulzfeld, Germany), and Ccr2RFP/+Cx3cr1GFP/+ were bred at the Karolinska Institutet animal facility and used as described.13, 20 Male mice were used in the experiments and housed four per cage under temperature- and humidity-controlled conditions with a 12 h light–dark cycle and provided food and water ad libitum. All experiments were performed during the light cycle.
Surgery
An experimental aseptic tibia fracture model was used as described.21 Anaesthesia was induced in a dedicated chamber with 5 vol% isoflurane (Abbott Scandinavia AB, Stockholm, Sweden) for 2 min, and an open stabilised tibia fracture of the left hind leg was performed under 2.1 vol% isoflurane for 10–12 min. Briefly, after shaving and disinfecting the surgical field, a longitudinal incision was made and the muscles were dissociated from the periosteum. A 0.38 mm pin was then inserted in the intramedullary canal and osteotomy was performed. The wound was irrigated and sutured using 6–0 Prolene. During the procedure, non-invasive pulse oximetry (MouseSTAT™; Kent Scientific, Torrington, CT, USA), concentrations of O2, CO2, isoflurane (Capnomac Ultima; Datex-Ohmeda, Helsinki, Finland), and temperature (Harvard Apparatus, Holliston, MA, USA) were continuously monitored and maintained within the normal range. Analgesia (buprenorphine, 0.1 mg kg−1 s.c.) was given after anaesthesia induction and prophylaxis with MaR1 (Cayman Chemical, Ann Arbor, MI, USA) was given at 2 μg ml−1 in saline with 0.14% ethanol, i.p. (50 μl) 100 ng per mouse before skin incision. Control mice received analgesia (buprenorphine, 0.1 mg kg−1 s.c.) and a bolus dose of 0.14% ethanol in saline.
Cytokine detection
Blood from C57BL/6 and Ccr2RFP/+ Cx3cr1GFP/+ mice was collected via cardiac puncture under deep isoflurane anaesthesia at 24 h, 72 h, and 14 days after surgery, centrifuged at 2000 g for 9 min at 4°C, aliquoted and stored at –80°C for further analysis. MesoScale Discovery Technology (Gaithersburg, MD, USA) was used to analyse inflammatory markers in plasma with a SECTOR Imager 2400 apparatus following the manufacturer's instructions.
Behavioural tests
Contextual fear conditioning was performed in a dedicated chamber (Med Associates Inc., St Albans, VT, USA). Separate cohorts of C57BL/6 or Ccr2RFP/+Cx3cr1GFP/+ mice were trained before surgery as described.21 Briefly, an initial exploratory phase (100 s) was followed by two trials with a conditional stimulus (20 s auditory cue, 75–80 dB, 5 kHz) and an unconditional stimulus (2 s foot shock, 0.75 mA) separated by a 100 s inter-trial interval. After 72 h, mice were returned to the same chamber for the contextual assessment (i.e. no tone or shock cues). Freezing behaviour was automatically scored for 270 s using a video tracking software and defined as lack of movement for ≥2 s, excluding breathing. To avoid possible locomotion impairments, general activity was recorded in an open field (Aditech, Fjärås, Sweden) with an automated photobeam activity system during a 5 min exploration period.
Bone healing assessment
Tibias and femurs were collected 14 days after surgery and fixed overnight in 4% paraformaldehyde (PFA). Bones were decalcified for 1 month in 10% ethylenediaminetetraacetic acid (EDTA; pH 8.05), which was replaced every 2–3 days. After decalcification, pins were removed from the bones using forceps; bones were then passed through an ethanol gradient to xylene and were embedded in paraffin. Serial sections of 5 μm thickness were collected every 70 μm throughout the callous on Superfrost Ultra Plus slides (Menzel-Glaser, Braunschweig, Germany). Haematoxylin and eosin staining was performed to identify the centre of the callous. Five consecutive serial sections were then stained for Safranin O/Fast Green to identify cartilaginous regions within the callous. Cartilaginous regions were then quantified using ImageJ software (Bethesda, MD, USA) using a colour threshold of the area using a Zeiss Axioscope 2, equipped with an Axiocam digital camera (Carl Zeiss).
Immunofluorescence
Mice were killed under deep isoflurane anaesthesia and perfused transcardially with ice-cold 0.1 M phosphate-buffered saline (PBS) followed by 4% PFA in 0.1 M PBS at pH 7.4 (VWR International, East Grinstead, UK). Brains were harvested and post-fixed in 4% PFA in 0.1 M PBS at 4°C, and cryoprotected in 0.1 M PBS containing 15% sucrose for 24 h and then 30% sucrose for a further 48 h. Brain tissue was freeze-mounted in optimal cutting temperature (OCT) embedding medium (VWR International), and 25 μm thick coronal sections were cut sequentially and mounted on Superfrost Plus slides (Menzel-Glaser). Sections were permeabilised in PBS with 0.5% Triton X-100 and blocked with 5% bovine serum albumin (BSA) in PBS for 60 min at room temperature to block non-specific binding. The following primary antibodies were used: rabbit anti-glial fibrillary acidic protein (GFAP) (1:100, cat. #Z0334; Dako, Kista, Sweden), goat anti-Iba-1 (1:2000, cat. #ab107159; Abcam, Cambridge, UK), rabbit anti-Claudin-5 (1:100, cat. #34-1600; Invitrogen, Stockholm, Sweden) and goat anti-mouse podocalyxin (1:200, cat. #AF1556; R&D Systems, Minneapolis, MN, USA) at 4°C overnight. For secondary detection, (goat anti-rat, goat anti-rabbit, donkey anti-goat) conjugated with Alexa Fluor dyes (405, 488, and 555) from Invitrogen (1:500) were used. Immunolabelled sections were coverslipped with Prolong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) and analysed by confocal microscopy (LSM5 Exciter; Zeiss, Jena, Germany). Four high magnifications were chosen in three non-overlapping fields randomly acquired in hippocampal subregions using a counting frame size of 0.4 mm2. Images were processed and the area of the astrocytes, microglia/macrophages quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The area of the selected cells was converted into a binary image using the dilation method and the cell outline measured. Total immunoreactivity was calculated as percentage area density defined as the number of pixels (positively stained areas) divided by the total number of pixels (sum of positively and negatively stained area) in the imaged field. Quantitative analyses were performed in a blinded manner.
Bone marrow-derived macrophages
Primary culture
Bone marrow-derived macrophages (BMDMs) were cultured as described with minor modifications.13 In brief, femoral and tibia bone-marrow cells from C57BL/6 mice were collected, and single-cell suspensions were prepared and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% heat-inactivated FBS, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 2 mM l-glutamine (all reagents from Life Technologies, Stockholm, Sweden), and 20 ng ml−1 rmM-CSF (recombinant mouse macrophage colony-stimulating factor; R&D Systems) for 8 days. Cells were harvested using 2 mM EDTA and seeded in 48-well plates at 5×105 cells/well. Cells were then stimulated with 10 ng ml−1 lipopolysaccharide (LPS) from Escherichia coli endotoxin (0111:B4, 10 ng ml−1; Sigma-Aldrich, St. Louis, MO, USA), with or without MaR1 (10 nM) for 2 or 24 h, respectively. Nuclear factor-kappa B (NF-κB) activation (2 h after treatment) and nicotinamide adenine dinucleotide phosphate (NADPH) mediated superoxide generation (24 h after treatment) were then evaluated. Cultured medium was collected for measurement of tumour necrosis factor-alpha (TNF-α) using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Biosource Invitrogen, Carlsbad, CA, USA) with a sensitivity of <3 pg ml−1. Separate batches of BMDMs were stimulated with LPS (10 ng ml−1) or 20 ng mL−1 recombinant mouse interleukin (IL)-4, IL-10, and recombinant human transforming growth factor (TGF)-β1 (R&D Systems) for 24 h and macrophage activation surface marker expression was assessed using flow cytometry.
NF-κB activation
Cells were fixed with 4% PFA for 15 min at room temperature, blocked in 5% BSA (in PBS containing 0.3% Triton X-100) for 1 h, and stained with anti-phospho-NF-κB p65 (1:200, cat. #3033; Cell Signaling Technology, Danvers, MA, USA) for 24 h at 4°C. For secondary detection a goat anti-rabbit Cy3-conjugated secondary antibody (1:200; Jackson ImmunoResearch, Ely, UK) was used for 1.5 h at room temperature in the dark. Coverslips were then mounted using Prolong Gold antifade reagent with DAPI (Invitrogen). Four representative images per slice were taken with an Axioplan II epifluorescence microscope (Zeiss). Quantification was determined as the proportion of total cells exhibiting nuclear phospho-NF-ĸB p65.
Superoxide generation
Cells were incubated in Dulbecco's PBS (DPBS) at 37°C for 10 min after treatment and detached by gently repeated pipetting. Cell suspensions were collected in detection tubes followed by addition of lucigenin (5 μM; Sigma) and NADPH (100 μM; Sigma). Superoxide generation was detected every 3 s for 3 min by chemiluminescence reaction using a AutoLumat LB953 Multi-Tube Luminometer (Berthold Technologies, Germany).
Flow cytometry
Cells were detached with 2 mM EDTA for 45 min at 37°C, then stained with antibodies specific for CD86, PD-L1, PD-L2, and CD206, and isotype controls (all from BD Pharmingen, Stockholm, Sweden). Samples were detected using a Gallios flow cytometer (Beckman Coulter, Brea, CA, USA), and Kaluza version 1.1 software (Beckman Coulter) was used for analysis. The median fluorescence intensity (MFI) of CD86, PD-L1, PD-L2, and CD206 was quantified as the readout of macrophage activation.
Gene expression analysis
Mice were killed 72 h after surgery and perfused transcardially with ice-cold 0.1 M PBS to avoid blood contamination. The hippocampus was rapidly dissected on ice and stored at –80°C. Total RNA was then extracted using RNeasy Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. An Agilent 2100 bioanalyser (Agilent Technologies, Palo Alto, CA, USA) was used for the RNA quality control and the samples with RNA integrity number (RIN) higher than 5.5 were used for further microarray analysis. Hippocampal RNA was hybridised to the Affymetrix Mouse Gene 2.1 ST 16-Array Plate (Affymetrix, Santa Clara, CA, USA) at the Karolinska Institutet's Bioinformatics and Expression core facility. The functional annotation tool from the Database for Annotation, Visualization, and Integrated Discovery (DAVID) software was used to identify gene ontology (GO) categories and biological pathways of differentially expressed genes.22 A cut-off at P<0.05 and fold change >2 or >1.5 was used for analysis. This dataset is deposited and available at GEO (submission number GSE115440).
Human patient enrollment
CSF samples were collected via lumbar puncture from 11 older adult subjects (age ≥60 yr) before, 24 h after, and 6 weeks after non-cardiac, non-neurological surgery. They were prospectively enrolled in the Markers of Alzheimer's Disease and Neurocognitive Outcomes after Perioperative Care (MADCO-PC) study,23 which is registered with clinicaltrials.gov (NCT01993836) and approved by the Duke University Medical Center Institutional Review Board. All MADCO-PC subjects underwent informed consent before completing any study activities, and then completed a neurocognitive test battery before and 6 weeks after surgery. Patients were excluded if they were pregnant, taking anticoagulants in contravention of the American Society of Regional Anesthesia and Pain Medicine (ASRA) guidelines, taking chemotherapeutic drugs, or were correctional facility inmates.
Perioperative CSF samples
Before lumbar puncture, the lower back was sprayed with 20% benzocaine spray (available without prescription in the USA), and then an additional 2–5 ml of 1% lidocaine was injected subcutaneously at the planned lumbar puncture site. After waiting for several minutes, CSF samples were obtained via lumbar puncture at the L4–5 interspace (or one space more rostral or caudal) with a 25 g pencil point needle. First, 10 ml of CSF was gently aspirated with a 10 ml polypropylene syringe, and was then placed in a pre-chilled 15 ml polypropylene conical tube on ice. Within 1 h, this CSF sample was separated into 1 ml aliquots using low binding polypropylene pipette tips, which were then frozen at –80°C. Cognitive testing was performed, and continuous cognitive indices were calculated as described.23 As no prior study had measured MaR1 in human CSF, there were insufficient data to perform a sample size or power analysis calculation to evaluate how many patients (or samples) are necessary to determine MaR1 in human CSF. Thus, we simply used samples from 11 MADCO-PC subjects for this pilot study. Because a prior study that measured other lipid concentrations in human CSF found that several millilitres of CSF was necessary for successful detection of low abundance lipid molecules,24 we chose to measure MaR1 levels in 3 ml CSF from each subject's sample time point.
Human CSF lipidomic analysis
CSF samples (three aliquots of 1 ml each per subject at each time point) were shipped via overnight express on dry ice to the Lipid Core Research Facility at Wayne State University (Detroit, MI, USA). Liquid chromatography mass spectrometry (LC-MS) grade methanol was added to the internal standard supplemented samples to a final concentration of 15%. Samples were sonicated in a bath sonicator for 2 min and left on ice for 1 h in the dark, and were applied to pre-conditioned C18 solid phase extraction cartridges (StrataX C18, Torrance, CA, USA, 30 mg; Phenomenex; conditioned with 2 ml methanol followed by 2 ml water containing 15% methanol), washed with 2 ml 15% methanol in water followed by 2 ml hexane, and dried under vacuum. Cartridges were eluted directly into high-performance liquid chromatography (HPLC) autosampler vials with 1 ml methanol containing 0.1% formic acid. Eluates were evaporated to dryness under a gentle stream of nitrogen while maintaining external temperature at 25°C. The dried residue was immediately reconstituted in methanol, vials flushed with nitrogen, capped, and stored at –80°C until analysis. At the time of LC-MS analysis, samples were thawed to room temperature and an equal volume of 25 mM aqueous ammonium acetate was added, vortex mixed, and loaded in the autosampler maintained at 15°C. HPLC is performed on a Prominence XR system (Shimadzu, Kyoto, Japan) using a Luna C18 (3 μm, 2.1 × 150 mm) column. The mobile phase consisted of a gradient between A (methanol–water–acetonitrile, 10:85:5 v/v) and B (methanol–water–acetonitrile, 90:5:5 v/v), both containing 0.1% ammonium acetate. The gradient program with respect to the composition of B was as follows: 0–1 min, 50%; 1–8 min, 50–80; 8–15 min, 80–95%; and 15–17 min, 95%, with flow rate 0.2 ml min−1. The HPLC eluate was directly introduced to the electrospray ionization (ESI) source of a QTRAP5500 mass analyser (SCIEX), Redwood City, CA in the negative ion mode with the following conditions: curtain gas, GS1, and GS2: 35 psi; temperature: 600°C; ion spray voltage: –2500 V; collision gas: low; declustering potential: –60 V; entrance potential: –7 V. The eluate was monitored via multiple reaction monitoring (MRM) to detect unique molecular ions. MRM was scheduled to monitor each transition for 120 s around the established retention time for each lipid mediator. Optimised collisional energies (18–35 eV) and collision cell exit potentials (7–10 V) were used for each MRM transition. Mass spectra for each detected lipid mediator were recorded using the Enhanced Product Ion (EPI) feature to verify the identity of the detected peak in addition to MRM transition and retention time match with the standard. Data were collected using Analyst 1.6.2 software, and the MRM transition chromatograms were quantitated using MultiQuant software (both from SCIEX). The detection limit for MaR1 was 0.1 pg on the column, and the quantitation limit was 3 pg on the column. The internal standard signals in each chromatogram were used for normalisation for recovery and relative quantitation of each analyte.
Statistical analysis
Data from mice were expressed as mean (standard error of the mean, sem). Sample size calculation was performed with one-way analysis of variance (anova) omnibus fixed-effects analysis using G*Power v 3.1 (http://www.gpower.hhu.de). Based on our previous work and preliminary studies,13, 21 the effect size f of the primary analysis (trace fear conditioning and neuroinflammation) between interventions were larger than 1. Thus, a study analysing four groups with five or more individuals per group would have 80% power to detect an effect size f of 0.835, which corresponds to the critical F value of 3.24. Differences between groups were compared using Student's t-test (two groups), one-way anova (multiple groups), or two-way anova (multiple groups at multiple time points) followed by post hoc Bonferroni test (GraphPad version 7.0; GraphPad Software, San Diego, CA, USA). Statistical significance was defined as P<0.05. For human CSF data, Friedman's test was used to evaluate the change in CSF MaR1 levels over the full study period, and Wilcoxon signed rank tests were used to assess change between pairs of time points, because of the small sample size and skewed distribution of MaR1 levels in this cohort.
Results
Pro-resolving and anti-inflammatory effects of maresin 1
C57BL/6 and Ccr2RFP/+Cx3cr1GFP/+ mice were continuously monitored during surgery for haemodynamic changes. No significant differences were observed in average heart rate (HR) and HR variability, and the percentage oxygen saturation (SpO2) remained >95% throughout the procedure (not shown). All mice were included in the study.
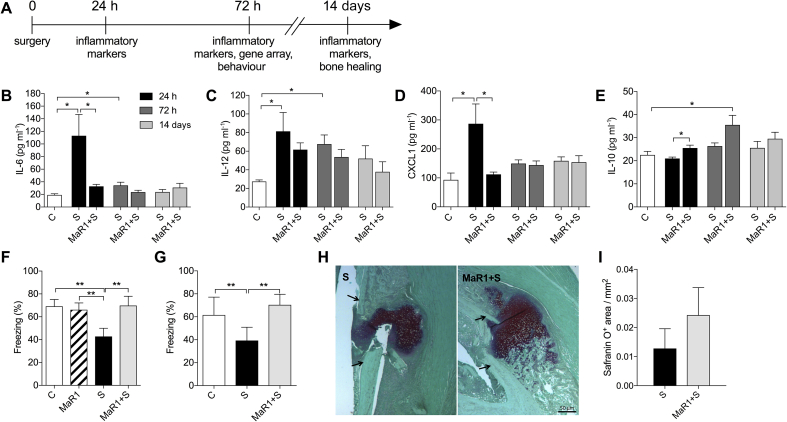
We evaluated systemic changes in the expression of inflammatory cytokines and the process of bone healing up to 14 days after surgery (Fig. 1a). Surgery induced a systemic inflammatory response at 24 h, with significantly elevated levels of IL-6 [113 (34) pg ml−1; P=0.02], IL-12 [81 (20) pg ml−1; P=0.03], and CXCL1 [287 (68) pg ml−1; P=0.01] compared with control mice (Fig. 1b–d). MaR1 prophylaxis reduced the levels of IL-6 and CXCL1 to baseline [33 (3) pg ml−1, P=0.04; and 112 (8) pg ml−1, P=0.02] compared with the vehicle-treated surgery group (Fig. 1b,d) and attenuated IL-12 (P=0.77), which remained up-regulated in plasma up to 72 h after surgery [68 (7) pg ml−1, P<0.01 vs Control; Fig. 1c]. Notably, MaR1 induced a sustained IL-10 response both at 24 h [26 (1) pg ml−1] and 72 h [36 (4) pg ml−1] after surgery compared with vehicle-treated mice with osteotomy [21 (1) pg ml−1, P=0.038 and 26 (1) pg ml−1, P=0.097, respectively; Fig. 1e]. All of these cytokines levels returned to baseline by day 14 (Fig. 1b–e).
Fig 1.
Anti-inflammatory and pro-resolving effects of maresin 1 prophylaxis. (a) Study design and endpoints. (b–e) Time course of plasma levels of interleukin (IL)-6, IL-12, CXCL1, and IL-10 at 24 h, 72 h, and 14 days after orthopaedic surgery. (f) Cognitive evaluation of mice using contextual fear conditioning. Training was performed before surgery and hippocampus-dependent memory was assessed at 72 h. MaR1 prevented surgery-induced cognitive dysfunction in both wild-type mice and (g) Ccr2RFP/+Cx3cr1GFP/+ transgenic mice. (h–i) Effects of MaR1 on bone healing 14 days after stabilised tibia fracture surgery. Arrows indicate the original cortical bone. Scale bar: 50 μm. Data expressed as mean (sem); *P<0.05; n=5–6 per group (**P<0.01, n=8–10 for behaviour). C, Control; MaR1, Maresin 1; S, Surgery; sem, standard error of the mean.
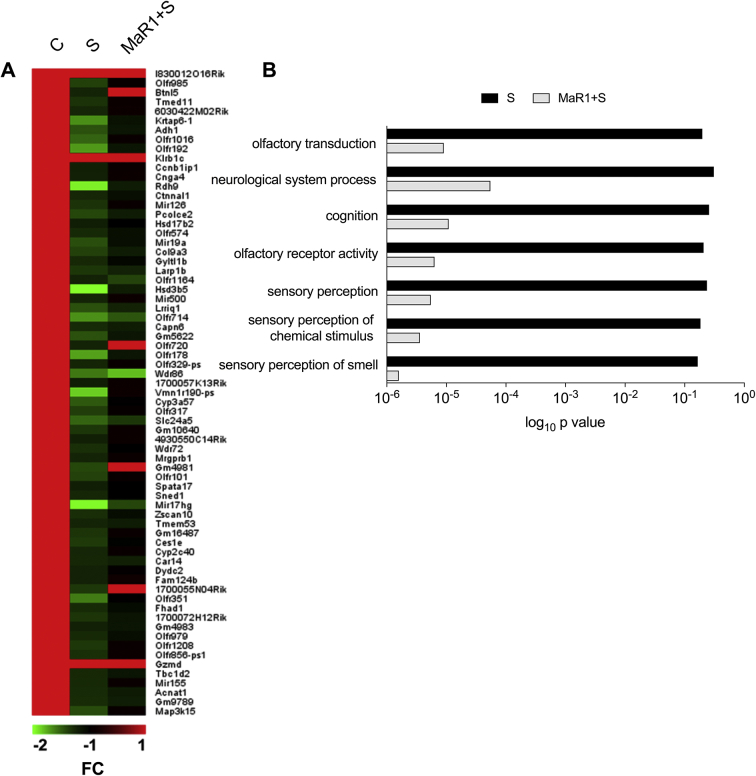
Systemic inflammation after infection or injury is well appreciated to affect behaviour and cognitive function. We tested hippocampal memory function using contextual fear conditioning in wild-type C57BL/6 and Ccr2RFP/+Cx3cr1GFP/+ mice. Training was performed before any intervention, and working memory was established for the context after tone/shock pairing. Mice were tested in the same context but with no cues 72 h after surgery. Freezing time was significantly improved in animals receiving MaR1 treatment in both wild-type and Ccr2RFP/+Cx3cr1GFP/+ mice (Fig. 1f and g; P<0.01), indicating better memory outcome after treatment. There was a significant effect on freezing time analysed using anova. Locomotor activity at 72 h was also measured using an open field, and no differences in exploratory time were observed amongst groups (not shown). We also performed gene expression analyses in hippocampal tissues showing a protective effect of MaR1 on biological pathways of relevance to neurological system processes and cognition (Supplementary Fig. S1a and b).
Resolvins exert dual anti-inflammatory and pro-resolution effects, which help stimulate pathways to terminate inflammation and influence tissue repair.19 We evaluated the effects of MaR1 on bone healing and callus formation after fracture and tibial pinning. Safranin O/Fast Green staining showed no statistically significant difference in callus formation in mice treated with MaR1 compared with vehicle on day 14 (P=0.35; Fig. 1h and i).
Maresin 1 prevented neuroinflammation and macrophage infiltration after surgery
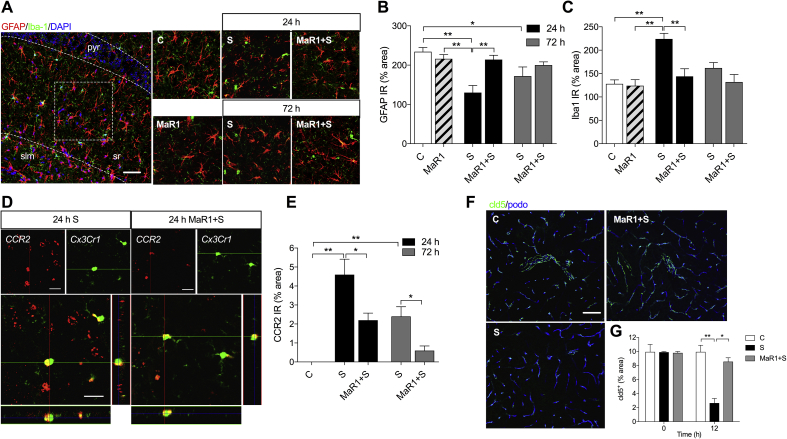
We previously described changes in glial activation, including microglia and astrocytes, using this model of orthopaedic surgery.13, 21 Here we evaluated the effects of MaR1 on neuroinflammation by measuring changes in GFAP and Iba-1 immunoreactivity in the hippocampus at 24 and 72 h after surgery. GFAP expression in the hippocampus was acutely dysregulated in the surgical group at 24 h [GFAP IR: 130 (18)% area in the Surgery group and 234 (11)% area in the Control group; P<0.01], returning to normal levels at 72 h [GFAP IR: 172 (23)% area; Fig. 2a and b]. Mice treated with MaR1 (1 ng per mouse) retained the physiological features of classical astrocytes, with longer and more complex processes than mice treated with vehicle.
Fig 2.
Maresin 1 prevents hippocampal neuroinflammation by regulating macrophage infiltration and claudin-5 (cld-5) expression at the blood–brain barrier. (a) Representative confocal images immunostained for GFAP and Iba1 at 24 h and 72 h. (b, c) Quantification of GFAP and Iba1 immunostaining. Surgery affected astrocytic processes and microglia/macrophage activation peaking at 24 h, with mild changes at 72 h. MaR1 pretreatment significantly reduced surgery-induced neuroinflammation. (d) Representative z-stack images from Ccr2RFP/+Cx3cr1GFP/+ at 24 h show macrophage infiltration (red) and residency microglia (green) in the CA1–CA3 hippocampus region. (e) Treatment with MaR1 reduced macrophage infiltration into hippocampus at 24 and 72 h after surgery. (f, g) Representative images for cld-5 immunostaining. MaR1 prevented cld-5 downregulation on the hippocampal vasculature, restoring the expression to control levels. Scale bar: 50 μm. Data expressed as mean (sem). *P<0.05, **P<0.01; n=3–5 per group. C, Control; DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; MaR1, Maresin 1; slm, stratum lacunosum-moleculare; pyr, pyramidale; sr, stratum radiatum; S, Surgery; sem, standard error of the mean.
MaR1 also improved microglial/macrophage activation as measured by morphological changes in Iba-1 immunoreactivity. Surgery induced more stout microglia in the hippocampus at 24 h [Iba1 IR: 224 (12)% area in the Surgery group and 128 (9)% area in the Control group; P<0.01], whereas mice treated with MaR1 retained a more ramified morphology reminiscent of the physiological state [Iba1 IR: 144 (16)% area; Fig. 2a and c]. Fewer activated microglia were evident at 72 h after surgery, with levels returning to baseline (Fig. 2a and c).
We used Ccr2RFP/+Cx3cr1GFP/+ knock-in mice to differentiate between monocyte-derived macrophages and resident microglia cells. At 24 h after surgery, the peak of neuroinflammation, we recorded an increase in CCR2+ cells in the vehicle-treated surgery group [CCR2 IR: 4.6 (0.8)% area at 24 h, P<0.01 vs Control and 2.4 (0.5)% area at 72 h, P<0.01 vs Control; Fig. 2d and e], indicating the presence of monocyte-derived macrophages in the CA1–CA3 area of the hippocampus. Prophylaxis with MaR1 in Ccr2RFP/+Cx3cr1GFP/+ mice limited macrophage infiltration in the brain parenchyma [CCR2 IR: 2.2 (0.4)% area at 24 h, P=0.04 vs vehicle-treated group, and 0.6 (0.2)% area at 72 h, P=0.02 vs vehicle-treated group; Fig. 2d and e]. Notably, macrophage infiltration was preceded by a reduction in claudin-5 (cld-5) expression, a key tight-junction protein critical to blood–brain barrier (BBB) integrity, at 12 h after surgery [cld5+ IR: 2.7 (0.7)% area in the Surgery group and 10 (1)% area in the Control group, P<0.01; Fig. 2f and g]. Mice treated with MaR1 were protected from changes in cld-5 (Fig. 2f and g).
Maresin 1 reduced LPS-induced NF-κB nuclear translocation and superoxide generation in BMDMs
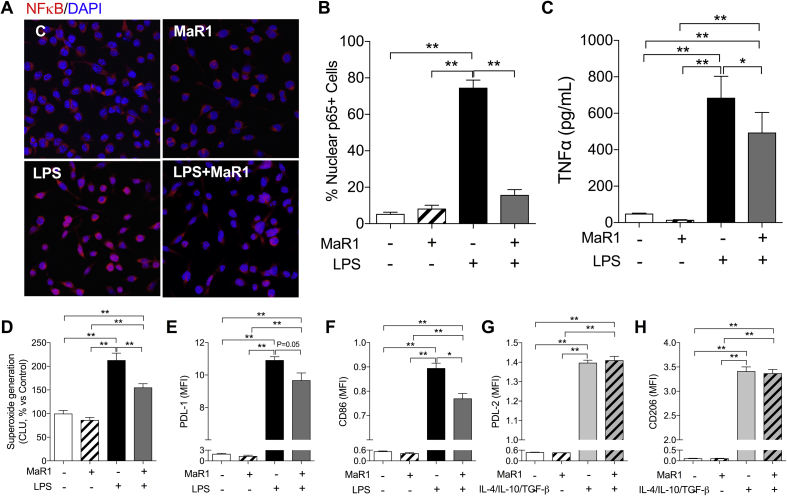
To better investigate the effects of MaR1 on CCR2+ monocytes, we cultured BMDMs in the presence of LPS with or without MaR1. LPS stimulation induced evident NF-κB p65 nuclear translocation [nuclear p65+ cells: 75 (4)% in the LPS group and 5 (1)% in the Control group, P<0.01; Fig. 3a and b] and TNF-α release in the culture medium [685 (52) pg ml−1 in the LPS group and 49 (1) pg ml−1 in the Control group, P<0.01; Fig. 3c]. Co-incubation with MaR1 (10 nM) significantly attenuated LPS-induced NF-κB p65 nuclear translocation in BMDMs [nuclear p65+ cells: 16 (3)%, P<0.01 vs LPS group, Fig. 3a and b], and partly reduced TNF-α levels in the culture medium [494 (49) pg ml−1, P=0.01 vs LPS group; Fig. 3c].
Fig 3.
Maresin 1 effects on bone marrow-derived macrophages (BMDMs). (a) Representative images of immunostaining of NF-κB in BMDMs. Stimulated with 10 ng ml−1 LPS for 2 h. (b) 2 h of LPS stimulation (10 ng ml−1) significantly increased NF-κB nuclear translocation in BMDMs. (c) LPS caused significant increase of TNF-α release from BMDMs after 24 h stimulation, which was reduced by co-application of MaR1 (10 nM). (d) NADPH oxidase mediated superoxide production in BMDMs was significantly increased after 24 h of LPS stimulation, which was inhibited by MaR1 co-application. (e–h) MaR1 reduced LPS-induced PD-L1 and CD86 expression in BMDMs at 24 h. No significant effect of MaR1 on PD-L2 and CD206 expression in BMDMs stimulated by M2 polarisation cytokines (IL-4/IL-10/TGF-β). Data are expressed as mean (sem). *P<0.05, **P<0.01; n=3–5 per group. CLU, chemiluminescence unit; IL, interleukin; LPS, lipopolysaccharide; MaR1, Maresin 1; MFI, median fluorescence intensity; NADPH, nicotinamide adenine dinucleotide phosphate; sem, standard error of the mean; TNF, tumour necrosis factor.
MaR1 significantly decreased LPS-induced NADPH oxidase activity in BMDMs at 24 h, indicated by the lower superoxide generation [Chemiluminescence Unit, % Control: 213 (15)% in the LPS group and 155 (8)% in the LPS+MaR1 group, P<0.01; Fig. 3d]. In addition, enhancement of PD-L1 and CD86 by LPS stimulation was significantly reduced by co-incubation of MaR1 (Fig. 3e and f). MaR1 treatment did not significantly affect PD-L2 and CD206 changes in response to anti-inflammatory macrophage stimulation (IL-4/IL-10/TGF-β; Fig. 3g and h).
Human CSF maresin 1 levels before and after non-cardiac surgery
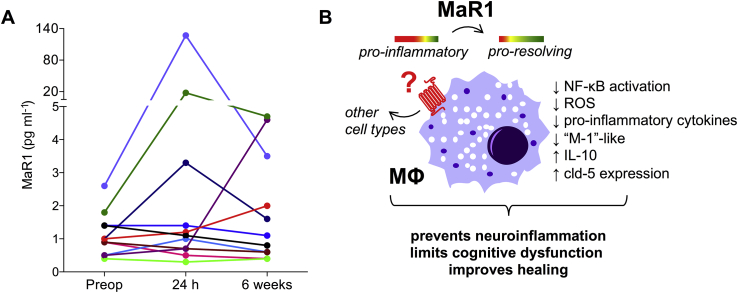
To determine the potential role of endogenous MaR1 in resolving neuroinflammation in patients at risk for developing PNDs, we measured CSF MaR1 levels in 11 older adults before and after major non-cardiac, non-neurological surgery. Baseline preoperative data and postoperative cognitive performance data in these 11 patients are presented in Table 1. CSF MaR1 levels were detectable in all 11 patients before and after surgery, although we detected no statistically significant overall change in MaR1 levels in this pilot patient sample (Fig. 4a; P=0.53), nor between any pair of study time points. A scatterplot of these data is presented in Supplementary Fig. S2.
Table 1.
Patient characteristics and cognitive data. sd, standard deviation
| All (n=11) | |
|---|---|
| Age, range | 60–80 |
| Male sex, n (%) | 10 (91) |
| ASA physical status, n (%) | |
| 2 | 3 (27) |
| 3 | 7 (64) |
| 4 | 1 (9) |
| Surgical service, n (%) | |
| Thoracic | 1 (9) |
| General surgery | 3 (27) |
| Urology | 7 (64) |
| Surgery duration (min incision to end procedure), median (Q1, Q3) | 215 (117, 297) |
| Years of education, median (Q1, Q3) | 16 (14, 20) |
| Mini-Mental Status Exam Score | |
| Preoperative, median (Q1, Q3) | 29 (27, 30) |
| 6-week, median (Q1, Q3) | 29 (27, 29) |
| Cognitive index | |
| Preoperative, mean (sd) | 0.23 (0.80) |
| 6-week, mean (sd) | 0.29 (0.74) |
Fig 4.
Maresin 1 levels in CSF before, 24 h, and 6 weeks after major non-cardiac, non-neurologic surgery in older patients (age ≥60 yr). (a) Each line represents a single patient. Diagonal cross marks on the X and Y axes indicate non-linearity/scale discontinuity. n=11. (b) Working model for MaR1 protection in PNDs. Pre-treatment with MaR1 regulated excessive inflammation from circulating macrophage by reducing NF-κB activation, oxidative stress, pro-inflammatory cytokine release, overall contributing to a ‘M2-like’ switch. This systemic milieu did not impair fracture healing or cause signs of immunosuppression. In fact, these systemic effects prevented loss of cld-5 expression at the blood–brain barrier (BBB) and macrophage infiltration into the brain parenchyma. Overall, dampening the postoperative neuroinflammation leads to improved cognitive function. CSF, cerebrospinal fluid; IL, interleukin; MaR1, Maresin 1; NF-κB, nuclear factor-kappa B; PNDs, perioperative neurocognitive disorders; ROS, reactive oxygen species.
Discussion
In the present study, maresin 1, a novel SPM synthetised by macrophages during the inflammatory resolution phase, provides distinct anti-inflammatory and pro-resolving effects by limiting excessive neuroinflammation and facilitating postoperative recovery (Fig. 4b). Dysregulated inflammation and failed resolution is now a well-recognised phenomenon in a variety of disease models.25 Trauma initiates an immune response contributing to the overall extent of injury and outcome. We previously reported a key role of TNF-α in initiating a cytokine cascade, contributing to the onset of cognitive decline after surgery.26 TNF-α is one of the earliest cytokine to appear after damage and is responsible for the production of many mediators, including IL-1β and IL-6. Our results show that MaR1 attenuates release of systemic pro-inflammatory cytokines and TNF-α in macrophages. Besides reducing phosphorylation and nuclear translocation of the NF-κB p65 subunit, a critical component in the regulation of the inflammatory process, MaR1 also attenuated superoxide generation in activated macrophages. The synergism/crosstalk between pro-inflammatory cytokines and oxidative stress in the pathophysiology of cognitive decline remains to be further explored. MaR1 exerts protective effects after vascular injury through modulation of NADPH-oxidase 4 (NOX-4) and NF-κB activity in vascular smooth muscle cells.27 This is consistent with our current findings in BMDMs and previous reports that other SPMs reduced reactive oxygen species production.21, 28, 29, 30
Maresins are stereotypical pro-resolving mediators that exert dual actions after trauma or infection by limiting polymorphonuclear leukocyte entry and stimulating efferocytosis.2 MaR1 exerts potent actions in models of peritonitis,5 colitis,29 and acute respiratory distress syndrome,7 in some cases being superior to other resolvins. Recently, we described a role for MaR1 in preventing postoperative pain in this model of orthopaedic surgery, even when administered 2 weeks after surgery.31 Acute perioperative treatment with MaR1 and DHA also delayed the development of mechanical allodynia and cold allodynia. MaR1 was also shown to modulate transient receptor potential vanilloid 1 (TRPV1) currents in dorsal root ganglia neurones, thereby producing analgesia.6 It is possible that managing postoperative pain signalling with SPMs, including MaR1, may interfere with the onset of neuroinflammation and cognitive decline. In addition, we described the protective effects of MaR1 on BBB permeability and other cell types, including microglia and astrocytes. Although SPMs stereotypically interact with cell-surface G-protein coupled receptors,32 the target of MaR1 will likely contribute to novel opportunities for resolution pharmacology.
These findings have clinical relevance to several common complications in the postoperative period, including prevention of delirium and postoperative cognitive dysfunction (POCD), and improvement of bone healing. The regenerative properties of MaR1 were initially reported using a surgical model in plenaria, a worm with the intrinsic ability to regenerate.6 We found that in rodents MaR1 does not impair the process of bone repair but actually enhances callus formation. We also found that MaR1 increased the systemic levels of IL-10 with a long-lasting trend up to postoperative day 14. IL-10 has anti-inflammatory actions but its contribution to the healing process is not fully established.33 The possible anti-inflammatory actions that IL-10 has on the pro-inflammatory niche, potentially attenuating neuroinflammation and cognitive decline, might be different from the pro-resolving effects that a ‘resolution agonist’ have to restore physiological function in the tissue.19 Notably, maresins were recently shown to interact with stem cells, limiting chronic inflammation and improving wound healing in diabetes mellitus and spinal cord injury.34, 35
We describe an attenuation of classically associated M1 markers in macrophages stimulated by LPS, including expression of miR155 in hippocampus, which has an established role in controlling Toll-like receptors (TLR) and NF-κB signalling.36, 37 Other SPMs aside from MaR1 have been shown to modify miRNAs, reduce nuclear translocation of NF-κB, and downregulate phospho-IκB, both in murine and human macrophages.38 Although we could not detect significant changes in M2 markers in vitro, it has been shown that MaR1 enhances ‘M2-like’ polarisation in vivo.29 The total organic synthesis and complete stereochemistry assignment performed by Dalli and colleagues39 identified a precursor in the MaR1 biosynthesis pathway, termed 13,14-epoxy-maresin (13,14-eMaR), which accounts for the change in human macrophage polarisation toward a homeostatic and tissue-protective ‘M2-like’ phenotype. This switch to an ‘M2-like’ profile may account for the multiple organoprotective actions of MaR1 that were also evident in our model, including promotion of tissue healing and attenuation of CCR2+ cell infiltration in the hippocampus. This study further illustrates the bidirectional communication between the periphery and brain, and the importance of macrophages in mediating CNS pathology. BBB dysfunction may represent a key mechanism contributing to neurological dysfunction and complications after surgery,40 and provides a potential pathway for peripheral macrophage access to the brain. This ‘passive’ diffusion through a relative permeable barrier may account for the subsequent neuroinflammatory response.13, 14 MaR1 prevented damage to the BBB via regulation of the expression of tight junctions (cld-5) and expression of endothelial microRNAs, such as miR126, that are implicated in response to stress and inflammation.41 This is consistent with the effects of resolvins and maresins on endothelial function and vasculature homeostasis, regulating diapedesis and stimulating vasoprotective and anti-thrombotic mediators.19, 42 In addition to reducing macrophage trafficking and neuroinflammation after surgery, MaR1 also restored memory function in both wild-type and Ccr2RFP/+Cx3cr1GFP/+ mice, suggesting that block of peripheral cell influx into the brain is a potential avenue to treat surgery-induced cognitive disorders. Evidence of de novo synthesis of pro-inflammatory cytokines and chemokines (including MCP-1) in the hippocampus has been reported, suggesting ‘active’ recruitment of peripheral monocytes into the CNS.12, 14 The mechanisms whereby systemic trauma contributes to changes in brain physiology require further investigation. It is suggested that CCR2+ Ly6Chi monocytes (as labelled in the CCR2-RFP knock-in mouse) initiate and sustain neuroinflammatory responses.43 Specific CCR2 subsets may be vital to the recovery process and local anti-inflammatory actions.44
Lipoxins, resolvins, protectins, and maresins were recently identified in human peripheral blood and lymphoid organs through metabololipidomics.45 We detected MaR1 in the CSF of older patients before and after non-cardiac, non-neurological surgery. Although we found no evidence of overall change in MaR1 levels in this limited cohort, two patients exhibited markedly greater change than others. There are potentially important differences between the subset of patients with large 24 h increases in MaR1 and those without preoperative factors commonly associated with cognitive outcomes (age, baseline cognition, years of education). Future studies with larger sample sizes might identify patient and procedural factors associated with postoperative CSF MaR1 increase. Such future studies may provide novel insights into the role of SPMs as biomarkers (such as for PND severity) in the perioperative period.
Limitations in our study include the specific interrogation of MaR1 signalling after surgery. Although MaR1 is a primary product of monocytic cell types, the ligand–receptor interactions are not yet fully defined. MaR1 can lead to complex metabolites that exert different temporal functions in the resolution of inflammation.9 This bioactive metabolome impacts multiple signalling pathways (anti-inflammation, regeneration, anti-nociception, etc.) which require further investigation in the perioperative context. For example, we recently described the analgesic effects of MaR1 at significantly higher doses (500 ng) and different routes of administration, including intrathecal.31 This suggests a different mode of action and effects on cell types that could be harnessed for different therapeutic needs. Earlier strategies aimed at attenuating the neuroinflammatory response after surgery have focused on dampening the innate immune response, with potential implications to both host defense and organ function. SPMs display potent anti-inflammatory actions without being immunosuppressive.42 Although clinical studies are identifying inflammatory biomarkers implicated with cognitive impairment after surgery, the role of neuroinflammation in PNDs needs to be further established.
In summary, our results identify MaR1 as a novel regulator of postoperative inflammation and ensuing cognitive decline through the modulation of macrophage trafficking and activity. These findings suggest a novel therapeutic potential for SPMs in preventing surgery-induced cognitive decline, including facilitating postoperative recovery, and provide evidence for detection in human CSF after surgery.
Authors' contributions
Bone healing experiments: PTN, ASC.
Gene array: SM.
Cell culture assays: TY., GX., M. Carlström, X-MZ.
Human study: M. Cooter, MB.
Lipidomic analyses: KRM.
Neuroinflammation and behavioural experiments: TY, GX, NT.
Provided key reagents: M. Carlström, RAH, KA.
Data analysis: all authors.
Drafting of the manuscript: TY., NT.
All authors participated in the revision and gave approval to the final version of the manuscript. All authors contributed to the study design.
Acknowledgements
The authors thank M. Goiny (senior researcher, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden) for excellent technical support.
Handling editor: H.C. Hemmings Jr
Editorial decision: 30 October 2018
Footnotes
This article is accompanied by an editorial: Specialised pro-resolving mediators: the magic bullet for perioperative neurocognitive disorders? by Li et al., Br J Anaesth 2019:122:292–294, doi: https://doi.org/10.1016/j.bja.2018.11.020.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2018.10.062.
Funding
Research grant program of the European Society of Anaesthesiology, Loo och Has Ostermans Foundation for Medical Research, Karolinska Institutet Foundations and National Institute of Aging R01AG057525 and R21AG055877 to NT National Institute of Aging R03 AG050918, K76 AG057022, AG028716, a Jahnigen Scholars Award, and an IARS Mentored Research Award to MB. MB also acknowledges additional support from National Center for Advancing Translational Sciences Clinical and Translational Science Award UL1TR001117 (Contact PI: Dr. Ebony Boulware) and from National Institute of Aging P30 AG028716 (Contact PIs: Drs. Ken Schmader, Miriam Morey, and Heather Whitson). National Institute of Neurological Disorders and Stroke R35 097976 to KA. Lipidomic analysis is supported by National Center for Research Resources grant S10RR027926 (KRM).
Declaration of interest
The authors declare they have no conflicts of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig S1.
Effects of MaR1 on hippocampal gene expression. (A) Filtering data using a statistical significance threshold of P<0.05 and relative expression fold change cutoff of 1.5 vs control mice expression values yielded ∼100 differentially expressed genes, with most of them being down-regulated compared with control values. The heat map shows differentially expressed genes 72 h after surgery (S) and surgery + Maresin 1 (MaR1+S) related to their corresponding control (C) values. Colour code bar indicates control (red) or decreased (shades of green) gene expression fold changes. (B) Analysis of down-regulated genes in the surgery group for the enriched gene ontology terms and biological pathways using David revealed a strong prevalence of genes involved in olfactory sensing, neurological system processes and cognition. n=3 per group for gene array; FC: fold change expression (log2), C: Control, S: Surgery, MaR1: Maresin 1.
Supplementary Fig S2.
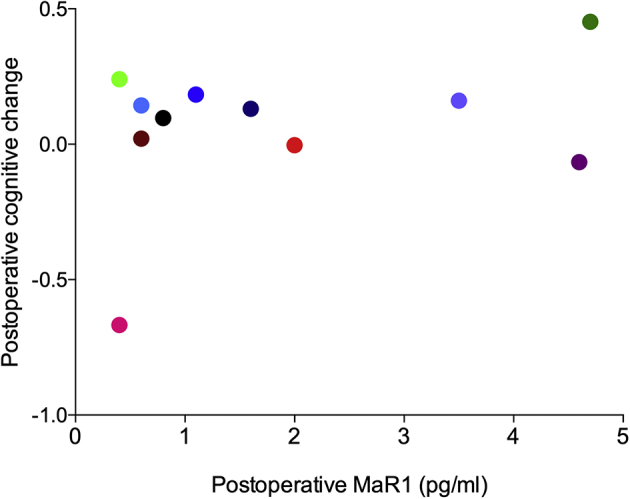
Postoperative changes in cognition and CSF MaR1 levels. Scatterplot representing on the Y axis the change in each patient's overall continuous cognitive index score from within 1 month before to 6 weeks after surgery. A positive score represents postoperative cognitive improvement and a negative score represents a decrement in postoperative cognition. Colours represent the same individual patient as reported in Fig. 4a in the main article.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 2.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serhan C.N., Levy B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128:2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perretti M., Leroy X., Bland E.J., Montero-Melendez T. Resolution pharmacology: opportunities for therapeutic innovation in inflammation. Trends Pharmacol Sci. 2015;36:737–755. doi: 10.1016/j.tips.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Serhan C.N., Yang R., Martinod K. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serhan C.N., Dalli J., Karamnov S. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdulnour R.E., Dalli J., Colby J.K. Maresin 1 biosynthesis during platelet–neutrophil interactions is organ-protective. Proc Natl Acad Sci U S A. 2014;111:16526–16531. doi: 10.1073/pnas.1407123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C.W., Colas R.A., Dalli J. Maresin 1 biosynthesis and proresolving anti-infective functions with human-localized aggressive periodontitis leukocytes. Infect Immun. 2015;84:658–665. doi: 10.1128/IAI.01131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colas R.A., Dalli J., Chiang N. Identification and actions of the maresin 1 metabolome in infectious inflammation. J Immunol. 2016;197:4444–4452. doi: 10.4049/jimmunol.1600837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epelman S., Lavine K.J., Randolph G.J. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry V.H., Newman T.A., Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- 12.D'Mello C., Le T., Swain M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terrando N., Eriksson L.I., Ryu J.K. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degos V., Vacas S., Han Z. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013;118:527–536. doi: 10.1097/ALN.0b013e3182834d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evered L., Silbert B., Knopman D. Recommendations for the nomenclature of cognitive changes associated with anesthesia and surgery. Br J Anaesth. 2018;65:1248–1257. doi: 10.1007/s12630-018-1216-x. [DOI] [PubMed] [Google Scholar]
- 16.Tan H., Cao J., Zhang J., Zuo Z. Critical role of inflammatory cytokines in impairing biochemical processes for learning and memory after surgery in rats. J Neuroinflamm. 2014;11:93. doi: 10.1186/1742-2094-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J., Feng X., Valdearcos M. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth. 2018;120:537–545. doi: 10.1016/j.bja.2017.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soriano S.G., Vutskits L., Jevtovic-Todorovic V., Hemmings H.C., Neurotoxicology B.J.A., Neuroplasticity Study G. Thinking, fast and slow: highlights from the 2016 BJA seminar on anaesthetic neurotoxicity and neuroplasticity. Br J Anaesth. 2017;119:443–447. doi: 10.1093/bja/aex238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M.D., Barde S., Yang T. Orthopedic surgery modulates neuropeptides and BDNF expression at the spinal and hippocampal levels. Proc Natl Acad Sci U S A. 2016;113:E6686–E6695. doi: 10.1073/pnas.1614017113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terrando N., Gomez-Galan M., Yang T. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J. 2013;27:3564–3571. doi: 10.1096/fj.13-230276. [DOI] [PubMed] [Google Scholar]
- 22.Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 23.Giattino C.M., Gardner J.E., Sbahi F.M. Intraoperative fontal alpha-band power correlates with preoperative neurocognitive function in older adults. Front Syst Neurosci. 2017;11:24. doi: 10.3389/fnsys.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Zhu M., Hjorth E. Resolution of inflammation is altered in Alzheimer's disease. Alzheimers Dement. 2015;11 doi: 10.1016/j.jalz.2013.12.024. 40–50 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Terrando N., Monaco C., Ma D., Foxwell B.M., Feldmann M., Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee A., Sharma A., Chen M., Toy R., Mottola G., Conte M.S. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PloS One. 2014;9:e113480. doi: 10.1371/journal.pone.0113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Titos E., Rius B., Gonzalez-Periz A. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol. 2011;187:5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- 29.Marcon R., Bento A.F., Dutra R.C., Bicca M.A., Leite D.F., Calixto J.B. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J Immunol. 2013;191:4288–4298. doi: 10.4049/jimmunol.1202743. [DOI] [PubMed] [Google Scholar]
- 30.Gu J., Luo L., Wang Q. Maresin 1 attenuates mitochondrial dysfunction through the ALX/cAMP/ROS pathway in the cecal ligation and puncture mouse model and sepsis patients. Lab Invest. 2018 doi: 10.1038/s41374-018-0031-x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L., Terrando N., Xu Z.Z. Distinct analgesic actions of DHA and DHA-derived specialized pro-resolving mediators on post-operative pain after bone fracture in mice. Front Pharmacol. 2018;9:412. doi: 10.3389/fphar.2018.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan C.N., Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013;13:632–640. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jetten N., Roumans N., Gijbels M.J. Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses. PloS One. 2014;9:e102994. doi: 10.1371/journal.pone.0102994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong S., Lu Y., Tian H. Maresin-like lipid mediators are produced by leukocytes and platelets and rescue reparative function of diabetes-impaired macrophages. Chem Biol. 2014;21:1318–1329. doi: 10.1016/j.chembiol.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francos-Quijorna I., Santos-Nogueira E., Gronert K. Maresin 1 promotes inflammatory resolution, neuroprotection, and functional neurological recovery after spinal cord injury. J Neurosci. 2017;37:11731–11743. doi: 10.1523/JNEUROSCI.1395-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tili E., Michaille J.J., Cimino A. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 37.Quinn S.R., O'Neill L.A. A trio of microRNAs that control Toll-like receptor signalling. Int Immunol. 2011;23:421–425. doi: 10.1093/intimm/dxr034. [DOI] [PubMed] [Google Scholar]
- 38.Li Y., Dalli J., Chiang N., Baron R.M., Quintana C., Serhan C.N. Plasticity of leukocytic exudates in resolving acute inflammation is regulated by MicroRNA and proresolving mediators. Immunity. 2013;39:885–898. doi: 10.1016/j.immuni.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalli J., Zhu M., Vlasenko N.A. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013;27:2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen M.A., Ryu J.K., Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. 2018;19:283–301. doi: 10.1038/nrn.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schober A., Nazari-Jahantigh M., Wei Y. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spite M., Norling L.V., Summers L. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saederup N., Cardona A.E., Croft K. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shechter R., London A., Varol C. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colas R.A., Shinohara M., Dalli J., Chiang N., Serhan C.N. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307:C39–C54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]