Abstract
The growth of wheat tillers and plant nitrogen-use efficiency (NUE) will gradually deteriorate in response to high plant density and over-application of N. Therefore, in this study, a 2-year field study was conducted with three levels of plant densities (75 ×104plants ha−1, D1; 300 ×104plants ha−1, D2; 525 ×104plants ha−1, D3) and three levels of N application rates (120 kg N ha−1, N1; 240 kg N ha−1, N2; 360 kg N ha−1, N3) to determine how to optimize plant density and N application to regulate tiller growth and to assess the contribution of such measures to enhancing grain yield (GY) and NUE. The results indicated that an increase in plant density significantly increased the number of superior tillers and the number of spikes per m2(SN), resulting in a higher GY and higher partial factor productivity of applied N (PFPN). However, there was no significant difference in GY and PFPN between plant densities D2 and D3. Increasing the N application rate significantly increased the vascular bundle number (NVB) and area (AVB), however, excess N application (N3) did not significantly improve these parameters. N application significantly increased GY, whereas there was a significant decrease in PFPN in response to an increase in N application rate. The two years results suggested that increasing the plant density (from 75 ×104plants ha−1to 336 ×104plants ha−1) in conjunction with the application of 290 kg N ha−1N will maximize GY, and also increase PFPN(39.7 kg kg−1), compared with the application of 360 kg N ha−1N. Therefore, an appropriate combination of increased planting density with reduced N application could regulate tiller number and favor the superior tiller group, to produce wheat populations with enhanced yield and NUE.
Keywords: Nitrogen, Plant density, Nitrogen-use efficiency, Grain yield, Superior tiller group
Introduction
Wheat is one of the most important food crops worldwide. In China, where wheat has accounted for more than 20% of the total sowing area in recent years, production has substantially increased during the past few decades (Chen et al., 2017). Nonetheless, owing to continual population growth, the demand for wheat will continue to increase. Moreover, arable areas have undergone a substantial decrease in extent due to urban expansion and environmental degradation (Wei et al., 2016), and wheat production currently faces severe constraints and difficult challenges (Li et al., 2016a). Therefore, it is essential to ensure food security through increasing yield per unit area, to enhance the total production on a diminishing area on cultivated farmland.
Tillering is an important agronomic trait in graminaceous crops such as wheat and rice, as it determines final spike number per m2 and plays an important role in determining grain yield (Naruoka et al., 2011; Wang et al., 2016b). When fewer tillers are produced, a smaller population is formed, resulting in yield reductions due to a smaller population of spikes per m2 (Mitchell et al., 2012; Moeller, Evers & Rebetzke, 2014). Conversely, an excess of surviving tillers can lead to a larger population, resulting in an increased risk of lodging risk due to reduced culm quality (Wang et al., 2009; Zheng et al., 2017). In addition, individual tillers exhibit heterogeneity. In this regard, Cai et al. (2014) reported decreases in grain number and kernel weight per spike as the tiller position shifts from low to high, and the flag leaves of low-position tillers have been shown to have a higher photosynthetic rate than high-position tillers (Xu et al., 2015a). There is also competition between low- and high-position tillers for limiting resources (Elhani et al., 2007; Ma et al., 2008). Early-emerging tillers generally intercept more radiation and shade the late-emerging tillers, and consequently dominate the asymmetric competition from the early growth stage (Moeller, Evers & Rebetzke, 2014). Late-emerging tillers generally make a minimal contribution to grain yield formation (Otteson et al., 2008), and indeed, these unproductive tillers can negatively affect grain yield by competing for resources such as nitrogen (N) and solar radiation (Richards et al., 2002; Berry et al., 2003). Hence, management of tiller emergence and heading, and utilization of early-emerging tillers, are key factors for the establishment of high-yield and high-efficiency populations.
The occurrence of tillers generally involves two developmental stages: the formation of axillary meristems in the leaf axil and the growth of tillers (Li et al., 2003; Bennett & Leyser, 2006). Tiller development is a result of complex interactions between endogenous signals and environmental factors. Internal factors regulating tiller emergence include numerous genetic factors (Xie, Mayes & Sparkes, 2016; Hyles et al., 2017), and endogenous hormones (Lin et al., 2016). External factors including light, plant density, and fertilizer application, have a significant effect on the growth of tillers (Assuero & Tognetti, 2010). The ratio of red to far-red light irradiance influences the outgrowth of tiller buds (Evers et al., 2006), whereas increasing planting density from 135 to 405 plants per m2 has been shown to significantly increase grain yield (Dai et al., 2014), although further increases in density do not affect grain yield. Although seeding rate has been shown to have no influence on main leaf development, it does have a considerable effect on tiller development (Chen et al., 2008). Increasing the seeding rate has been found to increase the proportion of yield obtained from the main spike and decreases the proportion from high-position tiller spikes (Otteson et al., 2008). Although low plant density produces a higher grain number and grain weight per spike (Li et al., 2016b), this is generally not sufficient to compensate for the lower spike density per m2 generated by a lower tiller density (Clerget et al., 2016). Therefore, an appropriate increase in plant density to balance yield component factors would appear to be an apposite agronomic management strategy for enhancing grain yield.
Nitrogen is an important and essential nutrient for all plants. N supply has considerable effects on plant growth in terms of the amount of biomass produced, the size and proportion of organs and their structure, and the progress of plant development (Lawlor, Lemaire & Gastal, 2001). N regulates rice tiller bud growth through regulating endogenous hormones and N metabolism (Liu et al., 2011). Nitrogen deficiency is associated with a reduced rate of leaf appearance and reduces the maximum rate of emergence for each tiller (Prystupa, Slafer & Savin, 2003), whereas an increase in N application rate increases tiller density and reduces tiller mortality (Ahmed et al., 2016). Increasing N application increases yield through an increase in grain yield in early-emerging tillers (Wang et al., 2016a). Furthermore, grain number per unit area is significantly increased in response to N application, mainly due to an increase in the contribution of grains produced by tillers (Terrile, Miralles & González, 2017). In China, farmers excessively apply N fertilizers because of their hope to sustain further grain yield increases, but grain yield does not keep synchronous increase with excessive N application (Meng et al., 2016). N application during the wheat growing season generally exceeds 320∼350 kg N ha−1; however, some farmers uses rates as high as 750 kg N ha−1 (Cui, Chen & Zhang, 2010; Lu et al., 2015; Zhang et al., 2015). Over-application of N contributes little to enhancing grain yield but can reduce nitrogen-use efficiency (NUE) and enhances the risk of environmental pollution (Cui, Chen & Zhang, 2010; Zhang et al., 2015; Mon et al., 2016; Tian et al., 2017). Therefore, achieving both high yield and high NUE simultaneously is a major challenge. Lu et al. (2014) have reported that optimal N management can optimize population quantity and quality to achieve high grain yield, and therefore a high-yielding wheat population can be obtained by amending N supply to regulate tiller development. Accordingly, N application rates should be calculated to produce the required quantity of tillers and modified over time to generate wheat crops with the desired NUE.
Although previous studies have either reported the effects of plant density on tiller development (e.g., tiller emergence, tiller mortality, and tiller production) or focused on N demand and supply, there have been few studies that have systematically investigated the relationship between these aspects, for example, the mechanisms underlying the differences in grain yield among different tiller spikes and how to manipulate tillers to establish high-yield and high-efficiency groups. Moreover, little is known regarding how plant density and N application interactively regulate tiller growth and wheat population development to produce higher grain yield and NUE. Therefore, the objectives of this study were as follows: (i) to quantify the variation between tillers regarding yield formation at different plant densities and N levels; and (ii) to build a model to determine the optimal planting density and N application strategies for realizing a high-yielding and high N efficient population structure.
Materials and Methods
Plant materials and growth conditions
The field experiments conducted in this study were carried out over two winter wheat growing seasons (2013–2014 and 2014–2015), at the experimental station of Shandong Agricultural University, Tai’an, China (36°09′N, 117°09′E, 128 m above sea level). This region has a warm and semi-humid continental monsoon climate, with an average total annual solar irradiance of 5.08 × 106 kJ cm−2, an average annual temperature of 13.7 °C, and an average annual rainfall of 631.5 mm. The rainfall and mean temperature distributions of winter wheat growing stages are shown in Fig. S1. The soil in the study area is classified as a Eutric Cambisol according to the World Reference Base for Soil Resources (2014). The top 30 cm of the soil contained 14.7 g kg−1 organic matter, 1.24 g kg−1 total N, 87.2 mg kg−1 available N, 9.6 mg kg−1 available P, and 85.3 mg kg−1 available K. The winter wheat cultivar used for the purposes of this study was Jimai 22 (JM22), which was grown in the experimental plots. Seeds were sown on October 10, 2013 and October 8, 2014, and plants were respectively harvested on June 9, 2014 and June 8, 2015. Disease, pests, and weeds in each treatment were well controlled by managers.
Treatments and experimental design
The experiments were laid out in a two-factor completely randomized design with three replicates. The main plots were assigned to three plant densities (75 × 104 plants ha−1, D1; 300 × 104 plants ha−1, D2; and 525 × 104 plants ha−1, D3). Subplots were assigned to three urea (N) fertilizer application rates (120 kg N ha−1, N1; 240 kg N ha−1, N2; and 360 kg N ha−1, N3). Each replicate plot (3 m × 3 m) consisted of 10 rows of wheat and two ridges. Row spacing, the width of rides, and a diagram of plant densities are shown in Fig. S2. Half the amount of N, 75 kg ha−1 P2O5, and 150 kg ha−1 K2O were mixed into the soil before planting the wheat. The remaining amount of N was applied at the jointing stage in each treatment.
Observations of wheat population dynamics
Uniform plants in a 1 m2 area per plot were selected and tagged with labels for observations of population dynamics at the following stages: three-leaf, over-wintering, stem elongation, heading time (50% of plants headed), and maturity. Twenty plants were randomly selected and tagged with labels in each treatment for observation of tiller number at over-wintering, stem elongation, heading time, and maturity. The main stem was denoted as 0; primary tillers on the main stem in emergence order were referred to as I, II, III, IV, and V. Secondary tillers on the primary tillers in emergence order were referred to as I1 and II1 (Fig. S3). The effective tiller rate (ETR) was calculated using the equation: ETR = the number of tiller spikes per plant at maturity/the maximal number of tillers per plant ×100%. The ineffective tiller rate (ITR) = 100–ETR.
Assessment of the grain-filling process
From 4 days after anthesis (DAA), 30 plants from each treatment in the field experiments were sampled at 4-day intervals until 36 DAA. Spikes were dried at 70 °C to constant weight, dehulled, and weighed. These data were used to characterize the grain-filling process using Richards’ growth equation, as described by Yang et al. (2004):
| (1) |
Grain filling rate (G) was calculated as the derivative of Eq. (1):
| (2) |
Integration of Eq. (2) gave the mean grain-filling rate (Eq. (3)) and the maximum grain-filling rate (Eq. (4)):
| (3) |
| (4) |
where W is the kernel weight (g), A is the final kernel weight (g), t is the time after anthesis (d), and B, k, and N are the coefficients determined by regression. The active grain-filling period (T) was defined as the period during which W constituted from 5% (t1) to 95% (t2) of A.
Assessment of first internode microstructure
The microstructure of the first internode was assessed according to the methods described by Zheng et al. (2017). Fifteen days after anthesis, three plants from each treatment were sampled for microstructural observations. The middle section of the first internode (2 cm in length) of plants from each treatment was fixed in formalin:acetic acid:ethanol for 24 h, dehydrated with ethanol, and embedded in paraffin. Approximately 4-µm-thick sections were obtained using a microtome (Leica, Wetzlar, Germany), and these were sequentially stained with 1% safranin and 0.5% fast green. The stained cross-sections were observed and photographed using a Nikon DS-V3 microscope (Nikon, Tokyo, Japan). The number and area of vascular bundles at the internode were measured and calculated using Image-Pro Plus (Version 6.0; Media Cybernetics, Rockville, MD, USA).
Measurements of single-stem biological yield and grain yield and its components
At maturity, the 20 tagged plants in each treatment were selected and divided into different spikes according to tillering order. The grains from individual spikes were oven dried at 70 °C to constant weight for determinations of grain number per spike and yield per spike. Euclidean distances were used to identify dissimilarities between the different tillers based on grain number and yield per spike.
Spikes in an area of 1 m2 from which no spikes had been sampled were harvested by hand to determine grain yield, number of kernels per spike, and 1,000-grain weight. Each measurement was performed on plants from three different plots.
Polynomial regression with response surface analysis and evaluation of nitrogen-use efficiency
Trend surface simulation was used to analyze the effect of plant densities and N application rates on grain yield. The quadratic polynomial trend surface equation is as follows:
| (5) |
where z is the grain yield (kg ha−1), x is plant density (plant ha−1), y is the N application rate (kg N ha−1), and a0, a1, a2, a3, a4 and a5 are the coefficients determined by regression. These coefficients were calculated by the least square method.
The partial factor productivity for the applied N (PFPN) was used to indicate NUE. PFPN was calculated using the following equation:
| (6) |
where YN is grain yield in the N-applied plot and N is the nitrogen application rate.
Statistical analysis and processing
Statistical analyses were carried out using Data Processing System software version 7.05 (DPS, Hangzhou, China). A multivariate ANOVA was conducted to determine the mean squares, degrees of freedom, and significance levels. Means were compared using LSD test and differences were considered significant at P < 0.05. The data for changes in the wheat population, effective tiller rate, grain number and yield per spike and the partial factor productivity for the applied N were averaged from the 2 years of the study. The trend surface simulation was analyzed using SPSS Statistics 24. Graphs were plotted using SigmaPlot 10.
Results
Results of variance analysis of the year (Yr), plant density (D), nitrogen application rate (N), and their interactions
Variance analysis was conducted using DPS7.05 to find the mean squares and significance (Tables 1 and 2). Gain yield (GY), spike number per m2 (SN), 100-grain weight (TGW), the effective tiller rate (ETR), partial factor productivity of applied N (PFP), yield per spike, grain number per spike, the maximum grain-filling rate (Gmax); the mean grain-filling rate (Gmean), the active grain-filling period (T), and the associated vascular bundle parameters showed no significant effects of year (Yr), the interaction between Yr, plant density (D) and nitrogen application rate (N). In contrast, GY, SN, TGW, ETR, PFP, and the associated grain-filling parameters were significantly influenced by D, N and D × N interactions (Tables 3 and 4).
Table 1. Analysis of variance of the effects of year (Yr), plant density (D), nitrogen application rate (N), and their interactions on the associated yield components parameters.
| Source of variation | GY | SN | TGW | ETR | PFP | WP | Yield per spike | Grain number per spike | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | P | MS | P | MS | P | MS | P | MS | P | MS | P | MS | P | MS | P | |
| Year (Yr) | 159,740 | 0.09 | 131 | 0.72 | 1.35 | 0.12 | 0.27 | 0.79 | 3.76 | 0.13 | 634 | 0.51 | 0.012 | 0.24 | 0.09 | 0.87 |
| Yr × D | 62,212 | 0.32 | 2,013 | 0.15 | 795 | 0.60 | 15,996 | 0.92 | 0.74 | 0.62 | 7,171 | 0.13 | 0.0097 | 0.32 | 1.90 | 0.54 |
| Yr × N | 5,353 | 0.91 | 16.17 | 0.98 | 101 | 0.87 | 231 | 0.90 | 0.25 | 0.85 | 563 | 0.68 | 0.0009 | 0.90 | 4.07 | 0.27 |
| Yr × D × N | 20,075 | 0.83 | 353 | 0.84 | 0.29 | 0.77 | 0.30 | 0.99 | 0.19 | 0.97 | 1,262 | 0.49 | 0.0055 | 0.63 | 2.51 | 0.52 |
Notes.
- Ms
- mean square
- GY
- grain yield
- SN
- spike number per m2
- TGW
- 100-grain weight
- ETR
- the effective tiller rate
- PFP
- partial factor productivity of applied N
- WP
- the wheat population at the jointing stage
The P value <0.05 was considered significant.
Table 2. Analysis of variance of the effects of year (Yr), plant density (D), nitrogen application rate (N), and their interactions on the associated grain-filling parameters.
| Tiller group | Source of variation | Gmax | Gmean | T | NBVB | NSVB | NTVB | ABVB | ASVB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | P | MS | P | MS | P | MS | P | MS | P | MS | P | MS | P | MS | P | ||
| Superior | Year (Yr) | 0.0001 | 0.81 | 0.0001 | 0.69 | 0.03 | 0.24 | 2.24 | 0.07 | 1.5 | 0.18 | 1.85 | 0.19 | 1,011,412 | 0.06 | 21,848 | 0.82 |
| Yr × D | 0.0002 | 0.41 | 0.0004 | 0.63 | 0.04 | 0.22 | 0.13 | 0.82 | 0.0001 | 0.99 | 0.52 | 0.61 | 633 | 0.99 | 206,240 | 0.61 | |
| Yr × N | 0.0001 | 0.94 | 0.0001 | 0.92 | 0.01 | 0.77 | 0.07 | 0.89 | 0.06 | 0.93 | 0.80 | 0.47 | 203,118 | 0.47 | 89,503 | 0.80 | |
| Yr × D × N | 0.0001 | 0.68 | 0.0002 | 0.94 | 0.03 | 0.27 | 0.05 | 0.99 | 0.64 | 0.54 | 0.38 | 0.83 | 152,063 | 0.68 | 147,978 | 0.83 | |
| Inferior | Year (Yr) | 0.0009 | 0.12 | 0.0001 | 0.91 | 0.0002 | 0.95 | 0.07 | 0.72 | 1.85 | 3.85 | 1.19 | 0.14 | 794,760 | 0.22 | 147,585 | 0.14 |
| Yr × D | 0.0006 | 0.19 | 0.0001 | 0.79 | 0.01 | 0.83 | 0.57 | 0.37 | 32.02 | 66.50 | 0.69 | 0.28 | 888,164 | 0.18 | 57,738 | 0.42 | |
| Yr × N | 0.0011 | 0.06 | 0.0002 | 0.26 | 0.02 | 0.67 | 0.13 | 0.79 | 130 | 270.50 | 0.07 | 0.87 | 277,122 | 0.58 | 11,855 | 0.83 | |
| Yr × D × N | 0.0002 | 0.72 | 0.0001 | 0.78 | 0.02 | 0.85 | 0.55 | 0.43 | 0.02 | 0.04 | 0.07 | 0.97 | 54,529 | 0.98 | 14,425 | 0.93 | |
Notes.
- Ms
- mean square
- D
- plant density
- N
- nitrogen application rate
- Gmax
- the maximum grain-filling rate
- Gmean
- the mean grain-filling rate
- T
- the active grain-filling period
- NBVB
- the number of big vascular bundles
- NSVB
- the number of small vascular bundle
- NTVB
- the number of total vascular bundle
- ABVB
- the area of big vascular bundle
- ASVB
- the area of small vascular bundle
The P value < 0.05 was considered significant.
Table 3. The split plot ANOVA table for the associated yield components parameters.
| Year | Source of variation | GY | SN | TGW | ETR | PFP | WP | Yield per spike | Grain number per spike |
|---|---|---|---|---|---|---|---|---|---|
| 2013–2014 | Block | 93,558 | 30 | 0.04 | 29.52 | 1.86 | 218 | 0.01 | 3.06 |
| D | 3,015,939** | 3,084,323** | 44.65** | 7,994.75** | 89.51** | 440,7628** | 7.04** | 1,419** | |
| D Error | 24,002 | 1,138 | 0.04 | 0.16 | 1.63 | 442 | 0.01 | 2.94 | |
| N | 23,218,384** | 15,850** | 13.54** | 122.94** | 2,876.23** | 869,123** | 0.20** | 201** | |
| D × N | 412,059* | 6,453** | 0.28* | 6.67** | 21.05** | 65,927** | 0.03* | 11.34* | |
| N Error | 111,701 | 726 | 0.08 | 0.54 | 2.96 | 1,140 | 0.01 | 4.18 | |
| 2014–2015 | Block | 123,787 | 544 | 0.08 | 15.64 | 3.54 | 934 | 0.01 | 3.53 |
| D | 4,998,595** | 250,523** | 39.66** | 8,001.76** | 70.96** | 4,674,104** | 7.79** | 1,306** | |
| D Error | 23,059 | 3,814 | 0.03 | 1.42 | 0.09 | 2,789 | 0.01 | 1.61 | |
| N | 47,088,641** | 15,844** | 14.10** | 109.14** | 2,946.75** | 898,100 | 0.19** | 183** | |
| D × N | 709,291** | 3,957** | 0.24* | 5.75* | 21.88** | 67,617** | 0.05** | 6.81* | |
| N Error | 48,952 | 510 | 0.13 | 2.70 | 0.17 | 1,932 | 0.01 | 2.46 |
Notes.
- D
- plant density
- N
- nitrogen application rate
- GY
- grain yield
- SN
- spike number per m2
- TGW
- 100-grain weight
- ETR
- the effective tiller rate
- PFP
- partial factor productivity of applied N
- WP
- the wheat population at the jointing stage
Data represent mean square. ** and * represent significance at the 0.01 and 0.05 probability level, respectively.
Table 4. The split plot ANOVA table for the associated grain-filling parameters and vascular bundle parameters.
| Year | Source of variation | Gmax | Gmean | T | NBVB | NSVB | NTVB | ABVB | ASVB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | I | S | I | S | I | S | I | S | I | S | I | S | I | S | I | ||
| 2013–2014 | Block | 0.0002 | 0.0001 | 0.0001 | 0.0001 | 0.0131 | 0.0003 | 0.04 | 1.81 | 0.15 | 1.44 | 0.26 | 0.70 | 211,898 | 516,335 | 496,285 | 153,037 |
| D | 0.14** | 0.14** | 0.02** | 0.05** | 5.08** | 7.47** | 79.59** | 21.92** | 121.59** | 16.78** | 388.59** | 73.37** | 66,419,449** | 43,232,960** | 34,199,792** | 5,948,765** | |
| D Error | 0.0001 | 0.0003 | 0.0005 | 0.0001 | 0.0348 | 0.0329 | 1.37 | 0.31 | 0.54 | 0.39 | 1.93 | 1.20 | 156,576 | 754,001 | 360,743 | 14,672 | |
| N | 0.22** | 0.178** | 0.0523** | 0.0709** | 6.753** | 18.92** | 61.81** | 30.04** | 51.81** | 65.33** | 226.81** | 182.26** | 75,444,130** | 40,140,666** | 19,331,029** | 3,226,157** | |
| D × N | 0.01** | 0.012** | 0.01** | 0.004** | 2.22** | 2.14** | 1.48* | 2.20** | 3.037* | 2.78** | 4.31* | 7.09** | 771,158* | 5,622,231** | 1,929,184* | 621,198** | |
| N Error | 0.0004 | 0.0007 | 0.0005 | 0.0002 | 0.02 | 0.04 | 0.26 | 0.20 | 0.80 | 0.41 | 0.81 | 0.54 | 200,765 | 725,536 | 513,907 | 78,317 | |
| 2014–2015 | Block | 0.0001 | 0.0001 | 0.0004 | 0.0001 | 0.05 | 0.0001 | 0.33 | 1.93 | 0.15 | 0.15 | 0.11 | 0.11 | 339,298 | 15,082 | 808,789 | 16,844 |
| D | 0.15** | 0.14** | 0.03* | 0.05** | 6.37** | 6.75** | 70.78** | 15.59** | 121.59** | 15.26** | 379.11** | 94.78** | 66,952,005** | 53,395,009** | 34,406,001** | 5,891,122** | |
| D Error | 0.0002 | 0.0004 | 0.002 | 0.0002 | 0.0105 | 0.1062 | 2.61 | 0.37 | 0.537 | 0.59 | 1.56 | 0.22 | 408,608 | 736,204 | 375,627 | 11,798 | |
| N | 0.22** | 0.19** | 0.05** | 0.07** | 6.21** | 20.58** | 57** | 29.15** | 51.81** | 64.93** | 236.78** | 180.11** | 80,329,210** | 34,688,599** | 18,879,826** | 3,751,582** | |
| D × N | 0.01** | 0.01** | 0.01* | 0.004** | 2.87** | 1.94** | 1.61* | 7.43** | 3.04* | 1.87* | 3.56* | 8.06** | 1,116,807* | 6,940,629** | 3,431,991** | 637,779** | |
| N Error | 0.0002 | 0.0001 | 0.001 | 0.0001 | 0.02 | 0.07 | 0.30 | 0.61 | 0.80 | 0.44 | 1.02 | 0.41 | 307,715 | 190,248 | 251,547 | 80,451 | |
Notes.
- D
- plant density
- N
- nitrogen application rate
- Gmax
- the maximum grain-filling rate
- Gmean
- the mean grain-filling rate
- T
- the active grain-filling period
- NBVB
- the number of big vascular bundles
- NSVB
- the number of small vascular bundle
- NTVB
- the number of total vascular bundle
- ABVB
- the area of big vascular bundle
- ASVB
- the area of small vascular bundle
- S
- superior tiller group
- I
- inferior tiller group
Data represent mean square. ** and * represent significance at the 0.01 and 0.05 probability level, respectively.
Grain yield and its components
Grain yield (GY), spike number per m2 (SN), grain number (GN), and 1,000-grain weight (TGW) were found to be significantly influenced by planting density and N application rate (Table 5). Although increasing plant density significantly enhanced GY and SN, both GN and TGW were significantly decreased at higher plant densities. As N application rate was increased from 120 to 240 kg ha−1, the 2-year average GY was significantly enhanced by 43.5%, 34.4%, and 35.2% under planting densities D1, D2, and D3, respectively. Compared with the D2N1 treatment, SN was significantly increased, by 5% and 12% in treatments D2N2 and D2N3, respectively. No significant differences in GY were observed between the N2 and N3 treatments across densities D1 and D2. However, compared with D3N2, the D3N3 treatment significantly decreased GY.
Table 5. Grain yield (GY), spike number (SN), grain number (GN), and 1000-grain weight (TGW) at different treatments.
| Treatment | GY (kg ha−1) | SN (×104 ha−1) | GN (No. spike−1) | TGW (g) |
|---|---|---|---|---|
| D1N1 | 6,999 d | 641 fg | 44.3 c | 43.0 b |
| D1N2 | 10,041 b | 628 g | 47.4 b | 44.6 a |
| D1N3 | 10,222 b | 687 f | 51.0 a | 44.5 a |
| D2N1 | 8,249 c | 935 d | 37.5 ef | 40.5 e |
| D2N2 | 11,083 a | 984 bc | 38.6 e | 42.6 c |
| D2N3 | 11,054 a | 1,046 a | 39.9 d | 42.4 c |
| D3N1 | 8,132 c | 877 e | 32.2 h | 39.7 f |
| D3N2 | 10,994 a | 1,025 ab | 34.0 g | 41.4 d |
| D3N3 | 10,127 b | 960 cd | 36.9 f | 40.8 e |
Notes.
Values followed by different letters within the columns are significantly different at the 0.05 probability level.
In addition, we found that that SN was only significantly positively correlated with GY (Table S1), whereas it was significantly negatively correlated with TGW and GN. Furthermore, we identified positive direct path coefficients for SN and TGW to GY, respectively, whereas the indirect path coefficients were negative for SN and TGW to GY (Tables S2 and S3).
Changes in the wheat population and effective tiller rate
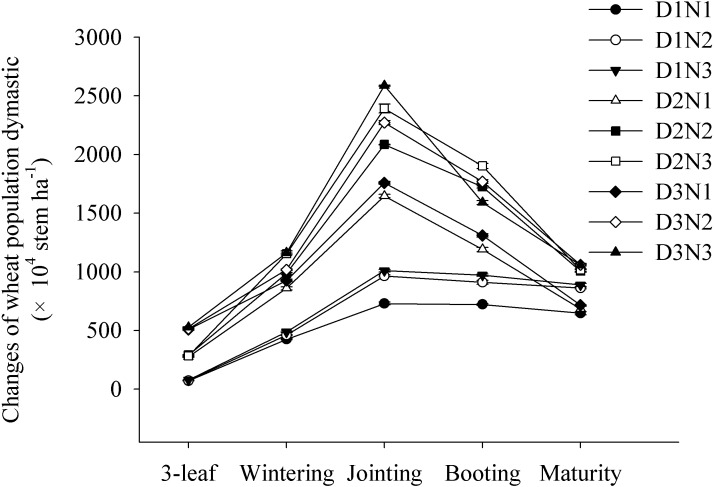
We found that the wheat populations rapidly increased from the three-leaf stage to the jointing stage, reaching a maximum before subsequently decreasing. Wheat populations increased significantly with increasing planting density and N application rate. At the jointing stage, compared with D1N1 treatment, the populations in treatments D2N1 and D3N1 increased by 125% and 141%, respectively. Similarly, compared with the D2N1 treatment, the populations obtained in the D2N2 and D2N3 treatments increased by 26.7% and 45.4%, respectively (Fig. 1).
Figure 1. Effects of plant densities and application of nitrogen rates on the changes of stems of winter wheat.
Symbols represent means ± standard error. Vertical bars indicate standard error.
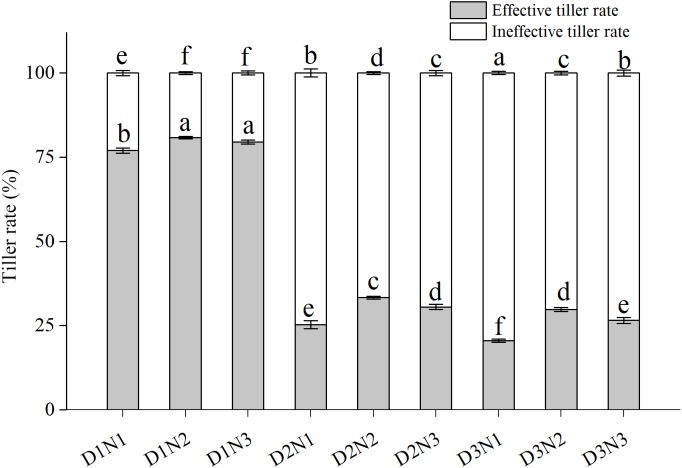
Effective tiller rate (ETR) tended to be higher than ineffective tiller rate (ITR) in the low plant density treatments (D1) (Fig. 2). ITR increased significantly with increases in plant density, with that in treatments D2N1 and D3N1 being more than doubling in both growing seasons compared with D1N1. Although application of N significantly increased ETR (P < 0.05), with that of D2N2 increasing by 24.1% compared with D2N1, the increase was less pronounced (20.8%) at the highest N application level (N3), and in both growing seasons, ETR in the D2N3 treatment was lower than that in the D2N2 treatment.
Figure 2. Effects of plant densities and application of nitrogen rates on the effective tiller rate and ineffective tiller rate.
Symbols represent means ± standard error. Vertical bars indicate standard error. Different letters within the columns are significantly different at the 0.05 probability level.
Grain number and yield per spike of different tillers
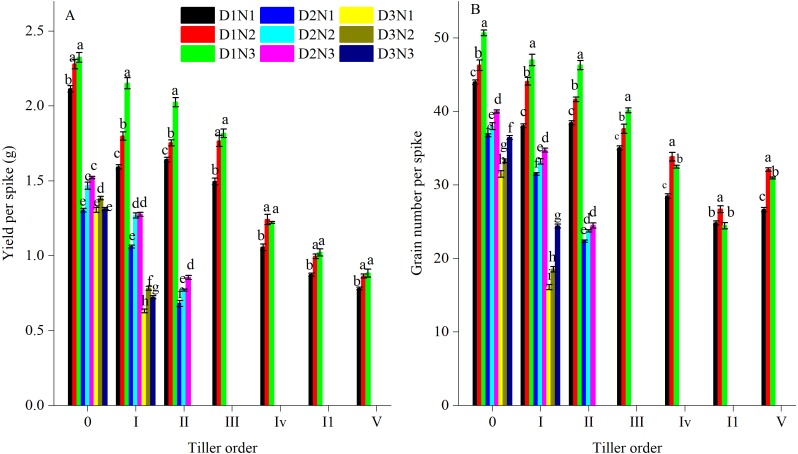
Grain number and yield per spike appeared to vary among different tiller orders (Fig. 3). The main stem had the highest GN and yield per spike. With the sequence of tiller emergence, GN and yield per spike of tillers gradually declined. For all tillers, increasing plant density resulted in a decrease in GN and yield per spike. For example, compared with treatment D1N2, yield per spike of I order tillers for D2N2 and D3N2 decreased by 29.5% and 56.5%, respectively. An increase in N application resulted in an increase in GN and yield per spike. In comparison with treatment D1N1, the yield per spike of order 0, I, and II tillers in response to treatment D1N2 was 7.8%, 13.0%, and 6.9%, respectively (Fig. 3A). Compared with the D1N2 treatment, the GN per spike of order I tillers for D2N2 and D3N2 decreased by 24.7% and 57.9%, respectively, whereas compared with the D1N1 treatment, the GN of order 0, I, and II tillers increased by 5.2%, 15.9%, and 8.3%, respectively, when N was applied at 240 kg N ha−1 (Fig. 3B). In addition, with an increase in plant density, there was a decrease in the number of fertile tillers. Under higher plant densities, only order I and II tillers were ear-bearing, and in the D3 treatment, only one order I tiller produced ears.
Figure 3. Effects of plant densities and application of nitrogen rates on yield per spike (A) and grain number per spike (B) of different tillers.
Different letters within the columns are significantly different at the 0.05 probability level.
Grain-filling process and microstructure of the first internode of superior and inferior tiller groups
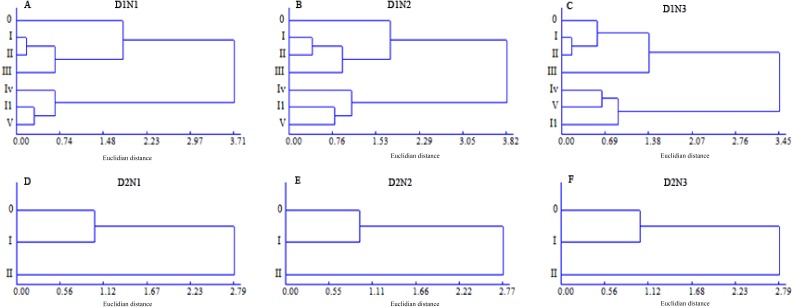
The main stem and tillers in density D1 plants were categorized into two groups, and defined as the superior tiller group, including order 0, I, II, and III tillers, and the inferior tiller group, including order IV, I1, and V tillers, whereas the superior tiller group for the density D2 plants included order 0 and I tillers and the inferior tiller group included order II tillers (Fig. 4). Plants at a density of D3 had just a single tiller; however, dissimilarities between GN and yield per spike meant that a distinction could be made between the main stem, as the superior group, and tiller I as the inferior tiller group. The grain-filling processes and the microstructure of the first internode of the two tiller groups were then investigated to identify the regulatory mechanisms whereby plant density and N application influence yield formation.
Figure 4. Dendrogram of the classification of different wheat tillers based on grain number and yield per spike.
The maximum grain-filling rate (Gmax) and the mean grain-filling rate (Gmean) of superior and inferior tiller groups decreased with an increase in plant density, but were enhanced by increasing N application (Table 6). For example, compared with treatment D2N1, the Gmax of the superior tiller group increased by 17.6% and 15.7% for treatments D2N2 and D2N3, respectively, whereas compared with treatment D1N1, the Gmean of the inferior tiller group increased by 15.0% and 19.2% for D1N2 and D1N3, respectively. Furthermore, we found that the active grain-filling period (T) of the inferior tiller group increased with increasing N application.
Table 6. The effects of plant density and nitrogen application on grain-filling parameters and vascular bundles.
| Tiller group | Treatment | Gmax (mg per kernel d−1) | Gmean (mg per kernel d−1) | T (d) | NBVB | NSVB | NTVB | ABVB (μm2) | ASVB (μm2) |
|---|---|---|---|---|---|---|---|---|---|
| Superior | D1N1 | 2.27 f | 1.47 de | 30.3 e | 23.5 d | 29.2 c | 52.7 c | 8,081.3 cd | 5,344.4 d |
| D1N2 | 2.56 a | 1.52 bc | 29.6 f | 25.7 c | 32.5 a | 58.2 b | 14,001.2 a | 9,279.7.1 a | |
| D1N3 | 2.50 b | 1.58 a | 31.8 c | 29.0 a | 33.2 a | 62.2 a | 14,128.2 a | 8,479.3 b | |
| D2N1 | 2.04 h | 1.33 f | 32.8 a | 21.2 f | 24.5 e | 45.7 e | 5,107.8 e | 3,891.6 e | |
| D2N2 | 2.40 c | 1.54 b | 30.7 d | 25.2 c | 27.0 d | 52.2 c | 10151.3 b | 5,709.3 d | |
| D2N3 | 2.36 d | 1.50 cd | 32.7 a | 27.0 b | 31.0 b | 58.0 b | 10113.8 b | 7,491.3 c | |
| D3N1 | 2.06 h | 1.33 f | 30.6 d | 18.2 g | 22.3 f | 40.5 f | 3,899.9 f | 2,961.8 f | |
| D3N2 | 2.25 g | 1.46 e | 32.1 b | 21.0 f | 24.5 e | 45.5 e | 7,844.2 d | 4,163.3 e | |
| D3N3 | 2.29 e | 1.46 e | 32.8 a | 22.3 e | 26.0 d | 48.3 d | 8,466.4 c | 4,279.7 e | |
| Inferior | D1N1 | 1.82 f | 1.20 e | 32.2 e | 18.5 b | 23.2 e | 41.7 e | 6,022.6 d | 1,369.8 d |
| D1N2 | 2.11 b | 1.38 b | 32.9 d | 19.7 a | 26.3 bc | 46.0 b | 10,126.3 b | 2,891.9 b | |
| D1N3 | 2.19 a | 1.43 a | 33.8 c | 20.3 a | 28.2 a | 49.0 a | 13,375.6 a | 3,526.1 a | |
| D2N1 | 1.74 g | 1.18 e | 31.8 f | 16.2 d | 21.5 f | 37.7 g | 4,136.1 f | 1,133.6 d | |
| D2N2 | 1.89 e | 1.27 d | 35.4 b | 19.8 a | 24.3 d | 44.1 d | 6,061.5 d | 2,023.3 c | |
| D2N3 | 1.99 c | 1.29 c | 35.9 a | 19.5 a | 25.3 c | 44.8 c | 6,735.1 cd | 2,114.2 c | |
| D3N1 | 1.71 h | 1.10 f | 32.8 d | 13.5 e | 19.5 g | 33.0 h | 4,960.3 e | 710.2 e | |
| D3N2 | 1.77 g | 1.19 e | 35.5 b | 17.3 c | 23.7 de | 41.0 f | 6,152.5 d | 1,128.8 d | |
| D3N3 | 1.94 d | 1.27 d | 35.4 b | 18.5 b | 26.7 b | 45.2 c | 7,146.4 c | 1,095.7 d |
Notes.
- Gmax
- the maximum grain-filling rate
- Gmean
- the mean grain-filling rate
- T
- the active grain-filling period
- NBVB
- the number of big vascular bundles
- NSVB
- the number of small vascular bundle
- NTVB
- the number of total vascular bundle
- ABVB
- the area of big vascular bundle
- ASVB
- the area of small vascular bundle
Values followed by different letters within the columns are significantly different at the 0.05 probability level.
On the basis of the results of cluster analysis, we examined the microstructures of the first internode in superior and inferior tiller groups. Our observations revealed that the number of large vascular bundles (NBVB), small vascular bundles (NSVB), and total number of vascular bundles (NTVB), as well as the area of the large vascular bundles (ABVB) and small vascular bundles (ASVB) of the superior tiller group, were all higher than those of the inferior tiller group (Table 6). An increase in plant density significantly decreased NBVB, NSVB, NTVB, ABVB, and ASVB, whereas an increase in N application significantly increased NBVB, NSVB, NTVB, ABVB, and ASVB. For example, compared with treatment D2N1, the NTVB of the superior and inferior tiller groups in the D2N2 treatment increased by 6.5 and 6.4 per culm, respectively. Compared with the D2N1 treatment, the ABVB of the superior tiller group increased significantly by 99% and 98% in the D2N2 and D2N3 treatments, and that of the inferior tiller group increased by 46.6% and 62.8%, respectively.
Relationships between individual yield per spike and vascular bundles
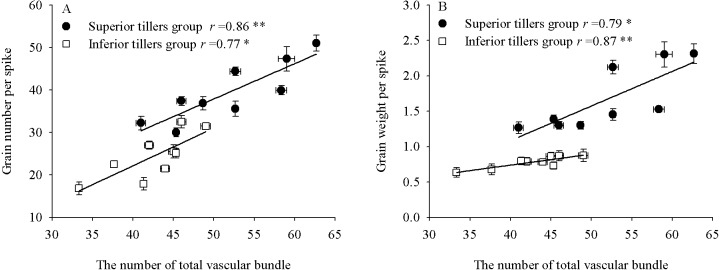
We observed significant correlations between individual yield and vascular bundles (Fig. 5). The grain number and yield per spike of the superior tiller group were significantly positively correlated with the NTVB (r = 0.86∗∗, P <0.01; r = 0.79*, P <0.05, respectively), as were the grain number and yield per spike of the inferior tiller group (r = 0.77 *, P <0.05; r = 0.87 **, P <0.01, respectively). Significant correlations were likewise observed between vascular bundle areas and grain-filling rates (Table 7).
Figure 5. The relationship between single grain yield and the total number vascular bundle of superior and inferior tiller groups.
Table 7. The relationship between the vascular bundle area and the grain-filling rate of superior and inferior tiller groups.
| Tiller group | Vascular bundle area | Gmax | Gmean |
|---|---|---|---|
| Superior tiller group | ABVB | 0.97** | 0.89** |
| ASVB | 0.91** | 0.80** | |
| Inferior tiller group | ABVB | 0.95** | 0.93** |
| ASVB | 0.93** | 0.94** |
Notes.
- Gmax
- the maximum grain-filling rate
- Gmean
- the mean grain-filling rate
- ABVB
- the area of big vascular bundle
- ASVB
- the area of small vascular bundle
Correlation coefficients (r) are calculated and asterisks (**) represent significance at the 0.01 probability level.
Trend surface analysis and evaluation of nitrogen-use efficiency
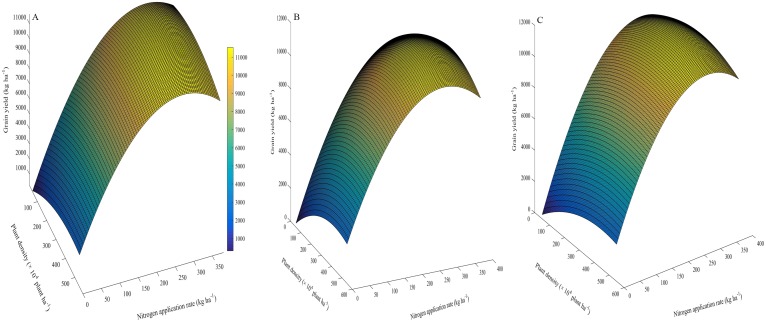
The effects of plant density and N application on GY are shown by the coefficients of second order polynomials (Fig. 6). These coefficients were calculated by the least square method, as given in the equations (Table 8). For example, the total determination coefficient R2 = 0.992 implies that variations of 99.2% for GY are attributable to plant density and N application rate. If the partial derivative of the equation is zero, two equations can be constructed as follows: 13.9 − 0.012y − 0.032x = 0 and 66 − 0.012x − 0.214y = 0, and 11.3 − 0.011y − 0.024x = 0 and 68 − 0.011x − 0.222y = 0, respectively. By solving the equation groups, the following results can be obtained: x = 326 (×10 4plant ha−1), y = 290 (kg N ha−1) in the first growing season, and x = 338 (×10 4 plant ha−1), y = 289 (kg N ha−1) in the second growing season, respectively. Similarly, the average results in two years can be obtained: x = 336 (×10 4plant ha−1), y = 290 (kg N ha−1). Via inputting the data of these two variables into the equation, the maximum GY and its partial factor productivity of applied N could be calculated: ZMaxGY = 11,524 (kg ha−1) and MaxGY PFP = 39.7 (kg kg−1).
Figure 6. 3D graphic surface optimization of grain yield versus plant density and nitrogen application rate (A, 2013–2014; B, 2014–2015; C, the average of the two years).
Table 8. The quadratic polynomial trend surface equations and PFP.
| Year | Equation | R2 | P | x | y | z | PFP |
|---|---|---|---|---|---|---|---|
| 2013–2014 | z= 13.9x+ 66y= 0.012xy= 0.016x2= 0.107y2= 421 | 0.992 | ** | 326 | 290 | 11,417 | 39.4 |
| 2014–2015 | z= 11.3x+ 68y= 0.011xy= 0.012x2= 0.111y2= 219 | 0.996 | ** | 338 | 289 | 11,438 | 39.6 |
| Mean | z= 12.6x+ 67y= 0.011xy= 0.014x2= 0.109y2= 320 | 0.991 | ** | 336 | 290 | 11,524 | 39.7 |
Notes.
- R2
- the coefficient of determination
- z
- represents the grain yield (kg ha−1)
- x
- represents plant density (plant ha−1)
- y
- represents the N application rate (kg N ha−1)
- PFP
- partial factor productivity of applied N (kg kg−1)
** represents significance at the 0.01 probability level.
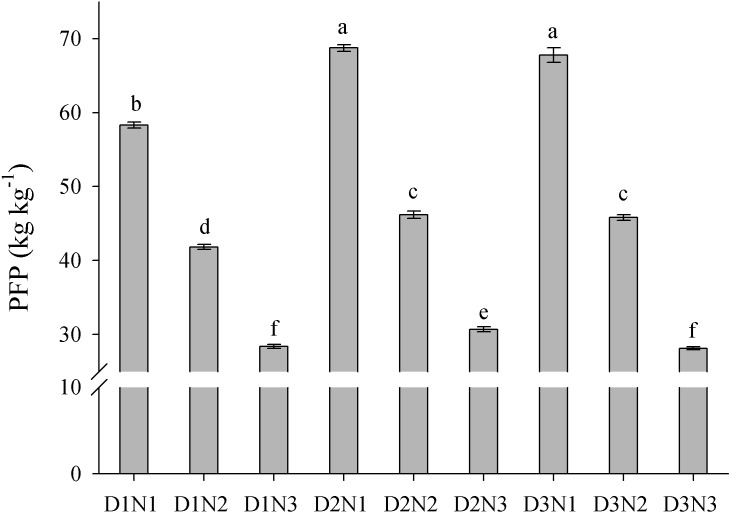
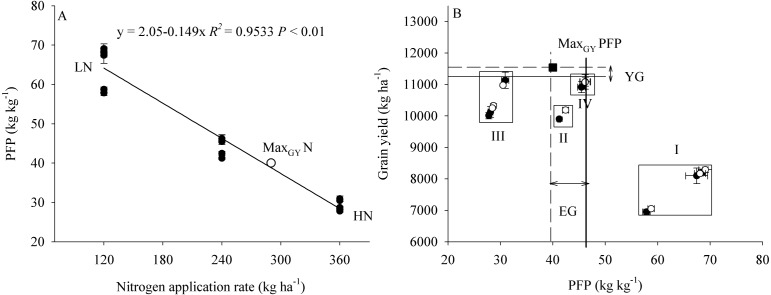
The results showed that an increase in plant density (D1 to D2) significantly increased PFP, but there was no significant difference in PFP between densities D2 and D3 (Fig. 7). In contrast, an increase in N application significantly decreased PFP. Compared with treatment D2N3, the average PFPN in the 2 years of the study was increased by 50.4% in response to treatment D2N2. Furthermore, we found that PFP was significantly negatively correlated with the rate of N application (Fig. 8A). Taking GY and NUE into consideration, treatments could be classified into four groups: (I) lower GY with higher NUE (D1N1, D2N1, and D3N1), (II) high yield with high NUE (D1N2), (III) higher yield with lower NUE (D1N3, D2N3, and D3N3), and (IV) higher yield with high NUE (D2N2 and D3N2) (Fig. 8B). These results indicate that increasing plant density and decreasing N application could be a useful strategy for achieving high GY and NUE.
Figure 7. Effects of plant densities and application of nitrogen rates on the PFP.
PFP represents the partial factor productivity of applied N. Different letters within the columns are significantly different at the 0.05 probability level.
Figure 8. The relationship between PFP and nitrogen application rate and grain yield.
PFP represents the partial factor productivity of applied N. MaxGYN represents the nitrogen application rate, which achieve the maximum grain yield calculated by the second order polynomials trend surface. MaxGYPFP represents the partial factor productivity of applied N, which achieve the maximum grain yield. YG represents yield gap between the maximum grain yield and D2N2 treatment. EG represents the PFP gap between the maximum grain yield and D2N2 treatment.
Discussion
Effects of plant density and nitrogen application on grain yield and its components
Yield variability is largely controlled by plant density and N fertilizer input (Sukumaran et al., 2015; Liu et al., 2016). In the current study, we found that plant density, N application rate, and their interactions significantly affected GY. Grain yield increased with an increase in plant density and N application, which can be explained in terms of an increase in SN, whereas, both GN and TGW were reduced by increasing plant density. Previously, Li et al. (2016b) observed that SN was significantly increased by an increase in plant density, resulting in higher grain yield, whereas TGW and GN were decreased, which is partially consistent with our results. Large changes in yield can only be determined by variation in the grain number per m2, which is primarily associated with SN (Slafer, Savin & Sadras, 2014). In the present study, path analysis revealed that SN appears to be the most important factor determining GY, thereby indicating that preferentially optimizing the SN is an important measure for increasing grain yield.
Spike number includes the number main stem and tiller spikes and is determined by seeding rate and tiller generation and survival (Lloveras et al., 2004; Dreccer et al., 2013). Tillers arise from tiller buds initiated from the axillary meristems in the axils of leaves on the main shoot and tiller shoots, and the development of wheat tillers is regulated by numerous factors. Regarding genetic characteristics, multi-tiller or inhibition genes have been shown to control the development of tillers (Xue et al., 2013; Hendriks et al., 2016). Environmental factors and agronomic management, such as planting density and N application, also affect the initiation and cessation of tillering (Evers et al., 2011; Huang et al., 2013). The results of the present study indicate that an increase in plant density increases the effective tiller rate and decreases the number of high-position tiller spikes. In contrast with the low plant density (D1) treatments, we found that only the main stem and order I and II tillers survive to constitute the final population under density D2 (300 plants m−2).
Plant density-regulated tiller development may be related mainly to the variation in light within the canopy structure. In previous studies, light interception has been shown to increase with an increase in plant density, leading to less photosynthetically active radiation penetrating to the lower vegetation layer (Mao et al., 2014; Xue et al., 2015). Given that the development of tillers is asynchronous, the low-position tillers develop earlier than the high-position tillers. The former occupy the upper space and preempt the uppermost light source, shading the late-emerging tillers (Wang et al., 2016a). The cessation of tillering is induced when the fraction of light intercepted by the canopy exceeds a threshold (Evers et al., 2006). Plant density affects not only light quantity but also light quality, and the ratio of red to far-red light at the base of the canopy is reduced by higher plant densities (Rondanini et al., 2017), resulting in the inhibition of bud growth (Holalu & Finlayson, 2017). In addition, leaf area and leaf number both decrease as the tiller position shifts from low to high (Lafarge, Broad & Hammer, 2002). These factors may contribute to decreases in the photosynthetic activity of leaves on the late emerging tillers, due to the lack of photosynthetically active radiation and photosynthetic area, thereby resulting in lower sucrose levels, which would suppress tiller bud outgrowth (Mason et al., 2014; Kebrom & Mullet, 2015).
Nitrogen is one of the essential nutrient elements for crop growth and yield formation. In the present study, we found that the number of stems per m2 was significantly increased by an increase in N application, and the effective tiller rate was also increased. Therefore, the final SN was increased by a high N supply (N2 and N3), compared with low N application (N1). N deficiency inhibits tiller bud elongation (Luo et al., 2017), whereas N application has been shown to promote tillering via the accumulation of microRNA393 (miR393) in response to upregulation of OsmiR393 (Li et al., 2016c). In addition to genetic factors, an interaction between N and certain hormones is known to play a role in tiller development (Domagalska & Leyser, 2011; Xu et al., 2015b). Nitrogen application promotes cytokinin biosynthesis, and inhibits its degradation, thereby inducing tiller bud development (Liu et al., 2011), and also affects auxin transport and strigolactone synthesis to regulate axillary bud activation (Jong et al., 2014). However, supra-optimal N application may result in the vigorous vegetative growth (Nasim et al., 2016; Ata-Ul-Karim et al., 2017). In the present study, we found that our highest N application rate (N3) decreased the effective tiller rate compared with N2 treatments, and, notably, a high N supply combined with a high plant density (D3N3) resulted in lodging (Fig. S4).
Plant density and N application have significant effects on culm development. Vascular bundle number (NVB) and area (AVB) are the major anatomical features influencing stem-breaking strength and transportation (Tian et al., 2015). We found that whereas increasing plant density significantly decreased NVB and AVB at the first internode, an increase in N application increased NVB and AVB, and GN and yield per spike also increased with excessive N application, Moreover, the results of correlation analysis indicated that the number vascular bundle was significantly positively correlated with yield per spike, and that vascular bundle area was significantly positively correlated with grain filling rate. These results indicate that an appropriate combination of increased planting density and N application could regulate culm quality to facilitate the transport of photoassimilates to the grain sink, resulting in an acceleration of the grain-filling rate and enhancement of yield per spike.
Optimized plant density and nitrogen application to enhance grain yield and nitrogen-use efficiency
Nutrient competition, mainly with respect to N, occurs between tillers (Assuero et al., 2012), and is intensified by increased plant density. Therefore, it should be possible to optimize plant density and N application to regulate tiller development and spike number, thereby achieving high yield and high NUE via a trade-off between the three yield components. Cluster analysis showed that the low- and high-position tiller spikes can be classified into superior and inferior tiller groups, respectively. The yield per spike, grain-filling rate and NVB and AVB in the superior tiller group were all higher than those in the inferior tiller group. In the present study, we found that increasing plant density reduced the number of high-position tillers, and that under a high plant density (300 × 104 plant ha−1) the spikes consisted of the superior group. Increasing plant density increased the partial factor productivity of applied N (PFP), because the density of roots in soil was increased by increasing plant density, which enhances N uptake (Dai et al., 2014). Although increasing N application increased GY, the PFP was significantly decreased. We found that PFP was significantly negatively correlated with the N application rate, which indicates that increasing NUE should properly reduce N application. Second order polynomial trend surface analysis revealed that a plant density of 336 × 104 plants ha−1 and an N application rate of 290 kg N ha−1 could produce the maximum GY (MaxGY). Although the yield gap between MaxGY and that obtained with treatment D2N2 was 453 kg ha−1, the corresponding PFP (39.7 kg kg−1) was obviously high than that for N application at 360 kg N ha−1.
Conclusion
Wheat spikes were classified into superior (including the main stem and low-position tillers) and inferior (high-position tillers) groups. The superior groups had higher grain number and yield per spike owing to a larger number of vascular bundles and faster grain-filling rate. We proposed and verified a technical approach to achieve a higher grain yield and high plant nitrogen-use efficiency through optimizing plant density and N application to regulate tiller growth, which appropriately increasing plant densities (from 75 to 336 plants m−2) and reducing nitrogen application (from 360 to 290 kg N ha−1), the number of inferior tillers can be reduced, thereby optimizing superior tiller development to generate a rational population structure.
Supplemental Information
Each plot (3 × 3 m) consisted of 10 rows of wheat and two ridges (A). A schematic diagram showing 75 ×104 (B), 300 ×104 (C), and 525 ×104 (D) plant ha−1 over two years.
The main stem was denoted as 0; primary tillers on the main stem in emergence order were referred to as I, II, III, IV, and V. Secondary tillers on the primary tillers in emergence order were referred to as I 1 and II 1.
GY, grain yield; SN, spike number; GN, grain number; GW grain weight. Correlation coefficients (r) are calculated and asterisks (∗∗) represent significance at the 0.01 probability level and asterisks (∗) represent significance at the 0.05 probability level.
GY, grain yield; SN, spike number; GN, grain number; GW grain weight. Correlation coefficients (r) are calculated and asterisks (∗∗) represent significance at the 0.01 probability level.
GY, grain yield; SN, spike number; GN, grain number; GW grain weight.
Funding Statement
The research report here was supported by the National Natural Science Foundation of China (31801295, 31601257), the Shandong Province Natural Science Foundation (Doctoral Fund, ZR2017BC106), the Project funded by China Postdoctoral Science Foundation (2018M632701), the National Key Research and Development Program of China (2016YFD0300400), the National Basic Research Program of China (973 Program, NO. 2015CB150404), and the Opening fund of the State Key Laboratory of Crop Biology (2016KF04). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Dongqing Yang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Tie Cai analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper.
Yongli Luo performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper.
Zhenlin Wang contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The raw data are available in Supplemental Files.
References
- Ahmed et al. (2016).Ahmed S, Humphreys E, Salim M, Chauhan BS. Growth, yield and nitrogen use efficiency of dry-seeded rice as influenced by nitrogen and seed rates in Bangladesh. Field Crops Research. 2016;186:18–31. doi: 10.1016/j.fcr.2015.11.001. [DOI] [Google Scholar]
- Assuero et al. (2012).Assuero SG, Lorenzo M, Pérez Ramírez NM, Velázquez LM, Tognetti JA. Tillering promotion by paclobutrazol in wheat and its relationship with plant carbohydrate status. New Zealand Journal of Agricultural Research. 2012;55(4):347–358. doi: 10.1080/00288233.2012.706223. [DOI] [Google Scholar]
- Assuero & Tognetti (2010).Assuero SG, Tognetti JA. Tillering regulation by endogenous and environmental factors, and its agricultural management. The American Journal of Plant Science and Biotechnology. 2010;4:35–48. [Google Scholar]
- Ata-Ul-Karim et al. (2017).Ata-Ul-Karim ST, Liu X, Lu Z, Zheng H, Cao W, Zhu Y. Estimation of nitrogen fertilizer requirement for rice crop using critical nitrogen dilution curve. Field Crops Research. 2017;201:32–40. doi: 10.1016/j.fcr.2016.10.009. [DOI] [Google Scholar]
- Bennett & Leyser (2006).Bennett T, Leyser O. Something on the side: axillary meristems and plant development. Plant Molecular Biology. 2006;60(6):843–854. doi: 10.1007/s11103-005-2763-4. [DOI] [PubMed] [Google Scholar]
- Berry et al. (2003).Berry PM, Spink JH, Foulkes MJ, Wade A. Quantifying the contributions and losses of dry matter from non-surviving shoots in four cultivars of winter wheat. Field Crops Research. 2003;80:111–121. doi: 10.1016/S0378-4290(02)00174-0. [DOI] [Google Scholar]
- Cai et al. (2014).Cai T, Xu HC, Peng DL, Yin YP, Yang WB, Ni YL, Chen XG, Xu CL, Yang DQ, Cui ZY, Wang ZL. Exogenous hormonal application improves grain yield of wheat by optimizing tiller productivity. Field Crops Research. 2014;155:172–183. doi: 10.1016/j.fcr.2013.09.008. [DOI] [Google Scholar]
- Chen et al. (2008).Chen CC, Karnes N, Dave W, Malvern W. Hard red spring wheat response to row spacing, seeding rate, and nitrogen. Agronomy Journal. 2008;100(5):1296–1302. doi: 10.2134/agronj2007.0198. [DOI] [Google Scholar]
- Chen et al. (2017).Chen Y, Zhang Z, Tao F, Wang P, Wei X. Spatio-temporal patterns of winter wheat yield potential and yield gap during the past three decades in North China. Field Crops Research. 2017;206:11–20. doi: 10.1016/j.fcr.2017.02.012. [DOI] [Google Scholar]
- Clerget et al. (2016).Clerget B, Bueno CS, Domingo AJ, Layaoen HL, Vial L. Leaf emergence, tillering, plant growth, and yield in response to plant density in a high-yielding aerobic rice crop. Field Crops Research. 2016;199:52–64. doi: 10.1016/j.fcr.2016.09.018. [DOI] [Google Scholar]
- Cui, Chen & Zhang (2010).Cui ZL, Chen XP, Zhang FS. Current nitrogen management status and measures to improve the intensive wheat–maize system in China. AMBIO. 2010;39(5–6):376–384. doi: 10.1007/s13280-010-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai et al. (2014).Dai XL, Xiao LL, Jia DY, Kong HB, Wang YC, Li CX, Zhang Y, He MR. Increased plant density of winter wheat can enhance nitrogen—uptake from deep soil. Plant and Soil. 2014;384(1–2):141–152. doi: 10.1007/s11104-014-2190-x. [DOI] [Google Scholar]
- Domagalska & Leyser (2011).Domagalska MA, Leyser O. Signal integration in the control of shoot branching. Nature Reviews Molecular Cell Biology. 2011;12(4):211–221. doi: 10.1038/nrm3088. [DOI] [PubMed] [Google Scholar]
- Dreccer et al. (2013).Dreccer MF, Chapman SC, Rattey AR, Neal J, Song Y, Christopher JJ, Reynolds M. Developmental and growth controls of tillering and water-soluble carbohydrate accumulation in contrasting wheat (Triticum aestivum L.) genotypes: can we dissect them? Journal of Experimental Botany. 2013;64(1):143–160. doi: 10.1093/jxb/ers317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhani et al. (2007).Elhani S, Martos V, Rharrabti Y, Royo C, García del Mora LF. Contribution of main stem and tillers to durum wheat (Triticum turgidum L. var. durum) grain yield and its components grown in Mediterranean environments. Field Crops Research. 2007;103(1):25–35. doi: 10.1016/j.fcr.2007.05.008. [DOI] [Google Scholar]
- Evers et al. (2011).Evers JB, Van der Krol AR, Vos J, Struik PC. Understanding shoot branching by modelling form and function. Trends in Plant Science. 2011;16(9):464–467. doi: 10.1016/j.tplants.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Evers et al. (2006).Evers JB, Vos J, Andrieu B, Struik PC. Cessation of tillering in spring wheat in relation to light interception and red: far-red ratio. Annals of Botany. 2006;97(4):649–658. doi: 10.1093/aob/mcl020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks et al. (2016).Hendriks PW, Kirkegaard JA, Lilley JM, Gregory PJ, Rebetzke GJ. A tillering inhibition gene influences root—shoot carbon partitioning and pattern of water use to improve wheat productivity in rainfed environments. Journal of Experimental Botany. 2016;67(1):327–340. doi: 10.1093/jxb/erv457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holalu & Finlayson (2017).Holalu SV, Finlayson SA. The ratio of red light to far red light alters Arabidopsis axillary bud growth and abscisic acid signalling before stem auxin changes. Journal of Experimental Botany. 2017;68(5):943–952. doi: 10.1093/jxb/erw479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2013).Huang M, Yang CL, Ji QM, Jiang LG, Tan JL, Li YQ. Tillering responses of rice to plant density and nitrogen rate in a subtropical environment of southern China. Field Crops Research. 2013;149:187–192. doi: 10.1016/j.fcr.2013.04.029. [DOI] [Google Scholar]
- Hyles et al. (2017).Hyles J, Vautrin S, Pettolino F, Macmillan C, Stachurski Z, Breen J, Berges H, Wicker T, Spielmeyer W. Repeat-length variation in a wheat cellulose synthase-like gene is associated with altered tiller number and stem cell wall composition. Journal of Experimental Botany. 2017;68(7):1519–1529. doi: 10.1093/jxb/erx051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong et al. (2014).Jong M de, George G, Ongaro V, Williamson L, Willetts B, Ljung K, McCulloch H, Leyser O. Auxin and strigolactone signaling are required for modulation of Arabidopsis shoot branching by nitrogen supply. Plant Physiology. 2014;166(1):384–395. doi: 10.1104/pp.114.242388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom & Mullet (2015).Kebrom TH, Mullet JE. Photosynthetic leaf area modulates tiller bud outgrowth in sorghum. Plant, Cell & Environment. 2015;38(8):1471–1478. doi: 10.1111/pce.12500. [DOI] [PubMed] [Google Scholar]
- Lafarge, Broad & Hammer (2002).Lafarge TA, Broad IJ, Hammer GL. Tillering in grain sorghum over a wide range of population densities: identification of a common hierarchy for tiller emergence, leaf area development and fertility. Annals of Botany. 2002;90(1):87–98. doi: 10.1093/aob/mcf152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor, Lemaire & Gastal (2001).Lawlor DW, Lemaire G, Gastal F. Nitrogen, plant growth and crop yield. In: Lea PJ, Morot-Gaudry JF, editors. Plant nitrogen. Springer; Berlin, Heidelberg: 2001. pp. 343–367. [Google Scholar]
- Li et al. (2016a).Li X, Li NJ, You LZ, Ke XL, Liu HJ, Huang ML, Waddington SR. Patterns of cereal yield growth across China from 1980 to 2010 and their implications for food production and food security. PLOS ONE. 2016a;11(7):e0159061. doi: 10.1371/journal.pone.0159061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2003).Li XY, Qian Q, Fu ZM, Wang YH, Xiong GS, Zeng DL, Wang XQ, Liu XF, Teng S, Hiroshi F, Yuan M, Luo D, Han B, Li JY. Control of tillering in rice. Nature. 2003;422(6932):618–621. doi: 10.1038/nature01518. [DOI] [PubMed] [Google Scholar]
- Li et al. (2016c).Li X, Xia KF, Liang Z, Chen KL, Gao CX, Zhang MY. MicroRNA393 is involved in nitrogen-promoted rice tillering through regulation of auxin signal transduction in axillary buds. Scientific Reports. 2016c;6:32158. doi: 10.1038/srep32158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2016b).Li Y, Cui ZY, Ni YL, Zheng MJ, Yang DQ, Jin M, Chen J, Wang ZL, Yin YP. Plant density effect on grain number and weight of two winter wheat cultivars at different spikelet and grain positions. PLOS ONE. 2016b;11(5):e0155351. doi: 10.1371/journal.pone.0155351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2016).Lin X, Wang D, Gu S, White PJ, Han K, Zhou J, Jin SP. Effect of supplemental irrigation on the relationships between leaf ABA concentrations, tiller development and photosynthate accumulation and remobilization in winter wheat. Plant Growth Regulation. 2016;79(3):331–343. doi: 10.1007/s10725-015-0137-8. [DOI] [Google Scholar]
- Liu et al. (2016).Liu H, Wang ZH, Yu R, Li FC, Li KY, Cao HB, Yang N, Li MH, Dai J, Zan YL, Li Q, Xue C, He G, Huang DL, Huang M, Liu JS, Qiu WH, Zhao HB, Mao H. Optimal nitrogen input for higher efficiency and lower environmental impacts of winter wheat production in China. Agriculture, Ecosystems & Environment. 2016;224:1–11. doi: 10.1016/j.agee.2016.03.022. [DOI] [Google Scholar]
- Liu et al. (2011).Liu Y, Ding YF, Wang QS, Meng DX, Wang SH. Effects of nitrogen and 6-benzylaminopurine on rice tiller bud growth and changes in endogenous hormones and nitrogen. Crop Science. 2011;51(2):786–792. doi: 10.2135/cropsci2010.04.0217. [DOI] [Google Scholar]
- Lloveras et al. (2004).Lloveras J, Manent J, Viudas J, López A, Santiveri P. Seeding rate influence on yield and yield components of irrigated winter wheat in a Mediterranean climate. Agronomy Journal. 2004;96(5):1258–1265. doi: 10.2134/agronj2004.1258. [DOI] [Google Scholar]
- Lu et al. (2015).Lu DJ, Lu FF, Pan JX, Cui ZL, Zou CQ, Chen XP, He MR, Wang ZL. The effects of cultivar and nitrogen management on wheat yield and nitrogen use efficiency in the North China Plain. Field Crops Research. 2015;171:157–164. doi: 10.1016/j.fcr.2014.10.012. [DOI] [Google Scholar]
- Lu et al. (2014).Lu DJ, Lu FF, Yan P, Cui ZL, Chen XP. Elucidating population establishment associated with N management and cultivars for wheat production in China. Field Crops Research. 2014;163(1):81–89. doi: 10.1016/j.fcr.2014.03.022. [DOI] [Google Scholar]
- Luo et al. (2017).Luo L, Pan S, Liu X, Wang H, Xu G. Nitrogen deficiency inhibits cell division-determined elongation, but not initiation, of rice tiller buds. Israel Journal of Plant Sciences. 2017;64:32–40. doi: 10.1080/07929978.2016.1275367. [DOI] [Google Scholar]
- Ma et al. (2008).Ma SC, Xu BC, Li FM, Huang ZB. Ecological significance of redundancy in tillers of winter wheat (Triticum aestivum) and effect of reducing redundancy on water efficiency. Acta Ecologica Sinica. 2008;28(1):321–326. [Google Scholar]
- Mao et al. (2014).Mao LL, Zhang LZ, Zhao XH, Liu SD, Werf WVD, Zhan SP, Spierzt H, Li ZH. Crop growth, light utilization and yield of relay intercropped cotton as affected by plant density and a plant growth regulator. Field Crops Research. 2014;155:67–76. doi: 10.1016/j.fcr.2013.09.021. [DOI] [Google Scholar]
- Mason et al. (2014).Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA. Sugar demand, not auxin, is the initial regulator of apical dominance. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(16):6092–6097. doi: 10.1073/pnas.1322045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng et al. (2016).Meng QF, Yue SC, Hou P, Cui ZL, Chen XP. Improving yield and nitrogen use efficiency simultaneously for maize and wheat in China: a review. Pedosphere. 2016;26(2):137–147. doi: 10.1016/S1002-0160(15)60030-3. [DOI] [Google Scholar]
- Mitchell et al. (2012).Mitchell JH, Chapman SC, Rebetzke GJ, Bonnett DG, Fukai S. Evaluation of a reduced-tillering (tin) gene in wheat lines grown across different production environments. Crop & Pasture Science. 2012;63(2):128–141. doi: 10.1071/CP11260. [DOI] [Google Scholar]
- Moeller, Evers & Rebetzke (2014).Moeller C, Evers JB, Rebetzke G. Canopy architectural and physiological characterization of near-isogenic wheat lines differing in the tiller inhibition gene tin. Frontiers in Plant Science. 2014;5:617. doi: 10.3389/fpls.2014.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon et al. (2016).Mon J, Bronson KF, Hunsaker DJ, Thorp KR, White JW, French AN. Interactive effects of nitrogen fertilization and irrigation on grain yield, canopy temperature, and nitrogen use efficiency in overhead sprinkler-irrigated durum wheat. Field Crops Research. 2016;191:54–65. doi: 10.1016/j.fcr.2016.02.011. [DOI] [Google Scholar]
- Naruoka et al. (2011).Naruoka Y, Talbert LE, Lanning SP, Blake NK, Martin JM, Sherman JD. Identification of quantitative trait loci for productive tiller number and its relationship to agronomic traits in spring wheat. Theoretical and Applied Genetics. 2011;123(6):1043–1053. doi: 10.1007/s00122-011-1646-0. [DOI] [PubMed] [Google Scholar]
- Nasim et al. (2016).Nasim W, Ahmad A, Ahmad S, Nadeem M, Masood N, Shahid M, Mubeen M, Hoogeboom G. Response of sunflower hybrids to nitrogen application grown under different agro-environments. Journal of Plant Nutrition. 2016;40(1):82–92. doi: 10.1080/01904167.2016.1201492. [DOI] [Google Scholar]
- Otteson et al. (2008).Otteson BN, Mergoum M, Ransom JK, Schatz B. Tiller contribution to spring wheat yield under varying seeding and nitrogen management. Agronomy Journal. 2008;100(2):406–413. doi: 10.2134/agronj2007.0109. [DOI] [Google Scholar]
- Prystupa, Slafer & Savin (2003).Prystupa P, Slafer GA, Savin R. Leaf appearance, tillering and their coordination in response to N ×P fertilization in barley. Plant and Soil. 2003;255(2):587–594. doi: 10.1023/A:1026018702317. [DOI] [Google Scholar]
- Richards et al. (2002).Richards RA, Rebetzke GJ, Condon AG, Van Herwaarden AF. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Science. 2002;42(1):111–121. doi: 10.2135/cropsci2002.1110. [DOI] [PubMed] [Google Scholar]
- Rondanini et al. (2017).Rondanini DP, Menendez YC, Gomez NV, Miralles DJ, Botto JF. Vegetative plasticity and floral branching compensate low plant density in modern spring rapeseed. Field Crops Research. 2017;210:104–113. doi: 10.1016/j.fcr.2017.05.021. [DOI] [Google Scholar]
- Slafer, Savin & Sadras (2014).Slafer GA, Savin R, Sadras VO. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crops Research. 2014;157:71–83. doi: 10.1016/j.fcr.2013.12.004. [DOI] [Google Scholar]
- Sukumaran et al. (2015).Sukumaran S, Reynolds MP, Lopes MS, Crossa J. Genome-wide association study for adaptation to agronomic plant density: a component of high yield potential in spring wheat. Crop Science. 2015;55:1–11. doi: 10.2135/cropsci2015.03.0139. [DOI] [Google Scholar]
- Terrile, Miralles & González (2017).Terrile II, Miralles DJ, González FG. Fruiting efficiency in wheat (Triticum aestivum, L.): trait response to different growing conditions and its relation to spike dry weight at anthesis and grain weight at harvest. Field Crops Research. 2017;201:86–96. doi: 10.1016/j.fcr.2016.09.026. [DOI] [Google Scholar]
- Tian et al. (2015).Tian BH, Liu LY, Zhang LX, Song SX, Wang JG, Wu LF, Li HJ. Characterization of culm morphology, anatomy and chemical composition of foxtail millet cultivars differing in lodging resistance. Journal of Agricultural Science. 2015;153(8):1437–1448. doi: 10.1017/S0021859614001105. [DOI] [Google Scholar]
- Tian et al. (2017).Tian GL, Gao LM, Kong YL, Hu XY, Xie KL, Zhang RQ, Ling N, Shen QR, Guo SW. Improving rice population productivity by reducing nitrogen rate and increasing plant density. PLOS ONE. 2017;12(8):e0182310. doi: 10.1371/journal.pone.0182310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2009).Wang FH, He ZH, Sayre K, Li SD, Si JS, Feng B, Kong LA. Wheat cropping systems and technologies in China. Field Crops Research. 2009;111(3):181–188. doi: 10.1016/j.fcr.2008.12.004. [DOI] [Google Scholar]
- Wang et al. (2016a).Wang Y, Ren T, Lu JW, Ming R, Li PF, Hussai S, Cong RH, Li XK. Heterogeneity in rice tillers yield associated with tillers formation and nitrogen fertilizer. Agronomy Journal. 2016a;108(4):1717–1725. doi: 10.2134/agronj2015.0587. [DOI] [Google Scholar]
- Wang et al. (2016b).Wang ZQ, Liu YX, Shi HR, Mo HJ, Wu FK, Lin Y, Ga S, Wang JR, Wei YM, Liu CJ, Zheng YL. Identification and validation of novel low-tiller number QTL in common wheat. Theoretical and Applied Genetics. 2016b;129:603–612. doi: 10.1007/s00122-015-2652-4. [DOI] [PubMed] [Google Scholar]
- Wei et al. (2016).Wei X, Zhang Z, Wang P, Tao F. Recent patterns of production for the main cereal grains: implications for food security in China. Regional Environmental Change. 2016;17(1):1–12. [Google Scholar]
- World Reference Base for Soil Resources (2014).World Reference Base for Soil Resources International soil classification system for naming soils and creating legends for soil maps. FAO, RomeWorld soil resources report. No. 106. 2014
- Xie, Mayes & Sparkes (2016).Xie Q, Mayes S, Sparkes DL. Optimizing tiller production and survival for grain yield improvement in a bread wheat × spelt mapping population. Annals of Botany. 2016;117(1):51–66. doi: 10.1093/aob/mcv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2015a).Xu HC, Cai T, Wang ZL, He MR. Physiological basis for the differences of productive capacity among tillers in winter wheat. Journal of Integrative Agriculture. 2015a;14(10):1958–1970. doi: 10.1016/S2095-3119(15)61094-2. [DOI] [Google Scholar]
- Xu et al. (2015b).Xu J, Zha M, Li Y, Ding YF, Chen L, Ding CQ, Wang SH. The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L.) Plant Cell Reports. 2015b;34(9):1647–1662. doi: 10.1007/s00299-015-1815-8. [DOI] [PubMed] [Google Scholar]
- Xue et al. (2015).Xue HY, Han YC, Li YB, Wang GP, Feng L, Fan ZY, Du WL, Yang BF, Cao CG, Mao SC. Spatial distribution of light interception by different plant population densities and its relationship with yield. Field Crops Research. 2015;184:17–27. doi: 10.1016/j.fcr.2015.09.004. [DOI] [Google Scholar]
- Xue et al. (2013).Xue JJ, Wu SH, Zhang HY, Xu PZ, Wu XJ. Genetic analysis and gene mapping of multi-tiller and dwarf mutant d63 in rice. Rice Science. 2013;20(3):179–184. doi: 10.1016/S1672-6308(13)60130-4. [DOI] [Google Scholar]
- Yang et al. (2004).Yang JC, Zhang JH, Ye YX, Wang ZQ, Zhu QS, Liu LJ. Involvement of abscisic acid and ethylene in the responses of rice grains to water stress during filling. Plant, Cell & Environment. 2004;27(8):1055–1064. doi: 10.1111/j.1365-3040.2004.01210.x. [DOI] [Google Scholar]
- Zhang et al. (2015).Zhang X, Davidson EA, Mauzerall DL, Searchinger TD, Dumas P, Shen Y. Managing nitrogen for sustainable development. Nature. 2015;528(7580):51–59. doi: 10.1038/nature15743. [DOI] [PubMed] [Google Scholar]
- Zheng et al. (2017).Zheng MJ, Chen J, Shi YH, Li Y, Yin YP, Yang DQ, Luo YL, Pang DW, Xu X, Li WQ, Ni J, Wang YY, Wang ZL, Li Y. Manipulation of lignin metabolism by plant densities and its relationship with lodging resistance in wheat. Scientific Reports. 2017;7:41805. doi: 10.1038/srep41805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each plot (3 × 3 m) consisted of 10 rows of wheat and two ridges (A). A schematic diagram showing 75 ×104 (B), 300 ×104 (C), and 525 ×104 (D) plant ha−1 over two years.
The main stem was denoted as 0; primary tillers on the main stem in emergence order were referred to as I, II, III, IV, and V. Secondary tillers on the primary tillers in emergence order were referred to as I 1 and II 1.
GY, grain yield; SN, spike number; GN, grain number; GW grain weight. Correlation coefficients (r) are calculated and asterisks (∗∗) represent significance at the 0.01 probability level and asterisks (∗) represent significance at the 0.05 probability level.
GY, grain yield; SN, spike number; GN, grain number; GW grain weight. Correlation coefficients (r) are calculated and asterisks (∗∗) represent significance at the 0.01 probability level.
GY, grain yield; SN, spike number; GN, grain number; GW grain weight.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in Supplemental Files.