Abstract
Schistosomiasis is a neglected tropical disease, caused by parasitic worms, which affects almost 200 millions people worldwide. For over 40 years, chemotherapeutic treatment has relied on the administration of praziquantel, an efficacious drug against schistosomiasis. However, concerns about developing drug resistance require the discovery of novel drug compounds. Currently, the drug-screening process is mostly based on the visual evaluation of drug effects on worm larvae in vitro by a trained operator. This manual process is extremely labor-intensive, has limited throughput and may be affected by subjectivity of the operator evaluation. In this paper, we introduce a microfluidic platform with integrated electrodes for the automated detection of worm larvae viability using an impedance-based approach. The microfluidic analysis unit consists of two sets of electrodes and a channel of variable geometry to enable counting and size detection of single parasite larvae, and the collective evaluation of the motility of the larvae as an unbiased estimator for their viability. The current platform also allows for multiplexing of the analysis units resulting in increased throughput. We used our platform to record size and motility variations of Schistosoma mansoni larvae exposed to different concentrations of mefloquine, a drug with established in vitro antischistosomal properties. The developed platform demonstrates the potential of integrated microfluidic platforms for high-throughput antischistosomal drug screening.
Keywords: Microfluidics, Electrical impedance spectroscopy, Schistosoma mansoni, drug-screening, Schistosomiasis
Schistosomiasis is one of the major neglected tropical diseases and affects just under 190 million people worldwide, predominantly children living in rural areas with poor sanitary conditions1,2. This disease is caused by parasitic worms (helminths) of the genus Schistosoma. If left untreated, the infection slowly becomes chronic, resulting in fibrosis of the liver, intestines and/or bladder, anemia, growth and cognitive stunting, malnutrition, urogenital cancers and, eventually, death3,4. Preventative chemotherapy is recommended by the World Health Organization as the most cost effective control strategy for schistosomiasis5. For over 40 years, therapeutic treatment has relied on the administration of praziquantel due to its high safety and efficacy against the adult worm infection. However, the use of a single compound to treat millions of people annually raises concerns with respect to the emergence of drug-resistant worms6.
The use of abundantly available larval-stage worms or Newly Transformed Schistosomula (NTS) of Schistosoma mansoni for pre-screening of drug candidates has been established a decade ago. The ability to acquire large sample numbers of these larvae in a cost-effective way and the concurrent reduction in the use of live animals to obtain relatively low quantities of adult worms have further promoted the development of automated and high-throughput approaches for antischistosomial drug screening7–9. Fluorescence- and luminescence-based assays have been developed for automated detection of larvae viability10–12. In addition, automatic image acquisition and image analysis using bright-field microscopy have been used for evaluating worm viability, based on morphology and motility, for hit/non-hit screening of drug compounds9,13,14. However, the need for massive computational power and high-cost equipment ultimately limits the application of microscopy-based systems for dose-response assays and continuous viability monitoring. Moreover, both fluorescence/luminescence-based approaches and image-based detection require a relatively large number of larvae to provide reliable viability detection, which reduces the actual throughput that can be attained with these methods. As an alternative method, microcalorimetry was shown to be a suitable method for the real-time monitoring of adult-stage schistosomes; however, this approach was not sensitive enough to measure NTS heat production15. As a consequence, the current gold standard for antischistosomal drug screening still consists of a phenotypic evaluation of the worm larvae by a trained operator using manual microscopy. This procedure is extremely labor intensive, and the operator assessment may be affected by a high level of subjectivity and, therefore, feature low reproducibility16,17. Consequently, there is an urgent need for identifying novel methods for replacing the manual microscopic evaluation of NTS larvae viability to advance the current drug screening pipeline.
Electrical impedance spectroscopy (EIS) is a non-invasive and label-free method for the investigation of the dielectric properties of samples. Integration of electrodes in microfluidic devices has enabled highly sensitive low-volume impedance measurements for a wide variety of biological samples in solution, ranging from single cells18 to multicellular aggregates19, and to multicellular organisms20. Impedance cytometers, realized by integrating a set of electrodes within a microfluidic channel, were used for multi-parametric assessments across different frequencies for differentiation of single cells, based on cell size, membrane integrity, and internal properties of cells21–25. The use of microfluidic cell traps or immobilization of cells enabled long-term, real-time EIS measurements of cells26–28. Integrated microfluidic devices have been used for parallelized measurements of microtissue spheroid size29. Microfluidic methods also have been used to study multicellular organisms. Microstructures enabled the detection, trapping and on-chip fluorescence characterization of S. haematobium eggs in urine30. Microfluidic devices for real-time drug screening of Caenorhabditis elegans nematodes based on worm locomotion31 and with electrophysiological and impedance readouts have also been developed32–34. Platforms, based on electrical cell-substrate impedance sensing (ECIS) methodology, have been used to monitor cell growth, spreading and cell attachment to the surface of electrodes35. The xCelligence system (ACEA Biosciences), a commercially available platform, based on the ECIS principle and featuring interdigitated microelectrodes at a bottom of a 96-well microtiter plate, was used to measure the motility of helminths36. This system was also adopted to study different aspects of the schistosomal life cycle (eggs, cercariae, adults). However, the large sensing volume requires a relatively large number of samples as compared to the standard method and is not sensitive enough to detect the small movements of NTS larvae of S. mansoni37.
In this paper, we present a parallelized and integrated microfluidic platform for the automated assessment of the viability of NTS by means of EIS. The platform was used to detect variations in size and motility of the larvae after being exposed to different concentrations of a test compound at two time points. The use of a small detection volume (6 nL) for measuring larvae motility enabled viability detection of NTS with only a low number of worm larvae (~10 NTS). The platform was operated via gravity flow to reduce the complexity of the experimental setup and to avoid the use of external pumping systems. The chip comprises four analysis units to allow for simultaneous execution of measurement replicates and to enable increased throughput. We recorded size and motility variations of NTS after 24-hour and 72-hour exposures to mefloquine, a rapidly-acting antimalarial compound, known for its antischistosomal efficacy in vitro. The obtained results indicate that impedance-based size and motility analysis can provide dose-dependent viability and activity patterns of the larvae and can be used for drug screening applications.
In this paper, we present a parallelized and integrated microfluidic platform for the automated assessment of the viability of NTS by means of EIS. The platform was used to detect variations in size and motility of the larvae after being exposed to different concentrations of a test compound at two time points. The use of a small detection volume (6 nL) for measuring larvae motility enabled viability detection of NTS with only a low number of worm larvae (~10 NTS). The platform was operated via gravity flow to reduce the complexity of the experimental setup and to avoid the use of external pumping systems. The chip comprises four analysis units to allow for simultaneous execution of measurement replicates and to enable increased throughput. We recorded size and motility variations of NTS after 24-hour and 72-hour exposures to mefloquine, a rapidly-acting antimalarial compound, known for its antischistosomal efficacy in vitro. The obtained results indicate that impedance-based size and motility analysis can provide dose-dependent viability and activity patterns of the larvae and can be used for drug screening applications.
Experimental section
Device design and loading
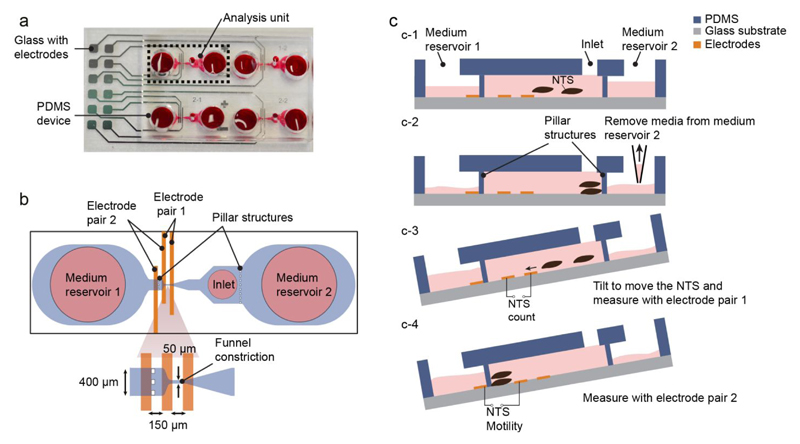
The platform consists of two parts: a top poly(dimethylsiloxane) (PDMS) layer, containing the microfluidic structures that define the four analysis units, and a bottom glass slide with patterned platinum electrodes (Figure 1a). The two parts are bonded together using plasma bonding. The fabrication process of the chip is shown in Figure S1.
Figure 1.
(a) Photograph of the microfluidic platform, which consists of a top PDMS part, aligned and bonded to a platinum-patterned glass slide; (b) schematic of the analysis unit marked in (a). The unit is composed of two medium reservoirs, separated from the sensing region by pillar structures. The sensing region is composed of: a funnel constriction, aligned with an electrode pair (electrode pair 1), which allows for the passage of single NTS and is used for counting the number of loaded larvae; a larger sensing area, aligned with a second electrode pair (electrode pair 2), which is used to measure the collective motility of the NTS. (c) Loading and operation of the chip (c1- c4). First, 20 µL of NTS suspension are loaded through the inlet with a pipette; 5 µL of medium are removed from medium reservoir 1 to generate a hydrostatic pressure and ensure that no NTS are in the sensing area before starting the measurement. The platform is then tilted to drive the NTS through the funnel constriction for counting and EIS size estimation, and to subsequently measure their motility
A schematic of one analysis unit is shown in Figure 1b. An analysis unit consists of an inlet port for loading the NTS solution and two medium reservoirs on either side. The sensing part of the analysis unit is formed by a channel with a funnel-like constriction (50 µm) to allow the passage of single NTS larvae, and by two pillar structures to retain the NTS within the channel but to enable a flow of the solution between the medium reservoirs and the inlet region. An electrode pair around the funnel constriction (electrode pair 1) is used to count the number of loaded NTS larvae and to measure the relative size of single NTS, while a second electrode pair (electrode pair 2) is used to measure the combined motility of NTS that have been loaded into the sensing region of the analysis unit as a proxy for their viability.
Figure 1c shows the experimental procedure. Each analysis unit of the device is loaded with 20 µL of NTS suspension (~10 NTS per 20 μl) through the inlet port. After loading the NTS in the chip, 5 µL of the medium is removed from medium reservoir 2 to generate a flow towards the reservoir and to keep the NTS away from the sensing region of the analysis unit. The platform is then tilted to generate a hydrostatic pressure difference between the medium reservoirs. This movement enables single NTS to pass through the funnel constriction and over electrode pair 1. The NTS are then stopped by the pillar structure between the sensing region and reservoir 1 and are then kept between the electrodes pair 2 for measuring their motility. The sensing region between electrode pair 2 can accommodate up to 15 NTS. Size and motility measurements were performed at three tilting angles (10°, 15°, 20°) with the same NTS sample (Figure S2). No significant changes in both, size and motility recordings were observed. An angle of ~15° was used for the majority of experiments. The developed loading methodology is simple, requires a small volume of NTS solution, and can be performed without additional fluidic systems.
Sample preparation
S. mansoni cercariae were harvested from infected intermediate host snails (Biomphalaria glabrata) and transformed into NTS using a transformation method described previously7. The resulting NTS suspension was adjusted to a concentration of 500 NTS/mL, and 20 μL of NTS suspension were added to each analysis unit to perform the measurements. We used standard samples as they also are used for visual inspection. Culture medium components for NTS were obtained as follows: Medium 199 RPMI 1640, supplemented with 1% penicillin /streptomycin, purchased from Lubioscience (Lucerne, Switzerland), and 5% fetal calf serum (iFCS), which was purchased from Connectorate AG (Dietikon, Switzerland). No additional purification or modification of the samples was performed.
For the experiment with exposure of the NTS to DMSO, the larvae were incubated with 30% DMSO at 37°C and 5% CO2 and measured after 30 minutes of exposure. For the drug dose-response experiments, the NTS were incubated in culture medium with serially diluted concentrations (0.78, 1.56, 3.3, 6.25, 12.5, 25 µM) of mefloquine (Sigma-Aldrich, Buchs, Switzerland) in a 96-well plate in triplicates for 72 hours. The viability of the NTS was assessed microscopically and with EIS after 24 and 72 hours after addition of mefloquine. The visual scores were given based on a previously described viability scale (3 = motile, no changes to morphology; 2 = reduced motility and/or some damage to tegument noted; 1 = severe reduction to motility and/or damage to tegument observed; 0 = dead)7,38.
Data acquisition and analysis
Detailed information on the experimental setup is reported in Figure S3. Briefly, the microfluidic platform was placed on a custom-made PCB, which was used to switch between the electrode pairs of different analysis units in an automated way. A custom-made software was used to select the analysis units and to program the switching protocol and recording duration for the electrode pairs for each unit. The sensing electrodes of the selected analysis units were connected to an impedance spectroscope (HF2, Zurich Instruments AG, Zurich, Switzerland) for user-defined recording durations. The system allowed for short recording durations at each electrode pair (2ms) and fast switching between three analysis units in a round-robin fashion. All the recordings were then combined to reconstruct the total signal for each analysis unit for both, counting and motility data. The total time required for parallelized size and motility measurements for a given condition in all three analysis units amounts to 5 min on average.
A multi-frequency AC signal with an amplitude of 300 mV at 48 kHz and 333 kHz carrier frequencies was applied to the center electrode (Figure 1a). The circuit was then closed by connecting either the right or the left electrode (electrode pair 1 or 2, respectively) to a trans-impedance amplifier (HF2TA, Zurich Instruments AG, Zurich Switzerland) for signal acquisition through current-to-voltage conversion. The magnitude signal of the output voltage, recorded at electrode pairs 1 and 2, was then used for further analysis.
The recorded data was filtered in MATLAB (The MathWorks Inc., Natick, USA) using a band-pass filter with 0.2 - 4 Hz cut-off frequencies for the signal trace, acquired with electrode pair 1, and a 0.5 Hz high-pass filter for the traces acquired with electrode pair 2. As different samples might exhibit varying baseline values, due to differences in the conductivity of the solution or variations in the electrode alignment with the fluidic structure, the acquired traces were normalized with the signal mean to reduce the influence of any of these effects. The location and count of the transient negative peaks in the signal, which were evoked by the passage of NTS and recorded with the electrode pair 1, were extracted from the filtered data by applying a threshold greater than -0.1 mV. The absolute peak-amplitude was then calculated from the raw data to minimize signal distortion caused by the filter. To quantify the fluctuations induced by motion of the larvae between electrode pair 2, we calculated the power of the filtered and normalized signal in a 1-3 Hz bandwidth. This approach minimizes the effect of readout noise, which is present at higher frequencies, while it preserves the signal power that is related to the contraction and expansion of the NTS between the electrodes. The calculated power of the measured fluctuations was normalized by the number of loaded larvae to be able to compare measurements with a different number of NTS in the analysis units.
Results & Discussion
Count and motility measurement
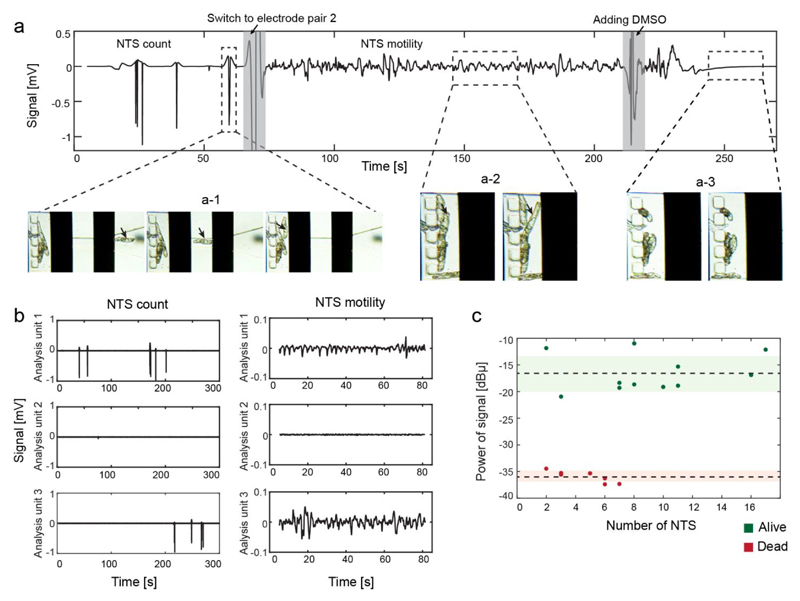
A representative output signal, recorded from a single analysis unit, is shown in Figure 2a. The passage of a NTS over the electrode pair 1 induced a transient reduction of the voltage-converted current between the electrode pair resulting in a peak. Figure 2a-1 shows the peaks generated by the passage of five individual NTS through the funnel structure. Electrode pair 2 was then selected to record the motility of the NTS. The movement of the NTS between the electrodes caused fluctuations in the signal around the mean value (Figure 2a-2). DMSO was added to the device to detect the difference in the motility signal recorded from alive and dead NTS. As the NTS stopped moving, the signal stabilized around the mean value, and no further signal variation was detected (Figure 2a-3).
Figure 2.
(a) Magnitude of the output signal after high-pass filtering. First, electrode pair 1 was selected and NTS flowing through the channel constriction (denoted by the arrow in a-1) induced a transient peak in the trace. Once all the NTS have passed, electrode pair 2 was switched on, and the movement of the NTS between the electrodes (a-2) caused fluctuations in the recorded signal. After addition of DMSO, the NTS died and stopped moving (a-3), and a flat line was recorded. (b) Signal recordings from three different analysis units in parallel. Analysis units 1 and 3 were loaded with NTS, while only medium was present in analysis unit 2. No crosstalk was observed between the analysis units. (c) Signal power in a 1-3 Hz bandwidth as a function of the number of NTS between the electrodes. The x-axis displays number of larvae used in each measurement. To compare the measurements, the signal power was normalized to the respective number of NTS in the analysis unit. The dotted line indicates the mean value of the signal power for alive (green) and dead (red) NTS. The shaded areas show the standard deviation. The signal power for live and dead NTS falls within narrow ranges and were significantly different (at least 4 times SD) from each other.
The multiplexing capability of the platform allowed us to record from three analysis units simultaneously. Therefore, it was crucial to test that the signal of each analysis unit could be reconstructed without cross talk. Figure 2b shows the counting and motility recordings of three different analysis units. Analysis unit 1 and 3 were loaded with NTS, whereas analysis unit 2 contained only medium. Peaks and motility signals were only detected in analysis units 1 and 3 hosting NTS, which indicates that there was no cross talk between the chambers as there were no signals detected in unit 2, which did not contain any larvae.
To compare motility recordings from measurements with different numbers of loaded NTS, the calculated signal power was normalized by the number of NTS in the respective analysis unit. The number of NTS in the sensing region was obtained in an automated way by counting the number of peaks recorded with electrode pair 1 prior to the motility measurement by using a peak detection algorithm. The normalized signal power of live and dead NTS as a function of the number of NTS in the chip is shown in Figure 2c. Signal power values of the live (-16.5 ± 3.4 dBµ) and dead NTS (35.8 ± 1.1 dBµ) fall in two narrow ranges for different number of worms. The two mean values were significantly different from each other (at least 4 times standard deviation), which evidences that a robust and accurate estimation of viability can be obtained even by using a small number of NTS. A lower limit of 3 NTS in the chip was chosen to reduce the dependence of the motility estimation on the behavior of individual larvae.
Impedance-based motility detection
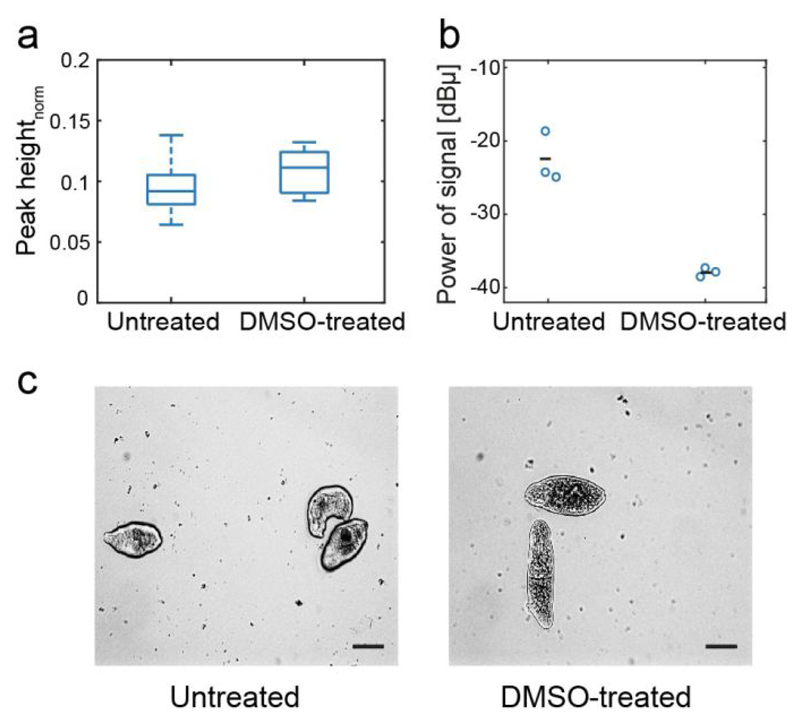
To validate the ability of the platform to detect the status of the NTS in the chip, we measured the viability of DMSO-treated and untreated NTS. As described above, DMSO affects the viability of the NTS if used at high concentrations. We analyzed two conditions: (i) NTS incubated for 30 minutes in standard medium with 30 % DMSO; (ii) NTS incubated in standard conditions (untreated). Replicate measurements of the NTS for the two test conditions were acquired simultaneously in the platform. The impedance signal at lower frequencies (< 1 MHz) can be used to extract information on the size of the particles passing over the electrodes39. Here, we used the peak height recorded at 48 kHz to estimate the size of the NTS under different conditions (Figure 3a). No significant size difference was observed for treated and untreated NTS, which was confirmed visually. However, the NTS exhibited a large difference in motility under the two conditions (Figure 3b). The movement of the larvae between electrode pair 2 in the analysis unit results in fluctuations of the impedance signal, which were used to measure the larvae motility. DMSO treatment killed the NTS, which therefore did not move anymore so that no signal fluctuations were observed. The DMSO treatment resulted in a significant decrease in signal power in comparison to the untreated NTS, which confirms the validity of signal power as indicator of NTS motility and, consequently, viability.
Figure 3.
(a) Normalized absolute amplitude of the peaks, generated by the passage of the NTS through the funnel constriction, of untreated and DMSO-treated NTS. The number of NTS was nuntreated = 35 in the untreated and ntreated = 13 in the DMSO-treated case. The peaks were measured with a 48 kHz carrier signal. No significant increase in size was detected by the EIS measurements, as has been confirmed by the micrographs displayed in (c). Scale bar 50 µm. (b) Normalized signal power, measured at 333 kHz for untreated and DMSO-treated NTS. Three replicates were acquired for each condition. Each circle represents a measurement of an analysis unit, and the mean is represented by the black line. (c) Micrographs of untreated and DMSO-treated NTSs.
Impedance – based readouts for drug dose-response assay
We measured the variations in the viability of NTS that were exposed to different concentrations of a test compound using our impedance-based readout of size and motility, and compared the results to the standard visual-evaluation method. Although praziquantel is the most widely administered drug, it is known for moderate efficacy against NTS in vitro40. Therefore, mefloquine was selected as a test compound, as this drug has shown potent antischistosomal properties, and is a good benchmark to test new screening methods owing to its fast activity and good dose-response effect in vitro41. As a control, we measured also the viability of untreated NTS and NTS incubated with the highest concentration of the drug vehicle, 0.5% DMSO (Figure S4).
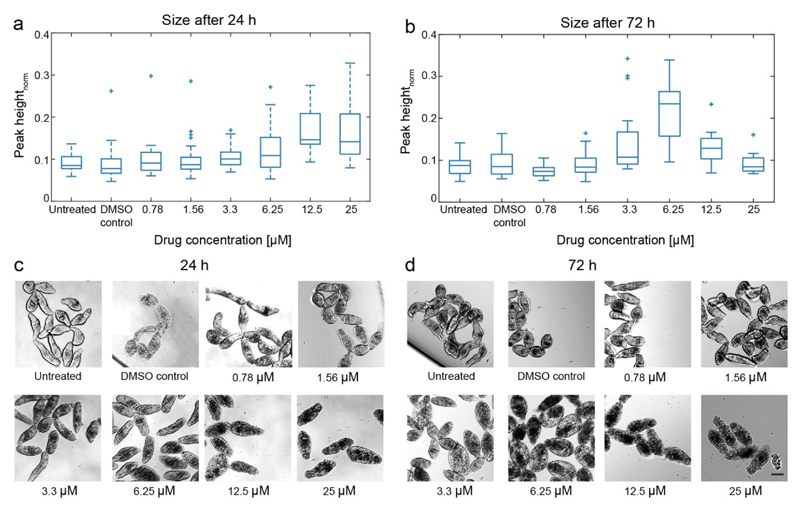
Figure 4 shows the relative size of the NTS, as extracted from the impedance measurements, and representative microscopic images for comparison after 24 hours and 72 hours of incubation with mefloquine. After 24 hours incubation in the presence of mefloquine, NTS size, as well as the spread of the NTS size distribution increased, with increasing compound concentrations. However, after 72 hours, the NTS showed a similar size for both the lowest and the highest drug concentration tested, which indicates a subsequent shrinking of the NTS at high drug concentrations. A significant swelling of the NTS was detected for 3.3 and 6.25 µM mefloquine concentrations after 72 hours as compared to that of a 24 hour incubation. The impedance-based size readout correlated well with visual observation, shown by the micrographs recorded at 24 and 72 hours (Figure 4c and 4d, respectively), which proves that impedance measurements can be used to provide a qualitative phenotypical evaluation.
Figure 4.
(a) EIS-size measurements of NTS, incubated with six different concentrations of mefloquine (nuntreated = 26, nDMSO control = 37, n0.78 =22, n1.56 = 32, n3.3 = 47, n6.25 = 23, n12.5= 15, n25 = 21) after 24 h. The size increased with increasing drug concentration. (b) Impedance-based size detection of NTS (nuntreated = 42, nDMSO control = 29, n0.78 =25, n1.56 = 23, n3.3 = 22, n6.25 = 20, n12.5= 11, n25 = 8) after 72 h of incubation. A drastic increase in NTS size for 6.25 µM of mefloquine was detected. As controls, also NTS incubated in pure medium and 0.5% DMSO were measured. (c),(d) – Micrographs of NTS after 24 h and 72 h of incubation with different concentrations of mefloquine and the DMSO control. Scale bar 50 µm.
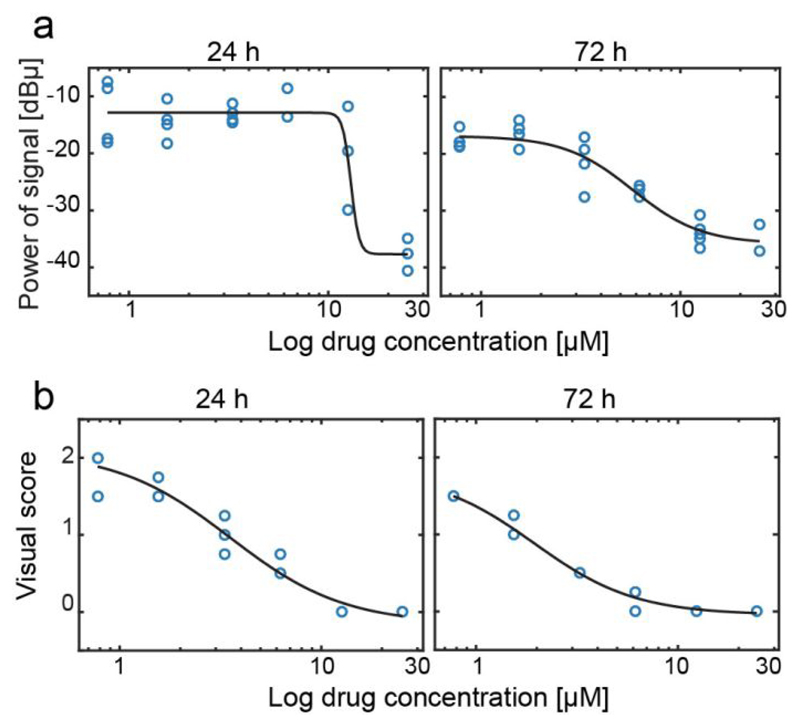
We measured the motility of NTS after incubation with mefloquine to detect NTS viability at different time points and as a function of drug concentration (Figure 5a). After 24 hours, no signal fluctuations could be detected for the NTS incubated with the highest drug concentration (25 µM), indicating that the NTS were no more motile. A high variability in signal power was observed between different replicates for 12.5 µM, which indicated that NTS viability was reduced and that not all NTS were still alive. The large variability is an effect of the low number of NTS in the analysis unit, so that the signal is highly dependent on the individual condition of the loaded NTS. After 72 hours of incubation, a drop in motility was observed for all drug concentrations. NTS incubated with 12.5 µM and 25 µM showed a very low signal power, which indicates loss of viability. A large reduction (~60%) in motility was also detected for the 6.25 µM mefloquine concentration, while, for lower concentrations, the signal power indicated still relatively motile NTS.
Figure 5.
(a) Signal power recorded from NTS incubated with different concentrations of mefloquine (0.78, 1.56, 3.3, 6.25, 12.5, 25 µM), for 24 h and 72 hours. Each circle represents a measurement of an analysis unit. The total numbers of samples that have been used for each concentration are given in the caption of figure 4. (b). Visual score evaluation of NTS (n = 200), incubated with mefloquine for 24 hours and 72 hours. A sigmoidal fit (in black) was used to calculate the IC50 values for the impedance-based evaluation and for the standard evaluation of NTS viability.
The current gold standard for assessing drug efficacy is based on visual evaluation of NTS’s morphology and motility. The visual scores at 24 and 72 hours are plotted in Figure 5b. The visual score was recorded with a higher number of NTS cultured under the same conditions. The trends of impedance–based motility scores and visual scores show good correlation for both time points.
We used the motility detection values to extract an IC50 value of mefloquine for the two time points. IC50 corresponds to the drug concentration at which 50% of the larvae were no more viable. A sigmoid fit (shown in black in Figure 5) was used to calculate the IC50 both by using the impedance-based evaluations and the visual scores. The IC50 value obtained from impedance motility recordings after 24 hours of incubation with the drug was 12.5 ± 0.01 µM, and decreased to 5.75 ± 0.07 µM after 72 hours. The IC50 values obtained from the visual evaluation were 3.46 ± 0.07 µM after 24 hours and 1.94 ± 0.05 µM after 72 hours, respectively. Differences in IC50 values obtained by the two methods were expected, as the differences in the readout methods are considerable38. Visual scoring also includes phenotypical evaluation of the larvae, such as morphology, tegument appearance, and opacity of the larvae, as key parameters for the evaluation, so that lower visual viability scores can be expected for sub-lethal drug concentrations in comparison to an impedance-based evaluation, which is solely based on motility. Moreover, the morphology of the NTSs changes at higher drug concentration, and it is difficult to detect subtle movements of the larvae by eye, which, however, are detectable through an impedance. Upon comparing the motility values, obtained from the impedance chip to those obtained through visual scoring (Figure S5), we observed that for a number of drug concentrations (12.5 µM, 6.25 µM at 24h and 6.25 µM and 3.3 µM at 72 h post drug exposure), we were able to detect fluctuations and motility of the NTS, which were missed by the classical visual evaluation method. In all these cases, the evaluators gave an unjustified low viability score. Nevertheless, the IC50 values obtained with the two different methods fall within the same order of magnitude, and only a slight increase in IC50 values was obtained by using the motility information alone.
Conclusion
Objective and quantitative methods for monitoring of NTS–stage schistosomes are needed to move towards high-throughput approaches for antischistosomal drug screening. In this paper, we have shown an integrated microfluidic platform that can be used to assess the viability of NTS using an impedance-based analysis method. The use of a microfluidic approach reduces the amount of NTS needed for the analysis, as compared to viability detection based on fluorescence/luminescence image detection or the standard visual evaluation method. The device features simple operation for loading the NTS and to detect their viability. Operation of the device is based on a pump-free microfluidic approach, so that the platform can be used with standard laboratory equipment. The impedance-based readout enables both an automated counting of loaded parasites, for comparing measurements between different analysis units, and subsequent NTS size and motility detection. Drug-dose responses of the larvae to different concentrations of mefloquine obtained via impedance detection showed good agreement with those obtained by standard visual evaluation of NTS, demonstrating that NTS motility could be used as a reliable estimator of viability.
Finally, we showed that impedance-based detection can be easily parallelized to increase throughput. The multiplexing capability of EIS recordings and the simplicity of the experimental setup enable further up-scaling. A higher throughput can be achieved either by increasing the number of analysis units on the platform or by stacking multiple platforms, which is possible due to the fact that optical access is not required for viability evaluation. Moreover, the use of EIS measurements can be extended to continuous monitoring of NTS viability, which could provide more insights into the dynamics of action of the drugs under evaluation, to allow the selection of fast-acting compounds. The presented platform paves the way towards the use of impedance-based detection for objective and automated screening of antischistosomal drug candidates.
Supplementary Material
Supporting information is available free of charge. The files include steps for device fabrication, setup description and data that support the presented results.
Acknowledgments
The work was financially supported by Swiss National Science Foundation (SNF 2-77079-16 Infected body-on-chip) and Swiss SystemX.ch. The authors would like to thank the clean room facility at D-BSSE, ETH Zurich, for their help and support.
Footnotes
Just Accepted
“Just Accepted” manuscripts have been peer-reviewed and accepted for publication. They are posted online prior to technical editing, formatting for publication and author proofing. The American Chemical Society provides “Just Accepted” as a service to the research community to expedite the dissemination of scientific material as soon as possible after acceptance. “Just Accepted” manuscripts appear in full in PDF format accompanied by an HTML abstract. “Just Accepted” manuscripts have been fully peer reviewed, but should not be considered the official version of record. They are citable by the Digital Object Identifier (DOI®). “Just Accepted” is an optional service offered to authors. Therefore, the “Just Accepted” Web site may not include all articles that will be published in the journal. After a manuscript is technically edited and formatted, it will be removed from the “Just Accepted” Web site and published as an ASAP article. Note that technical editing may introduce minor changes to the manuscript text and/or graphics which could affect content, and all legal disclaimers and ethical guidelines that apply to the journal pertain. ACS cannot be held responsible for errors or consequences arising from the use of information contained in these “Just Accepted” manuscripts.
Conflict of Interest
The authors declare no competing financial interest.
References
- (1).Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulkader RS, Abdulle AM, Abebo TA, Abera SF, et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Colley DG, Bustinduy AL, Secor WE, King CH. Human Schistosomiasis. Lancet (London, England) 2014;383(9936):2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Gryseels B. Schistosomiasis. Infect Dis Clin North Am. 2012;26(2):383–397. doi: 10.1016/j.idc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- (4).Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL, Olveda RM, Kurtis JD, McGarvey ST. Helminth Infection and Cognitive Impairment among Filipino Children. Am J Trop Med Hyg. 2005;72(5):540–548. [PMC free article] [PubMed] [Google Scholar]
- (5).Webster JP, Molyneux DH, Hotez PJ, Fenwick A. The Contribution of Mass Drug Administration to Global Health: Past, Present and Future. Philos Trans R Soc B Biol Sci. 2014;369(1645) doi: 10.1098/rstb.2013.0434. 20130434–20130434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wang W, Wang L, Liang Y-S. Susceptibility or Resistance of Praziquantel in Human Schistosomiasis: A Review. Parasitol Res. 2012;111(5):1871–1877. doi: 10.1007/s00436-012-3151-z. [DOI] [PubMed] [Google Scholar]
- (7).Keiser J. In Vitro and in Vivo Trematode Models for Chemotherapeutic Studies. Parasitology. 2010;137(3):589–603. doi: 10.1017/S0031182009991739. [DOI] [PubMed] [Google Scholar]
- (8).Peak E, Hoffmann KF. Cross-Disciplinary Approaches for Measuring Parasitic Helminth Viability and Phenotype. An Acad Bras Cienc. 2011;83(2):649–662. doi: 10.1590/S0001-37652011000200024. [DOI] [PubMed] [Google Scholar]
- (9).Mansour NR, Paveley R, Gardner JMF, Bell AS, Parkinson T, Bickle Q. High Throughput Screening Identifies Novel Lead Compounds with Activity against Larval, Juvenile and Adult Schistosoma Mansoni. PLoS Negl Trop Dis. 2016;10(4):e0004659. doi: 10.1371/journal.pntd.0004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Mansour NR, Bickle QD. Comparison of Microscopy and Alamar Blue Reduction in a Larval Based Assay for Schistosome Drug Screening. PLoS Negl Trop Dis. 2010;4(8):e795. doi: 10.1371/journal.pntd.0000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Peak E, Chalmers IW, Hoffmann KF. Development and Validation of a Quantitative, High-Throughput, Fluorescent-Based Bioassay to Detect Schistosoma Viability. PLoS Negl Trop Dis. 2010;4(7):e759. doi: 10.1371/journal.pntd.0000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lalli C, Guidi A, Gennari N, Altamura S, Bresciani A, Ruberti G. Development and Validation of a Luminescence-Based, Medium-Throughput Assay for Drug Screening in Schistosoma Mansoni. PLoS Negl Trop Dis. 2015;9(1):e0003484. doi: 10.1371/journal.pntd.0003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Paveley RA, Mansour NR, Hallyburton I, Bleicher LS, Benn AE, Mikic I, Guidi A, Gilbert IH, Hopkins AL, Bickle QD. Whole Organism High-Content Screening by Label-Free, Image-Based Bayesian Classification for Parasitic Diseases. PLoS Negl Trop Dis. 2012;6(7):e1762. doi: 10.1371/journal.pntd.0001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lee H, Moody-Davis A, Saha U, Suzuki BM, Asarnow D, Chen S, Arkin M, Caffrey CR, Singh R. Quantification and Clustering of Phenotypic Screening Data Using Time-Series Analysis for Chemotherapy of Schistosomiasis. Series on Advances in Bioinformatics and Computational Biology. 2012;13:S4. doi: 10.1186/1471-2164-13-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Manneck T, Braissant O, Haggenmüller Y, Keiser J. Isothermal Microcalorimetry to Study Drugs against Schistosoma Mansoni. J Clin Microbiol. 2011;49(4):1217–1225. doi: 10.1128/JCM.02382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Ramirez B, Bickle Q, Yousif F, Fakorede F, Mouries M-A, Nwaka S. Schistosomes: Challenges in Compound Screening. Expert Opin Drug Discov. 2007;2(sup1):S53–S61. doi: 10.1517/17460441.2.S1.S53. [DOI] [PubMed] [Google Scholar]
- (17).Abdulla M-H, Ruelas DS, Wolff B, Snedecor J, Lim K-C, Xu F, Renslo AR, Williams J, McKerrow JH, Caffrey CR. Drug Discovery for Schistosomiasis: Hit and Lead Compounds Identified in a Library of Known Drugs by Medium-Throughput Phenotypic Screening. PLoS Negl Trop Dis. 2009;3(7):e478. doi: 10.1371/journal.pntd.0000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Sun T, Morgan H. Single-Cell Microfluidic Impedance Cytometry: A Review. Microfluid Nanofluidics. 2010;8:423–443. doi: 10.1007/s10404-010-0580-9. [DOI] [Google Scholar]
- (19).Schmid YRF, Bürgel SC, Misun PM, Hierlemann A, Frey O. Electrical Impedance Spectroscopy for Microtissue Spheroid Analysis in Hanging-Drop Networks. ACS Sensors. 2016;1(8):1028–1035. doi: 10.1021/acssensors.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Bakhtina NA, Korvink JG. Microfluidic Laboratories for C. Elegans Enhance Fundamental Studies in Biology. RSC Adv. 2014;4(9):4691–4709. doi: 10.1039/C3RA43758B. [DOI] [Google Scholar]
- (21).Haandbæk N, Bürgel SC, Heer F, Hierlemann A. Characterization of Subcellular Morphology of Single Yeast Cells Using High Frequency Microfluidic Impedance Cytometer. Lab Chip. 2014;14(2):369–377. doi: 10.1039/c3lc50866h. [DOI] [PubMed] [Google Scholar]
- (22).Cheung KC, Di Berardino M, Schade-Kampmann G, Hebeisen M, Pierzchalski A, Bocsi J, Mittag A, Tárnok A. Microfluidic Impedance-Based Flow Cytometry. Cytom Part A. 2010;77A(7):648–666. doi: 10.1002/cyto.a.20910. [DOI] [PubMed] [Google Scholar]
- (23).McGrath JS, Honrado C, Spencer D, Horton B, Bridle HL, Morgan H. Analysis of Parasitic Protozoa at the Single-Cell Level Using Microfluidic Impedance Cytometry. Sci Rep. 2017;7(1):2601. doi: 10.1038/s41598-017-02715-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Cheung K, Gawad S, Renaud P. Impedance Spectroscopy Flow Cytometry: On-Chip Label-Free Cell Differentiation. Cytometry A. 2005;65(2):124–132. doi: 10.1002/cyto.a.20141. [DOI] [PubMed] [Google Scholar]
- (25).Holmes D, Pettigrew D, Reccius CH, Gwyer JD, van Berkel C, Holloway J, Davies DE, Morgan H. Leukocyte Analysis and Differentiation Using High Speed Microfluidic Single Cell Impedance Cytometry. Lab Chip. 2009;9(20):2881. doi: 10.1039/b910053a. [DOI] [PubMed] [Google Scholar]
- (26).Malleo D, Nevill JT, Lee LP, Morgan H. Continuous Differential Impedance Spectroscopy of Single Cells. Microfluid Nanofluidics. 2010;9(2–3):191–198. doi: 10.1007/s10404-009-0534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Zhu Z, Frey O, Franke F, Haandbæk N, Hierlemann A. Real-Time Monitoring of Immobilized Single Yeast Cells through Multifrequency Electrical Impedance Spectroscopy. Anal Bioanal Chem. 2014:7015–7025. doi: 10.1007/s00216-014-7955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Chawla K, Bürgel SC, Schmidt GW, Kaltenbach H-M, Rudolf F, Frey O, Hierlemann A. Integrating Impedance-Based Growth-Rate Monitoring into a Microfluidic Cell Culture Platform for Live-Cell Microscopy. Microsystems Nanoeng. 2018;4(1):1–9. doi: 10.1038/s41378-018-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Bürgel SC, Diener L, Frey O, Kim J-Y, Hierlemann A. Automated, Multiplexed Electrical Impedance Spectroscopy Platform for Continuous Monitoring of Microtissue Spheroids. Anal Chem. 2016;88(22):10876–10883. doi: 10.1021/acs.analchem.6b01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Xiao Y, Lu Y, Hsieh M, Liao J, Wong PK. A Microfiltration Device for Urogenital Schistosomiasis Diagnostics. PLoS One. 2016;11(4):e0154640. doi: 10.1371/journal.pone.0154640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Carr JA, Parashar A, Gibson R, Robertson AP, Martin RJ, Pandey S. A Microfluidic Platform for High-Sensitivity, Real-Time Drug Screening on C. Elegans and Parasitic Nematodes. Lab Chip. 2011;11(14):2385–2396. doi: 10.1039/c1lc20170k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hu C, Dillon J, Kearn J, Murray C, O’Connor V, Holden-Dye L, Morgan H. NeuroChip: A Microfluidic Electrophysiological Device for Genetic and Chemical Biology Screening of Caenorhabditis Elegans Adult and Larvae. PLoS One. 2013;8(5):e64297. doi: 10.1371/journal.pone.0064297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Lockery SR, Hulme SE, Roberts WM, Robinson KJ, Laromaine A, Lindsay TH, Whitesides GM, Weeks JC. A Microfluidic Device for Whole-Animal Drug Screening Using Electrophysiological Measures in the Nematode C. Elegans. Lab Chip. 2012;12(12):2211–2220. doi: 10.1039/c2lc00001f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Zhu Z, Chen W, Tian B, Luo Y, Lan J, Wu D, Chen D, Wang Z, Pan D. Using Microfluidic Impedance Cytometry to Measure C. Elegans Worms and Identify Their Developmental Stages. Sensors Actuators B Chem. 2018;275:470–482. doi: 10.1016/J.SNB.2018.07.169. [DOI] [Google Scholar]
- (35).Wegener J, Keese CR, Giaever I. Electric Cell–Substrate Impedance Sensing (ECIS) as a Noninvasive Means to Monitor the Kinetics of Cell Spreading to Artificial Surfaces. Exp Cell Res. 2000;259(1):158–166. doi: 10.1006/excr.2000.4919. [DOI] [PubMed] [Google Scholar]
- (36).Rinaldi G, Loukas A, Brindley PJ, Irelan JT, Smout MJ. Viability of Developmental Stages of Schistosoma Mansoni Quantified with XCELLigence Worm Real-Time Motility Assay (XWORM) Int J Parasitol Drugs Drug Resist. 2015;5(3):141–148. doi: 10.1016/j.ijpddr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Smout MJ, Kotze AC, McCarthy JS, Loukas A, Behnke J. A Novel High Throughput Assay for Anthelmintic Drug Screening and Resistance Diagnosis by Real-Time Monitoring of Parasite Motility. PLoS Negl Trop Dis. 2010;4(11):e885. doi: 10.1371/journal.pntd.0000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Panic G, Flores D, Ingram-Sieber K, Keiser J. Fluorescence/Luminescence-Based Markers for the Assessment of Schistosoma Mansoni Schistosomula Drug Assays. Parasites and Vectors. 2015;8(1):624. doi: 10.1186/s13071-015-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Gawad S, Schild L, Renaud PH. Micromachined Impedance Spectroscopy Flow Cytometer for Cell Analysis and Particle Sizing. Lab Chip. 2001;1(1):76–82. doi: 10.1039/b103933b. [DOI] [PubMed] [Google Scholar]
- (40).Caffrey CR. Chemotherapy of Schistosomiasis: Present and Future. Curr Opin Chem Biol. 2007;11(4):433–439. doi: 10.1016/j.cbpa.2007.05.031. [DOI] [PubMed] [Google Scholar]
- (41).Keiser J, Manneck T, Vargas M. Interactions of Mefloquine with Praziquantel in the Schistosoma Mansoni Mouse Model and in Vitro. J Antimicrob Chemother. 2011;66(8):1791–1797. doi: 10.1093/jac/dkr178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.