This mendelian randomization study evaluates the relationship between thyroid traits and atrial fibrillation.
Key Points
Question
Are free thyroxine (FT4) and thyrotropin levels within the reference range, triiodothyronine (FT3):FT4 ratio, hypothyroidism, thyroid peroxidase antibody levels, or hyperthyroidism on a direct pathway for atrial fibrillation (AF)?
Findings
This mendelian randomization study of 55 114 individuals with AF and 482 295 referents found that genetically increased FT3:FT4 ratio and hyperthyroidism were associated with increased risk of AF, and thyrotropin within the reference range and hypothyroidism were inversely associated with risk of AF. There was no support for a direct involvement of FT4 within the reference range or thyroid peroxidase antibody levels in AF.
Meaning
Low thyrotropin, as an early sign of an overactive thyroid gland, with a concomitant increased FT3:FT4 ratio are genetically associated with AF.
Abstract
Importance
Increased free thyroxine (FT4) and decreased thyrotropin are associated with increased risk of atrial fibrillation (AF) in observational studies, but direct involvement is unclear.
Objective
To evaluate the potential direct involvement of thyroid traits on AF.
Design, Setting, and Participants
Study-level mendelian randomization (MR) included 11 studies, and summary-level MR included 55 114 AF cases and 482 295 referents, all of European ancestry.
Exposures
Genomewide significant variants were used as instruments for standardized FT4 and thyrotropin levels within the reference range, standardized triiodothyronine (FT3):FT4 ratio, hypothyroidism, standardized thyroid peroxidase antibody levels, and hyperthyroidism. Mendelian randomization used genetic risk scores in study-level analysis or individual single-nucleotide polymorphisms in 2-sample MR for the summary-level data.
Main Outcomes and Measures
Prevalent and incident AF.
Results
The study-level analysis included 7679 individuals with AF and 49 233 referents (mean age [standard error], 62 [3] years; 15 859 men [29.7%]). In study-level random-effects meta-analysis, the pooled hazard ratio of FT4 levels (nanograms per deciliter) for incident AF was 1.55 (95% CI, 1.09-2.20; P = .02; I2 = 76%) and the pooled odds ratio (OR) for prevalent AF was 2.80 (95% CI, 1.41-5.54; P = .003; I2 = 64%) in multivariable-adjusted analyses. The FT4 genetic risk score was associated with an increase in FT4 by 0.082 SD (standard error, 0.007; P < .001) but not with incident AF (risk ratio, 0.84; 95% CI, 0.62-1.14; P = .27) or prevalent AF (OR, 1.32; 95% CI, 0.64-2.73; P = .46). Similarly, in summary-level inverse-variance weighted random-effects MR, gene-based FT4 within the reference range was not associated with AF (OR, 1.01; 95% CI, 0.89-1.14; P = .88). However, gene-based increased FT3:FT4 ratio, increased thyrotropin within the reference range, and hypothyroidism were associated with AF with inverse-variance weighted random-effects OR of 1.33 (95% CI, 1.08-1.63; P = .006), 0.88 (95% CI, 0.84-0.92; P < .001), and 0.94 (95% CI, 0.90-0.99; P = .009), respectively, and robust to tests of horizontal pleiotropy. However, the subset of hypothyroidism single-nucleotide polymorphisms involved in autoimmunity and thyroid peroxidase antibodies levels were not associated with AF. Gene-based hyperthyroidism was associated with AF with MR-Egger OR of 1.31 (95% CI, 1.05-1.63; P = .02) with evidence of horizontal pleiotropy (P = .045).
Conclusions and Relevance
Genetically increased FT3:FT4 ratio and hyperthyroidism, but not FT4 within the reference range, were associated with increased AF, and increased thyrotropin within the reference range and hypothyroidism were associated with decreased AF, supporting a pathway involving the pituitary-thyroid-cardiac axis.
Introduction
In observational longitudinal studies, increased free thyroxine (FT4) within the reference range,1 subclinical hyperthyroidism,2,3 and overt primary hyperthyroidism2,4 are associated with increased risk of atrial fibrillation (AF), whereas overt and subclinical hypothyroidism are associated with reduced risk of AF.2 Risk of AF is highest at the time of diagnosis of hyperthyroidism but risk persists despite antithyroid treatment.4,5,6,7 This raises the question of whether FT4 is on the causal pathway or instead a biomarker for the hyperthyroid-AF association.
Regulation of thyroid function is complex and involves not only the thyroid itself but also the pituitary and hypothalamus as well as feedback mechanisms. Produced and released by the pituitary, thyrotropin induces the thyroid to release thyroxine, which circulates in equilibrium between a sequestered, protein-bound form and the bioavailable free form, ie, FT4. Free thyroxine levels are regulated by feedback onto the pituitary to suppress thyrotropin production. In thyroidal and peripheral tissues, FT4 is converted to the active free triiodothyronine (FT3) hormone, a process that can be assessed by the circulating FT3:FT4 ratio.8 In the myocytes, FT3 binds to nuclear receptors with positive inotropic (cardiac contractility) and chronotropic effects (heart rate and rhythm).9 Thus, FT4 levels are only 1 measure of thyroid function and its association with AF may not reflect a direct relationship even if there is causality along the pituitary-thyroid-cardiac axis.
The physiologic diversity of influences on thyroid function is reflected in the genes implicated by genome-wide association studies (GWAS) of FT4 levels within the reference range,10,11,12 FT3:FT4 ratio,13 thyrotropin levels within the reference range,10,11,12 hypothyroidism,12,14,15 concentration of thyroid peroxidase antibodies16,17 (TPOAb, a marker of autoimmune thyroid disease), and hyperthyroidism.12 Genes implicated in FT4 levels are involved in thyroid development, thyroid hormone action and transport, intracellular mechanisms of hormone action, and metabolism of thyroid hormones.12
The mendelian randomization (MR) instrumental variable design mimics a randomized clinical trial by leveraging allelic randomization during meiosis and subsequent irreversible exposure to genotype at conception and is typically less likely affected by confounding or reverse causation than conventional observational analyses.18 If circulating FT4 levels are directly involved in the development of AF, then genetic variation influencing FT4 should also be associated with AF risk with effects that are quantitatively consistent with the observational associations.19 Similar arguments may be made for other measures of thyroid function, such as circulating thyrotropin.
We used MR to explore whether FT4 levels within the reference range, FT3:FT4 ratio, thyrotropin levels within the reference range, hypothyroidism, TPOAb, or hyperthyroidism may be on a causal pathway for AF. Mendelian randomization was implemented with genetic risk scores in study-level analysis from 11 studies with 56 912 participants and in a 2-sample strategy using summary-level analysis from 66 studies of 537 409 participants within the AF Genetics (AFGen) Consortium. To account for potential pleiotropy among instruments for the related traits of thyroid function, MR instruments for FT4, thyrotropin, and hypothyroidism were stratified according to involvement in other thyroid traits.
Methods
The analytic approach consisted of 2 parts. First, we assessed the relevance of FT4 levels in AF by performing MR with a genetic risk score of 4 FT4-associated single-nucleotide polymorphisms (SNPs) among population-based cohorts and population-based randomized clinical trials. Second, we assessed potential relevance of an expanded set of thyroid instruments for FT4 and thyrotropin levels within the reference range, FT3:FT4 ratio, hypothyroidism, TPOAb, and hyperthyroidism (determined by low thyrotropin levels) by performing an MR through the 2-sample method within published summary-level GWAS data for thyroid traits10,11,13,14,15,16,17,20 and AF21,22 (eTable 1 in the Supplement). See the eMethods in the Supplement for study information, study-specific funding and disclosure information, genetic instruments and genotyping, and study-specific characteristics of genotyping methodology.
Participants
A total of 56 912 participants with 7679 AF cases (2093 prevalent and 5586 incident) and 49 233 referents (eTable 2 in the Supplement) were included from 11 cross-sectional and prospective cohort studies of European ancestry contributing to the AFGen Consortium. Mean age ranged from 53 to 76 years. Among the 11 studies, FT4 (nanograms per deciliter) and thyrotropin (milli-international units per liter) levels were available respectively in 7 studies (n = 26 089) and 8 studies (n = 27 916). All protocols were approved by local institutional review boards. Participant consent was obtained if the local institutional review boards required this. Analyses were not restricted to thyroid measures within the reference range.
Summary-Level Analysis
Instrument-exposure associations (ie, FT4, thyrotropin, FT3:FT4 ratio, hypothyroidism, TPOAb, and hyperthyroidism) were from GWAS for various thyroid traits among individuals of European ancestry.12,13,14,15,16,17 The instrument-AF associations for common variants (minor allele frequency, >1%) came from a GWAS of AF from the AFGen Consortium among individuals of European ancestry, including 66 studies with a total of 55 114 individuals with AF (7672 incident, 47442 prevalent) and 482 295 referents.21,22 In the AFGen GWAS, we identified 30 of 31 SNPs for FT4 within the reference range,12 1 of 1 SNP for FT3:FT4 ratio (the DIO1, rs2235544),13 56 of 61 SNPs for thyrotropin within the reference range,12 15 of 30 SNPs for hypothyroidism,14,23 4 of 5 SNPs for TPOAb concentration,16,17 and 8 of 8 SNPs for hyperthyroidism (which was defined by low thyrotropin levels in population-based cohorts in which patients receiving medication for autoimmune thyroiditis were excluded).12 For the rare variant (minor allele frequency, 0.4%) in the TTR gene, the association with AF came from an exome-wide association study by meta-analysis including 22 346 cases and 132 086 referents.21
Atrial fibrillation diagnosis used cohort-specific definitions, including physician adjudication, questionnaire self-report, electrocardiography, and diagnosis codes for AF or flutter (International Classification of Diseases, Ninth Revision: 427.3, 427.31 or 427.32; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision: I48) present in hospitalization discharges or death certificates as previously described.22 For analyses of incident AF, individuals with baseline AF were excluded.
Instrument strength in MR was estimated with an approximated F statistic24 (eMethods in the Supplement). The instruments for FT4 in study-level analyses all had instrument strength above the minimum required threshold of 10 (F > 10), except for 1 SNP (AADAT, rs7694879). The genetic instruments for summary-level analyses all had instrument strengths (F) larger than 10 (ie, for FT4, range: 29-394; FT3:FT4 ratio, 21; thyrotropin, 29-576; hypothyroidism, 23-105; TPOAb, 10-19; and hyperthyroidism, 30-94). Mendelian randomization results are presented in SD units of the biomarkers (eMethods in the Supplement). We performed associations for 6 primary hypotheses (FT4, FT3:FT4 ratio, thyrotropin, hypothyroidism, TPOAb, and hyperthyroidism). While tests of these primary hypotheses involved multiple secondary analyses, the tests were correlated, we therefore considered a 2-tailed P < .008 (= .05/6) to be significant.
Study-Level MR Using a Genetic Risk Score
Instrumental estimates were combined across studies using inverse-variance weighted random-effects (IVW-RE) meta-analyses. First, we examined the observational associations of FT4 or thyrotropin with prevalent or incident AF using logistic or Cox regression, respectively. A genetic risk score for FT4 levels (GRSFT4) was created by summing the allele count of 4 genotyped alleles (DIO1, LHX3, AADAT, and FOXE1) or the maximum likelihood dose of imputed alleles at each of the variants, weighted by the effect size, ie, the β coefficients. Mendelian randomization instrumental effects were derived by the instrumental variable ratio (Wald) estimator, which was the ratio of the meta-analysis β coefficient from the genetic risk score on AF association divided by the meta-analysis β coefficient from the genetic risk score on FT4 association, with standard errors calculated using the δ method.25
Two-Sample Summary-Level MR
For the individual SNPs, MR instrumental estimates were determined by instrumental variable ratio. To determine the primary overall instrumental estimate, IVW fixed-effects MR was applied across the individual instrumental estimates and their standard errors, assuming all genetic variants are valid instruments with no pleiotropy.26 The between-instrument heterogeneity Cochran Q statistic and the I2MR index were used to assess heterogeneity in the MR.27 By several approaches, we performed sensitivity analyses to investigate potential pleiotropic bias26,28: IVW-RE MR, MR-Egger regression, weighted median MR, funnel plots, and leave-1-variant-out analysis for IVW-RE, where 1 variant at a time is left out.
Results
FT4 in Study-Level Analysis
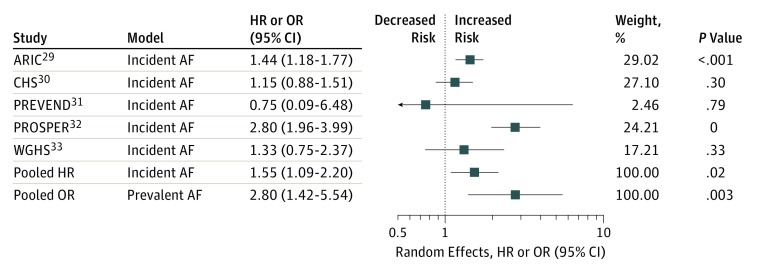
Characteristics of studies are presented in eTable 2 in the Supplement. In meta-analysis of multivariable-adjusted, observational study–level data, the pooled hazard ratio of FT4 (nanograms per deciliter; to convert to picomoles per liter, multiply by 12.871) for incident AF was 1.55 (95% CI, 1.09-2.20; P = .02; I2 = 76%) with no evidence of participation bias (P for Egger = .94) and the pooled odds ratio (OR) for prevalent AF was 2.80 (95% CI, 1.42-5.54; P = .003; I2 = 64%) with evidence of participation bias (P for Egger = .003) (Figure 1 and eTable 3 in the Supplement).29,30,31,32,33 Meanwhile, each increment in the GRSFT4 was associated with a 0.082 SD (standard error, 0.007) increase of standardized FT4 (P < .001) in multivariable-adjusted random-effects meta-analyses (eTables 4 and 5 in the Supplement) with only minimal potential pleiotropic effects on thyrotropin in multivariable-adjusted random-effects meta-analyses (eTable 6 in the Supplement). In the MR analysis, the instrumental estimates of FT4 (per SD) were nonsignificant for incident or prevalent AF with an overall risk ratio of 0.84 (95% CI, 0.62-1.14; P = .27) across 7 studies and OR of 1.32 (95% CI, 0.64-2.73, P = .46) across 5 studies in multivariable-adjusted random-effects meta-analyses, respectively (eTables 7 and 8 in the Supplement). Results were similar if analyses were restricted to studies that had complete information on GRSFT4, FT4 and AF (eTable 9 in the Supplement), if the TTR variant was included in the GRSFT4 (eTables 7 and 8 in the Supplement), or if the TTR variant was analyzed alone (eTables 7, 8, and 10 in the Supplement).
Figure 1. Observational Meta-analyses of Free Thyroxine (FT4) on Atrial Fibrillation (AF) (Study Level).
See eTable 3 in the Supplement for details. HR indicates hazard ratio; OR, odds ratio.
FT4 in Summary-Level Analysis
The individual instrument-exposure and instrument-outcome associations are shown in eTable 11 in the Supplement. In summary-level MR analysis, the IVW-RE OR for combined prevalent and incident AF per SD of FT4 within the reference range was 1.01 (95% CI, 0.89-1.14; P = .88) (Table and eFigure 1A in the Supplement). Results were similar for prevalent and incident AF separately in summary-level IVW fixed-effects and MR-Egger analysis (eTables 12, 13, and 14 in the Supplement). Weighted median analysis was significant with OR for AF per SD of FT4 of 0.87 (95% CI, 0.79-0.95; P = .001) (eTable 12 in the Supplement).
Table. Two-Sample Mendelian Randomization Estimates of Relationship Between Genetically Predicted Thyroid Function and Combined Incident and Prevalent Atrial Fibrillation Using CHARGE AF Genetics Consortium in European Individualsa.
| Instrument | Exposure | MR Method | No. of SNP | OR (95% CI) | P Value | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2MR % (95% CI) | P Value | ||||||
| FT4 | FT4 | IVW-FE | 30 | 1.00 (0.95-1.05) | .93 | 81 (73-86) | <.001 |
| FT4 | IVW-RE | 30 | 1.01 (0.89-1.14) | .88 | 81 (73-86) | <.001 | |
| DIO1 | FT3:FT4 ratio | IVR | 1 | 1.33 (1.08-1.63) | <.001 | NA | NA |
| Thyrotropin | Thyrotropin | IVW-FE | 56 | 0.88 (0.89-0.91) | <.001 | 27 (0-48) | .04 |
| Thyrotropin | IVW-RE | 56 | 0.88 (0.84-0.92) | <.001 | 27 (0-48) | .04 | |
| Hypothyroidism | Hypothyroidism | IVW-FE | 15 | 0.95 (0.91-0.97) | <.001 | 52 (13-73) | .01 |
| Hypothyroidism | IVW-RE | 15 | 0.94 (0.90-0.99) | <.001 | 52 (13-73) | .01 | |
| TPOAb | TPOAb levels | IVW-FE | 4 | 0.76 (0.49-1.18) | .22 | 0 (0-85) | .43 |
| TPOAb levels | IVW-RE | 4 | 0.76 (0.49-1.18) | .22 | 0 (0-85) | .43 | |
| Hyperthyroidism | Hyperthyroidism | IVW-FE | 8 | 1.05 (1.03-1.08) | <.001 | 77 (54-88) | <.001 |
| Hyperthyroidism | IVW-RE | 8 | 1.04 (0.99-1.10) | .13 | 77 (54-88) | <.001 | |
Abbreviations: AF, atrial fibrillation; FT4, free thyroxine; FT3, free triiodothyronine; GWAS, genome-wide association studies; IVR, instrumental variable ratio (Wald) estimator; IVW-FE, inverse-variance weighted fixed-effects MR; IVW-RE, inverse-variance weighted random-effects MR; MR, mendelian randomization; NA, not applicable; OR, odds ratio; SNP, single-nucleotide polymorphism; TPOAb, thyroid peroxidase antibody levels.
From GWAS of 66 studies with 55 114 AF cases and 482 295 referents.
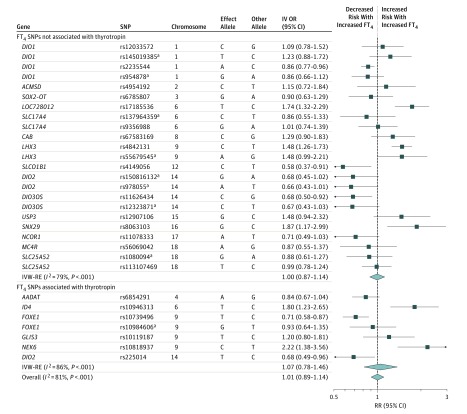
Estimates for the individual FT4 instruments in summary-level analysis were not homogeneous (Figure 2), which was reflected in the I2MR values of 81% (95% CI, 73%-86%; P < .001) (Table). There was no difference in the point estimate when parsing out the FT4 SNPs into those associated with thyrotropin (IVW-RE OR, 1.00; 95% CI, 0.78-1.46; P = .68) and those not (IVW-RE OR, 1.00; 95% CI, 0.87-1.14; P = .96) (P for interaction = 0.70) (Figure 2). FT4 SNPs were distributed symmetrically about the combined effect size in the funnel plot (eFigure 1B in the Supplement), and MR-Egger did not show evidence of horizontal pleiotropy (P for MR-Egger = .63) (eTable 12 in the Supplement). Leave-1-variant-out analysis did not identify variants with exaggerated influence on the combined effect estimate (eTable 15 in the Supplement). Subanalysis of identical instruments (n = 4) as in the study-level analysis revealed a similar point estimate (eTable 16 in the Supplement).
Figure 2. Summary-Level Analysis of FT4 SNPs and Atrial Fibrillation.
The mendelian randomization effect estimate is risk of atrial fibrillation (IV OR) per standardized FT4.
aIndependent SNPs within a locus as defined by Teumer et al.12
FT4 indicates free thyroxine; IV OR, instrumental variable odds ratio; IVW-RE, inverse variance-weighted random-effects; RR, risk ratio; SNP, single-nucleotide polymorphism.
FT3:FT4 Ratio
In 2-sample summary-level MR, a genetically predicted 1 SD–increase in FT3:FT4 ratio by the C allele was associated with increased AF with an OR of 1.33 (95% CI, 1.08-1.63; P = .006) (Table and eTable 12 in the Supplement). Results were similar for incident and prevalent AF separately (eTables 13 and 14 in the Supplement).
Thyrotropin
In random-effects meta-analysis of observational study-level data, the pooled hazard ratio of thyrotropin (milli-international units per liter) for incident AF was 1.00 (95% CI, 0.98-1.01; P = .87; I2 = 23%) with P = .06 for participation bias, and the pooled OR for prevalent AF was 0.99 (95% CI, 0.97-1.02; P = .52; I2 = 0%) with no evidence of participation bias (P = .52) in multivariable-adjusted analyses (eTable 17 in the Supplement).
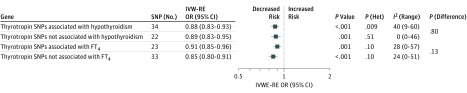
Instrument-exposure and instrument-outcome associations are shown in eTable 18 in the Supplement. A genetically predicted 1 SD–increase in thyrotropin was inversely associated with AF with IVW-RE OR of 0.88 (95% CI, 0.84-0.92; P < .001; I2MR = 27%) (Table and eFigure 2A and B in the Supplement). Results were similar with the MR-Egger and weighted median analyses (eTable 12 in the Supplement). The thyrotropin variants were distributed symmetrically about the combined effect size in the funnel plot (eFigure 2C in the Supplement), and MR-Egger did not show evidence of horizontal pleiotropy (P for MR-Egger = .95) (eTable 12 in the Supplement). Leave-1-variant-out analysis did not identify variants with exaggerated influence on the combined effect estimate (eTable 19 in the Supplement). Results were similar for incident and prevalent AF separately (eTables 13 and 14 in the Supplement). The IVW-RE association of thyrotropin levels with AF was stronger among thyrotropin SNPs that were also identified in GWAS for hypothyroidism vs those that were not and was stronger among thyrotropin SNPs that were not associated with FT4 vs those that were, but none of these differences was statistically significant (Figure 3).
Figure 3. Summary-Level Analysis of SNP (Thyrotropin) and Atrial Fibrillation.
The mendelian randomization effect estimate is risk of atrial fibrillation per standardized thyrotropin. FT4 indicates free thyroxine; het, heterogeneity; IV OR, instrumental variable odds ratio; IVW-RE, inverse variance-weighted random-effects; OR, odds ratio; SNP, single-nucleotide polymorphism.
Hypothyroidism and TPOAb
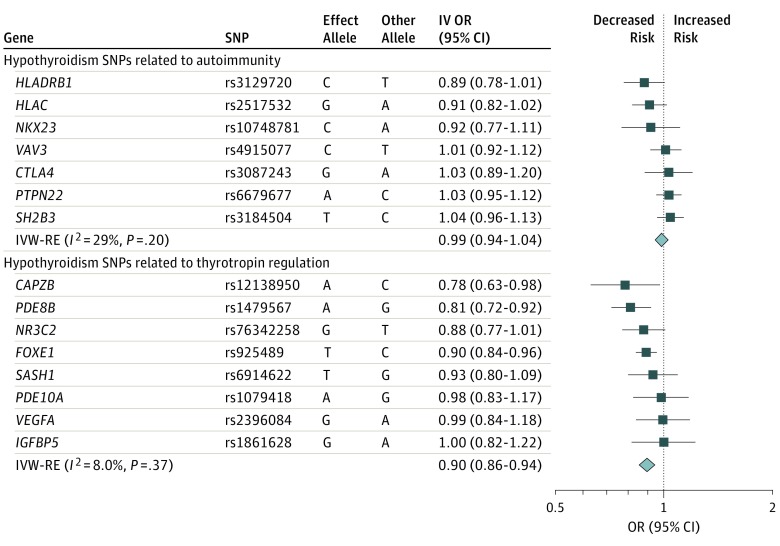
Genetically predicted hypothyroidism was inversely associated with AF with an IVW-RE OR of 0.94 (95% CI, 0.90-0.99; P = .009; I2MR = 52%) (Table and eFigure 3A, eTable 12, eTable 20 in the Supplement). This association was significantly stronger among hypothyroid SNPs that were also identified for association with thyrotropin (OR, 0.90; 95% CI, 0.86-0.94) than those that were also identified with autoimmune function (OR, 0.99; 95% CI, 0.94-1.04; P for difference between effects of autoimmune SNPs vs thyrotropin SNPs = .006) (Figure 4). Results were similar for prevalent AF (eTable 14 in the Supplement) and in the same direction but not significant for incident AF (eTable 13 in the Supplement). Leave-1-variant-out analysis did not identify variants with exaggerated influence on the combined effect estimate (eTable 21 in the Supplement). Genetically predicted 1 SD–increase in TPOAb was not associated with AF in the MR analyses (eTable 12, eTable 20, and eFigure 4 in the Supplement).
Figure 4. Summary-Level Analysis of SNP (Hypothyroidism) and Atrial Fibrillation.
The mendelian randomization effect estimate is risk of atrial fibrillation per allele per risk of hypothyroidism. IV OR indicates instrumental variable odds ratio; IVW-RE, inverse variance-weighted random-effects; OR, odds ratio; SNP, single-nucleotide polymorphism.
Hyperthyroidism
Genetically predicted hyperthyroidism was associated with AF with an IVW-RE OR of 1.04 (95% CI, 0.99-1.10; P = .13; I2MR = 77%) (Table and eTable 12, eTable 22, eFigure 5A in the Supplement) with similar but significant results in IVW fixed-effects and weighted median analysis. Funnel plot (eFigure 5B in the Supplement) and MR-Egger showed evidence of pleiotropy (P for MR-Egger = .045), and the MR-Egger OR was 1.31 (95% CI, 1.05-1.63; P = .02). Results were similar for incident and prevalent AF (eTables 13 and 14 in the Supplement). Leave-1-variant-out analysis did not identify variants with exaggerated influence on the combined effect estimate (eTable 23 in the Supplement).
Discussion
In this MR study, the combined genetic association of FT4 instruments did not support a direct effect of FT4 levels in the reference range on AF. However, the pituitary-thyroid-cardiomyocyte axis was implicated through the significant gene-based effects on AF of FT3:FT4 ratio, hyperthyroidism, thyrotropin, and hypothyroidism, the latter largely limited to instruments with effects on thyrotropin as opposed to autoimmune function. The result diverges sharply from observational associations of FT4 with AF, although those associations may have been influenced by inclusion of individuals with overt or subclinical thyroid disease,1,2,3 an explanation that would be supported by the gene-based findings here for hypothyroidism, hyperthyroidism, and thyrotropin.
The hypothalamic-pituitary-thyroid-cardiac axis represents a series of phenotypes that are dependent on phenotypes upstream the axis as well as downstream through negative feedback mechanisms. This vertical pleiotropy was reflected by overlap of some of the instruments between some pairs of thyroid traits and by subgenomewide significant association of instruments with a second thyroid trait. By stratifying the MR analysis according to unique vs shared instruments, we endeavored to parse out influences on AF along the axis. These analyses supported the idea that gene-based effects on AF of thyrotropin, including those associated with thyroid disease, were qualitatively different from those of FT4 within the normal range.
However, a larger question is why, in spite of very strong instruments, gene-based effects of FT4 were largely null compared with significant associations upstream of FT4 represented by thyrotropin? Some answers may be found by contrasting the instrumental associations with thyroid measures conditional on thyroid status. The FT4 SNPs derive from associations among individuals with FT4 levels within the reference range and no evidence of thyroid disease.10,11,12 Further, when these SNPs were combined into a genetic risk score, they were not associated with thyroid disease in the forms of Graves disease, hypothyroidism, or hyperthyroidism.12 By contrast, the instruments for thyrotropin within the reference range were associated with hypothyroidism and hyperthyroidism.12 These distinctions may emphasize a diseased thyroid state rather than normal variation in thyroid function as driving the instrumental associations with AF. Such an interpretation is consistent with variability in observational associations between thyrotropin and AF, where the proportion of individuals with subclinical or overt thyroid disease may not have been adequately controlled.2 Notably, the MR results for thyrotropin in this report are consistent with a large Danish observational study finding (N = 586 460) that hyperthyroidism and hypothyroidism were associated with increased and decreased risk of AF, respectively, and that there was an inverse linear association between thyrotropin and risk of AF in a sample that included individuals with thyrotropin outside of the normal range.2
Similarly, significant instrumental associations of FT3:FT4 ratio downstream of FT4 need to be reconciled with the null result for FT4. Here, we suggest that conversion of FT4 to FT3 (and therefore the value of the FT3:FT4 ratio) may be sufficiently regulated to limit the extent to which variation of FT4 within the normal range may be propagated downstream. Teumer et al12 examined this relationship for 3 independent genomewide significant SNPs for FT4 levels mapping to 2 genes, AADAT (rs6854291) and SLC17A4, (9356988, rs137964359). While the SNP at AADAT was associated with FT3 and FT3:FT4 ratio, the 2 SNPs at SLC17A4 were null suggesting at least partially independent control of FT3 with respect to FT4. Incidentally, the MR association of variation in the deiodinase (DIO1, rs2235544) may recommend pharmacologic inhibition of deiodinase-1 to block conversion of FT4 to FT3 in therapeutic protection from AF, although such treatment could potentially put patients at risk for other diseases as deiodinase-1 is expressed in many tissues.34
Limitations
The study has several limitations. Structurally, samples contributing to the study-level MR were also included in the much larger AF GWAS used for the summary-level MR, implying some dependence in the concordant results, even as the methodologies were different. The European ancestry of the samples also limits generalizability to other ancestries. In addition to the vertical pleiotropy considered above, there was evidence for horizontal pleiotropy that was addressed through current best practices for MR sensitivity analysis,19 all of which supported the primary conclusions. However, as with all MR studies, we could not address unobserved pleiotropy. Given the divergence of the observational and MR instrumental findings for FT4, there remains a need for more analysis, especially downstream of FT4 along the hypothalamic-pituitary-thyroid-cardiac axis. Our ability to draw mechanistic inferences downstream of FT4 was limited by the availability of only 1 instrument for the FT3:FT4 ratio and the lack of any instruments for FT3, although this hormone, as opposed to FT4, is transported into cardiomyocytes and thought to be more biologically relevant.9
Conclusions
In conclusion, we demonstrated that genetically increased FT3:FT4 ratio and hyperthyroidism are associated with increased AF with inverse associations for genetically predicted hypothyroidism and increased thyrotropin supporting relevance of the pituitary-thyroid-cardiac axis in AF susceptibility. However, genetically increased FT4 within the reference range was not associated with AF, and the current lack of additional genetic instruments, particularly for FT3, makes it difficult to infer a specific causal agent in the link between thyroid function and AF.
eMethods.
eReferences.
eTable 1. Study design of mendelian randomization analyses
eTable 2. Baseline characteristics of study cohorts
eTable 3. Meta-analyses of the observational association between fT4 and prevalent and incident atrial fibrillation in study-level analyses
eTable 4. Meta-analyses of the association of GRS(fT5) with standardized fT4 in study-level analyses
eTable 5. Association between fT4 instruments and fT4 in study-level analyses
eTable 6. Association between fT4 instruments and thyrotropin in study-level analyses
eTable 7. Association between fT4 instruments and incident atrial fibrillation in study-level analyses across all studies
eTable 8. Association between fT4 instruments and prevalent atrial fibrillation in study-level analyses
eTable 9. Meta-analyses of the instrumental variable estimates between GRS(fT4) and prevalent and incident atrial fibrillation (AF) in study-level analyses, which had information on GRS(fT4) and ft4, and AF
eTable 10. Meta-analyses of the instrumental variable estimates between TTR and prevalent and incident atrial fibrillation (AF) in study-level analyses, which had information on TTR and fT4, and AF
eTable 11. Published association between fT4 and fT3/fT4 instruments, thyroid phenotype, and AF in summary-level analyses in AFGen
eTable 12. Mendelian randomization estimates of genetically predicted thyroid function on prevalent and incident atrial fibrillation using publicly available data on SNP-thyroid function and CHARGE AFGen consortium in Europeans
eTable 13. Mendelian randomization estimates of genetically predicted thyroid function on incident atrial fibrillation using publicly available data on SNP-thyroid function and CHARGE AFGen consortium in Europeans
eTable 14. Mendelian randomization estimates of genetically predicted thyroid function on prevalent atrial fibrillation using publicly available data on SNP-thyroid function and CHARGE AFGen consortium in Europeans
eTable 15. Leave-one-out analysis for IVW-RE MR of fT4 on prevalent and incident AF in summary-level analyses
eTable 16. Association between fT4 instruments, thyroid phenotype, and incident and prevalent AF in summary-level analyses from AFGen with identical SNPs as used in the study-level analyses
eTable 17. Meta-analyses of the observation association between thyrotropin and prevalent and incident atrial fibrillation in study-level analyses
eTable 18. Association between thyrotropin instruments and incident and prevalent atrial fibrillation in summary-level analyses
eTable 19. Leave-one-out analysis for IVW-RE MR of thyrotropin on prevalent and incident AF in summary-level analyses
eTable 20. Association between hypothyroidism instruments and TPOAb and incident and prevalent atrial fibrillation in summary-level analyses
eTable 21. Leave-one-out analysis for IVW-RE MR of hypothyroidism on prevalent and incident AF in summary-level analyses
eTable 22. Association between hyperthyroidism instruments and incident and prevalent atrial fibrillation in summary-level analyses
eTable 23. Leave-one-out analyses for IVW-RE MR of hypothyroidism on prevalent and incident AF in summary-level analyses
eFigure 1. fT4-associated SNPs with risk of atrial fibrillation
eFigure 2. Thyrotropin-associated SNPs with risk of atrial fibrillation
eFigure 3. Hypothyroidism-associated SNPs with risk of atrial fibrillation
eFigure 4. Forest plot of TPOAb-associated SNPs with risk of atrial fibrillation.
eFigure 5. Hyperthyroidism-associated SNPs with risk of atrial fibrillation
References
- 1.Baumgartner C, da Costa BR, Collet TH, et al. ; Thyroid Studies Collaboration . Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. 2017;136(22):2100-2116. doi: 10.1161/CIRCULATIONAHA.117.028753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selmer C, Olesen JB, Hansen ML, et al. . The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ. 2012;345:e7895. doi: 10.1136/bmj.e7895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappola AR, Fried LP, Arnold AM, et al. . Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295(9):1033-1041. doi: 10.1001/jama.295.9.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dekkers OM, Horváth-Puhó E, Cannegieter SC, Vandenbroucke JP, Sørensen HT, Jørgensen JO. Acute cardiovascular events and all-cause mortality in patients with hyperthyroidism: a population-based cohort study. Eur J Endocrinol. 2017;176(1):1-9. doi: 10.1530/EJE-16-0576 [DOI] [PubMed] [Google Scholar]
- 5.Osman F, Franklyn JA, Holder RL, Sheppard MC, Gammage MD. Cardiovascular manifestations of hyperthyroidism before and after antithyroid therapy: a matched case-control study. J Am Coll Cardiol. 2007;49(1):71-81. doi: 10.1016/j.jacc.2006.08.042 [DOI] [PubMed] [Google Scholar]
- 6.Metso S, Auvinen A, Salmi J, Huhtala H, Jaatinen P. Increased long-term cardiovascular morbidity among patients treated with radioactive iodine for hyperthyroidism. Clin Endocrinol (Oxf). 2008;68(3):450-457. [DOI] [PubMed] [Google Scholar]
- 7.Ryödi E, Salmi J, Jaatinen P, et al. . Cardiovascular morbidity and mortality in surgically treated hyperthyroidism - a nation-wide cohort study with a long-term follow-up. Clin Endocrinol (Oxf). 2014;80(5):743-750. doi: 10.1111/cen.12359 [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Zhou Y, Zhou M, Yin Q, Wang S. Diagnostic values of free triiodothyronine and free thyroxine and the ratio of free triiodothyronine to free thyroxine in thyrotoxicosis. Int J Endocrinol. 2018;2018:4836736. doi: 10.1155/2018/4836736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116(15):1725-1735. doi: 10.1161/CIRCULATIONAHA.106.678326 [DOI] [PubMed] [Google Scholar]
- 10.Porcu E, Medici M, Pistis G, et al. . A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. 2013;9(2):e1003266. doi: 10.1371/journal.pgen.1003266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor PN, Porcu E, Chew S, et al. ; UK0K Consortium . Whole-genome sequence-based analysis of thyroid function. Nat Commun. 2015;6:5681. doi: 10.1038/ncomms6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teumer A, Chaker L, Groeneweg S, et al. ; Lifelines Cohort Study . Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat Commun. 2018;9(1):4455. doi: 10.1038/s41467-018-06356-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panicker V, Cluett C, Shields B, et al. . A common variation in deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and triiodothyronine. J Clin Endocrinol Metab. 2008;93(8):3075-3081. doi: 10.1210/jc.2008-0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson N, Tung JY, Kiefer AK, et al. . Novel associations for hypothyroidism include known autoimmune risk loci. PLoS One. 2012;7(4):e34442. doi: 10.1371/journal.pone.0034442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickrell JK, Berisa T, Liu JZ, Ségurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016;48(7):709-717. doi: 10.1038/ng.3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultheiss UT, Teumer A, Medici M, et al. . A genetic risk score for thyroid peroxidase antibodies associates with clinical thyroid disease in community-based populations. J Clin Endocrinol Metab. 2015;100(5):E799-E807. doi: 10.1210/jc.2014-4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medici M, Porcu E, Pistis G, et al. . Identification of novel genetic loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 2014;10(2):e1004123. doi: 10.1371/journal.pgen.1004123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133-1163. doi: 10.1002/sim.3034 [DOI] [PubMed] [Google Scholar]
- 19.Zheng J, Baird D, Borges MC, et al. . Recent developments in mendelian randomization studies. Curr Epidemiol Rep. 2017;4(4):330-345. doi: 10.1007/s40471-017-0128-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gudmundsson J, Sulem P, Gudbjartsson DF, et al. . Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat Genet. 2012;44(3):319-322. doi: 10.1038/ng.1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christophersen IE, Rienstra M, Roselli C, et al. ; METASTROKE Consortium of the ISGC; Neurology Working Group of the CHARGE Consortium; AFGen Consortium . Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet. 2017;49(6):946-952. doi: 10.1038/ng.3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roselli C, Chaffin MD, Weng LC, et al. . Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50(9):1225-1233. doi: 10.1038/s41588-018-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denny JC, Crawford DC, Ritchie MD, et al. . Variants near FOXE1 are associated with hypothyroidism and other thyroid conditions: using electronic medical records for genome- and phenome-wide studies. Am J Hum Genet. 2011;89(4):529-542. doi: 10.1016/j.ajhg.2011.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill D, Del Greco M F, Rawson TM, et al. . Age at menarche and time spent in education: a mendelian randomization study. Behav Genet. 2017;47(5):480-485. doi: 10.1007/s10519-017-9862-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson JR, Minelli C, Del Greco M F. Mendelian randomization using public data from genetic consortia. Int J Biostat. 2016;12(2):/j/ijb.2016.12.issue-2/ijb-2015-0074/ijb-2015-0074.xml. doi: 10.1515/ijb-2015-0074 [DOI] [PubMed] [Google Scholar]
- 26.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304-314. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greco M FD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926-2940. doi: 10.1002/sim.6522 [DOI] [PubMed] [Google Scholar]
- 28.Bowden J, Del Greco M F, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data mendelian randomization. Stat Med. 2017;36(11):1783-1802. doi: 10.1002/sim.7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 30.Fried LP, Borhani NO, Enright P, et al. . The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263-276. doi: 10.1016/1047-2797(91)90005-W [DOI] [PubMed] [Google Scholar]
- 31.Vermond RA, Geelhoed B, Verweij N, et al. . Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol. 2015;66(9):1000-1007. doi: 10.1016/j.jacc.2015.06.1314 [DOI] [PubMed] [Google Scholar]
- 32.Shepherd J, Blauw GJ, Murphy MB, et al. ; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk . Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi: 10.1016/S0140-6736(02)11600-X [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Chasman DI, Zee RY, et al. ; Women’s Genome Health Study Working Group . Rationale, design, and methodology of the Women’s Genome Health Study: a genome-wide association study of more than 25,000 initially healthy american women. Clin Chem. 2008;54(2):249-255. doi: 10.1373/clinchem.2007.099366 [DOI] [PubMed] [Google Scholar]
- 34.Maia AL, Goemann IM, Meyer EL, Wajner SM. Deiodinases: the balance of thyroid hormone: type 1 iodothyronine deiodinase in human physiology and disease. J Endocrinol. 2011;209(3):283-297. doi: 10.1530/JOE-10-0481 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eReferences.
eTable 1. Study design of mendelian randomization analyses
eTable 2. Baseline characteristics of study cohorts
eTable 3. Meta-analyses of the observational association between fT4 and prevalent and incident atrial fibrillation in study-level analyses
eTable 4. Meta-analyses of the association of GRS(fT5) with standardized fT4 in study-level analyses
eTable 5. Association between fT4 instruments and fT4 in study-level analyses
eTable 6. Association between fT4 instruments and thyrotropin in study-level analyses
eTable 7. Association between fT4 instruments and incident atrial fibrillation in study-level analyses across all studies
eTable 8. Association between fT4 instruments and prevalent atrial fibrillation in study-level analyses
eTable 9. Meta-analyses of the instrumental variable estimates between GRS(fT4) and prevalent and incident atrial fibrillation (AF) in study-level analyses, which had information on GRS(fT4) and ft4, and AF
eTable 10. Meta-analyses of the instrumental variable estimates between TTR and prevalent and incident atrial fibrillation (AF) in study-level analyses, which had information on TTR and fT4, and AF
eTable 11. Published association between fT4 and fT3/fT4 instruments, thyroid phenotype, and AF in summary-level analyses in AFGen
eTable 12. Mendelian randomization estimates of genetically predicted thyroid function on prevalent and incident atrial fibrillation using publicly available data on SNP-thyroid function and CHARGE AFGen consortium in Europeans
eTable 13. Mendelian randomization estimates of genetically predicted thyroid function on incident atrial fibrillation using publicly available data on SNP-thyroid function and CHARGE AFGen consortium in Europeans
eTable 14. Mendelian randomization estimates of genetically predicted thyroid function on prevalent atrial fibrillation using publicly available data on SNP-thyroid function and CHARGE AFGen consortium in Europeans
eTable 15. Leave-one-out analysis for IVW-RE MR of fT4 on prevalent and incident AF in summary-level analyses
eTable 16. Association between fT4 instruments, thyroid phenotype, and incident and prevalent AF in summary-level analyses from AFGen with identical SNPs as used in the study-level analyses
eTable 17. Meta-analyses of the observation association between thyrotropin and prevalent and incident atrial fibrillation in study-level analyses
eTable 18. Association between thyrotropin instruments and incident and prevalent atrial fibrillation in summary-level analyses
eTable 19. Leave-one-out analysis for IVW-RE MR of thyrotropin on prevalent and incident AF in summary-level analyses
eTable 20. Association between hypothyroidism instruments and TPOAb and incident and prevalent atrial fibrillation in summary-level analyses
eTable 21. Leave-one-out analysis for IVW-RE MR of hypothyroidism on prevalent and incident AF in summary-level analyses
eTable 22. Association between hyperthyroidism instruments and incident and prevalent atrial fibrillation in summary-level analyses
eTable 23. Leave-one-out analyses for IVW-RE MR of hypothyroidism on prevalent and incident AF in summary-level analyses
eFigure 1. fT4-associated SNPs with risk of atrial fibrillation
eFigure 2. Thyrotropin-associated SNPs with risk of atrial fibrillation
eFigure 3. Hypothyroidism-associated SNPs with risk of atrial fibrillation
eFigure 4. Forest plot of TPOAb-associated SNPs with risk of atrial fibrillation.
eFigure 5. Hyperthyroidism-associated SNPs with risk of atrial fibrillation