Abstract
Background
People who suffer from paralysis have difficulties participating in society. Particularly burdensome is the locked-in syndrome (LIS). LIS-patients are not able to move and speak but are cognitively healthy. They rely on assistive technology (AT) to interact with the world and may benefit from neurotechnological advances. Optimal research and design of such aids requires a well-defined target population. However, the LIS population is poorly characterized and the number of patients in this condition is unknown.
Objective
Here we estimated and described the LIS-patient population in the Netherlands to define the target population for assistive (neuro)technology.
Methods
We asked physicians in the Netherlands if they had patients suffering from severe paralysis and communication problems in their files. Physicians responding affirmatively were asked to fill out a questionnaire on the patients’ status.
Results
We sent out 9570 letters to general practitioners (GPs), who reported 83 patients. After first screening, the GPs of 46 patients received the questionnaire. Based on the responses, 26 patients were classified as having LIS. Extrapolation of these numbers resulted in a prevalence of 0.73 patients per 100 000 inhabitants. Notable results from the questionnaire were the percentage of patients with neuromuscular disease (>50%) and living at home (>70%).
Conclusions
We revealed an etiologically diverse group of LIS-patients. The functioning and needs of these patients were, however, similar and many relied on AT. By characterizing the LIS population, our study may contribute to optimal development of assistive (neuro)technology.
Keywords: Locked-in-syndrome, brain-stem stroke, neuromuscular disease, assistive technology, prevalence, Brain-computer interface
Introduction
Disabilities and the Locked-in Syndrome
According to the World Health Organization (WHO),1 one billion people, about 15% of the world’s population, have some form of disability. The term disability is used by the International Classification of Functioning, Disability and Health as an umbrella for all impairments, activity limitations and participation restrictions. Ranging from hearing impairments to paraplegia, disabilities can be diverse, but they all influence the personal and professional life of the affected individual. The number of people with a disability is rising because of global aging, increasing number of chronic health conditions and other reasons.1 As a result, an increasing number of people have difficulties participating in society and gaining access to employment, and global healthcare expenses are rising.
Particularly burdensome types of disabilities are forms of paralysis, with people suffering from locked-in syndrome (LIS) being the most severe cases. First described by Alexandre Dumas as "a corpse with living eyes" in “The Count of Monte-Cristo” (1844), LIS was only introduced as a medical term by Plum and Posner in 1966.2 According to the American Congress of Rehabilitation Medicine (ACRM), LIS is now defined by the following five criteria: 1) Sustained eye opening and preserved vertical eye movement, 2) Preserved higher cortical functions, 3) Aphonia or severe hypophonia, 4) Quadriplegia or quadriparesis, and 5) Primary mode of communication that uses vertical eye movements or blinking.3 Patients that fit all five criteria are considered classic LIS. Besides classic LIS, a distinction is made between incomplete LIS, which is the same as classic LIS, but with additional voluntary movement other than vertical eye movements, and total or complete LIS, which is defined by total immobility with no communication possible in the presence of sustained consciousness.4 Although the ACRM-definition leaves room for multiple causes (including trauma5 and neuromuscular diseases (NMDs), such as ALS6), stroke is considered the most frequent cause of LIS.2,7 For many care-related decisions, however, the level of functioning of the patient is more relevant than its cause. Therefore, we use the ACRM-definition and include patients based purely on their level of functioning (fLIS), independent of etiology.
Assistive technology
Assistive technology (AT), such as glasses, wheelchairs and advanced communication aids, may help people with disabilities to regain more independence and partake in everyday life. The more severe a disability, the more the need for AT. In the case of paralysis, AT is very important and may even be life sustaining. Many severely paralyzed patients rely for example on feeding tubes, communication aids, wheelchairs and mechanical ventilation.8
New developments within the field of neurotechnology are relevant for patients with disabilities, including those suffering from paralysis. Neurotechnology encompasses all technologies that record from, act upon or interact with the nervous system. A relatively new area in neurotechnology is that of Brain-Computer Interfaces (BCIs). A BCI is a device that facilitates communication between the brain and a machine using predominantly neuroelectrical signals. Recorded signals are translated into commands to control devices and can be used for one of several types of applications, such as to research, replace, assist, repair or augment human cognitive or sensorimotor functions.9,10 Several techniques can be used to acquire brain signals used for BCIs. The most widely used method is EEG. This noninvasive method uses electrodes on the scalp to record voltage fluctuations caused by synchronous activity of millions of neurons.11 Using P300, an event related potential associated with an infrequent stimulus, brain signals are used to detect which character in a matrix a user wants to select. Despite being the most successful EEG-based BCI technique12–14, the clinical application is only slowly realized and for LIS-patients, EEG-BCI performance is lower than for less severely paralyzed people.15 A more recent development is that of functional Near-Infrared Spectroscopy (fNIRS), which measures the hemodynamic responses associated with neural activity.16,17 Although promising, this technique has not found its way to home application yet. Implantable BCIs, utilizing the brain signal recorded with electrodes on or in the brain, have the potential to become a useful solution to the daily obstacles of people suffering from LIS, due to their high signal quality and potential 24/7 availability. The possibilities of implantable BCIs have predominantly been investigated and demonstrated in a laboratory setting.18–21 Recently, the first successful home-use of an implantable BCI by an ALS-patient was described by our group22 bridging the gap between research and the application of BCIs at home.
The translation from knowledge gained in research to implementation in AT for LIS-patients is difficult. The potential user-group seems very diverse, and the number of patients in this condition is still unknown.7,23–25 Only a limited number of studies has attempted to characterize the LIS population, often concentrating on quality-of-life.8,26,27 In the Netherlands, a patient group description was presented in a prevalence study by Kohnen and colleagues in 2013.28 Focusing on the number of LIS-patients living in nursing homes, they found two patients with classic LIS. During recruitment efforts for our aforementioned implantable BCI-study22 we learned, however, that a considerable number of people with LIS live at home with their family, instead of in a nursing home. The current study aims to describe the size and characteristics of the total LIS population in the Netherlands in order to obtain a more complete picture of the potential user-group of neurotechnology and BCIs in particular.
Method
Prevalence calculation and extrapolation
Healthcare in the Netherlands is well-organized and all inhabitants of the Netherlands are obliged to have a general practitioner (GP). Through a dedicated website on quality ratings of medical care in the Netherlands (www.zorgkaartnederland.nl; Patient federation NPCF) the names and contact details of 8783 individual GPs were found. This number is in accordance with the registered number of GPs (8804, except locum GPs).29 We contacted all GPs, thereby covering the entire population (16 900 726) of the Netherlands.30
At the beginning of June 2014 a letter was sent to 8783 GPs, with the question whether they have had or currently had any people with severe paralysis and communication problems on file. A year later, a second letter was sent to all GPs who did not respond affirmatively in the first round (including non-responders and GPs without eligible patients) and to new GPs in the updated GP-database (8843 in total). GPs responding affirmatively to either one of the letters were subsequently called, within a 3 month period (May – July 2015) in order to verify their answer and to inquire about the patient. When patients fit the main criteria for this study (motor paralysis, no speech) their GP was sent an elaborate questionnaire (supplementary materials) on the patients’ status, living environment, needs and disease history. Patients for whom we received answers on the questionnaire were rated as either classic LIS, incomplete LIS, total LIS or non-LIS according to the ACRM 1995 criteria by two independent raters (EGMP, EJA). Discrepancies between ratings were discussed until consensus. Notably, no name or other identifiable information was requested, to guarantee anonymity of the patients.
Since the GPs covered the entire Dutch population, population estimates can be made by extrapolating the results of the GP sample to the population level.31 Beneficial to this extrapolation is the comparable size of GP practices (±2000 patients) and the role GPs play in the Dutch healthcare system. In the Netherlands, GPs provide primary care and their consultation and referral are mandatory for further treatment. In return, the GP is informed on the treatment and status of the patient by the specialist. The extrapolation to population level was done by dividing the number of found patients by the GP response rate (i.e. the number of responding GPs divided by the total number of GPs that received a letter). This resulted in an estimated total number of LIS-patients. Dividing the total number of patients by the whole population and multiplying it by 100 000 resulted in the prevalence number (number of patients per 100 000 inhabitants).
Patient population description
In addition to the GPs, we sent letters to all rehabilitation and elderly-care physicians in the Netherlands, to increase the likelihood to find LIS-patients. Furthermore, many people made themselves known to us because of our media appearances and recruitment efforts for a BCI-study. All these patients and physicians were contacted and asked to fill out the same, aforementioned, questionnaire for verification and description of the population. The patients found in this way were not added to the prevalence calculation, but their questionnaire data were used complementary to the ‘GP-patients’ to describe the patient population. Fisher’s exact test was used to investigate a relationship between the primary communication method and remaining functions of the patient.
Biases and ethics
For both the prevalence calculation and the description of the patient population, we checked for instances where the same patient may have been reported twice, by indexing the patients on date-of-birth and physician-location (patient names were unknown), and discarded or combined records accordingly. Furthermore, we checked the prevalence calculation sample for a sample bias induced by regional population differences within the Netherlands and the location of the GPs, by calculating the percentage of GPs that replied per Dutch province.
The medical ethical committee of the University Medical Center Utrecht assessed the present study, and reported that no ethical approval was required since the study did not classify as medical research according to the Dutch Medical Scientific Research Act (WMO, article 1b).
Results
Locked-in syndrome patients in the Netherlands
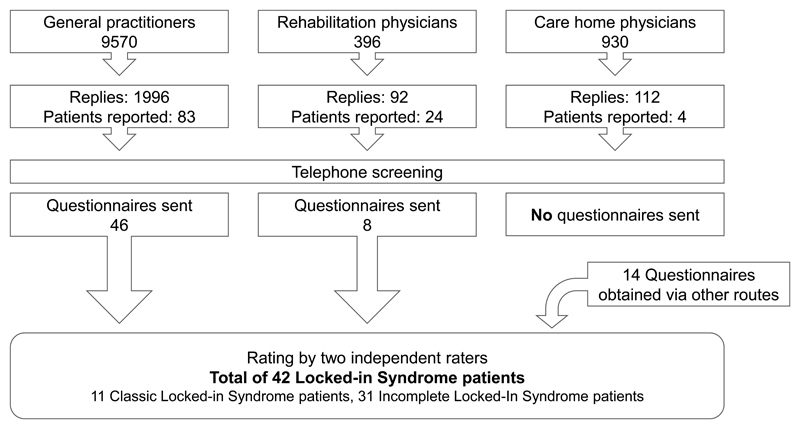
In two rounds, we sent letters to 9570 individual GPs. Of those, 65 were not deliverable due to for instance address change. A total of 1996 GPs replied, a response rate of 21%, of whom 83 affirmatively. Those GPs were contacted by telephone for primary screening to determine the nature of the patient’s disorder. After screening, 46 GPs received a questionnaire (by mail or administered by phone) of which 36 were completed and 10 were not returned (due to time constraints of the GP or for other reasons). Two patients were reported by both their current and former GP. Of these, the questionnaire of their previous GP was discarded. The remaining 34 cases were rated by two independent raters and resulted in classification of 18 incomplete, 8 classic and no complete LIS-patients. The other 8 patients did not qualify for a diagnosis of LIS. By projecting this number of 26 patients to the entire Dutch population, using the ratio of responding versus total number of GPs, we obtain an estimated total number of 124 LIS-patients in the Netherlands, and a prevalence of 0.73 LIS-patients per 100 000 inhabitants.
Description of the patient population found
We sent out another 1326 letters to rehabilitation and elderly-care physicians. The response rates were 23% and 12%, respectively, and resulted in another 28 physicians reporting patients. However, most of the patients were either no longer in contact with the reporting physician, or had already passed away. Therefore only 8 questionnaires were filled out. In addition, 14 questionnaires were sent to patients with whom we came in contact via other routes (media attention and patient organizations). Together with the GP and physician efforts, this resulted in a total of 68 sent questionnaires, of which 45 were completed and returned. Of those, 42 confirmed the status of LIS, as verified by the two raters (see figure 1 for a breakdown of the recruitment and patient selection).
Figure 1.
Flowchart of patient recruitment and selection. Note that the total number of General Practitioners addressed is a combination of the GPs extracted from the database when the first letter was sent and the new and changed records of the database at the time point the second letter was sent. In the end, in total, only 42 filled-out questionnaires were received, note that 10 GPs did not return the questionnaire (i.e. because of lack of time).
The most common cause of LIS in our population was NMD (55%), followed by stroke (26%). The mean age at the time the questionnaire was filled out was 53.1 years (sd 12.4). The median age at onset of LIS for stroke and traumatic events was 36 years (range 11-57). Median age at the moment of NMD diagnosis was 47 years (range 0-73). For the latter, onset of LIS could not be determined since the disease gradually progressed into LIS, making a timestamp rather unreliable.
The questionnaire responses confirmed our hypothesis that most LIS-patients live at home (71.4%), and another significant portion (11.9%) living independently with an assistance schedule. The latter typically consisted of fixed care moments across the day, plus response to calls (medical and home care). See table 1 for more details on the patient population. Notably, patients living at home were not necessarily those who needed less complicated care. In fact, a third of the patients living at home (26% of the entire group) was invasively ventilated (via tracheostomy). Many of these people also used percutaneous endoscopic gastrostomy (PEG, 60% of the entire group, 67% of patients living at home) and/or a uretic catheter (55% entire group, 57% patients living at home).
Table 1. Demographics and disease characteristics of the reported patient population.
| n | % | |
|---|---|---|
| Total | 42 | 100.0 |
| Demographics | ||
| Age (years) | ||
| <25 | 1 | 2.4 |
| 25 – 40 | 4 | 9.5 |
| 41 – 65 | 33 | 73.8 |
| >65 | 4 | 14.3 |
| Gender | ||
| Male | 24 | 57.1 |
| Female | 18 | 42.9 |
| Living situation | ||
| At home | 30 | 71.4 |
| Nursing home | 6 | 14.3 |
| Independent with support | 5 | 11.9 |
| Other | 1 | 2.4 |
| Disease characteristics Cause | ||
| Stroke | 11 | 26.2 |
| Cerebral palsy | 2 | 4.8 |
| Neuro muscular disease (NMD) | 23 | 54.8 |
| of which ALS | 14 | 33.3 |
| Trauma | 2 | 4.8 |
| Other | 4 | 9.5 |
| Age (years) at onset | ||
| At birth | 3 | 7.1 |
| 0 - 15 | 2 | 4.8 |
| 16 – 30 | 5 | 11.9 |
| 31 - 45 | 10 | 23.8 |
| 46 – 60 | 20 | 47.6 |
| >60 | 1 | 2.4 |
| Unknown | 1 | 2.4 |
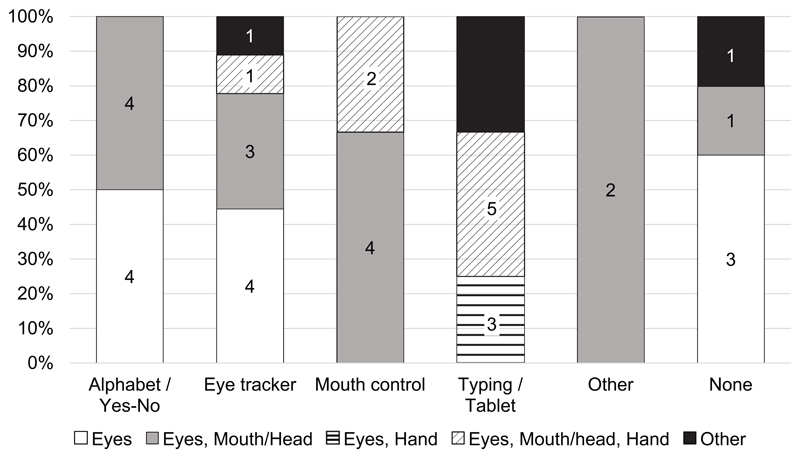
For communication, most of the patients relied on AT, but 12% reported to have no specific AT for communication (which could indicate use of yes/no system) and 19% used the alphabet system, which involves a caretaker articulating or pointing at letters and the patient selecting the letter he wants to use with, for example, an eye blink. In that respect, it is interesting to note that 41 of the 42 patients still had remaining voluntary movements of the eyes, whereas the prime use of an eye-tracker was only reported by 9 patients. The primary method for communication was dependent on remaining voluntary movements (Fisher’s exact test (20, N=42) = 34.35, p < 0.001), in that people with less remaining functioning relied on tailored systems, such as eye trackers for people with only eye movements and tablets or typing devices when people still have hand functioning (Table 2 and Figure 2).
Table 2.
Functioning and assistive technology use of the reported patient population. Note that for remaining voluntary movement multiple options are possible, i.e. eyes and hand. Furthermore, not all questionnaires were filled out completely for PEG 1 and for uretic catheter 5 answers were unknown.
| n | % | |
|---|---|---|
| Total | 42 | 100.0 |
| Functioning & Disabilities | ||
| Remaining voluntary movements (more than one possible) | ||
| Eyes | 41 | 97.6 |
| Mouth/face/head | 25 | 59.5 |
| Hand(s)/fingers(s) | 14 | 33.3 |
| Leg | 4 | 9.5 |
| None | 0 | 0 |
| Percutaneous endoscopic gastrostomy (PEG) | ||
| Yes | 25 | 59.5 |
| No | 16 | 38.1 |
| Uretic catheter | ||
| Yes | 23 | 54.8 |
| No | 14 | 33.3 |
| Mechanic ventilation | ||
| No ventilation | 25 | 59.5 |
| Non-invasive ventilation | 6 | 14.3 |
| Invasive ventilation | 11 | 26.2 |
| Main method for communication | ||
| Letter card / yes-no | 8 | 19.0 |
| Eye tracker | 9 | 21.4 |
| Chin or mouth joystick | 6 | 14.3 |
| Typing (Lightwriter or tablet) | 12 | 28.6 |
| Other | 2 | 4.8 |
| None | 5 | 11.9 |
Figure 2.
Distribution of the primary method for communication and the remaining motor functions of the LIS-patients of this study. The method for communication and the remaining motor functions are related as shown by Fisher’s exact test (see text). Columns are given as percentages, the numbers in the columns represent the number of patients for that specific sub group. The division of the patients over the categories has been made relatively coarsely and the category mouth/head, for example, includes mouth, face and neck movements. The categories "Other" contain responses that did not fit within one of the larger categories specified (i.e. eyes & leg). The total number of patients was 42.
Possible biases and confounds
To evaluate whether there was a regional bias for GPs to respond to the mailed invitations, the response rates were separated per province, and were corrected for the number of inhabitants (Table 3). This analysis showed that the response rates per province were in the same order of magnitude, ranging from 17% to 28%, showing that responses came from all areas and that we found patients in all parts of the Netherlands.
Table 3.
Response of general practitioners, and the distribution of all reported Locked-in patients over the Dutch provinces. Provinces are ordered by their population (highest first).
| Province | #GPs | Response | Response rate | #Patients |
|---|---|---|---|---|
| Zuid-Holland | 1920 | 366 | 19% | 7 |
| Noord-Holland | 1552 | 304 | 20% | 6 |
| Noord-Brabant | 1277 | 301 | 24% | 7 |
| Gelderland | 1140 | 290 | 25% | 7 |
| Utrecht | 738 | 191 | 26% | 3 |
| Overijssel | 578 | 160 | 28% | 3 |
| Limburg | 602 | 114 | 19% | 3 |
| Friesland | 344 | 58 | 17% | 1 |
| Groningen | 298 | 54 | 18% | 0 |
| Drenthe | 284 | 57 | 20% | 1 |
| Flevoland | 221 | 39 | 18% | 2 |
| Zeeland | 202 | 54 | 27% | 2 |
Discussion
Strengths and limitations
Estimates of the prevalence and characteristics of people who qualify for a functional definition of LIS are quite scarce and incomplete. Many live at home, and do not consult medical specialists on a frequent basis and are thus difficult to find through the medical channels. In this study we attempted to obtain an assessment of the prevalence of LIS by querying all GPs. In the Netherlands, all inhabitants need to be registered with a GP before they can access specialist treatment. We obtained an estimate of the number of LIS-patients in the Netherlands, and found a prevalence of 0.73 cases per 100 000 inhabitants. Since we adopted a functional definition of LIS rather than a definition in terms of cause, the present estimate is intended to indicate the size of the Dutch population with severe physical and communication limitations in mentally fully-abled people. This bears relevance for development of new technologies to ameliorate the capabilities of people with LIS. A previous study has estimated the number of LIS-patients in nursing homes in the Netherlands in 2013.28 In that study 2 classic LIS-patients and 7 incomplete LIS-patients were found in total, by contacting all long-term care institutions. Their response rate was quite high (91.4%) suggesting exhaustive numbers. Assuming that the number of LIS-patients in nursing homes was approximately constant during the few years between the studies, the fact that we found 6 LIS-patients living in nursing homes suggests that, in spite of relatively low physician response rates, we succeeded in identifying the majority of that population.
The response rate in GPs was 21%, which was lower than we expected. One can assume that the number of requests for survey participation is high for GPs. The motivation to reply with a “no”-answer might therefore be lower, whereas GPs with a LIS-patient on file may have been more likely to respond, possibly resulting in an overestimation of the number of LIS-patients. It could also be argued that non-responders were those who had no relevant patients, but we discovered that several LIS-patients who were found via other channels were not reported by their GP. Later on, 10 GPs did not return the questionnaire, likely resulting in a loss of patients for prevalence estimation (these GPs answered affirmatively to the first letter) and an underestimation of the LIS prevalence. Notably, the rehabilitation and elderly-care physicians also showed a limited response rate (23% and 12% respectively). A reason for the lower response rate for elderly-care physicians, compared to the GPs and rehabilitation physicians, might be that we addressed the same physicians with a similar question shortly (within 2 years) after the study of Kohnen. Overall, responses and reported patients came from all over the Netherlands, indicating no specific regional bias.
Patient population
The most notable finding resulting from the questionnaires was the high (>50%) proportion of NMD-patients in our LIS population. In literature, stroke is often mentioned as the most common cause of LIS4,7,25,28, although it is recognized that the functional definition of LIS is also met by other conditions, such as ALS26 and trauma25. In our study, we asked physicians to report severely paralyzed people with no speech, regardless of etiology. Putting the question in this manner has led to physicians reporting a wide range of paralyzed patients in terms of cause and levels of paralysis, including several paraplegic patients (with no loss of speech), suggesting that reporting of LIS was not constrained by the text-book association with stroke. Important outcomes of this approach are that we could investigate the most frequent etiologies of LIS, and the conclusion that LIS in the Netherlands is most often the result of NMD.
The detailed questionnaires indicated a high percentage of LIS-patients living at home (71%), and living independently with some professional assistance (12%). This proportion corresponds to the findings of other studies that questioned members of the French locked-in association (ALIS) and found that 61-64% of them live at home.23,32 Both studies did indicate, however, that their study may be subject to selection bias, as only a part of the ALIS-members replied. The patients who replied may have been those that were more independent or had more personal care. It is unlikely that the current study suffers from this type of bias, as we approached GPs and other professionals. Therefore, we conclude that a major proportion of Dutch LIS patients live at home with their family. Clearly, this has consequences for the daily living activities and medical care considerations around these patients.
A significant percentage of the LIS-patients we found were reported to both live at home and receive invasive mechanical ventilation (21% of the LIS-patients for whom we received a filled-out questionnaire) and therefore require intensive 24/7 care, which is generally performed by their family members and one or more professional caretakers. The total number of invasively ventilated patients in the Netherlands is 415 and the number of ventilated patients living at home (both invasive and non-invasive) has doubled in the last 9 years.33 The fact that many Dutch long-term care patients receive a personal health budget from the government, which allows them to choose who their care provider is and how their care is delivered, may be associated with the fact that most LIS-patients stay at home. It may be speculated that the possibility to live at home affects their choices for treatment and life sustaining support such as PEG and invasive ventilation.
The primary communication methods used among our LIS-patients are diverse and correlated with the remaining voluntary movements, indicating that communication aids used by most LIS-patients utilize the specific abilities of an individual (see table 2 for remaining voluntary movement). Strikingly, 19% of the LIS-patients used a lettercard or closed questions as a primary method for communication and another 12% of the patients did not have an AT for communication at all. Whether this means that these patients use multiple methods, none, or only communicate without AT remains unknown. These numbers suggest, however, that at least 19% of the patients are severely limited in their communication, in that they are dependent on another person to ask them the correct questions, and/or that they cannot initiate communication by themselves. Notably, for 98% of our LIS-patients, it was reported that they have voluntary eye movements. This, clearly, largely includes the people that depend on a caretaker for their communication and the use of an eye-tracker as the main method of communication by only 21% of the entire group thus seems remarkably low. Whether this indicates that eye-trackers do not always offer the best solution or that a substantial number of patients do not have access to them, remains to be determined. Neurotechnology, and especially BCIs, have the potential to serve all of these patients, especially those not helped by conventional communication tools, and cover the broad variety of personally tailored devices and technologies34. It should be noted that it has been reported that BCIs may no longer be possible in completely locked-in ALS-patients.35
Implications for neurotechnology
The patients found and characterized in the present study constitute the most severe cases of people in need of AT. This subgroup of patients would therefore likely benefit the most from development of new technologies. As a result of the present study, the Dutch population of LIS-patients is now well-defined in terms of numbers and characteristics. This is important, as the development of new technologies, such as neurotechnology, depends on such a well-defined target market. In turn, optimally designed AT, tailored to the needs of the population, and may result in less abandonment of devices by the user. About thirty percent of AT is abandoned for reasons related to the device, the user and his environment.36,37 The demands people have for AT are high. Not only do they need to be reliable, safe and versatile, users also want AT to be pretty and give a sense of freedom and not create an extra barrier between them and their environment.38 Neurotechnology, and especially implanted neurotechnology devices, meet these requirements as they are, for a large part, invisible.
A consequence of our finding that NMD is the major cause of LIS, is that the total number of LIS-patients might be growing when more NMD-patients receive life-sustaining support6 and progress into LIS. This number may be a function of cultural trends with regard to end-of-life decisions. In The Netherlands, only 1.3% of ALS-patients choose invasive ventilation (personal communication with Home-Ventilation Centers), whereas in Japan, one-third of the ALS-population is invasively ventilated.39,40 Within Europe, North Italy stands out with 10.6% of invasively ventilated ALS-patients.41
Because of the progressive nature of NMD, NMD-patients are subject to continuous change of their body and motor skills, while stroke and trauma related LIS-patients are generally stable and might even rehabilitate.42 It may be speculated that a fear of increasing function loss in NMD-patients leads them to be more open towards new and invasive techniques. Indeed, most patients who were interested in our implantable BCI22 were NMD-patients. Stable LIS-patients with intact eye-movements and/or other intact motor functionality may prefer to maintain status quo, until neurotechnology specifications improve (real-time speech, regaining hand function). For now, BCIs and invasive neurotechnology seem especially appropriate for patients with progressive (NMD) conditions, who face becoming (completely) locked-in. Future studies should further investigate this topic.
Conclusion
Our extensive investigation of prevalence of LIS in the Netherlands revealed a small and diverse group, with an estimated prevalence rate of 0.73. Although the underlying etiology differs between patients, most of the problems and needs of these patients are similar. One of the most urgent needs is a means to communicate when speech has become impossible. A wide variety of tailored systems is used, including basic eye–blink communication and letter cards, leaving room for the development of a well-designed AT that suits the communication needs of many of these patients. This gap may be filled by neurotechnology, and the present study helps the development of better AT for LIS-patients by quantifying and defining the target market. In turn, optimal AT may lead to a higher quality-of-life for patients with LIS by offering them more control and independence.
Acknowledgements
The authors would like to thank all patients and physicians questioned for this study. This research is supported by the European Union (ERC-Adv 320708) and the Dutch Technology Foundation STW, which is part of the Netherlands Organisation for Scientific Research (NWO), and which is partly funded by the Ministry of Economic Affairs (STW 12803).
References
- 1.WHO. Media centre: Disability and health. 2015:4–7. (Fact Sheet No.352) http://www.who.int/mediacentre/factsheets/fs352/en/
- 2.Posner JB, Saper CB, Schiff ND, Plum F. Plum and Posner’s Diagnosis of Stupor and Coma. 4th ed. Oxford University Press; 2007. [Google Scholar]
- 3.American Congress of Rehabilitation Medicine. Recommendations for use of uniform nomenclature pertinent to patients with severe alterations in consciousness. Arch Phys Med Rehabil. 1995;76(2):205–209. doi: 10.1016/S0003-9993(95)80031-X. [DOI] [PubMed] [Google Scholar]
- 4.Smith E, Delargy M. Locked-in syndrome. Br Med J. 2005 Feb;330:3–6. doi: 10.1136/bmj.g7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrai R, Grippo A, Fossi S, et al. Transient post-traumatic locked-in syndrome: A case report and a literature review. Neurophysiol Clin. 2009;39(2):95–100. doi: 10.1016/j.neucli.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi H, Kato S. Total manifestations of amyotrophic lateral sclerosis. ALS in the totally locked-in state. J Neurol Sci. 1989;93(1):19–35. doi: 10.1016/0022-510X(89)90158-5. [DOI] [PubMed] [Google Scholar]
- 7.Bauer G, Gerstenbrand F, Rumpl E. Varieties of the locked-in syndrome. J Neurol. 1979;221(2):77–91. doi: 10.1007/BF00313105. [DOI] [PubMed] [Google Scholar]
- 8.Snoeys L, Vanhoof G, Manders E. Living with locked-in syndrome: an explorative study on health care situation, communication and quality of life. Disabil Rehabil. 2013;35(9):713–718. doi: 10.3109/09638288.2012.705950. [DOI] [PubMed] [Google Scholar]
- 9.Wolpaw JR, Wolpaw EW. Brain-Computer Interfaces: Principles and Practice. Oxford University Press; 2012. http://www.amazon.com/Brain-Computer-Interfaces-Principles-Jonathan-Wolpaw/dp/0195388852. [Google Scholar]
- 10.Brunner C, Birbaumer N, Blankertz B, et al. BNCI Horizon 2020: towards a roadmap for the BCI community. Brain-Computer Interfaces. 2015;2(1):1–10. doi: 10.1080/2326263X.2015.1008956. [DOI] [Google Scholar]
- 11.Jackson AF, Bolger DJ. The neurophysiological bases of EEG and EEG measurement: A review for the rest of us. Psychophysiology. 2014;51(11):1061–1071. doi: 10.1111/psyp.12283. [DOI] [PubMed] [Google Scholar]
- 12.Farwell LA, Donchin E. Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephalogr Clin Neurophysiol. 1988;70(6):510–523. doi: 10.1016/0013-4694(88)90149-6. [DOI] [PubMed] [Google Scholar]
- 13.Sellers EW, Vaughan TM, Wolpaw JR. A brain-computer interface for long-term independent home use. Amyotroph Lateral Scler. 2010;11(5):449–455. doi: 10.3109/17482961003777470. [DOI] [PubMed] [Google Scholar]
- 14.Holz EM, Botrel L, Kaufmann T, Kübler A. Long-term independent brain-computer interface home use improves quality of life of a patient in the locked-in state: A case study. Arch Phys Med Rehabil. 2015;96(3):S16–S26. doi: 10.1016/j.apmr.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Kübler A, Birbaumer N. Brain-computer interfaces and communication in paralysis: Extinction of goal directed thinking in completely paralysed patients? Clin Neurophysiol. 2008;119(11):2658–2666. doi: 10.1016/j.clinph.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhary U, Birbaumer N, Ramos-Murguialday A. Brain–computer interfaces for communication and rehabilitation. Nat Rev Neurol. 2016;12(9):513–525. doi: 10.1038/nrneurol.2016.113. [DOI] [PubMed] [Google Scholar]
- 17.Sitaram R, Zhang H, Guan C, et al. Temporal classification of multichannel near-infrared spectroscopy signals of motor imagery for developing a brain-computer interface. Neuroimage. 2007;34(4):1416–1427. doi: 10.1016/j.neuroimage.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy PR, Kirby MT, Moore MM, King B, Mallory A. Computer control using human intracortical local field potentials. IEEE Trans Neural Syst Rehabil Eng. 2004;12(3):339–344. doi: 10.1109/TNSRE.2004.834629. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg LR, Serruya MD, Friehs GM, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442(7099):164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Collinger JL, Degenhart AD, et al. An Electrocorticographic Brain Interface in an Individual with Tetraplegia. PLoS One. 2013;8(2):1–8. doi: 10.1371/journal.pone.0055344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collinger JL, Wodlinger B, Downey JE, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381(9866):557–564. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vansteensel MJ, Pels EGM, Bleichner MG, et al. Fully Implanted Brain–Computer Interface in a Locked-In Patient with ALS. N Engl J Med. 2016;375(21) doi: 10.1056/NEJMoa1608085. NEJMoa1608085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruno M-A, Bernheim JL, Ledoux D, Pellas F, Demertzi A, Laureys S. A survey on self-assessed well-being in a cohort of chronic locked-in syndrome patients: happy majority, miserable minority. BMJ Open. 2011;1(1):e000039. doi: 10.1136/bmjopen-2010-000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.León-Carrión J, van Eeckhout P, Domínguez-Morales MDR, Pérez-Santamaría FJ. The locked-in syndrome: a syndrome looking for a therapy. Brain Inj. 2002;16(7):571–582. doi: 10.1080/02699050110119781. [DOI] [PubMed] [Google Scholar]
- 25.Patterson JR, Grabois M. Locked-in syndrome: a review of 139 cases. Stroke. 1986;17(4):758–764. doi: 10.1161/01.STR.17.4.758. [DOI] [PubMed] [Google Scholar]
- 26.Laureys S, Pellas F, Van Eeckhout P, et al. The locked-in syndrome: What is it like to be conscious but paralyzed and voiceless? Prog Brain Res. 2005;150(5):495–511. doi: 10.1016/S0079-6123(05)50034-7. [DOI] [PubMed] [Google Scholar]
- 27.Rousseau MC, Pietra S, Blaya J, Catala A. Quality of life of ALS and LIS patients with and without invasive mechanical ventilation. J Neurol. 2011;258(10):1801–1804. doi: 10.1007/s00415-011-6018-9. [DOI] [PubMed] [Google Scholar]
- 28.Kohnen RF, Lavrijsen JCM, Bor JHJ, Koopmans RTCM. The prevalence and characteristics of patients with classic locked-in syndrome in Dutch nursing homes. J Neurol. 2013;260(6):1527–1534. doi: 10.1007/s00415-012-6821-y. [DOI] [PubMed] [Google Scholar]
- 29.NIVEL. Cijfers Uit de Registratie van Huisartsen. 2015. [in Dutch] [Google Scholar]
- 30.Centraal Bureau voor de Statistieken. Bevolking; Kerncijfers. Den Haag/Heerlen: 2016. [in Dutch] www.cbs.nl. [Google Scholar]
- 31.Nijhof SL, Maijer K, Bleijenberg G, Uiterwaal CSPM, Kimpen JLL, van de Putte EM. Adolescent chronic fatigue syndrome: prevalence, incidence, and morbidity. Pediatrics. 2011;127(5):e1169–75. doi: 10.1542/peds.2010-1147. [DOI] [PubMed] [Google Scholar]
- 32.Rousseau M-C, Baumstarck K, Alessandrini M, Blandin V, Billette de Villemeur T, Auquier P. Quality of life in patients with locked-in syndrome: Evolution over a 6-year period. Orphanet J Rare Dis. 2015;10:88. doi: 10.1186/s13023-015-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vereniging Samenwerkingsverband Chronische Ademhalingsondersteuning. Landelijke registratie chronisch beademden in Nederland. [in Dutch] http://www.vsca.nl/thuisbeademing/registratiegegevens-2016/. Published 2016.
- 34.Söderholm S, Meinander M, Alaranta H. Augmentative and alternative communication methods in locked-in syndrome. J Rehabil Med. 2001;33(5):235–239. doi: 10.1080/165019701750419644. [DOI] [PubMed] [Google Scholar]
- 35.Bensch M, Martens S, Halder S, et al. Assessing attention and cognitive function in completely locked-in state with event-related brain potentials and epidural electrocorticography. J Neural Eng. 2014;11(2):26006. doi: 10.1088/1741-2560/11/2/026006. [DOI] [PubMed] [Google Scholar]
- 36.Phillips B, Zhao H. Predictors of assistive technology abandoment. AssistTechnol. 1993;5(1):35–45. doi: 10.1080/10400435.1993.10132205. [DOI] [PubMed] [Google Scholar]
- 37.Scherer MJ, Federici S. Why people use and don’t use technologies: Introduction to the special issue on assistive technologies for cognition/cognitive support technologies. NeuroRehabilitation. 2015;37(3):315–319. doi: 10.3233/NRE-151264. [DOI] [PubMed] [Google Scholar]
- 38.Nijboer F. Technology transfer of brain-computer interfaces as assistive technology: Barriers and opportunities. Ann Phys Rehabil Med. 2015;58(1):35–38. doi: 10.1016/j.rehab.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Atsuta N, Watanabe H, Ito M, et al. Age at onset influences on wide-ranged clinical features of sporadic amyotrophic lateral sclerosis. J Neurol Sci. 2009;276(1–2):163–169. doi: 10.1016/j.jns.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 40.Tagami M, Kimura F, Nakajima H, et al. Tracheostomy and invasive ventilation in Japanese ALS patients: Decision-making and survival analysis: 1990-2010. J Neurol Sci. 2014;344(1–2):158–164. doi: 10.1016/j.jns.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 41.Chiò A, Calvo A, Ghiglione P, Mazzini L, Mutani R, Mora G. Tracheostomy in amyotrophic lateral sclerosis: a 10-year population-based study in Italy. J Neurol Neurosurg Psychiatry. 2010;81(10):1141–1143. doi: 10.1136/jnnp.2009.175984. [DOI] [PubMed] [Google Scholar]
- 42.Casanova E, Lazzari RE, Lotta S, Mazzucchi A. Locked-in syndrome: Improvement in the prognosis after an early intensive multidisciplinary rehabilitation. Arch Phys Med Rehabil. 2003;84(6):862–867. doi: 10.1016/S0003-9993(03)00008-X. [DOI] [PubMed] [Google Scholar]