Abstract
Purpose
To better characterize the association between overall survival (OS) from metastatic thyroid cancer and the rate of structural disease progression
Methods
In this retrospective study, the average tumor volume doubling time (midDT) of two dominant lung metastases was used to group patients into 6 clinically relevant cohorts. Overall survival was calculated from the time of metastasis diagnosis and from the time the pulmonary lesions crossed over the 1 cm diameter.
Results
Tumor growth rate was remarkably constant in lung metastasis from thyroid cancer over a median follow up period of 8.5 years (median r = 0.92, r2 = 0.85). Patients with midDT ≤ 1 year had worse overall survival than those with higher midDT (Log rank p = 0.01). The 5 year overall survival from the 1 cm diameter time point was 20% for midDT ≤1 year (n = 15), 50% for midDT 1–2 years (n= 19), 53% for midDT 2–3 years (n=9), 80 % for midDT 3–4 years (n=6), and 80% for midDT ≥ 4 years or negative (n=12). Within the midDT ≤ 1 year group, the 2 year overall survival from the 1 cm diameter point was 88% in the MKI-treated group (n=8) as opposed to 43% in the non-treated group (n=7) (p = 0.13).
Discussion
MidDT of lung metastasis is a good prognostic indicator of overall survival in patients with metastatic thyroid cancer. Unlike Thyroglobulin DT, midDT alone can be used to predict eligibility for MKI therapy.
Keywords: Structural doubling time, metastatic thyroid cancer, lung metastasis, radioactive iodine refractory thyroid cancer, multi-kinase inhibitor therapy, overall survival
PRECIS
The average tumor volume doubling time (midDT) of lung metastasis from follicular cell derived thyroid cancer is an accessible, easily calculated and reliable prognostic indicator of overall survival in patients with metastatic differentiated thyroid cancer. Unlike thyroglobulin doubling time, midDT can be used to properly select patients with progressive metastatic radioactive iodine refractory thyroid cancer who are likely to have a survival benefit from multi-kinase therapy
The development of distant metastasis in follicular cell derived thyroid carcinoma is associated with poor clinical outcomes, particularly in older patients. Ten year overall survival rates of approximately 50% are seen in patients who present with distant metastasis at presentation while the median progression free survival is only 3.7 years after the identification of distant metastases (1, 2). Better outcomes have been described in young patients with small volume radioactive iodine (RAI) avid pulmonary metastases (3), while a variety of clinical factors including aggressive tumor histology, marked FDG avidity, poor RAI avidity, older age, larger volume metastases, and metastatic foci outside the lungs have been associated with much poorer clinical outcomes (2–6).
While these various clinicopathological risk factors provide some insight into the likely course of disease progression, they cannot accurately predict the rate of structural disease progression or progression free survival within an individual patient. Previous studies have demonstrated that initial risk stratification estimates can be significantly improved when data that is accumulated during follow-up is used to modify risk estimates over time (7, 8). For example, Miyauchi et al demonstrated that the doubling time of serum thyroglobulin values (TgDT) obtained during follow-up was a powerful predictor of local regional recurrence, distant metastasis, and disease specific survival (9).
While serum thyroglobulin values reflect total body disease burden, it is the size of the metastatic lesions and the rate of structural disease progression that is used to define if and when clinicians should consider initiation of systemic therapies. We recognized that patient survival is the product of the tumor burden and tumor growth rate. In that equation, we believe that tumor growth rate is a stronger indicator of survival than tumor burden itself. Thus, larger tumors that are stable are unlikely to cause a patient’s demise, while smaller tumor with rapid growth curve are more likely to lead to a bad outcome. Therefore, it is important to define the tumor growth rate for any individual patient in order to more precisely predict clinical outcomes so that systemic therapy can be offered to the appropriate patient at the appropriate time. From a clinical standpoint, it would be helpful to know the anticipated overall survival from both (1) the time of discovery of the distant metastasis and (2) the time that the pulmonary nodule crossed the 1 cm diameter boundary commonly used as the minimal threshold required to initiate systemic therapy.
Measuring tumor growth rate was traditionally done using either RECIST criteria in clinical trials or using tumor diameter change over a specific period of time in clinical practice. Alternatively, growth rate can be defined based on time needed for a tumor to double over time, herein referred to as doubling time. Doubling time assumes that tumor growth is constant overtime. It can be measured based on tumor diameters or volume. RECIST criteria have not been validated in clinical practice. Since metastatic lesions growth is associated with an increase in tumor volume rather than a uni-dimensional increase in size and since tumor structural progression usually demonstrate a pattern of exponential growth that remains constant over time until large burdens of disease are achieved, we chose to define the tumor growth rate as tumor volume doubling time which can visually be presented as straight lines on a semi log graph (10–13). Therefore, the primary objective of this study was to determine the rate of structural disease progression (as measured by tumor volume doubling time) in patients with lung metastasis from follicular cell derived thyroid cancer. We hypothesized that the tumor volume doubling time (TVDT) of the metastatic tumor foci would remain constant over time and be a significant predictor of overall survival for individual patients.
Materials and Methods
Patients:
After obtaining institutional review board (IRB) approval, we retrospectively reviewed the charts of 199 patients with follicular cell derived thyroid cancer patients presenting with pulmonary metastases identified either at diagnosis or during follow-up. These patients were followed at Memorial Sloan Kettering between 1992 and 2016. Their clinical characteristics were previously described in a prior study (2). All patients were treated with total thyroidectomy and RAI ablation at thyroid cancer diagnosis. Patients received repeat RAI therapies and/or repeat surgery and/or external beam radiation therapy (EBRT) to the neck and/or bone metastasis and/or multi-kinase inhibitor (MKI) therapies during the course of their followup and that at the discretion of their treating physician.
To accurately measure lung volume doubling times, we included patients with one or more measurable lung lesions on at least four consecutive CTs of the chest. For patients who were started on MKI therapy, doubling times were calculated prior to MKI therapy initiation.
We excluded patients with 1) anaplastic or medullary thyroid cancer, 2) inadequate follow up, 3) those with non-measurable disease (nodules less than 5 mm and stable or decreasing in size with time, nodules that are not clearly demarcated at diagnosis or with follow up, nodules obscured by effusions); 4) age less than 18 years at the time of metastatic thyroid cancer diagnosis, 5) concomitant second primary cancer, 6) chronic TSH elevation. From 199 potential patients, 88 patients were ultimately inc luded in the study.
Lung Nodules Evaluation
We included any measurable lung lesion, even those less than 1cm. When more than one nodule was present (85/88 patients), we measured the two largest measurable lung lesions on serial CT.
Standard dose CT images were acquired using spiral CT scanners. Over the years, the collimation used for each CT varied as the technology field advanced. To avoid variability in the measurements over the years, we selected 5 mm CT slice thickness for determining nodule sizes. All the nodules were measured by one physician (MMS). Measurements were made using the same PACS workstation. Longitudinal and transverse measurements were obtained by manually positioning electronic calipers on the CT image that showed the largest detectable size of the selected nodule. Assuming that each metastatic nodule is ellipsoid in shape, we then calculated the tumor volume (mL) for each nodule at each given time point using the following formula: π/6 x longest diameter × smallest diameter × smallest diameter.
Laboratory studies:
Suppressed thyroglobulin levels were collected if obtained within 3 months of nodules’ measurements. Thyroglobulin levels were not recorded if: 1) they were measured outside the 3 months window, 2) interfering antithyroglobulin levels were concomitantly measured, or 3) the associated TSH level exceeded 5 mIU/L. Recorded thyroglobulin levels were standardized to CRM 457.
Doubling time measurements:
Using the same concept previously used by Miyauchi et al to define Tg DT (10) and assuming that changes in tumor volume are exponential (as shown in the results section), we computed a regression line, log y= log a +bx, using a non linear square regression with x defined as time (in years) after initial CT chest and y defined as tumor volume. TVDT was calculated as (log 2) / b. We then calculated the average TVDT for each given patient (referred to herein as midDT) using the average of the calculated TVDTs of the two selected nodules.
We then calculated the Tg DT for each patient with metastatic thyroid cancer using at least 3 consecutive thyroglobulin levels obtained within a 3 months window of nodules’ measurements. We then correlated midDT and Tg DT.
Statistical Methods
Continuous data are presented as means and standard deviations or median and ranges, as appropriate for each variable.
The first objective of this study was to prove that tumor growth is exponential and constant over time. For this purpose, we analyzed the measured tumor volumes for each given nodule over time. One hundred forty six (n = 146) structurally progressive nodules were included in this analysis. We calculated the correlation coefficent (r) and the coefficent of determination (r2) for each given nodule.
The second objective was to determine if the lung metastasis TVDT could predict overall survival from metastatic differentiated thyroid cancer. Based on previously defined thyroglobulin and calcitonin doubling time prognostic breakpoints, we grouped patients into 6 clinically relevant groups based on their midDT: those with midDT ≤ 1 years, those with midDT between 1–2 years, those with midDT between 2–3 years, those with midDT between 3–4 years, those with midDT ≥ 4 years and those with negative midDT (9, 14–15). For this analysis, we grouped those with midDT > 4 years and midDT negative together. Using Kaplan Meir survival analysis and log rank testing, we calculated overall survival both from: 1) the diagnosis of the lung metastases but also,( 2) from the time the largest nodule was 1 cm in order to estimate survival from the earliest time point that patients were likely to be considered for entry in a clinical trial.
We then grouped patients according to their Tg DT: Those with Tg DT ≤ 1 year, those with Tg DT between 1–2 years, those with DT 2–3 years, those with Tg DT 3–4 years, and those with DT g ≥ 4 years or negative. We repeated the overall survival analysis from the time the lung metastasis diagnosis was established, and then from 1 cm time point.
Few patients with RAIR structurally progressive metastatic thyroid cancer were treated with MKI therapy alone or in combination with RAI. We were interested in demonstrating whether patients with shorter structural doubling times benefited from MKI therapy, when offered, irrespective of the drug (s) used and estimated response to that therapy. For each midDT group, we examined MKI therapy’s effect on their overall survivals using Kaplan Meir survival curve and log rank analyses.
Because patients with midDT ≤ 1 years who were treated with MKI seemed to have a survival benefit over those who were not treated, we then compared the clinical characteristics of patients of MKI- treated to those of non –treated patients using Pearson Chi square and ANOVA. We then selected 3 patients with midDT ≤ 1 year and who were treated with MKI therapyand compared the tumor volume regression lines before and after MKI therapy.
All analyses were performed using SPSS software (Version 24; SPSS, Inc., Chicago, IL). A p-value of ≤0.05 was considered statistically significant.
Results
Patient Characteristics:
The clinicopathological characteristics of the 88 patients are summarized in Table 1.The patients were a median of 54 years old at the time of distant metastasis diagnosis, with papillary (43%) or poorly differentiated histologies (38%), and predominantly sub centimeter pulmonary metastases (72%). The majority had only lung metastasis with only 22% showing additional metastatic lesions outside the lung at the time of lung metastases diagnosis. The primary tumor size median was 3.4 cm (range 0.7–16).
Table 1:
Clinical characteristics of the cohort
| N | ||
|---|---|---|
| Age at diagnosis ( years) | 88 | |
| Mean +/− SD | 52 +/− 17 | |
| Median | 54 | |
| Range | 18–86 | |
| Presence of Distant metastasis at cancer diagnosis | 47% | |
| Age at distant metastasis diagnosis | ||
| Mean +/− SD | 56 +/− 16 | |
| Median | 59 | |
| Range | 20–90 | |
| Gender | ||
| Female | 52% | 46 |
| Histology | ||
| Papillary other than FV-PTC | 43% | 38 |
| Follicular and FV-PTC | 10% | 9 |
| Hurthle Cell | 9% | 8 |
| Poorly Differentiated | 38% | 33 |
| Size of primary tumor (cm)(if known) | 141 | |
| Mean +/− SD | 3.9 +/− 2.8 | |
| Median | 3.4 | |
| Range | 0.7–16 | |
| Thyroid Cancer stage at diagnosis | 84 | |
| I | 20% | 17 |
| II | 11% | 9 |
| III | 11% | 9 |
| IV | 58% | 49 |
| Extrathyroidal extension (ETE) | 63 | |
| None | 32% | 20 |
| Minimal | 43% | 27 |
| Gross | 25% | 16 |
| Vascular invasion (VI) | 61 | |
| None | 20% | 12 |
| Present | 80% | 49 |
| Initial Thyroid Surgery | 88 | |
| Total | 93% | 82 |
| Subtotal/Hemithyroidectomy | 7% | 6 |
| EBRT to Neck | 88 | |
| Yes | 17% | 15 |
| Local Recurrence before DM diagnosis in M0 pts | 47 | |
| Yes | 36% | 17 |
| RAI adjuvant therapy | ||
| 85% | 75 | |
| RAI adjuvant therapy activity (mCi) | 74 | |
| Mean +/− SD | 161 +/− 85 | |
| Median | 150 | |
| Range | 29–493 | |
| RAI Avidity at time of DM diagnosis | 88 | |
| RAI Avid ( RAIA) | 52% | 46 |
| RAI Refractory ( RAIR) | 48% | 42 |
| FDG-PET Avidity | 66 | |
| FDG-PET (+) | 46% | 30 |
| FDG-PET (−) | 54% | 36 |
| Distant Metastasis Distribution at DM diagnosis | ||
| Lung only | 78% | 69 |
| Lung and Bone | 16% | 14 |
| Lung and Other( including Bone) | 4% | 4 |
| Lung and Liver | 2% | 1 |
| Lung Metastasis Size Category at DM diagnosis | 88 | |
| Micro ( < 1cm) | 72% | 63 |
| Macro (≥ 1cm) | 28% | 25 |
| Follow up since DM diagnosis ( years) | ||
| Mean +/− SD | 9.1+/− 5.0 | |
| Median | 8.5 | |
| Range | 0.7–29.4 | |
| Clinical Outcome at End of Follow Up | ||
| Partial Response/Stable Disease (PR/SD) | 15% | 13 |
| Disease Progression (DP) | 85% | 75 |
| Status at end of Follow up | ||
| Alive | 44% | 39 |
| Dead | 56% | 49 |
| Cause of Death | 49 | |
| Thyroid Cancer | 84% | 41 |
| Myelodysplastic Syndrome | 6% | 2 |
| Unknown | 10% | 5 |
The patients were followed for a median of 8.5 years (range 0.7–29.4). The majority of patients (85%) had disease progression by the end of the follow up period and more than half of the patients died (56%). Of the 49 patients who died, the primary cause of death was thyroid cancer (84%) followed by myelodysplastic syndrome in 6% of the cases. The cause of death was not known in 5 patients.
Distribution of tumor volume doubling time of lung metastasis:
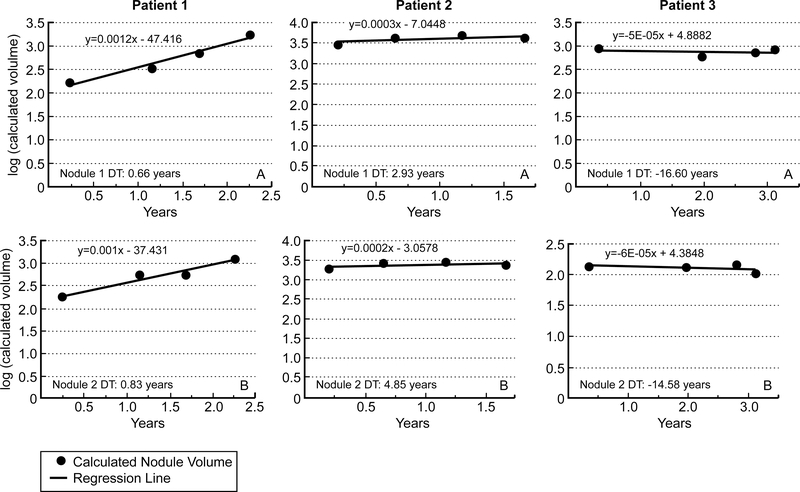
Eighty eight patients had lung lesions attributed to thyroid cancer metastasis. All but 3 patients had at least two measurable lung lesions. In keeping with RECIST criteria, we elected to measure no more that 2 nodules in one organ (16). Over time, some of the lesions grew, other remained stable and other decreased in size. Figure 1 depicts the regression lines of tumor volume change with time of the two selected nodule (largest nodule = nodule 1 in panel A, second largest nodule = nodule 2 in panel B) in three representative patients. In the 146 progressive metastatic lung lesions, the tumor growth rate was remarkably constant over time with a median follow up period of 8.5 years (median r = 0.92, r2 = 0.85).
Figure 1: Representative regression lines of the two largest nodules in 3 patients with lung metastasis from thyroid cancer.
Panels A represents regression lines of the largest measured nodule while Panel B represents regression lines in the second largest nodule measured. Patient 1 presents with rapid tumor growth rate in both lung lesions, Patient 2 with slow lung lesions structural progression and patient 3 with decline in lung lesion volume with time. DT= Tumor Volume Doubling Time.
For the cohort of 88 patients, the median size of the largest nodule was 1cm and grew to up to 1.6 cm with a median doubling time of 1.3 years. The median size of the second largest nodule was 0.76 cm and grew to about 1.2 cm with an equivalent median DT of 1.4 years (Table 2). The median average TVDT of nodule 1 and nodule 2 (midDT) was equivalent to median TVDT of either nodules (1.5 years versus 1.3 years for nodule 1 and 1.4 years for nodule 2).
Table 2:
Characteristics of measured lung nodules and Thyroglobulin parameters
| First measurement | Last measurement | Doubling Time (years) | midDT( years) | |
|---|---|---|---|---|
| Nodule 1 (mm) (n=88) | 1.5 8.5 +/− 77.5 −202.0 to 481.3 |
|||
| Median | 10.0 | 15.6 | 1.3 | |
| Mean +/− SD | 11.6 +/− 6.6 | 17.2 +/− 9.5 | 16.9 +/− 122.9 | |
| Range | 2.7–35.2 | 2.7–52.4 | −276.9 to 915.4 | |
| Nodule 2 (mm) (n=84) | ||||
| Median | 7.6 | 11.7 | 1.4 | |
| Mean +/− SD | 8.5 +/− 4.6 | 12.0 +/− 6.2 | −0.1 +/− 58.6 | |
| Range | 1.0 – 30.4 | 3.1–40.3 | −359.0 to 322.9 | |
| Thyroglobulin (ng/ml)(n=79) | ||||
| Median | 38 | 156 | 0.8 | |
| Mean +/− SD | 1184 +/− 5190 | 5289 +/− 19814 | −8.1 +/− 50.0 | |
| Range | 0.3– 44500.0 | 0.2 – 158000.0 | −319.0 to 24.3 | |
The doubling time of nodule 1 correlated moderately well with that of nodule 2 (Pearson correlation coefficient r = 0.48, r2 = 0.23 p < 0.001). Assuming that the tumor growth of two dominant lesions is more representative of the overall clinical course than the doubling time of each separate nodule individually, we used midDT as our primary outcome measure.
In general, midDT ranged between −202.0 and 481.3 years. Table 3 summarizes the distribution of patients based on biochemical and structural doubling time (midDT). Based on midDT, 79% of patients has progressive disease in the lung from the time of distant metastasis diagnosis, with 16 patients with rapidly progressive disease ( midDT ≤ 1 year), 38 patients with slowly progressive disease ( midDT between 1–4 years) and 16 patients with very slowly progressive disease in the lungs (midDT ≥ 4 years). A negative midDT was recorded in 18 patients, ranging between −0.93 and – 202.0 years (median negative midDT: −11.5 years) reflecting the stability or slight decline in average tumor volume overtime after RAI therapy and TSH suppression therapy.
Table 3:
Distribution of the patients according to Thyroglobulin doubling time and structural doubling time (midDT) groups
| DT groups | Tg DT status n = 79 patients | midDT status n = 88 patients |
|---|---|---|
| ≤ 1 year | 31 (39%) | 16 (18%) |
| 1–2 years | 14 (18%) | 19 (22%) |
| 2–3 years | 5 (6%) | 11 (12%) |
| 3–4 years | 4 (5%) | 8 (9%) |
| ≥ 4 years | 10 (13%) | 16 (18%) |
| Negative value | 15 (19%) | 18 (21%) |
At the time of distant metastasis diagnosis, half of the patients were classified as having RAI avid lung metastasis. The median midDT were similar in the RAIA patients (n= 46, median midDT 1.43 years) and RAIR patients ( n=42 patients, median midDT= 1.50 years). Similarly, median midDTs were similar if FDG-PET avid disease (n=30 patients, median midDT = 1.41 years) and FDG-PET negative metastatic thyroid cancer (n=36 patients, median midDT 1.65 years). Thus, we anticipate that the effect of intervening therapies on midDT was minimal.
Using chi square analysis, we did not show a statistically significant correlation between midDT and patient age, gender, RAI avidity, FDG-PET positivity or presence of other distant metastatic site. However, not surprisingly, patients with Hurthle cell or poorly differentiated thyroid cancer were more likely to have shorter midDT that those with more differentiated tumors (p <0.001).
Thyroglobulin doubling time:
81 patients had serial thyroglobulin levels that fit our inclusion criteria. Median thyroglobulin level at first CT was 38 ng/ml (range 0.3 – 44500.0) and rose to a median of 156 ng/ml (range 0.2–158000.0) (Table 2). The median Tg DT was 0.8 years.
Tg DT and midDT showed a moderate correlation (Pearson correlation coefficient r = 0.50, r2 = 0.25, p < 0.001). A similar proportion of patients had progressive disease (DT positive) based on TgDT as by midDT (81% versus 79%) (Table 3). Table 4 shows similar distribution of TgDT and midDT with the shortest midDT (≤ 2 years), longest (midDT ≥ 4 years) and negative doubling times, with more variability in the distribution of TgDT and midDT when DT measured between 2–4 years.
Table 4:
Comparison of Thyroglobulin Doubling time and structural doubling time (midDT)
| midDT |
||||||
|---|---|---|---|---|---|---|
| Tg DT | ≤ 1 year | 1–2 years | 2–3years | 3–4 years | ≥ 4 years | Negative |
| ≤ 1 year | 12 | 8 | 3 | 2 | 5 | 1 |
| 1–2 years | 1 | 7 | 0 | 1 | 2 | 3 |
| 2–3 years | 0 | 1 | 1 | 1 | 1 | 1 |
| 3–4 years | 0 | 1 | 3 | 0 | 0 | 0 |
| ≥ 4 years | 0 | 1 | 2 | 3 | 2 | 2 |
| Negative | 0 | 1 | 0 | 0 | 5 | 9 |
Association between overall survival and rate of disease progression:
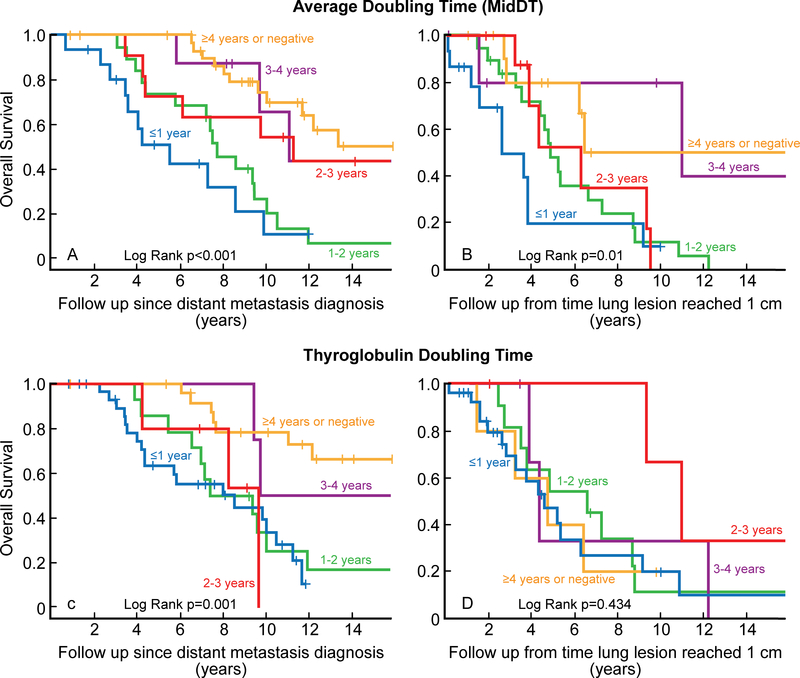
Figure 2 depicts the Kaplan Meier curves for overall survival in the cohort based on midDT and TgDT groups. Survival was measured from the time of distant metastasis diagnosis (panels A and C) and from time the lung lesion reached 1 cm (panels B and D). The median overall survival from the time of lung metastasis diagnosis was 9.8 years (95% CI: 8.8 – 10.9). The median overall survival from the 1 cm time point was 5.2 years (95% CI: 3.2 – 7.2).
Figure 2:
Overall survival based on midDT and Tg DT.
Patients with DT less than 1 year fared worse than patients with higher DT. This was true for both Tg DT and midDT from either time of lung metastasis diagnosis or from the time the largest lung lesion was 1 cm. The 5 year overall survival from the 1 cm time point was 20% for midDT ≤1 year (n = 15), 50% for midDT 1–2 years (n= 19), 53% for midDT 2–3 years (n=9), 80 % for midDT 3–4 years (n=6), and 86% for midDT ≥ 4 years (n=8) and 52% for those with negative midDT (n=4) (Table 5).
Table 5:
Overall Survival from the time the dominant lung nodule crossed the 1 cm point according to midDT
| Overall Survival |
||
|---|---|---|
| midDT | 5-year | 10-year |
| ≤ 1 year | 19.4 % | 10.2% |
| 1–2 years | 45.2% | 11.5% |
| 2–3 years | 52.5% | -- |
| 3–4 years | 80.2% | 80.2% |
| ≥ 4 years | 85.6% | 57.5% |
| Negative | 52.3% | -- |
Initial observation of the effect of MKI therapy on overall survival and tumor volume doubling time.
Twenty seven of the studied patients were treated with MKI therapy in the course of their follow up: 9 (33%) with. midDT ≤ 1 year, 8 (30%) had midDT between 1–2 years, 4 (15%) had a midDT between 2–3 years, 1(3%) patient had midDT from 3–4 years, and 5 had midDT ≥ 4 years or negative (19%).
Of the 15 patients with midDT ≤ 1 year, 8 were treated with MKI and could be followed from the time the largest lung nodule was 1 cm. Median follow up from 1cm time point was 2.4 years. The 2 year overall survival from the 1 cm time point was 88% in the MKI-treated group (n=8) as opposed to 43% in the non-treated group (n=7) (p = 0.13). There were no difference in the clinical characteristics of the MKI and non MKI treated patients (data not shown).An MKI survival benefit was not demonstrated when similar analysis was performed on patients with midDT between 1–2 years, 2–3 years (data not shown) or 3–4 years (data not shown).
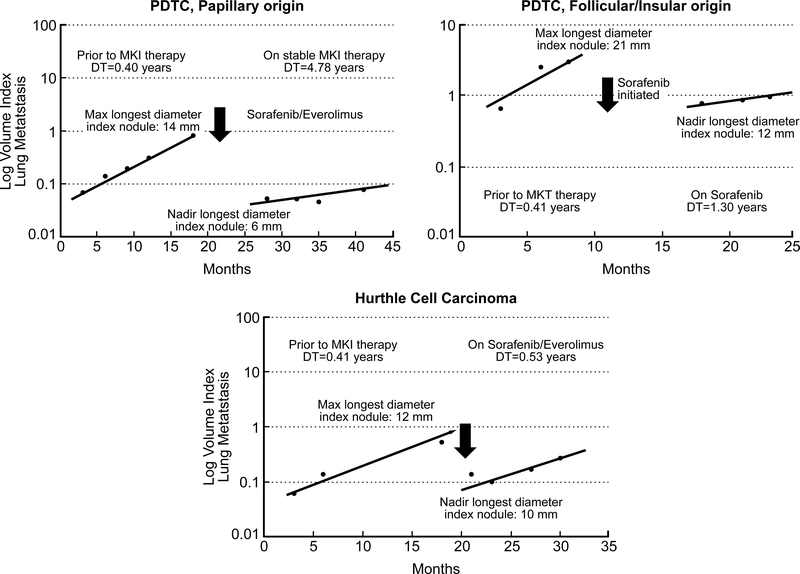
Figure 3 depicts the pattern of tumor volume regression lines before and after MKI therapy in 3patients with midDT < 1 year While all three patients showed an acute decrease in tumor volume within few weeks after MKI therapy, the doubling time or tumor growth rate of the index lesion on chronic MKI therapy varied widely among all them.
Figure 3:
Representative regression lines for dominant lung lesion tumor volume change in three patients before and after MKI therapy.
Discussion
In this study, we introduced midDT as a novel clinical marker used to define structural disease progression in lung metastasis of differentiated thyroid cancer. Given that tumors do not grow in a linear fashion, we estimate that change in tumor volume is a more accurate measure of growth than change the longest diameter of a given lesion. Tumor growth rate in the lungs is remarkably constant, allowing for reliable measurement of TVDT. Lung midDT is easily measured through serial, retrospective review of four consecutive chest CTs obtained at any time during the follow up of the patient. Using Miyauchi Aet al initial doubling time concept (9), the Kuma clinic in Tokyo, Japan published a Doubling Time and Progression Calculator that helps physician estimate tumor growth based on biochemical marker change and tumor volume change (http://www.kuma-h.or.jp/english, Kuma Hospital, Tokyo, Japan). Using the Kuma calculator, treating physicians in the community can easily and rapidly estimate the Tg DT and midDT during clinic visits. Furthermore, the selected clinically relevant midDT groups have been shown to predict overall prognosis from thyroid cancer, allowing physician to appropriately risk stratify patients in clinic, thus predicting early on those patients who more likely to progress rapidly as opposed to the slow progressors. Treatment approaches and frequency of the follow up imaging and visits can be tailored accordingly.
Metastatic thyroid cancer patients with midDT ≤ 1 year have a worse prognosis that those with higher midDT. Prior studies showed that the 1 year time point in doubling time equally predict worse prognosis when using thyroglobulin doubling time, and calcitonin and CEA doubling in medullary thyroid cancer. Our results further validate the prognostic value of TgDT in differentiated thyroid cancer. It is not surprising that, in this study, Tg DT did not correlate with midDT. After all, Tg DT is a biochemical estimate of total body tumor volume growth rate, while midDT estimated only tumor growth in a specific organ. While both Tg DT and midDT are prognostic indicators for metastatic thyroid cancer, biochemical disease progression alone is not an eligibility criterion for consideration for MKI therapy. On the other hand, structural progression alone, measured as midDT, can be used to select patients for MKI therapy. In the future, we anticipate that calculations of both lung midDT and TgDT can be jointly used to risk stratify patients and make treatment decisions. Thus, while the decision to treat is relatively straightforward for patients with both Tg DT and lung midDT ≤ 1 year, strong considerations would be made to treat those with Tg DT ≤ 1 year but with longer midDT ( ie midDT 1–4 years).
There continues to be a lack of uniform consensus regarding the ideal time for initiation of MKI therapy in RAIR metastatic follicular cell derived thyroid cancer. While the presence of clinically measurable disease ( ie lesions more than 1–1.5cm) are a minimum requirement for MKI therapy consideration, the inclusion criteria for the prospective randomized registration trials for Sorafenib and Lenvatinib required at least a 20% increase in the sum of the longest diameters of target lesions defined by RECIST criteria over either a 13 months (lenvatinib) or 14 months (sorafenib) (17, 18). Assuming that tumor growth rate is constant overtime (as is shown in this study) and using uni-dimensional tumor growth patterns, this would approximately equate to a doubling time of 4 years. On the other hand, based primarily on expert opinion, the 2015 American Thyroid Association thyroid cancer management guidelines suggest that a 20% increase in the longest tumor diameter over a 6 month period should prompt consideration of MKI therapies. This correlates with a doubling time of approximately 2 years (≈ 40% growth over 1 year) (19). The NCCN guidelines cautiously avoided defining a specific tumor growth rate or tumor size that would prompt use of MKI therapy in RAI refractory differentiated thyroid cancer or medullary thyroid cancer (20).
Thus, based on the above summarized criterion, structurally progressive clinically measurable lung lesions with tumor diameter doubling time of 2–4 years or less would be considered for MKI therapy initiation, while lesions with tumor diameter doubling times > 4 years would be observed. However, RECIST criteria are not validated in the clinical setting outside of prospective clinical trials. Also, these uni-dimensional growth rate patterns can underestimate tumor volume growth over time and have not been correlated with overall survival from metastatic RAIR thyroid cancer. Furthermore, using the above eligibility criteria for study entry, neither Lenvatinib nor Sorafenib therapy in RAIR metastatic thyroid cancer showed a significant survival benefit when compared to placebo. Thus, these eligibility criteria alone are not enough to select patients who are likely to benefit from MKI therapy. It is important to note that, due to its retrospective design, this study was not intended to provide a head to head comparison of one tumor growth rate definition over another.
This study not only validates the use of midDT as prognostic indicator in patients with lung metastasis from differentiated thyroid cancer in the clinical practice setting, but our preliminary observations also hints towards a survival benefit with MKI therapy in patients with midDT ≤ 1 year. It is not surprising that survival benefit in the MKI treated patients was not statistically significant given the small number of studied patients. When examining the regression lines before and after MKI therapy, it became evident that despite an initial significant drop in tumor size and volume (which may be considered as response to therapy) the long term TVDT after the lung lesion reached nadir size was quite varied in the 3 studied patients. We postulate that response to MKI therapy and long term survival after MKI therapy is more a function of that long term TVDT rather than the acute tumor shrinkage seen in the first weeks after MKI therapy initiation. To further examine the efficacy of MKI therapies in thyroid cancer, we are in the process of conducting further studies examining MKI therapy effect on overall survival and the change in TVDT before and after specific MKI therapy in a larger cohort of patients. In the interim, our data supports the use of midDT as a useful clinical marker to properly select patients likely to benefit from the initiation of MKI therapy. We postulate that early selection and treatment of patients with RAIR metastatic thyroid cancer and midDT ≤ 1 year would improve treatment response and overall survival with MKI therapy. In addition, proper patient selection would prevent exposing patients with slow TVDT to toxic, potentially fatal and costly drugs that are unlikely to benefit them clinically.
This study is a retrospective review of patient charts and therefore is inherent to all associated biases including selection bias. We assumed that the measured lung lesions were thyroid cancer metastasis based on the overall clinical picture. We were careful not to measure ground glass nodules. In a handful of patients, a wedge biopsy was performed to confirm lung metastasis from thyroid cancer. In others, prior RAI avidity confirmed lung metastasis from thyroid cancer. Nonetheless, it is possible that some stable or slowly progressive lung lesions may represent inflammatory changes and/or indolent bronchoalveolar cancer.
Many of the studied patients had disease considered to be RAI avid at the time of lung metastasis diagnosis on the basis of uptake on the first posttherapy scan. They were treated with repeat RAI therapies. We previously showed that more than 50% of the patients eventually present with disease progression despite repeat RAI therapies and are ultimately considered RAI refractory (6). In this study, 85% of patients had progressive disease at the end of the follow up. However, we recognize that in the truly RAI responsive tumors, repeat RAI therapy may have influenced the tumor volume doubling times. This is especially true for patients with RAI responsive tumors that are predicted to have negative midDT or midDT > 4 years after these therapies. To eliminate transient RAI response in partial RAI responders, we purposely elected not measure lung volumes within 18 months after RAI therapy.
In conclusion, the average tumor volume doubling time (midDT) of lung metastasis is an accessible, easily calculated and reliable prognostic indicator of overall survival in patients with metastatic differentiated thyroid cancer and can be used to identify patients with RAIR metastatic thyroid cancer likely to benefit from MKI therapy.
Acknowledgements
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
Footnotes
Disclosure Summary Statement: No competing financial interests exist for Dr Sabra. Drs Sherman and Tuttle are consultants for ESAI Pharmaceutical and Bayer Inc.
References
- 1-.Hundahl SA, Fleming ID, Fremgen AM, Menck HR 1998. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see comments]. Cancer 83:2638–2648 [DOI] [PubMed] [Google Scholar]
- 2-.Sabra M, Ghossein R, Tuttle M 2016. Time course and predictors of structural disease progression in pulmonary metastases arising from follicular cell derived thyroid cancer. Thyroid 26: 518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3-.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M 2006. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91:2892–2899 [DOI] [PubMed] [Google Scholar]
- 4-.Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, Tuttle RM, Drucker W, Larson SM 2006. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab 91:498–505 [DOI] [PubMed] [Google Scholar]
- 5-.Farooki A, Leung V, Tala H, Tuttle RM 2012. Skeletal-related events due to bone metastases from differentiated thyroid cancer. J Clin Endocrinol Metab 97:2433–2439 [DOI] [PubMed] [Google Scholar]
- 6-.Sabra MM, Dominguez JM, Grewal RK, Larson SM, Ghossein RA, Tuttle RM, Fagin JA 2013. Clinical outcomes and molecular profile of differentiated thyroid cancers with radioiodine-avid distant metastases. J Clin Endocrinol Metab 98:E829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7-.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A 2010. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 20:1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8-.Momesso DP, Tuttle RM. Update on differentiated thyroid cancer staging 2014. Endocrinol Metab Clin North Am 43:401–421. [DOI] [PubMed] [Google Scholar]
- 9-.Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Shimoyamate-dori C, Takamura Y, Higashiyama T, Fukushima M, Kihara M, Tomoda C, Inoue H, Yabuta T, Masuoka H 2011. Prognostic impact of serum thyroglobulin doubling-time under TSH suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid 7:707–716. [DOI] [PubMed] [Google Scholar]
- 10-.Collins VP, Loeffler RK, Tivey H 1956. Observations on growth rates of human tumors. Am J Roentgenol Radium Ther Nucl Med 76:988–1000. [PubMed] [Google Scholar]
- 11-.Friberg S, Mattson S 1997. On the growth rates of human malignant tumors: implications for medical decision making. J Surg Oncol 65:284–297. [DOI] [PubMed] [Google Scholar]
- 12-.Norton L, Simon R, Brereton HD, Bogden AE 1976. Predicting the course of Gompertzian growth. Nature 264:542–545. [DOI] [PubMed] [Google Scholar]
- 13-.Loberg RD, Bradley DA, Tomlins SA, Chinnaiyan AM, Pienta KJ 2007. The lethal phenotype of cancer: the molecular basis of death due to malignancy. CA Cancer J Clin 57:225–241. [DOI] [PubMed] [Google Scholar]
- 14-.Barbet J, Campion L, Kraeber-Bodere F, Chatal JF 2005. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab 90:6077–6084. [DOI] [PubMed] [Google Scholar]
- 15-.Miyauchi A, Onishi T, Morimoto S, Takai S, Matsuzuka F, Kuma K, Maeda M, Kumahara Y 1984. Relation of doubling time of plasma calcitonin levels to prognosis and recurrence of medullary thyroid carcinoma. Ann Surg 199:461–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16-.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J 2009. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. [DOI] [PubMed] [Google Scholar]
- 17-.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Pena C, Molnar I, Schlumberger MJ 2014. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18-.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI 2015. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621–630. [DOI] [PubMed] [Google Scholar]
- 19-.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L 2016. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20-.Network NCC. NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma version 1.2016. (Last accessed on November 12, 2016).