Abstract
Background
General anaesthesia combined with epidural analgesia may have a beneficial effect on clinical outcomes. However, use of epidural analgesia for cardiac surgery is controversial due to a theoretical increased risk of epidural haematoma associated with systemic heparinization. This review was published in 2013, and it was updated in 2019.
Objectives
To determine the impact of perioperative epidural analgesia in adults undergoing cardiac surgery, with or without cardiopulmonary bypass, on perioperative mortality and cardiac, pulmonary, or neurological morbidity.
Search methods
We searched CENTRAL, MEDLINE, and Embase in November 2018, and two trial registers up to February 2019, together with references and relevant conference abstracts.
Selection criteria
We included all randomized controlled trials (RCTs) including adults undergoing any type of cardiac surgery under general anaesthesia and comparing epidural analgesia versus another modality of postoperative pain treatment. The primary outcome was mortality.
Data collection and analysis
We used standard methodological procedures as expected by Cochrane.
Main results
We included 69 trials with 4860 participants: 2404 given epidural analgesia and 2456 receiving comparators (systemic analgesia, peripheral nerve block, intrapleural analgesia, or wound infiltration). The mean (or median) age of participants varied between 43.5 years and 74.6 years. Surgeries performed were coronary artery bypass grafting or valvular procedures and surgeries for congenital heart disease. We judged that no trials were at low risk of bias for all domains, and that all trials were at unclear/high risk of bias for blinding of participants and personnel taking care of study participants.
Epidural analgesia versus systemic analgesia
Trials show there may be no difference in mortality at 0 to 30 days (risk difference (RD) 0.00, 95% confidence interval (CI) −0.01 to 0.01; 38 trials with 3418 participants; low‐quality evidence), and there may be a reduction in myocardial infarction at 0 to 30 days (RD −0.01, 95% CI −0.02 to 0.00; 26 trials with 2713 participants; low‐quality evidence). Epidural analgesia may reduce the risk of 0 to 30 days respiratory depression (RD −0.03, 95% CI −0.05 to −0.01; 21 trials with 1736 participants; low‐quality evidence). There is probably little or no difference in risk of pneumonia at 0 to 30 days (RD −0.03, 95% CI −0.07 to 0.01; 10 trials with 1107 participants; moderate‐quality evidence), and epidural analgesia probably reduces the risk of atrial fibrillation or atrial flutter at 0 to 2 weeks (RD −0.06, 95% CI −0.10 to −0.01; 18 trials with 2431 participants; moderate‐quality evidence). There may be no difference in cerebrovascular accidents at 0 to 30 days (RD −0.00, 95% CI −0.01 to 0.01; 18 trials with 2232 participants; very low‐quality evidence), and none of the included trials reported any epidural haematoma events at 0 to 30 days (53 trials with 3982 participants; low‐quality evidence). Epidural analgesia probably reduces the duration of tracheal intubation by the equivalent of 2.4 hours (standardized mean difference (SMD) −0.78, 95% CI −1.01 to −0.55; 40 trials with 3353 participants; moderate‐quality evidence). Epidural analgesia reduces pain at rest and on movement up to 72 hours after surgery. At six to eight hours, researchers noted a reduction in pain, equivalent to a reduction of 1 point on a 0 to 10 pain scale (SMD −1.35, 95% CI −1.98 to −0.72; 10 trials with 502 participants; moderate‐quality evidence). Epidural analgesia may increase risk of hypotension (RD 0.21, 95% CI 0.09 to 0.33; 17 trials with 870 participants; low‐quality evidence) but may make little or no difference in the need for infusion of inotropics or vasopressors (RD 0.00, 95% CI −0.06 to 0.07; 23 trials with 1821 participants; low‐quality evidence).
Epidural analgesia versus other comparators
Fewer studies compared epidural analgesia versus peripheral nerve blocks (four studies), intrapleural analgesia (one study), and wound infiltration (one study). Investigators provided no data for pulmonary complications, atrial fibrillation or flutter, or for any of the comparisons. When reported, other outcomes for these comparisons (mortality, myocardial infarction, neurological complications, duration of tracheal intubation, pain, and haemodynamic support) were uncertain due to the small numbers of trials and participants.
Authors' conclusions
Compared with systemic analgesia, epidural analgesia may reduce the risk of myocardial infarction, respiratory depression, and atrial fibrillation/atrial flutter, as well as the duration of tracheal intubation and pain, in adults undergoing cardiac surgery. There may be little or no difference in mortality, pneumonia, and epidural haematoma, and effects on cerebrovascular accident are uncertain. Evidence is insufficient to show the effects of epidural analgesia compared with peripheral nerve blocks, intrapleural analgesia, or wound infiltration.
Plain language summary
Epidural analgesia for heart surgery with or without the heart lung machine in adults
Review question
We set out to determine from randomized controlled trials the effect of epidural pain relief on the number of deaths following surgery and risk of heart, lung, or nerve complications in adults undergoing heart surgery.
This review was first published in 2013, and it was updated in 2019.
Background
For epidural pain relief, a local anaesthetic, an opioid, or a mixture of both drugs is given through a catheter in the epidural space, which is the space immediately outside the membrane surrounding the cord. Epidural analgesia could reduce the risk of complications after surgery, such as lung infections including pneumonia, difficulty in breathing (respiratory failure), heart attack, and irregular heart rhythm caused by atrial fibrillation. A concern is that for cardiac surgery, the blood has to be thinned to reduce blood clotting, which may increase the chance of bleeding around the spinal cord. The collection of blood puts pressure on the spinal cord and can cause permanent nerve damage and disability.
Study characteristics
We included randomized controlled trials involving adults undergoing any type of cardiac surgery under general anaesthesia with or without cardiopulmonary bypass where researchers compared epidural pain relief around the time of surgery against other forms of pain relief. Surgeries performed were coronary artery bypass grafting or valvular procedures and surgeries for congenital heart disease. The average age of participants was between 43 and 75 years. Outcomes were measured up to one year after surgery.
We included 69 studies with 4860 participants. Where stated, the studies were funded by governmental resources (five studies), charity (eight), institutional resources (23), or in part by the industry (two). In all, 31 trials did not mention the source of funding. The evidence is current to November 2018.
Key results
When researchers compared epidural analgesia versus systemic pain relief (e.g. by an analgesic given directly into a vein), they could not detect any difference in the number of deaths in the first 30 days after surgery (38 studies, 3418 participants). There might be a difference in the number of people experiencing heart attacks (26 studies, 2713 participants). These findings were supported by low‐quality evidence. We found a small reduction in the risk of respiratory depression with epidural pain relief (21 studies, 1736 participants), but not in the risk of pneumonia (10 studies, 1107 participants) (low‐ or moderate‐quality evidence). The reduced risk of respiratory depression was more obvious when cardiopulmonary bypass was needed for cardiac surgery. Epidural analgesia reduced the risk of atrial fibrillation or atrial flutter early in recovery at zero to two weeks (18 studies, 2431 participants; moderate‐quality evidence). The number of cerebrovascular accidents was not clearly different (18 studies, 2232 participants), and no lasting neurological complications or epidural haematomas were reported (53 studies, 3982 participants; very low‐ or low‐quality evidence). Although epidural analgesia may have reduced the duration of tracheal intubation, this was noted mainly in older studies, and clinical practices have changed since that time (40 trials, 3353 participants; moderate‐quality evidence).
We found only six studies that compared epidural pain relief versus application of local anaesthetic on the body surface to produce peripheral nerve blocks directly into the space around the lungs (intrapleural analgesia) and onto the surgical wound (wound infiltration). These studies provided low‐ or very low‐quality evidence and did not report on many of the outcomes for this review. Study authors reported no heart attacks and no epidural haematomas.
Quality of the evidence
We rated the quality of evidence as moderate, low, or very low. We included too few participants in our review to rule out any differences in the number of patient deaths between epidural analgesia and systemic analgesia, nor to see any increase in epidural haematomas.
Summary of findings
Background
Description of the condition
The addition of thoracic epidural analgesia to general anaesthesia has been suggested to benefit patients after cardiac surgery (Svircevic 2013). However, this regional anaesthetic technique is controversial because the insertion of an epidural catheter in patients requiring full heparinization for cardiopulmonary bypass may lead to an epidural haematoma. The benefits of practicing off‐pump surgery instead of operating with the aid of cardiopulmonary bypass are not recognized by everyone, except perhaps for decreased risk of cerebrovascular accident and for high‐risk patients (Kowalewski 2016). Some clinicians argue that cardiopulmonary bypass induces a more severe inflammatory response. Also, using cardiopulmonary bypass usually requires more complete heparinization than off‐pump surgery. For this reason, we decided to evaluate all our outcomes while subgrouping the data by with or without cardiopulmonary bypass.
Description of the intervention
Epidural analgesia is a technique by which a local anaesthetic or an opioid or a mixture of both drugs is given in the epidural space (Guay 2016a; Guay 2016b; Salicath 2018). Epidural analgesia produces a superior quality of analgesia and may reduce the risk of postoperative complications such as pneumonia, respiratory failure, and myocardial infarction (Guay 2006; Guay 2014; Guay 2016a; Guay 2016b). Epidural analgesia may also shorten the duration of tracheal intubation as well as the time spent in an intensive care unit, which could have economic benefits (Guay 2016b).
How the intervention might work
High thoracic epidural analgesia may provide cardioprotective effects. High thoracic epidural analgesia increases myocardial oxygen availability, as reported in Lagunilla 2006, and reduces myocardial oxygen consumption (Hutchenson 2006). The latter is attributed to an attenuation of sympathetic response to the surgical stimuli (Kirno 1994). An influence on inflammatory response to the surgical stress and/or the cardiopulmonary bypass has also been reported (Volk 2003).
Why it is important to do this review
A possible complication of epidural analgesia includes spinal cord compression caused by a haematoma, which can result in paraplegia (Bos 2018). Systemic anticoagulation is needed for cardiac surgery and may increase the incidence of epidural haematoma related to the use of an epidural catheter (Horlocker 2018). While reviewing the literature, Landoni and colleagues found 25 cases of epidural haematoma out of 88,820 positioned epidurals in patients undergoing cardiac surgery, for an estimated risk of catheter‐related epidural haematoma of 3 per 10,000 procedures (95% confidence interval (CI) 2 to 4 per 10,000 procedures) (Landoni 2015). For the general population, the incidence of haematoma related to an epidural would be 1 per 10,000 procedures (95% CI 0 to 6 per 10,000 procedures) (Moen 2004). Although the incidence found by Landoni and colleagues may seem relatively low, the consequences of this complication may sometimes be catastrophic. In their large trial, Moen and colleagues reported 33 spinal haematomas related to neuraxial blocks. Only 6 of 33 patients made a full recovery, and 27 suffered permanent neurological damage (Moen 2004). It is therefore mandatory to have a clear view of the real benefits of epidural analgesia in cardiac surgery patients, so that patients and clinicians can make an informed decision when choosing the mode of postoperative analgesia.
This is an update of a previously published Cochrane review (Svircevic 2013).
Objectives
To determine the impact of perioperative epidural analgesia in adults undergoing cardiac surgery, with or without cardiopulmonary bypass, on perioperative mortality and cardiac, pulmonary, or neurological morbidity.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). We excluded observational studies, quasi‐randomized trials, cross‐over trials, and cluster‐randomized trials. We did not exclude studies on the basis of language of publication or publication status.
Types of participants
We included adult participants undergoing general anaesthesia for all types of cardiac surgery with or without cardiopulmonary bypass.
Types of interventions
We included trials that compared cardiac surgery including one group of participants with and one group of participants without epidural analgesia (Table 5). We excluded studies that compared cardiac surgery with participants with and participants without spinal anaesthesia. We included studies in which investigators administered epidural analgesia as a single shot block or as a continuous infusion for any duration and containing a local anaesthetic alone (extended duration or not), or in combination with an opioid (extended duration or not), or an opioid alone. We did not exclude studies in which trialists added an adjuvant other than an opioid to the solution. We excluded trials comparing nerve blocks versus systemic analgesia. For the comparator, we included all other modes of analgesia and divided them into:
1. Postoperative analgesia.
| Study | Regional blockade | Comparator |
| Aguero‐Martinez 2012 | TEA (T3‐T4) with 10 mL bupivacaine 0.5% and morphine 5 mg administered at least 1 hour before IV heparin | Low doses of opioids |
| Bach 2002 | TEA (T12‐L1) inserted the evening before surgery Bupivacaine 0.25% 10 mL Bupivacaine 0.25% ((body height (cm) − 100) × 10‐1 = mL/h) for 18 hours Catheter removed on the second or third day after surgery when coagulation parameters had returned to normal range |
Not reported |
| Bakhtiary 2007 | TEA (T1‐T3; soft multi‐port) inserted the day before surgery 6 mL ropivacaine 0.16% plus sufentanil 1 mcg/mL Ropivacaine 0.16% plus sufentanil 1 mcg/mL at 2 to 5 mL/h started before surgery and continued for 3 days after surgery |
Metamizole and piritramide |
| Barrington 2005 | TEA (T1‐T3) (20‐gauge; Portex, Hythe, Kent, UK) inserted 4 cm cephalad the day before surgery using a midline approach and a loss of resistance to saline technique Ropivacaine 1% 5 mL and fentanyl 50 mcg (adjusted for T1 to T6 sensory block) Ropivacaine 0.2% and fentanyl 2 mcg/mL 5 mL/h started 1 hour after induction and continued until morning of postoperative day 3 (adjusted on pain scores) |
IV morphine infusion and infiltration of chest drain sites |
| Bektas 2015 | TEA (T2‐T4) inserted 5 cm into the epidural space 1 day before surgery Lidocaine 60 mg Levobupivacaine 0.25% 0.1 mL/kg/min and fentanyl 2 mcg/kg/min bolus for T1‐L2 sensory block Levobupivacaine 0.25% 0.1 mL/kg/h and fentanyl 2 mcg/mL |
IV PCA with morphine for 24 hours |
| Berendes 2003 | TEA (C7‐T1) with a median approach and a hanging drop technique inserted the day before surgery 2 mL of 0.5% bupivacaine with epinephrine Bupivacaine 0.5% at 6 to 12 mL/h plus sufentanil 15 to 25 mcg started just before surgery and kept for 4 days |
Not reported |
| Brix‐Christensen 1998 | TEA (T3‐T4) inserted at least 12 hours before surgery Bupivacaine 0.5% 8 mL 30 minutes before induction of anaesthesia Continuous infusion with bupivacaine 2 mg/mL and fentanyl 5 mcg/mL at 5 mL/h during and after surgery until the second postoperative day |
IV morphine |
| Caputo 2011 | TEA (T2‐T4) inserted before surgery Bupivacaine 0.5% 5 + 5 mL Bupivacaine 0.125% plus clonidine 0.0003% 10 mL/hour started after induction and continued for 72 hours (adjusted for T1 to T10 sensory block and on pain scores) |
IV PCA with morphine |
| Celik 2015 | TEA (T5‐T6) inserted the day before surgery Levobupivacaine 2 mcg/mL and fentanyl 10 mcg/mL started at ICU admission at 5 mL/h and maintained for 24 hours |
IV fentanyl infusion at 8 mcg/kg/h for 24 hours |
| Cheng‐Wei 2017 | TEA PCEA with 0.075% bupivacaine and 2 mcg/mL fentanyl |
Wound infusion with 0.15% bupivacaine infused continuously at 2 mL/h through a catheter embedded in the wound plus IV PCA |
| de Vries 2002 | TEA (T3‐T4) placed immediately before induction of anaesthesia
Test dose with 3 to 4 mL of lidocaine 2% with epinephrine 1:200,000 8 to 10 mL bupivacaine 0.25% with sufentanil 25 mcg/10 mL Bupivacaine 0.125% and sufentanil 25 mcg/50 mL given at 8 to 10 mL/h |
Piritramide 0.2 mg/kg intramuscularly on request |
| Dohle 2001 | TEA (T4‐T5) 18G, midline approach, catheter advanced 3 cm past the needle tip Test dose with 3 mL 2% lidocaine Loading with 8 mL 0.5% bupivacaine injected through the catheter, followed by infusion of 0.25% bupivacaine at the rate of 6 mL/h |
Paravertebral blockade, left T4 to T5, loss of resistance with saline, catheter advanced 3 cm past the needle tip Test dose with 3 mL 2% lidocaine Loading with 8 mL 0.5% bupivacaine injected through the catheter, followed by an infusion of 0.25% bupivacaine at the rate of 6 mL/h |
| El‐Baz 1987 | TEA (T3‐T4), epidural catheter (American Pharmaseal Labs, Glendale, CA, USA) inserted by lateral approach Position of the catheter in the epidural space was confirmed by the catheter advancement test (El‐Baz 1984; "After eliciting a lack of resistance to the injection of air through the epidural needle, the ability to advance 20 cm of a soft epidural catheter, without stylet, beyond the vertebral lamina with minimal resistance was indicative of a successful epidural catheterization. After a successful advancement with minimal resistance, the epidural catheter was withdrawn 17‐18 cm leaving 2 ‐3 cm of the catheter in the epidural space and the tip near the spinal segment (T4 ‐ 5) that corresponded to the site of surgical incision. Subdural and intravascular catheterization were excluded by placing the proximal end of the epidural catheter below the site of injection for gravity drainage to assure the absence of cerebrospinal fluid or blood flow through the catheter") Morphine 0.1 mg/h started in ICU |
IV morphine on request |
| El‐Morsy 2012 | TEA (T3‐T4) inserted at least 2 hours before heparinization (change of level if blood in the needle or catheter) Test dose with 3 mL 1.5% lidocaine 0.125% bupivacaine with 1 mcg/mL fentanyl at 5 mL/h and continued until 24 hours postoperatively |
IV tramadol on demand |
| El‐Shora 2018 | TEA (T6‐T7) catheter inserted through a 17G Tuohy needle with loss of resistance technique Bupivacaine 0.125% plus fentanyl 1 mcg/mL 12 mL followed by 12 mL/h for 48 hours and started after surgery |
Ultrasound‐guided bilateral paravertebral blockade at T6‐T7 Bupivacaine 0.125% plus fentanyl 1 mcg/mL 6 mL per side followed by 6 mL/h for 48 hours and started after surgery |
| Fawcett 1997 | TEA (T2‐T4) inserted in operating room 15 mL bupivacaine 0.5% after CPB Bupivacaine 0.375% at 5 to 8 mL/h for 24 hours |
IV morphine infusion for 24 hours |
| Fillinger 2002 | TEA (T3‐T10), catheter inserted before induction of anaesthesia through an 18G Hustead needle using loss of resistance to saline technique and leaving 3 cm of catheter in the epidural space Test dose with 3 mL 1.5% lidocaine with 1:200,000 epinephrine Loading with morphine 20 mcg/kg and 0.5% bupivacaine in 5‐mg increments, to a total loading dose of 25 to 35 mg bupivacaine 0.5% bupivacaine with morphine 25 mcg/mL at 4 to 10 mL/h beginning after induction of anaesthesia (adjusted on haemodynamic parameters) Epidural catheters removed on the first postoperative day |
Intravenous morphine, intravenous meperidine, and oral oxycodone |
| Greisen 2012 | TEA (T2‐T4) inserted the day before surgery 5 to 7 mL 5.0 mg/mL bupivacaine (Marcaine, Astra, Södertälje, Sweden) together with sufentanil 2.5 mcg/mL Bupivacaine 2.5 mg/mL and sufentanil 1 mcg/mL 4 to 6 mL/h, by discretion of the attending anaesthesiologist, until end of surgery Changed to bupivacaine 1 mg/mL together with sufentanil 1 mcg/mL in ICU and continued after discharge from ICU until second postoperative day |
Not reported |
| Gurses 2013 | CEA (C6‐C7) (Braun Perifix 20 G) inserted 3 to 4 cm caudally (T2‐T4) at least 1 hour before heparin injection 0.075 mg/kg levobupivacaine hydrochloride (Chirocaine 5 mg/mL, Abbott Lab, Istanbul, Turkey) + 2 mcg/kg fentanyl (fentanyl citrate 50 mcg/mL, Abbott Lab, Istanbul, Turkey) in total 10 mL bolus 0.0375 mg/kg/h levobupivacaine + 0.5 mcg/kg/h fentanyl epidural infusion started with patient‐controlled analgesia instrument (Abbott Pain Management Provider, Abbott Laboratoires, North Chicago, IL, USA) |
Intramuscular diclofenac sodium (Dikloron 75 mg 10 amp, Mefar Drug Ltd, Istanbul, Turkey) |
| Hansdottir 2006 | TEA (T2‐T5) inserted the day before surgery using median hanging drop or loss of resistance technique, 3 to 5 cm into the epidural space Test dose with 4 mL lidocaine 1% PCEA with bupivacaine 0.1% and fentanyl 2 mcg/mL |
IV PCA with morphine |
| Heijmans 2007 | TEA (C7‐T1) by median approach and hanging drop technique Test dose of 2 mL lidocaine 2% Loading dose of 10 mL bupivacaine 0.25% with 2.5 mg morphine infused over 1 hour Bupivacaine 0.125% and morphine 0.2 mg/mL at 1.5 mL/h for 48 hours |
IV piritramide 0.15 mg/kg |
| Huh 2004 | TEA (T4‐T5) inserted the day before surgery Test dose with 3 mL lidocaine 2% and epinephrine 5 to 7 mL bupivacaine 0.15% and fentanyl 50 mcg before skin incision Bupivacaine 0.15% and fentanyl 10 mcg/mL through PCEA for 3 days after surgery |
IV meperidine, tramadol, and NSAIDs |
| Hutchenson 2006 | TEA (T2‐T4) inserted 3 cm the day before surgery with fluoroscopic guidance Bupivacaine 0.5% 200 mcg/cm body height Bupivacaine 0.25% 200 mcg/cm body height per hour |
Not reported |
| Jakobsen 2012 | TEA (T3‐T4) Test dose of 3 mL 2% lidocaine Bolus dose of 5 to 7 mL, guided by primary patient heights, of 0.5% bupivacaine (Marcaine; Astra, Södertälje, Sweden) and sufentanil 2.5 mcg/mL Bupivacaine 2.5 mg/mL/sufentanil 1 mcg/mL, 4 to 6 mL/h during surgery Bupivacaine 1 mg/mL and sufentanil 1 mcg/mL postoperatively and continued after discharge from ICU until second postoperative day |
Participants in both groups received intravenous morphine or alfentanil according to the department’s general guidelines (i.e. morphine 0.05 mg/kg, or alfentanil 25 mcg, if rapid pain relief was needed) All participants in both groups received additional oral or intravenous paracetamol 1 g every 6 hours |
| Kendall 2004 | TEA (T1‐T4) inserted after induction through a paramedian approach and loss of resistance technique 2 mL 0.5% bupivacaine plus epinephrine 0.1 mL/kg 0.1% bupivacaine plus fentanyl 5 mcg/mL followed by infusion at 0.1 mL/kg/h kept for 48 hours |
IV PCA with morphine |
| Kilickan 2006 | TEA (T1‐T5) inserted the day before surgery (3 attempts only) Test dose with 3 to 4 mL 2% lidocaine, position confirmed with injection of contrast material and X‐ray Bupivacaine 20 mg after anaesthesia induction Bupivacaine 0.125% 4 to 10 mL/h intraoperatively and postoperatively for 3 days, adjusted for a sensory blockade from T1 to T10 |
IV PCA with morphine |
| Kilickan 2008 | TEA (T1‐T5) inserted the day before surgery (3 attempts only) Test dose with 3 to 4 mL 2% lidocaine, position confirmed with injection of contrast material and X‐ray Bupivacaine 20 mg 60 minutes before induction of anaesthesia Bupivacaine 20 mg/h intraoperatively and postoperatively for 3 days |
IV PCA with Dolantin |
| Kirno 1994 | TEA (T3‐T4; Perifix, B. Braun, Melsungen AG, Germany) at least 12 hours before surgery Mepivacaine 20 mg/mL (Carbocain, Astra, Södertälje, Sweden) was injected to achieve a T1‐T5 block |
Not reported |
| Kirov 2011 | TEA (T2‐T4) Test dose of 1 mL 2% lidocaine Ropivacaine 0.75% 1 mg/kg and fentanyl 1 mcg/kg for surgery Ropivacaine 0.2% and fentanyl 2 mcg/mL at 3 to 8 mL/h (VAS score < 30 mm at rest) or via PCEA after surgery |
IV fentanyl 10 mcg/mL at 3 to 8 mL/h |
| Konishi 1995 | TEA (T7‐T10) inserted the day before surgery Butorphanol 0.5 to 1.0 mg or Morphine 2.5 mg |
Fentanyl, pentazocine, and minor tranquillizers |
| Kundu 2007 | TEA (C7‐T2) inserted 3 to 4 cm cephaladly before anaesthesia induction with hanging drop technique in left lateral decubitus position Lidocaine 1% 5 mL Bupivacaine 0.25% 5 mL plus fentanyl 10 mcg Bupivacaine 0.25% 5 mL plus fentanyl 10 mcg every 2 hours |
Not reported |
| Kunstyr 2001 | TEA (T1‐T5) inserted at least 60 minutes before heparinization 10 mL bupivacaine 0.5% Bupivacaine 0.125% plus sufentanil 1 mcg/mL infused at 3 to 8 mL/h after surgery |
|
| Lenkutis 2009 | TEA (T1‐T2) Lidocaine 2% 7 to 8 mL Bupivacaine 0.25% at 8 mL/h during surgery Bupivacaine 0.25% and fentanyl 5 mcg/mL at 5 to 7 mL/h for at least 84 hours postoperatively |
IM/IV pethidine 0.1 to 0.4 mg/kg |
| Liem 1992 | TEA (T1‐T2) inserted the day before surgery by paramedian approach and hanging drop technique
Test dose with 2 mL 2% lidocaine Loading with 0.375% bupivacaine plus sufentanil 5 mcg/mL at a dose of 0.05 mL/cm body length administered over a 10‐minute period 0.125% bupivacaine plus sufentanil 1 mcg/mL at 0.05 mL/cm body length/h started before induction and continued for 72 hours |
IV nicomorphine |
| Loick 1999 | TEA (C7‐T1) inserted the day before surgery by median approach and hanging drop technique Test dose with 2 mL bupivacaine 0.5% with adrenaline Loading before induction with 8 to 12 mL bupivacaine 0.375% and 16 to 24 mcg sufentanil into the epidural space in increments to block the somatosensory level C7‐T6 PCEA with bupivacaine 0.75% plus sufentanil 1 mcg/mL if < 65 years of age, and without adjuvant if ≥ 65 years (duration unclear, possibly 48 hours) |
PCA with piritramide |
| Lundstrom 2005 | TEA (T1‐T3) inserted the day before surgery by median approach using hanging drop technique Test dose with 2 mL 2% lidocaine Loading with 8 to 10 mL bupivacaine 0.5% (adjusted for sensory block T1‐T8) before induction Bupivacaine 0.125% and morphine 25 mcg/mL at 5 mL/h plus 4 mL every hour started after induction Bupivacaine 0.25% 4 mL on request after surgery (adjusted for T1‐T8) Catheters removed on day 4 or 5 |
Morphine IV for 24 hours, then orally |
| Lyons 1998 | TEA (C7‐T1) Bupivacaine 0.5% 0.1 mL/kg Bupivacaine 0.1% and fentanyl 2 mcg/mL, infusion for 72 hours |
Not reported |
| Mehta 1998 | TEA (T4‐T5 or T5‐T6) 16G, median approach, loss of resistance to saline, catheter inserted 3 to 4 cm past the needle tip On first demand for pain relief, participants in the TEA group received 8 mL 0.25% bupivacaine hydrochloride Maximum of 3 doses was given over the next 12 hours, if required |
Intrapleural catheter: 16G epidural catheter inserted in intercostal space 6 to 7 cm in left anterior axillary line by the operating surgeon, 6 to 8 cm in intrapleural space, directed posteriorly and anchored with a skin suture before thoracotomy closure On first demand for pain relief, participants in the intrapleural group received 20 mL 0.25% bupivacaine hydrochloride Before injection of intrapleural bupivacaine, participants were positioned supine with a one‐third left lateral tilt and with the intercostal chest tube clamped after exclusion of any air leak. The chest tube was kept clamped for 20 minutes after the injection Maximum of 3 doses was given over the next 12 hours, if required |
| Mehta 2008 | TEA (C7‐T1) hanging drop technique in the sitting position, catheter inserted 4 cm beyond needle tip Lidocaine 2% 3 mL Bupivacaine 0.5% 8 mL Bupivacaine 0.25% at 0.1 mL/kg/h |
Paravertebral blockade Loss of resistance to saline at left T4‐T5 Lidocaine 2% 3 mL Bupivacaine 0.5% 8 mL Bupivacaine 0.25% at 0.1 mL/kg/h |
| Mehta 2010 | TEA (C7‐T1) using hanging drop technique in sitting position inserted at least 2 hours before heparinization; intervention postponed in cases of bloody tap 3 mL 2% lidocaine without epinephrine; adequacy and level of the block established by confirming loss of pin‐prick sensation and warm/cold discrimination 8 to 10 mL 0.25% bupivacaine (aim at T4 sensory block) Bupivacaine infusion (0.125%) with fentanyl citrate (1 mcg/mL) at the rate of 5 mL/h was commenced and continued until postoperative day 3 to provide intraoperative and postoperative analgesia |
Not reported |
| Mishra 2004 | No details available | Not reported |
| Moore 1995 | TEA (T1‐T5) Bupivacaine 0.5% in 2 mL increments for sensory block from T1 to L2 Bupivacaine 0.375% at 5 to 8 mL/h started before induction Bupivacaine 0.25% at 5 to 8 mL/h for at least 24 hours |
IV papaveretum |
| Nagaraja 2018 | TEA (C7‐T1) inserted (3 to 4 cm caudally) the day before surgery through an 18G Tuohy needle 0.25% plain bupivacaine 15 mL before surgery followed by 0.125% plain bupivacaine at 0.1 mL/kg/h for 48 hours post extubation |
Ultrasound‑guided (in‐plane) erector spinae plane lock Catherer inserted 5 cm cephaladly the day before surgery through an 18G Tuohy needle. 3 cm lateral to T6 spinous process (T5 transverse process) with hydrodissection below the erector spinae muscle with 5 mL normal saline, 0.25% plain bupivacaine, 15 mL in each catheter before surgery, followed by 0.125% plain bupivacaine at 0.1 mL/kg/h for 48 hours post extubation, through each catheter |
| Neskovic 2013 | TEA (T2‐T4) inserted 30 minutes before surgery and at least 2 hours before the first dose of heparin Test dose 10 to 15 mL 0.125 or 0.25% bupivacaine with fentanyl 0.125 or 0.25% bupivacaine with fentanyl at 5 to 10 mL/h |
Not reported |
| Nygard 2004 | TEA (T1‐T3) inserted the day before surgery by the median approach and hanging drop technique Test dose with 2 mL 2% lidocaine Loading with 8 to 10 mL bupivacaine 05% before induction (adjusted for T1 to T8) Bupivacaine 0.125% with morphine 25 mcg/mL at 5 mL/h started after induction and continued for 4 days Additional bolus doses of 4 mL bupivacaine 0.5% hourly during the operation |
Morphine IV for 24 hours, then orally |
| Obersztyn 2018 | TEA (T1‐T3) with hanging drop technique, catheters inserted 3 to 4 cm into the epidural space at least 6 hours before surgery Before surgery: 9 to 11 mL 0.25% bupivacaine with fentanyl in a concentration of 10 mcg/mL, followed by 0.19% (more exactly, 0.1875%) bupivacaine and fentanyl at 6 mL/h during surgery and 0.125% bupivacaine plus fentanyl 6.25 mcg/mL at 2 to 8 mL/h after surgery until discharge fro the ICU (mean 18.8 hours) |
IV morphine |
| Onan 2011 | TEA (T2‐T4; side‐holed 18 G epidural catheter) by using a median approach and a loss of resistance technique with saline solution Test dose with 3 to 4 mL 2% lidocaine 20 mg bolus 0.25% bupivacaine through the epidural catheters 1 hour before surgery 0.25% bupivacaine infused at a rate of 20 mg/h during surgery 0.125% bupivacaine at 4 to 10 mL/h after surgery (adjusted for T1‐T10) Epidural catheters removed at 24 hours postoperatively |
Not reported |
| Onan 2013 | TEA (T1‐T5) inserted the night before surgery 3 cm into epidural space Test dose with 3 to 4 mL 2% lidocaine Sensory blockade tested with ice plus X‐ray after injection of contrast material Bolus of 20 mg 0.25% bupivacaine 1 hour before surgery 20 mg/h 0.25% bupivacaine intraoperatively Bupivacaine 0.25% 10 to 20 mL/h during first 24 hours after surgery (adjusted according to pain scores) |
Acetaminophen (500 mg) and tramadol (1 mg/kg) used as rescue medications |
| Palomero 2008 | TEA (T3‐T6) inserted the day before surgery Bolus of 6 to 8 mL 0.33% bupivacaine 0.175% bupivacaine 6 to 8 mL/h for 48 hours Catheter withdrawn after check of coagulation status |
Morphine 0.5 to 1 mL/h |
| Petrovski 2006 | TEA; no details | Not reported |
| Priestley 2002 | TEA (T1‐T4; 18G side‐holed epidural catheter) inserted the evening before surgery Test dose with 2% lidocaine 3 to 4 mL Loading with 4 mL ropivacaine 1% and fentanyl 100 mcg (adjusted for T1‐T6) Ropivacaine 1% and fentanyl 5 mcg/mL at 3 to 5 mL/h started before induction and continued for 48 hours |
Continuous morphine infusion for 24 hours, followed by PCA with morphine |
| Rein 1989 | TEA (T4‐T5) Bupivacaine 0.5% 10 mL at induction of anaesthesia and 4 mL every hour during surgery Bupivacaine 0.5% at 4 mL/h for 24 hours |
Morphine |
| Royse 2003 | TEA (T1‐T3) inserted the night before the operation 8 mL bupivacaine 0.5% with fentanyl 20 mcg before induction Ropivacaine 0.2% with fentanyl 2 mcg/mL at 5 to 14 mL/h (for T1‐T10 sensory block) and continued until postoperative day 3, 6H00 AM |
PCA with morphine |
| Scott 2001 | TEA (T2‐T4) inserted before induction Loading with bupivacaine 0.5% 2 boluses of 5 mL (for T1‐T10) Bupivacaine 0.125% and 0.0006% clonidine at 10 L/h started after induction and continued for 96 hours (adjusted on pain scores and sensory block) |
Target controlled infusion of alfentanil for 24 hours followed by PCA with morphine for another 48 hours (adjusted on pain scores) |
| Sen 2017 | TEA (T2‐T4) inserted 4 to 6 cm into epidural space the day before surgery Lidocaine 2% with epinephrine 5 mcg/mL 3 mL Bupivacaine 0.1% and fentanyl 2 mcg/mL at 0.1 mL/kg/h started after induction |
IV fentanyl 0.5 to 2 mcg/kg/h IV tramadol 100 mg as rescue analgesia |
| Sharma 2010 | TEA (C7‐T2) inserted at least 2 hours before heparinization and using hanging drop technique via midline approach Test dose 3 mL 2% lignocaine without epinephrine Loading with 8 to 10 mL bupivacaine 0.25% (for sensory block until at least T4) before induction Bupivacaine 0.125% with 1 mcg/mL fentanyl citrate at 5 mL/h started after induction and continued until third postoperative day |
IV continuous infusion of tramadol |
| Stenseth 1994 | TEA (T4‐T6) inserted the day before surgery Test dose with lidocaine 10 mL bupivacaine 0.5% before induction 4 mL bupivacaine 0.5% hourly during surgery Bupivacaine 0.5% at 3 mL/h plus 4 mL every 4 hours after surgery |
IV morphine on request |
| Stenseth 1996 | TEA (T4‐T6) inserted the day before surgery Test dose with lidocaine 10 mL bupivacaine 0.5% before induction (for at least T1‐T2 block) 4 mL bupivacaine 0.5% hourly during surgery Bupivacaine 0.5% at 3 mL/h plus 4 mL every 4 hours after surgery Morphine epidurally 4 to 6 mg 3 to 4 times a day for the next 2 days, supplemented with bupivacaine 5 mg/mL when needed until third postoperative day |
IV morphine on request |
| Stritesky 2006 | TEA (T2‐T4) 1 hour before surgery with an 18G Tuohy needle and hanging drop or loss of resistance technique, with catheter inserted 4 cm past the needle tip 10 mL bupivacaine 0.25% plus fentanyl 100 mcg for loading (half through the needle and half through the catheter) Bupivacaine 0.25% and fentanyl 1 mcg/mL at 8 to 12 mL/h during surgery and for 48 hours |
Not reported |
| Svircevic 2011 | TEA (T2‐T4) at least 4 hours before heparinization Test dose with lidocaine 2% 3 mL 0.1 mL/kg administered of a solution of 0.08 mg/mL morphine and 0.125% bupivacaine, followed by continuous infusion of 4 to 8 mL/h of the same solution started before induction Epidural catheter removed before transfer to the general ward (median 22 hours) |
Morphine IV infusion |
| Tenenbein 2008 | TEA (T2‐T5) inserted at least 4 hours before systemic heparinization 2.5 mL test dose of 2% lidocaine, with 1:200,000 epinephrine on insertion 3 mL test dose of 2% lidocaine before surgery 0.75% ropivacaine 5 mL with hydromorphone 200 mcg followed by an infusion of ropivacaine 0.75% at 5 mL/h during surgery 0.2% ropivacaine with hydromorphone 15 mcg/mL for 48 hours after surgery |
IV PCA with morphine Indomethacin suppositories (100 mg) postoperatively, and twice‐daily naproxen (500 mg) |
| Tenling 1999 | TEA (T3‐T5; 16G), inserted the day before surgery through the lateral approach and loss of resistance technique with saline 0.9% Test dose of 2 to 3 mL lidocaine 1% 8 to 12 mL bupivacaine 0.5% the morning of the operation (for T1‐T8 sensory block) Bupivacaine 0.5% at 4 to 8 mL/h until ICU admission Bupivacaine 0.2% and sufentanil 1 mcg/mL at 3 to 7 mL/h from arrival to ICU until the day after the operation |
IV ketobemidone |
| Usui 1990 | TEA (T6‐T7) inserted 4 cm past needle tip 24 hours before surgery and kept for 1 or 2 days after extubation Morphine 3 mg given after surgery and repeated as required |
Morphine 10 mg IV as required Additional co‐analgesia as required |
| Volk 2003 | TEA (C7‐T3) inserted the day before surgery Lidocaine 2% for T1‐T6 sensory block Bupivacaine 0.5% 6 to 10 mL hourly during surgery Bupivacaine 0.25% at 6 to 12 mL/h for at least 24 hours |
IV patient‐controlled analgesia with piritramide |
| Yang 1996 | TEA (T4‐T5) inserted 3 cm cephalad in the right lateral decubitus position Lidocaine 2% 3 mL Bupivacaine 0.375% and fentanyl 5 mcg/mL 0.06 mL/cm of body length Bupivacaine 0.25% with fentanyl 5 mcg/mL 0.03 mL/cm of body length every hour |
Not reported |
| Yilmaz 2007 | TEA (T3‐T6) inserted cranially 3 to 4 cm 16 to 24 hours before systemic heparinization (Perifix 18G, Braun) Loading with morphine 5 mcg/kg and 6 mL bupivacaine 0.25% at least 45 minutes before surgical incision 6 mL bupivacaine 0.12% with fentanyl 2.5 mcg/kg every 6 hours for 48 hours, after which catheters were withdrawn |
IV fentanyl 0.7 mcg/kg/h |
| Yung 1997 | TEA or upper lumbar epidural inserted 24 hours before surgery Lidocaine 1.5% 25 to 30 mL with ketamine 15 mg, morphine 1 mg/10 kg for surgery Morhine 1 mg in 10 mL normal saline every 12 hours for 5 days for postoperative analgesia |
IV meperidine HCl |
| Zawar 2015 | TEA (C7‐T2) catheters inserted 4 to 5 cm cranially using hanging drop technique If a “bloody tap” was to occur, the operation was postponed for 24 hours and participant was excluded from the study Bolus of 6 to 14 mL ropivacaine 0.75% for T1‐T10 sensory block (sensory loss to cold pack and needle prick) Infusion of 5 to 15 mL/h ropivacaine 0.2% for 72 hours after surgery |
IV tramadol hydrochloride 100 mg 8 hourly |
| Zhou 2010 | TEA (T4‐T6) inserted in lateral decubitus position the day before surgery Bolus 8 to 20 mL lidocaine 1% PCEA with ropivacaine 0.125% and fentanyl 2 mcg/mL at 4 mL/h plus 2 mL bolus (lockout time 20 minutes) |
IV PCA with fentanyl |
CEA: cervical epidural analgesia; CPB: cardiopulmonary bypass; ICU: intensive care unit; NSAIDs: non‐steroidal anti‐inflammatory drugs; PCA: patient‐controlled analgesia; PCEA: patient‐controlled epidural analgesia; TEA: thoracic epidural analgesia; VAS: visual/verbal analogical pain score.
all forms of systemic analgesia (opioid‐based regimen or other), regardless of the route of administration (intravenous (with or without a self‐administered patient‐controlled device), intramuscular, or oral analgesia);
peripheral nerve blocks;
intrapleural analgesia; and
wound infiltration.
Types of outcome measures
Primary outcomes
Risk of mortality (0 to 30 days, six months, and one year)
Secondary outcomes
Risk of myocardial infarction (0 to 30 days; study author's definitions (Table 6))
-
Risk of pulmonary complications
Riisk of atrial fibrillation or atrial flutter during surgery and up to two weeks after surgery
-
Risk of neurological complications
Cerebrovascular accident (0 to 30 days; study author's definitions (Table 8))
Risk of serious neurological complications from epidural analgesia (lasting (> 3 months) sensory or motor deficit) or epidural haematoma (with or without epidural analgesia) (0 to 30 days)
Duration of tracheal intubation (Table 9)
Pain scores (rest and movement at 6 to 8, 24, 48, and 72 hours)
-
Haemodynamic support (in hospital)
Hypotension or need for vasopressor boluses
Inotropic or vasopressor infusions
2. Diagnostic criteria for myocardial infarction.
| Study | Criteria |
| Aguero‐Martinez 2012 | New pathological Q wave (duration ≥ 0.04 second and depth ≥ 25% of the R wave or QRS complex) in more than 1 derivation. Non‐specific changes that included elevation of the ST segment > 1.5 mm from the isoelectric line in 2 or more leads of the same region, ST depression > 2 mm in the precordial leads, or reversal of the T wave for longer than 48 hours; absence of R wave in the precordial leads. Ventricular or atrioventricular conduction defects Enzymatic criteria: 5 times normal values: troponin > 1 mcg/mL, CK > 250 U/L, CK‐MB > 133 U/L, LDH > 800 U/L, LDH 1/LDH 2 > 1 in blood samples collected between postoperative days 2 and 3, and GOT > 90 U/L Echocardiographic criteria: new segmental motility disorders Anatomopathological criteria: in dead patients |
| Bakhtiary 2007 | Unspecified |
| Barrington 2005 | Transmural infarction defined as new Q waves |
| Bektas 2015 | 1. Cardiac biomarkers (with troponins preferred) rise > 10 times 99% upper reference limit (URL) from normal preoperative level 2. New pathological Q waves or new left bundle branch block (LBBB) and/or imaging or angiographic evidence of new occlusion of native vessels or grafts, new regional wall motion abnormality, or loss of viable myocardium |
| Caputo 2011 | New Q waves of 0.04 ms and/or reduction in R waves > 25% in at least 2 contiguous leads on ECG |
| Celik 2015 | ECG monitored (ST analysis); CK‐MB and troponin I levels measured at fourth and 24th hours |
| de Vries 2002 | Myocardial infarction defined as a new Q wave on ECG and CK 180 U/L with CK‐MB 10% of total |
| Dohle 2001 | Myocardial infarction assessed by ECG changes and CK‐MB values |
| Fillinger 2002 | New Q waves of at least 0.04 second duration or postoperative elevation of serum creatine phosphokinase confirmed by creatine phosphokinase isoenzyme pattern |
| Hansdottir 2006 | New Q waves or CK‐MB isoenzyme concentration ≥ 50 |
| Heijmans 2007 | Myocardial infarction not mentioned in the report |
| Jakobsen 2012 | Perioperative myocardial infarction, defined as new Q waves of 0.04 ms and/or reduction in R waves > 25% in at least 2 contiguous leads on ECG |
| Kendall 2004 | ECG changes (new Q wave, or loss of R wave progression, or new permanent left bundle branch block) and increase in creatinine kinase myocardial fraction (CK‐MB) to > 120 units per litre |
| Kilickan 2006 | Unspecified |
| Liem 1992 | CK‐MB values ≥ 80 IU/L and evidence of new Q waves or bundle branch block on postoperative ECG |
| Loick 1999 | Unclear |
| Lyons 1998 | Unclear |
| Mehta 1998 | Incidence of perioperative myocardial infarction also analysed by an independent cardiologist, as per the appearance of new Q waves in the ECG and increase in creatine phosphokinase‐myocardial band isoenzyme (CPK‐MB) levels to > 70 ng/mL in the first 12 hours postoperatively |
| Mehta 2010 | 2‐lead ECG and CPK, CPK‐MB levels |
| Neskovic 2013 | New ECG changes with positive enzymes (CK‐MB and troponin) |
| Obersztyn 2018 | ECG and elevated serum enzymes |
| Onan 2011 | Unspecified |
| Onan 2013 | Unspecified |
| Palomero 2008 | Myocardial infarction defined by analysis of the ECG (new Q waves or increases in ST segment > 3 mm) |
| Priestley 2002 | New Q waves (assessed by the blinded cardiologist) on a 12‐lead ECG on days 0, 1, 2, and 4 and assessment of venous blood levels of troponin T and creatine kinase‐MB fraction on arrival in the ICU, and again at 4, 12, and 24 hours and on postoperative day 2 |
| Scott 2001 | Q waves, ST segment increase of 3 mm, and a myocardial specific serum creatinine kinase level ≥ 60 ng/mL |
| Stenseth 1994 | Unspecified |
| Stenseth 1996 | Unspecified |
| Svircevic 2011 | Creatine kinase muscle–brain isoenzymes > 75 units per litre (5 times upper limit of normal level) and peak creatine kinase muscle–brain isoenzyme/creatine kinase ratio > 10% or new Q wave infarction |
| Zawar 2015 | Myocardial infarction defined as developing ECG changes, new Q waves on postoperative ECG ≥ 0.03 second in duration in 2 or more adjacent leads lasting until discharge, rise in creatine phosphokinase‑MB and troponin I, and new regional wall motion abnormalities |
CK: creatinine kinase; CK‐MB: creatinine kinase muscle brain; ECG: electrocardiogram; GOT: glutamic‐oxaloacetic transaminase; LBBB: left bundle branch block; LDH: lactate dehydrogenase; URL: upper reference limit.
3. Diagnostic criteria for pulmonary complications.
| Study | Criteria |
| Aguero‐Martinez 2012 | Respiratory depression |
| Barrington 2005 | Respiratory depression: reintubation |
| Berendes 2003 | Respiratory depression: need of ICU 24 hours due to intermittent respiratory insufficiency |
| Caputo 2011 | Pneumonia: presence of purulent sputum associated with fever and requiring antibiotic therapy according to positive sputum culture |
| Celik 2015 | Respiratory depression: PaCO₂ and PO₂ measurements at baseline and at first, sixth, and 12th hours were followed Pneumonia: fever, C‐reactive protein, leukocyte values, and chest radiography were assessed |
| de Vries 2002 | Respirarory depression: respiratory acidosis Pneumonia: criteria of the Centers for Disease Control and Prevention |
| El‐Baz 1987 | Respiratory depression: respiratory insufficiency requiring intubation and ventilatory support |
| Fillinger 2002 | Respiratory depression: need for mechanical ventilation for 24 hours after surgery or clinical decision to initiate mechanical ventilation after initial tracheal extubation Pneumonia: positive sputum culture and chest radiograph changes |
| Hansdottir 2006 | Respiratory depression: postoperative mechanical ventilation for longer than 24 hours or need for non‐invasive positive‐pressure ventilation Pneumonia: defined as pulmonary infiltrate with positive microbial cultures from sputum or fever, high leukocyte count, or high levels of C‐reactive protein |
| Kunstyr 2001 | Respiratory depression: 8 or fewer breaths per minute and PaCO₂ > 55 kPa |
| Liem 1992 | Respiratory depression: no details provided |
| Lundstrom 2005 | Respiratory depression: constant hypoxaemia on third night after surgery |
| Mehta 2010 | Respiratory depression: no details provided |
| Neskovic 2013 | Respiratory depression: need for re‐intubation Pneumonia: febrile state, with new chest radiography findings |
| Obersztyn 2018 | Respiratory depression: need for respiratory support after extubation |
| Onan 2013 | Pneumonia: no details provided |
| Royse 2003 | Respiratory depression: need for non‐invasive respiratory support or re‐intubation |
| Scott 2001 | Respiratory depression: respiratory failure requiring tracheal re‐intubation or prolonged mechanical ventilation (> 24 hours) Pneumonia: combination of increased white cell count, pyrexia, productive sputum, radiological signs, and positive bacterial growth on culture |
| Tenenbein 2008 | Respiratory depression: no details provided Pneumonia: no details provided |
| Yilmaz 2007 | Pneumonia: respiratory infection |
| Yung 1997 | Respiratory depression: re‐intubation |
| Zawar 2015 | Respiratory depression: re‐intubation |
ICU: intensive care unit; kPa: kilopascal; PaCO₂: partial pressure of carbon dioxide; PaO₂: partial pressure of oxygen.
4. Diagnostic criteria for neurological complications: cerebrovascular accident.
| Study | Neurological complication |
| Aguero‐Martinez 2012 | Neurological complication: any new‐onset psychiatric or neurological disorder with altered consciousness with or without focalization |
| Barrington 2005 | Stroke |
| Bektas 2015 | Stroke: All participants were postoperatively managed in the cardiac surgery intensive care unit. Postoperative stroke was suspected when a patient showed focal neurological deficits or delayed recovery of mental status after surgery. Such patients were referred to stroke neurologists and were evaluated by computed tomography. Post coronary artery bypass grafting, stroke was diagnosed as: 1) newly developed neurological deficits within 14 days of coronary artery bypass grafting; and 2) Low‐density lesions on postoperative computed tomography that were not observed preoperatively. Strokes that occurred within 24 hours after coronary artery bypass grafting were defined as immediate, whereas all others were considered delayed |
| Caputo 2011 | Stroke/transient ischaemic attack: diagnosis of stroke was made if evidence showed new neurological deficit with morphological substrate confirmed by computed tomography or nuclear magnetic resonance imaging |
| Celik 2015 | Stroke: neurological findings of participants (hemiparesis, hemiplegia, etc.) were followed |
| Fillinger 2002 | Neurological event: new sensorimotor neurological events |
| El‐Shora 2018 | Stroke |
| Hansdottir 2006 | Stroke: defined as a new central neurological deficit |
| Heijmans 2007 | Stroke |
| Jakobsen 2012 | Transitory Ischaemic attack lasting less than 24 hours |
| Neskovic 2013 | Stroke: new motor or sensory deficit after surgery |
| Onan 2013 | Cerebrovascular accident |
| Palomero 2008 | Focal neurological dysfunction defined as a sensory or motor deficit affecting 1 or more limbs appearing 5 days after surgery |
| Royse 2003 | Stroke |
| Scott 2001 | Cerebrovascular accident defined as a new motor or sensory deficit affecting 1 or more limbs and present on awakening from anaesthesia or occurring within the next 5 days |
| Stenseth 1996 | Hemiparesis |
| Svircevic 2011 | Stroke: a new motor or sensory deficit of central origin, persisting longer than 24 hours, preferably confirmed by computed tomography, resulting in a drop of 2 points on the Rankin scale |
| Tenenbein 2008 | Stroke or transient ischaemic attack |
| Zawar 2015 | Stroke was documented if diagnosed on computed tomography scan or magnetic resonance imaging |
5. Criteria for tracheal extubation.
| Study | Criteria |
| Aguero‐Martinez 2012 | Adequate response to verbal commands, pulse oximetry 95% with FiO₂ 0.5, PaCO₂ 45 mmHg in spontaneous respiration, respiratory rate between 10 and 20/min, regular thoracic movements with tidal volume > 5 mL/kg, temperature > 36°C, stable haemodynamic parameters, and no surgical complications |
| Bakhtiary 2007 | Not reported |
| Barrington 2005 | Anaesthesiologist tracheally extubated participants in the operating room if extubation criteria—respiratory rate 10 to 20 breaths/min, responsiveness to voice, end‐tidal CO₂ 50 mmHg, SaO₂ 94% with a fraction of inspired oxygen of 1.0, haemodynamic stability, minimal chest drain output (not requiring transfusion or consideration for surgical re‐exploration), and temperature 35.9°C—were achieved within 30 minutes. For participants not extubated in the operating room, postoperative management of ventilation and extubation followed existing unit guidelines. Participants were required to respond appropriately to voice, have an acceptable ventilatory pattern and arterial blood gas analysis, and be haemodynamically stable |
| Berendes 2003 | Weaning the participant from the respirator and extubation were performed according to standard procedures |
| Caputo 2011 | Not reported |
| Celik 2015 | Not reported |
| de Vries 2002 | Extubation criteria were normothermia, haemodynamic stability, ability to respond to verbal commands, and respiratory rate of at least 8 breaths/min with peripheral oxygen saturation of at least 94% |
| Dohle 2001 | Extubated whenever they qualified for extubation |
| El‐Baz 1987 | Not reported |
| El‐Morsy 2012 | Extubation criteria included an adequate level of consciousness and muscle strength, stable cardiovascular status, normothermia, adequate pulmonary function (PaO₂ > 80 mmHg with fraction of inspired oxygen ≤ 0.4), and minimal thoracotomy tube output |
| Fillinger 2002 | Endotracheal extubation was managed by ICU staff following standardized criteria. ICU extubation criteria included an adequate level of consciousness and muscle strength, stable cardiovascular status, normothermia, adequate pulmonary function (PaO₂ 80 mmHg with fraction of inspired oxygen 0.4), and minimal thoracostomy tube output |
| Gurses 2013 | Participants were extubated when they completely recovered and regained muscular power (Aldrete’s recovery score = 9, PaCO₂ < 45 mmHg, PaO₂ > 100 mmHg, FiO₂ = 0.4, and pH between 7.35 and 7.45, together with stable haemodynamic and metabolic parameters) |
| Hansdottir 2006 | Participants underwent extubation when they fulfilled the following criteria.
|
| Huh 2004 | Participants were extubated when they were awake (eyes opened and able to follow orders), were haemodynamically stable, and had normal arterial blood gases with FiO₂ ≤ 0.3 |
| Jakobsen 2012 | Extubation was performed when the participant was awake and without pain following objective criteria such as a spontaneous respiratory rate of 10 to 16, a core temperature of 36°C, a normal acid/base balance with pH between 7.34 and 7.45, PaO₂ of 10 kPa with FiO₂ 40% and maximum positive end‐expiratory pressure 5 cm H₂O, PaCO₂ 6 kPa, drainage 100 mL/h in the 2 following hours, together with stable haemodynamics, which were considered present with 20% change in cardiac index, SvO₂, and mean arterial pressure over the last hour |
| Kendall 2004 | Intermittent positive‐pressure ventilation was continued until the participant met the following minimum criteria for extubation: haemodynamic stability with blood loss < 120 mL/h, core temperature > 36°C, responsive, co‐operative, and pain‐free |
| Kilickan 2006 | Participants were extubated when they met set criteria as assessed by the ICU nursing staff: not in pain or agitated, cardiovascular stability without inotropes, systolic pressure > 90 mmHg, core temperature > 36.4°C, spontaneous ventilation with PaO₂ > 12 kPa on FiO₂ < 0.4 and PaCO₂ < 7 kPa, blood loss from chest drains < 60 mU/h, urine output > 1 mL/kg/h |
| Kilickan 2008 | Participants were extubated when they met set criteria as assessed by the ICU nursing staff: not in pain or agitated, cardiovascular stability without inotropes, systolic pressure > 90 mmHg, core temperature > 36.4°C, spontaneous ventilation with PaO₂ > 12 kPa on FiO₂ < 0.4 and PaCO₂ < 7 kPa, blood loss from chest drains < 60 mU/h, urine output > l mL/kg/h |
| Kirov 2011 | Extubation criteria were the following: a co‐operative, alert participant; adequate muscular tone; SpO₂ > 95% with FiO₂ 0.5; PaCO₂ < 45 mmHg; stable haemodynamics without inotrope/vasopressor support; absence of arrhythmias; and body temperature > 35°C. Temporary pacing was not regarded as a contraindication to extubation |
| Konishi 1995 | Not reported in partial translation |
| Kunstyr 2001 | Not reported |
| Lenkutis 2009 | Participants were extubated according to conventional clinical criteria: bleeding < 50 mL/h, stable haemodynamics, SpO₂ > 95% on FiO₂ 50%, awake enough to follow commands |
| Liem 1992 | Participants were extubated when they fulfilled the following criteria: responsive to verbal stimuli; respiratory rate per minute ≥ 10 and ≤ 25; SaO₂ ≥ 95%; breathing adequately via endotracheal tube with 5 L/min of oxygen (pH 7.30 to 7.40; PaO₂ ≥ 10 kPa; PaCO₂ ≤ 6.5 kPa); rectal temperature ≥ 36°C and temperature "p" ("p" not defined in report) ≥ 31°C; haemodynamically stable; chest and mediastinal tube output ≤ 2 mL/kg/h; and urine output ≥ 0.5 mL/kg/h |
| Loick 1999 | Participants were tracheally extubated as soon as they fulfilled extubation criteria: sufficient spontaneous ventilation, existing protective reflexes |
| Mehta 1998 | Not reported |
| Mehta 2008 | After surgery, participants were transferred to the recovery room and were extubated when they qualified for extubation |
| Mehta 2010 | Extubation criteria included haemodynamic stability with systolic blood pressure ≥ 100 mmHg (without inotropes and/or vasopressors), core temperature ≥ 36°C, spontaneous ventilation with PaO₂ ≥ 100 mmHg on FiO₂ = 0.4 and PaCO₂ ≤ 40 mmHg, blood loss from chest drains < 50 mL/h, and urine output > 1 mL/kg/h |
| Onan 2013 | All participants were extubated in the ICU after rewarming and haemodynamic stabilization. Participants were extubated using clinical criteria together with analytical criteria (PaO₂) with the participant breathing through a T piece. The decision was made by the consultant on call |
| Palomero 2008 | Extubation time was calculated starting from the moment the participant was transferred to the ICU |
| Petrovski 2006 | Not reported |
| Priestley 2002 | Participants in the ICU were weaned from positive‐pressure ventilation and were extubated when they met set criteria as assessed by the ICU nursing staff: participant responsive to voice, oxygen saturation > 94% on inspired oxygen concentration < 50%, respiratory rate < 20 breaths/min and no obvious respiratory distress, PaCO₂ < 50 mmHg, pH > 7.3, tidal volume > 7 mL/kg on pressure support < 12 cm H₂O above end‐expiratory pressure, temperature > 36.0°C, chest tube drainage < 100 mL/h, haemodynamic stability (i.e. not requiring significant inotropic support and no uncontrolled arrhythmia) |
| Royse 2003 | Extubation was performed when the participant was awake, co‐operative, normothermic (core body temperature 36°C), pH 7.3, and PaO₂ > 75 mmHg on 40% inspired oxygen |
| Sharma 2010 | Once participants were awake with adequate spontaneous ventilation and a stable haemodynamic state, they were weaned off the ventilator and the trachea was extubated. Extubation criteria were as follows: haemodynamic stability with mean arterial pressure > 60 mmHg (without or with minimal inotropes and/or vasopressors), core temperature ≥ 36°C, spontaneous ventilation with PaO₂ > 100 mmHg on FiO₂ ≤ 0.4 and PaCO₂ < 40 mmHg, blood loss from chest drains < 50 mL/h, and urine output > 1 mL/kg/h |
| Stenseth 1996 | Participants were extubated when awake, with adequate spontaneous ventilation (PaCO₂ < 6 kPa, PaO₂ > 10 kPa at FiO₂ = 0.6), and when in a stable haemodynamic state |
| Svircevic 2011 | Participants were extubated as soon as extubation criteria were met: core temperature > 36°C, difference core/skin temperature < 5°C, haemodynamic stability without the use of major doses of vasoactive medication, chest drain output < 1.5 mL/kg/h, presence of deglutition reflex, breathing minute volume > 80 mL/kg/min, breathing frequency > 10/min and < 20/min, oxygen saturation > 94% with FiO₂ ≤ 40% |
| Tenenbein 2008 | Postoperatively, participants' tracheas were extubated when they were haemodynamically stable, awake, and able to follow commands, with oxygen saturation ≥ 97%, FiO₂ ≤ 60%, and end‐tidal CO₂ ≤ 50 |
| Tenling 1999 | Participants were tracheally extubated when they were awake and haemodynamically stable and had carbon dioxide tension < 5.5 kPa while spontaneously breathing, oxygen tension > 10 kPa, FiO₂ < 0.45, and body temperature > 37.0°C |
| Usui 1990 | Extubation was considered once participants demonstrated the ability to breathe under continuous positive airway pressure |
| Yilmaz 2007 | Criteria for tracheal extubation were: stayed awake without stimulation, respiratory rate < 30 breaths/min, PaO₂ > 100 mmHg with FiO₂ ≤ 40% and PaCO₂ < 45 mmHg, stable haemodynamic and metabolic variables, and drainage < 100 mL/h |
| Zawar 2015 | Not reported |
cm H₂O: centimetre of water; CO₂: carbon dioxide; FiO₂: fraction of inspired oxygen; ICU: intensive care unit; kPa: kilopascal; PaCO₂: partial pressure of carbon dioxide; PaO₂: partial pressure of oxygen; pH: acidity or alkalinity of a solution on a logarithmic scale on which 7 is neutral; SaO₂: oxygen saturation; SpO₂: pulse oximetry; SvO₂: venous oxygen saturation.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (2018, Issue 11), Ovid MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE (1946 to 19 November 2018), Embase (1974 to 19 November 2018), the Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO host), and Web of Science (Science Citation Index (SCI)/Social Sciences Citation Index (SSCI)) (19 November 2018). We applied no language or publication status restriction. The exact search strategies can be found in Appendix 1.
Searching other resources
We screened reference lists from retrieved randomized trials, reviews, meta‐analyses, and systematic reviews (Appendix 2), to identify additional trials.
We searched for conference abstracts from 2012 to 2017: American Society of Regional Anesthesia spring meetings, and European Society of Anaesthesiology, European Society of Regional Anaesthesia, and American Society of Anesthesiologists (December 2017) meetings.
We searched the World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch), as well as ClinicalTrials.gov (http://www.clinicaltrials.gov), to identify trials in progress (February 2019). For trials in progress, we did not retain trials past the date of completion and not updated within the last two years. We did this to avoid listing registered trials that are unlikely to ever be completed by study authors.
Data collection and analysis
Selection of studies
We independently screened the lists of all titles and abstracts identified by the search above. We retrieved and independently read articles of interest to determine their eligibility for inclusion. We resolved discrepancies by discussion. We examined for classification trials that might be included and that we found through sources other than electronic databases (included, excluded, or awaiting classification). We documented the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009). We listed all reasons for exclusion in a Characteristics of excluded studies table.
Data extraction and management
We independently extracted data. For selected studies, we entered the following variables into our data extraction form: risk of bias as measured with the Cochrane tool; and outcomes and factors chosen a priori for assessment of heterogeneity (Higgins 2011a; Higgins 2011b). We extracted dichotomous data as the number of participants experiencing the event and the total number of participants in each treatment group. We extracted continuous data as means, standard deviations, and total numbers of participants. When data were not available in these formats, we extracted data as P values, numbers of participants, and direction of effect. We did not consider medians as equivalent to means, and we did not estimate standard deviations from quartiles or ranges. We entered first the site where the study was performed and the date of data collection (to facilitate exclusion of duplicate publications), then whether the study was included in the review or the reason for exclusion. After we reached agreement, one review author entered into the comprehensive meta‐analysis the data and moderators for heterogeneity exploration (Comprehensive Meta‐Analysis 2007). Also, after we reached agreement, we entered the risk of bias evaluation into Review Manager 5 (Review Manager 2014). We resolved disagreements by discussion. We contacted all study authors for additional information. We entered data for analysis into Review Manager in the format required to include the maximal number of studies (events and total numbers of participants for each group; means, standard deviations, and numbers of participants included in each group; or generic inverse variance, if necessary). When possible, we entered the data into an intention‐to‐treat analysis.
Assessment of risk of bias in included studies
We independently assessed the quality of included studies by using the Cochrane 'Risk of bias’ tool found in RevMan 5 (Higgins 2011a), to examine random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, or other risks of bias. We resolved disagreements by discussion. We assessed risk of bias on the basis of information presented in the reports or according to additional information received from study authors, while making no assumptions. We judged trials without a published protocol to be at low risk of bias for selective reporting when researchers provided in the results section the results for all measurements prespecified in the methods section.
Measures of treatment effect
We planned to report results as risk ratios (RRs) and to provide 95% confidence intervals (95% CIs) for dichotomous data (McColl 1998). Due to the large number of trials with zero cells, we analysed dichotomous data as risk differences (RDs). We reported results for continuous data (time of tracheal intubation) as mean differences (MDs) with 95% CIs. For continuous data, because some data were extracted from different scales (days, hours, or minutes), and some data were available only as P values, we reported results as standardised mean differences (SMDs) with 95% CIs. For results reported as SMDs, we gave equivalence on a clinical scale. For SMDs, we considered 0.2 a small effect, 0.5 a medium effect, and 0.8 a large effect (Pace 2011). When an effect was found, we calculated using the odds ratio the number to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) (Cates 2016; Deeks 2002). When we were not able to demonstrate an effect, we calculated the number of participants required in a large trial to make sure that enough participants were included in the retained studies to justify a conclusion on the absence of effect (Brant 2017; Pogue 1998).
Unit of analysis issues
We included only parallel‐group trials. If a study contained more than two groups, we fused two groups (by using the appropriate formula for adding standard deviations when required) when we thought they were equivalent (taking our factors for heterogeneity exploration into account), or we separated them and split the control group in half if we thought they were different.
Dealing with missing data
We contacted all study authors for additional information. We made no imputation.
Assessment of heterogeneity
We considered clinical heterogeneity before pooling results, and we examined statistical heterogeneity before carrying out any meta‐analysis. We quantified statistical heterogeneity by using the I² statistic. We quantified the amount of heterogeneity as low (I² < 25%), moderate (I² = 25% to 74%), or high (I² = 75% or higher), depending on the value obtained for the I² statistic (Higgins 2003).
Assessment of reporting biases
We assessed publication bias by using a funnel plot, followed by Duval and Tweedie’s trim and fill technique (Borenstein 2009; Duval 2000a; Duval 2000b). This technique not only assesses whether publication bias is likely, it also yields an estimate of effect size after correction for the possibility of publication bias when such bias is detected.
Data synthesis
We analysed data using Review Manager 5 and Comprehensive Meta‐Analysis Version 2.2.044 with fixed‐effect (I² < 25%) or random‐effects models (I² ≥ 25%) (Comprehensive Meta‐Analysis 2007; Review Manager 2014). For dichotomous data, we planned to provide results as RRs (values best understood by clinicians; McColl 1998), but due to the large number of trials with zero cells, we had to give results as RDs. For some continuous data, we had to enter data as P values, numbers of participants, and direction of effect using the RevMan 5 calculator (see Measures of treatment effect ). In such cases, MDs cannot be obtained. We then presented our results as SMDs and gave clinical equivalents calculated as follows: SMD multiplied by a typical SD on a clinical scale of one of the included trials (Higgins 2011b). For results in which the intervention produced an effect, we calculated the NNTB or the NNTH by using the odds ratio (http://www.nntonline.net/visualrx/) (Cates 2016). If an effect could not be demonstrated, we also calculated the number of participants required in a large trial to ensure that enough participants were included in the retained studies to justify a conclusion based on absence of effect (Brant 2017; Pogue 1998).
Subgroup analysis and investigation of heterogeneity
We divided all our outcomes as cardiac surgery with cardiopulmonary bypass and as off‐pump surgery (Kowalewski 2016). We looked at year of publication as a factor for heterogeneity so we could take into account changes in clinical practice and types of drugs used over time. We analysed subgroup differences using Review Manager (Chi²), and we considered a P value < 0.05 as significant for subgroup differences. We evaluated the effect of time by examining meta‐regressions between effect size and year of publication (pneumonia and duration of tracheal intubation), using Comprehensive Meta‐Analysis 2007.
Sensitivity analysis
We performed a sensitivity analysis on risk of bias.
'Summary of findings’ table and GRADE
We judged the quality of the body of evidence according to the GRADE system and presented this assessment in ’Summary of findings’ tables for each comparison for all of our outcomes: mortality (0 to 30 days), myocardial infarction, respiratory complications (respiratory depression or pneumonia), atrial fibrillation or atrial flutter, neurological complications (cerebrovascular accident or epidural haematoma), duration of tracheal intubation, pain at six to eight hours, and haemodynamic support (GRADEpro GDT; Schünemann 2013). For risk of bias, we judged the quality of evidence as high when we derived most information from studies at low risk of bias, and we downgraded quality when we obtained most information from studies at high or unclear risk of bias (allocation concealment and blinding of outcome assessors). For inconsistency, we downgraded the quality of evidence when the I² statistic was 75% or higher without satisfactory explanation. We did not downgrade the quality of evidence for indirectness because outcomes were based on direct comparisons performed on the population of interest and were not surrogate markers. For imprecision, we downgraded the quality of evidence when the confidence interval around the effect size was large or overlapped an absence of effect and failed to exclude an important benefit or harm, or when the number of participants was less than the number required in a large trial. For publication bias, we downgraded the quality of evidence when correcting for the possibility of publication bias as assessed by Duval and Tweedie’s fill and trim analysis changed the conclusion. It is noteworthy that although factors influencing the quality of evidence are additive – such that the reduction or increase in each individual factor is added together with the other factors to reduce or increase the quality of evidence for an outcome – grading the quality of evidence involves judgements that are not exclusive. Therefore, GRADE is not a quantitative system for grading the quality of evidence. Each factor for downgrading or upgrading reflects not discrete categories but a continuum within each category and among categories (Schünemann 2013). When the body of evidence is intermediate with respect to a particular factor, the decision about whether a study falls above or below the threshold for upgrading or downgrading the quality depends on judgment. Reviewers may decide not to downgrade, even if they have some uncertainty around a specific category, when they already downgraded for another factor and further lowering the quality of evidence for this outcome would seem inappropriate (Schünemann 2013).
When the quality of the body of evidence is high, further research is very unlikely to change our confidence in the estimate of effect. When quality is moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. When quality is low, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. When the quality is very low, any estimate of effect is very uncertain. Studies with low quality and very low quality of evidence are considered equivalent to observational studies.
Results
Description of studies
Results of the search
We identified 574 titles from the electronic search: 69 from CENTRAL, 106 from MEDLINE, 256 from EMBASE, 42 from CINHAL, and 101 from the Web of Science. We identified two additional trials from the other sources. We reviewed 107 trials for potential eligibility. Of these 107 trials, we excluded 38 for various reasons (see Figure 1Excluded studies, Characteristics of excluded studies, and Characteristics of ongoing studies).
1.
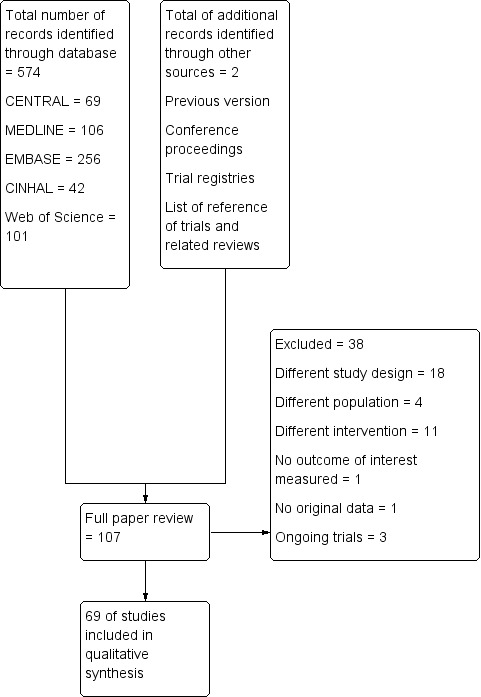
Study flow diagram for update in 2019.
Included studies
We included 69 trials with 4860 participants: 2404 given epidural analgesia and 2456 given comparators. Trials were published between 1988 and 2018.
Source of funding
Of the 66 included studies:
five were funded by governmental resources;
eight by charity;
23 by departmental/institutional resources; and
two in part by the industry; and
31 trials did not specify their sources of funding.
Setting
The trials were conducted at university hospitals (n = 66) or in tertiary care centre hospitals (n = 3).
The trials were conducted in Australia (n = 3); Bangladesh (n = 1); Canada (n = 1); China (n = 2); Cuba (n = 1); Czech Republic (n = 2); Denmark (n = 5); Egypt (n = 2); Germany (n = 5); India (n = 9); Italy and UK (n = 1); Japan (n = 2); Korea (n = 1); Lithuania (n = 1); Macedonia (n = 1); Norway (n = 3); Poland (n = 1); Russia (n = 1); Serbia (n = 1); Spain (n = 1); Sweden (n = 3); Taiwan (n = 2); Turkey (n = 8); The Netherlands (n = 4); UK (n = 5); and USA (n = 3).
Participants
The mean (or median) age of participants varied between 43.5 years and 74.6 years (Characteristics of included studies).
The types of surgeries performed were:
coronary artery bypass grafting (CABG) (n = 62);
mainly CABG (n = 1);
CABG or valve procedures (n = 4);
heart surgery for participants older than 15 years of age with congenital disease (n = 1); and
various cardiac procedures (n = 1).
The surgeries were performed:
with cardiopulmonary bypass (n = 50);
with off‐pump surgery (n = 15); and
on some participants with and some participants without cardiopulmonary bypass (n = 4).
Interventions
See Table 5.
Investigators administered epidural analgesia as a single injection block (n = 3); or as a continuous epidural analgesia with patient‐controlled analgesia (n = 7) or without patient‐controlled analgesia (n = 51); or as repeated injections through a catheter (n = 6).
The solution contained a local anaesthetic alone (n = 23); an opioid alone (n = 3); or a mixture of a local anaesthetic and an opioid (n = 41).
Two studies added clonidine and one added ketamine. A majority of studies added no other adjuvant to the solution (n = 64).
Local anaesthetics used were bupivacaine (n = 55); bupivacaine and ropivacaine (n = 1); ropivacaine (n = 7); levobupivacaine (n = 3); or mepivacaine (n = 1).
Opioids used were fentanyl (n = 24); morphine (n = 10); morphine or butorphanol (n = 1); sufentanil (n = 9); or hydromorphone (n = 1).
Mishra 2004 and Petrovski 2006 provided no details.
Comparators
See Table 5.
Researchers compared epidural analgesia versus systemic analgesia alone (n = 63), paravertebral blockade (n = 3), erector spinae plane block (n = 1), intrapleural analgesia (n = 1), or wound local anaesthetic infusion (n = 1).
Systemic analgesia consisted of morphine (intravenous (IV) patient‐controlled analgesia (PCA) (n = 7), IV infusion (n = 4), or on request (n = 9)); morphine or alfentanil (n = 2); fentanyl (IV PCA (n = 1) or infusion (n = 3)); nicomorphine (n = 1); piritramide (n = 5); tramadol (n = 4); meperidine (n = 3); meperidine and tramadol (n = 1); fentanyl and tramadol (n = 1); ketobemidone (n = 1); papaveretum (n = 1); diclofenac (n = 1); or various opioids (n = 4). The other trials did not provide details on systemic analgesia.
Study authors performed paravertebral blockade with bupivacaine infusion (n = 3).
Others performed erector spinae plane block with bupivacaine infusion (n = 1).
Some researchers provided intrapleural analgesia with repeated injections of bupivacaine (n = 1).
Others provided wound infusion with 0.15% bupivacaine (n = 1).
Excluded studies
We excluded 35 trials for the following reasons: different study design (n = 18), different study population (n = 4), different intervention (n = 11), or lack of original data in the publication (n = 1).
See Characteristics of excluded studies.
Studies awaiting classification
We have no studies awaiting classification.
Ongoing trials
We identified three ongoing trials (CTRI/2012/04/002608; CTRI/2018/05/013902; NCT03719248).
Risk of bias in included studies
2.
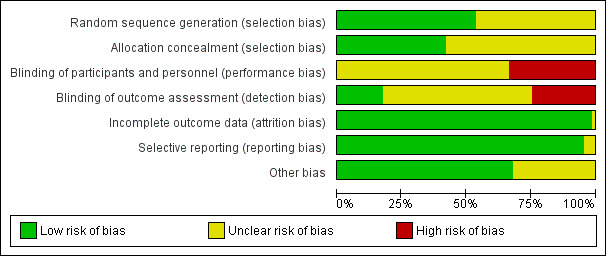
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
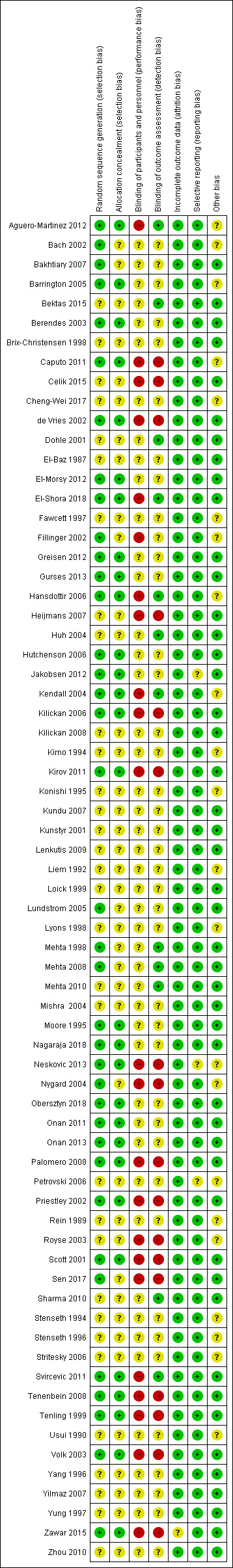
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We judged that no trials were at low risk of bias for all domains. Overall, we judged that the following percentages of included trials were at low risk of bias: 54% for random sequence generation, 42% for allocation concealment, 0% for blinding of participants and of personnel taking care of study participants, 17% for blinding of outcome assessment, 99% for attrition bias, 96% for selective reporting bias, and 68% for other risks of bias (Figure 2).
We judged random sequence generation as causing low risk of bias for 37 trials and unclear/high risk of bias for the other 32 trials.
Allocation
We judged 29 trials as having low risk and 40 trials as having unclear/high risk of bias for allocation concealment.
Blinding
We judged all 69 trials as having unclear/high risk of bias for blinding of study participants and personnel taking care of participants.
We judged 12 trials as having low risk of bias and the other 57 trials as having unclear/high risk of bias for blinding of outcome assessment.
Incomplete outcome data
We judged one trial as having high/unclear risk and the other 68 trials as having low risk of attrition bias.
Selective reporting
None of the trials published a protocol; therefore we judged this domain using the methods section of trial reports. We judged three trials as having unclear/high risk and the other 66 trials as having low risk of reporting bias.
Other potential sources of bias
We judged 47 trials to have low risk and 22 trials to have unclear/high risk of other bias.
We judged five trials as having low risk of bias for random sequence generation, allocation concealment, and blinding of outcome assessment (Aguero‐Martinez 2012; El‐Shora 2018; Hansdottir 2006; Kendall 2004; Svircevic 2011).
Risk of bias for each study
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, and selective reporting; and unclear/high risk of bias for blinding of participants and personnel and for other risks of bias (the group given systemic analgesia contained more aged participants).
We judged this trial to have low risk of bias for random sequence generation, incomplete outcome data, and selective reporting; and unclear/high risk of bias for allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (control group consisted of 27 participants, 13 of whom received a dopexamine infusion; supported in part by the industry).
We judged this trial to have low risk of bias for random sequence generation, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, and selective reporting; and unclear/high risk of bias for blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (prevalence of cerebrovascular and peripheral vascular disease was more frequent in the epidural group).
We judged this trial to have low risk of bias for blinding of outcome assessment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk for random sequence generation, allocation concealment, and blinding of participants and personnel.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, and selective reporting; and unclear/high risk of bias for blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (groups had similar demographic characteristics except for lung disease/chronic obstructive airways disease, which was more common in the epidural group, i.e. 23% vs 12%).
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (conference abstract with limited information).
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for blinding of outcome assessment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, and blinding of participants and personnel.
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel.
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (groups well balanced except for cardiopulmonary bypass time: 107 minutes for the epidural analgesia group vs 78 minutes for the no epidural group).
We judged this trial to have low risk of bias for random sequence generation, incomplete outcome data, and selective reporting; and unclear/high risk of bias for allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (groups had similar demographic data, except for the fact that 11 participants in the epidural group had a history of a myocardial infarction within the three months immediately preceding surgery compared with two participants in the systemic analgesia group (P < 0.005)).
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, and selective reporting; and unclear/high risk of bias for blinding of participants and personnel.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, and selective reporting; and unclear/high risk of bias for blinding of participants and personnel and other risks of bias (groups had similar demographic data, except for a higher incidence of off‐pump coronary artery bypass grafting in the epidural group and longer cardiopulmonary bypass time in the systemic analgesia group).
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for blinding of outcome assessment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, and blinding of participants.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel, blinding of outcome assessment, and selective reporting.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, and selective reporting; and unclear/high risk of bias for blinding of participants and personnel and other risks of bias (not in intention‐to‐treat analysis).
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation. allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (no details on preoperative demographic data of groups).
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (some participants had laparotomy to take the gastroepiploic artery used for coronary grafting).
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (not in intention‐to‐treat analysis; groups differed for time of surgery and number of mammary artery bypasses).
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (conference abstract with limited information).
We judged this trial to have low risk of bias for random sequence generation, blinding of outcome assessment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for allocation concealment and blinding of participants and personnel.
We judged this trial to have low risk of bias for random sequence generation, blinding of outcome assessment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for allocation concealment and blinding of participants and personnel.
We judged this trial to have low risk of bias for blinding of outcome assessment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, and blinding of participants and personnel.
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, and incomplete outcome data; and unclear/high risk of bias for blinding of participants and personnel, blinding of outcome assessment, selective reporting, and other risks of bias (not in intention‐to‐treat analysis).
We judged this trial to have low risk of bias for random sequence generation, incomplete outcome data, and selective reporting; and unclear/high risk of bias for allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (not in intention‐to‐treat analysis).
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, selective reporting, and other risks of bias (conference abstract with limited information).
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (not in intention‐to‐treat analysis).
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (not in intention‐to‐treat analysis; epidural group had longer bypass time; supported in part by industry).
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for blinding of outcome assessment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, and blinding of participants and personnel.
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (not in intention‐to‐treat analysis).
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (not in intention‐to‐treat analysis).
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (groups had similar demographic data, except for pulmonary disease).
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data and selective reporting; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other risks of bias (additional co‐analgesia for the group given systemic analgesia only).
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
We judged this trial to have low risk of bias for random sequence generation, allocation concealment, selective reporting, and other risks of bias; and unclear/high risk of bias for blinding of participants and personnel, blinding of outcome assessment, and incomplete outcome data.
We judged this trial to have low risk of bias for incomplete outcome data, selective reporting, and other risks of bias; and unclear/high risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Epidural analgesia compared with systemic analgesia for cardiac surgery with or without cardiopulmonary bypass in adults.
| Epidural analgesia compared with systemic analgesia for cardiac surgery with or without cardiopulmonary bypass in adults | ||||||
| Patient or population: adults undergoing cardiac surgery with or without cardiopulmonary bypass Settings: trials were conducted in university hospitals (n = 60) or at a tertiary care centre (n = 3). Trials were conducted in Australia (n = 3); Bangladesh (n = 1); Canada (n = 1); China (n = 2); Cuba (n = 1); Czech Republic (n = 2); Denmark (n = 5); Egypt (n = 1); Germany (n = 5); India (n = 6); Italy and UK (n = 1); Japan (n = 2); Korea (n = 1); Lithuania (n = 1); Macedonia (n = 1); Norway (n = 3); Poland (n = 1); Russia (n = 1); Serbia (n = 1); Spain (n = 1); Sweden (n = 3); Taiwan (n = 1); Turkey (n = 8); The Netherlands (n = 4); UK (n = 5); and USA (n = 3) Intervention: epidural analgesia Comparison: systemic analgesia | ||||||
| Outcomes | Illustrative comparative risks (95% CI)* | Risk difference or relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic analgesia | Epidural analgesia | |||||
| Mortality (0 to 30 days) | Study population | RD 0.00 (‐0.01 to 0.01) | 3418 (38 studies) | ⊕⊕⊝⊝ lowa | ||
| 6 per 1000 | 7 per 1000 (4 to 13) | |||||
|
Myocardial infarction (0 to 30 days) |
Study population | RD ‐0.01 (‐0.02 to 0.00) | 2713 (26 studies) | ⊕⊕⊝⊝ lowa | ||
| 40 per 1000 |
28 per 1000 (21 to 39) |
|||||
|
Pulmonary complications (0 to 30 days) |
Respiratory depression | RD ‐0.03 (‐0.05 to ‐0.01) | 1736 (21 studies) | ⊕⊕⊝⊝ lowb | NNTB 32 (95% CI 22 to 102) |
|
| Study population | ||||||
| 70 per 1000 |
42 per 1000 (30 to 57) |
|||||
| Pneumonia | RD ‐0.03 (‐0.07 to 0.01) | 1107 (10 studies) | ⊕⊕⊕⊝ moderatec | |||
| Study population | ||||||
| 148 per 1000 |
79 per 1000 (59 to 105) |
|||||
|
Atrial fibrillation or atrial flutter (0 to 2 weeks) |
Study population | RD ‐0.06 (‐0.10 to ‐0.01) | 2431 (18 studies) | ⊕⊕⊕⊝ moderatec | NNTB 14 (95% CI 8 to 90) |
|
| 327 per 1000 |
258 per 1000 (234 to 283) |
|||||
|
Risk of neurological complications (0 to 30 days) |
Cerebrovascular accident | RD ‐0.00 (‐0.01 to 0.01) | 2232 (18 studies) | ⊕⊝⊝⊝ very lowd | ||
| Study population | ||||||
| 12 per 1000 |
11 per 1000 (6 to 18) |
|||||
| Epidural haematoma |
RD 0.00 (‐0.01 to 0.01) |
3982 (53 studies) |
⊕⊝⊝⊝ lowa | |||
| Study population | ||||||
| 0 per 1000 |
0 per 1000 (0 to 2) |
|||||
| Duration of tracheal intubation | Mean duration of tracheal intubation was 0.78 SMD lower (‐1.01 to ‐0.55) |
3353 (40 studies) | ⊕⊕⊕⊝ moderatec | The difference was equivalent to 2.4 hourse and was more evident in older trials (see text) | ||
| Pain at rest at 6 to 8 hours after surgery | Mean pain scores were 1.35 SMD lower (‐1.98 to ‐0.72) | 502 (10 studies) |
⊕⊕⊕⊝ moderatef | The difference was equivalent to 1 on a score from 0 to 10e | ||
|
Haemodynamic support (in hospital) |
Hypotension or need for vasopressor boluses |
RD 0.21 (0.09 to 0.33) |
870 (17 studies) |
⊕⊝⊝⊝ lowg | The number needed to harm is 4 (95% CI 3 to 12) | |
| Study population | ||||||
| 451 per 1000 |
284 per 1000 (243 to 330) |
|||||
| Inotropic or vasopressor infusions |
RD 0.00 (‐0.06 to 0.07) |
1821 (23 studies) |
⊕⊝⊝⊝ lowh | |||
| Study population | ||||||
| 344 per 1000 |
338 per 1000 (302 to 376) |
|||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Confidence intervals were calculated using VassarStats (http://www.vassarstats.net/). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; RD: risk difference; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by one level for risk of bias and by one level for imprecision. bDowngraded by one level for risk of bias and by one level for possibility of publication bias.
cDowngraded by one level for risk of bias.
dDowngraded by one level for risk of bias, by one level for imprecision, and by one level for publication bias.
eThe equivalence was obtained by multiplying the SMD by a typical standard deviation of one of the included trials (Higgins 2011a).
fDowngraded by one level for heterogeneity.
gDowngraded by two levels for risk of bias.
Summary of findings 2. Epidural analgesia compared with peripheral nerve blocks for cardiac surgery in adult.
| Epidural analgesia compared with peripheral nerve blocks for cardiac surgery without cardiopulmonary bypass in adults | ||||||
|
Patient or population: adults undergoing cardiac surgery without cardiopulmonary bypass Settings: trials were conducted in university hospitals in Egypt (n = 1) or India (n = 3) Intervention: epidural analgesia Comparison: peripheral nerve blocks (erector spinae plane block (n = 1) or paravertebral blockade (n = 3)) | ||||||
| Outcomes | Illustrative comparative risks (95% CI)* | Risk difference or relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Peripheral nerve block | Epidural analgesia | |||||
| Mortallity (0 to 30 days | Study population | RD ‐0.03 (‐0.08 to 0.02) |
145 (1 study) |
⊕⊝⊝⊝ very lowa |
||
| 43 per 1000 |
13 per 1000 (2 to 72) |
|||||
|
Myocardial infarction (0 to 30 days) |
Study population | RD 0.00 (‐0.07 to 0.07) |
76 (2 studies) |
⊕⊝⊝⊝ very lowa |
||
| 0 per 1000 |
0 per 1000 (0 to 90) |
|||||
|
Pulmonary complications (0 to 30 days) |
We found no data for this outcome (respiratory depression or pneumonia) | |||||
|
Atrial fibrillation or atrial flutter (0 to 2 weeks) |
We found no data for this outcome | |||||
|
Risk of neurological complications (0 to 30 days) |
Cerebrovascular accident | |||||
| Study population |
RD 0.00 (‐0.03 to 0.03) |
145 (1 study) |
⊕⊝⊝⊝ very lowa |
|||
| 0 per 1000 |
0 per 1000 (0 to 49) |
|||||
| Epidural haematoma | ||||||
| Study population |
RD 0.00 (‐0.03 to 0.03) |
271 (4 studies) |
⊕⊕⊝⊝ lowb |
|||
| 0 per 1000 |
0 per 1000 (0 to 27) |
|||||
| Duration of tracheal intubation | Study population |
MD ‐0.08 hour (‐0.54 to 0.38 hour) |
271 (4 studies) |
⊕⊝⊝⊝ very lowa |
||
| 6.82 ± 2.14 hours (mean ± SD) | 6.67 ± 2.31 hours (mean ± SD) | |||||
|
Pain at rest at 6 to 8
hours after surgery (score from 0 to 10) |
Study population |
MD 0.12 (‐0.42 to 0.66) |
90 (2 studies) |
⊕⊝⊝⊝ very lowa |
||
| 2.20 ± 0.79 (mean ± SD) |
1.80 ± 0.22 (mean ± SD) |
|||||
|
Haemodynamic support (in hospital) |
Hypotension or need for vasopressor boluses |
RD 0.05 (‐0.08 to 0.18) |
40 (1 study) |
⊕⊕⊝⊝ lowb |
||
| Study population | ||||||
| 50 per 1000 | 0 per 1000 (0 to 161) |
|||||
| Inotropic or vasopressor infusions | ||||||
| We found no data for this outcome | ||||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Confidence intervals were calculated using VassarStats (http://www.vassarstats.net/) with no continuity correction. CI: confidence interval; MD: mean difference; RD: risk difference; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by one for risk of bias and by two levels for imprecision.
bDowngraded by two levels for imprecision.
Summary of findings 3. Epidural analgesia compared with intrapleural analgesia for cardiac surgery in adults.
| Epidural analgesia compared with intrapleural analgesia for cardiac surgery in adults | ||||||
|
Patient or population: adults undergoing cardiac surgery without cardiopulmonary bypass Settings: university hospital in India Intervention: epidural analgesia Comparison: intrapleural analgesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Risk difference or relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intrapleural analgesia | Epidural analgesia | |||||
|
Mortality (0 to 30 days) |
We found no data for this outcome | |||||
|
Myocardial infarction (0 to 30 days) |
Study population |
RD 0.00 (‐0.07 to 0.07) |
50 (1 study) |
⊕⊕⊝⊝ lowa |
||
| 0 per 1000 |
0 per 1000 (0 to 71) |
|||||
|
Pulmonary complications (0 to 30 days) |
We found no data for this outcome (respiratory depression or pneumonia) | |||||
| Atrial fibrillation or atrial flutter) (0 to 2 weeks) | We found no data for this outcome | |||||
|
Risk of neurological
complications (0 to 30 days) |
Cerebrovascular accident | |||||
| We found no data for this outcome | ||||||
| Epidural haematoma | ||||||
| Study population |
RD 0.00 (‐0.07 to 0.07) |
50 (1 study) |
⊕⊕⊝⊝ lowa |
|||
| 0 per 1000 | 0 per 1000 | |||||
| Duration of tracheal intubation | Study population |
MD ‐0.30 (‐1.20 to 0.60 hour) |
15 (1 study) |
⊕⊕⊝⊝ very lowb |
17 participants in the epidural analgesia group and 14 in the intrapleural analgesia group were extubated in the operating room Means and SDs given by study authors are those for the rest of the participants |
|
| 4.1 ± 0.59 hours (mean ± SD) |
3.8 ± 1.13 hours (mean ± SD) | |||||
|
Pain at rest at 6 to 8 hours (score from 0 to 10) |
Study population |
MD 0.84 (0.31 to 1.37) |
50 (1 study) |
⊕⊕⊝⊝ lowa |
||
| 4.52 ± 1.08 | 3.68 ± 0.82 | |||||
|
Haemodynamic support (in hospital) |
We found no data for this outcome | |||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Confidence intervals were calculated using VassarStats (http://www.vassarstats.net/). CI: confidence interval; MD: mean difference; RD: risk difference; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels for imprecision.
bDowngraded by one level for risk of bias and by two levels for imprecision.
Summary of findings 4. Epidural analgesia compared with wound infiltration for cardiac surgery in adults.
| Epidural analgesia compared with wound infiltration for adults undergoing cardiac surgery without cardiopulmonary bypass | ||||||
|
Patient or population: adults undergoing cardiac surgery without cardiopulmonary bypass Settings: university hospital in Taiwan Intervention: epidural analgesia Comparison: wound infiltration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Wound infiltration | Epidural analgesia | |||||
| Mortallity (0 to 30 days) | We found no data for this outcome | |||||
| Myocardial infarction (0 to 30 days) | We found no data for this outcome | |||||
|
Pulmonary complications (0 to 30 days) |
We found no data for this outcome (respiratory depression or pneumonia) | |||||
| Atrial fibrillation or atrial flutter (0 to 2 weeks) | We found no data for this outcome | |||||
| Risk of neurological complications (0 to 30 days) | We found no data for this outcome (cerebrovascular accident or epidural haematoma) | |||||
| Duration of tracheal intubation | One trial with 37 participants published as a conference abstract reported no difference in time to tracheal extubation between epidural analgesia and intravenous patient‐controlled analgesia plus wound infusion (numbers and P value not provided) (very low quality)a | |||||
|
Pain at rest at 6 to 8 hours (score from 0 to 10) |
One trial with 37 participants published as a conference abstract reported lower pain scores with epidural analgesia (numbers and P value not provided) (very low quality)a | |||||
|
Haemodynamic support (in hospital) |
We found no data for this outcome | |||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by one level for risk of bias and by two levels for imprecision.
Comparison 1: epidural analgesia compared with systemic analgesia
Primary outcomes
1. Risk of mortality
1a. Mortality at 0 to 30 days
Thirty‐eight trials with 3418 participants reported on mortality from 0 to 30 days after surgery: in hospital, at two weeks, or at 28 to 30 days. We obtained data from published reports or from study authors (n = 3; Bektas 2015; Celik 2015; Tenenbein 2008).
We did not find a difference in mortality at 0 to 30 days (risk difference (RD) 0.00, 95% confidence interval (CI) −0.01 to 0.01; Analysis 1.1; Table 1). There was no evidence of a small‐study effect. With correction for the impact of asymmetry in the funnel plot, the RD would be 0.00 (95% CI −0.002 to 0.01). For trials judged as having low risk of bias for blinding of outcome assessment, the RD would be 0.00 (95% CI −0.01 to 0.01). Based on an incidence of death at one month of 1%, 34,318 participants (17,159 per group) would be required in a large trial to eliminate a 25% difference (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality of evidence by one level for risk of bias, and by one level for imprecision, and we rated evidence as low quality.
1.1. Analysis.
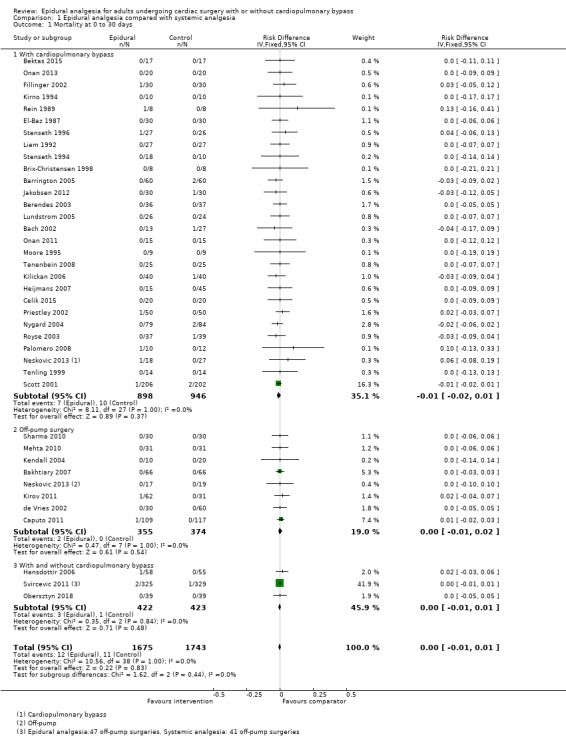
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 1 Mortality at 0 to 30 days.
1b. Mortality at six months
Seven trials with 407 participants gave results for mortality at six months (RD −0.00, 95% CI −0.03 to 0.03; Analysis 1.2). We obtained data from published reports or from study authors (n = 3; Bektas 2015; Celik 2015; Tenenbein 2008). We found no evidence of a small‐study effect. Correction for asymmetry of the funnel plot leads to an estimated RD of −0.02 (95% CI −0.04 to 0.01). For trials judged as at low risk of bias for blinding of outcome assessment, the RD would be 0.00 (95% CI −0.11 to 0.11). We downgraded the quality of evidence by one level for risk of bias and by two levels for imprecision, and we rated evidence as very low quality.
1.2. Analysis.
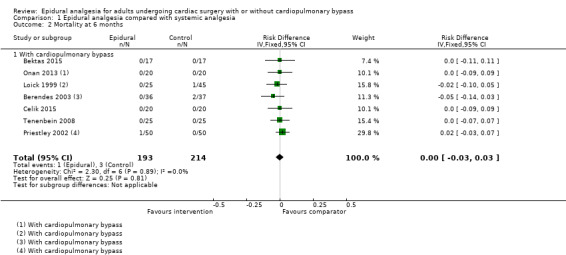
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 2 Mortality at 6 months.
1c. Mortality at one year
Five trials with 849 participants reported on mortality at one year after surgery (RD −0.01, 95% CI −0.03 to 0.00; Analysis 1.3). We obtained data from published reports or from study authors (n = 3; Bektas 2015; Celik 2015; Tenenbein 2008). We found no evidence of a small‐study effect. Correction for publication bias does not change the estimate. For trials judged as at low risk of bias for blinding of outcome assessment, the RD would be −0.01 (95% CI −0.03 to 0.01). Based on a 3% mortality rate, 2416 participants (1208 per group) would be required to eliminate a 50% difference in a large trial (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality of evidence by one level for risk of bias and by two levels for imprecision, and we rated evidence as very low quality for absence of effect.
1.3. Analysis.
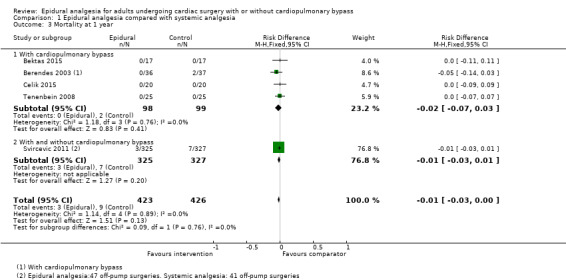
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 3 Mortality at 1 year.
Secondary outcomes
1. Risk of myocardial infarction (0 to 30 days)
Twenty‐six trials with 2713 participants gave results for myocardial infarction from 0 to 30 days: in hospital, at 30 days, or at an unspecified time point. The definition used by the study authors can be found in Table 6. We obtained data from published reports or from study authors (n = 3; Bektas 2015; Celik 2015; Neskovic 2013). Epidural analgesia may reduce myocardial infarction at 0 to 30 days (RD −0.01, 95% CI −0.02 to 0.00; Analysis 1.4; Table 1). We found no statistically significant evidence of a small‐study effect. The impact of asymmetry in the funnel plot leads to a trim and fill analysis estimate of RD −0.01 (95% CI −0.02 to 0.00) (fixed‐effect model). For trials judged as at low risk of bias for blinding of outcome assessment, the RD would be −0.00 (95% CI −0.03 to 0.02). Based on a 4% rate of myocardial infarction, 5640 participants (2820 per group) would be required in a large trial to eliminate a 30% difference (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality of evidence by one for risk of bias and by one for imprecision, and we rated evidence as low quality.
1.4. Analysis.
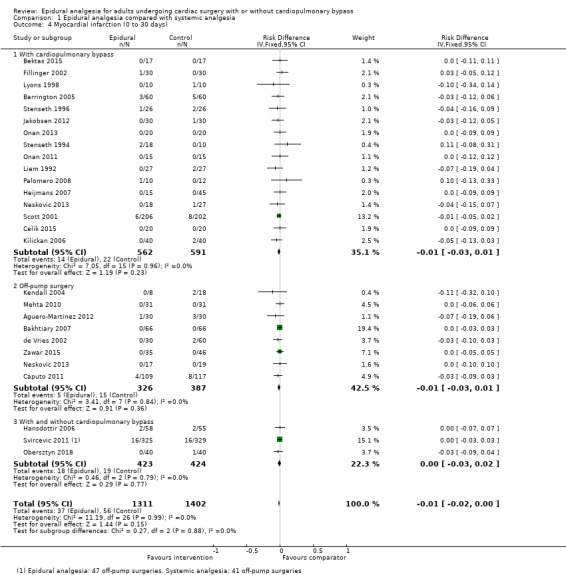
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 4 Myocardial infarction (0 to 30 days).
2. Risk of pulmonary complications
2a. Respiratory depression (0 to 30 days)
Twenty‐one trials with 1736 participants gave results for respiratory depression. Definitions used by study authors can be seen in Table 7. We obtained data from published reports or from study authors (n = 4; Bektas 2015; Celik 2015; Neskovic 2013; Tenenbein 2008). Results show that epidural analgesia decreases the risk of respiratory depression after cardiac surgery (RD −0.03, 95% CI −0.05 to −0.01; Analysis 1.5; Table 1). Egger's regression intercept indicates that a small‐study effect might be present (P = 0.01; two‐tailed). The asymmetry of the funnel plot leads to a trim and fill estimate of RD −0.01 (95% CI −0.03 to 0.00). For trials judged as at low risk of bias for blinding of outcome assessment, the RD would be 0.01 (95% CI −0.05 to 0.06). A decreased risk of respiratory depression may apply only for cardiac surgery with cardiopulmonary bypass (RD −0.04, 95% CI −0.07 to −0.01) ‐ not for off‐pump surgery (RD −0.01, 95% CI −0.05 to 0.02). The NNTB is 32 (95% CI 22 to 102) (Appendix 3). Based on a 7.5% incidence, 932 participants (466 per group) would be required in a large trial to eliminate a 50% difference (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality by one level for risk of bias and by one level for the possibility of publication bias that would change the conclusion, and we rated the quality of evidence as low.
1.5. Analysis.
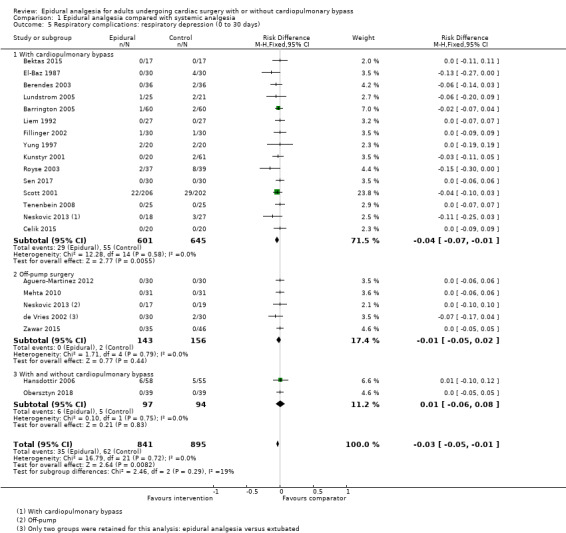
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 5 Respiratory complications: respiratory depression (0 to 30 days).
2b. Pneumonia (0 to 30 days)
Ten trials with 1107 participants gave results for pneumonia. The definition used by study authors can be found in Table 7. We obtained data from published reports or from study authors (n = 4; Celik 2015; de Vries 2002; Neskovic 2013; Tenenbein 2008). There might be no difference in the risk of pneumonia (RD −0.03, 95% CI −0.07 to 0.01; Analysis 1.6; Table 1). We found no evidence of a small‐study effect. The asymmetry of the funnel plot leads to a trim and fill estimate of RD −0.04 (95% CI −0.06 to −0.01). For trials judged as at low risk of bias for blinding of outcome assessment, the RD would be −0.05 (95% CI −0.12 to 0.01). Heterogeneity (I²) was 57% with no differences between subgroups. Trials were published between 2001 and 2015. The P value for effect size versus year of publication was 0.12 (residual P value 0.40). Based on a 16% incidence, 406 participants (203 per group) would be required in a large trial to eliminate a 50% difference (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality by one level for risk of bias, and we rated the quality of evidence as moderate.
1.6. Analysis.
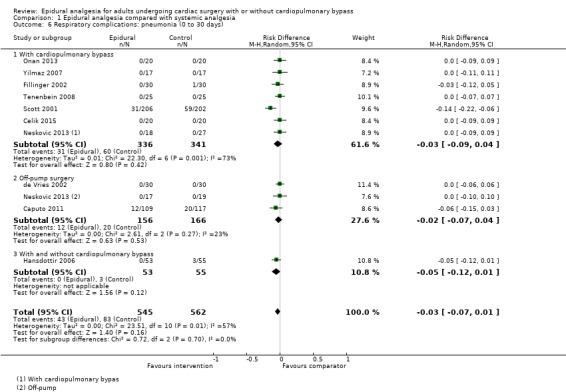
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 6 Respiratory complications: pneumonia (0 to 30 days).
3. Risk of atrial fibrillation or atrial flutter during surgery and within two weeks after surgery
Eighteen trials with 2431 participants reported on atrial fibrillation or atrial flutter. Epidural analgesia reduces the risk of atrial fibrillation or atrial flutter (RD −0.06, 95% CI −0.10 to −0.01; Analysis 1.7; Table 1). We obtained data from published reports or from study authors (n = 2; Bektas 2015; Neskovic 2013). We found no evidence of a small‐study effect. The asymmetry of the funnel plot leads to a trim and fill estimate of RD −0.07 (95% CI −0.12 to −0.02). The NNTB is 14 (95% CI 8 to 90) (Appendix 3). For trials judged as at low risk of bias for blinding of outcome assessment, the RD would be −0.09 (95% CI −0.17 to −0.01). Based on an incidence of 34%, 714 participants (357 per group) would be required in a large trial to eliminate a 25% difference (alpha 0.05; beta 0.2; one‐sided test). We downgraded the evidence by one level for risk of bias, and we rated the quality of evidence as moderate.
1.7. Analysis.
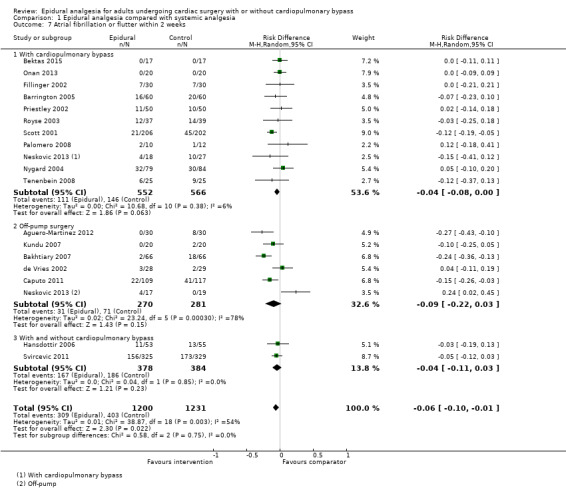
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 7 Atrial fibrillation or flutter within 2 weeks.
4. Risk of neurological complications
4a. Cerebrovascular accident (0 to 30 days)
Eighteen trials with 2232 participants reported on the risk of cerebrovascular accident. Study authors' definitions can be found in Table 8. We obtained data from published reports or from study authors (n = 4; Bektas 2015; Celik 2015; Neskovic 2013; Tenenbein 2008). The effect of epidural analgesia on cerebrovascular accident was uncertain (RD −0.00, 95% CI −0.01 to 0.01; Analysis 1.8; Table 1). We found no evidence of a small‐study effect. The asymmetry of the funnel plot leads to an estimated RD of −0.01 (95% CI −0.02 to −0.01). For trials judged as at low risk of bias for blinding of outcome assessment, the RD is −0.00 (95% CI −0.01 to 0.02). Based on an incidence of 1.5%, 22,778 participants (11,389 per group) would be required in a large trial to eliminate a 25% difference (alpha 0.05; beta 0.2; one‐sided test). We downgraded the level of quality by one for risk of bias, by two levels for imprecision, and by one level to correct for the possibility that publication bias would change the conclusion, and we rated the quality of evidence as very low.
1.8. Analysis.
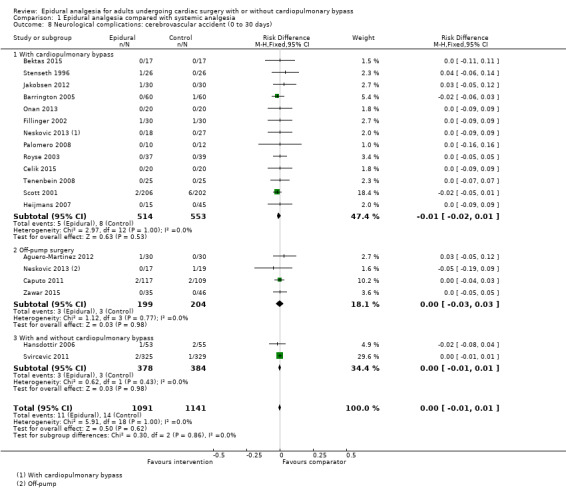
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 8 Neurological complications: cerebrovascular accident (0 to 30 days).
4b. Risk of serious neurological complications from epidural analgesia or epidural haematoma (0 to 30 days)
One trial reported one transient quadriparesis appearing on emergence of general anaesthesia for the epidural group (Tenenbein 2008). The participant awoke with quadriparesis (unable to move hands or legs). Computerized tomography (CT scan) showed that the tip of the epidural catheter had gone cephalad and was located at the cervical level (C3‐4), where the participant had a large osteophyte and cervical stenosis. By the time the CT scan was done, neurological function was returning, and the participant made a complete recovery. Study authors attributed this occurrence to local anaesthetic effect, which has been concentrated at the cervical level because of spinal stenosis.
Study authors reported no episodes of epidural haematoma in any of the included studies. Researchers clearly reported the information for 53 trials with 3982 participants (RD 0.00, 95% CI −0.01 to 0.01; Analysis 1.9; Table 1). We obtained information from published reports or from study authors (n = 3; Celik 2015; de Vries 2002; Neskovic 2013). For trials judged as at low risk of bias for outcome assessment, the RD is unchanged. We downgraded the quality by one for risk of bias and by one for imprecision, and we rated the quality of evidence as low.
1.9. Analysis.
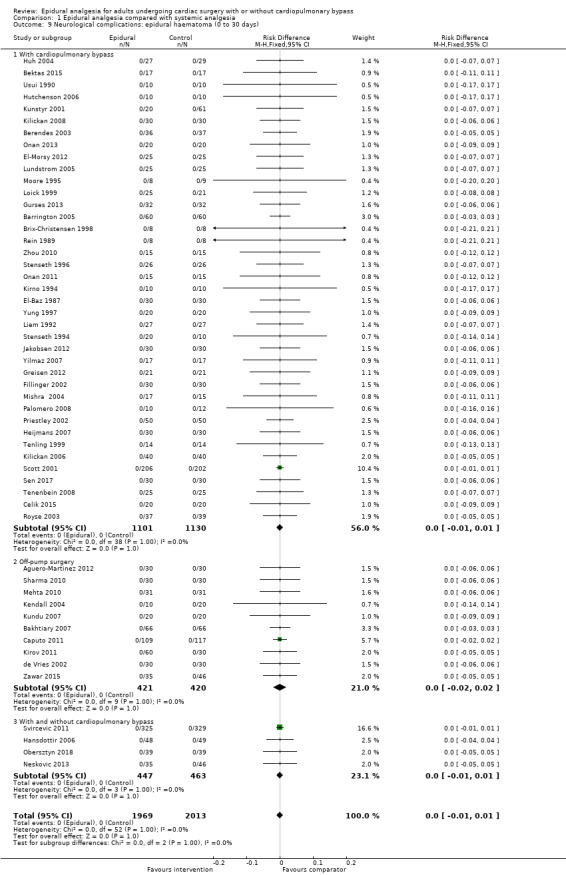
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 9 Neurological complications: epidural haematoma (0 to 30 days).
5. Duration of tracheal intubation
Forty trials with 3353 participants gave results for time to tracheal extubation. We obtained data from reports (n = 36) or from study authors (n = 2; Celik 2015; Neskovic 2013). For seven trials, results were not available because means and standard deviations (SDs) had to be extracted as P values (Barrington 2005; Caputo 2011; Jakobsen 2012; Kirov 2011; Priestley 2002; Stritesky 2006; Svircevic 2011). Epidural analgesia reduces the time of tracheal intubation (standardised mean difference (SMD) −0.78, 95% CI −1.01 to −0.55; Analysis 1.10; Table 1). A small‐study effect might be present (P = 0.0003; two‐sided test; Egger's regression intercept). The asymmetry of the funnel plot leads to a corrected estimate (SMD −0.29, 95% CI −0.56 to −0.03). For trials judged as at low risk of bias for blinding of outcome assessment, the SMD would be −0.75 (95% CI −1.25 to −0.25). We noted no difference between surgeries performed with or without cardiopulmonary bypass (P = 0.15 for heterogeneity between the first two subgroups). The effect was more evident in older trials: the P value for the meta‐regression effect size versus the year of publication was less than 0.0001 (Figure 4). With inclusion of Kendall 2004 (SD in the control group 3.1 hours), the difference would be equivalent to 2.4 hours. Considering only the trials for which means and SDs were available would lead to an estimate of mean difference (MD) of −2.91 hours (95% CI −3.61 to −2.21; 33 studies with 2062 participants; Analysis 1.11). For these trials, the mean duration of tracheal intubation was 6.1 hours for epidural analgesia and 9.1 hours for systemic analgesia (Appendix 4). We downgraded the quality of evidence by one level for risk of bias, and we judged the quality of evidence as moderate.
1.10. Analysis.
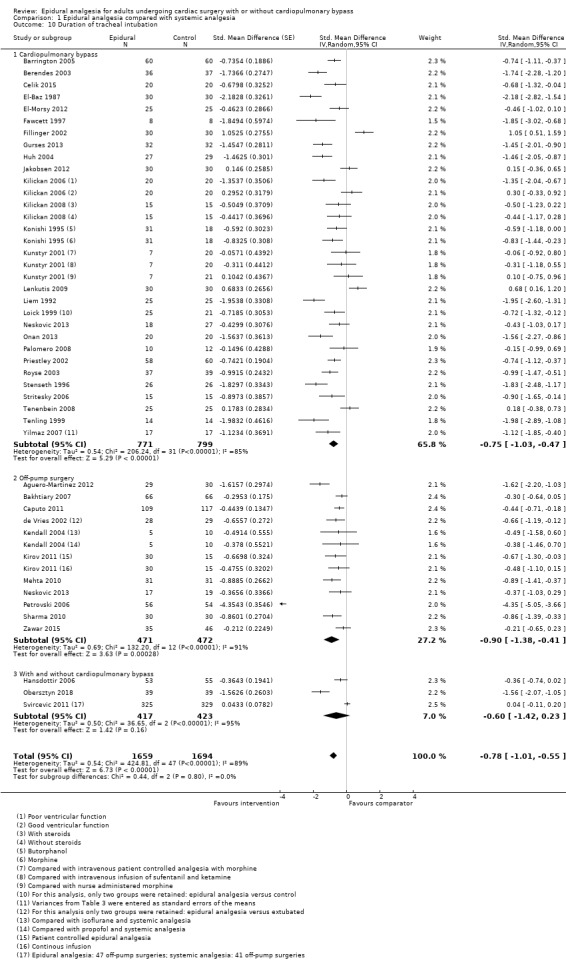
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 10 Duration of tracheal intubation.
4.
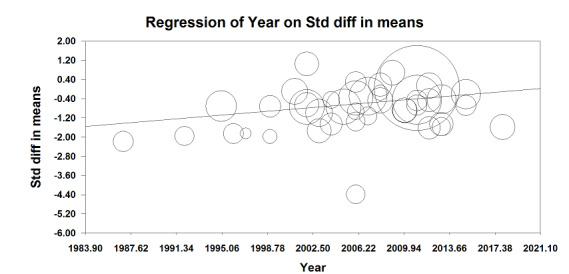
Meta‐regression.
Effect of epidural analgesia on tracheal extubation versus year of publication.
The effect was more evident in older trials: P value for the meta‐regression effect size versus year of publication was < 0.0001.
1.11. Analysis.
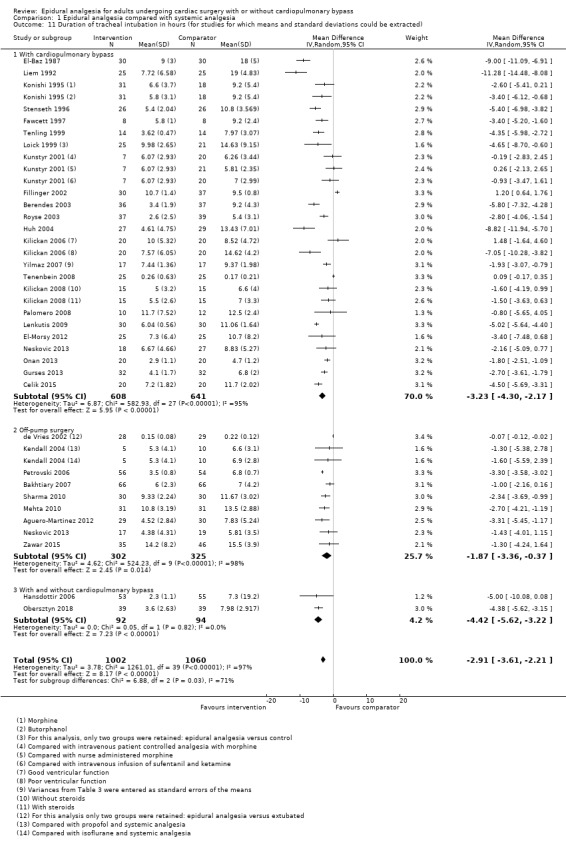
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 11 Duration of tracheal intubation in hours (for studies for which means and standard deviations could be extracted).
6. Pain
6a. Pain at six to eight hours
Pain at rest at six to eight hours
From 10 trials with 502 participants, epidural analgesia may reduce pain at rest at six to eight hours (SMD −1.35, 95% CI −1.98 to −0.72; Analysis 1.12). For five trials, data were available as means and SDs (MD −2.26, 95% CI −4.84 to 0.32; Analysis 1.13). For trials judged as having low risk of bias for blinding of outcome assessment, SMD is −2.35 (95% CI −4.04 to −0.66), Egger's regression intercept showed the possibility of a small‐study effect (P = 0.001; two‐tailed). Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. Based on data from Mehta 2010 (SD 0.7), the difference would be equivalent to 1 on a score from 0 to 10. In trials for which data were available as means and SDs, mean pain scores were 1.9 for epidural analgesia and 4.2 for systemic analgesia (Appendix 5). We downgraded the quality of evidence by one level for heterogeneity and rated it as moderate.
1.12. Analysis.
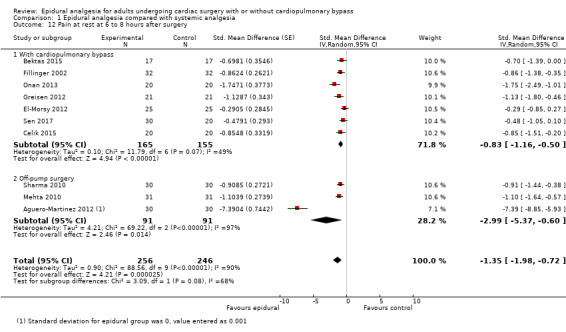
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 12 Pain at rest at 6 to 8 hours after surgery.
1.13. Analysis.
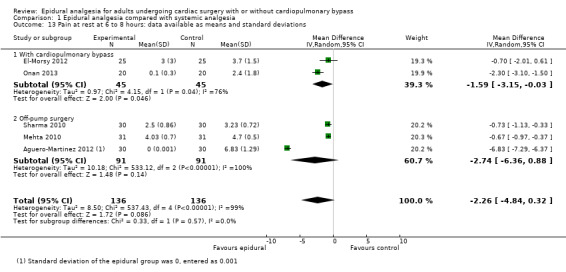
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 13 Pain at rest at 6 to 8 hours: data available as means and standard deviations.
Pain on movement or coughing at six to eight hours
From five trials with 342 participants, epidural analgesia may reduce pain on movement at six to eight hours (SMD −1.39, 95% CI −2.16 to −0.62; Analysis 1.14). We found no statistically significant evidence of small‐study effect. Correcting the asymmetry of the funnel plot gives an estimated SMD of −0.97 (95% CI −1.86 to −0.08). For trials with data available as means and SDs, the MD is −2.46 (95% CI −4.37 to −0.54; Analysis 1.15). For trials judged as at low risk of bias for blinding of outcome assessment (available only for off‐pump surgery), the SMD is −1.01 (95% CI −1.24 to −0.78).
1.14. Analysis.
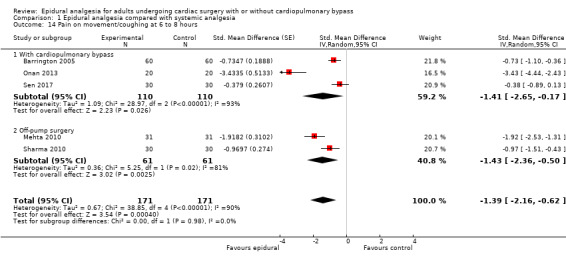
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 14 Pain on movement/coughing at 6 to 8 hours.
1.15. Analysis.
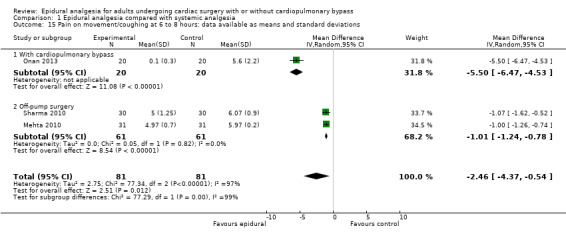
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 15 Pain on movement/coughing at 6 to 8 hours: data available as means and standard deviations.
6b. Pain at 24 hours
Pain at rest at 24 hours
From 22 trials with 2033 participants, epidural analgesia may reduce pain at rest at 24 hours (SMD −0.93, 95% CI −1.22 to −0.65; Analysis 1.16). The difference was higher for off‐pump surgery (P < 0.00001 for heterogeneity between subgroups; Analysis 1.16). Egger's regression intercept showed the possibility of a small‐study effect (P = 0.001; two‐tailed). Correcting the asymmetry of the funnel plot gives an estimated SMD of −0.43 (95% CI −0.74 to −0.13). For trials with data available as means and SDs, the MD is −1.53 (95% CI −2.51 to −0.55; Analysis 1.17). For trials judged as at low risk of bias for blinding of outcome assessment, the SMD is −1.37 (95% CI −2.19 to −0.54).
1.16. Analysis.
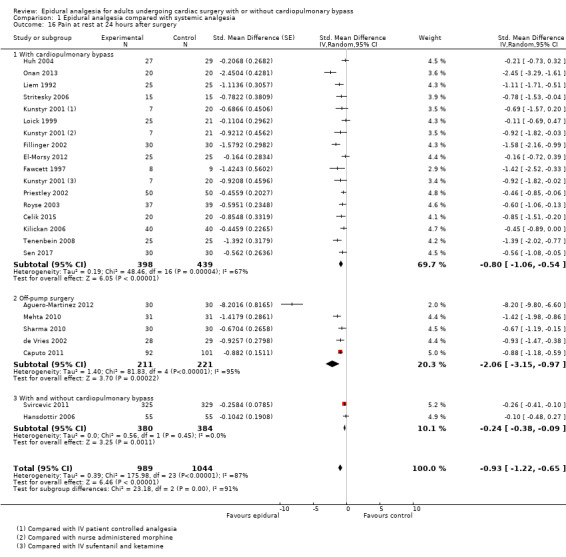
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 16 Pain at rest at 24 hours after surgery.
1.17. Analysis.
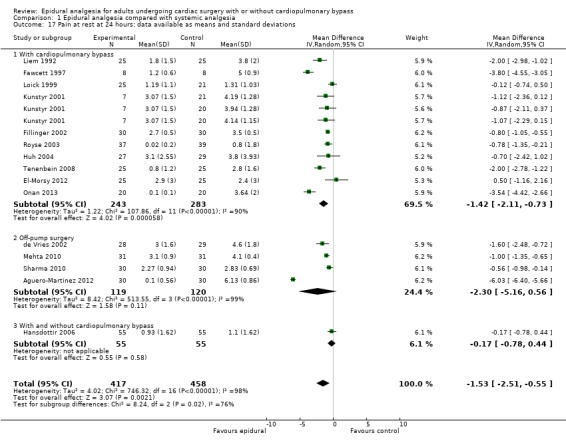
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 17 Pain at rest at 24 hours: data available as means and standard deviations.
Pain on movement or coughing at 24 hours
From 12 trials with 842 participants, epidural analgesia may reduce pain on movement at 24 hours (SMD −0.83, 95% CI −1.18 to −0.49; Analysis 1.18). Egger's regression intercept showed the possibility of a small‐study effect (P = 0.02; two‐tailed). We found no evidence of publication bias. For trials with data available as means and SDs, the MD is −1.74 (95% CI −2.63 to −0.86; Analysis 1.19). For trials judged as at low risk of bias for blinding of outcome assessment, the SMD is −0.59 (95% CI −1.28 to 0.11).
1.18. Analysis.
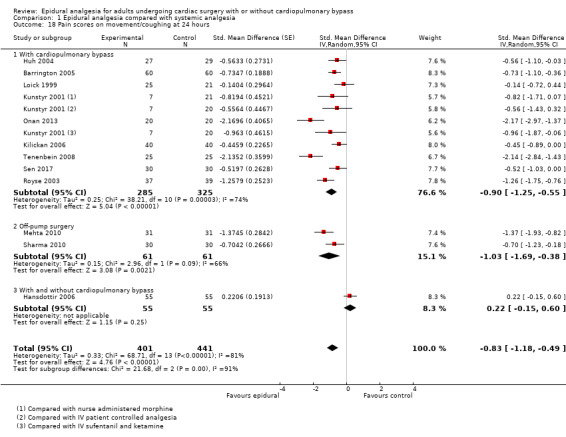
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 18 Pain scores on movement/coughing at 24 hours.
1.19. Analysis.
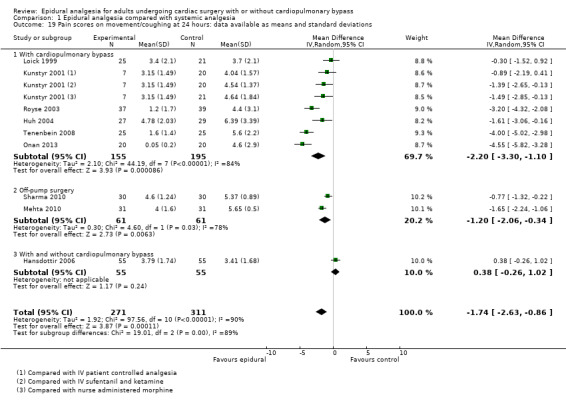
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 19 Pain scores on movement/coughing at 24 hours: data available as means and standard deviations.
6c. Pain at 48 hours
Pain at rest at 48 hours
From 15 trials with 1649 participants, epidural analgesia may reduce pain at rest at 48 hours (SMD −1.01, 95% CI −1.37 to −0.64; Analysis 1.20). Egger's regression intercept showed the possibility of a small‐study effect (P = 0.01; two‐tailed). Correcting the asymmetry of the funnel plot gives an estimated SMD of −0.38 (95% CI −0.78 to 0.02). For trials with data available as means and SDs, the MD is −1.31 (95% CI −1.99 to −0.64; Analysis 1.21). For trials judged as at low risk of bias for blinding of outcome assessment, the SMD is −1.34 (95% CI −2.16 to −0.53).
1.20. Analysis.
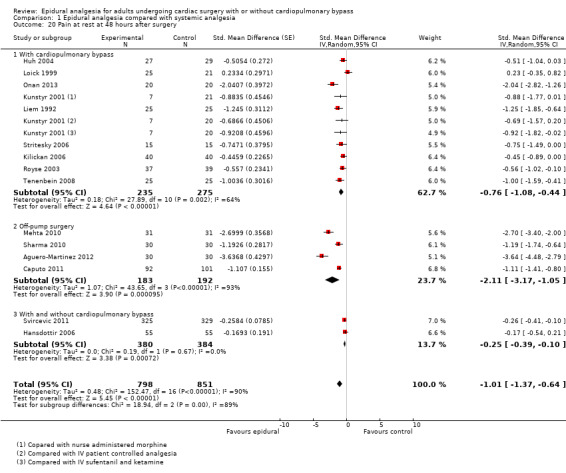
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 20 Pain at rest at 48 hours after surgery.
1.21. Analysis.
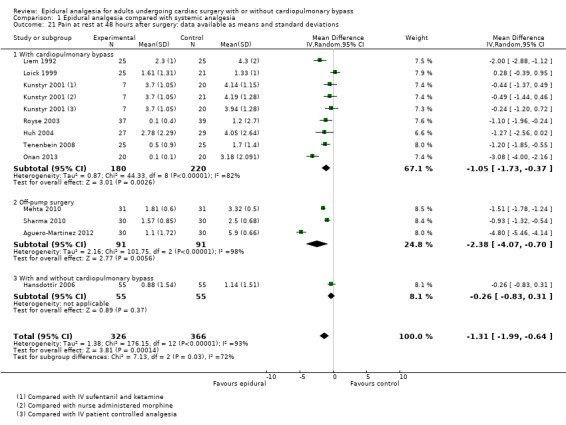
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 21 Pain at rest at 48 hours after surgery: data available as means and standard deviations.
Pain on movement or coughing at 48 hours
From 10 trials with 700 participants, epidural analgesia may reduce pain on movement at 48 hours (SMD −0.83, 95% CI −1.31 to −0.35; Analysis 1.22). Egger's regression intercept showed the possibility of a small‐study effect (P = 0.04; two‐tailed). Correcting the asymmetry of the funnel plot gives an estimated SMD of −1.06 (95% CI −1.49 to −0.64). For trials with data available as means and SDs, the MD is −1.30 (95% CI −2.00 to −0.60; Analysis 1.23). For trials judged as at low risk of bias for blinding of outcome assessment, the SMD is −0.71 (95% CI −1.76 to 0.34).
1.22. Analysis.
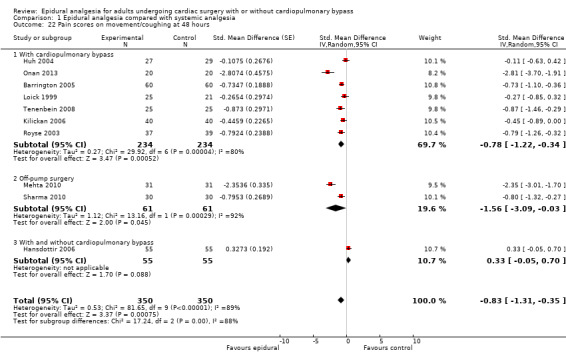
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 22 Pain scores on movement/coughing at 48 hours.
1.23. Analysis.
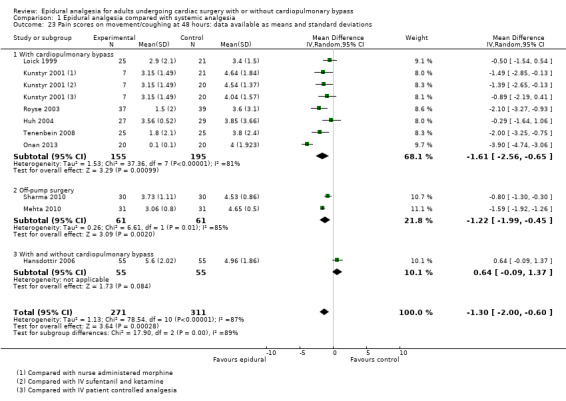
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 23 Pain scores on movement/coughing at 48 hours: data available as means and standard deviations.
6d. Pain at 72 hours
Pain at rest at 72 hours
From 12 trials with 897 participants, epidural analgesia may reduce pain at rest at 72 hours (SMD −1.09, 95% CI −1.57 to −0.62; Analysis 1.24). We found no statistically significant evidence of a small‐study effect. Correcting the asymmetry of the funnel plot gives an estimated SMD of −1.20 (95% CI −1.71 to −0.69). For trials with data available as means and SDs, the MD is −1.02 (95% CI −1.41 to −0.63; Analysis 1.25). For trials judged as at low risk of bias for blinding of outcome assessment, the SMD is −1.10 (95% CI −1.96 to −0.24).
1.24. Analysis.
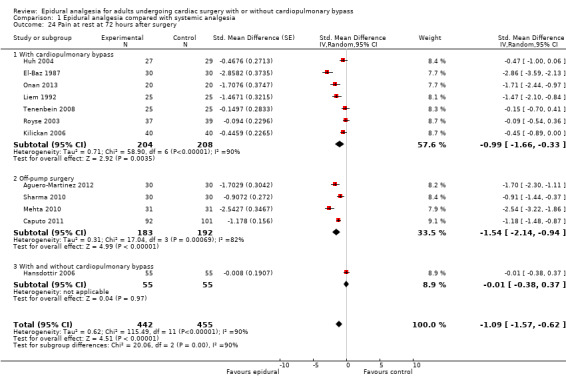
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 24 Pain at rest at 72 hours after surgery.
1.25. Analysis.
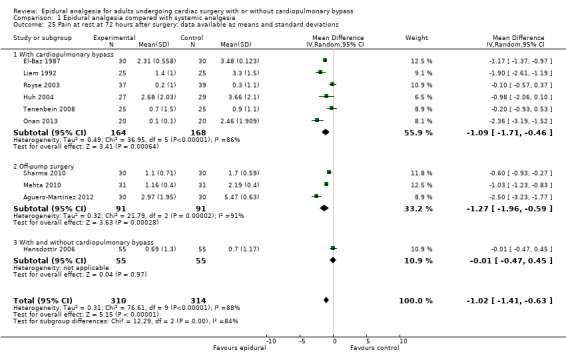
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 25 Pain at rest at 72 hours after surgery: data available as means and standard deviations.
Pain on movement or coughing at 72 hours
From nine trials with 654 participants, epidural analgesia may reduce pain on movement at 72 hours (SMD −0.62, 95% CI −1.13 to −0.11; Analysis 1.26). We found no statistically significant evidence of small‐study effect. Correcting the asymmetry of the funnel plot gives an estimated SMD of −0.82 (95% CI −1.24 to −0.39). For trials with data available as means and SDs, the MD is −0.90 (95% CI −1.49 to −0.30; Analysis 1.27). For trials judged as at low risk of bias for blinding of outcome assessment, the SMD is −0.86 (95% CI −1.87 to 0.15).
1.26. Analysis.
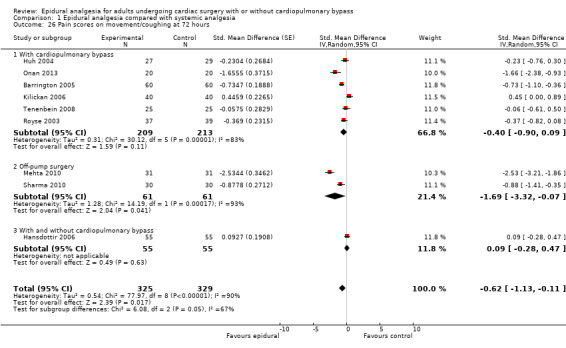
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 26 Pain scores on movement/coughing at 72 hours.
1.27. Analysis.
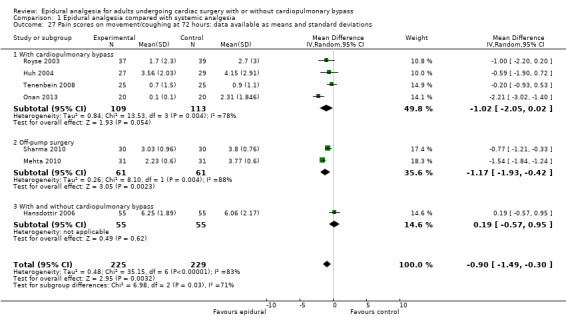
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 27 Pain scores on movement/coughing at 72 hours: data available as means and standard deviations.
7. Haemodynamic support (in hospital)
7a. Hypotension or need for vasopressor boluses
From 17 trials with 870 participants, epidural analgesia may increase the risk of hypotension and/or the need for vasopressor boluses (RD 0.21, 95% CI 0.09 to 0.33; Analysis 1.28). Egger's regression intercept showed the possibility of a small‐study effect (P = 0.01; two‐tailed). Correcting the asymmetry of the funnel plot gives an estimated RD of 0.13 (95% CI 0.02 to 0.24). We judged that only one trial was at low risk of bias for blinding of the outcome assessor (RD −0.07, 95% CI −0.17 to 0.04). From an incidence of 30% in the systemic analgesia group, the number needed to harm is 4 (95% CI 3 to 12). From an incidence of 30%, 480 participants (240 per group) would be required in a large trial to eliminate a 25% increase in incidence (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality by two levels for risk of bias and rated it as low.
1.28. Analysis.
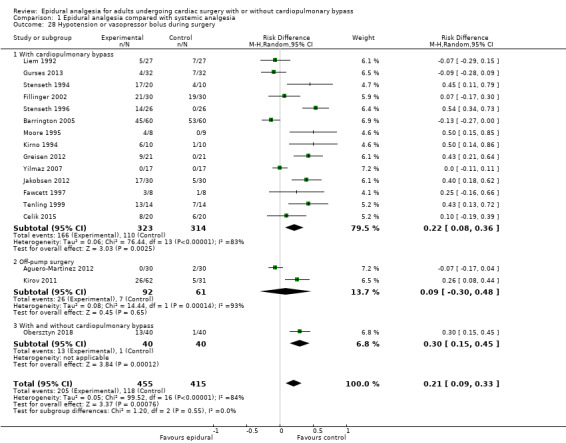
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 28 Hypotension or vasopressor bolus during surgery.
7b. Inotropic or vasopressor infusions
From 23 trials with 1821 participants, epidural analgesia makes little or no difference in the need for vasopressor or inotropic infusions (RD 0.00, 95 CI −0.06 to 0.07; Analysis 1.29). Criteria used by study authors are provided in Table 10. We found no evidence of a small‐study effect. Correcting the asymmetry of the funnel plot gives an estimated RD of 0.05 (95% CI −0.02 to 0.12). For trials judged as at low risk of bias for binding of outcome assessment, the RD is −0.06 (95% CI −0.17 to 0.05). From an incidence of 34%, 396 participants (198 per group) would be required in a large trial to eliminate a 25% increase in incidence (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality by two levels for risk of bias and rated it as low.
1.29. Analysis.
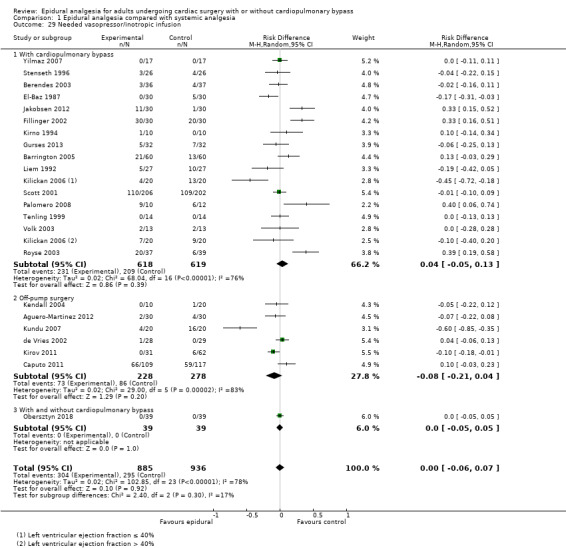
Comparison 1 Epidural analgesia compared with systemic analgesia, Outcome 29 Needed vasopressor/inotropic infusion.
6. Criteria for hypotension or use of inotropics/vasopressors.
| Study | Definition |
| Aguero‐Martinez 2012 | Change > 20% of basal value after local anaesthetic injection |
| Bach 2002 | Mean arterial blood pressure < 55 mmHg |
| Barrington 2005 | Mean arterial blood pressure < 65 mmHg |
| Berendes 2003 | Unspecified |
| Celik 2015 | Intraoperative hypotension |
| de Vries 2002 | Mean arterial blood pressure < 60 mmHg |
| Fawcett 1997 | Mean arterial blood pressure < 60 mmHg |
| Dohle 2001 | Unspecified |
| Fillinger 2002 | Unspecified |
| Greisen 2012 | Unspecified |
| Gurses 2013 | Mean arterial blood pressure < 50 mmHg |
| Jakobsen 2012 | Mean arterial blood pressure < 60 mmHg |
| Kendall 2004 | Variation > 20% from baseline |
| Kilickan 2006 | Systolic arterial blood pressure < 80 mmHg |
| Kirno 1994 | Unspecified |
| Kirov 2011 | Mean arterial blood pressure < 60 mmHg |
| Kundu 2007 | Unspecified |
| Liem 1992 | Change in mean arterial blood pressure ≥ 20% of baseline value |
| Moore 1995 | Mean blood arterial pressure < 50 mmHg |
| Palomero 2008 | Mean blood arterial pressure < 50 mmHg |
| Royse 2003 | Systolic arterial blood pressure ≤ 100 mmHg |
| Scott 2001 | Mean arterial blood pressure ≤ 40 mmHg |
| Stenseth 1994 | Mean arterial blood pressure < 65 mmHg |
| Stenseth 1996 | Mean arterial blood pressure ≤ 65 mmHg |
| Tenling 1999 | Mean arterial blood pressure decreased > 30% from baseline |
| Tenenbein 2008 | Mean arterial blood pressure < 55 mmHg |
| Volk 2003 | Unspecified |
| Yilmaz 2007 | Mean arterial blood pressure < 50 mmHg |
Comparison 2: epidural analgesia compared with peripheral nerve blocks
Primary outcomes
1. Risk of mortality
From one trial with 145 participants, epidural analgesia makes little or no difference for mortality at 0 to 30 days (RD −0.03, 95% CI −0.08 to 0.02; Analysis 2.1). We judged this trial as having low risk of bias for blinding of outcome assessment. We downgraded the quality by one level for risk of bias and by two levels for imprecision. We judged the quality as very low.
2.1. Analysis.
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 1 Mortality at 0 to 30 days.
We found no data for this outcome at six months nor at one year.
Secondary outcomes
1. Risk of myocardial infarction (0 to 30 days)
Two trials with 76 participants compared epidural analgesia versus paravertebral blockade for off‐pump cardiac surgery. Results show no myocardial infarction at 0 to 30 days (RD 0.00, 95% CI −0.07 to 0.07; Analysis 2.2; Table 2). We judged the two trials as having low risk of bias for blinding of outcome assessment. Based on a 4% rate of myocardial infarction, 5640 participants (2820 per group) would be required in a large trial to eliminate a 30% difference (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality of evidence by one level for risk of bias and by two levels for imprecision, and we rated the quality as very low.
2.2. Analysis.
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 2 Myocardial infarction (0 to 30 days).
2. Risk of pulmonary complications
2a. Respiratory depression (0 to 30 days)
We found no data for this outcome.
2b. Pneumonia (0 to 30 days)
We found no data for this outcome.
3. Risk of atrial fibrillation or atrial flutter during surgery and within two weeks after surgery
We found no data for this outcome.
4. Risk of neurological complications
4a. Cerebrovascular accident (0 to 30 days)
From one trial with 145 participants, epidural analgesia makes little or no difference in the risk of cerebrovascular accident at 0 to 30 days (RD 0.00, 95% CI −0.03 to 0.03). We judged this trial as at low risk of bias for blinding of outcome assessment.
4b. Risk of serious neurological complications from epidural analgesia or epidural haematoma (0 to 30 days)
Mehta 2008 reported two participants with epidural analgesia who experienced transient numbness and no participants with epidural haematoma. Dohle 2001 reported that one participant reported pain at the epidural catheter insertion site and no complications of paravertebral blockade. For epidural haematoma, the RD is 0.00 (95% CI −0.03 to 0.03; four trials with 271 participants; Analysis 2.4; Table 2). For trials judged as at low risk of bias for blinding of outcome assessment, the RD is 0.00 ( −0.03 to 0.03). We downgraded the quality by two levels for imprecision and rated the quality as low.
2.4. Analysis.
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 4 Neurological complications: epidural haematoma (0 to 30 days).
5. Duration of tracheal intubation
Four trials with 271 participants compared epidural analgesia versus paravertebral blockade or erector spinae plane blockade. We did not find a difference for time to tracheal extubation (MD −0.08 hour, 95% CI −0.54 to 0.38 hour; Analysis 2.5; Table 2). We found no evidence of a small‐study effect. Correcting the asymmetry of the funnel plot gives an estimated MD of −0.05 hour (95% CI −0.50 to 0.40). For trials judged as at low risk of bias for blinding of outcome assessment, the MD is −0.18 hour (95% CI −1.41 to 1.05). We downgraded the quality of evidence by one for risk of bias and by two levels for imprecision, and we rated the quality as very low.
2.5. Analysis.
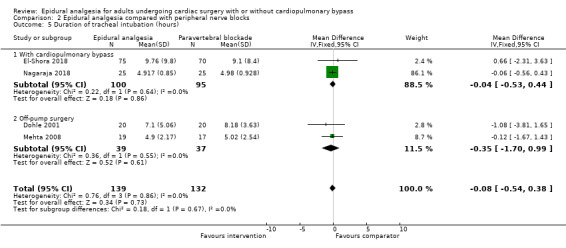
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 5 Duration of tracheal intubation (hours).
6. Pain
6a. Pain at six to eight hours
Pain at rest at six to eight hours
From two trials with 90 participants, epidural analgesia makes little or no difference in pain at rest at six to eight hours (MD 0.12, 95% CI −0.42 to 0.66; Analysis 2.6). For the trial judged as at low risk of bias for blinding of outcome assessment, the MD is 0.80 (95% CI −0.61 to 2.21). We downgraded the quality by one level for risk of bias and by two levels for imprecision and rated it as very low.
2.6. Analysis.
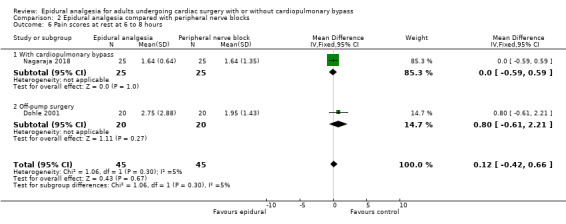
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 6 Pain scores at rest at 6 to 8 hours.
Pain on movement or coughing at six to eight hours
From two trials with 90 participants, epidural analgesia makes little or no difference in pain on movement at six to eight hours (MD −0.15, 95% CI −0.69 to 0.39; Analysis 2.6). For the trial judged as at low risk of bias for blinding of outcome assessment, the MD is −0.40 (95% CI −1.57 to 0.77).
6b. Pain at 24 hours
Pain at rest at 24 hours
From three trials with 231 participants, epidural analgesia makes little or no difference in pain at rest at 24 hours (MD 0.11, 95% CI −0.41 to 0.63; Analysis 2.8). We found no evidence of a small‐study effect. Correcting the asymmetry of the funnel plot gives an estimated MD of 0.29 (95% CI −0.25 to 0.82). For the two trials judged as at low risk of bias for blinding of outcome assessment, the MD is −0.10 (95% CI −0.51 to 0.31).
2.8. Analysis.
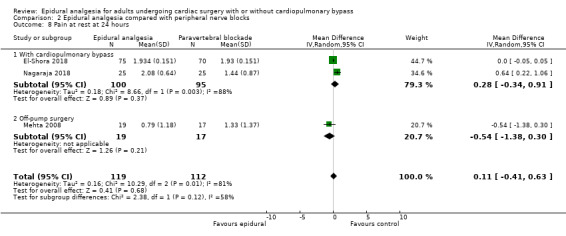
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 8 Pain at rest at 24 hours.
Pain on movement or coughing at 24 hours
From two trials with 86 participants, epidural analgesia makes little or no difference in pain on movement or coughing (MD 0.31, 95% CI −0.62 to 1.24). For the trial judged as at low risk of bias, the MD is −0.24 (95% CI −1.11 to 0.63).
6c. Pain at 48 hours
Pain at rest at 48 hours
From two trials with 195 participants, epidural analgesia makes little or no difference in pain at rest at 48 hours after surgery (MD 0.51, 95% CI −0.77 to 1.80). For the trial judged as at low risk of bias for blinding of outcome assessment, the MD is ‐0.11 (95% CI −0.15 to ‐0.77).
Pain on movement or coughing at 48 hours
From one trial with 50 participants, pain on movement or on coughing at 48 hours may be greater with epidural analgesia than with bilateral erector spinae block (MD 1.36, 95% CI 0.76 to 1.96). We judged this trial to be at unclear risk of bias for blinding of outcome assessment.
6d. Pain at 72 hours
Pain at rest at 72 hours
We found no data for this outcome.
Pain on movement or coughing at 72 hours
We found no data for this outcome.
7. Haemodynamic support (in hospital)
7a. Hypotension or need for vasopressor boluses
From one trial with 40 participants, epidural analgesia makes little or no difference in risk of hypotension (RD 0.05, 95% CI −0.08 to 0.18). We judged this trial to be at low risk of bias for blinding of outcome assessment. From an incidence of 5% with epidural analgesia, 1720 participants per group would be required in a large trial to eliminate a 25% decrease with peripheral nerve block (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality by two levels for imprecision and rated it as low.
7b. Inotropic or vasopressor infusions
We found no data for this outcome.
Comparison 3: epidural analgesia compared with intrapleural analgesia
Primary outcomes
1. Risk of mortality
We found no data for this outcome at 0 to 30 days, at six months, or at one year.
Secondary outcomes
1. Risk of myocardial infarction (0 to 30 days)
One small trial with 50 participants reported no myocardial infarction in either group (RD 0.00, 95% CI −0.07 to 0.07; Analysis 3.1; Table 3; Mehta 1998). We judged this trial to be at low risk of bias for blinding of outcome assessment. We downgraded the evidence by two levels for imprecision and rated it as low.
3.1. Analysis.
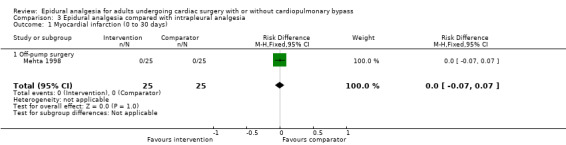
Comparison 3 Epidural analgesia compared with intrapleural analgesia, Outcome 1 Myocardial infarction (0 to 30 days).
2. Risk of pulmonary complications
2a. Respiratory depression (0 to 30 days)
We found no data for this outcome.
2b. Pneumonia (0 to 30 days)
We found no data for this outcome.
3. Risk of atrial fibrillation or atrial flutter during surgery and up to two weeks after surgery
We found no data for this outcome.
4. Risk of neurological complications
4a. Cerebrovascular accident (0 to 30 days)
We found no data for this outcome.
4b. Risk of serious neurological complications from epidural analgesia or epidural haematoma (0 to 30 days)
One trial with 50 participants reported no epidural haematoma (RD 0.00, 95% CI −0.07 to 0.07; Analysis 3.2; Table 3; Mehta 2008). We judged this trial to be at low risk of bias for blinding of outcome assessment. We downgraded the quality by two levels for imprecision and rated the quality as low.
3.2. Analysis.
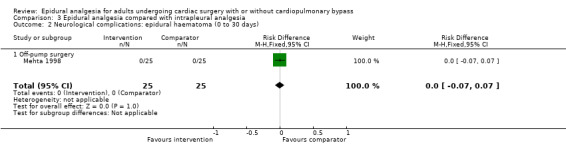
Comparison 3 Epidural analgesia compared with intrapleural analgesia, Outcome 2 Neurological complications: epidural haematoma (0 to 30 days).
5. Duration of tracheal intubation
One small trial with 50 participants reported that 17 in the epidural analgesia group and 14 in the intrapleural analgesia group were extubated in the operating room, and the remainder were extubated in the post‐anaesthesia care unit after a mean time of 3.8 ± 1.13 hours (mean ± SD) of ventilation in the epidural group and 4.1 ± 0.59 hours in the intrapleural group (MD −0.30 hour, 95% CI −1.20 to 0.60 hour; 15 participants; Analysis 3.3; Table 3; Mehta 2008). We judged this trial as being at low risk of bias for blinding of outcome assessment. We downgraded the quality by one level for risk of bias and by two levels for imprecision, and we rated the quality as very low.
3.3. Analysis.
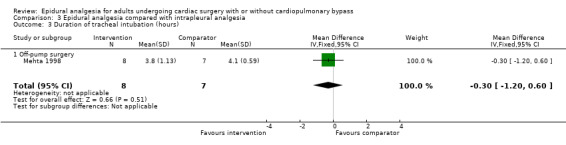
Comparison 3 Epidural analgesia compared with intrapleural analgesia, Outcome 3 Duration of tracheal intubation (hours).
6. Pain
6a. Pain at six to eight hours
Pain at rest at six to eight hours after surgery
From one trial with 50 participants, pain may be greater with epidural analgesia compared with paravertebral blockade for off‐pump surgery (MD 0.84, 95% CI 0.31 to 1.37). We judged this trial to be at low risk of bias for blinding of outcome assessment. We downgraded the quality by two levels for imprecision, and we rated the quality as low.
Pain on movement or coughing at six to eight hours after surgery
We found no data for this outcome.
6b. Pain at 24 hours
We found no data for this outcome.
6c. Pain at 48 hours
We found no data for this outcome.
6d. Pain at 72 hours
We found no data for this outcome.
7. Haemodynamic support (in hospital)
We found no data for this outcome.
Comparison 4: epidural analgesia compared with wound infiltration
Primary outcomes
1. Risk of mortality
We found no data for this outcome at 0 to 30 days, at six months, or at one year.
Secondary outcomes
1. Risk of myocardial infarction (0 to 30 days)
We found no data for this outcome.
2. Risk of pulmonary complications
2a. Respiratory depression (0 to 30 days)
We found no data for this outcome.
2b. Pneumonia (0 to 30 days)
We found no data for this outcome.
3. Risk of atrial fibrillation or atrial flutter during surgery and up to two weeks after surgery
We found no data for this outcome.
4. Risk of neurological complications
4a. Cerebrovascular accident (0 to 30 days)
We found no data for cerebrovascular accident.
4b. Risk of serious neurological complications from epidural analgesia or epidural haematoma (0 to 30 days)
We found no data for this outcome.
5. Duration of tracheal intubation
One small trial with 37 participants published as a conference abstract reported no difference in time to tracheal extubation between epidural analgesia and intravenous patient‐controlled analgesia plus wound infusion (Cheng‐Wei 2017). Data were not suitable for extraction (Table 4). We judged this trial to be at low risk of bias for blinding of outcome assessment. We downgraded the quality by one level for risk of bias and by two levels for imprecision and rated the quality as very low.
6. Pain
6a. Pain at six to eight hours
One small trial with 37 participants and judged as at unclear risk of bias for blinding of outcome assessment reported: "Both groups achieved satisfactory pain relief postoperatively. However, thoracic patient‐controlled epidural analgesia further reduced the verbal analogue pain scores both at rest and during motion significantly as compared to continuous local infusion combined with patient controlled analgesia". Data were unsuitable for extraction.
6b. Pain at 24 hours
We found no suitable data for extraction for this outcome.
6c. Pain at 48 hours
We found no suitable data for extraction for this outcome.
6d. Pain at 72 hours
We found no suitable data for extraction for this outcome.
7. Haemodynamic support (in hospital)
We found no data for this outcome
Discussion
Summary of main results
There may be no difference in mortality between epidural analgesia and systemic analgesia. Review authors found that the number of participants included was insufficient to exclude a difference in mortality between epidural analgesia and systemic analgesia, particularly at one year after surgery (Analysis 1.3). This is important because if indeed epidural analgesia would reduce the mortality rate at one year by half or more (three events for 423 participants for epidural analgesia, or 71 per 10,000 surgeries vs nine events for 426 participants for systemic analgesia or 211 events per 10,000 surgeries; Analysis 1.3), then a risk of three spinal haematomas per 10,000 epidural blocks could be justified (Landoni 2015). Although collecting enough participants to demonstrate a reduced mortality rate at one year may prove difficult (Choi 2009), a satisfactory answer to that important question could possibly be obtained with large well‐designed retrospective trials (propensity score analysis).
There may be a difference in the risk of myocardial infarction. This is similar to what we found for patients undergoing abdominal aortic surgery (Guay 2016b). Although both populations shared many risk factors, in the present review, a vast majority of participants were undergoing coronary artery bypass grafting. Therefore, these participants should have had improved coronary artery blood flow after surgery, which may have offered a certain degree of protection that was added to any potential advantages of epidural analgesia. Many of the patients who undergo heart surgery have coronary artery bypass grafting (CABG) surgery, which may be protective against myocardial infarction.
Epidural analgesia reduces the risk of respiratory depression, but we did not find a difference for risk of pneumonia (Analysis 1.6). Reduced risk of respiratory depression was however more evident for participants undergoing cardiac surgery with cardiopulmonary bypass ‐ a procedure usually performed through a median sternotomy; off‐pump surgery may be performed by a mini‐thoracotomy. The clinical relevance of this finding is unclear because all these patients are usually closely monitored during the period of higher risk for respiratory depression, no matter the mode of postoperative analgesia used.
Epidural analgesia also reduces the risk of atrial fibrillation or atrial flutter after surgery. However, one trial compared four treatment groups: epidural analgesia with or without amiodarone versus no epidural analgesia with or without amiodarone (Nygard 2004). Researchers administered amiodarone at 1800 mg orally the day before surgery and 900 mg IV per 24 hours started after anaesthesia induction and continued for three days. From the morning of the first postoperative day, 24‐hour Holter recordings were obtained by a standard three‐channel tape recorder, and this continued for five days. The incidence of new‐onset atrial fibrillation requiring treatment was 20/48 (42%; 95% confidence interval (CI) 28 to 57%) for control, 22/44 (50%; 95% CI 35 to 65%) for epidural analgesia, 10/36 (28%; 05% CI 14 to 45%) for amiodarone, and 10/35 (29%; 95% CI 14 to 46%) for epidural analgesia plus amiodarone. This small trial suggests that epidural analgesia might not add much to a potent prophylaxis such as the one that can be obtained with amiodarone or other drugs (De Oliveira 2012). The Healthcare Improvement Scotland Committee recommended against the use of epidural analgesia for the sole purpose of decreasing the incidence of atrial fibrillation: "The choice of anaesthetic agent or technique and analgesia should be based on factors other than atrial fibrillation prophylaxis" (Healthcare Improvement Scotland Committee 2018).
We did not find an increase in the incidence of epidural haematoma, but the number of participants included in the analysis is clearly insufficient to evaluate this (Analysis 2.4; Table 1). Furthermore, pooling results from small randomized controlled trials (RCTs) to evaluate the risks of a rare event might not be appropriate. Small trials including such a severe complication might have been terminated and never published. From other authors (Landoni 2015), the risk of epidural haematoma in patients undergoing cardiac surgery would be 3 per 10,000 compared with 1 per 10,000 for the general population (Moen 2004). In its latest recommendation, the American Society of Regional Anesthesia (ASRA) (joint recommendation with the European Society of Anaesthesiology) stated: "Currently, insufficient data and experience are available to determine if the risk of neuraxial haematoma is increased when combining neuraxial techniques with the full anticoagulation of cardiac surgery" (Horlocker 2018). The ASRA also recommends the following precautions if a neuraxial block is performed in this specific population: "1) Neuraxial blocks should be avoided in a patient with known coagulopathy from any cause. 2) Surgery should be delayed 24 hours in the event of a traumatic tap. 3) Time from instrumentation to systemic heparinization should exceed 60 minutes. 4) Heparin effect and reversal should be tightly controlled (smallest amount of heparin for the shortest duration compatible with therapeutic objectives). 5) Epidural catheters should be removed when normal coagulation is restored, and patients should be closely monitored postoperatively for signs and symptoms of hematoma formation" (Chaney 1997; Horlocker 2018 ). It is noteworthy that these recommendations also apply to paravertebral blockade (a deep block). The ASRA considers that precautions for any block performed at a "non‐compressible" site should be identical to those followed for neuraxial blocks. Numerous cases of substantial internal haemorrhage (some with poor prognosis) have been reported when deep blocks (including paravertebral blockade) are performed in individuals with altered haemostasis/coagulation (Horlocker 2018; Thomas 1999). Bilateral erector spinae plane analgesic blocks have been proposed for postoperative analgesia after cardiac surgery through median sternotomy (Tsui 2018; Nagaraja 2018). However, this block is not without complications in itself (Ueshima 2018), and more randomized clinical trials will be required before an opinion can be made on the usefulness of this new block for cardiac surgery. Although intrapleural analgesia may sound attractive (Mehta 1998), it cannot be used for patients without pleural drainage and might be less effective in patients with large postoperative blood loss (more than 200 mL/h through the intercostal chest tube for the first five hours postoperatively; Mehta 1998).
Epidural analgesia reduces the duration of tracheal intubation, but this effect is more evident in older trials (P < 0.0001 for the meta‐regression effect size vs year of publication), making epidural analgesia less likely to have an important beneficial impact on costs in 2018 for patients undergoing cardiac surgery (Fillinger 2002). This might be related to a change in clinical practice. Fast‐track protocols including more systematic use of co‐analgesic drugs, favouring short‐acting drugs over long‐acting ones and promoting early tracheal extubation, are used more often nowadays. Furthermore, other modalities of postoperative pain treatment such as peripheral nerve blocks (Analysis 2.5; Table 2), intrapleural analgesia (Analysis 3.3; Table 3), or wound infusion (Table 4) might be equally effective in reducing the duration of tracheal intubation.
Overall completeness and applicability of evidence
The number of participants included in the review is insufficient to eliminate a difference in mortality between epidural analgesia and systemic analgesia. The number of trials comparing epidural analgesia versus other techniques of regional anaesthesia is very limited.
Quality of the evidence
We rated the quality of evidence as moderate for reduction of respiratory depression, reduction of atrial fibrillation or atrial flutter, duration of tracheal intubation, and pain reduction. We rated the quality of evidence as low for no difference in mortality, reduced risk of myocardial infarction, no difference in risk of pneumonia, and haemodynamic support requirements, and as very low for uncertainty of differences in cerebrovascular accidents.
Potential biases in the review process
Conclusions of this review are limited by an insufficient number of participants/trials to eliminate a difference in mortality between epidural analgesia and systemic analgesia. Although the exact content of solutions infused varied widely, all but three studies included a local anaesthetic, and all were performed at the thoracic (or low cervical) level. It seems therefore unlikely that variations in techniques/drugs used in included trials could explain the lack of effect of epidural analgesia on most of the studied outcomes.
We found no published protocol for any of the included trials. Therefore, we were unable to judge whether or not trialists adhered to their protocol.
The three ongoing trials may change the results of this review.
Agreements and disagreements with other studies or reviews
In agreement with epidural analgesia for abdominal aortic surgery, epidural analgesia for cardiac surgery may reduce the risk of postoperative myocardial infarction (Guay 2016b). Many of the patients undergoing heart surgery are having coronary artery bypass graft (CABG) surgery, which may in itself be protective against myocardial infarction.
Others have also reported a reduction in the risk of arrhythmia (Barbosa 2016; Zhang 2015).
While reviewing randomized and case‐matched studies, Landoni and colleagues reported a reduction in all‐cause mortality at the longest follow‐up available (risk ratio (RR) 0.65, 95% CI 0.48 to 0.86; 57 trials including 6383 participants; Landoni 2015). Inclusion of non‐randomized trials, which are expected to be at higher risk of bias, and lack of a clear time point for mortality may explain in part the differences between their results and ours.
Authors' conclusions
Implications for practice.
Compared with systemic analgesia, epidural analgesia may reduce the risk of myocardial infarction, respiratory depression, atrial fibrillation/atrial flutter, duration of tracheal intubation, and pain in adults undergoing cardiac surgery. There might be little or no difference in mortality, pneumonia, and epidural haematoma. Effects on risks of cerebrovascular accident are uncertain. Evidence is insufficient to show the effects of epidural analgesia compared with peripheral nerve blocks, intrapleural analgesia, or wound infiltration.
Implications for research.
It is actually unclear whether benefits of epidural analgesia justify its potential risk for adults undergoing cardiac surgery. The risk of spinal haematoma might be higher than in the general population (3 per 10,000 vs 1 per 10,000; Landoni 2015; Moen 2004). Although rare, this complication can be devastating, with more than 75% of patients who experience it suffering permanent neurological damage. This potential increase in complications could however be justified if epidural analgesia would reduce postoperative mortality. The number of participants included in our review is clearly insufficient to justify any statement on the effects of epidural analgesia on mortality at one year. Collecting data for a large randomized controlled trial on epidural analgesia is difficult, if possible at all (Choi 2009). Therefore, large well‐designed retrospective trials evaluating potential differences in mortality between epidural analgesia and systemic analgesia at one year would be useful (Analysis 1.3).
Trials comparing superficial regional anaesthetic techniques versus systemic analgesia for postoperative pain, risk of respiratory depression, myocardial infarction, arrhythmia, and duration of tracheal intubation could be interesting (Horlocker 2018; Nagaraja 2018; Tsui 2018). As opposed to deep blocks (non‐compressible sites), superficial blocks offer the advantage of being performed at a compressible site, thus potentially limiting the consequences of inadvertent vascular puncture in heparinized patients. Erector spinae blocks might be one of these "superficial blocks" deserving further exploration (Nagaraja 2018; Tsui 2018).
What's new
| Date | Event | Description |
|---|---|---|
| 19 November 2018 | New citation required and conclusions have changed | Conclusions unchanged for outcomes included in the previous version New conclusions provided for new outcomes, along with new comparisons |
| 19 November 2018 | New search has been performed | Review updated in 2018 by new authors: Joanne Guay and Sandra Kopp Methodology updated 38 new trials included; 3 new comparisons and 3 outcomes (duration of tracheal intubation, pain, haemodynamic support requirements) added |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 6, 2013
| Date | Event | Description |
|---|---|---|
| 26 September 2017 | New search has been performed | Review undertaken by 2 new review authors |
| 22 September 2017 | Amended | Change made to review authors Previous review authors replaced by Joanne Guay and Sandra Kopp |
| 1 July 2013 | Amended | Contact details for Geert J. van der Heijden amended |
Acknowledgements
We would like to thank Maria Oslaida Agüero Martínez (Aguero‐Martinez 2012), Amr Arafat (El‐Shora 2018), Gianni Angelini (Caputo 2011), Şerife Bektaş (Bektas 2015), Adrianus J de Vries (de Vries 2002), CJ Jakobsen (Jakobsen 2012), Mikhail Kirov (Kirov 2011), Turkan Kudsioglu (Celik 2015), Stephen Kowalski (Tenenbein 2008), Yatin Mehta (Mehta 2008), Vojislava Neskovic (Neskovic 2013), Miguel Angel Palomero Rodriguez (Palomero 2008), Colin Royse (Royse 2003), and Roar Stenseth (Stenseth 1994; Stenseth 1996), who provided additional information on their trials or informed us that data were no longer available. We also thank Mina Nishimori for her help and Hironobu Hayashi for the translation of Usui 1990.
We are also in debt to Liz Bickerdike and Jane Cracknell for their editorial help.
We thank our reviewers: Stephanie Weibe (Content Editor), Nathan L Pace (Statistical Editor), R Peter Alston (Peer Reviewer), Padma G Nainar (Peer Reviewer), Janne Vendt (Information Specialist), Janet Wale (Consumer Editor), and Andrew Smith (Co‐ordinating Editor).
We also thank Vesna Svircevic, Martijn M Passier, Arno P Nierich, Diederik van Dijk, Cor J Kalkman, and Geert J van der Heijden, who authored the previous published version (Svircevic 2013).
Appendices
Appendix 1. Search strategies
Search date 19 November 2018
Cochrane Central Register of Controlled Trials: November 2018, Issue 11 of 12
#1 MeSH descriptor: [Analgesia, Epidural] explode all trees 1909 #2 MeSH descriptor: [Anesthesia, Epidural] explode all trees 1918 #3 (epidural* or peridural* or subarachnoid* or extradural* or neuraxial*) 13410 #4 (#1 or #2 or #3) 13538 #5 MeSH descriptor: [Cardiac Surgical Procedures] explode all trees 12271 #6 MeSH descriptor: [Cardiopulmonary Bypass] explode all trees 2615 #7 MeSH descriptor: [Coronary Artery Bypass] explode all trees 5206 #8 ((coronary or bypass or heart or cardio* or cardiac* or valve) next (surg* or graft* or bypass or plasty or replacement)) or cabg 21798 #9 (#5 or #6 or #7 or #8) 26170 #10 (#9 and #4) 306 (242 trials) #11 #10 Publication Year from 2012 to 2018 = 69
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
1 (Cardiac Surgical Procedures or cardiopulmonary bypass or Coronary Artery Bypass or ((coronary or heart or cardio* or cardiac* or valve) adj5 (surg* or graft* or bypass or plasty or replacement)) or cabg).af. (288226) 2 (Epidural* or peridural* or extradural* or subarachnoid* or neuraxial* or (Anesthesia, Epidural or Analgesia, Epidural)).af. (83065) 3 (randomized controlled trial or controlled clinical trial or randomi? or placebo or drug therapy or randomly or trial or groups).af. (4258550) 4 1 and 2 and 3 (429) 5 limit "2012 ‐Current" (106)
Embase <1974 to 19 November 2018>
1 (heart surgery) or (cardiopulonary bypass) or (heart valve surgery) or ((coronary) or (heart) or (cardio* or cardiac* or valve) adj5 (surg* or graft* or bypass or plasty or replacement)) or (cabg) (541,226) 2 (epidural or peridural or extradural or subarachnoid or neuraxial) and (an?esth or analg) or (epidural anesthesia or epidural analgesia) (28,907) 3 (double blind procedure or single blind procedure) or (placebo ) or (crossover procedure) or (controlled adj3 (study or design or trial)) or (allocat* or trial or random*) not ((exp animal or animal or nonhuman) not (exp human cell)) (2,347,081) 4 1 and 2 and 3 (775) 5 limit 4 to yr="2012 ‐Current" (256)
Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO host)
|
CINAHL, EBSCOhost S1 |
(MM "Heart Surgery+") OR (MM "Cardiopulmonary Bypass+") OR CABG OR ((coronary or heart or cardiac* OR cardio* or valve) N3 (surg* or graft* OR bypass OR shunt or plasty or replacement))) | 63,212 |
| S2 | (MM "Analgesia, Epidural+") OR (MM "Anesthesia, Epidural+") OR (MM "Epidural Analgesia Administration (Iowa NIC)") OR (epidural* OR peridural* OR extradural* OR subarachnoid* OR neuraxial*) | 18,984 |
| S3 | (MH "Placebos") OR ( (MM "Randomized Controlled Trials") OR (MM "Random Assignment") OR (MH "Prospective Studies") OR (MH "Multicenter Studies") OR (MH "Clinical Trials") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Double‐Blind Studies") ) OR ( placebo* or multicenter or prospective or ((random* or control*) and trial*) ) | 755,253 |
| S4 | S1 AND S2 AND S3 | 103 |
| S5 | S1 AND S2 AND S3 Published: 2012‐2018 | 42 |
Web of Science (SCI/SSCI)
| # 5 | 101 | #4 Timespan=2012‐2018 |
| # 4 | 331 | #3 AND #2 AND #1 |
| # 3 | 4,539,758 | TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*) |
| # 2 | 19,362 | TS=((epidural* OR peridural* OR extradural* OR subarachnoid* OR neuraxial*) AND (an?esth* or analg*)) |
| # 1 | 191,475 | TS=(((coronary or heart or cardio* or cardiac* or valve) NEAR/5 (surg* or graft* or bypass or plasty or replacement)) or cabg) |
Appendix 2. List of reviews checked for additional trials
Baidya 2014
Baidya DK, Khanna P, Maitra S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: a systematic review and meta‐analysis. Interactive Cardiovascular and Thoracic Surgery 2014;18(5):626‐35. (DOI: 10.1093/icvts/ivt551; PubMed: 24488821)
Barbosa 2016
Barbosa FT, de Sousa Rodrigues CF, Castro AA, da Cunha RM, Barbosa TRBW. Is there any benefit in associating neuraxial anesthesia to general anesthesia for coronary artery bypass graft surgery? Revista Brasileira de Anestesiologia 2016;66(3):304‐9. (DOI: 10.1016/j.bjane.2013.09.015; PubMed: 27108829)
Barbosa 2016a
Barbosa FT, da Cunha RM, da Silva Ramos FW, Camello de Lima FJ, Barros Rodrigues AK, do Nascimento Galvão AM, et al. Revista Brasileira de Anestesiologia 2016;66(2):183‐193. (DOI: 10.1016/j.bjane.2014.05.012; PubMed: 25746164)
Bigeleisen 2015
Bigeleisen PE, Goehner N. Novel approaches in pain management in cardiac surgery. Current Opinion in Anaesthesiology 2015;28(1):89‐94. (DOI: 10.1097/ACO.0000000000000147; PubMed: 25500688)
Bignami 2010
Bignami E, Landoni G, Biondi‐Zoccai GG, Boroli F, Messina M, Dedola E, et al. Epidural analgesia improves outcome in cardiac surgery: a meta‐analysis of randomized controlled trials. Journal of Cardiothoracic and Vascular Anesthesia 2010;24(4):586‐97. (DOI: 10.1053/j.jvca.2009.09.015; PubMed: 20005129)
Bignami 2017
Bignami E, Castella A, Pota V, Saglietti F, Scognamiglio A, Trumello C, et al. Perioperative pain management in cardiac surgery: a systematic review. Minerva Anestesiologica 2017. (DOI: 10.23736/S0375‐9393.17.12142‐5; PubMed: 29027773)
Bracco 2007
Bracco D, Hemmerling T. Epidural analgesia in cardiac surgery: an updated risk assessment. Heart Surgery Forum 2007;10(4):E334‐7.
Bracco 2008
Bracco D, Hemmerling TM. Thoracic epidural analgesia in cardiac surgery: impact on postoperative morbidity. Techniques in Regional Anesthesia and Pain Management 2008;12:32‐40.
Chaney 2006
Chaney MA. Intrathecal and epidural anesthesia and analgesia for cardiac surgery. Anesthesia and Analgesia 2006;102:45‐64. (MEDLINE: 16368803)
Chaparro 2013
Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database of Systematic Reviews 2013;(7):CD008307. (PubMed: 23881791)
Gu 2012
Gu WJ, Wei CY, Huang DQ, Yin RX. Meta‐analysis of randomized controlled trials on the efficacy of thoracic epidural anesthesia in preventing atrial fibrillation after coronary artery bypass grafting. BMC Cardiovascular Disorders 2012;12:67. (DOI: 10.1186/1471‐2261‐12‐67; PubMed: 22900930)
Hemmerling 2013a
Hemmerling TM, Romano G, Terrasini N, Noiseux N. Anesthesia for off‐pump coronary artery bypass surgery. Annals of Cardiac Anaesthesia 2013;16(1):28‐39. (PubMed: 23287083)
Huang 2016
Huang AP, Sakata RK. Pain after sternotomy ‐ review. Brazilian Journal of Anesthesiology 2016;66(4):395‐401. (DOI: 10.1016/j.bjane.2014.09.013; PubMed: 27343790)
Jakobsen 2015
Jakobsen CJ. High thoracic epidural in cardiac anesthesia: a review. Seminars in Cardiothoracic and Vascular Anesthesia 2015;19(1):38‐48. (PubMed: 25201889)
Konstantatos 2008
Konstantatos A, Silvers AJ, Myles PS. Analgesia best practice after cardiac surgery. Anesthesiology Clinics 2008;26(3):591‐602. (PubMed: 18765224)
Kooij 2014
Kooij FO, Schlack WS, Preckel B, Hollmann MW. Does regional analgesia for major surgery improve outcome? Focus on epidural analgesia. Anesthesia and Analgesia 2014;119(3):740‐4. (DOI: 10.1213/ANE.0000000000000245.; PubMed: 25137006)
Landoni 2015
Landoni G, Isella F, Greco M, Zangrillo A, Royse CF. Benefits and risks of epidural analgesia in cardiac surgery. British Journal of Anaesthesia 2015;115(1):25‐32. (DOI: 10.1093/bja/aev201; PubMed: 26089444)
Liu 2004
Liu SS, Block BM, Wu CL. Effects of perioperative central neuraxial analgesia on outcome after coronary artery bypass surgery: a meta‐analysis. Anesthesiology 2004;101:153‐61. (MEDLINE: 15220785)
Mehta 2012
Mehta Y, Arora D, Vats M. Epidural analgesia in high risk cardiac surgical patients. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2012;4(1):11‐4. (PubMed: 23440670)
Mehta 2014
Mehta Y, Arora D. Benefits and risks of epidural analgesia in cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2014;28(4):1057‐63. (DOI: 10.1053/j.jvca.2013.07.016; PubMed: 24315759)
Meissner 1997
Meissner A, Rolf N, Van Aken H. Thoracic epidural anesthesia and the patient with heart disease: benefits, risks, and controversies. Anesthesia and Analgesia 1997;85(3):517‐28. (PubMed: 9296403)
Neskovic 2003
Nešković V, Milojević P. High thoracic epidural anesthesia for coronary artery bypass graft surgery [Visoka torakalna epiduralana anestezija u koronarnoj hirurgili]. Medicinski Pregled 2003;LVI(3‐4):152‐6.
Popping 2014
Popping DM, Elia N, Van Aken HK, Marret E, Schug SA, Kranke P, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta‐analysis of randomized controlled trials. Annals of Surgery 2014;259(6):1056‐67. (DOI: 10.1097/SLA.0000000000000237; PubMed: 24096762)
Ronald 2006
Ronald A, Abdulaziz KA, Day TG, Scott M. In patients undergoing cardiac surgery, thoracic epidural analgesia combined with general anaesthesia results in faster recovery and fewer complications but does not affect length of hospital stay. Interactive Cardiovascular and Thoracic Surgery 2006;5(3):207‐16. (DOI: 10.1510/icvts.2005.125054; PubMed: 17670549)
Royse 2009
Royse CF. High thoracic epidural anaesthesia for cardiac surgery. Current Opinion in Anaesthesiology 2009;22(1):84‐7. (DOI: 1097/ACO.0b013e32831a40b6; PubMed: 19295296)
Ruppen 2006
Ruppen W, Derry S, McQuay HJ, Moore RA. Incidence of epidural haematoma and neurological injury in cardiovascular patients with epidural analgesia/anaesthesia: systematic review and meta‐analysis. BMC Anesthesiology 2006;6:10. (DOI: 10.1186/1471‐2253‐6‐10; PubMed: 16968537)
Scarfe 2016
Scarfe AJ, Schuhmann‐Hingel S, Duncan JK, Ma N, Atukorale YN, Cameron AL. Continuous paravertebral block for post‐cardiothoracic surgery analgesia: a systematic review and meta‐analysis. European Journal of Cardio‐Thoracic Surgery 2016;50(6):1010‐8. (DOI: 10.1093/ejcts/ezw168; PubMed: 27242357)
Smith 2017
Smith LM, Cozowicz C, Uda Y, Memtsoudis SG, Barrington MJ. Neuraxial and combined neuraxial/general anesthesia compared to general anesthesia for major truncal and lower limb surgery: a systematic review and meta‐analysis. Anesthesia and Analgesia 2017;124(6):1931‐45. (DOI: 10.1213/ANE.0000000000002069; PubMed: 28537970)
Sondekoppam 2014
Sondekoppam RV, Arellano R, Ganapathy S, Cheng D. Pain and inflammatory response following off‐pump coronary artery bypass grafting. Current Opinion in Anaesthesiology 2014;27(1):106‐15. (DOI: 10.1097/ACO.0000000000000036; PubMed: 24322210)
Wardhan 2015
Wardhan R. Update on paravertebral blocks. Current Opinion in Anaesthesiology 2015;28(5):588‐92. (DOI: 10.1097/ACO.0000000000000235; PubMed: 26308511)
Wei 2013
Wei G, Xuan Y, Zheng H, Wang J. Effectiveness and safety of thoracic epidural analgesia for postoperative complications after cardiac surgery: a systematic review. Chinese Journal of Evidence‐Based Medicine 2013;13(10):1229‐35. (DOI: 10.7507/1672‐2531.20130211)
Williams 2002
Williams J. Thoracic epidural anaesthesia for cardiac surgery. Canadian Journal of Anesthesia 2002;49:7R.
Yeung 2016
Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. The Cochrane Database of Systematic Reviews 2016;2:CD009121. (DOI: 10.1002/14651858.CD009121.pub2.; PubMed: 26897642)
Zhang 2015
Zhang S, Wu X, Guo H, Ma L. Thoracic epidural anesthesia improves outcomes in patients undergoing cardiac surgery: meta‐analysis of randomized controlled trials. European Journal of Medical Research 2015;20:25. (DOI: 10.1186/s40001‐015‐0091‐y; PubMed: 25888937)
Appendix 3. Numbers needed to treat for additional beneficial outcome or harmful effect
1. Comparison 1: risk of respiratory complications: respiratory depression
Odds ratio: 0.56 (95% CI 0.37 to 0.86)
For the control group, there were 62 events out of 856 participants included for an incidence of 7.5%.
From Cates 2016:
NNTB = 32 (95% CI 22 to 102)
2. Comparison 1: risk of atrial fibrillation or atrial flutter during surgery and up to 2 weeks after surgery.
Odds ratio: 0.69 (95% CI 0.50 to 0.95)
For the control group, there were 403 events out of 1231 participants included for an incidence of 32.7%.
From Cates 2016:
NNTB = 14 (95% CI 8 to 90)
3. Comparison 1: hypotension or need for vasopressor
For the control group, there were 118 events out of 398 participants for an incidence of 30%.
Odds ratio: 3.16 (95% CI 1.49 to 6.71)
From Cates 2016:
NNTH = 4 (95% CI 3 to 12)
Appendix 4. Comparison 1: duration of tracheal intubation for trials for which means and standard deviations were available
Tracheal intubation Comparison 1: trials for which means and standard deviations were available
| Study | Epidural analgesia | Systemic analgesia | ||||
| Mean | SD | N | Mean | SD | N | |
| El‐Baz 1987 | 9.00 | 3.00 | 30 | 18.000 | 5.000 | 30 |
| Liem 1992 | 7.72 | 6.58 | 25 | 19.000 | 4.830 | 25 |
| Konishi 1995 (1) | 6.60 | 3.70 | 31 | 9.200 | 5.400 | 18 |
| Konishi 1995 (2) | 5.80 | 3.10 | 31 | 9.200 | 5.400 | 18 |
| Stenseth 1996 | 5.40 | 2.04 | 26 | 10.800 | 3.569 | 26 |
| Fawcett 1997 | 5.80 | 1.00 | 8 | 9.200 | 2.400 | 8 |
| Loick 1999 | 9.98 | 2.65 | 25 | 14.630 | 9.150 | 21 |
| Tenling 1999 | 3.62 | 0.47 | 14 | 7.970 | 3.070 | 14 |
| Kunstyr 2001 (4) | 6.07 | 2.93 | 7 | 7.000 | 2.990 | 20 |
| Kunstyr 2001 (5) | 6.07 | 2.93 | 7 | 5.810 | 2.350 | 21 |
| Kunstyr 2001 (6) | 6.07 | 2.93 | 7 | 6.260 | 3.440 | 20 |
| Fillinger 2002 | 10.70 | 1.40 | 30 | 9.500 | 0.800 | 30 |
| Berendes 2003 | 3.40 | 1.90 | 36 | 9.200 | 4.300 | 37 |
| Royse 2003 | 2.60 | 2.50 | 37 | 5.400 | 3.100 | 39 |
| Huh 2004 | 4.61 | 4.75 | 27 | 13.430 | 7.010 | 29 |
| Hansdottir 2006 | 2.30 | 1.10 | 53 | 7.300 | 19.200 | 55 |
| Kilickan 2006 (7) | 7.57 | 6.05 | 20 | 14.620 | 4.200 | 20 |
| Kilickan 2006 (8) | 10.00 | 5.32 | 20 | 8.520 | 4.720 | 20 |
| Petrovski 2006 | 3.50 | 0.80 | 56 | 6.800 | 0.700 | 54 |
| Yilmaz 2007 | 7.44 | 1.36 | 17 | 9.370 | 1.980 | 17 |
| Tenenbein 2008 | 0.26 | 0.63 | 25 | 0.170 | 0.210 | 25 |
| Kilickan 2008 (10) | 5.00 | 3.20 | 15 | 6.600 | 4.000 | 15 |
| Kilickan 2008 (11) | 5.50 | 2.60 | 15 | 7.000 | 3.300 | 15 |
| Palomero 2008 | 11.70 | 7.52 | 10 | 12.500 | 2.400 | 12 |
| Lenkutis 2009 | 6.04 | 0.56 | 30 | 11.060 | 1.640 | 30 |
| El‐Morsy 2012 | 7.30 | 6.40 | 25 | 10.700 | 8.200 | 25 |
| Onan 2013 | 2.90 | 1.10 | 20 | 4.700 | 1.200 | 20 |
| Neskovic 2013 (12) | 6.67 | 4.66 | 18 | 8.830 | 5.270 | 27 |
| Gurses 2013 | 4.10 | 1.70 | 32 | 6.800 | 2.000 | 32 |
| Celik 2015 | 7.20 | 1.82 | 20 | 11.700 | 2.020 | 20 |
| de Vries 2002 (13) | 0.15 | 0.08 | 28 | 0.220 | 0.120 | 29 |
| Kendall 2004 (14) | 5.30 | 4.10 | 5 | 6.900 | 2.800 | 10 |
| Kendall 2004 (15) | 5.30 | 4.10 | 5 | 6.600 | 3.100 | 10 |
| Bakhtiary 2007 | 6.00 | 2.30 | 66 | 7.000 | 4.200 | 66 |
| Sharma 2010 | 9.33 | 2.24 | 30 | 11.670 | 3.020 | 30 |
| Mehta 2010 | 10.80 | 3.19 | 31 | 13.500 | 2.880 | 31 |
| Aguero‐Martinez 2012 | 4.52 | 2.84 | 29 | 7.830 | 5.240 | 30 |
| Neskovic 2013 (16) | 4.38 | 4.31 | 17 | 5.810 | 3.500 | 19 |
| Zawar 2015 | 14.20 | 8.20 | 35 | 15.500 | 3.900 | 46 |
| Obersztyn 2018 | 3.60 | 2.63 | 39 | 7.983 | 2.917 | 39 |
SD: standard deviation.
(1) Morphine (2) Butorphanol (3) For this analysis, only two groups were retained: epidural analgesia versus control (4) Compared with intravenous infusion of sufentanil and ketamine (5) Compared with nurse administered morphine (6) Compared with intravenous patient controlled analgesia with morphine (7) Poor ventricular function (8) Good ventricular function (9) Variances from Table 3 were entered as standard errors of the means (10) Without steroids (11) With steroids (12) With cardiopulmonary bypass
(13) For this analysis only two groups were retained: epidural analgesia versus extubated
(14)Compared with isoflurane and systemic analgesia (15) Compared with propofol and systemic analgesia
(16) Off‐pump
| Epidural analgesia | Systemic analgesia | |
| Mean (hours) | 6.1 | 9.1 |
| Std. deviation | 3.0 | 4.0 |
Appendix 5. Pain scores at rest at 6 to 8 hours for trials with data available as means and SDs
Comparison 1
| Study | Epidural analgesia | Systemic analgesia | ||||
| Mean | SD | N | Mean | SD | N | |
| El‐Morsy 2012 | 3.00 | 3.000 | 25 | 3.70 | 1.50 | 25 |
| Onan 2013 | 0.10 | 0.300 | 20 | 2.40 | 1.80 | 20 |
| Mehta 2010 | 4.03 | 0.700 | 31 | 4.70 | 0.50 | 31 |
| Sharma 2010 | 2.50 | 0.860 | 30 | 3.23 | 0.72 | 30 |
| Aguero‐Martinez 2012 | 0.00 | 0.001 | 30 | 6.83 | 1.29 | 30 |
| Epidural analgesia | Systemic analgesia | |
| Mean | 1.92 | 4.17 |
| SD | 1.80 | 1.70 |
N: number of participants; SD: standard deviation.
Comparison 2
| Study | Epidural analgesia | Peripheral nerve block | ||||
| Mean | SD | N | Mean | SD | N | |
| Nagaraja 2018 | 1.64 | 0.64 | 25 | 1.64 | 1.35 | 25 |
| Dohle 2001 | 2.75 | 2.88 | 20 | 1.95 | 1.43 | 20 |
| Epidural analgesia | Peripheral nerve block | |
| Mean | 2.195 | 1.795 |
| SD | 0.7849 | 0.2192 |
N: number of participants; SD: standard deviation.
Data and analyses
Comparison 1. Epidural analgesia compared with systemic analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at 0 to 30 days | 38 | 3418 | Risk Difference (IV, Fixed, 95% CI) | 0.00 [‐0.01, 0.01] |
| 1.1 With cardiopulmonary bypass | 28 | 1844 | Risk Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 1.2 Off‐pump surgery | 8 | 729 | Risk Difference (IV, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 1.3 With and without cardiopulmonary bypass | 3 | 845 | Risk Difference (IV, Fixed, 95% CI) | 0.00 [‐0.01, 0.01] |
| 2 Mortality at 6 months | 7 | 407 | Risk Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 2.1 With cardiopulmonary bypass | 7 | 407 | Risk Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 3 Mortality at 1 year | 5 | 849 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.00] |
| 3.1 With cardiopulmonary bypass | 4 | 197 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 3.2 With and without cardiopulmonary bypass | 1 | 652 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 4 Myocardial infarction (0 to 30 days) | 26 | 2713 | Risk Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.02, 0.00] |
| 4.1 With cardiopulmonary bypass | 16 | 1153 | Risk Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 4.2 Off‐pump surgery | 8 | 713 | Risk Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 4.3 With and without cardiopulmonary bypass | 3 | 847 | Risk Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 5 Respiratory complications: respiratory depression (0 to 30 days) | 21 | 1736 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.05, ‐0.01] |
| 5.1 With cardiopulmonary bypass | 15 | 1246 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 5.2 Off‐pump surgery | 5 | 299 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.02] |
| 5.3 With and without cardiopulmonary bypass | 2 | 191 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.06, 0.08] |
| 6 Respiratory complications: pneumonia (0 to 30 days) | 10 | 1107 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.07, 0.01] |
| 6.1 With cardiopulmonary bypass | 7 | 677 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.09, 0.04] |
| 6.2 Off‐pump surgery | 3 | 322 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.04] |
| 6.3 With and without cardiopulmonary bypass | 1 | 108 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.12, 0.01] |
| 7 Atrial fibrillation or flutter within 2 weeks | 18 | 2431 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.10, ‐0.01] |
| 7.1 With cardiopulmonary bypass | 11 | 1118 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.08, 0.00] |
| 7.2 Off‐pump surgery | 6 | 551 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.22, 0.03] |
| 7.3 With and without cardiopulmonary bypass | 2 | 762 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 8 Neurological complications: cerebrovascular accident (0 to 30 days) | 18 | 2232 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 8.1 With cardiopulmonary bypass | 13 | 1067 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 8.2 Off‐pump surgery | 4 | 403 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 8.3 With and without cardiopulmonary bypass | 2 | 762 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.01] |
| 9 Neurological complications: epidural haematoma (0 to 30 days) | 53 | 3982 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 9.1 With cardiopulmonary bypass | 39 | 2231 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 9.2 Off‐pump surgery | 10 | 841 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 9.3 With and without cardiopulmonary bypass | 4 | 910 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10 Duration of tracheal intubation | 40 | 3353 | Std. Mean Difference (Random, 95% CI) | ‐0.78 [‐1.01, ‐0.55] |
| 10.1 Cardiopulmonary bypass | 27 | 1570 | Std. Mean Difference (Random, 95% CI) | ‐0.75 [‐1.03, ‐0.47] |
| 10.2 Off‐pump surgery | 11 | 943 | Std. Mean Difference (Random, 95% CI) | ‐0.90 [‐1.38, ‐0.41] |
| 10.3 With and without cardiopulmonary bypass | 3 | 840 | Std. Mean Difference (Random, 95% CI) | ‐0.60 [‐1.42, 0.23] |
| 11 Duration of tracheal intubation in hours (for studies for which means and standard deviations could be extracted) | 33 | 2062 | Mean Difference (IV, Random, 95% CI) | ‐2.91 [‐3.61, ‐2.21] |
| 11.1 With cardiopulmonary bypass | 23 | 1249 | Mean Difference (IV, Random, 95% CI) | ‐3.23 [‐4.30, ‐2.17] |
| 11.2 Off‐pump surgery | 9 | 627 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐3.36, ‐0.37] |
| 11.3 With and without cardiopulmonary bypass | 2 | 186 | Mean Difference (IV, Random, 95% CI) | ‐4.42 [‐5.62, ‐3.22] |
| 12 Pain at rest at 6 to 8 hours after surgery | 10 | 502 | Std. Mean Difference (Random, 95% CI) | ‐1.35 [‐1.98, ‐0.72] |
| 12.1 With cardiopulmonary bypass | 7 | 320 | Std. Mean Difference (Random, 95% CI) | ‐0.83 [‐1.16, ‐0.50] |
| 12.2 Off‐pump surgery | 3 | 182 | Std. Mean Difference (Random, 95% CI) | ‐2.99 [‐5.37, ‐0.60] |
| 13 Pain at rest at 6 to 8 hours: data available as means and standard deviations | 5 | 272 | Mean Difference (IV, Random, 95% CI) | ‐2.26 [‐4.84, 0.32] |
| 13.1 With cardiopulmonary bypass | 2 | 90 | Mean Difference (IV, Random, 95% CI) | ‐1.59 [‐3.15, ‐0.03] |
| 13.2 Off‐pump surgery | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐2.74 [‐6.36, 0.88] |
| 14 Pain on movement/coughing at 6 to 8 hours | 5 | 342 | Std. Mean Difference (Random, 95% CI) | ‐1.39 [‐2.16, ‐0.62] |
| 14.1 With cardiopulmonary bypass | 3 | 220 | Std. Mean Difference (Random, 95% CI) | ‐1.41 [‐2.65, ‐0.17] |
| 14.2 Off‐pump surgery | 2 | 122 | Std. Mean Difference (Random, 95% CI) | ‐1.43 [‐2.36, ‐0.50] |
| 15 Pain on movement/coughing at 6 to 8 hours: data available as means and standard deviations | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐2.46 [‐4.37, ‐0.54] |
| 15.1 With cardiopulmonary bypass | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐5.5 [‐6.47, ‐4.53] |
| 15.2 Off‐pump surgery | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.24, ‐0.78] |
| 16 Pain at rest at 24 hours after surgery | 22 | 2033 | Std. Mean Difference (Random, 95% CI) | ‐0.93 [‐1.22, ‐0.65] |
| 16.1 With cardiopulmonary bypass | 15 | 837 | Std. Mean Difference (Random, 95% CI) | ‐0.80 [‐1.06, ‐0.54] |
| 16.2 Off‐pump surgery | 5 | 432 | Std. Mean Difference (Random, 95% CI) | ‐2.06 [‐3.15, ‐0.97] |
| 16.3 With and without cardiopulmonary bypass | 2 | 764 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.38, ‐0.09] |
| 17 Pain at rest at 24 hours: data available as means and standard deviations | 15 | 875 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐2.51, ‐0.55] |
| 17.1 With cardiopulmonary bypass | 10 | 526 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐2.11, ‐0.73] |
| 17.2 Off‐pump surgery | 4 | 239 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐5.16, 0.56] |
| 17.3 With and without cardiopulmonary bypass | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.78, 0.44] |
| 18 Pain scores on movement/coughing at 24 hours | 12 | 842 | Std. Mean Difference (Random, 95% CI) | ‐0.83 [‐1.18, ‐0.49] |
| 18.1 With cardiopulmonary bypass | 9 | 610 | Std. Mean Difference (Random, 95% CI) | ‐0.90 [‐1.25, ‐0.55] |
| 18.2 Off‐pump surgery | 2 | 122 | Std. Mean Difference (Random, 95% CI) | ‐1.03 [‐1.69, ‐0.38] |
| 18.3 With and without cardiopulmonary bypass | 1 | 110 | Std. Mean Difference (Random, 95% CI) | 0.22 [‐0.15, 0.60] |
| 19 Pain scores on movement/coughing at 24 hours: data available as means and standard deviations | 9 | 582 | Mean Difference (IV, Random, 95% CI) | ‐1.74 [‐2.63, ‐0.86] |
| 19.1 With cardiopulmonary bypass | 6 | 350 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐3.30, ‐1.10] |
| 19.2 Off‐pump surgery | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.06, ‐0.34] |
| 19.3 With and without cardiopulmonary bypass | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.26, 1.02] |
| 20 Pain at rest at 48 hours after surgery | 15 | 1649 | Std. Mean Difference (Random, 95% CI) | ‐1.01 [‐1.37, ‐0.64] |
| 20.1 With cardiopulmonary bypass | 9 | 510 | Std. Mean Difference (Random, 95% CI) | ‐0.76 [‐1.08, ‐0.44] |
| 20.2 Off‐pump surgery | 4 | 375 | Std. Mean Difference (Random, 95% CI) | ‐2.11 [‐3.17, ‐1.05] |
| 20.3 With and without cardiopulmonary bypass | 2 | 764 | Std. Mean Difference (Random, 95% CI) | ‐0.25 [‐0.39, ‐0.10] |
| 21 Pain at rest at 48 hours after surgery: data available as means and standard deviations | 11 | 692 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐1.99, ‐0.64] |
| 21.1 With cardiopulmonary bypass | 7 | 400 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.73, ‐0.37] |
| 21.2 Off‐pump surgery | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐2.38 [‐4.07, ‐0.70] |
| 21.3 With and without cardiopulmonary bypass | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.83, 0.31] |
| 22 Pain scores on movement/coughing at 48 hours | 10 | 700 | Std. Mean Difference (Random, 95% CI) | ‐0.83 [‐1.31, ‐0.35] |
| 22.1 With cardiopulmonary bypass | 7 | 468 | Std. Mean Difference (Random, 95% CI) | ‐0.78 [‐1.22, ‐0.34] |
| 22.2 Off‐pump surgery | 2 | 122 | Std. Mean Difference (Random, 95% CI) | ‐1.56 [‐3.09, ‐0.03] |
| 22.3 With and without cardiopulmonary bypass | 1 | 110 | Std. Mean Difference (Random, 95% CI) | 0.33 [‐0.05, 0.70] |
| 23 Pain scores on movement/coughing at 48 hours: data available as means and standard deviations | 9 | 582 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [0.00, ‐0.60] |
| 23.1 With cardiopulmonary bypass | 6 | 350 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐2.56, ‐0.65] |
| 23.2 Off‐pump surgery | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.99, ‐0.45] |
| 23.3 With and without cardiopulmonary bypass | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.09, 1.37] |
| 24 Pain at rest at 72 hours after surgery | 12 | 897 | Std. Mean Difference (Random, 95% CI) | ‐1.09 [‐1.57, ‐0.62] |
| 24.1 With cardiopulmonary bypass | 7 | 412 | Std. Mean Difference (Random, 95% CI) | ‐0.99 [‐1.66, ‐0.33] |
| 24.2 Off‐pump surgery | 4 | 375 | Std. Mean Difference (Random, 95% CI) | ‐1.54 [‐2.14, ‐0.94] |
| 24.3 With and without cardiopulmonary bypass | 1 | 110 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.38, 0.37] |
| 25 Pain at rest at 72 hours after surgery: data available as means and standard deviations | 10 | 624 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.41, ‐0.63] |
| 25.1 With cardiopulmonary bypass | 6 | 332 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.71, ‐0.46] |
| 25.2 Off‐pump surgery | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐1.96, ‐0.59] |
| 25.3 With and without cardiopulmonary bypass | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.47, 0.45] |
| 26 Pain scores on movement/coughing at 72 hours | 9 | 654 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐1.13, ‐0.11] |
| 26.1 With cardiopulmonary bypass | 6 | 422 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.90, 0.09] |
| 26.2 Off‐pump surgery | 2 | 122 | Std. Mean Difference (Random, 95% CI) | ‐1.69 [‐3.32, ‐0.07] |
| 26.3 With and without cardiopulmonary bypass | 1 | 110 | Std. Mean Difference (Random, 95% CI) | 0.09 [‐0.28, 0.47] |
| 27 Pain scores on movement/coughing at 72 hours: data available as means and standard deviations | 7 | 454 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.49, ‐0.30] |
| 27.1 With cardiopulmonary bypass | 4 | 222 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐2.05, 0.02] |
| 27.2 Off‐pump surgery | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.93, ‐0.42] |
| 27.3 With and without cardiopulmonary bypass | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.57, 0.95] |
| 28 Hypotension or vasopressor bolus during surgery | 17 | 870 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [0.09, 0.33] |
| 28.1 With cardiopulmonary bypass | 14 | 637 | Risk Difference (M‐H, Random, 95% CI) | 0.22 [0.08, 0.36] |
| 28.2 Off‐pump surgery | 2 | 153 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [‐0.30, 0.48] |
| 28.3 With and without cardiopulmonary bypass | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.3 [0.15, 0.45] |
| 29 Needed vasopressor/inotropic infusion | 23 | 1821 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.07] |
| 29.1 With cardiopulmonary bypass | 16 | 1237 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.05, 0.13] |
| 29.2 Off‐pump surgery | 6 | 506 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.21, 0.04] |
| 29.3 With and without cardiopulmonary bypass | 1 | 78 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
Comparison 2. Epidural analgesia compared with peripheral nerve blocks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at 0 to 30 days | 1 | 145 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.08, 0.02] |
| 1.1 With cardiopulmonary bypass | 1 | 145 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.08, 0.02] |
| 2 Myocardial infarction (0 to 30 days) | 2 | 76 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 2.1 Off‐pump surgery | 2 | 76 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 3 Neurological complications: cerebrovascular accident (0 to 30 days) | 1 | 145 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 3.1 With cardiopulmonary bypass | 1 | 145 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 4 Neurological complications: epidural haematoma (0 to 30 days) | 4 | 271 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 4.1 With cardiopulmonary bypass | 2 | 195 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 4.2 Off‐pump surgery | 2 | 76 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 5 Duration of tracheal intubation (hours) | 4 | 271 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.54, 0.38] |
| 5.1 With cardiopulmonary bypass | 2 | 195 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.53, 0.44] |
| 5.2 Off‐pump surgery | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐1.70, 0.99] |
| 6 Pain scores at rest at 6 to 8 hours | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.42, 0.66] |
| 6.1 With cardiopulmonary bypass | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.59, 0.59] |
| 6.2 Off‐pump surgery | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [‐0.61, 2.21] |
| 7 Pain scores on movement/coughing at 6 to 8 hours | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.69, 0.39] |
| 7.1 With cardiopulmonary bypass | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.69, 0.53] |
| 7.2 Off‐pump surgery | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.57, 0.77] |
| 8 Pain at rest at 24 hours | 3 | 231 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.41, 0.63] |
| 8.1 With cardiopulmonary bypass | 2 | 195 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.34, 0.91] |
| 8.2 Off‐pump surgery | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.38, 0.30] |
| 9 Pain on movement/coughing at 24 hours | 2 | 86 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.62, 1.24] |
| 9.1 With cardiopulmonary bypass | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.72 [0.22, 1.22] |
| 9.2 Off‐pump surgery | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐1.11, 0.63] |
| 10 Pain at rest at 48 hours | 2 | 195 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.77, 1.80] |
| 10.1 With cardiopulmonary bypass | 2 | 195 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.77, 1.80] |
| 11 Pain at rest on movement/coughing at 48 hours | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.36 [0.76, 1.96] |
| 12 Hypotension or need for vasopressor | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.08, 0.18] |
| 12.1 Off‐pump surgery | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.08, 0.18] |
2.3. Analysis.
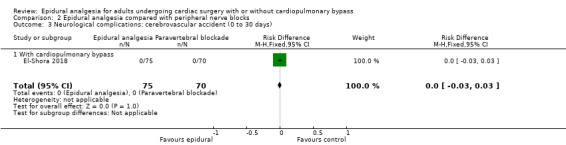
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 3 Neurological complications: cerebrovascular accident (0 to 30 days).
2.7. Analysis.
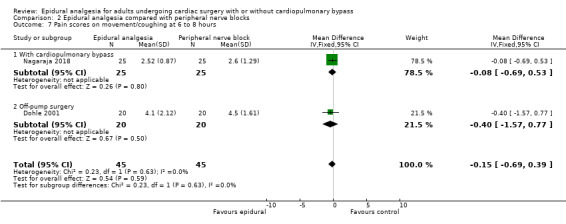
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 7 Pain scores on movement/coughing at 6 to 8 hours.
2.9. Analysis.
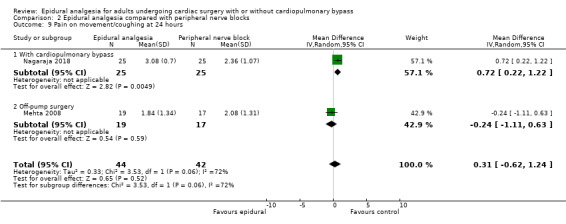
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 9 Pain on movement/coughing at 24 hours.
2.10. Analysis.
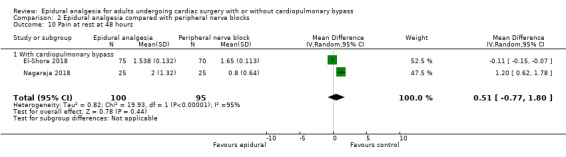
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 10 Pain at rest at 48 hours.
2.11. Analysis.
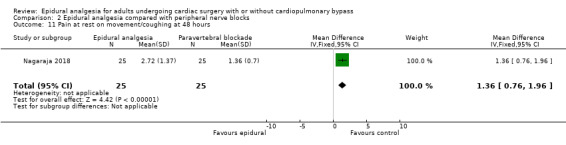
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 11 Pain at rest on movement/coughing at 48 hours.
2.12. Analysis.
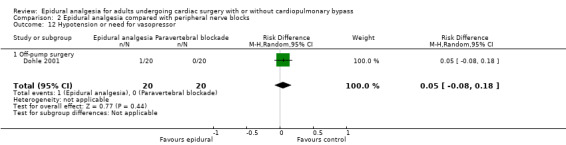
Comparison 2 Epidural analgesia compared with peripheral nerve blocks, Outcome 12 Hypotension or need for vasopressor.
Comparison 3. Epidural analgesia compared with intrapleural analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Myocardial infarction (0 to 30 days) | 1 | 50 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 1.1 Off‐pump surgery | 1 | 50 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 2 Neurological complications: epidural haematoma (0 to 30 days) | 1 | 50 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 2.1 Off‐pump surgery | 1 | 50 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 3 Duration of tracheal intubation (hours) | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.20, 0.60] |
| 3.1 Off‐pump surgery | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.20, 0.60] |
| 4 Pain scores at rest at 6 hours | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [0.31, 1.37] |
| 4.1 Off‐pump surgery | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [0.31, 1.37] |
3.4. Analysis.
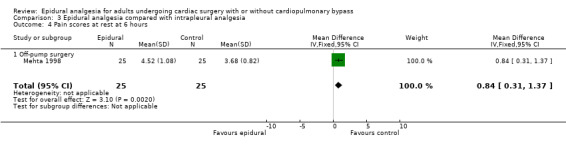
Comparison 3 Epidural analgesia compared with intrapleural analgesia, Outcome 4 Pain scores at rest at 6 hours.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aguero‐Martinez 2012.
| Methods | Parallel RCT Ethics committee: approved by the institutional ethics committee Informed consents: written informed consents obtained Site: Surgical‐Clinic Hermanos Ameijeiras Hospital, Cuba, Havana Setting: university hospital Dates of data collection: between September 2008 and March 2010 Funding: departmental Registration: RPCEC00000131 2012 |
|
| Participants | Adults undergoing off‐pump CABG; mean age: 60.2; sex distribution: 11 females and 49 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: IV midazolam 0.05 mg/kg Induction: midazolam, fentanyl, lidocaine, and atracurium Maintenance: propofol, isoflurane, and atracurium Surgery: off‐pump CABG |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: information received from study authors Conflict of interest: no conflict of interest DOI: n/a The trial also contains a third group with intrathecal analgesia not retained in the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized with a computer‐generated table |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "patients were not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed epidural reported |
| Other bias | Unclear risk | The group given systemic analgesia included more aged participants |
Bach 2002.
| Methods | Parallel RCT Ethics committee: approved by the ethics committee Informed consents: written informed consents obtained Site: University of Saarland, Homburg/Saarland, Germany Setting: university hospital Dates of data collection: unspecified Funding: supported in part by the industry Registration: unspecified |
|
| Participants | 40 participants: mean age 63.0 years; sex distribution: 12 females and 28 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: 1 mg of flunitrazepam orally on the day of surgery Induction: fentanyl 10 mcg/kg, midazolam 40 mcg/kg, etomidate 0.15 mg/kg, and pancuronium 0.1 mg/kg Maintenance: fentanyl/midazolam infusion (10/75 mcg/kg/h) and pancuronium Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: supported in part by the industry DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "drawing lots" |
| Allocation concealment (selection bias) | Unclear risk | Assigned the day before surgery; "randomizing box’ contained 20 lots of each group" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant died at 8 hours after surgery, as included in the review |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Control group consisted of 27 participants; 13 of them received a dopexamine infusion Supported in part by the industry |
Bakhtiary 2007.
| Methods | Parallel RCT Ethics committee: approved by the institutional review board Informed consents: written informed consents obtained Site: Johann Wolfgang Goethe University Hospital, Main, Germany Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: not reported |
|
| Participants | 132 participants; mean age 65 years; sex distribution: 20 females and 112 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: oral midazolam 7.5 mg Induction: propofol, remifentanil, and cisatracurium Maintenance: propofol, remifentanil, and cisatracurium Surgery: off‐pump CABG |
|
| Outcomes |
Relevant to this review
Other
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI: 10.1016/j.jtcvs.2007.03.043 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized to receive either GA or combined GATEA |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No failed epidural reported Groups had similar demographic data |
Barrington 2005.
| Methods | Parallel randomized controlled trial Ethics committee: approved by the ethics committee Informed consents: written informed consents obtained Site: St. Vincent’s Hospital, Melbourne, Australia Setting: university hospital Dates of data collection: from December 1999 to March 2002 Funding: grants from the Australian Society of Anaesthetists and the Australian and New Zealand College of Anaesthetists Registration: unspecified |
|
| Participants | 120 participants scheduled for elective coronary artery bypass grafting surgery; mean age 62.5 years; sex distribution: 16 females and 104 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: temazepam, ranitidine, and morphine Induction: midazolam, fentanyl, propofol, and rocuronium Maintenance: propofol Surgery: CABG with CPB using a membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: none reported DOI: 10.1213/01.ANE.0000146437.88485.47 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized the day before surgery to 2 groups. The random allocation sequence was computer‐generated in permuted blocks of 4 and was enclosed in sequentially numbered opaque sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Twelve‐lead ECGs were recorded before surgery and on postoperative days 1 and 5 and were assessed by 2 observers blinded to group allocation and postoperative clinical course. No mention of blinding for any other outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. Two participants with failed epidural were kept in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Unclear risk | All participants were included in the intention‐to‐treat analysis. Prevalence of cerebrovascular and peripheral vascular disease was more frequent in the epidural group |
Bektas 2015.
| Methods | Parallel RCT Ethics committee: approved by the Turkey High Education and Research Hospital Ethics Committee Informed consents: written informed consents obtained Site: Turkiye Yuksek Ihtisas Education and Research Hospital, Ankara, Turkey Setting: university hospital Dates of data collection: between 15 February 2009 and 10 August 2011 Funding: departmental/institutional Registration: not registered |
|
| Participants | 34 participants; mean age: 55 years; sex distribution: 10 females and 24 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: midazolam Induction: fentanyl, midazolam, and rocuronium Maintenance: fentanyl, midazolam, and rocuronium Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: information received from study authors Conflict of interest: "the authors declare that there is no conflict of interests regarding the publication of this paper" DOI: org/10.1155/2015/658678 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote. "randomly divided into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote. "patient selection, data collection and evaluation were performed by separate workers unaware of each other" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "patient selection, data collection and evaluation were performed by separate workers unaware of each other" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "outcomes were evaluated by another doctor" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural Groups had similar demographic data |
Berendes 2003.
| Methods | Parallel RCT Ethics committee: approved by the ethics committee Informed consents: written informed consents obtained Site: Munster, Germany Setting: university hospital Dates of data collection: from 1 February 2000 through 31 August 2000 Funding: supported in part by grant Be‐1‐1‐1/97‐5 to the Faculty of Medicine, Westfalische Wilhelms‐Universitat Munster, Innovative Medizinische Forschung, Munster, Germany Registration: unspecified |
|
| Participants | 73 participants: mean age 60.0 years; sex distribution: 20 females and 53 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: midazolam, sufentanil, and pancuronium Maintenance: propofol and sufentanil Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: none reported DOI: 10.1001/archsurg.138.12.1283 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomization |
| Allocation concealment (selection bias) | Low risk | Administered through a sequential opaque envelope technique |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "blinded for primary outcome measure: echographic examination for global and regional myocardial function |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No failed epidural Groups had similar demographic characteristics |
Brix‐Christensen 1998.
| Methods | Parallel RCT Ethics committee: approved by the Regional Ethical Committee on Human Research Informed consents: informed consents were obtained from each patient Site: Aarhus University Hospital, 8000 Aarhus C, Denmark Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 16 participants; mean age: 58.5 years; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: morphine, scopolamine, and diazepam Induction: midazolam, fentanyl, and pancuronium Maintenance: midazolam, enflurane, and fentanyl (group systemic analgesia only) or epidural analgesia Surgery: CABG with CPB using a hollow fibre oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to 2 groups; no details were provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural mentioned Groups had similar demographic data |
Caputo 2011.
| Methods | Parallel RCT Ethics committee: approved by the Central and South Bristol Research Ethics Committee (registration number E5471) Informed consents: written informed consents were obtained Site: Yale School of Medicine, New Haven, CT, USA; and University of Bristol, Bristol, UK; and Clinica Montevergine, Mercogliano, Italy Setting: university hospital Dates of data collection: August 2003 to November 2007 Funding: funded by the British Heart Foundation Registration: unspecified |
|
| Participants | 226 participants; mean age 65.7 years; sex distribution: 22 females and 204 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: benzodiazepines Induction: fentanyl, propofol, and pancuronium or vecuronium Maintenance: isoflurane or propofol Surgery: off‐pump CABG |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: information received from study authors Conflict of interest: none declared DOI: 10.1093/icvts/ivt001 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized treatment allocations were generated using Stata version 8. Participants were stratified by the consultant team via 1:1 allocation using blocks of varying sizes |
| Allocation concealment (selection bias) | Low risk | Allocation details were concealed in sequentially numbered, opaque sealed envelopes. These were prepared by the clinical trials and evaluation unit |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were included in the analysis No participants withdrew from the trial |
| Selective reporting (reporting bias) | Low risk | All results were reported |
| Other bias | Unclear risk | Intention‐to‐treat Epidural anaesthesia: 18 not performed and 9 failed epidural Systemic analgesia: 3 participants received epidural analgesia Groups had similar demographic characteristics, except that lung disease/chronic obstructive airways disease was more common in the epidural group (23% vs 12%) |
Celik 2015.
| Methods | Parallel RCT Ethics committee: approved by the hospital scientific committee Informed consents: obtained Site: Kardiyovasküler Cerrahi Kliniği, İstanbul, Türkiye Setting: university hospital Dates of data collection: 2009 Funding: institutional/departmental Registration: not registered |
|
| Participants | 40 participants; mean age; 58 years; sex distribution: 12 females and 28 males Incusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: fentanyl, midazolam, and pancuronium Maintenance: fentanyl and propofol Surgery: CABG, classified as with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Conflict of interest: no conflict of interest Correspondence: information received from study authors DOI: 10.4274/haseki.2163 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospectively randomized; no details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "only the anaesthesiologist knew the treatment group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the study was not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the study was not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Analysed in intention‐to‐treat Groups well balanced |
Cheng‐Wei 2017.
| Methods | Parallel RCT Ethics committee: not reported Informed consents: not reported Site: Far Eastern Memorial Hospital, New Taipei City, Taiwan Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 37 participants; mean age: not reported; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction and maintenance: not reported Surgery: off‐pump CABG or valve surgery |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Conflict of interest: not reported Correspondence: email sent 16 March 2018; no reply DOI: n/a Conference abstract, limited information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Conference abstract; limited details provided |
| Selective reporting (reporting bias) | Low risk | Conference abstract; limited details provided |
| Other bias | Unclear risk | Conference abstract; limited details provided |
de Vries 2002.
| Methods | Parallel RCT Ethics committee: approved by the ethics committee Informed consents: obtained Site: Groningen, The Netherlands Setting: university hospital Dates of data collection: January 1996 to January 1999 Funding: departmental/institutional Registration: not registered |
|
| Participants | 90 participants, for the 2 groups included in this review: mean age: 58.5 years; sex distribution: 18 females and 42 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: midazolam and sufentanil Maintenance: sufentanil and isoflurane or propofol Surgery: off‐pump CABG |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: information received from study authors Conflict of interest: no conflict of interest DOI: 10.1053/jcan.2002.29645 The trial includes a third group not retained for this review: high opoid dose and mandatory postoperative mechanical ventilation for a specific duration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "90 patients were randomly divided into 3 groups"; "computer‐generated table" |
| Allocation concealment (selection bias) | Low risk | Quote: "envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five participants were excluded from analysis: 3 for surgical reasons and 2 because the epidural technique failed |
| Selective reporting (reporting bias) | Low risk | All results were reported |
| Other bias | Low risk | Not in intention‐to‐treat Groups had similar demographic data |
Dohle 2001.
| Methods | Parallel RCT Ethics committee: approved Informed consents: obtained Site: New Delhi, India Setting: university hospital Dates of data collection: not reported Funding: unspecified Registration: unspecified |
|
| Participants | 41 participants, for participants included in the analysis: mean age: 56 years; sex distribution: 7 females and 33 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: lorazepam and morphine Induction and maintenance: midazolam, fentanyl (total dose 5 mcg/kg), nitrous oxide, isoflurane, and vecuronium Surgery: off‐pump CABG |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: 10.1053/jcan.2001.23271 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized study"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "an independent observer who was blinded to the analgesia technique recorded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One failed epidural |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Not in intention‐to‐treat Groups had similar demographic data |
El‐Baz 1987.
| Methods | Parallel RCT Ethics committee: not reported Informed consents: not reported Site: Chicago, IL, USA Setting: university hospital Dates of data collection: not reported Funding: unspecified Registration: unspecified |
|
| Participants | 60 participants: mean age: 59 years; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: thiopental and succinylcholine Maintenance: nitrous oxide, halothane, and pancuronium Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: none reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote. "patients were randomly divided into two equal groups of 30 patients"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural mentioned Groups had similar demographic characteristics |
El‐Morsy 2012.
| Methods | Parallel RCT Ethics committee: approved by the institutional ethics committee Informed consents: written informed consents obtained Site: Mansoura University, Egypt Setting: university hospital Dates of data collection: unspecified Funding: departmental resources Registration: unspecified |
|
| Participants | 50 participants; mean age: 69 years; sex distribution: 5 females and 45 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication with midazolam and tramadol Induction: fentanyl, thiopental, and pancuronium Maintenance: sevoflurane, fentanyl, and pancuronium Surgery: CABG with CPB using a membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Conflict of interest: none declared Correspondence: email sent 16 March 2018; no reply DOI: 10.4103/1658‐354X.93048 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly enrolled (sealed envelope) |
| Allocation concealment (selection bias) | Low risk | Participants were randomly enrolled (sealed envelope) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural analgesia mentioned Participants in both groups were comparable with regard to demographic data, number of grafts, and time of surgery |
El‐Shora 2018.
| Methods | Parallel RCT Ethics committee: approved by the ethics committee Informed consents: written informed consents obtained Site: Egypt Setting: 2 centres from university hospital Dates of data collection: from March 2016 to March 2017 Funding: departmental/institutional Registration: PACTR201603001502110 (www.pactr.org). |
|
| Participants | 145 participants: mean age 43.5 years; sex distribution: 68 females and 77 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: midazolam, fentanyl, propofol, lidocaine, and pancuronium Maintenance: isoflurane and pancuronium Surgery: valve or CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 18 November 2018; study authors asked us to extract the information from the trial Conflict of interest: none DOI: 10.1055/s‐0038‐1668496 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked stratified randomization was used to assign participants to 2 groups via 1:1 allocation Randomization sequence was generated randomly online using https://www.randomizer.org/; block size ranged from 4 to 6 participants. Randomization was stratified by participating centres |
| Allocation concealment (selection bias) | Low risk | On the day before surgery, participants were sorted to 1 of the 2 groups based on blocked single‐blinded randomization |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single‐blinded Cardiac anaesthesia specialist, not participating in data collection or patient follow‐up, performed the block designated for each participant (either bilateral thoracic paravertebral or thoracic epidural block) A nurse collected the data without pre‐knowledge of participants' assigned groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants were excluded from the paravertebral group: 3 for in‐hospital mortality and 2 for reoperation within 24 hours One participant in the epidural group died in hospital, but data for this participant were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All results were reported |
| Other bias | Low risk | Groups well balanced “intention‐to‐treat” principle |
Fawcett 1997.
| Methods | Parallel RCT Ethics committee: approved by the Local Research Ethics Committee Informed consents: written informed consents obtained Site: St. George's Hospital Medical School, Cranmer Terrace, London, UK, and Queen's Medical Centre, University of Nottigham, Nottingham, UK Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 16 male participants; mean age: 61.5 years; sex distribution: 16 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: morphine and hyoscine Induction: fentanyl, thiopentone, and suxamethonium Maintenance: nitrous oxide, pancuronium, and midazolam Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned"; no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | No failed epidural Groups well balanced except for CPB time: 107 minutes for TEA vs 78 minutes for no epidural |
Fillinger 2002.
| Methods | Parallel RCT Ethics committee: approved by the Dartmouth College Committee for Protection of Human Subjects (institutional review board) Informed consents: written, informed consents were obtained from all participants Site: Dartmouth‐Hitchcock Medical Center, Lebanon; and Dartmouth Medical School, Hanover, NH, USA Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 60 participants; mean age: 62.8 years; sex distribution: 10 females and 50 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: fentanyl and midazolam Induction: fentanyl, midazolam, thiopental, and pancuronium or vecuronium Maintenance: isoflurane Surgery: CABG with CPB using a membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI: 10.1053/jcan.2002.29639 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerized randomization" (as classified by previous review authors) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "nonblinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "nonblinded" Except for ECG: recordings were reviewed by one of the study authors and a cardiologist, both of whom were blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Two participants in the epidural group were withdrawn from treatment in the operating room: 1 because of inability to place the catheter and 1 because of intravascular migration of an initially functioning catheter Both were included in subsequent analyses as intention‐to‐treat Groups had similar demographic data, except that 11 participants in the epidural group had a history of myocardial infarction within the 3 months immediately preceding surgery compared with 2 participants in the systemic analgesia group (P < 0.005) |
Greisen 2012.
| Methods | Parallel RCT Ethics committee: approved by the regional ethics committee and the Danish Medicines Agency Informed consents: written as well as oral information was obtained Site: Department of Anaesthesiology and Intensive Care, Aarhus University Hospital‐Skejby, Denmark Setting: university hospital Dates of data collection: from 1 March 2007 to 31 March 2009 Funding: departmental resources Registration: EudraCT 2005‐000617‐35 |
|
| Participants | 42 participants; mean age: 71.4 years; sex distribution: 17 females and 25 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: benzodiazepine and paracetamol Maintenance: propofol or sevoflurane Surgery: CABG or valve replacement or both with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Conflict of interest: none Correspondence: letter sent 16 March 2018; no reply DOI: 10.1111/j.1399‐6576.2012.02731.x |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by standard envelope method |
| Allocation concealment (selection bias) | Low risk | Randomized by standard envelope method |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants had displaced catheters when they arrived for surgery All participants in both groups completed the study |
| Selective reporting (reporting bias) | Low risk | All results were reported |
| Other bias | Low risk | All participants who intended to receive an epidural catheter had an epidural catheter successfully placed Groups well balanced |
Gurses 2013.
| Methods | Parallel RCT Ethics committee: approved by the Ethics Committee of the Medical School, Pamukkale University Informed consents: written informed consent was received from each individual before entry into the study Site: School of Medicine, Pamukkale University, Denizli, Turkey Setting: university hospital Dates of data collection: between July 2010 and January 2011 Funding: funded solely by the institution of the authors Registration: unspecified |
|
| Participants | 64 participants; mean age: 62.3; sex distribution: 18 females and 46 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: thiopental and rocuronium Maintenance: sevoflurane and rocuronium Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: no conflict of interest DOI: 10.12659/MSM.883861 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to study groups by the closed envelope method |
| Allocation concealment (selection bias) | Low risk | Randomly assigned to study groups by the closed envelope method |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Study groups were similar in terms of demographic variables |
Hansdottir 2006.
| Methods | Parallel RCT Ethics committee: the Human Ethics Committee of the Sahlgrenska Academy, Goteborg University, Goteborg, Sweden, approved the study protocol Informed consents: all participants gave written informed consent Site: Goteberg, Sweden Setting: university hospital Dates of data collection: from 1 April 2002 to 31 December 2003 Funding: support was provided solely from institutional and/or departmental sources Registration: unspecified |
|
| Participants | 113 participants; mean age: 66.5 years; sex distribution: 37 females and 76 males Inclusion criteria
Exclusion criteria
Aspirin treatment was not considered a contraindication to placement of a thoracic epidural catheter |
|
| Interventions |
Intervention
Comparator
Premedication: midazolam Induction and maintenance: propofol, remifentanil, and atracurium Surgery: CABG, valve procedures, or both with CPB using a membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned the day before surgery to 1 of 2 regimens |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The infusion bag (250 mL) of the patient‐controlled analgesia pump was changed only once during the postoperative treatment period (72 h) by the nursing team, which was neither blinded to treatment nor involved in assessment of patients The decision to allow hospital discharge was made by the surgical team not blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Atelectasis was defined as new area(s) of lobar or sublobar atelectatic consolidation with an air bronchogram by a radiologist blinded to treatment Evaluation of quality of recovery score, level of mobilization, pain, degree of sedation, lung function, and eligibility for hospital discharge was performed between 1:00 and 3:00 PM each day by either of two investigators. These investigators were blinded to the assigned treatment The blinded investigators were not involved in nursing of the participants Less than 5% of epidural participants revealed by mistake to the blinded observer the presence of the epidural catheter |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 113 participants were randomized, 110 participants received allocated treatment, and 97 participants were eventually analysed |
| Selective reporting (reporting bias) | Low risk | All results were reported |
| Other bias | Unclear risk | Three participants were excluded because of inability to place the epidural catheter. In 1 of these participants, the catheter was positioned intradurally, and another participant did not co‐operate. A malfunctioning epidural catheter was considered in 7 participants after extubation. Three of these participants had the epidural catheter replaced in the ICU, and 4 were treated with intravenous patient‐controlled analgesia with morphine These 7 participants were analysed on an intention‐to‐treat basis Groups had similar demographic data, except for a higher incidence of off‐pump CABG in the epidural group and a longer cardiopulmonary bypass time in the systemic analgesia group |
Heijmans 2007.
| Methods | Parallel RCT Ethics committee: the study was approved by the authors’ hospital’s Medical Ethics Committee Informed consents: written informed consents were obtained Site: Maastricht, The Netherlands Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 60 participants; mean age: 60 years; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: midazolam Induction and maintenance: propofol, remifentanil, or alfentanil and pancuronium Surgery; CABG with CPB using a hollow‐fibre membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI: 10.1053/j.jvca.2007.02.008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "60 patients scheduled to undergo coronary artery bypass surgery were randomized"; no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the study was blinded for the opioid infusion, except in the thoracic epidural group" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the study was blinded for the opioid infusion, except in the thoracic epidural group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results were reported |
| Other bias | Low risk | No failed epidural mentioned Groups had similar demographic data |
Huh 2004.
| Methods | Parallel RCT Ethics committee: approved by the clinical committee Informed consents: unspecified Site: Ulsan University College of Medicine, Asan Medical Center, Seoul, Korea Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 56 participants; mean age: 57.8; sex distribution: 13 females and 43 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: midazolam Induction: midazolam, fentanyl, and vecuronium Maintenance: vecuronium Surgery: various cardiac surgery with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: 10.4097/kjae.2004.47.4.521 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized; no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pain scores were evaluated by a blinded observer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 participants enrolled, 27 and 29 analysed for TEA and control groups, respectively |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Hutchenson 2006.
| Methods | Parallel RCT Ethics committee: approved by the Human Review Committee of the University of Goettingen (N* 15 II 94) Informed consents: written informed consent obtained Site: University of Goettingen, Germany, and Department of Anaesthesia and Perioperative Medicine, Medical University Charleston, SC, USA Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 20 participants: mean age 61.0; sex distribution 20 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: flunitrazepam 2 mg orally Induction: sufentanil, midazolam, and pancuronium Maintenance: midazolam, sufentanil Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply
Email: riekeh@musc.edu Conflict of interest: none DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned (envelope method) |
| Allocation concealment (selection bias) | Low risk | Randomly assigned (envelope method) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural reported Groups well balanced |
Jakobsen 2012.
| Methods | Parallel RCT Ethics committee approval: approved by the Central Denmark Region Committee on Biomedical Research Ethics and the Danish Medicine Agency Informed consents: written informed consents were obtained from all participants Site: Aarhus University Hospital, Skejby, Aarhus, Denmark Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: Eudra CT 2005‐000617‐35 |
|
| Participants | 60 participants; mean age: 71.3 years; sex distribution: 21 females and 39 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction and maintenance: propofol or sevoflurane, sufentanil, and rocuronium Surgery: CABG, valve procedure, or both with CPB, using a hollow‐fibre membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Conflict of interest: study authors declare that they have no competing interests Correspondence: email sent 16 March 2018; no reply DOI: 10.1053/j.jvca.2012.05.007 and 10.1053/j.jvca.2012.05.008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by the standard envelope method with blocks of 20 participants was performed immediately before insertion of the epidural catheter the day before surgery |
| Allocation concealment (selection bias) | Low risk | Randomization by the standard envelope method with blocks of 20 participants was performed immediately before insertion of the epidural catheter the day before surgery |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sixty‐three patients were approached; 2 declined, and 1 was excluded because surgery was changed to off‐pump CABG surgery |
| Selective reporting (reporting bias) | Unclear risk | Quote: "there were no significant differences in blood loss, urine output, administration of crystalloids and adverse events (not shown)" |
| Other bias | Low risk | Groups well balanced for preoperative characteristics One participant in the TEA group did not receive a functional epidural, but because the protocol was based on an intention‐to‐treat principle, this participant was analysed in the TEA group |
Kendall 2004.
| Methods | Parallel RCT Ethics committee: approved by local research ethics committee Informed consents: obtained Site: Liverpool, UK Setting: university hospital Dates of data collection: not reported Funding: this work was entirely funded by The Cardiothoracic Centre, Thomas Drive, Liverpool, L14 3PE Registration: unspecified |
|
| Participants | 30 participants: mean age: 64.1 years; sex distribution: 8 females and 22 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: diazepam Induction: etomidate or propofol and fentanyl Maintenance: isoflurane (N = 10) or propofol (N = 10) Surgery: off‐pump CABG General anaesthesia: propofol or isoflurane or epidural: isoflurane with bupivacaine |
|
| Outcomes |
Relevant to this review
Other
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: none reported DOI: 10.1111/j.1365‐2044.2004.03713.x |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated to 1 of 3 groups, using a shuffled, sealed envelope technique |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "single‐blinded"; no sham block mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "single‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "three patients were excluded from the study and further analysis. Their treatment was re‐randomized and reallocated, providing 30 complete data sets for analysis" |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Not in intention‐to‐treat: (quote): "two participants required cardiopulmonary bypass to complete arterial revascularization, one in the isoflurane group and one in the epidural group. Two participants were found to have inadequate postoperative epidural analgesia. One participant in the propofol group had incomplete troponin T data. These participants were excluded from the study and further analysis" Groups had similar demographic characteristics |
Kilickan 2006.
| Methods | Parallel RCT Ethics committee: approved by the institutional review committee Informed consents: patient consents were obtained Site: Kocaeli University School of Medicine, Kocaeli, Turkey Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified | |
| Participants | 80 participants; mean age: 59.9 years; sex distribution: 16 females and 64 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: midazolam Induction: midazolam, fentanyl, and vecuronium Maintenance: nitrous oxide, propofol, and fentanyl Suregry: CABG with CPB |
|
| Outcomes |
Relevant to this review
Other
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly distributed sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural Groups well balanced |
Kilickan 2008.
| Methods | Parallel RCT Ethics committee: approved by the institutional review committee Informed consents: unspecified Site: Istanbul Bilim University School of Medicine, Istanbul, Turkey Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 60 participants: mean age: 61.8 years; sex distribution: 15 females and 45 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Interevention
Comparator
Premedication: midazolam Induction: midazolam, fentanyl, and vecuronium Maintenance: nitrous oxide, propofol, and fentanyl Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Other
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated; no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural mentioned Groups well balanced |
Kirno 1994.
| Methods | Parallel RCT Ethics committee: approved Informed consents: obtained Site: Goteberg, Sweden Setting: university hospitals Dates of data collection: unspecified Funding: this work was supported by grants from the Swedish Medical Research Council (No. 08682,09047, and 09720), the Medical Faculty at the University of Goteborg, the Medical Society of Goteborg, and the Swedish Heart‐Lung Foundation Registration: unspecified |
|
| Participants | 20 participants: mean age: not reported; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: thiopental, pancuronium, and fentanyl Maintenace: nitrous oxide in oxygen and fentanyl Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: none reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized to 2 groups; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | No failed epidural reported No details on preoperative groups' demographic data |
Kirov 2011.
| Methods | Parallel RCT Ethics committee: study protocol and informed consent form were approved by the Ethics Committee of Northern State Medical University, Arkhangelsk, Russian Federation Informed consents: written informed consent was obtained from every patient Site: Northern State Medical University, Troitsky Avenue 51, Arkhangelsk, 163000, Russian Federation; University of Tromsø, MH‐Breivika, Tromsø, 9038, Norway; and University Hospital of North Norway, Sykehusveien 38, Tromsø, 9038, Norway Setting: university hospital Dates of data collection: from January 2008 to September 2009 Funding: supported by a grant from the Government of Arkhangelsk region, “Young Pomor scientists”, and departmental funds Registration: NCT01384175 |
|
| Participants | 93 participants; for the participants included in the analysis, mean age: 55.6 years; sex distribution: 42 females and 48 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: diazepam Induction: fentanyl, propofol, and pipecuronium Maintenance: propofol, fentanyl, and pipecuronium Surgery: off‐pump CABG |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: information received from study authors Conflict of interest: study authors declare that they have no competing interests DOI: 10.1186/1471‐2253‐11‐17 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized to 3 groups, using the envelope method |
| Allocation concealment (selection bias) | Low risk | Participants were randomized to 3 groups, using the envelope method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was withdrawn from analysis in each group due to protocol violation (transfer to CPB) |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural reported Groups well balanced |
Konishi 1995.
| Methods | Parallel RCT Ethics committee: unspecified Informed consents: unspecified Site: New Tokyo Hospital, Matsudo, Japan Setting: university hospital Dates of data collection: October 1993 to March 1994 Funding: unspecified Registration: unspecified |
|
| Participants | 97 participants: mean age 64 years: sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction and maintenance: low‐dose fentanyl, nitrous oxide, and isoflurane Surgery: mainly CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote. "divided"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | No failed epidural mentioned Some participants had laparotomy to take the gastroepiploic artery used for coronary grafting |
Kundu 2007.
| Methods | Parallel RCT Ethics committee: approved by institutional ethics committee Informed consents: informed written consents were taken from all participants Site: National Institute of Cardiovascular Diseases (NICVD), Dhaka, Bangladesh Setting: university hospital Dates of data collection: between July 2006 and March 2007 Funding: unspecified Registration: unspecified |
|
| Participants | 40 participants: mean age: 51.2; sex distribution: 6 females and 34 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: diazepam Induction: midazolam, morphine, and pancuronium bromide Maintenance: halothane, midazolam, morphine, and pancuronium bromide Surgery: off‐pump CABG |
|
| Outcomes |
Relevant to this review
Other
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly selected and divided in two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All results reported |
| Selective reporting (reporting bias) | Low risk | No loss to follow‐up |
| Other bias | Low risk | No failed epidural mentioned Groups well balanced |
Kunstyr 2001.
| Methods | Parallel RCT Ethics committee: not reported Informed consents: not reported Site: Department of Cardiology at General University Hospital, Medical Faculty of Charles University, Prague, Czech Republic Setting: university hospital Dates of data collection: from autumn 1998 to spring 1999 Funding: unspecified Registration: unspecified |
|
| Participants | 81 participants; mean age: 61.6 years; sex distribution: 5 females and 76 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator 1
Comparator 2
Comparator 3
Premedication: morphine; atropine, and midazolam Induction: sufentanil, midazolam, and pipecuronium Maintenance: isoflurane, sufentanil, and midazolam Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants were withdrawn from the study and were excluded from analysis in the "ketamine" group due to diplopia, 2 were excluded due to TEA catheter dislodgement, and 3 for technical PCA pump problems (low battery) |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Not in intention‐to‐treat Groups well balanced |
Lenkutis 2009.
| Methods | Parallel RCT Ethics committee: approved by the local Ethics Committee of Kaunas Medical University Informed consents: obtained the day before surgery Site: Clinic of Cardiothoracic and Vascular Surgery, Kaunas University Hospital, Kaunas, Lithuania Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 60 participants; mean age: 65.4 years; sex distribution: 27 females and 33 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: midazolam and morphine Induction: fentanyl, midazolam, etomidate, and rocuronium Maintenance: sevoflurane, midazolam, and fentanyl for the systemic analgesia group Surgery: CABG with CPB using a hollow‐fibre membranous oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: 10.1177/0267659109348724 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural reported Groups well balanced |
Liem 1992.
| Methods | Parallel RCT Ethics committee: approved by the hospital ethics committee. Informed consents: oral informed consents obtained Site: Nijmegen, The Netherlands Setting: university hospital Dates of data collection: not reported Funding: supported by a grant from the Janssen Research Foundation, Beerse, Belgium Registration: unspecified |
|
| Participants | 54 participants; mean age: 59.6 years; sex distribution: 15 females and 39 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: midazolam, sufentanil, etomidate, and pancuronium Maintenance: midazolam, sufentanil, and pancuronium Surgery: CABG with CPB using a membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: none reported other than the grant received DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | On the day before surgery, participants were assigned randomly to an epidural or systemic analgesia group; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "the x‐rays were reviewed for atelectasis in a double blind manner" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Re‐sternotomy was necessary in 2 participants (1 in each group). In 1 TEA group participant, the epidural catheter was dislocated. These participants were excluded from the study |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Not in intention‐to‐treat With the exception of time of surgery and number of mammary artery bypasses, no significant differences were observed |
Loick 1999.
| Methods | Parallel RCT Ethics committee: approved by the local ethical committee Informed consents: all study participants gave written consent Site: Munster, Germany Setting: university hospital Dates of data collection: unspecified Funding: departmental/institutional Registration: unspecified |
|
| Participants | 70 participants, for the participants included in this review: mean age: 61.9 years; sex distribution: 9 females and 37 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: sufentanil, propofol, and pancuronium Maintenance: sufentanil and propofol Surgery: CABG with CPB using hollow fibre oxygenator |
|
| Outcomes |
Relevant to this review
Other
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: none reported DOI: n/a The trial contains a third group given IV clonidine and not retained for analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote. "The patients were randomly allocated to one of the following three study groups"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Post hoc, two patients in the control group, who underwent repeat thoracotomy due to surgical bleeding, were excluded from the study" |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Epidural blockade was performed successfully in all participants without any observed complications The groups were comparable with respect to previous myocardial infarction, preoperative medication of β‐blockers, and vasoactive substances. All participants had 2‐ to 3‐vessel coronary artery disease, and all, except 1 in each group, received a left internal mammary artery graft to bypass stenosis of the left descending artery |
Lundstrom 2005.
| Methods | Parallel RCT Ethics committee: approved by the local ethics committee Informed consents: written informed consents obtained Site: Rigshospitalet, Copenhagen, Denmark Setting: university hospital Dates of data collection: from 3 January 2000 to 12 December 2000 Funding: this research was supported by The Danish Heart Foundation, Copenhagen, Denmark, by research grants No. 99‐2‐3‐79‐22764 and 99‐1‐5‐92‐22709 Registration: unspecified |
|
| Participants | 50 participants; mean age: 64.6 years; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: midazolam, fentanyl, and pancuronium Maintenance: midazolam, pancuronium, and fentanyl or epidural analgesia Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Other
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization list was generated from a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "the data analyses were blinded in relation to any clinical information" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No failed epidural reported No statistically significant differences between demographic data for the 2 groups |
Lyons 1998.
| Methods | Parallel RCT Ethics committee: not reported Informed consents: not reported Site: Harefield Hospital, Middlesex, UK Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 20 participants; mean age: not reported; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction and maintenance: propofol and isoflurane plus fentanyl or epidural Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Other
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a Conference abstract; limited information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote. "randomized"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Conference abstract; limited information |
Mehta 1998.
| Methods | Parallel RCT Ethics committee: approved Informed consents: written informed consents obtained Site: New Delhi, India Setting: university hospital Funding: unspecified Registration: unspecified |
|
| Participants | 50 participants: mean age 54.4 years; sex distribution: 3 females and 47 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: lorazepam and morphine Induction: morphine, diazepam, vecuronium bromide, and thiopentone sodium Maintenance: morphine dose of 0.15 mg/kg, nitrous oxide, isoflurane, and vecuronium Surgery: CABG; off‐pump surgery performed with a 4‐ to 6‐inch left anterior thoracotomy incision through the fourth intercostal space |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly divided into two groups using computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All analgesic dosing was administered based on the VAS score, as noted by the blinded nurse observer" Myocardial infarction was assessed by a cardiologist blinded to the treatment group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural reported Groups had similar demographic data |
Mehta 2008.
| Methods | Parallel RCT Ethics committee: approved by institutional review board Informed consents: informed consents obtained Site: Escorts Heart Institute and Research Centre, New Delhi, India Setting: university hospital Dates of data collection: 2006 to 2007 Funding: departmental Registration: not registered |
|
| Participants | 36 participants; mean age: 53.9; sex distribution: 2 females and 34 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: oral lorazepam 2 mg and morphine sulphate 0.1 mg/kg with glycopyrrolate 0.2 mg IM Induction: midazolam, fentanyl Maintenance: isoflurane in oxygen and air, and vecuronium bromide Surgery: off‐pump robotic‐assisted CABG |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: information received from study authors Conflict of interest: none DOI: 10.4103/0971‐9784.41576 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote. "randomised"; "chit system" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent observer who was blinded to the analgesic techniques recorded visual analogue scale scores |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All results reported |
| Selective reporting (reporting bias) | Low risk | No loss to follow‐up |
| Other bias | Low risk | Analysed in intention‐to‐treat Groups had similar demographic data |
Mehta 2010.
| Methods | Parallel RCT Ethics committee: approved by institutional ethics board Informed consents: written informed consents obtained Site: Indraprastha Apollo Hospital, New Delhi, India Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 62 participants; mean age: 58.3 years; sex distribution: 5 females and 57 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: lorazepam, pantoprazole, inhaled levo‐salbutamol sulphate, and ipratropium bromide Induction: midazolam, fentanyl, thiopental, and pancuronium bromide Maintenance: midazolam, fentanyl, and pancuronium bromide Surgery: off‐pump CABG |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent observer who was blinded to the analgesic techniques recorded visual analogue scale" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural Groups well balanced |
Mishra 2004.
| Methods | Parallel RCT Ethics committee: not reported Informed consents: not reported Site: All India Institute of Medical Sciences, New Delhi, India Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 31 participants; mean age: not reported; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction and maintenance: not reported Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a Conference abstract, limited information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No indication of other bias |
Moore 1995.
| Methods | Parallel RCT Ethics committee: approved by the ethics committee (reference No. 90/3496) Informed consents: written informed consents obtained Site: Hammersmith Hospital, London, UK Setting: university hospital Dates of data collection: not reported Funding: financial support for this study from Hammersmith and Acton Special Trustees and Hammersmith and Queen Charlotte's Special Health Authority Registration: unspecified |
|
| Participants | 18 participants: mean age: 57.1 years; sex distribution: 1 female and 16 males and 1 unclear Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: diazepam, papaveretum, and hyoscine Induction: sufentanil, thiopentone, and pancuronium Maintenance: sufentanil Surgery: CABG with CPB using a bubble oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: none reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated by selection of a sealed envelope |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant had a severe haemorrhage; data from this participant were not presented |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Not in intention‐to‐treat Groups had similar demographic data |
Nagaraja 2018.
| Methods | Parallel RCT Ethics committee: approved by the ethics committee Informed consents: written informed consents obtained Site: Departments of Cardiac Anaesthesiology and CTVS, Sri Jayadeva Institute of Cardiovascular Sciences and Research, Bengaluru, Karnataka, India Setting: university hospital Dates of data collection: not reported Funding: departmental/institutional Registration: unspecified |
|
| Participants | 50 participants undergoing cardiac surgery; mean age 47.5 years; sex distribution: 22 females and 28 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Interevention
Comparator
Standardized general anaesthesia Surgery: CABG through median sternotomy |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 18 November 2018; no reply Conflict of interest: none DOI: 10.4103/aca.ACA_16_18 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed to two groups of 25 each using the closed envelope method" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed to two groups of 25 each using the closed envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Neskovic 2013.
| Methods | Parallel RCT Ethics committee: obtained Informed consents: not reported Site: Clinic for Anesthesiology and Intensive Care, Military Medical Academy, Belgrade, Serbia; Faculty of Medicine of the Military Medical Academy, University of Defence, Belgrade, Serbia; and Dedinje Cardiovascular Institute, Belgrade, Serbia Setting: university hospital Dates of data collection: February 2002 to October 2005 Funding: departmental (academic trial; part of a PhD thesis) Registration: not registered |
|
| Participants | 82 participants; mean age: 54.8 years; sex distribution: 13 females and 68 males and 1 unclear Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: midazolam, propofol, fentanyl, and pancuronium Maintenance: propofol, fentanyl, and pancuronium Surgery: CABG with or without CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: information received from study authors Conflict of interest: none DOI: 10.2298/VSP1305439N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was done by: (quote) "envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open label study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One of the participants had incomplete data and was excluded from further statistical analysis |
| Selective reporting (reporting bias) | Unclear risk | Initially, the study was designed for a larger number of participants, but for technical reasons, enrolment of patients was stopped earlier (82 participants) Otherwise, all results were reported |
| Other bias | Unclear risk | Not in intention‐to‐treat: there were 3 conversions from off‐pump to standard surgery and cardiopulmonary bypass; these participants were assigned to different groups according to the anaesthetic technique applied Groups well balanced except for LVEF |
Nygard 2004.
| Methods | Parallel RCT Ethics committees: approved by the Scientific Ethics Committees for Copenhagen and Frederiksberg (KF 02‐124/98), the Danish Data Protection Agency, and the Danish Medicines Agency Informed consents: written informed consents obtained Site: Copenhagen, Denmark Setting: university hospital Dates of data collection: not reported Funding: supported by The Danish Heart Foundation (grant no. 99‐2‐3‐79‐22764 and no. 99‐1‐5‐92‐22709) and an unrestricted grant from AstraZeneca, Denmark Registration: unspecified |
|
| Participants | 163 participants; mean age: 64.4 years; sex distribution: 18 females and 145 females Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: midazolam, fentanyl, and pancuronium Maintenance: isoflurane and fentanyl or epidural analgesia Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Other
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: none other than the grants received DOI: 10.1053/j.jvca.2004.08.006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 4 groups; randomization was 1:1:1:1. Randomization list was generated from a computerized table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the study was conducted in an open manner" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the study was conducted in an open manner" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 196 patients included, 163 were evaluated: 18 patients had surgery cancelled, and 4 patients had a change in surgical procedure. One withdrew consent preoperatively, and 6 withdrew consent postoperatively. One patient had a stroke before surgery, and in 1 patient placement of the epidural catheter was unsuccessful. Two patients were excluded because of protocol violations |
| Selective reporting (reporting bias) | Low risk | Appears to be free of other sources of bias. Sample size calculation stated |
| Other bias | Unclear risk | Not in intention‐to‐treat Groups had similar demographic data |
Obersztyn 2018.
| Methods | Parallel RCT Ethics committee: approved by the ethics committee Informed consents: written informed consents obtained Site: Medical University of Silesia in Katowice, Poland Setting: university hospital Dates of data collection: 18‐month period Funding: departmental/institutional Registration: unspecified |
|
| Participants | 80 participants: mean age 59.6 years; sex distribution: 20 females and 60 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: oral midazolam Induction: etomidate, fentanyl, and pancuronium Maintenance: isoflurane, fentanyl, and 1 dose of morphine before wound closure Surgery: CABG with or without CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 18 November 2018; no reply Conflict of interest: none DOI: 10.5114/kitp.2018.76471 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assessed by the anaesthesiologists at least 12 hours before transfer to the operating theatre. Randomization was performed at this stage, by tossing a coin" |
| Allocation concealment (selection bias) | Low risk | From the information above, group treatment was unknown at enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study was discontinued if the following complications occurred: myocardial insufficiency requiring placement of an intra‐aortic balloon pump or other methods of mechanical support, symptoms of acute myocardial ischaemia, requirement for increased doses of inotropic drugs (dopamine and/or dobutamine up to 5 mcg/kg/min was acceptable), cumulative time of extracorporeal circulation exceeding 180 minutes, marked drainage, deterioration in blood gases or other problems requiring elective extubation, and other circumstances not listed in the protocol that could affect postoperative sedation or elective extubation. For patients with any complications mentioned above, only the operation period was analysed Analysis of the postoperative period was performed for 39 participants in each group because 2 participants were excluded from participation in the study according to the methodology In group I, 1 participant (operated on with the use of extracorporeal circulation) was excluded because of a serious haemorrhage that occurred immediately after transfer from the surgical theatre and required reoperation One exclusion occurred in group II (also in a participant operated on with the use of extracorporeal circulation) because of perioperative myocardial infarction diagnosed both in ECG and with elevated serum enzymes |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Onan 2011.
| Methods | Parallel RCT Ethics committee: approved Informed consents: obtained Site: Kocaeli, Turkey Setting: university hospital Dates of data collection: not reported Funding: unspecified Registration: unspecified |
|
| Participants | 30 participants; mean age: 59.0 years; sex distribution: 3 females and 27 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: midazolam Induction: midazolam, fentanyl, and rocuronium Maintenance: nitrous oxide, propofol, fentanyl, and rocuronium Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: not reported DOI: 10.1053/j.jvca.2011.06.004 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed immediately before surgery using sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Randomization was performed immediately before surgery using sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural reported Groups had similar demographic data |
Onan 2013.
| Methods | Parallel RCT Ethics committee: approved by the Institutional Ethics Committee Informed consents: informed written consents were obtained from each patient Site: Istanbul Florence Nightingale Hospital, Istanbul, Turkey Setting: unspecified Dates of data collection: between April 2009 and March 2010 Funding: unspecified Registration: unspecified |
|
| Participants | 40 participants; mean age: 58.5 years; sex distribution: 4 females and 36 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: midazolam Induction: midazolam, fentanyl, and rocuronium Maintenance: nitrous oxide, rocuronium, propofol, and fentanyl Surgery: CABG with CPB using a membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: study authors acknowledge no conflict of interest in the submission DOI: 10.1111/jocs.12086 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed immediately before surgery using sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Randomization was performed immediately before surgery using sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural reported Groups well balanced |
Palomero 2008.
| Methods | Parallel RCT Ethics committee: approved by the La Paz Hospital Human Research Ethics Committee Informed consents: written informed consents were obtained from all patients Site: La Paz University Hospital, Madrid, Spain; and Gregorio Marañon University Hospital, Madrid, Spain Setting: university hospital Dates of data collection: not reported Funding: departmental Registration: unspecified |
|
| Participants | 22 participants; mean age: 65.3 years; sex distribution: 4 females and 18 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: propofol, fentanyl, and vecuronium Maintenance: propofol, sevoflurane, and fentanyl or epidural analgesia Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: information received from study authors Conflict of interest: no conflict of interest DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "assigned a computer‐generated randomization code" |
| Allocation concealment (selection bias) | Low risk | Quote: "final randomization was performed by a physician not belonging to the hospital team the day before surgery, using the randomization code" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the study was not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the study was not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural reported Groups well balanced, except Euroscore was higher in the TEA group (5.4 vs 3.8) |
Petrovski 2006.
| Methods | Parallel RCT Ethics committee: not reported Informed consents: not reported Site: Special Hospital for Cardiac Surgery Filip II, Skopje, Macedonia Setting: private hospital Dates of data collection: between March 2003 and March 2004 Funding: unspecified Registration: unspecified |
|
| Participants | 110 participants; mean age: not reported; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: not reported Maintenance: not reported Surgery: off‐pump CABG |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 18 November 2018; no reply Conflict of interest: not reported DOI: n/a Conference abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly selected"; no details |
| Allocation concealment (selection bias) | Unclear risk | "not reported" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "not reported" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "not reported" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Unclear risk | "conference abstract"; "limited information" |
| Other bias | Unclear risk | "conference abstract"; "limited information" |
Priestley 2002.
| Methods | Parallel RCT Ethics committee: approved by the local hospital Research and Ethics Committee Informed consents: all participants gave written informed consent Site: Westmead Hospital, Westmead, Australia Setting: university hospital Dates of data collection: unspecified Funding: this study was supported by the Australian and New Zealand College of Anaesthetists Registration: unspecified |
|
| Participants | 100 participants; mean age: 59 years; sex distribution: 14 females and 86 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: lorazepam, morphine, and midazolam Induction: fentanyl, propofol, and pancuronium Maintenance: fentanyl, propofol, and pancuronium Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: none reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization via sealed envelopes was used; participants at high risk were randomized separately |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "blinding of participants or investigators was not considered feasible" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "blinding of participants or investigators was not considered feasible" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fifty participants were enrolled into each group, and data were analysed on an intention‐to‐treat basis. A per‐protocol analysis was also performed, and 12 participants were excluded from such analysis: 4 failed epidural blocks, 3 surgical complications required reoperation (2 systemic analgesia, 1 epidural analgesia), 1 underwent reintubation (epidural) for respiratory failure, and 4 had protocol violations (3 systemic analgesia and 1 epidural analgesia) |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups had similar demographic data |
Rein 1989.
| Methods | Parallel RCT Ethics committee: approved by the ethical committee of the hospital Informed consents: obtained from all participants Site: Trondheim, Norway Setting: university hospital Dates of data collection: unspecified Funding: this study was supported by a grant from the Norwegian Council for Cardiovascular Diseases Registration: unspecified |
|
| Participants | 16 participants: age: 60.3 years; sex distribution: 16 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: morphine and scopolamine Induction: thiopentone and pancuronium Maintenance: nitrous oxide, diazepam, and fentanyl or epidural analgesia Surgery: CABG with CPB using a bubble oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: none reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "16 male patients were allocated at random to two groups"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant died 9 hours postoperatively and was excluded from final analyses |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Not in intention‐to‐treat Groups had similar demographic data |
Royse 2003.
| Methods | Parallel RCT Ethics committee: approved by institutional ethics committee Informed consents: informed written consents obtained Site: Melbourne, Victoria, Australia Setting: university hospital Dates of data collection: between 1998 and 2001 Funding: the study is supported by grants from the National Heart Foundation of Australia; Australian Society of Anaesthetists; and AstraZeneca Pty, Ltd Registration: unspecified |
|
| Participants | 76 participants; mean age: 64.7 years; sex distribution: 16 females and 60 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction and maintenance: midazolam, propofol, and alfentanil Surgery: CABG with CPB using a membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: information received from study authors Conflict of interest: none other than the grant received DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants were withdrawn: 1 withdrew from the study after randomization, deciding not to participate in research; 2 had failed epidurals; and 1 from the control group requested the epidural |
| Selective reporting (reporting bias) | Low risk | All results were reported |
| Other bias | Unclear risk | Not in intention‐to‐treat Epidural group had significantly longer cardiopulmonary bypass time Supported in part by the industry |
Scott 2001.
| Methods | Parallel RCT Ethics committee: the hospital ethics committee approved the study Informed consents: all participants gave written informed consent Site: Glasgow, Scotland, UK Setting: university hospital Dates of data collection: unspecified Funding: departmental/institutional Registration: unspecified |
|
| Participants | 408 participants; mean age: 59 years; sex distribution: 56 females and 352 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: temazepam, ranitidine, and metoclopramide Induction and maintenance: propofol, alfentanil, and pancuronium Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: none reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to one of two regimens...by using cards drawn from a sealed envelope" |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was conducted in an open manner; therefore, neither the anaesthesiologists nor the nurses taking measurements were blinded to participants’ treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was conducted in an open manner; therefore, neither the anaesthesiologists nor the nurses taking measurements were blinded to participants’ treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 participants had insufficient data |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | In intention‐to‐treat for remaining 408 participants Groups had similar demographic data |
Sen 2017.
| Methods | Parallel RCT Ethics committee: approved by the ethical committee of the hospital Informed consents: obtained Site: Amrita Institute of Medical Sciences, Amrita Vishwa Vidyapeetham, Kochi, Kerala, India Setting: university hospital Dates of data collection: unspecified Funding: departmental resources Registration: unspecified |
|
| Participants | 60 participants; mean age: not reported; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: lorazepam, ranitidine, allopurinol, vitamin C, vitamin A, and vitamin E Induction and maintenance: fentanyl, midazolam, and pancuronium Surgery: CABG with CPB using a membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: no conflicts of interest DOI: 10.4103/0259‐1162.186613 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated to 2 equal groups by computer‐generated random sequence of numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was a prospective, randomized, non‐blinded comparative study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was a prospective, randomized, non‐blinded comparative study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural reported Groups well balanced |
Sharma 2010.
| Methods | Parallel RCT Ethics committee: approved by the institutional ethical review board Informed consents: informed written consents obtained Site: Indraprastha Apollo Hospitals, New Delhi, India Setting: university hospital Dates of data collection: unspecified Funding: departmental/institutional Registration: unspecified |
|
| Participants | 60 participants; mean age: 58.1 years; sex distribution: 4 females and 56 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: lorazepam and pantoprazole Induction: midazolam, fentanyl, propofol, and vecuronium Maintenamce: isoflurane Surgery: off‐pump CABG |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: none declared DOI: 10.4103/0971‐9784.58831 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized into two groups of 30 each"; no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Any untoward complications of epidural analgesia such as paresis, hypotension, urinary retention (after removal of Foley’s catheter), respiratory depression, and pruritus were noted every‐4‐hourly by a blinded observer Pain assessment was done using a 10‐cm visual analogue scale at rest and on coughing (10 cm = maximum pain and 0 = no pain) by a blinded observer Richter scale was observed by a blinded radiologist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural reported Groups had similar characteristics |
Stenseth 1994.
| Methods | Parallel RCT Ethics committee: approved by the Ethics Committee of the University of Trondheim Informed consents: obtained Site: Trondheim, Norway Setting: university hospital Dates of data collection: unspecified Funding: departmental/institutional Registration: unspecified |
|
| Participants | 28 participants: mean age: 54.9 years; sex distribution: 28 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intevention
Comparator
Premedication: morphine and scopolamine Induction: thiopentone and pancuronium Maintenance: fentanyl, diazepam, and nitrous oxide Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: data no longer available Conflict of interest: none reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants were excluded from the final analysis due to surgical problems |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Not in intention‐to‐treat Groups had similar demographic data |
Stenseth 1996.
| Methods | Parallel RCT Ethics committee: approved by the Ethics Committee of the University of Trondheim Informed consents: obtained Site: Trondheim, Norway Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 52 participants: mean age: 55.2 years; sex distribution: 52 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: morphine and scopolamine Induction and maintenance: diazepam, thiopentone, nitrous oxide, and pancuronium Surgery: CABG with CPB using a bubble oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: data are no longer available Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized into two groups"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants were excluded from the analysis because of complications experienced |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Not in intention‐to‐treat Groups had similar demographic data |
Stritesky 2006.
| Methods | Parallel RCT Ethics committee: approved by the ethics committee Informed consents: written informed consents obtained Site: Cardiovascular Clinic, Prague, Czech Republic Setting: private clinic Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 30 participants: mean age 69 years; sex distribution: 9 females and 21 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: midazolam, morphine, and atropine Induction: fentanyl, midazolam, thiopental, and atracurium Maintenance: midazolam, nitrous oxide, isoflurane, sufentanil or epidural anaesthesia, and atracurium Surgery: CABG with CPB with a membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 18 Novembre 2018; no reply Conflict of interest: none reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided"; no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced, except for pulmonary disease |
Svircevic 2011.
| Methods | Parallel: 2‐centre RCT Ethics committee: local human research ethics committees of the 2 participating centres (METC Isala Clinics, Zwolle, The Netherlands; and METC MST, Enschede, The Netherlands) approved of the study Informed consents: written informed consent was obtained from all participants Site: Utrecht, The Netherlands Setting: university hospitals Dates of data collection: from March 2004 to September 2007 Funding: the health renewal project provided from institutional sources: Isala Clinics Hospital 02/19. Registration number: 100000461 Registration: ISRCTN50434243 |
|
| Participants | 654 participants; mean age: 64.5 years; sex distribution: 111 females and 543 males Patients scheduled for elective cardiac surgery, including off‐pump procedures |
|
| Interventions |
Intervention
Comparator
Induction: remifentanil, etomidate, and pancuronium Maintenace: propofol or sevoflurane and remifentanil Surgery: CABG with or without CPB |
|
| Outcomes |
Relevant to this review
Other
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: none reported DOI: 10.1097/ALN.0b013e318201d2de |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random allocation sequence was concealed and computer‐generated in permuted unequal blocks, accessible through an Internet site |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "It was not possible for either the patient or the care providers to be blinded for treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All components of the primary endpoint were evaluated by an independent event committee blinded for randomization, consisting of a cardiologist, cardiothoracic surgeon, nephrologist, pulmonologist, and a neurologist" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was excluded because his surgery was cancelled, and one participant withdrew his consent after randomization |
| Selective reporting (reporting bias) | Low risk | All results were reported |
| Other bias | Low risk | Intention‐to‐treat Groups had similar demographic data |
Tenenbein 2008.
| Methods | Parallel RCT Ethics committee: approved by the Ethics Review Board of the University of Manitoba Health Sciences Centre Informed consents: all participants gave written informed consent Site: University of Manitoba, Canada Setting: university hospital Dates of data collection: between July 1, 2003, and June 30, 2004 Funding: supported by the Health Sciences Centre Research Foundation Registration: not registered |
|
| Participants | 50 participants; mean age: 60.5 years; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Control
Premedication: diazepam Induction: sufentanil, sodium thiopental, or propofol and rocuronium Maintenance: isoflurane Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: information received from study authors Conflict of interest: no conflicts of interest DOI: 10.1007/BF03021489 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "prospective, randomized, controlled trial"; "Randomization occurred immediately after enrolment"; "assigned a sealed envelope that contained the group assignment" |
| Allocation concealment (selection bias) | Low risk | Quote: "assigned a sealed envelope that contained the group assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "this was not a blinded study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "this was not a blinded study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Due to incomplete data collection, two patients were excluded from each group"; "unable to insert an epidural in one patient, and the epidural was not use, postoperatively in another patient, because of quadriparesis on emergence" |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Quote: "intention to treat principle" Preoperatively, groups were similar, except for higher mean ejection fractions in the control group (59.1 ± 8.9% vs 52.9 ± 7.5%; P < 0.01) |
Tenling 1999.
| Methods | Parallel RCT Ethics committee: approved by the ethics committee of Uppsala University Informed consents: obtained Site: Uppsala, Sweden Setting: university hospital Dates of data collection: not reported Funding: the study was supported by grants from the Swedish Medical Research Council (5315), the E. K. G. Selander Foundation, the Uppsala County Association Against Heart and Lung Diseases, and Uppsala University Registration: unspecified |
|
| Participants | 29 participants: mean age: 61.6 years; sex distribution: 1 female and 28 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: morphine and scopolamine Induction: fentanyl, thiopental, and pancuronium Maintenance: nitrous oxide, isoflurane, and fentanyl or epidural analgesia Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: none reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was achieved with sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "unblinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "unblinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was excluded from the analyses (reoperation) |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Not in intention‐to‐treat Groups had similar demographic data |
Usui 1990.
| Methods | Parallel RCT Ethics committee: not reported Informed consents: not reported Site: Japan Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 20 participants; mean age: not reported; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction: morphine and pancuronium Maintenance: nitrous oxide, morphine, and pancuronium Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "divided randomly"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups had similar demographic data Additional co‐analgesia for the systemic analgesia group only |
Volk 2003.
| Methods | Parallel RCT Ethics committee: approved Informed consents: written informed consents obtained Site: Germany Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 26 participants; mean age: 66 years; sex distribution: 5 females and 21 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: oral midazolam 0.1 mg/kg Induction: etomidate 0.2 mg/kg Maintenance: midazolam, sufentanil, and pancuronium Surgery: CABG with CPB with a membrane oxygenator |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 18 November 2018; no reply Conflict of interest: not reported DOI: 10.1016/S1043‐4666(03)00090‐5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced, except perhaps for sex distribution |
Yang 1996.
| Methods | Parallel RCT Ethics committee: unspecified Informed consents: unspecified Site: Baogang Hospital, Baotou, China Setting: university hospital Dates of data collection: unspecified Funding: unspecified Registration: unspecified |
|
| Participants | 21 participants; mean age: not reported; sex distribution: not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: morphine and scopolamine Induction: thiopental, fentanyl, and pancuronium Maintenance: enflurane, pancuronium, and fentanyl or epidural analgesia Surgery: heart surgery for congenital heart disease with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI:n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"randomly divided"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural mentioned Groups similar for preoperative cathecholamine values |
Yilmaz 2007.
| Methods | Parallel RCT Ethics committee: approved by the ethics committee Informed consents: not reported Site: Yeditepe University Hospital, Department of Anesthesiology and Reanimation Anabilim; Psychiatry Ersek Chest Cardiovascular Center; Anesthesia Clinic and Special Swiss Hospital Breast Cardiovascular Anesthesiology, Turkey Setting: university hospital Dates of data collection: not reported Funding: unspecified Registration: unspecified |
|
| Participants | 34 participants; mean age: 55.7 years; sex distribution: 7 females and 27 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: atropine and midazolam Induction: fentanyl, midazolam, and pancuronium Maintenance: fentanyl, midazolam, and isoflurane Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomized"; no details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural mentioned Groups well balanced |
Yung 1997.
| Methods | Parallel RCT Ethics committee: not reported Informed consents: obtained Site: Veterans General Hospital‐Taipei and National Yang‐Ming University School of Medicine, Taipei, Taiwan, ROC Setting: university hospital Dates of data collection: from June 1995 to December 1995 Funding: unspecified Registration: unspecified |
|
| Participants | 40 participants; mean age: 66.6 years; sex distribution: 5 females and 35 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction and maintenance: etomidate, vecuronium, fentanyl, and isoflurane Surgery: CABG with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: not reported DOI: n/a |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly selected and randomly divided into two groups"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural reported Groups well balanced |
Zawar 2015.
| Methods | Parallel RCT Ethics committee: approved by hospital research ethics committee Informed consents: written informed consents obtained Site: Institute of Critical Care Anesthesiology, Medanta ‐ The Medicity, Gurgaon, Haryana, India Setting: tertiary care hospital Dates of data collection: between December 2011 and November 2014 Funding: departmental resources Registration: unspecified |
|
| Participants | 81 participants; mean age: 74.6 years; sex distribution: 9 females and 72 males Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention
Comparator
Premedication: lorazepam and pantoprazole Induction: thiopentone sodium, fentanyl sulfate, and midazolam Maintenance: isoflurane, fentanyl, midazolam, and pancuronium or vecuronium bromide Surgery: off‐pump CABG |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: email sent 16 March 2018; no reply Conflict of interest: no conflicts of interest DOI: 10.4103/0971‐9784.159810 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized by computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was a non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was a non‐blinded study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Five protocol violations were reported in participants allocated to the study group. Two epidural catheters were accidentally dislodged during shifting of the participant; 1 participant developed severe hypotension requiring a bolus of epinephrine during catheter placement without any clinical consequences; 1 off‐pump CABG was converted to open CABG due to haemodynamic instability during surgery; and 1 participant withdrew consent from the trial. None of the participants had (quote:) “bloody tap” during epidural catheter placement |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Not in intention‐to‐treat Groups well balanced |
Zhou 2010.
| Methods | Parallel RCT Ethics committee: not reported Informed consents: not reported Site: Shandong University, Jinan, China Setting: university hospital Dates of data collection: from July 2007 to July 2009 Funding: departmental resources Registration: unspecified |
|
| Participants | 30 participants; mean age: not reported; sex distribution: 11 females and 19 males Inclusion criteria
Excusion criteria
|
|
| Interventions |
Intervention
Comparator
Induction and maintenance: not reported Surgery: unclear if surgeries were performed with or without cardiopulmonary bypass, classified as with CPB |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Correspondence: letter sent 16 March 2018; no reply Conflict of interest: no conflict of interest DOI: 10.16252/j .cnki .issn1004‐0501‐2010.03.021 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; no details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | No failed epidural reported No significant differences between the 2 groups in general characteristics (P > 0.05) |
APTT: activated partial thromboplastin time; ASA: American Society of Anesthesiologists; AVR: aortic valve replacement; BMI: body mass index; CABG: coronary artery bypass grafting; CK‐MB: creatine kinase muscle/brain; CPB: cardiopulmonary bypass; ECG: electrocardiogram; EF: ejection fraction; FEV₁: forced expiratory volume in one second; FVC: forced vital capacity; GA: general anaesthesia; GATEA: general anaesthesia plus thoracic epidural analgesia; ICU: intensive care unit; INR: international normalized ratio; kg/m²: kilogram per square meter; IM: intramuscularly; IV: intravenously; LVEF: left ventricular ejection fraction; n/a: not available; NSAID: non‐steroidal anti‐inflammatory drug; NYHA: New York Heart Association; PaCO₂: partial pressure of carbon dioxide; PaO₂: partial pressure of oxygen; PCA: patient‐controlled analgesia; PCEA: patient‐controlled epidural analgesia; PT: prothrombin time; RCT: randomized controlled trial; TEA: thoracic epidural analgesia.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amat‐Santos 2012 | Different study design: not an RCT: "depending on the preference of the anaesthesiologist responsible for the case" |
| Anderson 2005 | Different study design: not an RCT: "the lack of randomization is a limitation" |
| Casalino 2006 | Different study design: not an RCT: case series of 144 patients |
| Chae 1998 | Different study design: classified as "no adequate sequence generation" by original review authors |
| Chakravarthy 2005 | Different study design: prospective audit of cases conducted over a 13‐year period |
| Crescenzi 2009 | Different study design: not an RCT: case‐matched, non‐randomized study |
| Djaiani 2000 | No original data |
| El‐Morsy 2012a | Different study population: children |
| Jideus 2001 | Different study design: classified as not randomized by previous review authors |
| Joachimsson 1989 | Different study design: not an RCT: "two groups of consecutive patients meeting the inclusion criteria were investigated" |
| Kaunienė 2016 | No outcome of interest measured |
| Kessler 2002 | Different study design: not an RCT and different intervention: "use of TEA alone was applied in awake patients with multi‐vessel coronary artery disease who underwent CABG via median sternotomy" |
| Kessler 2005 | Different study design: classified as "no adequate sequence generation" by previous review authors |
| Kunstyr 2008 | Different study population: pulmonary endarterectomy with cardiopulmonary bypass |
| Kurtoglu 2009 | Different intervention: compares general vs epidural anaesthesia for minimally invasive direct coronary artery bypass |
| Lagunilla 2006 | Different intervention: "In the post‐operative period, 0.2% ropivacaine with 5 mg/ml fentanyl was used for analgesia in all patients, employing a patient controlled system" |
| Liang 2012 | Different intervention: comparison between epidural anaesthesia perioperatively and postoperatively |
| Liem 1998 | Different study design: not an RCT: case report |
| Martinez 2012 | Different intervention: general anaesthesia compared with epidural anaesthesia or intrathecal morphine for beating heart surgery |
| Novikov 2011 | Different study population: aorto‐femoral bypass |
| Olivier 2005 | Different intervention: comparison of 3 different epidural solutions |
| Orsolya 2015 | Different study population: robot‐assisted laparoscopic urogenital surgery |
| Ortega 2011 | Different intervention: all participants had epidural analgesia with bupivacaine alone or bupivacaine plus morphine |
| Ovezov 2011 | Different intervention: all participants had epidural analgesia |
| Rao 2016 | Different intervention: all participants had epidural anaesthesia |
| Salman 2012 | Different study design: not an RCT: "retrospective study" |
| Salvi 2004 | Different study design: not an RCT: retrospective review of prospectively collected data |
| Schmidt 2005 | Different intervention: all participants had epidural analgesia |
| Stenger 2013 | Different study design: not an RCT: retrospective cohort study of prospectively registered data using population‐based healthcare databases |
| Stenseth 1993 | Different intervention: all participants had epidural analgesia and were randomized to light or deep general anaesthesia |
| Thorelius 1996 | Different study design: not an RCT: classified as "no adequate sequence generation" by previous review authors |
| Thorelius 1997 | Different study design: not an RCT |
| Toda 2013 | Different study design: not an RCT: "in this prospective non‐randomized study" |
| Turfrey 1997 | Different study design: not an RCT: "Using computerised patient medical records, we analysed the frequency of respiratory, neurological, renal, gastrointestinal, haematological and cardiovascular complications in these two groups" |
| Yashiki 2005 | Different intervention: TEA vs general anaesthesia |
RCT: randomized controlled trial; TEA: thoracic epidural analgesia.
Characteristics of ongoing studies [ordered by study ID]
CTRI/2012/04/002608.
| Trial name or title | Non‐analgesic benefits of combined thoracic epidural analgesia in elderly off‐pump coronary artery bypass grafting patients |
| Methods | Randomized by sealed opaque envelope; blinded participants |
| Participants | Participants undergoing off‐pump coronary artery bypass grafting Inclusion criteria: comorbidities Exclusion criteria: patient refusal, signs of infection over the spine, coagulation disorders, on antiplatelet agent, low‐molecular‐weight heparin or heparin infusion, emergency cases, unstable angina, left main stem disease, dysrhythmias, on steroids, undergoing combined procedures, on intra‐aortic, balloon pulsation, on rosuvastatin |
| Interventions | Intervention: epidural analgesia (5 to 15 mL of ropivacaine 0.75% followed by 6 to 14 mL as an infusion) Comparator: unspecified |
| Outcomes | 1. Stress response 2. Hypercoagulability |
| Starting date | Registered: 27 April 2012 Last refreshed: 14 January 2019 Status: opened to recruitment |
| Contact information | Dr. Bhanu Prakash Medanta ‐ The Medicity, Sec‐38, Haryana, Gurgaon, 122001, Sonipat, Hatyana, India Email: doctorbhanu@yahoo.in |
| Notes | Found 6 February 2019 Funded by Industry (AstraZeneca Pharma India Ltd,, Avishkar, PB No. 2483, Bellary Road, Hebbal, Bangalore 560024) |
CTRI/2018/05/013902.
| Trial name or title | A study of central and mixed venous oxygen saturation with outcomes in open heart surgery patients between two groups conventional general anaesthesia and combined with perioperative thoracic epidural or intravenous analgesia |
| Methods | Randomized (computer generated; open list of random numbers; participant, investigator and outcome assessor blinded) |
| Participants | 80 adults with coronary artery disease aged from 40 to 70 years Inclusion criteria: requiring off‐pump open heart bypass surgery Exclusion criteria: abnormal coagulation profiles, requiring salvage coronary artery bypass grafting, cardiogenic shock, heart valve pathology, antiplatelet therapy continuing local infection; renal, metabolic, neurological, or psychiatric disorders |
| Interventions | Intervention: epidural analgesia (10 mL bupivacaine 025%) Comparator: intravenous analgesia |
| Outcomes | 1. Central venous and mixed venous oxygen saturation during intraoperative period and time for extubation and length of postoperative intensive care unit stay 2. Stress response, inotropic/vasodilatory support, pain outcome, and analgesia requirements 3. New arrhythmia, postoperative blood loss, perioperative myocardial infarction, neurological events, infective complication, if present 4. Any other adverse effects during the study period |
| Starting date | Started: 26 March 2013 Completed: 25 November 2013 Trial registered retrospectively: 5 May 2018 Unpublished results |
| Contact information | Dr. Chaitali Sen Professor and Head Institute of Post Graduate Medical Education & Research, Kolkata Department of Cardiac Anesthesiology IPGMER and SSKM Hospital, 242 A J C Bose Road Kolkata, West Bengal, 700020, India Email: chaitali03@rediffmail.com |
| Notes | Found on 6 February 2019 |
NCT03719248.
| Trial name or title | Thoracic epidural reduces risks of increased left ventricular mass index during coronary artery bypass graft surgery |
| Methods | Open‐label, parallel, randomized controlled trial |
| Participants | 80 ASA II to IV adults (65 to 75 years old) Inclusion criteria: aortic valve replacement with or without coronary artery bypass grafting Exclusion criteria: ejection fraction 0.3, myocardial infarction within the last 4 weeks, diabetes, severe pulmonary or arterial hypertension, a contraindication for epidural analgesia, administration of ticlopidine within 15 days before surgery and administration of platelet glycoprotein IIb/IIIa inhibitor, significant aortic insufficiency, emergency surgery, poor acoustic windows for adequate echocardiographic assessment, and/or did not undergo an echocardiogram before the operation |
| Interventions | Intervention: thoracic epidural Comparator: unspecified |
| Outcomes | 1. Cardiac function 2. Other haemodynamic variables 3. Myocardial ischaemia |
| Starting date | Started: 1 January 2017 Registered: 15 October 2018 Completed |
| Contact information | Not available |
| Notes | Found 6 February 2019 |
ASA: American Society of Anesthesiologists physical status.
Differences between protocol and review
We made the following changes to the published review (Svircevic 2013).
Two new review authors (Joanne Guay and Sandra Kopp) replaced authors from the previously published version (Vesna Svircevic, Martijn M Passier, Arno P Nierich, Diederik van Dijk, Cor J Kalkman and Geert J van der Heijden).
We clarified that we are excluding observational studies, quasi‐randomized trials, cross‐over trials, and cluster‐randomized trials.
We clarified that patients operated with or without cardiopulmonary bypass are included.
We clarified that studies in which investigators administered epidural analgesia as a single shot block or as a continuous infusion for any duration and containing a local anaesthetic alone (extended duration or not) or in combination with an opioid (extended duration or not) or an opioid alone were included.
For the comparator, we included all other modes of analgesia and divided them into the following: (1) all forms of systemic analgesia (opioid‐based regimen or other) regardless of the route of administration (intravenous (with or without a self‐administered patient‐controlled device), intramuscular, or oral analgesia), (2) peripheral nerve blocks, (3) intrapleural analgesia, and (4) wound infiltration.
Some time points were changed, and we are now evaluating the following: (1) mortality at 0 to 30 days, six months, and one year, (2) myocardial infarction at 0 to 30 days, (3) respiratory complications at 0 to 30 days, (4) atrial fibrillation or atrial flutter at zero to two weeks, (5) neurological complications at 0 to 30 days, and (6) duration of tracheal intubation.
We have clarified that the definition used for myocardial infarction was the one used by study authors.
-
We added three outcomes.
Duration of tracheal intubation: we think that resource utilization is an important factor in nowadays budgets.
Pain scores: we wanted to quantify the differences between epidural analgesia and other modalities of pain treatment.
Haemodynamic support: we wanted to quantify the additional risk or not of hypotensive episodes and the need for vasopressors or inotropic support.
We clarified supraventricular tachyarrhythmia as atrial fibrillation or atrial flutter.
We clarified respiratory complications as respiratory depression or pneumonia.
We clarified neurological complications as stroke or severe neurological complications from epidural analgesia.
We updated the methodology: provided clarification for use of fixed versus random effects models, use of risk difference for study with zero cells, a priori factors for heterogeneity exploration, numbers needed to treat for additional beneficial outcome, and rating of the quality of evidence as per the GRADE system.
Contributions of authors
Conceiving the update: Joanne Guay (JG) and Sandra Kopp (SK) Co‐ordinating the review: JG Undertaking manual searches: JG Screening search results: JG and SK Organizing retrieval of papers: JG Screening retrieved papers against inclusion criteria: JG and SK Appraising quality of papers: JG and SK Abstracting data from papers: JG and SK Writing to authors of papers for additional information: JG Obtaining and screening data on unpublished studies: JG and SK Managing data for the review: JG Entering data into Review Manager (Review Manager 2014): JG Analysing RevMan 5 statistical data: JG and SK Performing other statistical analysis not using RevMan 5: JG Interpreting data: JG and SK Making statistical inferences: JG Writing the review: JG and SK Serving as guarantor for the review (one author): JG Being responsible for reading and checking the review before submission: JG and SK
Sources of support
Internal sources
-
University of Sherbrooke, Canada.
University of Sherbrooke granted access to databases and major medical journals
-
Laval University, Canada.
Laval University granted access to databases and major medical journals
-
University of Quebec in Abitibi Temiscamingue, Canada.
University of Quebec in Abitibi Temiscamingue granted access to databases and major medical journals
External sources
No sources of support supplied
Declarations of interest
Joanne Guay: none known
Sandra Kopp: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aguero‐Martinez 2012 {unpublished data only}
- Aguero Martinez MO. Copy of our manuscript. Email to: J Guay, 4 December 2017.
- Aguero‐Martinez MO. Anesthetic multimodal methods for coronary artery surgery without extracorporeal circulation. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=RPCEC00000131 2012.
- Aguerro Martinez MO. Information on our trial. Email to: J Guay, 21 March 2018.
- Agüero Martínez MO, Jiménez Paneque RE. Multimodal analgesia for off‐pump CABG: a randomized clinical trial [Métodos anestésicos multimodales en el procedimiento quirúrgico de revascularización miocárdica sin circulación extracorpórea:ensayo clínico aleatorizado]. CORE: https://core.ac.uk/display/11816611. Accessed 5 December 2017. [oai:generic.eprints.org:500/core394 ]
Bach 2002 {published data only (unpublished sought but not used)}
- Bach F, Grundmann U, Bauer M, Buchinger H, Soltész S, Graeter T, et al. Modulation of the inflammatory response to cardiopulmonary bypass by dopexamine and epidural anesthesia. Acta Anaesthesiologica Scandinavica 2002;46(10):1227‐35. [PUBMED: 12421195] [DOI] [PubMed] [Google Scholar]
Bakhtiary 2007 {published data only (unpublished sought but not used)}
- Bakhtiary F, Therapidis P, Dzemali O, Ak K, Ackermann H, Meininger D, et al. Impact of high thoracic epidural anesthesia on incidence of perioperative atrial fibrillation in off‐pump coronary bypass grafting: a prospective randomized study. Journal of Thoracic and Cardiovascular Surgery 2007;134(2):460‐4. [DOI: 10.1016/j.jtcvs.2007.03.043; PUBMED: 17662790] [DOI] [PubMed] [Google Scholar]
Barrington 2005 {published data only (unpublished sought but not used)}
- Barrington MJ, Kluger R, Watson R, Scott DA, Harris KJ. Epidural anesthesia for coronary artery bypass surgery compared with general anesthesia alone does not reduce biochemical markers of myocardial damage. Anesthesia and Analgesia 2005;100(4):921‐8. [DOI: 10.1213/01.ANE.0000146437.88485.47; PUBMED: 15781499] [DOI] [PubMed] [Google Scholar]
Bektas 2015 {published and unpublished data}
- Bektas S. Information on our trial. Email to: J Guay, 11 April 2018.
- Bektas S. Information on our trial. Email to: J Guay, 11 April 2018.
- Bektas S. Information on our trial. Email to: J Guay, 21 March 2018.
- Bektas SG, Turan S, Karadeniz U, Ozturk B, Yavas S, Biricik D, et al. Does high thoracic epidural analgesia with levobupivacaine preserve myocardium? A prospective randomized study. BioMed Research International 2015;2015:658678. [DOI: 10.1155/2015/658678; PUBMED: 25918718] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berendes 2003 {published data only (unpublished sought but not used)}
- Berendes E, Schmidt C, Aken H, Hartlage MG, Wirtz S, Reinecke H, et al. Reversible cardiac sympathectomy by high thoracic epidural anesthesia improves regional left ventricular function in patients undergoing coronary artery bypass grafting: a randomized trial. Archives of Surgery 2003;138(12):1283‐90. [DOI: 10.1001/archsurg.138.12.1283; PUBMED: 14662525] [DOI] [PubMed] [Google Scholar]
Brix‐Christensen 1998 {published data only (unpublished sought but not used)}
- Brix‐Christensen V, Tonnesen E, Sanchez RG, Bilfinger TV, Stefano GB. Endogenous morphine levels increase following cardiac surgery as part of the antiinflammatory response?. International Journal of Cardiology 1997;62(3):191‐7. [PUBMED: 9476677] [DOI] [PubMed] [Google Scholar]
- Brix‐Christensen V, Tonnesen E, Sorensen IJ, Bilfinger TV, Sanchez RG, Stefano GB. Effects of anaesthesia based on high versus low doses of opioids on the cytokine and acute‐phase protein responses in patients undergoing cardiac surgery. Acta Anaesthesiologica Scandinavica 1998;42(1):63–70. [PUBMED: 9527747] [DOI] [PubMed] [Google Scholar]
Caputo 2011 {published and unpublished data}
- Angelini G. Information on our trial. Email to: J Guay, 16 March 2018.
- Caputo M, Alwair H, Rogers CA, Ginty M, Monk C, Tomkins S, et al. Myocardial, inflammatory, and stress responses in off‐pump coronary artery bypass graft surgery with thoracic epidural anesthesia. Annals of Thoracic Surgery 2009;87(4):119‐26. [CRSREF: 2991383; PUBMED: 19324137] [DOI] [PubMed] [Google Scholar]
- Caputo M, Alwair H, Rogers CA, Pike K, Cohen A, Monk C, et al. Thoracic epidural anesthesia improves early outcomes in patients undergoing off‐pump coronary artery bypass surgery: a prospective, randomized, controlled trial. Anesthesiology 2011;114(2):380‐90. [PUBMED: 21245735] [DOI] [PubMed] [Google Scholar]
- Rajakaruna C, Rogers C, Pike K, Alwair H, Cohen A, Tomkins S, et al. Superior haemodynamic stability during off‐pump coronary surgery with thoracic epidural anaesthesia: results from a prospective randomized controlled trial. Interactive Cardiovascular and Thoracic Surgery 2013;16(5):602‐7. [DOI: 10.1093/icvts/ivt001; PUBMED: 23357523] [DOI] [PMC free article] [PubMed] [Google Scholar]
Celik 2015 {published and unpublished data}
- Kudsioglu T. Information on our trial. Email to: J Guay, 11 April 2018.
- Çelik BB, Kudsioğlu T, Alıcıkuş Z, Yapıcı N, Orhan G, Tandoğar N, et al. The effects of thoracic epidural analgesia on postoperative pain and myocardial protection in coronary artery bypass surgery [Koroner arter cerrahisinde torakal epidural analjezinin postoperatif ağrı ve miyokard korunması űzerine etkileri]. Haseki Tip Bulteni 2015;53(1):72‐6. [DOI: 10.4274/haseki.2163] [DOI] [Google Scholar]
Cheng‐Wei 2017 {published data only (unpublished sought but not used)}
- Cheng‐Wei L, Tzu‐Yu L. Comparison of thoracic epidural bupivacaine with fentanyl and local infusion of bupivacaine combined with intravenous patient‐controlled analgesia for pain relief in patients undergoing minimally invasive cardiac surgery. http://www.asaabstracts.com/strands/asaabstracts/searchresults.htm?base=1&index=1&display=50&highlight=true&highlightcolor=0&bold=true&italic=false. Boston, MA; 21‐25 October 2017: American Society of Anesthesiologists Annual Meeting, 2017:A1161.
de Vries 2002 {published and unpublished data}
- Vries AJ. Information on our trial. Email to: J Guay, 12 April 2018.
- Vries AJ, Mariani MA, Maaten JM, Loef BG, Lip H. To ventilate or not after minimally invasive direct coronary artery bypass surgery: the role of epidural anesthesia. Journal of Cardiothoracic and Vascular Anesthesia 2002;16(1):21‐6. [DOI: 10.1053/jcan.2002.29645; PUBMED: 11854873] [DOI] [PubMed] [Google Scholar]
Dohle 2001 {published data only (unpublished sought but not used)}
- Dhole S, Mehta Y, Saxena H, Juneja R, Trehan N. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. Journal of Cardiothoracic and Vascular Anesthesia 2001;15(3):288‐92. [DOI: 10.1053/jcan.2001.23271; PUBMED: 11426357] [DOI] [PubMed] [Google Scholar]
El‐Baz 1987 {published data only (unpublished sought but not used)}
- El‐Baz N, Goldin M. Continuous epidural infusion of morphine for pain relief after cardiac operations. Journal of Thoracic and Cardiovascular Surgery 1987;93(6):878–83. [PUBMED: 2952842] [PubMed] [Google Scholar]
El‐Morsy 2012 {published data only (unpublished sought but not used)}
- El‐Morsy GZ, El‐Deeb A. The outcome of thoracic epidural anesthesia in elderly patients undergoing coronary artery bypass graft surgery. Saudi Journal of Anaesthesia 2012;6(1):16‐21. [DOI: 10.4103/1658-354X.93048; PUBMED: 22412771] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
El‐Shora 2018 {published and unpublished data}
- Arafat A. Information on our trial. Email to: J Guay 20 Novembre 2018.
- El‐Shora HA, Beleehy AA, Abdelwahab AA, Ali GA, Omran TE, Hassan EA, et al. Bilateral paravertebral block versus thoracic epidural analgesia for pain control post‐cardiac surgery: a randomized controlled trial. Thoracic and Cardiovascular Surgeon 2018 August 16 [Epub ahead of print]. [DOI: 10.1055/s-0038-1668496; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fawcett 1997 {published data only (unpublished sought but not used)}
- Edwards RE, Quin AC, Fawcett WJ, MacDonald IA, Hall GM. Effect of thoracic extradural anaesthesia started after cardiopulmonary bypass on catecholamine values. British Journal of Anaesthesia. London, 1‐5 June: European Society of Anaesthesiologists Annual Congress Abstracts, 1996; Vol. 76, issue Suppl 2:54.
- Fawcett WJ, Edwards RE, Quinn AC, MacDonald IA, Hall GM. Thoracic epidural analgesia started after cardiopulmonary bypass: adrenergic, cardiovascular and respiratory sequelae. Anaesthesia 1997;52(4):294‐9. [PUBMED: 9135178] [DOI] [PubMed] [Google Scholar]
- Fawcett WJ, Quinn AC, Edwards RE, Hall GM. Thoracic extradural anaesthesia improves respiratory function after cardiac surgery. British Journal of Anaesthesia. London. 1‐5 June: European Society of Anaesthesiologists Annual Congress Abstracts, 1996; Vol. 76 (Suppl 2):71.
Fillinger 2002 {published data only (unpublished sought but not used)}
- Fillinger MP, Yeager MP, Dodds TM, Fillinger MF, Whalen PK, Glass DD. Epidural anesthesia and analgesia: effects on recovery from cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2002;16(1):15‐20. [DOI: 10.1053/jcan.2002.29639; PUBMED: 11854872] [DOI] [PubMed] [Google Scholar]
Greisen 2012 {published data only (unpublished sought but not used)}
- Greisen J, Nielsen DV, Sloth E, Jakobsen CJ. High thoracic epidural analgesia decreases stress hyperglycemia and insulin need in cardiac surgery patients. Acta Anaesthesiologica Scandinavica 2013;57(2):171‐7. [DOI: 10.1111/j.1399-6576.2012.02731.x; EudraCT 2005‐000617‐35; PUBMED: 22762307 ] [DOI] [PubMed] [Google Scholar]
- Greisen J, Nielsen DV, Sloth E, Jakobsen CJ. High thoracic epidural analgesia reduces the need for insulin, preserves glucose metabolism and attenuates stress hyperglycaemia in cardiac surgery patients. Applied Cardiopulmonary Pathophysiology. 2012; Vol. 16:193‐4.
Gurses 2013 {published data only (unpublished sought but not used)}
- Gurses E, Berk D, Sungurtekin H, Mete A, Serin S. Effects of high thoracic epidural anesthesia on mixed venous oxygen saturation in coronary artery bypass grafting surgery. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 2013;19:222‐9. [DOI: 10.12659/MSM.883861; DOI: 10.12659/MSM.883861; PUBMED: 23531633] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansdottir 2006 {published data only (unpublished sought but not used)}
- Hansdottir V, Philip J, Olsen MF, Eduard C, Houltz E, Ricksten SE. Thoracic epidural versus intravenous patient‐controlled analgesia after cardiac surgery: a randomized controlled trial on length of hospital stay and patient‐perceived quality of recovery. Anesthesiology 2006;104(1):142‐51. [PUBMED: 16394700 ] [DOI] [PubMed] [Google Scholar]
Heijmans 2007 {published data only (unpublished sought but not used)}
- Heijmans J, Fransen E, Buurman W, Maessen J, Roekaerts P. Comparison of the modulatory effects of four different fast‐track anesthetic techniques on the inflammatory response to cardiac surgery with cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 2007;21(4):512‐8. [DOI: 10.1053/j.jvca.2007.02.008; PUBMED: 17678776] [DOI] [PubMed] [Google Scholar]
Huh 2004 {published data only (unpublished sought but not used)}
- Huh IY, Sim JY, Lim YJ, Choi IC. The effects of high thoracic epidural patient controlled analgesia after open heart surgery. Korean Journal of Anesthesiology 2004;47:521‐6. [DOI: 10.4097/kjae.2004.47.4.521] [DOI] [Google Scholar]
Hutchenson 2006 {published data only (unpublished sought but not used)}
- Hutchenson J, Sonntag H, Hill E, et al. High thoracic epidural anesthesia’s effect on myocardial blood flow, oxygen consumption, myocardial work and markers of ischemia during coronary artery bypass grafting:a randomised controlled trial. Anesthesia and Analgesia. San Diego, 2006; Vol. 102:SCA‐13.
- Rieke H, Abernathy JH, Kazmaier S, Mielck F, Hutchenson K, Hanekop G. High thoracic epidural anaesthesia in patients undergoing CABG reduces sympathetic effects on myocardial blood flow and myocardial oxygen uptake and its effect on myocardial ischemia. Anestezjologia i Ratownictwo 2011;5:150‐60. [Google Scholar]
Jakobsen 2012 {published and unpublished data}
- Greisen J, Nielsen DV, Ryhammer PK, Sloth E, Jacobsen CJ. High thoracic epidural analgesia supplement seems to protect renal function, evaluated by serum creatinine changes, in cardiac surgery patients: a randomized study. Cardiovascular System 2013, (1):11. [DOI: 10.7243/2052-4358-1-11] [DOI] [Google Scholar]
- Greisen J, Ryhammer PK, Nielsen DV, Sloth E, Jakobsen CJ. High thoracic epidural analgesia supplement may protect renal function in cardiac surgery patients. Applied Cardiopulmonary Pathophysiology. 2013; Vol. 17 Issue 2:179‐80.
- Jakobsen CJ. Information on our trial. Email to: J Guay, 16 March 2018.
- Jakobsen CJ, Bhavsar R, Nielsen DV, Ryhammer PK, Sloth E, Greisen J. High thoracic epidural analgesia in cardiac surgery: part 1 ‐ high thoracic epidural analgesia improves cardiac performance in cardiac surgery patients. Journal of Cardiothoracic and Vascular Anesthesia 2012;26(6):1039‐47. [DOI: 10.1053/j.jvca.2012.05.007; PUBMED: 22770758] [DOI] [PubMed] [Google Scholar]
- Jakobsen CJ, Kirkegaard H, Christiansen CL, Lorentzen AG, Severinsen IK, Sloth E. Epidural anaesthesia reduces NT‐proBNP after cardiac surgery. European Journal of Anaesthesiology. 2007; Vol. 24, issue Suppl 41:1‐18.
- Nielsen DV, Bhavsar R, Greisen J, Ryhammer PK, Sloth E, Jakobsen CJ. High thoracic epidural analgesia in cardiac surgery: part 2 ‐ high thoracic epidural analgesia does not reduce time in or improve quality of recovery in the intensive care unit. Journal of Cardiothoracic and Vascular Anesthesia 2012;26(6):1048‐54. [DOI: 10.1053/j.jvca.2012.05.008; PUBMED: 22770692] [DOI] [PubMed] [Google Scholar]
Kendall 2004 {published data only (unpublished sought but not used)}
- Kendall JB, Russell GN, Scawn ND, Akrofi M, Cowan CM, Fox MA. A prospective, randomised, single‐blind pilot study to determine the effect of anaesthetic technique on troponin T release after off‐pump coronary artery surgery. Anaesthesia 2004;59(6):545‐9. [DOI: 10.1111/j.1365-2044.2004.03713.x; PUBMED: 15144293] [DOI] [PubMed] [Google Scholar]
Kilickan 2006 {published data only (unpublished sought but not used)}
- Gonca S, Kilickan L, Dalcik C, Dalcik H, Bayindir O. The cardioprotective effects of thoracal epidural anesthesia are induced by the expression of vascular endothelial growth factor and inducible nitric oxide synthase in cardiopulmonary bypass surgery. Journal of Cardiovascular Surgery 2007;48(1):93‐102. [PUBMED: 17308528] [PubMed] [Google Scholar]
- Kilickan L, Solak M, Bayindir O. Thoracic epidural anesthesia preserves myocardial function during intraoperative and postoperative period in coronary artery bypass grafting operation. Journal of Cardiovascular Surgery 2005;46(6):559‐67. [PUBMED: 16424844] [PubMed] [Google Scholar]
- Kiliçkan L, Gonca S, Dalçik C, Dalçik H, Solak M, Bayindir O, et al. General anesthesia with thoracic epidural anesthesia in the cardiopulmonary bypass surgery reduces apoptosis by upregulating antiapoptotic protein Bcl‐2. Journal of Cardiovascular Surgery 2006;47(3):315‐22. [PUBMED: 16760868] [PubMed] [Google Scholar]
Kilickan 2008 {published data only (unpublished sought but not used)}
- Kilickan L, Yumuk Z, Bayindir O. The effect of combined preinduction thoracic epidural anaesthesia and glucocorticoid administration on perioperative interleukin‐10 levels and hyperglycemia: a randomized controlled trial. Journal of Cardiovascular Surgery 2008;49(1):87‐93. [PUBMED: 18212693] [PubMed] [Google Scholar]
Kirno 1994 {published data only (unpublished sought but not used)}
- Kirnö K, Friberg P, Grzegorczyk A, Milocco I, Ricksten SE, Lundin S. Thoracic epidural anesthesia during coronary artery bypass surgery: effects on cardiac sympathetic activity, myocardial blood flow and metabolism, and central hemodynamics. Anesthesia and Analgesia 1994;79(6):1075‐81. [PUBMED: 7978429] [PubMed] [Google Scholar]
Kirov 2011 {published and unpublished data}
- Kirov M. Information on our trial. Email to: J Guay, 16 March 2018.
- Kirov MY, Eremeev AV, Smetkin AA, Bjertnaes LJ. Epidural anesthesia and postoperative analgesia with ropivacaine and fentanyl in off‐pump coronary artery bypass grafting: a randomized, controlled study. BMC Anesthesiology 2011;11:17. [DOI: 10.1186/1471-2253-11-17; PUBMED: 21923942] [DOI] [PMC free article] [PubMed] [Google Scholar]
Konishi 1995 {published data only (unpublished sought but not used)}
- Konishi A, Kikuchi K. Postoperative management following open heart surgery by thoracic epidural anesthesia. Japanese Journal of Anesthesiology 1995;44(8):1113‐7. [PUBMED: 7474310] [PubMed] [Google Scholar]
Kundu 2007 {published data only (unpublished sought but not used)}
- Kundu RK, Beg AK, Hossain S, Haque N, Mahafuddoza, Chowdury A. Comparative study of haemodynamic status and arrhythmia between combined epidural with general anaesthesia and general anaesthesia alone in off‐pump coronary artery bypass (OPCAB) surgery. Journal of Bangladesh College of Physicians and Surgeons 2008;26:116‐20. [Google Scholar]
Kunstyr 2001 {published data only (unpublished sought but not used)}
- Kunstýř J, Stříteský M, Krištof J, Škrovina B, Kotulák T. Postoperative analgesia in cardiac surgery [Pooperační analgezie v kardiochirurgii]. Anesteziologie a Neodkladna Pece 2001;12(4):191‐4. [Google Scholar]
Lenkutis 2009 {published data only (unpublished sought but not used)}
- Lenkutis T, Benetis R, Sirvinskas E, Raliene L, Judickaite L. Effects of epidural anesthesia on intrathoracic blood volume and extravascular lung water during on‐pump cardiac surgery. Perfusion 2009;24(4):243‐8. [DOI: 10.1177/0267659109348724; PUBMED: 19808745] [DOI] [PubMed] [Google Scholar]
Liem 1992 {published data only (unpublished sought but not used)}
- Liem TH, Booij LH, Gielen MJ, Hasenbos MA, Egmond J. Coronary artery bypass grafting using two different anesthetic techniques: part 3 ‐ adrenergic responses. Journal of Cardiothoracic and Vascular Anesthesia 1992;6(2):162‐7. [PUBMED: 1533167] [DOI] [PubMed] [Google Scholar]
- Liem TH, Booij LH, Hasenbos MA, Gielen MJ. Coronary artery bypass grafting using two different anesthetic techniques: part 1 ‐ hemodynamic results. Journal of Cardiothoracic & Vascular Anesthesia 1992;6(2):148‐55. [PUBMED: 1533165] [DOI] [PubMed] [Google Scholar]
- Liem TH, Hasenbos MA, Booij LH, Gielen MJ. Coronary artery bypass grafting using two different anesthetic techniques: part 2 ‐ postoperative outcome. Journal of Cardiothoracic and Vascular Anesthesia 1992;6(2):156‐61. [PUBMED: 1533166] [DOI] [PubMed] [Google Scholar]
Loick 1999 {published data only (unpublished sought but not used)}
- Loick HM, Mollhoff T, Erren M, Berendes E, Rolf N, Aken H. Thoracic epidural anesthesia lowers catecholamine and YNF alpha release after CABG in humans. Anesthesia and Analgesia. Orlando, FL; March 7‐11, 1998: International Anesthesia Research Society; 72nd Clinical and Scientific Congress: Cardiovascular Anesthesia, 1998; Vol. 86, issue 2S:81S. [DOI: 10.1097/00000539-199802001-00081] [DOI]
- Loick HM, Schmidt C, Aken H, Junker R, Erren M, Berendes E, et al. High thoracic epidural anesthesia, but not clonidine, attenuates the perioperative stress response via sympatholysis and reduces the release of troponin T in patients undergoing coronary artery bypass grafting. Anesthesia and Analgesia 1999;88(4):701‐9. [PUBMED: 10195508] [DOI] [PubMed] [Google Scholar]
Lundstrom 2005 {published data only (unpublished sought but not used)}
- Lundstrom LH, Nygard E, Hviid LB, Pedersen FM, Ravn J, Aldershvile J, et al. The effects of thoracic epidural analgesia on the occurence of late postoperative hypoxemia in patients undergoing elective coronary bypass surgery: a randomized controlled trial. Chest 2005;128(3):1564‐70. [PUBMED: 16162759] [DOI] [PubMed] [Google Scholar]
Lyons 1998 {published data only (unpublished sought but not used)}
- Lyons FM, Riedel BJ, Haw M, Royston D. Myocardial protection during coronary artery bypass graft surgery with thoracic epidural analgesia. British Journal of Anaesthesia. 1980; Vol. 80, issue Suppl 1:55‐56.
Mehta 1998 {published data only (unpublished sought but not used)}
- Mehta Y, Swaminathan M, Mishra Y, Trehan N. A comparative evaluation of intrapleural pain relief after minimally invasive direct coronary artery bypass surgery. Journal of Cardiothoracic and Vascular Anesthesia 1998;12(2):162‐5. [PUBMED: 9583546 ; PUBMED: 9583546] [DOI] [PubMed] [Google Scholar]
Mehta 2008 {published and unpublished data}
- Mehta Y. Information on our trial. Email to: J Guay, 2 April 2018.
- Mehta Y, Arora D, Sharma KK, Mishra Y, Wasir H, Trehan N. Comparison of continuous thoracic epidural and paravertebral block for postoperative analgesia after robotic‐assisted coronary artery bypass surgery. Annals of Cardiac Anaesthesia 2008;11(2):91‐6. [DOI: 10.4103/0971-9784.41576; PUBMED: 18603748] [DOI] [PubMed] [Google Scholar]
Mehta 2010 {published data only (unpublished sought but not used)}
- Mehta Y, Vats M, Sharma M, Arora R, Trehan N. Thoracic epidural analgesia for off‐pump coronary artery bypass surgery in patients with chronic obstructive pulmonary disease. Annals of Cardiac Anaesthesia 2010;13(3):224‐30. [PUBMED: 20826963] [DOI] [PubMed] [Google Scholar]
Mishra 2004 {published data only (unpublished sought but not used)}
- Mishra S Chauhan S, Kiran U, Bhan A, Eisai AK. Does addition of epidural analgesia enhance "Fast tracking" in routine CABG patients?. Indian Journal of Thoracic and Cardiovascular Surgery. 2004; Vol. 20, issue Jan‐Mar:6, Abstract 14.
Moore 1995 {published data only (unpublished sought but not used)}
- Moore CM, Cross MH, Desborough JP, Burrin JM, Macdonald IA, Hall GM. Hormonal effects of thoracic extradural analgesia for cardiac surgery. British Journal of Anesthesia 1995;75(4):387‐93. [PUBMED: 7488474] [DOI] [PubMed] [Google Scholar]
Nagaraja 2018 {published data only}
- Nagaraja PS, Ragavendran S, Singh NG, Asai O, Bhavya G, Manjunath N, et al. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Annals of Cardiac Anaesthesia 2018;21(3):323‐7. [CENTRAL: 01629440; DOI: 10.4103/aca.ACA_16_18; EMBASE: 623313977; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neskovic 2013 {published and unpublished data}
- Neskovic V. Information on our trial. Email to: J Guay, 20 March 2018.
- Neskovic V. Information on our trial. Email to: J Guay, 24 March 2018.
- Neskovic V, Milojevic P, Unic‐Stojanovic D, Slavkovic Z. Blood transfusion in cardiac surgery ‐ does the choice of anesthesia or type of surgery matter?. Vojnosanitetski Pregled 2013;70(5):439‐44. [DOI: 10.2298/VSP1305439N; PUBMED: 23789281] [DOI] [PubMed] [Google Scholar]
Nygard 2004 {published data only (unpublished sought but not used)}
- Nygård E, Sørensen LH, Hviid LB, Pedersen FM, Ravn J, Thomassen L, et al. Effects of amiodarone and thoracic epidural analgesia on atrial fibrillation after coronary artery bypass grafting. Journal of Cardiothoracic Vascular Anesthesia 2004;18(6):709‐14. [DOI: 10.1053/j.jvca.2004.08.006; PUBMED: 15650978] [DOI] [PubMed] [Google Scholar]
Obersztyn 2018 {published data only}
- Obersztyn M, Trejnowska E, Nadziakiewicz P, Knapik P. Evaluation of thoracic epidural analgesia in patients undergoing coronary artery bypass surgery ‐ a prospective randomized trial. Kardiochirurgia I Torakochirurgia Polska 2018;15(2):72‐8. [DOI: 10.5114/kitp.2018.76471; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Onan 2011 {published data only (unpublished sought but not used)}
- Onan IS, Onan B, Korkmaz AA, Oklu L, Kilickan L, Gonca S, et al. Effects of thoracic epidural anesthesia on flow and endothelium of internal thoracic artery in coronary artery bypass graft surgery. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(6):1063‐70. [DOI: 10.1053/j.jvca.2011.06.004; PUBMED: 21835638] [DOI] [PubMed] [Google Scholar]
Onan 2013 {published data only (unpublished sought but not used)}
- Onan B, Onan IS, Kilickan L, Sanisoglu I. Effects of epidural anesthesia on acute and chronic pain after coronary artery bypass grafting. Journal of Cardiac Surgery 2013;28(3):248‐53. [DOI: 10.1111/jocs.12086; PUBMED: 23461638] [DOI] [PubMed] [Google Scholar]
Palomero 2008 {published and unpublished data}
- Palomero MA. Information on our trial. Email to: J Guay, 3 April 2018.
- Palomero Rodriguez MA. Information on our trial. Email to: J Guay, 21 March 2018.
- Palomero Rodriguez MA, Suarez Gonzalo L, Villar Alvarez F, Varela Crespo C, Moreno Gomez Limon I, Criado Jimenez A. Thoracic epidural anesthesia decreases C‐reactive protein levels in patients undergoing elective coronary artery bypass graft surgery with cardiopulmonary bypass. Minerva Anestesiologica 2008;74(11):619‐26. [PUBMED: 18971890] [PubMed] [Google Scholar]
Petrovski 2006 {published data only}
- Petrovski V, Slavevski D, Stoicovski E, Belostotskii V, Hristov N, Mitrev Z. The advantages of combined epidural and general anesthesia vs. conventional general anesthesia in off pump coronary artery bypass surgery. Anesthesia and Analgesia. May 1, 2006: Society of Cardiovascular Anesthesiologists 28th AnnualMeeting and Workshops, 2006:SCA68.
Priestley 2002 {published data only (unpublished sought but not used)}
- Priestley MC, Cope L, Halliwell R, Gibson P, Chard RB, Skinner M, et al. Thoracic epidural anesthesia for cardiac surgery: the effects on tracheal intubation time and length of hospital stay. Anesthesia and Analgesia 2002;94(2):275–82. [PUBMED: 11812684] [DOI] [PubMed] [Google Scholar]
Rein 1989 {published data only (unpublished sought but not used)}
- Rein KA, Stenseth R, Myhre HO, Levang OW, Krogstad A. The influence of thoracic epidural analgesia on transcapillary fluid balance in subcutaneous tissue. A study in patients undergoing aortocoronary bypass surgery. Acta Anaesthesiologica Scandinavica 1989;33(1):79‐83. [PUBMED: 2644753] [DOI] [PubMed] [Google Scholar]
Royse 2003 {published and unpublished data}
- Royse C. Information on our trial. Email to: J Guay, 16 March 2018.
- Royse C, Remedios C, Royse A. High thoracic epidural analgesia reduces the risk of long‐term depression in patients undergoing coronary artery bypass surgery. Annals of Thoracic and Cardiovascular Surgery 2007;13(1):32‐5. [PUBMED: 17392668 ] [PubMed] [Google Scholar]
- Royse C, Royse A, Soeding P, Blake D, Pang J. Prospective randomized trial of high thoracic epidural analgesia for coronary artery bypass surgery. Annals of Thoracic Surgery 2003;75(1):93‐100. [PUBMED: 12537199] [DOI] [PubMed] [Google Scholar]
Scott 2001 {published data only (unpublished sought but not used)}
- Scott NB, Turfrey DJ, Ray DAA, Nzewi O, Sutcliffe NP, Lal AB, et al. A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesthesia and Analgesia 2001;93(3):528‐35. [PUBMED: 11524314] [DOI] [PubMed] [Google Scholar]
- Turfrey D, Ray D. The effect of thoracic epidural analgesia on postoperative complications after cardiac surgery ‐ a prospective study. British Journal of Anaesthesia. 1998; Vol. 80, issue Suppl 2:17.
Sen 2017 {published data only (unpublished sought but not used)}
- Sen AC, Rajan S, Balachandran R, Kumar L, Nair SG. Comparison of perioperative thoracic epidural fentanyl with bupivacaine and intravenous fentanyl for analgesia in patients undergoing coronary artery bypass grafting surgery. Anesthesia, Essays and Researches 2017;11(1):105‐9. [DOI: 10.4103/0259-1162.186613; PUBMED: 28298766] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2010 {published data only (unpublished sought but not used)}
- Sharma M, Mehta Y, Sawhney R, Vats M, Trehan N. Thoracic epidural analgesia in obese patients with body mass index of more than 30 kg/m² for off pump coronary artery bypass surgery. Annals Cardiac Anaesthesia 2010;13(1):28‐33. [DOI: 10.4103/0971-9784.58831; PUBMED: 20075532 ] [DOI] [PubMed] [Google Scholar]
Stenseth 1994 {published data only (unpublished sought but not used)}
- Stenseth R. Information on our trial. Email to: J Guay, 28 May 2018.
- Stenseth R, Berg EM, Bjella L, Christensen O, Levang OW, Gisvold SE. Effects of thoracic epidural analgesia on coronary hemodynamics and myocardial metabolism in coronary artery bypass surgery. Journal of Cardlothoracic and Vascular Anesthesta 1995;9(5):503‐9. [PUBMED: 8547549] [DOI] [PubMed] [Google Scholar]
- Stenseth R, Bjella L, Berg EM, Christensen O, Levang OW, Gisvold SE. Thoracic epidural analgesia in aortocoronary bypass surgery. I: haemodynamic effects. Acta Anaesthesiologica Scandinavica 1994;38(8):826‐33. [PUBMED: 7887106] [DOI] [PubMed] [Google Scholar]
- Stenseth R, Bjella L, Berg EM, Christensen O, Levang OW, Gisvold SE. Thoracic epidural analgesia in aortocoronary bypass surgery. II: effects on the endocrine metabolic response. Acta Anaesthesiologica Scandinavica 1994;38(8):834‐9. [PUBMED: 7887107 ] [DOI] [PubMed] [Google Scholar]
Stenseth 1996 {published data only (unpublished sought but not used)}
- Stenseth R. Information on our trial. Email to: J Guay, 28 May 2018.
- Stenseth R, Bjella L, Berg EM, Christensen O, Levang OW, Gisvold SE. Effects of thoracic epidural analgesia on pulmonary function after coronary artery bypass surgery. European Journal of Cardiothoracic Surgery 1996;10(10):859‐65. [PUBMED: 8911839] [DOI] [PubMed] [Google Scholar]
Stritesky 2006 {published data only}
- Stritesky M, Rubes D, Semrad M, Lips M. A comparison of haemodynamic changes using thoracic epidural anesthesia versus standard balanced anaesthesia during on‐pump coronary artery bypass grafting. Anesteziologie a Intenzivni Medicina 2006;17:138‐44. [Google Scholar]
Svircevic 2011 {published data only (unpublished sought but not used)}
- Svircevic V, Nierich AP, Moons KG, Diephuis JC, Ennema JJ, Brandon Bravo Bruinsma GJ, et al. Thoracic epidural anesthesia for cardiac surgery: a randomized trial. Anesthesiology 2011;114(2):262‐70. [DOI: 10.1097/ALN.0b013e318201d2de; PUBMED: 21239976 ] [DOI] [PubMed] [Google Scholar]
Tenenbein 2008 {published and unpublished data}
- Kowalski S. Information on our trial. Email to: J Guay, 27 March 2018.
- Tenenbein PK, Debrouwere R, Maguire D, Duke PC, Muirhead B, Enns J, et al. Thoracic epidural analgesia improves pulmonary function in patients undergoing cardiac surgery. Canadian Journal of Anaesthesia 2008;55(6):344‐50. [DOI: 10.1007/BF03021489; PUBMED: 18566197] [DOI] [PubMed] [Google Scholar]
Tenling 1999 {published data only (unpublished sought but not used)}
- Tenling A, Joachimsson P‐O, Tyden H, Hedenstierna G. Thoracic epidural analgesia as an adjunct to general anaesthesia for cardiac surgery. Effects on pulmonary mechanics. Acta Anaesthesiologica Scandinavica 2000;44(9):1071‐6. [PUBMED: 11028725] [DOI] [PubMed] [Google Scholar]
- Tenling A, Joachimsson PO, Tydén H, Wegenius G, Hedenstierna G. Thoracic epidural anesthesia as an adjunct to general anesthesia for cardiac surgery: effects on ventilation‐perfusion relationships. Journal of Cardiothoracic and Vascular Anesthesia 1999;13(3):258‐64. [PUBMED: 10392674] [DOI] [PubMed] [Google Scholar]
Usui 1990 {published data only (unpublished sought but not used)}
- Usui K, Yasuda A, Tadokoro M, Kon‐nai T, Shoji Y, Yoshida S. Epidural morphine as a sedative after open heart surgery: in cases of coronary artery bypass graft. Kyobu Geka ‐ Japanese Journal of Thoracic Surgery 1990;43(1):19‐23. [PUBMED: 2304293] [PubMed] [Google Scholar]
Volk 2003 {published data only}
- Volk T, Dopfmer UR, Schmutzler M, Rimpau S, Schnitzler H, Konertz W, et al. Stress induced IL‐10 does not seem to be essential for early monocyte deactivation following cardiac surgery. Cytokine 2003;24(6):237‐43. [DOI: 10.1016/S1043-4666(03)00090-5; PUBMED: 14609565] [DOI] [PubMed] [Google Scholar]
Yang 1996 {published data only (unpublished sought but not used)}
- Yang C, Deng S. Effects of epidural block combined with general anesthesia on stress response during cardiopulmonary bypass. Chinese Journal of Anesthesiology 1996;16:492. [Google Scholar]
Yilmaz 2007 {published data only (unpublished sought but not used)}
- Yilmaz C, Çoruh T, Yapıcı N, Yılmaz Ö, Maçika H, Aykaç Z. The effects of preemptive analgesia with thoracic epidural on respiratory function tests, pain scores and hemodynamics in coronary artery bypass graft surgery. Anestezi Dergisi 2007;15:20‐7. [Google Scholar]
Yung 1997 {published data only (unpublished sought but not used)}
- Yung MC, Chang Y, Lai ST, Tsou MY, Chan KH. Improved postoperative pain relief via preemptive analgesia in relation to heart rate variability for coronary artery bypass grafting: a preliminary report. Zhonghua Yi Xue Za Zhi 1997;60(1):28‐35. [PUBMED: 9316325] [PubMed] [Google Scholar]
Zawar 2015 {published data only (unpublished sought but not used)}
- Zawar BP, Mehta Y, Juneja R, Arora D, Raizada A, Trehan N. Nonanalgesic benefits of combined thoracic epidural analgesia with general anesthesia in high risk elderly off pump coronary artery bypass patients. Annals of Cardiac Anaesthesia 2015;18(3):385‐91. [DOI: 10.4103/0971-9784.159810; PUBMED: 26139745] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2010 {published data only (unpublished sought but not used)}
- Zhou X, Hong C. Clinical study of postoperative thoracic epidural block in cardiac thoracic surgery [胸部硬膜外阻滞在心脏开胸手术术后镇痛的临床研究.]. Sichan Medical Journal 2010;31(3):344‐6. [10.16252/j .cnki .issn1004‐0501‐2010.03.021] [Google Scholar]
References to studies excluded from this review
Amat‐Santos 2012 {published data only}
- Amat‐Santos IJ, Dumont E, Villeneuve J, Doyle D, Rheault M, Lavigne D, et al. Effect of thoracic epidural analgesia on clinical outcomes following transapical transcatheter aortic valve implantation. Heart (British Cardiac Society) 2012;98(21):1583‐90. [DOI: 10.1136/heartjnl-2012-302185; PUBMED: 22791654] [DOI] [PubMed] [Google Scholar]
Anderson 2005 {published data only}
- Anderson RE, Ehrenberg J, Barr G, Brismar K, Owall A, Alserius T, et al. Effects of thoracic epidural analgesia on glucose homeostasis after cardiac surgery in patients with and without diabetes mellitus. European Journal of Anaesthesiology 2005;22(7):524‐9. [PUBMED: 16045142] [DOI] [PubMed] [Google Scholar]
Casalino 2006 {published data only}
- Casalino S, Mangia F, Stelian E, Novelli E, Diena M, Tesler UF. High thoracic epidural anesthesia in cardiac surgery: risk factors for arterial hypotension. Texas Heart Institute Journal 2006;33(2):148‐53. [PUBMED: 16878616] [PMC free article] [PubMed] [Google Scholar]
Chae 1998 {published data only}
- Chae BK, Lee HW, Sun K, Choi YH, Kim HM. The effect of combined epidural and light general anesthesia on stress hormones in open heart surgery patients. Surgery Today 1998;28(7):727‐31. [PUBMED: 9697266] [DOI] [PubMed] [Google Scholar]
Chakravarthy 2005 {published data only}
- Chakravarthy M, Thimmangowda P, Krishnamurthy J, Nadiminti S, Jawali V. Thoracic epidural anesthesia in cardiac surgical patients: a prospective audit of 2113 cases. Journal of Cardiothoracic and Vascular Anesthesia 2005;19(1):44‐8. [PUBMED: 15747268] [DOI] [PubMed] [Google Scholar]
Crescenzi 2009 {published data only}
- Crescenzi G, Landoni G, Monaco F, Bignami E, Luca M, Frau G, et al. Epidural anesthesia in elderly patients undergoing coronary artery bypass graft surgery. Journal of Cardiothoracic and Vascular Anesthesia 2009;23(6):807‐12. [PUBMED: 19376734] [DOI] [PubMed] [Google Scholar]
Djaiani 2000 {published data only}
- Djaiani G. Thoracic epidural anesthesia as an adjunct to general anesthesia for cardiac surgery: effects on ventilation‐perfusion relationships. Journal of Cardiothoracic and Vascular Anesthesia 2000;14(3):359‐60. [DOI: 10.1053/cr.2000.5816; PUBMED: 10890498] [DOI] [PubMed] [Google Scholar]
El‐Morsy 2012a {published data only}
- El‐Morsy GZ, El‐Deeb A, El‐Desouky T, Elsharkawy AA, Elgamal MA. Can thoracic paravertebral block replace thoracic epidural block in pediatric cardiac surgery? A randomized blinded study. Annals of Cardiac Anaesthesia 2012;15(4):259‐63. [DOI: 10.4103/0971-9784.101848; PUBMED: 23041682] [DOI] [PubMed] [Google Scholar]
Jideus 2001 {published data only}
- Jideus L, Joachimsson PE, Stridsberg M, Ericson M, Tyden H, Nilsson L, et al. Thoracic epidural anesthesia does not influence the occurrence of postoperative sustained atrial fibrillation. Annals of Thoracic Surgery 2001;72(1):65‐71. [PUBMED: 11465233] [DOI] [PubMed] [Google Scholar]
Joachimsson 1989 {published data only}
- Joachimsson PO, Nystrom SO, Tyden H. Early extubation after coronary artery surgery in efficiently rewarmed patients: a postoperative comparison of opioid anesthesia versus inhalational anesthesia and thoracic epidural analgesia. Journal of Cardiothoracic Anesthesia 1989;3(4):444‐54. [PUBMED: 2520917] [DOI] [PubMed] [Google Scholar]
Kaunienė 2016 {published data only (unpublished sought but not used)}
- Kaunienė A, Urbonaitė A, Lenkutis T, Širvinskas E, Švagždienė M. Effects of combined anesthesia on kidney function after coronary artery bypass graft surgery with cardiopulmonary bypass [Kombinuotos anestezijos poveikis inkstu funkcijaipo vainkinu arteriju suntavimo, naudojant dirtinekraujo apytaka]. Biomedicine 2016;26(4):31‐4. [DOI: 10.5200/sm-hs.2016.054] [DOI] [Google Scholar]
Kessler 2002 {published data only}
- Kessler P, Neidhart G, Lischke V, Bremerich DH, Aybek T, Dogan S, et al. Coronary bypass operation with complete median sternotomy in awake patients with high thoracic peridural anesthesia [Koronare bypassoperation mit kompletter medianer sternotomie am wachen patienten in hoher thorakaler periduralanasthesie]. Der Anaesthesist 2002;51(7):533‐8. [PUBMED: 12243038] [DOI] [PubMed] [Google Scholar]
Kessler 2005 {published data only}
- Kessler P, Aybek T, Neidhart G, Dogan S, Lischke V, Bremerich DH, et al. Comparison of three anesthetic techniques for off‐pump coronary artery bypass grafting: general anesthesia, combined general and high thoracic epidural anesthesia, or high thoracic epidural anesthesia alone. Journal of Cardiothoracic and Vascular Anesthesia 2005;19(1):32‐9. [PUBMED: 15747266] [DOI] [PubMed] [Google Scholar]
Kunstyr 2008 {published data only}
- Kunstyr J, Klein A, Lindner J, Rubes D, Blaha J, Jansa P, et al. Use of high‐thoracic epidural analgesia in pulmonary endarterectomy: a randomized feasibility study. Heart Surgery Forum 2008;11(4):E202‐8. [DOI: 10.1532/HSF98.2008i036; PUBMED: 18782697] [DOI] [PubMed] [Google Scholar]
Kurtoglu 2009 {published data only}
- Kurtoglu M, Ates S, Bakkaloglu B, Besbas S, Duvan I, Akdas H, et al. Epidural anesthesia versus general anesthesia in patients undergoing minimally invasive direct coronary artery bypass surgery. Anadolu Kardiyoloji Dergisi 2009;9(1):54‐8. [PUBMED: 19196575] [PubMed] [Google Scholar]
Lagunilla 2006 {published data only (unpublished sought but not used)}
- Lagunilla J, Garcia‐Bengochea JB, Fernandez AL, Alvarez J, Rubio J, Rodriguez J, et al. High thoracic epidural blockade increases myocardial oxygen availability in coronary surgery patients. Acta Anaesthesiologica Scandinavica 2006;50(7):780‐6. [PUBMED: 16879458] [DOI] [PubMed] [Google Scholar]
Liang 2012 {published data only}
- Liang Y, Chu H, Zhen H, Wang S, Gu M. A prospective randomized study of intraoperative thoracic epidural analgesia in off‐pump coronary artery bypass surgery. Journal of Anesthesia 2012;26(3):393‐9. [DOI: 10.1007/s00540-012-1325-6; PUBMED: 22274169] [DOI] [PubMed] [Google Scholar]
Liem 1998 {published data only}
- Liem TH, Williams JP, Hensens AG, Singh SK. Minimally invasive direct coronary artery bypass procedure using a high thoracic epidural plus general anesthetic technique. Journal of Cardiothoracic and Vascular Anesthesia 1998;12(6):668‐72. [PUBMED: 9854665] [DOI] [PubMed] [Google Scholar]
Martinez 2012 {published data only}
- Martinez A, Oslaida M, Jimenez Paneque R, Cordero Escobar I, Cruz Bouza R, Cabrera Pratts A. Neuroaxial anesthesia methods combined with general anesthesia for beating heart surgery. British Journal of Anaesthesia. 2012; Vol. 108 (S2):ii215‐ii277. [DOI: 10.1093/bja/aer485] [DOI]
Novikov 2011 {published data only}
- Novikov OV, Guleshov VA, Morozov UA, Komarov RN, Iavorovskij AG. Metabolic disorders as a result of intestine ischemia and its defensive factors due to aorta prosthetics and cross‐clamping of descending aorta before celiac trunk [Метаболические нарушения как результат повреждения кишечника и фаторы его защиты при операциях протезироьания аорты с уровнем пережатия выше чревного ствола]. Anesteziologiia i Reanimatologiia 2011;56(6):4‐7. [PUBMED: 22379904]22379904 [Google Scholar]
Olivier 2005 {published data only}
- Olivier JF, Le N, Choinière JL, Prieto I, Basile F, Hemmerling T. Comparison of three different epidural solutions in off‐pump cardiac surgery: pilot study. British Journal of Anaesthesia 2005;95(5):685‐91. [PUBMED: 16183682] [DOI] [PubMed] [Google Scholar]
Orsolya 2015 {published data only}
- Orsolya M, Attila‐Zoltan M, Gherman V, Zaharie F, Bolboaca S, Chira C, et al. The effect of anaesthetic management on neutrophil gelatinase associated lipocalin (NGAL) levels after robotic surgical oncology. Journal of B.U.ON. 2015;20(1):317‐24. [PUBMED: 25778333] [PubMed] [Google Scholar]
Ortega 2011 {published data only}
- Ortega RH, Alfonso OG, Menéndez PA, Font IM, Rodríguez JM, Martínez JM, et al. Postoperative analgesic efficacy of epidural morphine in myocardial bypass surgery [Efficacia analgesica postoperatoria de la morfina epidural en la cirurgia de revascularizacion miocardica]. Corsalud 2011;3(1):15‐25. [Google Scholar]
Ovezov 2011 {published data only}
- Obezov АМ, Кim SU, Danilin АМ, Prokoshev РV, Charenco DV. Combined inhalation and epidural anaesthesia, during aortocoronary bypass surgery on beating heart [Сочетанная ингаляционно‐эпидуральная анстезия при прямой реваскляризции миокарда на работающем сердце]. Anesteziologiia i Reanimatologiia 2011;54(6):8‐12. [PUBMED: 22379905] [PubMed] [Google Scholar]
Rao 2016 {published data only}
- Rao TR, Dal A, Dronumraju A, Kumar KK, J Ibrahim J. Coronary artery bypass surgery in "awake" patient: a prospective study. International Journal of Scientific Study 2016;3(10):18‐22. [DOI: 10.17354/ijss/2016/04] [DOI] [Google Scholar]
Salman 2012 {published data only}
- Salman N, Serter FT, Uçar HI, Günaydin S, Yorgancioğlu AC. Comparison between intravenous and thoracic epidural analgesia in single coronary artery bypass graft surgery. Turkish Journal of Medical Sciences 2012;42(4):581‐9. [DOI: 10.3906/sag-1107-9] [DOI] [Google Scholar]
Salvi 2004 {published data only}
- Salvi L, Sisillo E, Brambillasca C, Juliano G, Salis S, Marino MR. High thoracic epidural anesthesia for off‐pump coronary artery bypass surgery. Journal of Cardiothoracic and Vascular Anesthesia 2004;18(3):256‐62. [PUBMED: 15232802] [DOI] [PubMed] [Google Scholar]
Schmidt 2005 {published data only}
- Schmidt C, Hinder F, Aken H, Theilmeier G, Bruch C, Wirtz SP, et al. The effect of high thoracic epidural anesthesia on systolic and diastolic left ventricular function in patients with coronary artery disease. Anesthesia and Analgesia 2005;100(6):1561‐9. [DOI: 10.1213/01.ANE.0000154963.29271.36; PUBMED: 15920175] [DOI] [PubMed] [Google Scholar]
Stenger 2013 {published data only}
- Stenger M, Fabrin A, Schmidt H, Greisen J, Erik Mortensen P, Jakobsen CJ. High thoracic epidural analgesia as an adjunct to general anesthesia is associated with better outcome in low‐to‐moderate risk cardiac surgery patients. Journal of Cardiothoracic and Vascular Anesthesia 2013;27(6):1301‐9. [DOI: 10.1053/j.jvca.2012.12.001; PUBMED: 23906858] [DOI] [PubMed] [Google Scholar]
Stenseth 1993 {published data only}
- Stenseth R, Berg EM, Bjella L, Christensen O, Levang OW, Gisvold SE. The influence of thoracic epidural analgesia alone and in combination with general anesthesia on cardiovascular function and myocardial metabolism in patients receiving beta‐adrenergic blockers. Anesthesia and Analgesia 1993;77(3):463‐8. [PUBMED: 8103648] [DOI] [PubMed] [Google Scholar]
Thorelius 1996 {published data only}
- Thorelius J, Ekroth R, Hallhagen S, et al. Thoracolumbar epidural blockade as adjunct to high dose fentanyl/midazolam anesthesia in coronary surgery: effects of sternotomy. European Journal of Cardiothoracic Surgery 1996;10:754‐62. [DOI] [PubMed] [Google Scholar]
Thorelius 1997 {published data only}
- Thorelius J. Neuroendocrine blockade in coronary surgery. A clinical study with reference to myocardial metabolism and performance. Scandinavian Cardiovascular Journal 1997;31(3):185. [DOI: 10.3109/14017439709058093; PUBMED: 9264171] [DOI] [PubMed] [Google Scholar]
Toda 2013 {published data only}
- Toda A, Watanabe G, Matsumoto I, Tomita S, Yamaguchi S, Ohtake H. Monitoring brain oxygen saturation during awake off‐pump coronary artery bypass. Asian Cardiovascular and Thoracic Annals 2013;21(1):14‐21. [DOI: 10.1177/0218492312444908; PUBMED: 23430415] [DOI] [PubMed] [Google Scholar]
Turfrey 1997 {published data only}
- Turfrey DJ, Ray DA, Sutcliffe NP, Ramayya P, Kenny GN, Scott NB. Thoracic epidural anaesthesia for coronary artery bypass graft surgery. Effects on postoperative complications. Anaesthesia 1997;52(11):1090‐5. [PUBMED: 9404174] [DOI] [PubMed] [Google Scholar]
Yashiki 2005 {published data only}
- Yashiki N, Watanabe G, Tomita S, Nishida S, Yasuda T, Arai S. Thoracic epidural anesthesia for coronary bypass surgery affects autonomic neural function and arrhythmias. Innovations (Philadelphia, Pa.) 2005;1(2):83‐7. [DOI: 10.1097/01.gim.0000198517.54063.74; PUBMED: 22436549] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CTRI/2012/04/002608 {published data only}
- Prakash B. Non‐analgesic benefits of combined thoracic epidural analgesia in elderly off‐pump coronary artery bypass grafting patients. World Health Organization Clinical Trials Registry Platform. date first received 27 April 2012. [CTRI/2012/04/002608]
CTRI/2018/05/013902 {published data only}
- Sen C. A study of central and mixed venous oxygen saturation with outcomes in open heart surgery patients between two groups: conventional general anaesthesia and combined with perioperative thoracic epidural or intravenous analgesia. World Health Organization Clinical Trials Registry Platform. date first received 15 May 2018. [CTRI/2018/05/013902]
NCT03719248 {published data only}
- Elgebaly AS. Thoracic epidural reduces risks of increased left ventricular mass index during coronary artery bypass graft surgery. World Health Organization Clinical Trials Registry Platform. Accessed 25 October 2018. [NCT03719248]
Additional references
Barbosa 2016
- Barbosa FT, Cunha RM, Silva Ramos FW, Lima FJ, Rodrigues AK, do Nascimento Galvao AM, et al. Effectiveness of combined regional‐general anesthesia for reducing mortality in coronary artery bypass: meta‐analysis. Brazilian Journal of Anesthesiology 2016;66(2):183‐93. [PUBMED: 26952228] [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Publication bias. In: Borenstein M, Hedges LV, Higgins JPT, Rothstein HR editor(s). Introduction to Meta‐Analysis. 1st Edition. Chichester, UK: Wiley, 2009:277‐92. [Google Scholar]
Bos 2018
- Bos EM, Haumann J, Quelerij M, Vandertop WP, Kalkman CJ, Hollmann MW, et al. Haematoma and abscess after neuraxial anaesthesia: a review of 647 cases. British Journal of Anaesthesia 2018;120(4):693‐704. [PUBMED: 29576110] [DOI] [PubMed] [Google Scholar]
Brant 2017
- Brant R. Sample size calculator. www.stat.ubc.ca/˜rollin/stats/ssize/index.html. Accessed March 2018.
Cates 2016
- Cates C. Visual Rx Version 4. www.nntonline.net/visualrx. Accessed March 2018.
Chaney 1997
- Chaney MA. Intrathecal and epidural anesthesia and analgesia for cardiac surgery. Anesthesia and Analgesia 1997;84(6):1211‐21. [PUBMED: 9174295] [DOI] [PubMed] [Google Scholar]
Choi 2009
- Choi PT, Beattie WS, Bryson GL, Paul JE, Yang H. Effects of neuraxial blockade may be difficult to study using large randomized controlled trials: the PeriOperative Epidural Trial (POET) Pilot Study. Plos One 2009;4(2):e4644. [PUBMED: 19247497] [DOI] [PMC free article] [PubMed] [Google Scholar]
Comprehensive Meta‐Analysis 2007 [Computer program]
- Meta‐Analysis.com. Comprehensive Meta‐Analysis. Version 2.2.044 for Windows.. 2007.
De Oliveira 2012
- Oliveira GS Jr, Knautz JS, Sherwani S, McCarthy RJ. Systemic magnesium to reduce postoperative arrhythmias after coronary artery bypass graft surgery: a meta‐analysis of randomized controlled trials. Journal of Cardiothoracic and Vascular Anesthesia 2012;26(4):643‐50. [PUBMED: 22560953] [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575‐600. [PUBMED: 12111921] [DOI] [PubMed] [Google Scholar]
Duval 2000a
- Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):455‐63. [PUBMED: 10877304] [DOI] [PubMed] [Google Scholar]
Duval 2000b
- Duval S, Tweedie R. A non parametric ’trim and fill’ method accounting for publication bias in meta‐analysis. Journal of the American Statistical Association 2000;95:89‐98. [Google Scholar]
El‐Baz 1984
- El‐Baz NM, Faber LP, Jensik RJ. Continuous epidural infusion of morphine for treatment of pain after thoracic surgery: a new technique. Anesthesia and Analgesia 1984;63(8):757‐64. [PUBMED: 6465562] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guay 2006
- Guay J. The benefits of adding epidural analgesia to general anesthesia: a metaanalysis. Journal of Anesthesia 2006;20(4):335‐40. [PUBMED: 17072704] [DOI] [PubMed] [Google Scholar]
Guay 2014
- Guay J, Choi P, Suresh S, Albert N, Kopp S, Pace NL. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010108.pub2; PUBMED: 24464831] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guay 2016a
- Guay J, Nishimori M, Kopp S. Epidural local anaesthetics versus opioid‐based analgesic regimens for postoperative gastrointestinal paralysis, vomiting and pain after abdominal surgery. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD001893.pub2; PUBMED: 27419911] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guay 2016b
- Guay J, Kopp S. Epidural pain relief versus systemic opioid‐based pain relief for abdominal aortic surgery. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD005059.pub4; PUBMED: 26731032] [DOI] [PMC free article] [PubMed] [Google Scholar]
Healthcare Improvement Scotland Committee 2018
- Healthcare Improvement Scotland Committee. Cardiac arrhythmias in coronary heart disease: a national guideline. Available at https://www.sign.ac.uk/assets/sign152.pdf. 2018 September: 1‐69.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical research ed.) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glaziou P, et al. Chapter 12. Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horlocker 2018
- Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence‐Based Guidelines (Fourth Edition). Regional Anesthesia and Pain Medicine 2018;43(3):263‐309. [PUBMED: 29561531] [DOI] [PubMed] [Google Scholar]
Kowalewski 2016
- Kowalewski M, Pawliszak W, Malvindi PG, Bokszanski MP, Perlinski D, Raffa GM, et al. Off‐pump coronary artery bypass grafting improves short‐term outcomes in high‐risk patients compared with on‐pump coronary artery bypass grafting: meta‐analysis. Journal of Thoracic and Cardiovascular Surgery 2016;151(1):60‐77.e1‐58. [PUBMED: 26433633] [DOI] [PubMed] [Google Scholar]
Landoni 2015
- Landoni G, Isella F, Greco M, Zangrillo A, Royse CF. Benefits and risks of epidural analgesia in cardiac surgery. British Journal of Anaesthesia 2015;115(1):25‐32. [PUBMED: 26089444] [DOI] [PubMed] [Google Scholar]
McColl 1998
- McColl A, Smith H, White P, Field J. General practitioner's perceptions of the route to evidence based medicine: a questionnaire survey. BMJ 1998;316(7128):361‐5. [PUBMED: 9487174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moen 2004
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990‐1999. Anesthesiology 2004;101(4):950‐9. [PUBMED: 15448529] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ (Clinical research ed.) 2009;339:b2535. [PUBMED: 19622551] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pace 2011
- Pace NL. Research methods for meta‐analyses. Best Practice and Research. Clinical Anaesthesiology 2011;25(4):523‐33. [PUBMED: 22099918] [DOI] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet (London, England) 1998;351(9095):47‐52. [PUBMED: 9433436] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salicath 2018
- Salicath JH, Yeoh EC, Bennett MH. Epidural analgesia versus patient‐controlled intravenous analgesia for pain following intra‐abdominal surgery in adults. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD010434.pub2; PUBMED: 30161292] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group 2013. https://gdt.gradepro.org/app/handbook/handbook.html#h.svwngs6pm0f2, 2013. [Google Scholar]
Thomas 1999
- Thomas PW, Sanders DJ, Berrisford RG. Pulmonary haemorrhage after percutaneous paravertebral block. British Journal of Anaesthesia 1999;83(4):668‐9. [PUBMED: 10673891] [DOI] [PubMed] [Google Scholar]
Tsui 2018
- Tsui BCH, Navaratnam M, Boltz G, Maeda K, Caruso TJ. Bilateral automatized intermittent bolus erector spinae plane analgesic blocks for sternotomy in a cardiac patient who underwent cardiopulmonary bypass: a new era of cardiac regional anesthesia. Journal of Clinical Anesthesia 2018; Vol. 48:9‐10. [PUBMED: 29684728] [DOI] [PubMed]
Ueshima 2018
Zhang 2015
- Zhang S, Wu X, Guo H, Ma L. Thoracic epidural anesthesia improves outcomes in patients undergoing cardiac surgery: meta‐analysis of randomized controlled trials. European Journal of Medical Research 2015;20:25. [PUBMED: 25888937] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Svircevic 2007
- Svircevic V, Passier M, Nierich AP, Kalkman CJ, Dijk D, Heijden G, et al. Epidural analgesia for cardiac surgery. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006715] [DOI] [PubMed] [Google Scholar]
Svircevic 2013
- Svircevic V, Passier MM, Nierich AP, Dijk D, Kalkman CJ, Heijden GJ. Epidural analgesia for cardiac surgery. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD006715.pub2] [DOI] [PubMed] [Google Scholar]


