Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease of unknown origin characterized by progressive scarring of lung parenchyma due to aberrant activation of stromal cells producing excessive amounts of extracellular matrix. Growing evidence suggests that injury to the alveolar epithelium may represent a primary event that initiates and promotes fibrosis in IPF (1). In advanced disease, alveolar derangement is often accompanied by bronchiolization, with ectopic emergence of mucociliary epithelium in the dilated alveolar spaces, known as “honeycomb cysts” (2). Specific mechanisms underlying these structural changes are unknown, and no currently available therapies halt disease progression. The prognosis for patients with IPF remains poor, with a median survival of 2–4 years after diagnosis, comparable to aggressive types of cancer (3). Whereas some progress has been made in identifying systemic biomarkers of IPF (4), no biomarkers reflecting potentially targetable mechanistic aspects of alveolar derangement in IPF that correlate with disease aggressiveness have so far been described.
In this issue of the Journal, Prasse and colleagues (pp. 622–630) address this problem by transcriptional profiling of samples obtained by BAL of 212 patients with IPF from three independent cohorts (Freiburg, Siena, and Leuven) and correlating the obtained gene expression data with patients’ survival (5). BAL is a minimally invasive procedure that allows access to cells from the most distal regions of the respiratory tree, including the alveoli, the epicenter of IPF pathology. Because predominant cell types obtained by BAL are macrophages (normally >80% of BAL cells) followed by lymphocytes and other leukocytes, BAL has largely been used to evaluate the immune microenvironment in IPF lungs (6). In contrast to this approach, Prasse and colleagues (5) hypothesized that BAL samples from patients with IPF may harbor prognostically relevant information about structural changes in the alveoli, potentially concealed in the cumulative gene expression reflecting the status of diverse, including nonimmune, cells recovered by BAL.
By following this logic, the authors first identified 1,582 genes whose expression in BAL samples from patients with IPF correlated with shorter survival. Using biostatistical methods, this gene set was reduced to fewer than 10 genes, which, when combined with the Gender-Age-Physiology (GAP) index, known to predict IPF mortality on the basis of basic patient characteristics (7), estimated poor IPF outcomes more robustly than the GAP index alone. Notably, one-tenth of identified 1,582 IPF mortality-related genes were previously found upregulated in airway basal cells (ABCs) cultured from human airway epithelial samples obtained by bronchoscopy (8). Accordingly, cells expressing KRT5 (keratin 5), an ABC marker, and “related” protein KRT6 were detected in BAL samples from patients with IPF, but not healthy individuals, or patients with sarcoidosis or chronic obstructive pulmonary disease. Thus, the presence of ABCs or ABC-like cells in BAL samples may represent a novel, prognostically relevant feature of IPF.
ABCs are stem cells of the airway epithelium, normally absent in the alveolar region that is maintained by its local progenitors, that is, type II alveolar epithelial cells, which in IPF become the primary target of injury (1). In earlier studies, cells having ABC features have been found in the remodeled alveolar epithelium of IPF lungs (9, 10). Aberrant reepithelialization of the damaged alveolar epithelium by epithelial progenitors mobilized from adjacent bronchioles may lead to bronchiolization (9, 11), a characteristic feature of alveolar remodeling in IPF. In mice, KRT5/6-expressing ABC-like cells emerge in the lung parenchyma after severe injury caused by influenza virus infection (12), and their long-term persistence results in cysts with histologic features of bronchiolization (13), resembling IPF honeycomb lesions. Because the latter are most frequently found in patients with late-stage IPF, it is logical that the emergence of ABC-like cells in BAL samples, if it reflects alveolar bronchiolization driven by these cells as a stereotypic response to severe alveolar injury (14), correlates with shorter survival of patients.
The fact that an ABC signature can be detected in BAL samples from subjects with IPF with poorer prognosis implies a possibility that mobilization of these cells to sites of alveolar injury, if the above theory is correct, demarcates the transition to a more aggressive disease. Recruitment of ABCs from the bronchioles to injured alveoli and capturing these cells by BAL would require disassembly of hemidesmosomes, which normally keep ABCs firmly attached to the basement membrane (9). A similar process occurs in skin basal cells, when they migrate to cover the injured epidermis during the wound-healing process (15). Whereas in the skin such migration occurs within the same tissue compartment and leads to physiological repair, alveolar “colonization” by ABCs would lead to an aberrant, airway-like regeneration (11), making the resulting “alveolar” epithelium incapable of performing its respiratory function.
It should be noted, however, that many genes in the ABC signature detected by Prasse and colleagues (5) in the IPF BAL samples are not bona fide ABC markers; rather, they are molecular features of squamous metaplasia, an injury-associated histologic pattern, which can be produced by ABCs (16). Examples include calcium-binding protein S100A14, KRT6, stratifin, and neuregulin (16–18). The latter gene has been found earlier to be expressed in squamous cells in the remodeled alveolar epithelium in IPF (18). ABC-derived squamous metaplasia can promote a fibrotic response in subjacent fibroblasts (16), potentially relevant to IPF pathogenesis. Both disassembly of hemidesmosomes required for basal cell migration and squamous metaplasia are dependent on epidermal growth factor receptor signaling (15, 19), which could represent a candidate pathway of ABC-mediated alveolar remodeling in IPF.
Thus, the ABC signature in the IPF BAL samples observed by Prasse and colleagues (5) may represent an “echo” of regenerative crisis in the diseased lung, potentially mediated by ABCs or ABC-like cells mobilized in response to alveolar injury (Figure 1). Further studies, including those employing evaluation of BAL samples at single-cell resolution, as performed for IPF lung tissues (20), are needed to identify the cellular origin and targetable pathways of alveolar remodeling in IPF. The advantage of BAL as a discovery tool is that evaluation of cells sampled by this method from the primary site of IPF pathology can be performed within the lifetime of patients, so that the knowledge about patient-specific disease pathways learned using this approach can be translated into effective personalized therapies.
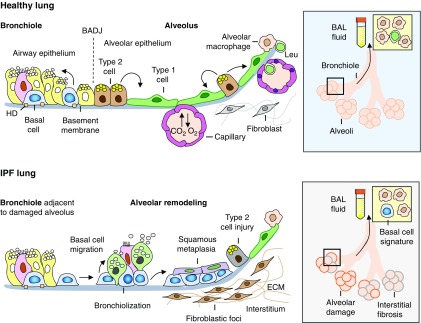
Figure 1.
The normal bronchoalveolar region of the human lung (top left) is composed of terminal bronchioles lined by the distal airway epithelium, which continue into the respiratory alveolar region lined by type 1 and type 2 alveolar epithelial cells. The bronchoalveolar duct junction (BADJ) demarcates a boundary between the most distal conducting airways and alveoli. The airway epithelium is maintained by basal stem cells, normally attached to the basement membrane via hemidesmosome (HD) integrins. The alveolar epithelium is maintained by type 2 alveolar epithelial cells capable of self-renewal and generating type 1 cells. The latter form a gas exchange unit with the pulmonary capillaries in the alveolar interstitium. Alveolar macrophages are the resident immune cells located on the alveolar surface. BAL enables the sampling of cells from the bronchoalveolar region (top right). Under normal conditions, the major components of the BAL fluid are alveolar macrophages followed by other leukocytes (Leu). In idiopathic pulmonary fibrosis (IPF), the alveolar epithelium becomes damaged (bottom left) with preferential injury to and loss of type 2 cells and acquisition of remodeling phenotypes, including bronchiolization, that is, emergence of pseudostratified mucociliary epithelium, and squamous metaplasia, paralleled by fibrotic changes in the alveolar interstitium. It is possible that basal cells from adjacent bronchioles can migrate to areas of alveolar injury, colonize the damaged alveolar epithelium in IPF, and contribute to disease progression by generating some of these lesions. Consistent with this theory, Prasse and colleagues (5) have found that in patients with IPF with particularly short survival, the BAL fluid contains basal-like cells (bottom right), and upregulation of the gene expression program of these cells in BAL samples is indicative of IPF mortality. Thus, the emergence of airway basal or basal-like cells in the alveolar region in IPF may represent a biomarker of regenerative crisis in the lung that determines disease aggressiveness. ECM = extracellular matrix.
Supplementary Material
Footnotes
Supported by NIH grants R01HL123544 and R01HL127393.
Originally Published in Press as DOI: 10.1164/rccm.201808-1557ED on September 5, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Zoz DF, Lawson WE, Blackwell TS. Idiopathic pulmonary fibrosis: a disorder of epithelial cell dysfunction. Am J Med Sci. 2011;341:435–438. doi: 10.1097/MAJ.0b013e31821a9d8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seibold MA, Smith RW, Urbanek C, Groshong SD, Cosgrove GP, Brown KK, et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucociliary pseudostratified epithelium. PLoS One. 2013;8:e58658. doi: 10.1371/journal.pone.0058658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010;35:496–504. doi: 10.1183/09031936.00077309. [DOI] [PubMed] [Google Scholar]
- 4.White ES, Xia M, Murray S, Dyal R, Flaherty CM, Flaherty KR, et al. Plasma surfactant protein-D, matrix metalloproteinase-7, and osteopontin index distinguishes idiopathic pulmonary fibrosis from other idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2016;194:1242–1251. doi: 10.1164/rccm.201505-0862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasse A, Binder H, Schupp JC, Kayser G, Bargagli E, Jaeger B, et al. BAL cell gene expression is indicative of outcome and airway basal cell involvement in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199:622–630. doi: 10.1164/rccm.201712-2551OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pesci A, Ricchiuti E, Ruggiero R, De Micheli A. Bronchoalveolar lavage in idiopathic pulmonary fibrosis: what does it tell us? Respir Med. 2010;104:S70–S73. doi: 10.1016/j.rmed.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Hackett NR, Shaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, et al. The human airway epithelial basal cell transcriptome. PLoS One. 2011;6:e18378. doi: 10.1371/journal.pone.0018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawanami O, Ferrans VJ, Crystal RG. Structure of alveolar epithelial cells in patients with fibrotic lung disorders. Lab Invest. 1982;46:39–53. [PubMed] [Google Scholar]
- 10.Chilosi M, Poletti V, Murer B, Lestani M, Cancellieri A, Montagna L, et al. Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of ΔN-p63. Lab Invest. 2002;82:1335–1345. doi: 10.1097/01.lab.0000032380.82232.67. [DOI] [PubMed] [Google Scholar]
- 11.Nettesheim P, Szakal AK. Morphogenesis of alveolar bronchiolization. Lab Invest. 1972;26:210–219. [PubMed] [Google Scholar]
- 12.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanegai CM, Xi Y, Donne ML, Gotts JE, Driver IH, Amidzic G, et al. Persistent pathology in influenza-infected mouse lungs. Am J Respir Cell Mol Biol. 2016;55:613–615. doi: 10.1165/rcmb.2015-0387LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor MS, Chivukula RR, Myers LC, Jeck WR, Waghray A, Tata PR, et al. A conserved distal lung regenerative pathway in acute lung injury. Am J Pathol. 2018;188:1149–1160. doi: 10.1016/j.ajpath.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margadant C, Frijns E, Wilhelmsen K, Sonnenberg A. Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol. 2008;20:589–596. doi: 10.1016/j.ceb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, et al. Squamous metaplasia amplifies pathologic epithelial–mesenchymal interactions in COPD patients. J Clin Invest. 2007;117:3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, Ma J, Sunkel B, Luo A, Ding F, Li Y, et al. S100A14: novel modulator of terminal differentiation in esophageal cancer. Mol Cancer Res. 2013;11:1542–1553. doi: 10.1158/1541-7786.MCR-13-0317. [DOI] [PubMed] [Google Scholar]
- 18.Plantier L, Crestani B, Wert SE, Dehoux M, Zweytick B, Guenther A, et al. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax. 2011;66:651–657. doi: 10.1136/thx.2010.151555. [DOI] [PubMed] [Google Scholar]
- 19.Shaykhiev R, Zuo W-L, Chao I, Fukui T, Witover B, Brekman A, et al. EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype. Proc Natl Acad Sci USA. 2013;110:12102–12107. doi: 10.1073/pnas.1303058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1:e90558. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.