Abstract
Linked Articles
This article is part of a joint Themed section with the British Journal of Pharmacology on Cardiotoxicity. The rest of the Themed section will appear in a future issue of BJP and will be available at http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1476‐5381
The number of survivors of childhood cancers has increased exponentially over the past few decades. However, these survivors are also at substantially increased long‐term risk of morbidity and mortality, especially from treatment‐related cardiotoxicity. Preventing these risks is now a priority when treating children and adolescents with cancer. Dexrazoxane reduces the risk of anthracycline‐induced cardiotoxicity among adults and children with cancer without reducing its antineoplastic effects or event‐free survival. Thus, it should be strongly considered as a part of therapy for children and adolescents treated with anthracyclines.
Keywords: cardiotoxicity, childhood cancer, dexrazoxane, survivor
Tables of Links
| LIGANDS | |
|---|---|
| BNP | Enalapril |
| Bortezomib | Etoposide |
| Carfilzomib | Fluorouracil |
| Carvedilol | IGF‐1 |
| Cyclophosphamide | Methotrexate |
| Daunorubicin | Prednisone |
| Dexrazoxane | Trastuzumab |
| Doxorubicin | Vincristine |
These Tables lists key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2, 3.
Introduction
Survival rates of childhood cancers have substantially improved over the past several decades as a result of new and improved treatments. The overall 5‐year survival rate has increased from 58% in the mid‐1970s to 83% today 4. Acute lymphoblastic leukaemia (ALL) alone has an overall cure rate between 85% and 90% 5. As a result of increased survival, however, these patients are at an increased risk of adverse treatment‐related effects. For example, chemotherapeutic agents such as anthracyclines and radiation therapy are extremely effective against numerous types of cancer, but they are also cardiotoxic.
Survivors of childhood cancer are at a significantly higher risk of cardiovascular morbidity and mortality as a result of treatment. Most of these survivors will experience one or more chronic health condition within 30 years of their diagnosis 6. These findings have been verified in numerous large‐scale, multicentre studies including randomized controlled trials. For example, the Childhood Cancer Survivor Study reported that the overall mortality rate in survivors of childhood cancer is 8.4 times higher than that of healthy controls 7. Recurrence and progression of the underlying disease account for almost 60% of the deaths in these patients, followed by secondary cancers (19%), circulatory diseases (7%), and respiratory diseases (3%) 7. The British Childhood Cancer Survivor Study also noted excess long‐term mortality from secondary primary malignancies, as well as circulatory disease, 25 years after the initial cancer diagnosis 8. In addition to the need to improve event‐free and overall survival in these patients, these findings support the need to focus on late effects as well as on quality of life. Therefore, preventing cardiotoxicity is of utmost importance in survivors of childhood cancer.
Effects of anthracycline‐related cardiotoxicity
Several treatments effectively manage and even cure various forms of paediatric cancer. With these treatments, however, come numerous adverse effects. Anthracyclines (e.g., doxorubicin, daunorubicin, and epirubicin) are among the most commonly used chemotherapeutic agents for treating children with any of several haematological and solid tumour malignancies. These drugs are unfortunately associated with well‐known cardiac conditions resulting from the irreversible and dose‐dependent loss of cardiomyocytes 9, 10, 11. Cardiotoxic effects include, but are not limited to, cardiomyopathy (initially dilated cardiomyopathy that may then progress to a restrictive cardiomyopathy), heart failure, myocardial infarction, conduction defects, valve disease, pericardial disease, and hypertension (Figure 1) 12, 13, 14, 15. An anthracycline cumulative dose of 300 mg m−2 or greater substantially increases this risk. However, subclinical echocardiographic abnormalities were found among patients treated with a cumulative dose of anthracyclines <100 mg m−2 16, indicating that there is no safe dose of these drugs 11, 17.
Figure 1.
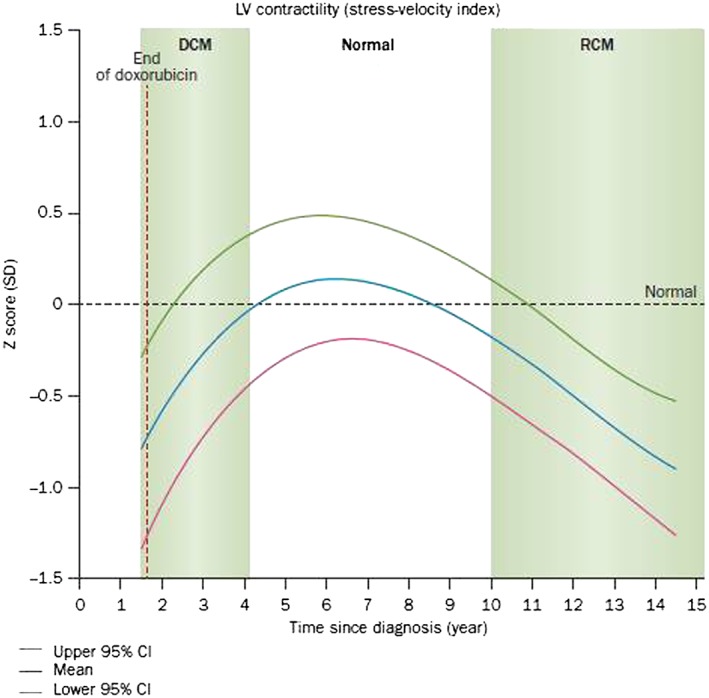
Progressive cardiac dysfunction after doxorubicin therapy in children treated for acute lymphoblastic leukaemia. The blue line corresponds to the overall group mean in this model. Green and red lines are the upper and lower 95% CI from the predicted mean ± 2 standard errors. Dilated cardiomyopathy (DCM) had echocardiographic signs of reduced left ventricular (LV) fractional shortening and contractility with LV dilation. In time, the pattern changed and the children showed signs consistent with a restrictive cardiomyopathy (RCM): normal to reduced LV dimension with significantly reduced LV thickness, fractional shortening and contractility. CI, confidence interval; SD, standard deviation. Permission obtained from American Society of Clinical Oncology © 13
Anthracycline‐associated cardiotoxicity reduces left ventricular (LV) wall thickness and mass, as well as LV fractional shortening 9, 12, 13. In survivors of malignant bone tumors treated with anthracyclines, after 22 years of follow‐up, adverse cardiac structural changes ultimately resulted in marked, progressive cardiac dysfunction 18. A cross‐sectional study by the Dana–Farber Cancer Institute evaluated serial echocardiograms (n = 499) from long‐term survivors of childhood acute lymphoblastic leukaemia treated with doxorubicin (n = 115) 13. The median follow‐up after completion of therapy was 11.8 years. Patients initially developed asymptomatic dilated cardiomyopathy soon after the completion of doxorubicin therapy as diagnosed by reductions in LV fractional shortening and contractility with LV dilation. Over time, this cardiomyopathy normalized but years later progressed to a restrictive cardiomyopathy diagnosed by significantly reduced LV wall thickness, fractional shortening and contractility with normal to reduced LV dimension (Figure 1) 13. Compared to their healthy siblings, survivors have up to five times the risk of heart failure, valvar disease, and pericardial disease. In addition, up to half of all survivors treated with anthracyclines will experience some form of cardiac dysfunction within 20 years after anthracycline treatment 15. Given the young age of this population at the time of diagnosis and treatment, survivors of childhood cancer are especially vulnerable to these risks at an early age.
| Continuing medical education (CME) objectives |
|---|
| 1. To recognize the potential adverse cardiotoxic effects that exist among survivors of childhood cancer |
| 2. To know the risk factors that place survivors of childhood cancer at highest risk of cardiotoxicity |
| 3. To recognize the importance of dexrazoxane as a cardioprotective medication against anthracycline‐induced cardiotoxicity |
| 4. To recognize the need for evidence‐based guidelines to screen for, prevent, and treat cardiotoxicity among survivors of childhood cancer |
Risk factors for anthracycline‐related cardiotoxicity
Survivors of childhood cancers are at the same risk for developing atherosclerotic disease as the general population. Unfortunately, those with a history of anthracycline treatment are at greater risk for cardiotoxicity because of the drugs' inherent adverse effects against cardiomyocytes. Risk factors such as age, body weight, female sex, radiotherapy, trisomy 21, genotype or phenotypic evidence of or susceptibility for preexisting cardiomyopathy, genotypic evidence of increased cardiotoxic risk from anthracycline chemotherapy, diabetes, hypertension, and others are examples of why a careful patient assessment prior to treating with anthracyclines are advised. Risk factors of anthracycline‐related cardiotoxicity include treatment‐related and both modifiable and nonmodifiable risk factors (Table 1).
Table 1.
Risk factors for anthracycline‐related cardiotoxicity
| Risk factors | Comment | References |
|---|---|---|
| Cumulative anthracycline dose | Cumulative doses >300 mg m−2 are associated with significantly elevated long‐term risk | 9, 13, 35, 36, 82 |
| Time after therapy | The incidence of clinically important cardiotoxicity increases progressively over decades | 9, 13, 35, 83 |
| Rate of anthracycline administration | Continuous infusion not cardioprotective in children | 25, 83 |
| Individual anthracycline dose | Higher individual doses are associated with increased late cardiotoxicity, even when cumulative doses are limited; no dose is risk‐free | 13, 35, 64 |
| Type of anthracycline | Liposomal encapsulated preparations may reduce cardiotoxicity. Data on anthracycline analogues and differences in cardiotoxicity are conflicting | 79, 84, 85 |
| Radiation therapy | Cumulative cardiac radiation dose >30 Gy before or concomitant with anthracycline treatment; as little as 5 Gy increased the risk | 28, 82, 83, 86 |
| Concomitant therapy | Trastuzumab, cyclophosphamide, bleomycin, vincristine, amsacrine and mitoxantrone, among others, may increase susceptibility or toxicity | 28, 85 |
| Preexisting cardiac risk factors | Hypertension; ischemic, myocardial and valvular heart disease; prior cardiotoxic treatment | 85 |
| Personal health habits | Smoking; consumption of alcohol, energy drinks, stimulants, prescription and illicit drugs | 49, 83 |
| Comorbidities | Diabetes, obesity, renal dysfunction, pulmonary disease, endocrinopathies, electrolyte and metabolic abnormalities, sepsis, infection, pregnancy, viruses, elite athletic participation, low vitamin D concentrations | 25, 26, 27, 40, 83, 85, 87 |
| Age | Young (<1 year) and advanced age at treatment are associated with elevated risk | 9, 35, 82, 83 |
| Sex | Females are at greater risk than males | 35, 64 |
| Complementary therapies | More information needs to be collected to assess risk | 83 |
| Additional factors | Trisomy 21; African–American ancestry | 36 |
Lipshultz SE, Franco VI, Miller TL, Colan SD, Sallan SE. (2015) Cardiovascular disease in adult survivors of childhood cancer. Annu Rev Med 66: 161–176. 11
Therapy‐related risk factors
A high cumulative dose of anthracyclines is the most important risk factor for cardiotoxicity among survivors 11. The risk of cardiotoxicity at cumulative doses >300 mg m−2 is 11 times as high as that at cumulative doses <300 mg m−2 11, 19, 20. Despite this increased risk, however, cardiotoxicity can develop at cumulative doses <240 mg m−2 11, 21.
Concomitant mediastinal or cranial radiation is an additional risk factor for cardiotoxicity 22. Among 294 survivors of Hodgkin lymphoma exposed to mediastinal radiation, 23 had coronary events after 6.5 years of follow‐up, of which 10 were myocardial infarctions 23. Cranial radiation has been associated with cardiovascular disease—specifically decreased LV mass—presumably in part through its detrimental effect on the hypothalamic and pituitary glands that lead to growth hormone deficiency 14, 24, 25, 26, 27. It is not clear whether the effect of cranial radiation on cardiotoxicity is additive or synergistic with anthracycline treatment 28. Longer follow‐up and greater cumulative doses of anthracycline increase the prevalence of cardiotoxicity in survivors; however, evidence of cardiotoxicity after relatively shorter follow‐up times is possible 17.
Nonmodifiable risk factors
Independent of the aforementioned therapy‐related risk factors, some patients appear to be inherently more susceptible to cardiotoxicity. For example, not all children exposed to anthracyclines will experience LV dysfunction or other forms of cardiac dysfunction, suggesting a possible genetic predisposition 29, 30, 31, 32, 33, 34. Krajinovic et al. 31 identified two genes that may modulate late‐onset chronic cardiotoxicity among children with ALL treated with doxorubicin. Patients with an A‐1629 T genotype variant in the ABCC5 gene, an ATP‐binding cassette transporter gene, had significant decreases in LV ejection and shortening fractions, suggesting impairment of cardiac function. By contrast, patients with a G‐894 T genotype variant of the NOS3 gene, an endothelial nitric oxide synthase gene, demonstrated an increase in the LV ejection fraction, suggestive of a potential protective effect 31.
Mutations of the haemochromatosis gene (HFE), which is associated with hereditary haemochromatosis, interfere with iron metabolism, leading to iron overload and consequently increased susceptibility to anthracycline‐related cardiotoxicity 32. In one study of 184 survivors of childhood ALL, 10% carried a mutation in the HFE C282Y allele. Those who were heterozygous for the HFE C282Y mutation had a nine‐fold higher risk of myocardial injury than that of noncarriers 32. In an additional study, patients homozygous for the G allele in CBR3, a gene that encodes for carbonyl reductase 3, were at increased risk of cardiomyopathy when exposed to low‐to‐moderate doses of anthracyclines 29. Such genetic predispositions may allow for genetic screening as well as guiding treatment of susceptible patients.
Children with pre‐existing cardiovascular disease or a family history of premature cardiovascular disease are at an increased risk of cardiotoxicity after anthracycline treatment 32. Additionally, females are more vulnerable than males to anthracycline‐induced cardiotoxicity at the same cumulative anthracycline dose 35 . One likely explanation for this sex‐related difference is that females have a higher percentage of body fat. Having a greater percentage of body fat could contribute to a higher incidence of cardiac damage because anthracyclines are poorly absorbed by fat. Therefore, at the same dose, females will have higher circulating concentrations that increase the effective dose to cardiomyocytes 35.
Younger age at diagnosis is also associated with increased risk of anthracycline‐related cardiotoxicity. Specifically, the risk is elevated for increased LV afterload and decreased LV mass and LV wall thickness 35. Patients with trisomy‐21 and African–Americans are also at increased risk of early anthracycline‐related cardiotoxicity 36.
Modifiable risk factors
Patients treated with anthracyclines are likely to be subject to incremental cancer and its therapy related risks, as well as to conventional risks for atherosclerotic disease found in the noncancer population. A rigorous initial cardiovascular assessment followed by close monitoring is advised. Nonpharmacological measures such as exercise, healthy lifestyles, control of risk factors and treatment of comorbidities may be helpful in this population at high risk for premature cardiovascular disease. Addressing modifiable risk factors, such as physical inactivity, hypertension, diabetes, obesity and smoking may be important in reducing the incidence of atherosclerosis and hypertensive cardiovascular disease in survivors of childhood cancer similar to the general population 37. Although the Centers for Disease Control and Prevention recommend that children aged 6–17 years should be physically active for at least 60 minutes a day 38, childhood cancer survivors are more likely to report having an inactive lifestyle than their healthy counterparts when matched for age and sex 39. Additionally, survivors also report watching more television than their siblings 39, 40. Among older childhood cancer survivors, a higher percentage of body fat, a history of methotrexate treatment, and either high or low LV mass were associated with lower maximum oxygen consumption 40. In females, maximum myocardial oxygen consumption was lower than that of their sibling controls 41.
Childhood obesity is epidemic in the USA, and survivors of childhood cancer are at an especially high risk, given their sedentary lifestyle 40. Miller et al. 40 found that male survivors had a higher percentage of body fat and truncal obesity than that of their siblings and were more likely to be overweight or obese. Although survivors are at greater risk, the prevalence of obesity in childhood cancer survivors is currently similar to that of the general population 42. One study of 7195 childhood cancer survivors found that 13% were obese and 28% were overweight 43. An additional study found that about a third of 441 cancer survivors were obese or overweight 44.
Survivors exposed to cranial irradiation were at increased risk of cardiotoxicity through its detrimental effects on the hypothalamus and pituitary glands and subsequent deficiency in growth hormone. These survivors also had substantially lower insulin‐like growth factor‐1 concentrations than those of unexposed survivors, placing them at greater risk for obesity 24. Obesity may increase the risk of cardiotoxicity in survivors because their cardiovascular systems might not be able to compensate for the conditions associated with obesity, such as ischemia or atherosclerosis.
Obese survivors of childhood cancer with sedentary lifestyles are at additional risk for insulin resistance and diabetes mellitus. This risk is especially present in survivors treated with hematopoietic stem cell transplantation. In one study of these survivors, the 10‐year cumulative incidence of diabetes was 18% 44. In an analysis of National Health and Nutrition Examination Survey data, Armenian et al. also noted that between 1995 and 2008, the prevalence of diabetes developing in survivors treated with stem cell transplantation increased from 8% to 17% in survivors but only from 6% to 9% in the general population 44. In another study of 7195 long‐term survivors of childhood cancer by Meacham et al. 45, diabetes was twice as likely to develop in survivors as it was in the general public. Radiation to the abdomen, head, and total body was associated with an even higher incidence of diabetes. Finally, a prospective study of 248 childhood cancer survivors found that, over a 13‐year period, 4% had diabetes, 4% had hyperinsulinemia, and 7% had glucose intolerance 46.
Although the rate of smoking among cancer survivors is less than that of the general population (17% vs. 20%, respectively) 47, smoking cessation is of utmost importance to long‐term survival. The prevalence of illicit drug use among childhood cancer survivors is similar to that of the general population, but additional general health education should be implemented in this susceptible population due to the cardiotoxic effects of these substances 48. Shultz et al. 49 found that of 117 survivors of acute myeloid leukaemia aged 18 years or older, 25% reported binge drinking, and less than 10% reported cocaine, heroin, or methamphetamine use. Males were more likely to abuse drugs than were females. Given that alcohol and cocaine are both risk factors for cardiomyopathy in the general population (Shultz et al., 2010), abuse is especially concerning among patients with anthracycline‐mediated cardiomyopathy.
Preventing anthracycline‐induced cardiotoxicity: dexrazoxane
Dexrazoxane can prevent late effects from anthracycline treatment. Studies on beagles in the early 1980s first documented dexrazoxane's ability to reduce chronic doxorubicin cardiotoxicity 50. It is believed to chelate iron and therefore interferes with iron‐mediated free radical generation, ultimately decreasing tissue damage caused by anthracyclines (Figure 2) 14, 51. Hasinoff et al. 52 recently examined the ability of dexrazoxane to protect against myocyte damage from doxorubicin when combined with either bortezomib or carfilzomib, proteasome inhibitors also known to be cardiotoxic. Dexrazoxane maintained its cardioprotective effects supporting the hypothesis that its mechanism acts by preventing iron‐based doxorubicin‐mediated oxidative stress and establishing that its cardioprotective effects were not exclusively due to targeting of topoisomerase II‐beta. Doxorubicin targets mitochondria and dexrazoxane abrogates these effects 53. Mitochondrial transcription in energy metabolism and apoptosis genes were significantly altered by doxorubicin administration but these changes were attenuated by pretreatment with dexrazoxane 54. Clinical trials conducted among women with breast cancer established its cardioprotective effectiveness in humans 55. Dexrazoxane is given at a 10:1 ratio by intravenous infusion immediately before anthracycline administration. Speyer et al.'s. 56 randomized controlled trial included 150 women with advanced breast cancer treated with fluorouracil, doxorubicin and cyclophosphamide with or without dexrazoxane (ICRF‐187) and demonstrated a significant difference in incidence of clinical heart failure between the two groups (two patients in the ICRF‐187 compared to 20 in the control group). The use of dexrazoxane for anthracycline cardioprotection has been determined to be cost effective in several studies 57, 58, 59, 60, 61.
Figure 2.
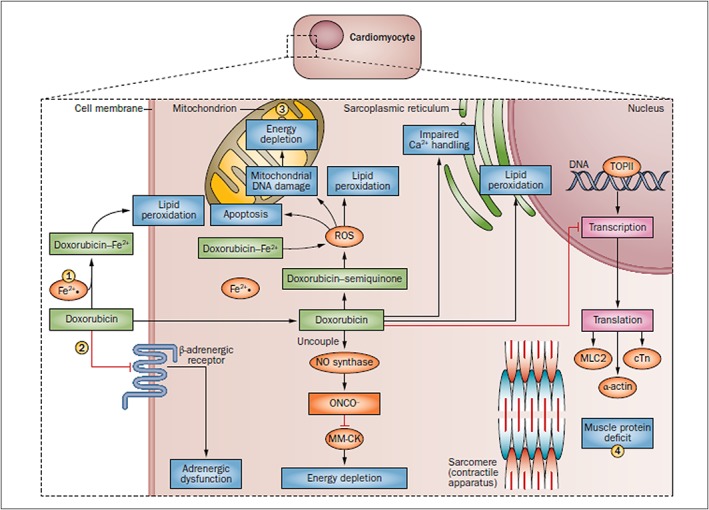
Potential opportunities for cardioprotection. Doxorubicin chemotherapy has a range of effects on cardiomyocytes. It induces lipid peroxidation at the cell and mitochondrial membranes by way of complexing with Fe2+ and induces apoptosis, mitochondrial DNA damage and energy depletion through its production of ROS. Furthermore, it impairs Ca2+ processing in the sarcoplasmic reticulum and inhibits the transcription of important muscle elements, weakening the heart muscle. It also downregulates adrenergic receptors and interrupts cell signalling. (1) Administration of dexrazoxane can prevent Fe2+ complex formation. (2) Intravenous immunoglobulin therapy can reduce inflammatory cytokines. (3) L‐carnitine can bolster mitochrondrial function. (4) Anti‐heart‐failure therapies, such as angiotensin‐converting‐enzyme inhibitors and β‐blockers, can prevent further damage. cTn, cardiac troponin; MLC2, myosin light chain 2; MM‐CK, myofibrillar isoform of the CK enzyme; ROS, reactive oxygen species; TOPII, topoisomerase 2 14
Since its approved use in women with breast cancer treated with anthracyclines, multiple clinical studies have found that dexrazoxane prevents cardiotoxicity among children and adolescents. Importantly, dexrazoxane is cardioprotective without decreasing the effectiveness of anthracyclines or compromising event‐free survival. Its use as a cardioprotectant among children and adolescents has also been endorsed by the American Heart Association and the American Academy of Pediatrics 62. A randomized, multicentre trial conducted by the Dana–Farber Cancer Institute's Childhood ALL Consortium Protocol 95–01 found that dexrazoxane was associated with a decrease in cardiac injury among children with ALL treated with doxorubicin 63. Patients were assigned to receive either doxorubicin with dexrazoxane or doxorubicin alone. Serum concentrations of cardiac troponin T, a well‐known marker for acute myocardial injury, were significantly higher in patients treated with doxorubicin than in those who also received dexrazoxane. Furthermore, at a median follow‐up of 2.7 years, event‐free survival did not differ significantly. Additional long‐term results from this same multicentre trial revealed that dexrazoxane provides long‐term cardioprotection, as determined by echocardiography, 5 years after completing doxorubicin chemotherapy with no significant difference in event‐free survival at 8.7 years of follow‐up 64.
Three large, consecutive, multicentre randomized control trials have studied the incidence of secondary malignant neoplasms (SMNs) among patients with high‐risk ALL treated with dexrazoxane and doxorubicin 65. After a median follow‐up of 3.8 years, only one SMN was observed among 553 patients. A recent report from the Children's Oncology Group concluded that dexrazoxane had no adverse effect on overall long‐term survival among patients with T‐cell ALL, T‐cell acute lymphoblastic lymphoma, or low‐, intermediate‐, or high‐risk Hodgkin lymphoma after a median follow‐up of 12.6 years (Figure 3) 66. Furthermore, the Pediatric Oncology Group 9404 randomized trial found that dexrazoxane was cardioprotective among children and adolescents with newly diagnosed T‐ALL or lymphoblastic non‐Hodgkin lymphoma without compromising antitumour efficacy 67. Additionally, this study also noted that dexrazoxane was not associated with an increased incidence of SMNs or toxicities.
Figure 3.
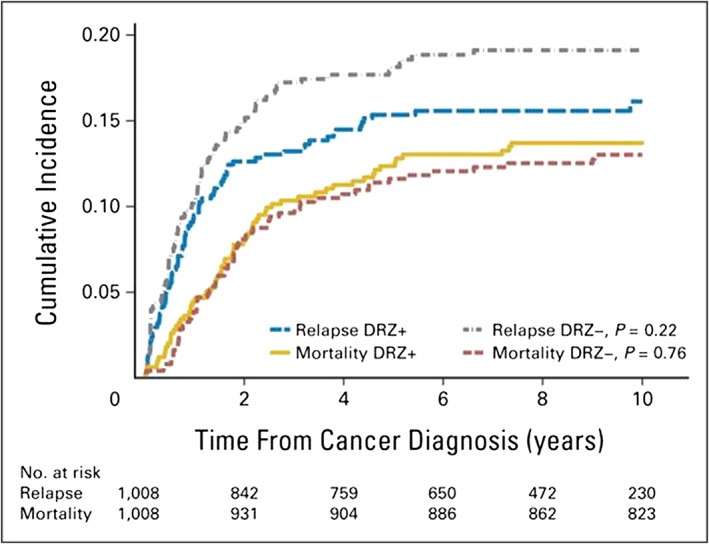
Cumulative incidence of relapse and overall mortality in the combined Children's Oncology Group randomized trials of dexrazoxane (DRZ). Cumulative incidences at 10 years were not significantly different by DRZ status for either outcome. DRZ+, exposed to DRZ; DRZ−, not exposed to DRZ. Permission obtained from American Society of Clinical Oncology © 66
Studies of children with osteosarcoma have also documented the effectiveness of dexrazoxane in combination with anthracyclines to prevent cardiotoxicity 68, 69. In a phase II trial among patients with HER2‐positive metastatic osteosarcoma, combining trastuzumab with doxorubicin and dexrazoxane did not increase the risk of acute myocardial injury 68. Schwartz et al., in patients with nonmetastatic osteosarcoma, found that dexrazoxane allowed doxorubicin therapy to be intensified without impairing tumor response or increasing the risk of SMNs 69.
Tebbi et al. 70 suggested that, in patients with low‐ and high‐risk Hodgkin lymphoma, dexrazoxane might increase the incidence of SMNs when added to standard treatment of doxorubicin, bleomycin, vincristine and etoposide (ABVE), or dose‐intensified ABVE with prednisone and cyclophosphamide followed by low‐dose radiation. Their conclusions were based on the results of Pediatric Oncology Group trials 9426 and 9425, in which 10 SMNs occurred among 478 patients randomly assigned to treatment with or without dexrazoxane. Based on these results the European Medicines Agency's Committee for Medicinal Products for Human Use reported in 2011 that dexrazoxane may be associated with SMNs in children 71. However, the statistical evidence of their findings was limited because the study was not designed to examine SMNs and dexrazoxane use, and several trials have also found that dexrazoxane has not been associated with an increase in SMNs 72. In fact, some studies have suggested that dexrazoxane may avert SMNs by reducing doxorubicin‐induced aneuploidy in part by its free radical reducing activity 73. Further, preclinical studies of dexrazoxane have not shown interference with the anticancer activity of doxorubicin or trastuzumab 74. The EMA should therefore re‐evaluate the safety and efficacy of dexrazoxane as a cardioprotective medication against anthracycline‐induced cardiotoxicity for children and adolescents.
Preventing anthracycline‐induced cardiotoxicity: other treatment options
Various medical therapies and techniques have been studied in an attempt to prevent or mitigate anthracycline‐induced cardiotoxicity. Several potentially cardioprotectve agents other than dexrazoxane have been developed and tested in animals in small clinical trials but await large scale clinical trials including statins, angiotensin‐converting enzyme (ACE) inhibitors, calcium channel blockers, angiotensin receptor antagonists, liposomal formations of anthracyclines, and beta‐adrenergic antagonists. In particular, several studies have tested ACE inhibitors. Enalapril, an ACE‐inhibitor commonly used to treat heart failure, LV dysfunction, and hypertension, has been studied among children with cancer treated with anthracyclines. An observational study found that enalapril delayed the progression of cardiotoxicity among survivors but did not prevent its progression 75. In a randomized, double blind, placebo‐controlled trial of survivors of childhood cancer, enalapril also improved LV wall stress but did not affect overall exercise tolerance among the participants 76. The beta‐blocker carvedilol, which is also used to treat heart failure, hypertension, and LV dysfunction after myocardial infarction among other conditions, is currently being studied to assess its potential in preventing heart failure among survivors of childhood cancer treated with anthracyclines (NCT02717507) 88. More studies are needed to assess whether additional medications may be cardioprotective in patients treated with anthracyclines.
Administering doxorubicin as a continuous infusion to lower peak blood concentrations has also been tested to prevent anthracycline‐induced toxicity in children and adolescents. The Dana–Farber Cancer Institute's ALL Consortium Protocol 91–01 compared continuous 48‐hour infusion to bolus dosing of doxorubicin among patients with high‐risk ALL 25. Both groups had marked abnormalities in LV structure and function from baseline after a median of 8 years of follow‐up. However, neither measures of cardiac function or event‐free survival differed between the two groups. Although doxorubicin administered continuously provides early cardioprotection in adults, its administration to protect the heart as a continuous infusion is not supported over bolus dosing in children and adolescents 25, 75, 77, 78, 79.
Additional cardioprotective techniques may include further decreasing the total cumulative dose of anthracycline or eliminating its use altogether. Given the additive adverse effects of radiation treatment, reducing or eliminating radiation exposure would also be ideal. Although these techniques can reduce cardiotoxicity, their benefits need to be balanced with any potential adverse effects and reductions in efficacy. Research is needed to determine how to reduce or potentially replace these techniques with newer, less‐toxic treatments that remain equally effective.
Follow‐up and monitoring of survivors with cardiotoxicity
Early detection of adverse effects, such as cardiotoxicity from cancer therapy, is necessary to be able to intervene quickly and effectively. Organizations, such as the Children's Oncology Group, have developed consensus‐based recommended guidelines for detecting adverse health effects among survivors of childhood cancer 80. Despite existing recommendations, studies are needed to establish more validated, evidence‐based guidelines for monitoring this population 14.
Recommendations for cardiotoxicity screening among childhood cancer survivors are primarily risk‐based and depend on age at time of diagnosis and treatment, cumulative dosage of both anthracycline and radiation therapy, and additional risk factors associated with cardiac dysfunction 81. Assessing cardiac function should begin with a detailed history and physical, focusing on cardiac symptoms and risk factors for cardiac disease. Modifiable risk factors for cardiac disease should be addressed, especially obesity, illicit drug use and physical inactivity, and are even more important in this vulnerable population 81.
Imaging techniques are often used to monitor cardiotoxicity during and after chemotherapy. However, serum cardiac biomarkers are increasingly being used. Cardiac troponin T (cTnT) and N‐terminal probrain natriuretic peptide (NT‐proBNP) concentrations have shown to be effective as markers of early anthracycline‐induced cardiotoxicity 63 and have been validated as surrogate markers of late LV structural status among long‐term survivors of childhood cancer 26. In a study of 134 children receiving an anthracycline dose of 300 mg m−2 for high‐risk ALL, elevations in serum cTnT concentrations during the first 90 days of anthracycline treatment were significantly associated with reduced LV end‐diastolic posterior wall thickness and LV mass and increased pathologic LV remodelling 4 years later. Similarly, elevations in serum concentrations of NT‐proBNP, indicative of cardiomyopathy, during the first 90 days of treatment were correlated with abnormal LV thickness‐to‐dimension ratios four years after therapy 26. Thus, as validated surrogate markers of late cardiotoxicity, serum biomarkers of cardiomyopathy (NT‐proBNP) and cardiomyocyte injury or death (cTnT) might allow studies to determine whether these markers can be used to tailor anticancer therapy and to determine whether overall outcomes are improved 11.
Imaging studies, such as echocardiography, are also often used during and after chemotherapy because a large percentage of survivors experience reduced LV function and fractional shortening within just a few years after completing therapy 9. Unfortunately, echocardiography during therapy does not detect early subtle cardiac damage or dysfunction that is associated with late cardiotoxicity in long‐term survivors 14, 17. Additionally, the frequency of screening and the best treatment options if an abnormality occurs are still debated.
Treating anthracycline‐induced cardiotoxicity
As the number of survivors of childhood cancer increase, so too will be the number of survivors with adverse effects from cancer therapy. Validated treatments for anthracycline‐induced cardiotoxicity remain scarce, and there is no consensus on their use 76. Once cardiotoxicity occurs, it is often irreversible and progressive (Figure 4) 14. Among survivors with reduced LV systolic performance, for example, there is often a decreased number of functional cardiomyocytes and stem cells for regenerating cardiac tissue 9. Drugs often used to treat cardiac disorders, such as heart failure and hypertension, have not been validated in effectively managing anthracycline‐induced cardiotoxicity, and have not been found to improve overall morbidity and mortality in this population when used to treat asymptomatic LV dysfunction 14.
Figure 4.
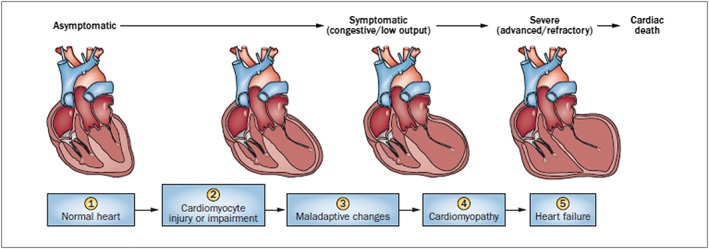
Stages in the course of paediatric ventricular dysfunction. (1) Primary prevention is possible at this stage by reducing risk factors in high‐risk populations (such as those receiving anticancer therapy). (2) Secondary prevention is possible at this stage to reduce the effects of the treatment‐induced injury. (3) Secondary prevention is also possible at this stage. (4) Clinically significant conduction and rhythm abnormalities might be observed. (5) Radical therapies might be required at this stage (such as heart transplant) if there is failure of medical management. Preventive strategies are progressively less effective as the toxicity increases. Treatment strategies have a greater impact when used to treat the more‐diseased heart, but have longer effects if initiated early. Biomarkers and surrogate end points are potentially useful at early stages to alter the course with interventions, and are potentially useful at later stages to aid decisions about transplantation 14
Advocating for a heart‐healthy lifestyle may be among the most important treatments for these patients 81. A healthy diet, supervised physical activity tailored to an individual survivor, extracurricular activities, and good mental health are likely to be as important among survivors as they are in the general population to reduce the risk for cardiac dysfunction. Lifestyle counseling and education are especially important among survivors of childhood cancer because of their increased vulnerability to late cardiac effects as adults. However, after addressing conventional risk factor for premature cardiovascular disease, childhood cancer survivors are likely to have additional cancer and cancer therapy‐related risk factors for premature cardiovascular disease.
Conclusions
The number of survivors of childhood cancer has grown exponentially and will continue to increase as cancer therapies continue to improve. These patients therefore remain at increased risk of morbidity and mortality from the adverse effects of therapy, especially cardiotoxicity. The need for anthracyclines as a component of treatment for most children, adolescents and young adults with cancer is well recognized. As the number of survivors continues to increase, the need to improve overall morbidity associated with therapy and ultimately their quality of life must also be recognized. An important preventive treatment is the use of dexrazoxane, which can prevent or reduce anthracycline‐induced cardiotoxicity as shown in many studies of adults and children with cancer, without decreasing antineoplastic effects or event‐free survival. Dexrazoxane should therefore be a concomitant treatment among children, adolescents and young adults treated with anthracyclines now and in the foreseeable future.
Competing Interests
The authors have nothing to disclose and have no conflicts of interest to report.
Supported in part by grants from the National Institutes of Health (HL072705, HL078522, HL053392, CA127642, CA068484, HD052104, AI50274, CA068484, HD052102, HL087708, HL079233, HL004537, HL087000, HL007188, HL094100, HL095127, HD80002), Chiron, Pfizer Pharmaceuticals, Inc., Lance Armstrong Foundation, Women's Cancer Association, Roche Diagnostics Corporation, the Scott Howard Fund, the Michael Garil Fund, the Parker Family Foundation, Sofia's Hope and the American Heart Association.
Hutchins, K. K. , Siddeek, H. , Franco, V. I. , and Lipshultz, S. E. (2017) Prevention of cardiotoxicity among survivors of childhood cancer. Br J Clin Pharmacol, 83: 455–465. doi: 10.1111/bcp.13120.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 2015; 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 5. Schrappe M, Nachman J, Hunger S, Schmiegelow K, Conter V, Masera G, et al. Educational symposium on long‐term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985–2000). Leukemia 2010; 24: 253–254. [DOI] [PubMed] [Google Scholar]
- 6. Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006; 355: 1572–1582. [DOI] [PubMed] [Google Scholar]
- 7. Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause‐specific late mortality among 5‐year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst 2008; 100: 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, et al. Long‐term cause‐specific mortality among survivors of childhood cancer. JAMA 2010; 304: 172–179. [DOI] [PubMed] [Google Scholar]
- 9. Lipshultz SE, Colan SD, Gelber RD, Perez‐Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med 1991; 324: 808–815. [DOI] [PubMed] [Google Scholar]
- 10. Lipshultz SE, Franco VI, Sallan SE, Adamson PC, Steiner RK, Swain SM, et al. Dexrazoxane for reducing anthracycline‐related cardiotoxicity in children with cancer: An update of the evidence. Prog Pediatr Cardiol 2014; 36: 39–49. [Google Scholar]
- 11. Lipshultz SE, Franco VI, Miller TL, Colan SD, Sallan SE. Cardiovascular disease in adult survivors of childhood cancer. Annu Rev Med 2015; 66: 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lipshultz SE. Exposure to anthracycline during childhood causes cardiac injury. Semin Oncol 2006; 33: S8–14. [DOI] [PubMed] [Google Scholar]
- 13. Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 2005; 23: 2629–2636. [DOI] [PubMed] [Google Scholar]
- 14. Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment‐related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol 2013a; 10: 697–710. [DOI] [PubMed] [Google Scholar]
- 15. Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009; 339: b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leger K, Slone T, Lemler M, Leonard D, Cochran C, Bowman WP, et al. Subclinical cardiotoxicity in childhood cancer survivors exposed to very low dose anthracycline therapy. Pediatr Blood Cancer 2015; 62: 123–127. [DOI] [PubMed] [Google Scholar]
- 17. Lipshultz SE, Diamond MB, Franco VI, Aggarwal S, Leger K, Santos MV, et al. Managing chemotherapy‐related cardiotoxicity in survivors of childhood cancers. Paediatr Drugs 2014; 16: 373–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brouwer CA, Gietema JA, Van Den Berg MP, Bink‐Boelkens MT, Elzenga NJ, Haaksma J, et al. Long‐term cardiac follow‐up in survivors of a malignant bone tumour. Ann Oncol 2006; 17: 1586–1591. [DOI] [PubMed] [Google Scholar]
- 19. Kremer LC, van Dalen EC, Offringa M, Ottenkamp J, Voûte PA. Anthracycline‐induced clinical heart failure in a cohort of 607 children: long‐term follow‐up study. J Clin Oncol 2001; 19: 191–196. [DOI] [PubMed] [Google Scholar]
- 20. Nysom K, Holm K, Lipsitz SR, Mone SM, Colan SD, Orav EJ, et al. Relationship between cumulative anthracycline dose and late cardiotoxicity in childhood acute lymphoblastic leukemia. J Clin Oncol 1998; 16: 545–550. [DOI] [PubMed] [Google Scholar]
- 21. Trachtenberg BH, Landy DC, Franco VI, Henkel JM, Pearson EJ, Miller TL, et al. Anthracycline‐associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol 2011; 32: 342–353. [DOI] [PubMed] [Google Scholar]
- 22. Harake D, Franco VI, Henkel JM, Miller TL, Lipshultz SE. Cardiotoxicity in childhood cancer survivors: strategies for prevention and management. Future Cardiol 2012; 8: 647–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heidenreich PA, Schnittger I, Strauss HW, Vagelos RH, Lee B, Mariscal CS, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkins disease. J Clin Oncol 2007; 25: 43–49. [DOI] [PubMed] [Google Scholar]
- 24. Landy DC, Miller TL, Lipsitz SR, Lopez‐Mitnik G, Lipsitz SR, Hinkle AS, et al. Cranial irradiation as an additional risk factor for anthracycline cardiotoxicity in childhood cancer survivors: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Pediatr Cardiol 2013; 34: 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lipshultz SE, Landy DC, Lopez‐Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol 2012; 30: 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lipshultz SE, Miller TL, Lipsitz SR, Neuberg DS, Dahlberg SE, Colan SD, et al. Continuous versus bolus infusion of doxorubicin in children with ALL: long‐term cardiac outcomes. Pediatrics 2012; 130: 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lipshultz SE, Miller TL, Scully RE, Lipsitz SR, Rifai N, Silverman LB, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high‐risk acute lymphoblastic leukemia: associations with long‐term echocardiographic outcomes. J Clin Oncol 2012; 30: 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giantris A, Abdurrahman L, Hinkle A, Asselin B, Lipshultz SE. Anthracycline‐induced cardiotoxicity in children and young adults. Crit Rev Oncol Hematol 1998; 27: 53–68. [DOI] [PubMed] [Google Scholar]
- 29. Blanco JG, Leisenring WM, Gonzalez‐Covarrubias VM, Kawashima TI, Davies SM, Relling MV, et al. Genetic polymoprhisms in the carbonyl reductase 3 gene CBR3 and the NAD(p)H:quinine oxidoreductase 1 gene NQO1 in patients who developed anthracycline‐related congestive heart failure after childhood cancer. Cancer 2008; 112: 2789–2795. [DOI] [PubMed] [Google Scholar]
- 30. Blanco JH, Sun CL, Landier W, Chen L, Esparza‐Duran D, Leisenring W, et al. Anthracycline‐related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes—a report from the Children's Oncology Group. J Clin Oncol 2012; 30: 1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krajinovic M, Elbared J, Drouin S, Bertout L, Rezgui A, Ansari M, et al. Polymorphisms of ABCC5 and NOS3 genes influence doxorubicin cardiotoxicity in survivors of childhood acute lymphoblastic leukemia. Pharmacogenomics J (Epub ahead of print) 2015. doi: 10.1038/tpj.2015.63. [DOI] [PubMed] [Google Scholar]
- 32. Lipshultz SE, Lipsitz SR, Kutok JL, Miller TL, Colan SD, Neuberg DS, et al. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin‐treated survivors of childhood high‐risk leukemia. Cancer 2013; 119: 3555–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Visscher H, Ross CJ, Rassekh SR, Barhdadi A, Dube MP, Al‐Saloos H, et al. Pharmacogenomic prediction of anthracycline‐induced cardiotoxicity in children. J Clin Oncol 2012; 30: 1422–1428. [DOI] [PubMed] [Google Scholar]
- 34. Visscher H, Ross CJ, Rassek SR, Sandor GS, Caron HN, van Dalen EC, et al. Validation of variants in SLC28A3 and UGT1A6 genetic markers predictive of anthracycline‐induced cardiotoxicity in children. Pediatr Blood Cancer 2013; 60: 1375–1381. [DOI] [PubMed] [Google Scholar]
- 35. Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 1995; 332: 1738–1743. [DOI] [PubMed] [Google Scholar]
- 36. Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol 1997; 15: 1544–1552. [DOI] [PubMed] [Google Scholar]
- 37. Steiner R. Increasing exercise in long‐term survivors of pediatric cancer and their siblings: should treatment be a family affair? Pediatr Blood Cancer 2013; 60: 529–530. [DOI] [PubMed] [Google Scholar]
- 38. Centers for Disease Control and Prevention . (2011). How much physical activity do children need? Centers for Disease Control and Prevention. Available at http://www.cdc.gov/physicalactivity/basics/children (last accessed 23 May 2016).
- 39. Ness KK, Leisenring WM, Huang S, Hudson MM, Gurney JG, Whelan K, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2009; 115: 1984–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller TL, Lipsitz SR, Lopez‐Mitnik G, Hinkle AS, Constine LS, Adams MJ, et al. Characteristics and determinants of adiposity in pediatric cancer survivors. Cancer Epidemiol Biomarkers Prev 2010; 19: 2013–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miller AM, Lopez‐Mitnik G, Somarriba G, Lipsitz SR, Hinkle AS, Constine LS, et al. Exercise capacity in long‐term survivors of pediatric cancer: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivor study. Pediatr Blood Cancer 2013; 60: 663–668. [DOI] [PubMed] [Google Scholar]
- 42. Messiah SE, Arheart KL, Lopez‐Mitnik G, Lipshultz SE, Miller TL. Ethnic group differences cardiometabolic disease risk factors independent of body mass index among American youth. Obesity (Silver Spring) 2013; 21: 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meacham LRL, Gurney JG, Mertens AC, Ness KK, Sklar CA, Robison LL, et al. Body mass index in long‐term adult survivors of childhood cancer—a report from the Childhood Cancer Survivor Study. Cancer 2005; 103: 1730–1739. [DOI] [PubMed] [Google Scholar]
- 44. Armenian SH, Sun CL, Vase T, Ness KK, Blum E, Francisco L, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood 2012; 120: 4505–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meacham LR, Sklar CA, Li S, Liu Q, Gimpel N, Yasui Y, et al. Diabetes mellitus in long‐term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med 2009; 169: 1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neville KA, Cohn RJ, Steinbeck KS, Johnston K, Walker JL. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J Clin Endocrinol Metab 2006; 91: 4401–4407. [DOI] [PubMed] [Google Scholar]
- 47. Emmons K, Li FP, Whitton J, Mertens AC, Hutchinson R, Diller L, et al. Predictors of smoking initiation and cessation among childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2002; 20: 1608–1616. [DOI] [PubMed] [Google Scholar]
- 48. Klosky JL, Howell CR, Li Z, Foster RH, Mertens AC, Robison LL, et al. Risky health behavior among adolescents in the childhood cancer survivor study cohort. J Pediatr Psychol 2012; 37: 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schultz KAP, Chen L, Chen Z, Zeltzer LK, Nicholson HS, Neglia JP. Health and risk behaviors in survivors of childhood acute myeloid leukemia: a report from the children's oncology group. Pediatric Blood & Cancer 2010; 55: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herman EH, Ferrans VJ, Myers CE, VanVleet JF. Comparison of the effectiveness of (±)‐1,2‐Bis(3,5‐dioxopiperazinyl‐1yl)propane (ICRF‐187) and N‐acetylcysteine in preventing chronic doxorubicin cardiotoxicity in beagles. Cancer Res 1985; 45: 276–281. [PubMed] [Google Scholar]
- 51. Hochster HS. Clinical pharmacology of dexrazoxane. Semin Oncol 1998; 25: 37–42. [PubMed] [Google Scholar]
- 52. Hasinoff BB, Patel D, Wu X. Molecular mechanisms of the cardiotoxicity of the proteasomal‐targeted drugs bortezomib and carfilzomib. Cardiovasc Toxicol. 2016. doi: 10.1007/s12012-016-9378-7. [DOI] [PubMed] [Google Scholar]
- 53. Lipshultz SE, Anderson LM, Miller TL, Gerschenson M, Stevenson KE, Neuberg DS, et al. Impaired mitochondrial function is abrogated by dexrazoxane in doxorubicin‐treated childhood acute lymphoblastic leukemia survivors. Cancer 2016; 122: 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vijay V, Moland CL, Han T, Fuscoe JC, Lee T, Herman EH, et al. Early transcriptional changes in cardiac mitochondria during chronic doxorubicin exposure and mitigation by dexrazoxane in mice. Toxicol Appl Pharmacol 2016; 295: 68–84. [DOI] [PubMed] [Google Scholar]
- 55. Speyer JL, Green MD, Kramer E, Rey M, Sanger J, Ward C, et al. Protective effect of the bispiperazinedione ICRF‐187 against doxorubicin‐induced cardiac toxicity in women with advanced breast cancer. N Engl J Med 1988; 319: 745–752. [DOI] [PubMed] [Google Scholar]
- 56. Speyer JL, Green MD, Zeleniuch‐Jacquotte A, Wernz JC, Rey M, Sanger J, et al. ICRF‐187 permits longer treatment with doxorubicin in women with breast cancer. J Clin Oncol 1992; 10: 117–127. [DOI] [PubMed] [Google Scholar]
- 57. Bates M, Lieu D, Zagari M, Spiers A, Williamson T. A pharmacoeconomic evaluation of the use of dexrazoxane in preventing anthracycline‐induced cardiotoxicity in patients with stage IIIB or IV metastatic breast cancer. Clin Ther 1997; 19: 167–184. [DOI] [PubMed] [Google Scholar]
- 58. Limat S, Demesmay K, Fagnoni P, Voillat L, Bernard Y, Deconinck E, et al. Cost effectiveness of cardioprotective strategies in patients with aggressive non‐Hodgkin's lymphoma. Clin Drug Investig 2005; 25: 719–729. [DOI] [PubMed] [Google Scholar]
- 59. Monsuez JJ. Detection and prevention of cardiac complications of cancer chemotherapy. Arch Cardiovasc Dis 2012; 105: 593–604. [DOI] [PubMed] [Google Scholar]
- 60. Paladio‐Hernandez J, Martinez‐Morales J. Cost‐effectiveness of cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline‐based chemotherapy in Mexico. Value Health 2015; 18: A454. [Google Scholar]
- 61. Tonkin K, Bates M, Lieu D, Arundell E, Williamson T, Zagari M. Dexrazoxane cardioprotection for patients receiving FAC chemotherapy: a pharmacoeconomic evaluation. Can J Oncol 1996; 6: 458–473. [PubMed] [Google Scholar]
- 62. Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long‐term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions (A scientific statement from the American Heart Association). Circulation 2013; 128: 1927–1995. [DOI] [PubMed] [Google Scholar]
- 63. Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin‐treated children with acute lymphoblastic leukemia. N Engl J Med 2004; 351: 145–153. [DOI] [PubMed] [Google Scholar]
- 64. Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin‐treated children with high‐risk acute lymphoblastic leukaemia: long‐term follow‐up of a prospective, randomized, multicentre trial. Lancet Oncol 2010; 11: 950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vrooman LM, Neuberg DS, Stevenson KE, Asselin BL, Athale UH, Clavell L, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana–Farber Cancer Institute ALL Consortium. Eur J Cancer 2011; 47: 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chow EJ, Asselin BL, Schwartz CL, Doody DR, Leisenring WM, Aggarwal S, et al. Late mortality after dexrazoxane treatment: A report from the Children's Oncology Group. J Clin Oncol 2015; 33: 2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Asselin BL, Devidas M, Chen L, Franco VI, Pullen J, Borowitz MJ, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T‐cell acute lymphoblastic leukemia or advanced‐stage lymphoblastic non‐Hodgkin lymphoma: A report of the Children's Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol 2016; 34: 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ebb D, Meyers P, Grier H, Bernstein M, Gorlick R, Lipshultz SE, et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: A report from the Children's Oncology Group. J Clin Oncol 2012; 30: 2545–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schwartz CL, Wexler LH, Krailo MD, Teot LA, Devidas M, Steinherz L, et al. Intensified chemotherapy with dexrazoxane cardioprotection in newly diagnosed nonmetastatic osteosarcoma: a report from the Children's Oncology Group. Pediatr Blood Cancer 2016; 63: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, et al. Dexrazoxane‐associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol 2007; 25: 493–500. [DOI] [PubMed] [Google Scholar]
- 71. Akam‐Venkata J, Franco VI, Lipshultz SE. Late cardiotoxicity: issues for childhood cancer survivors. Curr Treat Options Cardio Med 2016; 18: 47. [DOI] [PubMed] [Google Scholar]
- 72. Lipshultz SE, Lipsitz SR, Orav EJ. Dexrazoxane‐associated risk for secondary malignancies in pediatric Hodgkin's disease: a claim without compelling evidence. J Clin Oncol 2007; 25: 3179; author reply, 3180. [DOI] [PubMed] [Google Scholar]
- 73. Attia SM, Ahmad SF, Bakheet SA. Impact of dexrazoxane on doxorubicin‐induced aneuploidy in somatic and germinal cells of male mice. Cancer Chemother Pharmacol 2016; 77: 27–33. [DOI] [PubMed] [Google Scholar]
- 74. Smith TA, Phyu SM, Akabuogu EU. Effects of administered cardioprotective drugs on treatment responses of breast cancer cells. Anticancer Res 2016; 36: 87–93. [PubMed] [Google Scholar]
- 75. Lipshultz SE, Lipsitz SR, Sallan SE, Simbre VC 2nd, Shaikh SL, Mone SM, et al. Long‐term enalapril therapy for left ventricular dysfunction in doxorubicin‐treated survivors of childhood cancer. J Clin Oncol 2002; 20: 4517–4522. [DOI] [PubMed] [Google Scholar]
- 76. Silber JH, Cnaan A, Clark BJ, Paridon SM, Chin AJ, Rychik J, et al. Enalapril to prevent cardiac function decline in long‐term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol 2004; 22: 820–828. [DOI] [PubMed] [Google Scholar]
- 77. Casper ES, Gaynor JJ, Hajdu SI, Magill GB, Tan C, Friedrich C, et al. A prospective randomized trial of adjuvant chemotherapy with bolus versus continuous infusion of doxorubicin in patients with high‐grade extremity soft tissue sarcoma and an analysis of prognostic factors. Cancer 1991; 68: 1221–1229. [DOI] [PubMed] [Google Scholar]
- 78. Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, Valdivieso M, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med 1982; 96: 133–139. [DOI] [PubMed] [Google Scholar]
- 79. Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline‐induced myocardial damage: a review of the most promising strategies. Br J Haematol 2005; 131: 561–578. [DOI] [PubMed] [Google Scholar]
- 80. Children's Oncology Group (COG) . (2013). Long‐term follow‐up guidelines for survivors of childhood, adolescent, and young adult cancer. Available at www.survivorshipguidelines.org (last accessed 1 April 2016).
- 81. Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics 2008; 121: e387–e396. [DOI] [PubMed] [Google Scholar]
- 82. van der Pal HJ, van Dalen EC, Hauptmann M, Kok WE, Caron HN, van den Bos C, et al. Cardiac function in 5‐year survivors of childhood cancer: a long‐term follow‐up study. Arch Intern Med 2010; 170: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 83. Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol 2010; 28: 1276–1281. [DOI] [PubMed] [Google Scholar]
- 84. Van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev 2011; 1: CD003917 [DOI] [PubMed] [Google Scholar]
- 85. Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE. Anthracycline‐induced cardiotoxicity: course, pathophyiology, prevention and management. Expert Opin Pharmacother 2007; 8: 1039–1058. [DOI] [PubMed] [Google Scholar]
- 86. Adams MJ, Lipshultz SE. Pathophysiology of anthracycline‐ and radiation‐associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer 2005; 44: 600–606. [DOI] [PubMed] [Google Scholar]
- 87. Landy DC, Miller TL, Lopez‐Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, et al. Aggregating traditional cardiovascular disease risk factors to assess the cardiometabolic health of childhood cancer survivors: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Am Heart J 2012; 163: 295–301.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Children's Oncology Group (COG) ; National Cancer Institute (NCI) . Carvedilol in preventing heart failure in childhood cancer survivors In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2000. Available at https://clinicaltrials.gov/ct2/show/NCT02717507 NLM Identifier: NCT02717507 (last accessed 10 February 2016). [Google Scholar]


