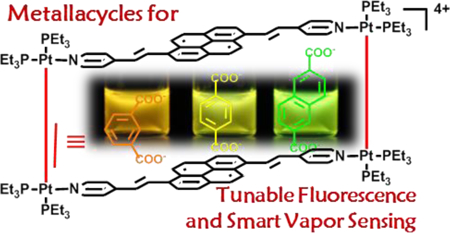
Abstract
Constructing polycyclic aromatics-based highly emissive fluorophores with good solubility, tunable aggregated structures and properties is of great importance for film fabrication, solution processing and relevant functionality studies. Herein, we describe a general strategy to endow conventional organic fluorophores with enhanced solubility and modulated fluorescent properties via the incorporation into coordination-driven self-assembled metallacycles. A widely used fluorophore, pyrene, was decorated with two pyridyl groups to yield functionalized pyrene (4). Mixing 4 with three aromatic dicarboxylates with different lengths and a 90° Pt(II) metal acceptor in a 2:2:4 stoichiometric ratio resulted in the formation of three metallacycles (1, 2, and 3). The metallacycles display good solubility in polar organic solvents, highly aggregation-dependent fluorescence, and size-dependent emissions at higher concentrations. Moreover, metallacycle 2- based, silica-gel supported film as fabricated is not only more emissive than the ligand, 4-based one, but also displays much improved sensing properties for amines in the vapor state as demonstrated by significantly increased response speed and decreased recovery time. The enhanced solubility, unique fluorescence behavior and multi-factor modulation character show that coordination-driven self-assembly can be utilized for the development of new fluorophores through simple modification of conventional fluorophores. The fluorophores synthesized this way possess not only complex topological structures but also good modularity and tunability in fluorescence behavior, which are important for grafting multi-stage energy transfer systems, both important for the development of high performance sensing materials.
Graphical Abstract
INTRODUCTION
Fluorescent molecules have been widely designed and synthesized for applications in probing, sensing and the fabrication of optoelectronic devices.1–4 Conventional organic fluorophores, especially those containing large pi structures, exhibit interesting photophysical behaviors such as being highly emissive, emission switching between monomer and excimer or exciplex due to aggregation, and micro-environment sensitivity.5–8 These fluorophores, however, may also have several drawbacks, including poor solubility, aggregation caused quenching (ACQ), and difficulties in the modulation of their photophysical behaviors.9–11 In order to obtain fluorophores with favorable properties, structural modifications are required, which often involve tedious organic synthesis.12–15
Metal-ligand coordination interactions are widely used for directing self-assembly due to their high directionality, predictability, and moderate bonding energy that enable self-repairing,16–28 thus supramolecular coordination complexes (SCCs) with diverse sizes and shapes, ranging from two-dimensional metallacycles to three-dimensional metallacages have been successfully prepared using this methodology.29–38 The rigidity and well-defined cavity of SCCs, on a molecular level, not only offers applications via host-guest interactions,39–48 but also allows modulation of interactions between functional moieties by altering the size and/or shape of the building blocks. Furthermore, SCCs are also endowed with good solubility and processability due to their discrete structures. Based on these advantages, we anticipated that the incorporation of conventional aromatic fluorophores within SCCs would give rise to facile tunability of their emission behaviors as well as good processability, which is desirable for sensing and the fabrication of optoelectronic devices.49–51
As a proof-of-concept, pyrene was selected as a model fluorophore in our study. Though pyrene is a well-known conventional organic fluorophore that possesses a number of unique photophysical properties, such as high fluorescence quantum yield, long fluorescence lifetime, tendency for excimer formation, and high polarity dependence of the fine structure of its monomer emission, the application of this fluorophore and its derivatives are limited partially due to emission at short wavelengths (379nm-485 nm).55, 56 To overcome this problem, functionalized pyrenyl derivatives have been obtained, mostly through covalent synthesis.57, 58 We herein describe the design, synthesis and photophysical properties of three pyrene-based self-assembled metallacycles. As shown in Scheme 1, 1,6-di(4-pyridylvinyl)pyrene (4) was obtained by the reaction of 4-vinylpyridine with 1,6-dibromopyrene, which can be used as an electron-rich donor for coordination. Three dicarboxylates with different distances between the two carboxylate groups were used to tune the size of the resulting metallacycles, that are emissive and, at the same time, exhibit improved solubility compared to the emissive precursor building blocks. In addition, the fluorescence properties of these metallacycles are highly aggregation-dependent: they exhibit similar photophysical behaviors at lower concentrations, but different in the aggregated state. Taking advantage of this feature, fluorescence emission, spanning the visible region, was obtained by varying the size and concentration of these metallacycles, as well as the temperature and solvent. Additionally, metallacycle 2 has brighter fluorescence than the ligand when deposited on silica-gel plate surface. Moreover, the fluorescence emission of the metallacycle based film is much more sensitive to the presence of aromatic amines in the air, the film possesses the potential to be developed into an important fluorescent aromatic amine sensor.
Scheme 1.
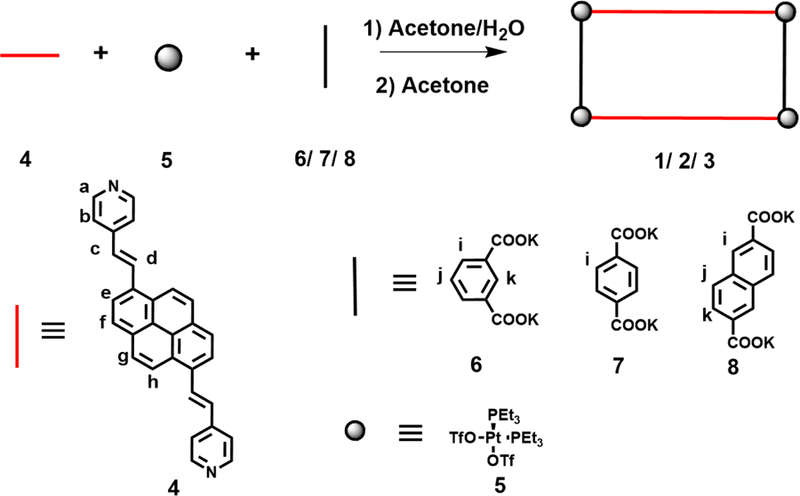
Formation of pyrene-based metallacycles via coordination-driven self-assembly
RESULTS AND DISCUSSION
Metallacycles 1–3 were prepared via a three-component coordination-driven self-assembly strategy.59–65Compound 4 and one of the dicarboxylates (6 for 1, 7 for 2 and 8 for 3) were mixed with 90° Pt (II) (5) in a molar ratio of 2:2:4 in a mixture of acetone/water (v/v, 8/2). The mixture was heated at 50 °C for 10 h, then the solvents were removed and acetone was added to the residue to acquire a homogenous solution. Addition of diethyl ether to this solution led to the precipitation of the desired product in excellent yields. These metallacycles exhibit good solubility in acetone, in sharp contrast to the precursor building block 4, which is not soluble at all under the same condition, indicating that the formation of SCC improves the solubility of 4.
The structure of the metallacycles was established by multinuclear NMR studies (31P and 1H), diffusion ordered NMR spectroscopy (DOSY), as well as electrospray ionization time-of-flight mass spectroscopy (ESI-TOF-MS). The 31P{1H} NMR spectra exhibit two doublets of approximately equal intensity, corresponding to two different chemical environments around the phosphorous center, indicating heteroligation of the Pt(II) center (δ = 6.97 and 1.88 ppm for 1, 6.72 and 1.66 ppm for 2, and 6.81 and 1.71 ppm for 3, respectively), Figure 1. With reference to the 1H NMR spectra of the metallacycles (Figures 1, S1, S4, S9, and S14), the pyridinyl protons showed significant downfield shifts compared to the free precursor 4. In the 1H NMR spectrum of 1, the shifts of Ha from 8.61 to 8.80 ppm and Hb from 7.84 to 8.02 ppm indicate the loss of electron density of the pyridyl unit upon coordination. Similar downfield shifts in both spectra of 2 and 3 were also observed for Ha (from 8.61 to 8.76 and 8.83 ppm) and Hb (from 7.84 to 7.97 and 8.02 ppm) of the pyridinyl moieties.
Figure 1.
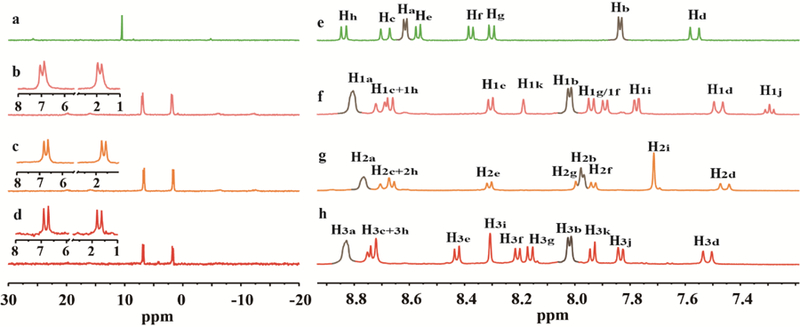
31P{1H} (a–d) and partial 1H NMR spectra (DMSO-d6, 293 K) of 5 (a), 4 (e), 1 (b and f), 2 (c and g) and 3 (d and h).
The results from the DOSY experiments are depicted in Figure S6, Figure S11, and Figure S16. All the metallacycles display a single vertical trace, suggesting the formation of one product in all cases. ESI-TOF-MS measurements further confirms the formation of the metallacycles, which show isotopically resolved peaks at m/z = 1006.57, 1006.57, and 1039.58 (Figure 2) that correspond to the [M-3OTf]3+ species of the metallacycles 1-3, respectively. These peaks are in good agreement with the calculated theoretical distributions. Additionally, the [M-2OTf]2+ species of the three metallacycles also matched well with the calculated values (Figures S7, S12, and S17).
Figure 2.
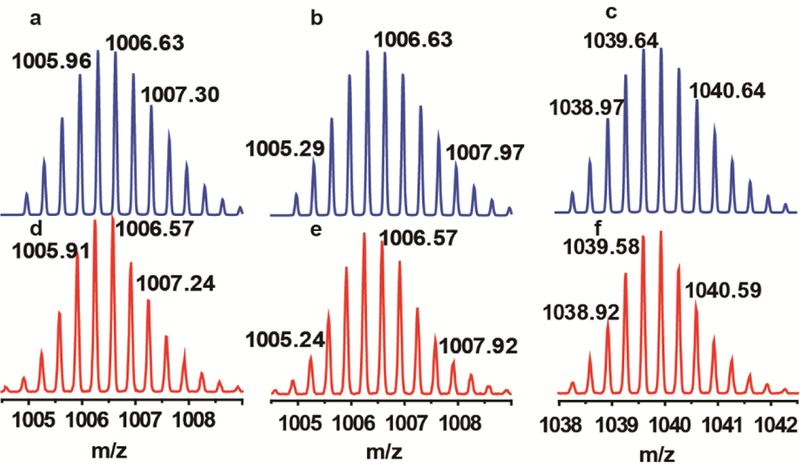
Experimental (red) and calculated (blue) ESI-TOF-MS spectra of metallacycles 1 [M − 3OTf]3+ (a and d), 2 [M − 3OTf]3+ (b and e), and 3 [M − 3OTf]3+ (c and f)
The photophysical behavior of 4 was investigated first. Absorption spectra of 4 in acetone with different concentrations (from 1×10−6 mol/L to saturated solution) exhibit a broad peak with the maximum wavelength at ~404 nm and a shoulder at ~426 nm. (Figure S18) The normalized absorption spectra of 4 show a similar absorption profile at different concentrations, indicating that 4 exists in the same state in solution. As shown in Figure S18, the intensities of the absorptance increase linearly along with increasing concentration of 4. The fluorescence emission spectra of 4 are broad with an emission maximum at ~462 nm, accompanied by a shoulder at ~485 nm. (Figure 3a) The emission spectra of 4 in acetone in a range from saturated solution to 5×10−8 mol/L exhibit the same profile. The fluorescence spectrum of 4 in the solid state (~574 nm) shows a significant red-shift relative to the solution state, that is attributed to the aggregation of the pyrene moieties.52–54 (Figure S19) These data suggest that 4 in the solution state exists in a monomeric state, even in saturated solution, and displays aggregation emission in the solid state. Both the absorption and fluorescence spectra of 4 are red- shifted by about 70 nm relative to the spectra of pyrene, which is an aggregation-based switch between a monomer with five peaks (379–400 nm) and a structureless excimer (maximum at 485 nm).53
Figure 3.
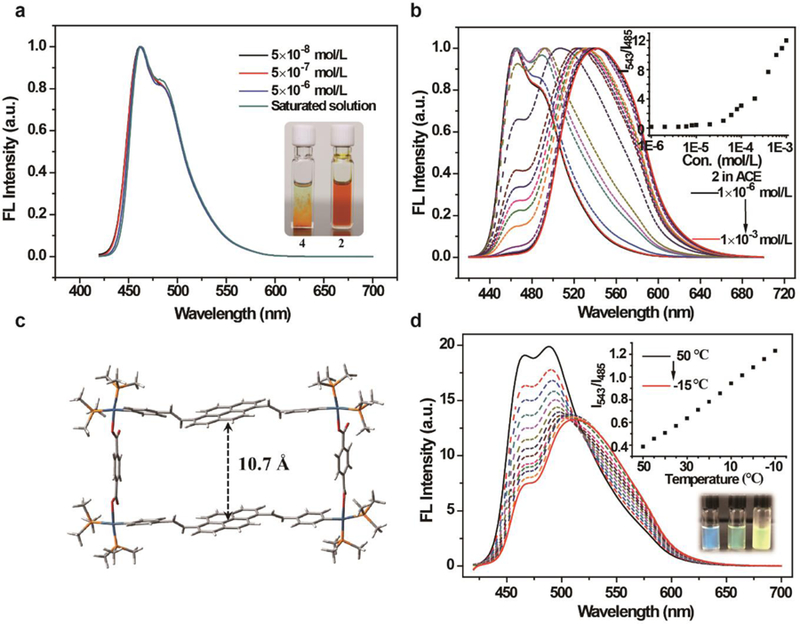
(a) Normalized fluorescence emission spectra of the acetone solution of ligand 4 recorded at different concentrations and at room temperature, where the inset is the fluorescent pictures of the acetone solutions of ligand 4 (~1.3 × 10−5 mol/L), and metallacycle 2 (1.0 × 10−3 mol/L) taken under UV light (365 nm); (b) Normalized fluorescence emission spectra of the acetone solution of metallacycle 2 recorded at different concentrations and at room temperature, where the inset is a plot of the intensity ratio of I543/I485 against concentration; (c) PM6-simulated geometrical structure of metallacycle 2; (d) Fluorescence emission spectra of metallacycle 2 in acetone with a concentration of 1.0 × 10−5 mol/L at temperatures from 50 °C to −10 °C (λex = 400 nm), where the inset is a plot of the intensity ratio of I543/I485 against temperature (top) and fluorescent pictures of the solution taken at 50 °C, 15 °C, and −10 °C, respectively, under UV light (365 nm, bottom).
Having established the photophysical behavior for the precursor, the optical characteristics of the metallacycles were investigated, and 2 was chosen as an example. The UV-Vis absorption and fluorescence emission spectra of 2 are depicted in Figure S20. At the concentration examined (1×10−6mol/L), the absorption spectrum is characterized by a broad peak with a maximum wavelength at ~402 nm and a shoulder at ~430 nm. The fluorescence emission is also broad with a maximum at ~464 nm, accompanied by a shoulder at ~485 nm. The emission maximum of 2 red-shifted to 581 nm in the solid state due to its aggregation. The absorption and fluorescence emission spectra of 2 show similar profiles to the free precursor 4 in dilute solution. However, 2 displays better solubility compared to 4 (>1×10−3 mol/L) (Insert picture of Figure 3a), which provides an opportunity to study the aggregation behavior of pyrene-based metallacycles and thus provide a further understanding of the fundamental properties of the functionalized pyrene.
To investigate the aggregation behavior of 2, its fluorescence emission in acetone at different concentrations (1×10−6 to 1×10−3 mol/L) was recorded (Figure 3b). The emission at shorter wavelengths decreases with increasing concentration, while a new emission peak at 543 nm appears and increases with concentration. The new emission peak is assigned to the aggregation state of the enlarged pyrenyl moiety. The intensity ratio of I543/I485 was plotted against concentration, where the ratio of I543/I485 remains constant in the concentration range of 1.0 × 10−6 mol/L to 2.0 × 10−5 mol/L and the fluorescence maximum occurs at ~464 nm with a shoulder at 485 nm, which suggests that the substituted pyrenyl moieties in 2 exist in the monomer state. However, from 2.0 × 10−5 mol/L to 1.0 × 10−3 mol/L, the ratio increases sharply and the emission band red-shifts, suggesting that the pyrenyl moeties start to aggregate at a concentration of 4.0 × 10−5 mol/L, likely as a result of intermolecular interactions. This insight is supported by the structural optimization study, which was performed with metallacycle 2 as an example using PM 6 semi-empirical calculations. PMe3 was used to replace PEt3 in order to simplify the calculation and the result is depicted in Figure 3c. As seen, the metallacycle has a tetragonal structure, where the distance between the two pyrenyl units is ~10.7 Å, which rules out the possibility of intra-molecular pi-pi stacking as that value is much larger than the distance for such interaction.12–14
Further examination of the emission of 2 indicates that, unlike the emission observed in the solid state, the new emission band is at shorter wavelengths, suggesting that the pyrenyl moieties in the metallacycles are loosely aggregated.56 The UV-Vis absorption spectra of 2 were also recorded at different concentrations (Figure S21). The fact that the absorption of 2 gradually red-shifts and the spectrum changes significantly with increasing concentration suggests aggregation in the ground state.
The temperature effect on the aggregation behavior of the metallacycles was examined using solutions in acetone (1.0 × 10−5 mol/L). With metallacycle 2 as an example, the fluorescence emission and absorption spectra are presented in Figure 3d and Figure S22, respectively. A decrease in temperature resulted in the aggregation of 2, as evidenced by the red-shift in the emission spectra as well as the appearance of a broad emission at longer wavelengths (Figure 3d). Similar to increasing the concentration, the ratio of I543/I485 increases by decreasing the temperature from 50 °C to −10 °C. These changes can be easily visualized by the red-shifts of the emission colors of the solution, from blue to green then to yellow, with decreasing temperature under 365 nm UV light illumination (Figure 3d). The temperature-dependent absorption spectra exhibit a broad and red-shiftd profile with decreasing temperature, suggesting aggregation in the ground state53 similar to the concentration effect (Figure S22).
To investigate the origin of the aggregation of 2, concentration- and temperature-dependent 1H NMR measurements in acetone-d6 were obtained. The results are given in Figures S23 and S24, respectively. By increasing the concentration from 22 μΜ to 0.65 mM, the intensities of the enlarged pyrene proton (H2c-H2h) signals increased while still remaining sharp and well-resolved (Figure S23). However, by further increasing the concentration to 4.2 mM, the signals broaden and become less-resolved (Figure S23), suggesting that the aggregation of 2 is due to the enlarged pyrenyl moieties.6, 66The temperature-dependent studies of 2 at a concentration of 0.65 mM showed that with decreasing temperature from 50 °C to 20 °C, the proton signals of the pyrenyl moiety (H2e-H2h) and ethenyl moiety (H2c and H2d) significantly downfield shifted, whereas the proton signals of the pyridinyl (H2a and H2b) and dicarboxylate ligand (H2i) exhibited little change. (Figure S24) All the above data indicate that the aggregation of 2 in solution is mainly due to the intermolecular pi-pi interaction between the functionalized pyrenyl moieties of the different metallacycles.
The solvent effect on the emission of the metallacycles was examined using a solution of 2 in CH2Cl2 (Figures S25 and S26). Both the absorption and emission are different from those observed in acetone. For the solution at a concentration of 1.0 × 10−6 mol/L, the emission spectrum is composed of both monomeric emission and emission from the aggregated state, indicating that 2 starts to aggregate at a much lower concentration in CH2Cl2 than acetone. The longest wavelength of the aggregated emission observed in CH2Cl2 exceeds 565 nm, which is 20-nm-red-shifted compared with that observed in acetone. In addition, the absorption spectra at concentrations of 2.0 × 10−6 mol/L to 6.0 × 10−5 mol/L are broad and red-shifted compared with that in acetone under the same condition, implying that metallacycle 2 readily forms aggregates in CH2Cl2 in the ground state in contrast to that in acetone.
Alkyl chains with different length or steric bulk are often introduced into conventional fluorophores through covalent organic synthesis to construct and control pi-pi stacking-driven molecular gels and aggregates, and these fluorophores always emit tunable emission due to their difference in aggregation ability.6, 14 Herein, modulation of aggregation behavior is achieved by regulating the sizes of the pyrene-based metallacycles via selection of the dicarboxylate building blocks. Metallacycles 1 and 3 might possess different aggregation compared to 2, and then might also exhibit different fluorescence emission behaviors. The concentration-dependent fluorescence emission of 1 and 3 in acetone were examined. (Figures S27, S28) The fluorescence of 1 and 3 is almost identical to those of 2 recorded in the same solvent at low concentrations (<1.0 × 10−5 mol/L), due to the monomeric emission, as a consequence of the similarities of the structures of the three metallacycles. However, their emission in the aggregated states is different from that of 2 under the same conditions. Figure 4 shows the plots of the longest wavelengths of the emission of each compound verus the concentration. The longest wavelengths emission of the three metallacycles shift sharply at concentrations from 1.0 × 10−5 mol/L to 2.0 × 10−4 mol/L, but are essentially the same at higher concentrations. The emisson maximum of 1, 2 and 3 appeared at ~560 nm, ~543 nm and ~530 nm, respectively. The distinct fluorescence color of the three metallacycles at low concentration (1.0 × 10−6 mol/L) and high concentation (6.0 × 10−4 mol/L) under UV light is displayed in the insert of Figure 4. Metallacycles with larger size display emission maxima at shorter wavelength in the aggregated state compared to the smaller metallacycle, implying that the metallacycles have different aggregation abitlities, probably due to differences in free volume fractions in the aggregated state as the molecular mobility is largely dependent on this value.67
Figure 4.
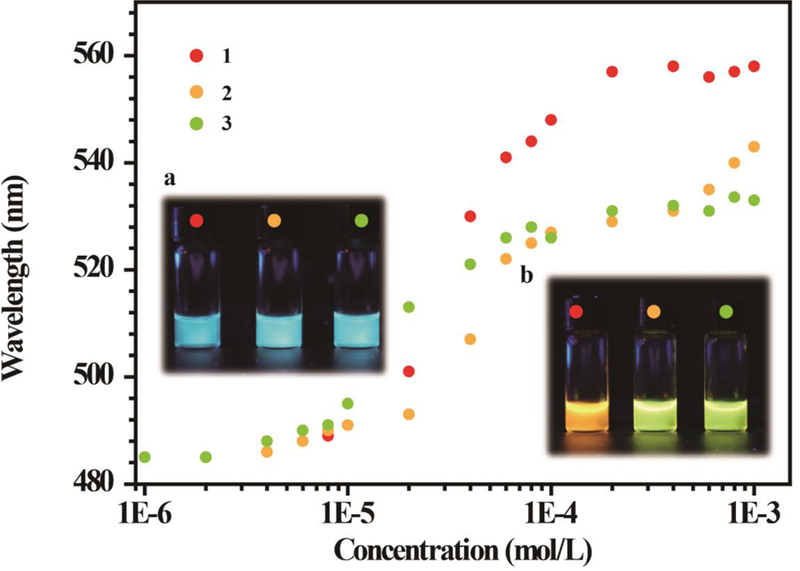
Plots of the maximum emission wavelengths of the acetone solutions of the three metallacycles 1, 2 and 3 as functions of concentration, where the excitation wavelength employed is 400 nm. The insets a and b are the fluorescent pictures of the solutions with a concentration of 1.0 × 10−6 mol/L and 6.0 × 10−4 mol/L, respectively, taken at room temperature and under UV light (365 nm).
Photochemical stability is one of the key features determining the performance of a film-based fluorescent sensor, and therefore the photochemical stabilities of the metallacycle based films, with 2 as an example, were examined before their sensing performance was studied. The fluorescence intensity of the film decreased only ~5% after 9 hour’s continuous light irritation (Figure S29). In contrast, the emission of the pyrene-based film reaches almost zero due to complete photo-bleaching as reported earlier, 68 suggesting that the metallacycle is photochemically suitable for sensing.
Substrate is another crucial factor for high performance film sensing.51 To our knowledge, for volatile small organic molecules (VSOMs), silica-gel plate is preferred as its surface is rough and it possesses high porosity, which are favorable for accumulation of analytes from the air, and allow mass transfer within the film adlayer: both are pre-requirements for superior sensing. To further explore the potential of the metallacycle for sensing, the fluorescence quantum yield (QY) of 2 and that of 4 in solution, powder, and the substrate supported states were determined respectively. The results are listed in Table S1. The QY of 2 is lower than that of 4 both in solution and in the powder state, a result partially attributable to the 90° Pt(II) induced fluorescence quenching as previously reported.28 However, in the substrate supported state, the QY of 2 exceeds that of 4, a property favorable for sensing. This is likely due to the specific structure of the metallacycle that screens the dense packing of the compound on the coarse surface of the substrate as the maximum emission of the 4-based film appears at 620 nm, but that of the 2-based film at 570 nm (Figure 6a).
Figure 6.
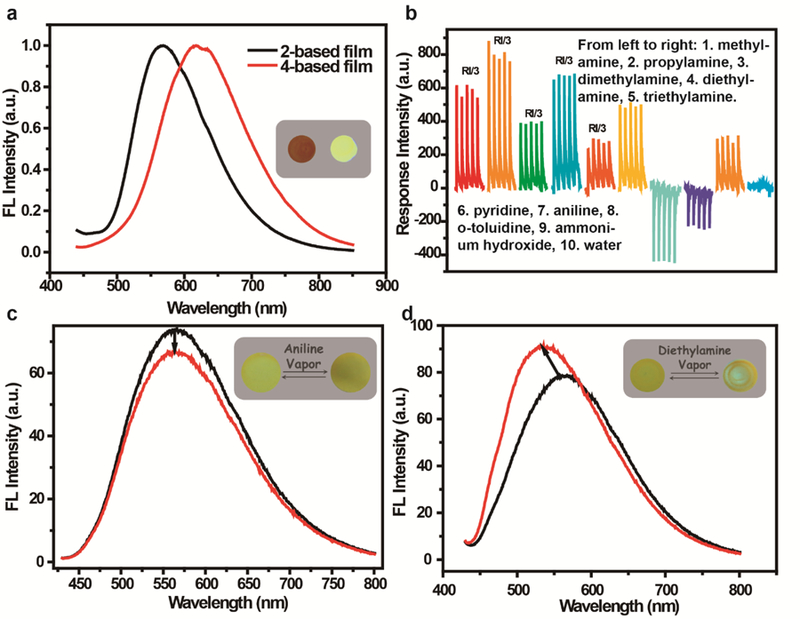
(a) Fluorescence spectra of metallacycle 2 and ligand 4-based, silica-gel plate supported films, where the inset is a picture of ligand 4 (left) and metallacycle 2 (right) deposited on a silica plate surface taken under UV light (365 nm); (b) Response traces of the metallacycle 2-based film to the presence of saturated vapors of the VSOMs under study and water; (c) Fluorescence emission spectra of metallacycle 2-based film with and without aniline vapor, where the inset is a picture of the film taken before and after treatment with aniline vapor under UV light (365 nm); (d) Fluorescence emission spectra of the film before and after treatment with Et2NH vapor, where the inset is a picture of the metallacycle 2-based film taken before and after treatment with Et2NH vapor under UV light (365 nm). Note: Presence of green spots in the inserted picture (d) is a result of fast response and fast recovery with treatment of Et2NH vapor.
The sensing performance of the fluorescent film was examined on a specially fabricated sensing platform, which affords not only steady state information of the sensing but also time-dependent information. The nature of the home-made sensing platform is shown in Figure 5a: made up of a gas injector, a film device, an air pump, and a signal processing unit. More specifically, the film-based device, as the core structure of the sensing platform, consists of a light source, a sensing chamber, a light sensitive element, and an electronic circuit, as schematically depicted in Figure 5b.
Figure 5.
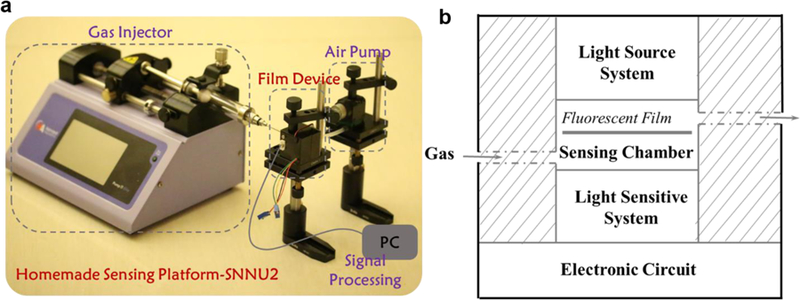
(a) A photo of the homemade sensing platform; (b) Schematic description of the relevant film devices.
The sensing performances of the film-based device for representative amines of different structures were systematically investigated, and shown in Figure 6b. The samples used are the saturated vapors of the analytes, and each sample was tested five times to ensure the quality of the measurements. The film shows different responses to the sample vapors, where aromatic amines, including aniline and o-toluidine, function as quenchers of the fluorescence emission of the film. The other amides enhance the emission of the film. The fluorescence emission spectrum of the film was recorded before and after treatment with the analytes. Figure 6c and Figure 6d depict the results obtained with aniline and Et2NH as representative analytes. For aromatic amine, the change observed is just the intensity of the emission, with no observable profile variation. For Et2NH, however, the change observed is both a blue shift and an intensity increase. Since the position of the maximum emission of each of the synthesized metallacycles is aggregation dependent, the observed blue shift and intensity increase could be a result of dis-aggregation of the metallacycle deposited on the substrate surface. For the fluorescence quenching, there may be no detectable structure change during the process of aromatic amine sensing. Water vapor shows little effect upon the film’s emission, suggesting the suitability of the film in an environment of high humidity.
As a control, the sensing performance of ligand 4-based film was also investigated, and the results are shown in Figure S30. This film shows similar responses to the analytes tested, indicating that the sensing performance of the 2-based film may be due to the ligand. However, further inspection of the response traces demonstrates that this film recovers much slower than that of the metallacycle-based film. Response speed and recovery time are two key parameters for practical sensors.50, 51 To have a quantitative comparison of the sensing performances of the two films, Et2NH was employed as a representative analyte. The response kinetics of the two films was determined and the results are shown in Figures 7a and S31. As seen, the response trace of the 2-based film is characterized by a sharp response with injection of the sample vapor, and faster recovery upon stopping the injection. In contrast, the trace of the ligand-based film shows a much slower response speed and a longer recovery time. Specifically, the response and recovery times of the two films are 3.8 s and 11.5 min, and 1.6 min and 88.3 min, respectively, indicating that the metalla-cyclization significantly enhances the sensing performance of the ligand, 4, in the film state likely due to less aggregation and molecular channel formation (Figure S31).
Figure 7.
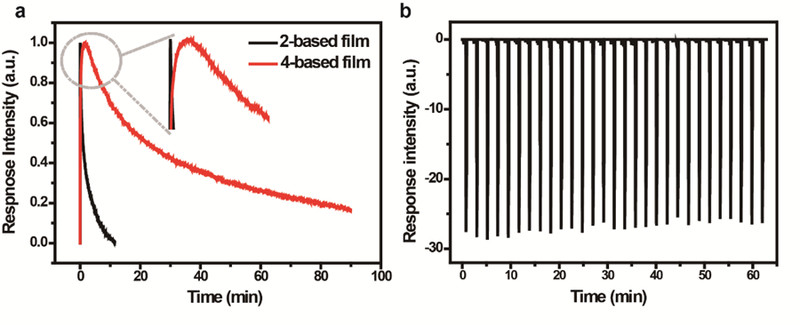
(a) Response traces of the metallacycle 2 and ligand 4-based film devices to the presence of saturated Et2NH vapor; (b) Response cycles of metallacycle 2-based film device to the presence of saturated aniline vapor (30 cycles).
To further investigate the performance of the metallacycle-based film device, sensing reversibility was also examined and the result is presented in Figure 7b. As seen 30 cycles showed no observable degradation in sensing when aniline was employed as an analyte, further indicating that the metallacycle-based film has the potential to be developed into an aromatic amine sensor.
CONCLUSION
In summary, three pyrene-based metallacycles (1, 2, 3) were synthesized via a coordination-driven self-assembly of a 90° organoplatinum(II) complex with a dipyridinyl derivative of pyrene (4) and three different dicarboxylates as ligands. Studies of 2 reveal that (1) it exhibits much improved solubility in organic solvents such as acetone and CH2Cl2 compared with that of the fluorescent ligand, 4, (2) rectangle 2 emits strongly both in solution and in the solid state, and the solution emission is highly concentration- and temperature-dependent with the colors ranging from blue to green and then to yellow in acetone solution, and (3) pi-pi stacking between the enlarged pyrenyl moieties of the different metallacycles is the likely driving force for the inter-metallacycle aggregation. All metallacycles exhibited similar properties in the solution state, while the emission wavelength in the aggregated states varies even though they possess the same fluorophore. Metalla-cyclization screens the dense packing of ligand 4 when deposited on a silica surface, allowing the film to be developed into a fluorescent aromatic amine sensor as evidenced by the superior performance of the metallacycle 2-based, silica supported film device.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Natural Science Foundation of China (21527802, 21673133, 21820102005), 111 project (B14041), Program for Changjiang Scholars and Innovative Research Team in University (IRT-14R33), Research Funds of Shaanxi Normal University (X2014YB04) and Program of China Scholarships Council (201706870032). X.C. thanks Miss Xiaohua (Sophie) Fang for her linguistic assistance during the preparation of this manuscript. X.L. is thankful for financial support from the U.S. National Science Foundation (CHE-1506722) and National Institutes of Health (1R01GM128037-01). P.J.S. thanks the NIH (Grant R01-CA215157) for financial support.
Footnotes
Notes
The authors declare no competing financial interest.
Associated content
The Supporting Information is available free of charge on the ACS Publications website at XXXX Experimental details and additional data (PDF)
REFERENCES
- (1).Ding L; Fang Y Chemically assembled monolayers of fluorophores as chemical sensing materials. Chem. Soc. Rev 2010, 39, 4258–4273. [DOI] [PubMed] [Google Scholar]
- (2).Maggini L; Bonifazi D Hierarchised luminescent organic architectures: design, synthesis, self-assembly, self-organisation and functions. Chem. Soc. Rev 2012, 41, 211–241. [DOI] [PubMed] [Google Scholar]
- (3).Stender AS; Marchuk K; Liu C; Sander S; Meyer MW; Smith EA; Neupane B; Wang G; Li J; Cheng J-X; Huang B; Fang N Single cell optical imaging and spectroscopy. Chem. Rev 2013, 113, 2469–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Zhu M; Yang C Blue fluorescent emitters: design tactics and applications in organic light-emitting diodes. Chem. Soc. Rev 2013, 42, 4963–4976. [DOI] [PubMed] [Google Scholar]
- (5).Figueira-Duarte TM; Müllen K Pyrene-based materials for organic electronics. Chem. Rev 2011, 111, 7260–7314. [DOI] [PubMed] [Google Scholar]
- (6).Babu SS; Praveen VK; Ajayaghosh A Functional π-gelators and their applications. Chem. Rev 2014, 114, 1973–2129. [DOI] [PubMed] [Google Scholar]
- (7).Yang Z; Cao J; He Y; Yang JH; Kim T; Peng X; Kim JS Macro-/micro-environment-sensitive chemosensing and biological imaging. Chem. Soc. Rev 2014, 43, 4563–4601. [DOI] [PubMed] [Google Scholar]
- (8).Stępień M; Gońka E; Żyła M; Sprutta N Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: synthetic routes, properties, and applications. Chem. Rev 2017, 117, 3479–3716. [DOI] [PubMed] [Google Scholar]
- (9).Birks JB Photophysics of aromatic molecules; Wiley: London, 1970. [Google Scholar]
- (10).Hong Y; Lam JWY; Tang BZ Aggregation-induced emission. Chem. Soc. Rev 2011, 40, 5361–5388. [DOI] [PubMed] [Google Scholar]
- (11).Mei J; Leung NLC; Kwok RTK; Lam JWY; Tang BZ Aggregation-induced emission: Together we shine, united we soar! Chem. Rev 2015, 115, 11718–11940. [DOI] [PubMed] [Google Scholar]
- (12).Chen S; Slattum P; Wang C; Zang L Self-assembly of perylene imide molecules into 1D nanostructures: methods, morphologies, and applications. Chem. Rev 2015, 115, 11967–11998. [DOI] [PubMed] [Google Scholar]
- (13).Würthner F; Saha-Möller CR; Fimmel B; Ogi S; Leowanawat P; Schmidt D Perylene bisimide dye assemblies as archetype functional supramolecular materials. Chem. Rev 2016, 116, 962–1052. [DOI] [PubMed] [Google Scholar]
- (14).Kobaisi MA; Bhosale SV; Latham K; Raynor AM; Bhosale SV Functional naphthalene diimides: synthesis, properties, and applications. Chem. Rev 2016, 116, 11685–11796. [DOI] [PubMed] [Google Scholar]
- (15).Miao R; Peng J; Fang Y Recent advances in fluorescent film sensing from the perspective of both molecular design and film engineering. Mol. Syst. Des. Eng 2016, 1, 242–257. [Google Scholar]
- (16).Lehn J-M Supramolecular chemistry: concepts and perspectives; VCH: Weinheim, Germany, 1995. [Google Scholar]
- (17).Leininger S; Olenyuk B; Stang PJ Self-assembly of discrete cyclic nanostructures mediated by transition metals. Chem. Rev 2000, 100, 853–908. [DOI] [PubMed] [Google Scholar]
- (18).Fujita M; Tominaga M; Hori A; Therrien B Coordination assemblies from a Pd(II)-cornered square complex. Acc. Chem. Res 2005, 38, 369–378. [DOI] [PubMed] [Google Scholar]
- (19).De S; Mahata K; Schmittel M Metal-coordination-driven dynamic heteroleptic architectures. Chem. Soc. Rev 2010, 39, 1555–1575. [DOI] [PubMed] [Google Scholar]
- (20).Lippert B; Sanz Miguel PJ Metallatriangles and metallasquares: the diversity behind structurally characterized examples and the crucial role of ligand symmetry. Chem. Soc. Rev 2011, 40, 4475–4487. [DOI] [PubMed] [Google Scholar]
- (21).Han M; Engelhard DM; Clever GH Self-assembled coordination cages based on banana-shaped ligands. Chem. Soc. Rev 2014, 43, 1848–1860. [DOI] [PubMed] [Google Scholar]
- (22).Mukherjee S; Mukherjee PS Template-free multicomponent coordination-driven self-assembly of Pd(II)/Pt(II) molecular cages. Chem. Commun 2014, 50, 2239–2248. [DOI] [PubMed] [Google Scholar]
- (23).Newkome GR; Moorefield CN From 1→3 dendritic designs to fractal supramacromolecular constructs: understanding the pathway to the Sierpiński gasket. Chem. Soc. Rev 2015, 44, 3954–3967. [DOI] [PubMed] [Google Scholar]
- (24).Brown CJ; Toste FD; Bergman RG; Raymond KN Supramolecular catalysis in metal-ligand cluster hosts. Chem. Rev 2015, 115, 3012–3035. [DOI] [PubMed] [Google Scholar]
- (25).Cook TR; Stang PJ Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev 2015, 115, 7001–7045. [DOI] [PubMed] [Google Scholar]
- (26).McConnell AJ; Wood CS; Neelakandan PP; Nitschke JR Stimuli-Responsive Metal-Ligand Assemblies. Chem. Rev 2015, 115, 7729–7793. [DOI] [PubMed] [Google Scholar]
- (27).Yam VW-W; Au VK-M; Leung SY-L Light-emitting self-assembled materials based on d8 and d10 transition metal complexes. Chem. Rev 2015, 115, 7589–7728. [DOI] [PubMed] [Google Scholar]
- (28).Saha ML; Yan X; Stang PJ Photophysical properties of organoplatinum(II) compounds and derived self-assembled metallacycles and metallacages: fluorescence and its applications. Acc. Chem. Res 2016, 49, 2527–2539. [DOI] [PubMed] [Google Scholar]
- (29).Fermi A; Bergamini G; Roy M; Gingras M; Ceroni P Turn-on phosphorescence by metal coordination to a multivalent terpyridine ligand: a new paradigm for luminescent sensors. J. Am. Chem. Soc 2014, 136, 6395–6400. [DOI] [PubMed] [Google Scholar]
- (30).Schouwey C; Holstein JJ; Scopelliti R; Zhurov KO; Nagornov KO; Tsybin YO; Smart OS; Bricogne G; Severin K Self-assembly of a giant molecular solomon link from 30 subcomponents. Angew. Chem. Int. Ed 2014, 53, 11261–11265. [DOI] [PubMed] [Google Scholar]
- (31).Wei P; Cook TR; Yan X; Huang F; Stang PJ A discrete amphiphilic organoplatinum(Ⅱ) metallacycle with tunable lower critical solution temperature behavior. J. Am. Chem. Soc 2014, 136, 15497–15500. [DOI] [PubMed] [Google Scholar]
- (32).Zhou Z; Yan X; Saha ML; Zhang M; Wang M; Li X; Stang PJ Immobilizing tetraphenylethylene into fused metallacycles: shape effects on fluorescence emission. J. Am. Chem. Soc 2016, 138, 13131–13134. [DOI] [PubMed] [Google Scholar]
- (33).Rickhaus M; Jentzsch AV; Tejerina L; Grüebner I; Jirasek M; Claridge TDW; Anderson HL Single-acetylene linked porphyrin nanorings. J. Am. Chem. Soc 2017, 139, 16502–16505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Yazaki K; Akita M; Prusty S; Chand DK; Kikuchi T; Sato H; Yoshizawa M Polyaromatic molecular peanuts. Nature Commun 2017, 8, 15914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Chen M; Wang J; Liu D; Jiang Z; Liu Q; Wu T; Liu H; Yu W; Yan J; Wang P Highly stable spherical metallo-capsule from a branched hexapodal terpyridine and its self-assembled berry-type nanostructure. J. Am. Chem. Soc 2018, 140, 2555–2561. [DOI] [PubMed] [Google Scholar]
- (36).Wang H; Qian X; Wang K; Su M; Haoyang W-W; Jiang X; Brzozowski R; Wang M; Gao X; Li Y; Xu B; Eswara P; Hao X-Q; Gong W; Hou J-L; Cai J; Li X Supramolecular Kandinsky circles with high antibacterial activity. Nature Commun 2018, 9, 1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kim DH; Singh N; Oh J; Kim E-H; Jung J; Kim H; Chi K-W Coordination-driven self-assembly of a molecular knot comprising sixteen crossings. Angew. Chem. Int. Ed 2018, 57, 5669–5673. [DOI] [PubMed] [Google Scholar]
- (38).Zhou J; Zhang Y; Yu G; Crawley MR; Fulong CRP; Friedman AE; Sengupta S; Sun J; Li Q; Huang F; Cook TR Highly emissive self-assembled BODIPY-platinum supramolecular triangles. J. Am. Chem. Soc 2018, 140, 7730–7736. [DOI] [PubMed] [Google Scholar]
- (39).Ronson TK; League AB; Gagliardi L; Cramer CJ; Nitschke JR Pyrene-edged FeII4L6 cages adaptively reconfigure during guest binding. J. Am. Chem. Soc 2014, 136, 15615–15624. [DOI] [PubMed] [Google Scholar]
- (40).Yamashina M; Sartin MM; Sei Y; Akita M; Takeuchi S; Tahara T; Yoshizawa M Preparation of highly fluorescent host–guest complexes with tunable color upon encapsulation. J. Am. Chem. Soc 2015, 137, 9266–9269. [DOI] [PubMed] [Google Scholar]
- (41).Zheng Y-R; Suntharalingam K; Johnstone TC; Lippard SJ Encapsulation of Pt(IV) prodrugs within a Pt(II) cage for drug delivery. Chem. Sci 2015, 6, 1189–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Roy B; Ghosh AK; Srivastava S; D’Silva P; Mukherjee PS A Pd8 tetrafacial molecular barrel as carrier for water insoluble fluorophore. J. Am. Chem. Soc 2015, 137, 11916–11919. [DOI] [PubMed] [Google Scholar]
- (43).Leenders SHAM; Becker R; Kumpulainen T; de Bruin B; Sawada T; Kato T; Fujita M; Reek JNH Selective co-encapsulation inside an M6L4 cage. Chem. Eur. -J 2016, 22, 15468–15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Zhang W-Y; Lin Y-J; Han Y-F; Jin G-X Facile separation of regioisomeric compounds by a heteronuclear organometallic capsule. J. Am. Chem. Soc 2016, 138, 10700–10707. [DOI] [PubMed] [Google Scholar]
- (45).Preston D; Lewis JEM; Crowley JD Multicavity [PdnL4]2n+ cages with controlled segregated binding of different guests. J. Am. Chem. Soc 2017, 139, 2379–2386. [DOI] [PubMed] [Google Scholar]
- (46).Cai L-X; Li S-C; Yan D-N; Zhou L-P; Guo F; Sun Q-F Water-soluble redox-active cage hosting polyoxometalates for selective desulfurization catalysis. J. Am. Chem. Soc 2018, 140, 4869–4876. [DOI] [PubMed] [Google Scholar]
- (47).Jiao J; Tan C; Li Z; Liu Y; Han X; Cui Y Design and assembly of chiral coordination cages for asymmetric sequential reactions. J. Am. Chem. Soc 2018, 140, 2251–2259. [DOI] [PubMed] [Google Scholar]
- (48).Marti-Centelles V; Lawrence AL; Lusby PJ High activity and efficient turnover by a simple, self-assembled “artificial Diels–Alderase”. J. Am. Chem. Soc 2018, 140, 2862–2868. [DOI] [PubMed] [Google Scholar]
- (49).Takashima Y; Martínez VM; Furukawa S; Kondo M; Shimomura S; Uehara H; Nakahama M; Sugimoto K; Kitagawa S Molecular decoding using luminescence from an entangled porous framework. Nature Commun 2011, 2, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Liu K; Shang C; Wang Z; Qi Y; Miao R; Liu K; Liu T; Fang Y Non-contact identification and differentiation of illicit drugs using fluorescent films. Nature Commun 2018, 9, 1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Qi Y; Xu W; Kang R; Ding N; Wang Y; He G; Fang Y Discrimination of saturated alkanes and relevant volatile compounds via the utilization of a conceptual fluorescent sensor array based on organoboron-containing polymers. Chem. Sci 2018, 9, 1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Birks JB Excimers and exciplexes. Nature 1967, 214, 1187–1190. [Google Scholar]
- (53).Winnik FM Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media. Chem. Rev 1993, 93, 587–614. [Google Scholar]
- (54).Lakowicz JR Principles of fluorescence spectroscopy, 3rd edition, Springer Science & Business Media: New York, 2006. [Google Scholar]
- (55).Zhai D; Xu W; Zhang L; Chang Y-T The role of “disaggregation” in optical probe development. Chem. Soc. Rev 2014, 43, 2402–2411. [DOI] [PubMed] [Google Scholar]
- (56).Chang X; Wang Z; Qi Y; Kang R; Cui X; Shang C; Liu K; Fang Y Dynamic chemistry-based sensing: a molecular system for detection of saccharide, formaldehyde, and the silver ion. Anal. Chem 2017, 89, 9360–9367. [DOI] [PubMed] [Google Scholar]
- (57).Mateo-Alonso A Pyrene-fused pyrazaacenes: from small molecules to nanoribbons. Chem. Soc. Rev 2014, 43, 6311–6324. [DOI] [PubMed] [Google Scholar]
- (58).Kohl B; Rominger F; Mastalerz M A Pyrene-fused N-heteroacene with eleven rectilinearly annulated aromatic rings. Angew. Chem. Int. Ed 2015, 54, 6051–6056. [DOI] [PubMed] [Google Scholar]
- (59).Zheng Y-R; Zhao Z; Wang M; Ghosh K; Pollock JB; Cook TR; Stang PJ A facile approach toward multicomponent supramolecular structures: selective self-assembly via charge separation. J. Am. Chem. Soc 2010, 132, 16873–16882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Li S; Huang J; Cook TR; Pollock JB; Kim H; Chi K-W; Stang PJ Formation of [3]catenanes from 10 precursors via multicomponent coordination-driven self-assembly of metallarectangles. J. Am. Chem. Soc 2013, 135, 2084–2087. [DOI] [PubMed] [Google Scholar]
- (61).Shi Y; Sánchez-Molina I; Cao C; Cook TR; Stang PJ Synthesis and photophysical studies of self-assembled multicomponent supramolecular coordination prisms bearing porphyrin faces. Proc. Natl. Acad. Sci. U S A 2014, 111, 9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Yan X; Cook TR; Wang P; Huang F; Stang PJ Highly emissive platinum(II) metallacages. Nature Chem 2015, 7, 342–348. [DOI] [PubMed] [Google Scholar]
- (63).Yu G; Cook TR; Li Y; Yan X; Wu D; Li S; Shen J; Tang G; Huang F; Chen X; Stang PJ Tetraphenylethene-based highly emissive metallacage as a component of theranostic supramolecular nanoparticles. Proc. Natl. Acad. Sci. U S A 2016, 113, 13720–13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Li Z; Yan X; Huang F; Sepehrpour H; Stang PJ Near-infrared emissive discrete platinum(II) metallacycles: synthesis and application in ammonia detection. Org. Lett 2017, 19, 5728–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Zhang M; Saha ML; Wang M; Zhou Z; Song B; Lu C; Yan X; Li X; Huang F; Yin S; Stang PJ Multicomponent platinum(II) cages with tunable emission and amino acid sensing. J. Am. Chem. Soc 2017, 139, 5067–5074. [DOI] [PubMed] [Google Scholar]
- (66).Yan N; Xu Z; Diehn KK; Raghavan SR; Fang Y; Weiss RG How do liquid mixtures solubilize insoluble gelators? Self-assembly properties of pyrenyl-linker-glucono gelators in tetrahydrofuran–water mixtures. J. Am. Chem. Soc 2013, 135, 8989–8999. [DOI] [PubMed] [Google Scholar]
- (67).Mao Z; Yang Z; Fan Z; Ubba E; Li W; Li Y; Zhao J; Yang Z; Aldreda MP; Chi Z The methylation effect in prolonging the pure organic room temperature phosphorescence lifetime. Chem. Sci 2019, 10, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Chang X; Wang G; Yu C; Wang Y; He M; Fan J; Fang Y Studies on the photochemical stabilities of some fluorescent films based on pyrene and pyrenyl derivatives. J. Photochem. Photobio. A 2015, 298, 9–16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


