Abstract
Background:
Oxidative stress and inflammation are considered to be important pathways leading to particulate matter (PM)-associated disease. In this exploratory study, we examined the effects of metals and oxidative potential (OP) in urban PM on biomarkers of systemic inflammation, oxidative stress and neural function.
Methods:
Fifty-three healthy non-smoking volunteers (mean age 28 years, twenty-eight females) were exposed to coarse (2.5–10 μm, mean 213 μg/m3), fine (0.15–2.5 μm, 238 μg/m3), and/or ultrafine concentrated ambient PM (<0.3 μm, 136 μg/m3). Exposures lasted 130 minutes, separated by ≥2 weeks. Metal concentrations and OP (measured by ascorbate and glutathione depletion in synthetic airway fluid) in PM were analyzed. Blood and urine samples were collected pre-exposure, and 1-hour and 21-hour post exposure for assessment of biomarkers. We used mixed-regression models to analyze associations adjusting for PM size and mass concentration.
Results:
Results for metals were expressed as change (%) from daily pre-exposure biomarker levels after exposure to a metal at a level equivalent to the mean concentration. Exposure to various metals (silver, aluminum, barium, copper, iron, potassium, lithium, nickel, tin, and/or vanadium) was significantly associated with increased levels of various blood or urinary biomarkers. For example, the blood inflammatory marker vascular endothelia growth factor (VEGF) increased 5.3% (95% confidence interval: 0.3%, 10.2%) 1-hr post exposure to nickel; the traumatic brain injury marker ubiquitin C-terminal hydrolase L1 (UCHL1) increased 11% (1.2%, 21%) and 14% (0.3%, 29%) 1-hr and 21-hr post exposure to barium, respectively; and the systemic stress marker cortisol increased 1.5% (0%, 2.9%) and 1.5% (0.5%, 2.8%) 1-hr and 21-hr post exposure to silver, respectively. Urinary DNA oxidation marker 8-hydroxy-deoxy-guanosine increased 14% (6.4%, 21%) 1-hr post exposure to copper; urinary neural marker vanillylmandelic acid increased 29% (3%, 54%) 1-hr post exposure to aluminum; and urinary cortisol increased 88% (0.9%, 176%) 1-hr post exposure to vanadium. Results for OP were expressed as change (%) from daily pre-exposure biomarker levels after exposure to ascorbate-related OP at a level equivalent to the mean concentration, or for exposure to glutathione-related OP at a level above the limit of detection. Exposure to ascorbate- or glutathione-related OP was significantly associated with increased inflammatory and neural biomarkers including interleukin-6, VEGF, UCHL1, and S100 calcium-binding protein B in blood, and malondialdehyde and 8-hydroxy-deoxy-guanosine in urine. For example, UCHL1 increased 9.4% (1.8%, 17%) in blood 21-hr post exposure to ascorbate-related OP, while urinary malondialdehyde increased 19% (3.6%, 35%) and 8-hydroxy-deoxy-guanosine increased 24% (2.9%, 48%) 21-hr post exposure to ascorbate- and glutathione-related OP, respectively.
Conclusion:
Our results from this exploratory study suggest that metal constituents and OP in ambient PM may influence biomarker levels associated with systemic inflammation, oxidative stress, perturbations of neural function, and systemic physiological stress.
INTRODUCTION
Particulate matter (PM) in urban ambient air is a complex mixture of various sizes and constituents. Some metals in PM, such as the transition metals iron (Fe), copper (Cu), nickel (Ni) and vanadium (V), have been found to be associated with cardiovascular and respiratory hospital admissions and mortality (Bell and HEI Health Review Committee 2012; Lippmann et al. 2013; Zhou et al. 2011), and increased heart rate and decreased lung function (Cakmak et al. 2014). Oxidative potential (OP) is a measure of the capacity of PM to deplete certain antioxidant molecules such as ascorbic acid (AA), glutathione (GSH) and dithiothreitol (DDT) in synthetic airway fluid (Ayres et al. 2008), and has been found to contribute to oxidative stress and inflammation in cultured human lung carcinoma cells (Crobeddu et al. 2017). Large variations in OP levels of PM from different regions or locations have been reported in the Netherlands and Belgium (Yang et al. 2015b), London (UK) (Kelly et al. 2011), and Canada (Weichenthal et al. 2016b; Weichenthal et al. 2016c). The transition metal contents appear to partially explain the differences in OP levels in these PM samples (Daher et al. 2014).
Oxidative stress and systemic inflammation are considered to be important pathways linked to PM exposure-associated disease (Brook et al. 2010; Nel 2005; Weichenthal et al. 2013). Measurement of OP involves redox activity of transition metals and organic chemicals, and reflects interactions between different metals in the reaction. Therefore, compared to PM mass, OP has been proposed as an exposure metric more closely related to biological responses to PM exposures (Borm et al. 2007). Indeed, in a study conducted in school children with asthma in Montreal (Canada) we found a stronger association of exhaled nitric oxide (a clinical biomarker for lung inflammation) with daily personal exposure to oxidative burden as measured by PM2.5-induced GSH depletion than with PM2.5 mass (Maikawa et al. 2016). In another study performed in Atlanta (USA), compared to PM mass, OP measured as PM2.5-induced DTT depletion was also found to have a stronger association with emergency department visits for asthma and congestive heart failure (Bates et al. 2015). Others have reported inconsistent results. For example, Tonne et al. reported no association between GSH depletion-based OP and carotid intima-media thickness (a measure of subclinical atherosclerosis) in London (UK) (Tonne et al. 2012), while Strak et al. reported no associations between AA- or GSH-related OP and acute respiratory response including exhaled nitric oxide levels and lung function in adults in the Netherlands (Strak et al. 2012). Although OP has been proposed as a useful parameter representing the overall oxidative toxicity potency of PM, evidence that OP leads to oxidative stress and inflammation in humans is still scarce.
We previously reported that human exposure to coarse, fine and ultrafine PM in a controlled environment was associated with increased blood pressure (Zhong et al. 2015), as well as biomarkers indicative of inflammation and oxidative stress (Behbod et al. 2013; Liu et al. 2015), perturbation of blood-brain barrier, and physiological stress response (Liu et al. 2017). In the present study, we explored the influence of metal constituents and OP (AA and GSH depletion) of urban PM on biomarkers of systemic inflammatory and neural function. The biomarkers examined in this study have been reported to be indicative of: a) oxidative stress: 8-hydroxy-deoxy-guanosine (8-OHdG)(Park and Floyd 1992), malondialdehyde (MDA)(Janero 1990), and brain-derived neurotrophic factor (BDNF)(Moylan et al. 2013); b) inflammation: endothelin-1 (ET-1, also a potent vasoconstrictor)(Haynes et al. 1996), interleukin-6 (IL-6)(Nishimoto and Kishimoto 2006), C-reactive protein (CRP)(Anderson et al. 1998), and vascular endothelial growth factor (VEGF, a growth factor that regulates endothelial progenitor cells from bone marrow to an injured site)(Haberzettl et al. 2012); c) traumatic brain injury or neurodegeneration: neuron-specific enolase (NSE), S100 calcium-binding protein B (S100B), and ubiquitin C-terminal hydrolase L1 (UCHL1) (Blyth et al. 2011; Lewis et al. 2010; Zetterberg et al. 2013); and d) systemic physical and mental stress: cortisol, homovanillic acid (HVA, end-product of dopamine metabolism) and vanillylmandelic acid (VMA, end-product of epinephrine and norepinephrine metabolism) (Frankenhaeuser et al. 1986; Fukuda et al. 1996; Sapolsky et al. 2000).
MATERIALS AND METHODS
The original study design was a single-blind randomized cross-over trial (Liu et al. 2015). The exposure technologist controlled the exposure levels, thus was not blinded, but did not carry out any data analyses. The participants were blinded to the randomization pattern of the exposures. The biomarker measures and PM mass and composition data were determined in blinded fashion. Exposure codes were unmasked only for statistical analyses. Exposure to clean air was used as a control for all participants in the original study, but in the present study it was not included because metals and OP were not determined in particle filters collected from exposure to clean air. Most of the participants had more than one exposure to concentrated ambient particles (CAPs).
Participants were non-smokers, 18–60 years of age, without a history of coronary artery disease, myocardial infarction, peripheral vascular disease, angina, heart failure, hypertension, diabetes mellitus, or ongoing upper respiratory infection. All participants were free of lipid abnormalities and respiratory tract infections. We excluded participants with baseline spirometry <75% of predicted normal values (forced vital capacity and forced expiratory volume in 1 second), those with clinically significant abnormalities in their resting electrocardiogram, or who were pregnant or breast-feeding. All participants provided informed written consent prior to participating in the study. The Research Ethics Boards of Health Canada, St. Michael’s Hospital, and the University of Toronto approved the study protocol. Detailed methods of participant recruitment were described by Liu et al. (2015).
Exposure facility
Details of the coarse, fine and ultrafine PM concentrator facility were described elsewhere (Rastogi et al. 2012). All participants were also exposed to high-efficiency particulate absorption (HEPA) filtered ambient air and/or medical air, but these exposures were not included in the present study. The controlled exposures to CAPs were derived from ambient air drawn from breathing height adjacent to a downtown street in Toronto, Canada. We used Harvard Ambient Fine, Coarse and Ultrafine Particle Concentrators to concentrate the particles for the exposure. Ambient aerosols were drawn through a size-selective inlet where particles >10 μm were removed. The fine PM concentrator delivered CAP 0.15–2.5 μm in mass median aerodynamic diameter (MMAD) (fine CAP), while the coarse PM concentrator delivered CAP 2.5–10 μm in MMAD (coarse CAP). In the airstream of the ultrafine particle concentrator, particles larger than 0.3 μm were removed by inertial impaction to deliver a concentrated ultrafine aerosol to the participant. Experimental exposures took place in an enclosed temperature-controlled exposure chamber. The exposure air stream was delivered directly to the participant who was seated at rest and breathing freely via an “oxygen type” facemask covering his/her nose and mouth. Each exposure lasted 130 minutes. PM in the airstream was collected on a Teflon filter (37 mm, 2 μm) during the 130-min exposure for determination of elemental contents and OP, and gravimetric determinations of mass concentrations analyzed. All exposures were carried out at the same time of the day. There was a minimum washout period of at least 2 weeks between exposures.
Characterization of metal constituents in CAPs
Preparation of airborne PM filter samples: The airborne particulate matter filter samples were prepared and digested in a clean-laboratory environment (Class 100). The total metal extraction procedure for Teflon filters was carried out using a mixture of nitric acid and hydrofluoric acid in closed vessels at a constant temperature. An ultrasonication procedure was carried out during the extraction and digestion period. The extracted solutions were then diluted using distilled deionized water and analyzed by an inductively coupled plasma-mass spectrometer (ICP-MS) for 36 elements.
The ICP-MS system (Perkin-Elmer Elan DRC-II) was equipped with GemTip cross-flow nebulizer, Ryton spray chamber, plasma torch with an alumina injector, a three-channel peristaltic pump attached on the instrument and a Cetac auto-sampler ADX-500 used for feeding sample solutions into the nebulizer and ICP plasma. In the ICP-MS analysis, indium (In) was used as an internal standard and the five-point external standard calibration curves were plotted linearly through zero for each analyte. The concentrations of the standard solutions for each analyte were 0, 10, 30, 50, 70 and 100 μg/L. All analytical results were standard blank subtracted. Results for the filter samples were also corrected for laboratory filter blank levels. The interferences of calcium oxides and hydroxides on the measurements of 57Fe, 59Co and 60Ni isotopes were corrected (Wu et al. 1996). Metal concentrations were expressed as μg/m3. Metals that were below the limit of detection were assigned a value corresponding to [limit of detection]/ √2. Only the metals presenting over 75% of detectable values were included in the current analysis.
Measurement of oxidative potential (OP) in CAPs
Detailed methods of OP analyses can be found in Maikawa et al. (2016). Briefly, PM collected on filters was extracted using high-pressure liquid chromatography grade methanol. A synthetic human respiratory tract lining fluid containing physiologically-relevant low molecular weight antioxidants (AA or GSH), was incubated with CAP samples for 4 hours at 37°C on a 96-well plate. Positive [non-ferrous dust, NIST PD-1 and Cu(ll), Gaithersburg, MD, USA] and negative (model carbon black, Arosperse 15B; NIST) controls were assessed in parallel with the CAP samples for inter-experimental standardisation. AA concentrations were measured using absorbance spectra in a UV-vis plate reader (Molecular Devices, SpectraMax 190). GSH concentration was determined using the oxidized glutathione-reductase-5,5’-dithio-bis(2-nitrobenzoic acid) recycling assay (Baker et al. 1990). OP was expressed as percentage depletion of AA or GSH over 4 hours per μg of CAP within the 200 μl of solution in the incubation well.
Measurement of biomarkers in blood and urine
At the time of enrollment, we measured the height and weight and calculated the body mass index (BMI) of volunteers using standard procedures. We collected urine and venous blood samples (20 ml) prior to, and at 1-hr and 21-hr after each exposure.
Blood tests:
We obtained fasting blood samples by venipuncture and stored plasma at −70°C. Biomarkers analyzed using ELISA kits were: CRP (Alpco Laboratory Products Company, Salem, NH, USA); IL-6, ET-1, VEGF, NSE and BDNF (R&D Systems, Minneapolis, MN, USA); S100B (Millipore, Billerica, MA, USA); UCHL1 (EnCor Biotechnology Inc., Gainesville, FL, USA); and total cortisol (DetectX Cortisol ELISA kit, Arbor Assays, Ann Arbor, MI, USA). MDA was measured using HPLC with an Agilent 1200 series system (Mississauga, ON, Canada) as previously described (Liu et al. 2015).
Urine tests:
We collected and stored urine samples at −20°C. Urine samples were clarified by centrifugation (5000 rpm, 5 mins in an Eppendorf 5804 centrifuge) prior to analyses. Biomarkers analyzed using ELISA kits were: VEGF (R&D Systems), 8-OHdG (8-OHdG kit from Cosmo Bio USA, Carlsbad, CA, USA); VMA and HVA (Eagle Biosciences Inc., Nashua, NH, USA); free cortisol (DetectX Cortisol ELISA kit, Arbor Assays). Creatinine concentrations were measured using a CREA kit (Roche Diagnostics, Laval, QC, Canada). Creatinine concentration was used to normalize urinary biomarker concentrations. All assays were carried out following the manufacturer instructions. HPLC analysis was used to measure urinary MDA, as previously described (Liu et al. 2015).
Since all exposures were carried out at the same time of the day, urine and blood samples were also collected at the same time of the day for each participant. This procedure eliminated potential diurnal effects on biomarkers which might have contributed to intra-individual variations.
Statistical analysis
We calculated percent change of biomarker values at 1-hr and 21-hr post exposure using the following equation:
Percent change of biomarker (%) = [(post-exposure value minus pre-exposure value)/pre-exposure value]*100.
This equation was used to adjust for potential day-to-day variations in participant factors such as diet, stress, exposure to ambient pollutants and environmental tobacco smoke, and other unknown factors that may have contributed to variations in systemic biomarker levels. We calculated the correlations between OP measurements and PM mass and metal concentrations using the Spearman rank order correlation method.
We used mixed-effects linear regression models (with restricted maximum likelihood estimation) to analyze associations of exposure to metals and OP with post-exposure percent changes in biomarkers. Mixed models accounted for the repeated measures, assuming random participant intercepts and fixed slopes. (Fixed intercepts and random slopes analysis was attempted, but models could not continue due to convergence issue). We used an autoregressive model of order-one to adjust for serial autocorrelation. OP as assessed by AA depletion was a continuous variable, while OP as assessed by GSH depletion was a binary variable (values above the limit of detection=1). Age, sex (binary variable, male=1), BMI, season [binary variable, warm season (May to October) =1], PM size (dummy variable, coarse particle as reference), and particle mass concentration were included in all models. Temperature was not adjusted for in the models as it was kept constant in the testing facility. The unit of regression coefficients for metals and AA-related OP was the mean change (95% confidence interval) of a biomarker per unit of a metal (1 ng/m3) or OP (1% AA depletion/μg CAP) during the 130-minute exposure. For the final results, regression coefficients were multiplied by the mean concentrations of metals or OP in the facility during the exposure (listed in Table 1 and Table S1) to represent the change (95% confidence interval) of a biomarker after exposure to a metal or AA-related OP at a level equivalent to the mean concentration. The unit of regression coefficient for GSH-related OP was the mean change (95% confidence interval) of a biomarker after exposure to GSH-related OP at values above the limit of detection. The statistical software used was R, version 3.2.4 (https://www.r-project.org/). A two tailed value of p<0.05 was considered statistically significant.
Table 1.
Characteristics of selective constituents in concentrated ambient particulate matter in the exposure airstream. Values are expressed as mean ± standard deviation. Number of samples is provided in parentheses.
| All CAPs Combined | Coarse CAPs | Fine CAPs | Ultrafine CAPs | |
|---|---|---|---|---|
| Total mass (μg/m3) | 198.0 ± 72.4 (136) | 212.6 ± 51.8 (77) | 238.4 ± 62.0 (29) | 120.0 ± 72.0 (30) |
| Element (ng/m3) | ||||
| Silver (Ag) | 2.5 ± 11.8 (125) | 0.7 ± 0.5 (72) | 1.3 ± 0.8 (25) | 8.2 ± 24.4 (28) |
| Aluminum (Al) | 5608±4152(125) | 8360 ± 2775 (72) | 3685± 2474 (25) | 251±343 (28) |
| Barium (Ba) | 285 ±213 (125) | 386± 179 (72) | 299± 158 (25) | 14.8 ± 9.2 (28) |
| Copper (Cu) | 287±313 (125) | 340 ±220 (72) | 240± 110 (25) | 193 ± 542 (28) |
| Iron (Fe) | 6394±4121 (125) | 8800 ± 2723 (72) | 6172±2550 (25) | 408 ± 274 (28) |
| Potassium (K) | 1813 ± 1348 (125) | 2412± 1283 (72) | 1827±772 (25) | 257± 136 (28) |
| Lithium (Li) | 2.7 ± 1.6 (125) | 3.5 ± 1.0 (72) | 3.0 ± 1.6 (25) | 0.5 ± 0.4 (28) |
| Nickel (Ni) | 32.5 ± 56.8 (125) | 33.5 ± 54.6 (72) | 30 ± 20.5 (25) | 32.6 ± 81.1 (28) |
| Tin (Sn) | 22.5± 16.1 (125) | 27.8 ± 15.5 (72) | 25.3 ± 14.3 (25) | 6.3 ± 5.4 (28) |
| Vanadium (V) | 8.1± 4.4 (125) | 9.9 ± 3.4 (72) | 8 ± 3.7 (25) | 3.5 ± 3.9 (28) |
| OP (% AA depletion/μg CAP in incubation well)a | 0.63 ± 0.72 (76) | 0.80 ± 0.39 (42) | 0.77± 0.34 (8) | 0.33± 1.07 (26) |
OP, oxidative potential. AA, ascorbic acid. CAP, concentrated ambient particles.
RESULTS
In total 53 participants were enrolled, including 28 females and 25 males. They self-identified as Asian (44%), Caucasian (42%) or other (14%). Their mean age (± standard deviation) was 28 years (± 9) and their mean BMI (± standard deviation) was 23.2 kg/m2 (± 2.7). Forty participants had more than one exposure to CAPs.
Table 1 presents the concentrations of total mass, selective metals, and OP in coarse, fine and ultrafine CAPs, and in all CAP exposures combined. Ninety-two percent of the coarse, fine and ultrafine PM filters were available for determining elemental constituents. Metals shown in Table 1 are those that have demonstrated consistent associations with biomarkers. Full list of results on all elements can be found in Supplementary Tables S1–S6. Filters of 35 coarse CAP, seventeen fine CAP and 4 ultrafine CAP exposures representing 44% of the total samples were unavailable for OP assays. Fifty-six percent of the GSH depletion values were below the limit of detection. For the samples with GSH depletion values above the limit of detection, the mean (± standard deviation) was 1.24% (± 0.62%) GSH depletion/μg CAP in incubation well. We therefore used GSH depletion as a binary variable (values above versus below the limit of detection) in statistical analyses.
Table 2 lists correlation coefficients between OP (measured as AA depletion) and metals in coarse, fine and ultrafine CAPs, and all CAP exposures combined. OP appears to have a stronger correlation with metals in fine and ultrafine CAPs compared to coarse CAP. AA-related OP was not correlated with PM mass (r=0.09).
Table 2.
Spearman correlation coefficients between metals (ng/μg CAP) and oxidative potential (OP) measurements (% AA depletion/μg CAP in incubation well).
| OP (% AAa depletion/μg CAP in incubation well) | ||||
|---|---|---|---|---|
| Element (ng/μg CAPb) | All CAPs combined (n=75) | Coarse CAP (n=42) | Fine CAP (n=8) | Ultrafine CAP (n=25) |
| Ag | 0.26* | 0.19 | −0.12 | 0.46* |
| Al | 0.31* | −0.18 | 0.60 | 0.16 |
| Ba | 0.46* | 0.18 | 0.64 | 0.33 |
| Cu | 0.54* | 0.14 | 0.62 | 0.70* |
| Fe | 0.45* | 0.14 | 0.74* | 0.27 |
| K | 0.37* | −0.11 | 0.76* | 0.38 |
| Li | 0.36* | −0.17 | 0.71* | 0.34 |
| Ni | 0.21 | −0.02 | 0.48 | 0.30 |
| Sn | 0.51* | 0.26 | 0.43 | 0.57* |
| V | 0.37* | −0.05 | 0.74* | 0.33 |
p<0.05.
AA, ascorbic acid
CAP, concentrated ambient particles
Table 3 presents percentage change in blood biomarkers relative to pre-exposure levels after exposure to metals in all CAPs, adjusted for particle size and mass concentration in the models, with a sample size of 125 observations. Ag, Ba, K, Ni, and Sn were significantly positively associated with certain blood biomarkers including VEGF, MDA, NSE, UCHL1, cortisol or BDNF respectively, at 1-hr or 21-hr post exposure. Ag, Cu and V were significantly negatively associated with ET-1 21-hr post exposure. There was also a trend towards a positive association between Fe and several blood biomarkers (MDA, UCHL1 and BDNF), as well as for V with NSE (p<0.1), but the confidence intervals included the null value, indicative of large uncertainty.
Table 3.
Mean percent change (95% confidence interval) in blood biomarkers post exposure to metals at the mean exposure concentration. Percent change was calculated using the equation: [(post-exposure value minus pre-exposure value)/pre-exposure value]*100. Models were adjusted for age, sex, BMI, season, type of particulate matter, mass concentration.
| Biomarker | Time post exposure |
Ag | Al | Ba | Cu | Fe | K | Li | Ni | Sn | V |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ET1 | 1-hr | 0.0 (−1.7, 1.8) | −15.6 (−37.4, 6.1) | −5.1 (−20, 9.7) | 1.0 (−7.0, 8.9) | −11.2 (−32.7, 10.4) | −2.88 (−19.7, 13.9) | −9.0 (−31, 13) | −4.7 (−9.4, 0.0)* | −5.3 (−18.8, 8.2) | −16.7 (−36.1, 2.7)* |
| 21-hr | −1.1 (−2, − 0.1)** | −4.5 (−17.0, 7.9) | −2.9 (−11, 5.8) | −4.6 (−9.0, − 0.2)** | −6.3 (−19.0, 6.4) | −0.57 (−10.0, 8.9) | −2.1 (−15, 10) | −1.7 (−4.4, 0.9) | −5.6 (−13.4, 2.2) | −14.0 (−24.9, −3.1)** | |
| IL6 | 1-hr | −3.4 (−6.8, 0.0)* | −4.6 (−46.3, 37.1) | −6.0 (−35.9, 23.8) | −11.9 (26.9, 3.1) | −11.5 (55.1, 32.1) | −5.4 (−36.7, 25.9) | 0.3 (−42.9, 43.4) | −4.5 (−13.5, 4.4) | 2.4 (−24.4, 29.2) | −30.4 (−68.2, 7.4) |
| 21-hr | −0.4 (−5.2, 4.5) | 29.2 (−29.7, 88.1) | 11.0 (−30, 52.0) | −7.9 (−29.2, 13.4) | 20 (−40.3, 80.3) | 18.7 (−26.1, 63.4) | 9.4 (−51, 70) | −4.8 (−17.5, 7.9) | 12.7 (−24.8, 50.2) | −6.4 (−59.6, 46.9) | |
| CRP | 1-hr | −18 (−68, 32.4) | 213 (−405, 831) | −88.0 (−525, 349) | 33.0 (−191, 257) | −109 (−746, 27) | 124 (−345, 593) | 68 (−568, 705) | −31 (−164, 103) | −150 (−543, 243) | −231 (−793, 331) |
| 21-hr | −7.2 (−20.7, 6.2) | 213 (−404, 831) | −18.7 (−128, 90.4) | 33.0 (−190, 257) | −109 (−746, 527) | 123 (−345, 592) | 19.1 (−144, 183) | −30 (−163, 102) | −150 (−543, 242) | −230 (−792, 331) | |
| VEGF | 1-hr | −0.4 (−2.3, 1.5) | −0.1 (−23.4, 23.2) | 3.7 (−12, 20.0) | 3.2 (−5.3, 11.6) | 7.9 (−15.6, 31.3) | 9.77 (−7.9, 27.4) | 3.4 (−20, 27) | 5.3 (0.3, 10.2)** | 0.0 (−14.6, 14.6) | −6.1 (27.1,14.9) |
| 21-hr | −1.5 (−3.6, 0.7) | 9.6 (−16.6, 35.9) | 10.3 (−8.6, 29.0) | 1.3 (−8.0, 10.7) | 9.9 (−17.8, 37.6) | 10.5 (−8.7, 29.7) | 2.4 (−24, 29) | −0.5 (−6.3, 5.3) | 3.8 (−13.1, 20.6) | −15.6 (−38.7, 7.5) | |
| MDA | 1-hr | 2.1 (−0.6, 4.7) | 7.6 (−26.2, 41.3 | −15.0 (−39, 8.5) | −6.6 (−18.7, 5.5) | −24.6 (−59.6, 10.3) | −7.29 (−31.8, 17.2) | 5 (−29, 39) | −1.5 (−8.9, 5.8) | −5.9 (−27.5, 15.6) | −3.8 (−33.9, 26.4) |
| 21-hr | 1.9 (−1.5, 5.2) | −0.3 (−41.7, 41.2) | 2.0 (−2.7, 54)* | 5.2 (−9.7, 20.2) | 35.6 (−5.6, 76.7)* | −22.8 (−53.9, 8.3) | 13 (−28, 56) | 5.8 (−3.1, 14.6) | 40.7 (15.6, 65.8)** | 3.8 (−33.4, 41.0) | |
| S100 | 1-hr | 0.4 (−0.5, 1.4) | −0.3 (−12.5, 11.9) | 2.7 (−5.9, 11.0) | 0.7 (−3.7, 5.2) | 4.5 (−8.1, 17.0) | 3.55 (−5.2, 12.3) | −1.2 (−13, 11) | 0.6 (−2.1, 3.3) | 2.8 (−4.9, 10.6) | 0.7 (−10.1, 11.4) |
| 21-hr | 0.5 (−0.7, 1.6) | 2.5 (−11.5, 16.4) | 2.0 (−7.8, 12.0) | −0.3 (−5.4, 4.7) | −2 (−16.5, 12.4) | 3.77 (−6.7, 14.3) | 9.5 (−4.7, 23) | −1.3 (−4.3, 1.7) | 2 (−6.9, 10.9) | −0.8 (−13.4, 11.8) | |
| NSE | 1-hr | 0.2 (−1.8, 2.1) | 6.3 (−17.4, 29.9) | 6.7 (−10.0, 24.0) | −0.1 (−8.5, 8.4) | 11.7 (−13.4, 36.8) | 3.77 (−14.1, 21.6) | −6.8 (−31, 18) | 1.1 (−4.1, 6.3) | 0.1 (−15.2, 15.5) | 20.1 (−1.0, 41.3)* |
| 21-hr | 0 (−1.3, 1.3) | 4.4 (−11.4, 20.1) | 5.9 (−5.4, 17.0) | −1.2 (−6.8, 4.4) | 5.0 (−11.5, 21.6) | 10.7 (−1.0, 22.3)* | 1.1 (−15.3, 17) | 1.6 (−1.8, 5.0) | 5.0 (−5.2, 15.2) | 2.4 (−11.8, 16.6) | |
| UCHL1 | 1-hr | 0.3 (−0.9, 1.5) | 11 (−3.1, 25.1) | 11.0 (1.2, 21.0)** | 0.9 (−4.2, 6.0) | 13.8 (−0.9, 28.5)* | 21.9 (12.0, 31.9)** | 8.6 (−6.1, 23) | 1.4 (−1.7, 4.5) | 9.9 (0.9, 18.9)** | 7.7 (−5.0, 20.5) |
| 21-hr | 0.4 (−1.2, 2.1) | 19.3 (−0.9, 39.5)* | 14.0 (0.3, 29.0)** | 3.0 (−4.2, 10.3) | 19.8 (−1.1, 40.8)* | 34.6 (20.8, 48.5)** | 17 (−3.6, 38) | 1.0 (−3.4, 5.3) | 14.0 (1.1, 26.9)** | 10.4 (−7.7, 28.5) | |
| Cortisol | 1-hr | 1.5 (0.0, 2.9)** | −1.3 (−18.8, 16.2) | −1.3 (−13.0, 11.0) | 3.0 (−3.5, 9.4) | 0.6 (−17.2, 18.4) | 1.67 (−10.6, 14.0) | 0.4 (−17, 17) | 2.1 (−1.8, 6.1) | −1.1 (−12.1, 9.9) | 1.5 (−13.6, 16.7) |
| 21-hr | 1.5 (0.1, 2.8)** | 3.6 (−13.2, 20.4) | 7.5 (−4.6, 19.0) | 1.8 (−4.2, 7.8) | 14.3 (−3.2, 31.9) | 9.32 (−3.3, 21.9) | 3.3 (−14, 21) | 2.5 (−1.1, 6.1) | 8.5 (−2.4, 19.3) | −1.3 (−16.5, 13.9) | |
| BDNF | 1-hr | −1.8 (−3.5, − 0.1)** | −0.6 (−22.2, 20.9) | 8.2 (−7.0, 23.0) | −4.3 (−12.1, 3.4) | 11.3 (−11.0, 33.6) | −1.05 (−16.9, 14.8) | 15 (−6.8, 37) | −1.2 (−5.8, 3.5) | 3.8 (−10.0, 17.5) | 13.8 (−5.4, 33.0) |
| 21-hr | −1.1 (−2.5, 0.3) | 1.9 (−15.6, 19.4) | 12.6 (0.2, 25.0)** | 1.8 (−4.5, 8.1) | 17.6 (−0.5, 35.7)* | 1.4 (−11.7, 14.6) | 14.9 (−3.1, 32.8) | −1 (−4.8, 2.8) | 6.4 (−4.8, 17.7) | 8.3 (−7.4, 24.1) |
P<0.1.
P<0.05
Bold font is for a demonstration of statistical significance levels at p<0.1 and p<0.05.
Regression results for metals across all CAP exposures and urinary biomarkers are shown in Table 4, with sample sizes varying between 123 and 124, depending on the variables. The most consistent findings for these regression results were significantly positive associations of Al, K and Li with VMA, Ni and V with HVA, and Li and V with cortisol, at 1-hr and/or 21-hr post exposure.
Table 4.
Percent change (95% confidence interval) in urinary biomarkers post exposure to metals at the mean exposure concentration. Percent change was calculated using the equation: [(post-exposure value minus pre-exposure value)/pre-exposure value]*100. Models were adjusted for age, sex, BMI, season, type of particulate matter, mass concentration.
| Biomarker | Time post exposure | Ag | Al | Ba | Cu | Fe | K | Li | Ni | Sn | V |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VEGF | 1 hr | 4.5 (−41.6, 50.5) | 238 (−324, 799) | 54.4 (−349, 458) | 40 (−162, 242) | 347 (−237, 931) | 128 (−298, 554) | 135 (−446, 717) | 22 (−99, 144) | 174 (−187, 534) | 151 (−357, 659) |
| 21 hr | 3.0 (−19.9, 25.8) | −7.9 (−285, 269) | −8.6 (−210, 193) | 15.7 (−82.9, 114) | 25.8 (−267, 319) | −99.6 (−308, 108) | −41.5 (−329, 246) | −3.9 (−64.3, 56.5) | 103 (−75.6, 281) | 161 (−87.1, 410) | |
| 8−OHdG | 1 hr | 0.3 (−1.6, 2.2) | 6.6 (−16.0, 29.1) | 2.6 (−13.8, 19.0) | 13.9 (6.4, 21.3)** | −5.2 (−29.2, 18.9) | 7.20 (−9.8, 24.2) | −8.4 (−32.1, 15.3) | −0.1 (−5.1, 4.9) | 3.1 (−11.4, 17.7) | −18.3 (−38.4, 1.9)* |
| 21 hr | −0.2 (−2.2, 1.9) | −2.4 (−27.0, 22.1) | 1.8 (−15.3, 18.9) | −0.6 (−9.8, 8.5) | −5.4 (−30.3, 19.6) | −5.66 (−22.5, 11.2) | −6.4 (−30.5, 17.7) | 3.8 (−1.9, 9.5) | 5.0 (−10.3, 20.3) | −13.4 (−34.1, 7.4) | |
| MDA | 1 hr | −0.6 (−3.9, 2.7) | 27.4 (−13.9, 68.7) | −12.9 (−42.9, 17.0) | −2.9 (−17.9, 12.1) | −14.8 (−58.6, 29) | 7.7 (−20.3, 35.7) | 26.4 (−13.8, 66.6) | 0.7 (−9.0, 10.4) | −14.8 (−41, 11.4) | 1.9 (−33, 36.9) |
| 21 hr | −0.7 (−4.8, 3.4) | 28.5 (−21.2, 78.2) | −8.2 (−44.1, 27.8) | −4.5 (−22.5, 13.4) | −2.8 (−55.2, 49.6) | 17.0 (−20.6, 54.7) | 20.8 (−30.8, 72.4) | 1.9 (−9.0, 12.7) | −7.0 (−39.2, 25.1) | 6.1 (−39.1, 51.3) | |
| VMA | 1 hr | 0.7 (−1.4, 2.9) | 28.6 (3.0, 54.2)** | −2.2 (−20.9, 16.5) | −3.2 (−12.6, 6.1) | 1.8 (−25.4, 29.0) | 26.3 (7.1, 45.4)** | 27.0 (0.5, 53.5)** | 1.0 (−4.6, 6.6) | −0.6 (−17.4, 16.1) | 8.4 (−15.1, 32.0) |
| 21 hr | 0.2 (−2, 2.3 | 28.0 (1.8, 54.3)** | −12.6 (−31.1, 5.9) | −2.7 (−12.3, 6.9) | −11.4 (−38.6, 15.8) | 25.0 (5.3, 44.7)** | 38.8 (12.3, 65.2)** | 1.3 (−4.5, 7.0) | −5.7 (−22.6, 11.3) | 18.5 (−5.4, 42.3) | |
| HVA | 1 hr | 1.7 (−2, 5.3) | 29.5 (−19.9, 78.9) | 5.0 (−34.6, 44.5) | 6.5 (−11.7, 24.8) | −0.1 (−58.7, 58.4) | 24.8 (−3.6, 53.2)* | 39.1 (−6.3, 84.4) | 21.2 (5.1, 37.3)** | −1.4 (−35.6, 32.8) | 35.9 (2.2, 69.6)** |
| 21 hr | −0.2 (−5.2, 4.8) | −7.9 (−68.8, 53.0) | −17.4 (−61.2, 26.4) | −1.7 (−23.6, 20.2) | −24.1 (−88.0, 39.8) | −14.6 (−60.5, 31.2) | 32.6 (−30.4, 95.6) | 8.9 (−4.2, 22.0) | −3.9 (−43.2, 35.5) | 53.2 (−1.3, 107.7)* | |
| Cortisol | 1 hr | −1.3 (−9.4, 6.9) | −17.4 (−118, 82.7) | 65.1 (−6.0, 136)* | 18.4 (−17.1, 54.0) | 71.6 (−32.3, 175.5) | −15.5 (−89.7, 58.8) | 51.7 (−50.4, 154) | −6.6 (−28.3, 15.1) | 38.4 (−25.4, 102.1) | 88.4 (0.9, 176.0)** |
| 21 hr | 0.5 (−6.8, 7.8) | 40.4 (−47.1, 127.9) | −7.5 (−64.7, 49.8) | −5.4 (−36.8, 25.9) | −0.2 (−82.9, 82.5) | 10.3 (−52.2, 72.8) | 86.5 (0.4, 173)** | −9.1 (−28.6, 10.4) | −14.1 (−66.5, 38.4) | 65.2 (−8.7, 139.2)* |
P<0.1.
P<0.05
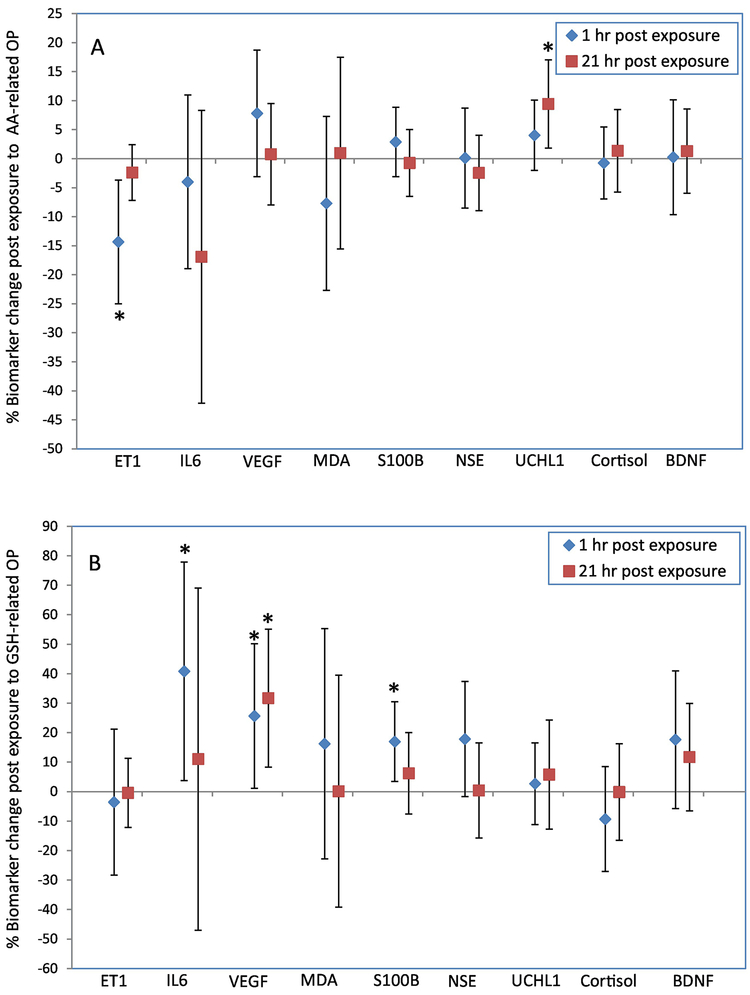
Figure 1 illustrates the associations between exposure to OP and percent change (95% confidence interval) in blood biomarkers adjusted for PM size and mass concentration, with a sample size of 74–76 observations depending on the variables. Numerical values can be found in Table S7. AA-related OP was significantly associated with a 9.4% increase in UCHL1 (1.8%, 17%) at 21-hr post exposure, but a 14% decrease in ET-1 (−25%, −3.7%) 1-hr post exposure. GSH-related OP at levels above the limit of detection was significantly associated with IL-6 [41% increase (3.8%, 78%), VEGF 26% increase (1.1%, 50%) and S100B [17% increase (3.4%, 30%)] at 1-hr post exposure. NSE at 1-hr post exposure also exhibited a trend towards positive associations with GSH-related OP [18% increase (−1.7%, 37%), p<0.1], but the confidence intervals included the null value, suggesting a large degree of uncertainty for this association. Regression results for CRP at 21-hr post exposure were a change of 63.8% (−25.7%, 153%) for an increase of AA depletion to the mean level, and a change of −80.6% (−292%, 131%) for a GSH depletion level above the limit of detection. Results for CRP at 1-hr post exposure were not available due to model convergence issue, likely because of a small sample size.
Figure 1.
Percentage change (95% confidence interval) in blood biomarkers 1-hr and 21-hr post exposure to AA-related OP at the mean exposure level (1A), and to GSH-related OP at levels above the limit of detection (1B). Percent change was calculated using the equation: [(post-exposure value minus pre-exposure value)/pre-exposure value]*100. Models were adjusted for age, sex, BMI, season, type of CAPs, mass concentration. *, p<0.05.
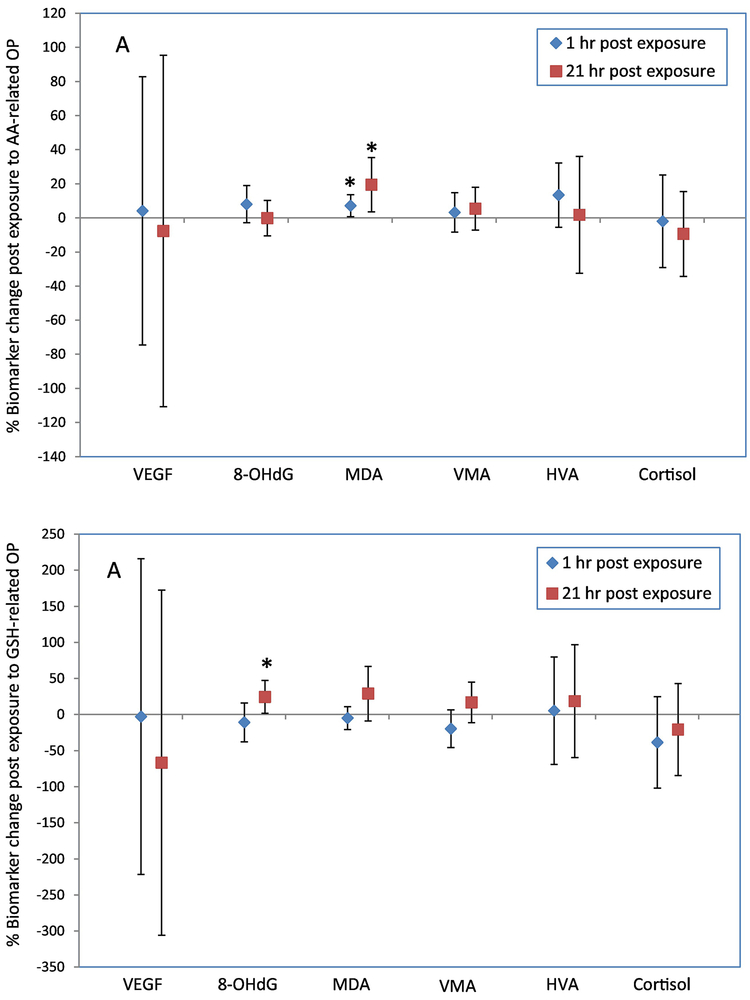
The associations between exposure to OP and percent change (95% confidence interval) in urinary biomarkers are presented in Figure 2, with a sample size of 76 observations. Numerical values are reported in Table S8. AA-related OP was significantly associated with urinary MDA [7.1% increase (0.65%, 13.6%) and 19% increase (3.6%, 35%) at 1-hr and 21-hr post exposure, respectively], but not with any other biomarkers. GSH-related OP (for levels above the limit of detection) was significantly associated with urinary 8-OHdG [24% increase (1.6%, 47%)] 21-hr post exposure.
Figure 2.
Percentage change (95% confidence interval) in urinary biomarkers 1-hr and 21-hr post exposure to AA-related OP at the mean exposure level (2A), and to GSH-related OP at levels above the limit of detection (2B). Percent change was calculated using the equation: [(post-exposure value minus pre-exposure value)/pre-exposure value]*100. Models were adjusted for age, sex, BMI, season, type of CAPs, mass concentration. *, p<0.05.
The mass concentration of CAP was significantly associated with 97% increase in blood MDA (39%, 155%) in models adjusted for AA-related OP, as well as 96% increase in MDA (35%, 157%) in models adjusted for GSH-related OP (Table S9). CAP mass concentration was also significantly associated with 52% increase in urinary 8-OHdG in models adjusted for AA-related OP or GSH-related OP (Table S10). Mass concentration was not significantly associated with other biomarkers.
DISCUSSION
In this study we sought to determine whether or not metal constituents in urban PM and their oxidative potential were associated with systemic biomarkers of inflammation, oxidative stress, and neural function. The results demonstrate that exposure to certain metals (Ag, Al, Ba, K, Li, Ni, Sn, and V) and OP of PM were significantly associated with increased inflammatory markers IL-6 and VEGF, oxidative stress markers 8-OHdG and MDA, and neural markers S100B, NSE, UCHL1, VMA, HVA and cortisol, and decreased inflammatory marker ET-1. These associations were robust after the adjustment for PM size and mass concentration in models. This suggests that the effects of metals and oxidative potential on biomarkers were independent of PM size and mass concentrations. Many of the biomarker changes were acute and started almost immediately post exposure, some lasted for 21 hours. The peak response time for biomarkers varied, likely due to various factors such as the location of production, the protein generation process, and the breakdown time of metabolites. We previously observed a significant influence of concentrated ambient PM mass on biomarkers of inflammation (increases in blood and urinary VEGF and a decrease in blood ET-1), oxidative stress (increases in urinary 8-OHdG and MDA) (Liu et al. 2015), and neural biomarkers (increases in blood UCHL1 and urinary VMA, and variations in blood and urinary cortisol) (Liu et al. 2017) in study participants. Although biological constituents endotoxin and β-glucan were contributing factors for these biomarker changes, they did not account for all of the effects of the PM. It should be noted that although the biomarkers we utilized for this study have been proposed as risk factors for certain health conditions, many of them have not been used for clinical disease diagnosis. Taken together, the results in the present study suggest that oxidative potential and metal species in urban PM may influence systemic inflammation, oxidative stress, and perturbation in biomarkers of neural function.
Adverse health effects of transition metals in PM have been relatively well studied in experimental animals and human population (Bell and HEI Health Review Committee 2012; Lippmann et al. 2013), as summarized by Chen and Lippmann (Chen and Lippmann 2009). In a panel study, Cakmak et al. followed 59 young healthy non-smoking participants for 10 days in a small Canadian city that had an industrial steel mill (Cakmak et al. 2014). The researchers observed significant associations between metal constituents such as Ca, Cd, Li, Pb, Sn, Sr, V and Zn in ambient PM2.5 and increases in heart rate and blood pressure, and/or decreases in lung function. In another panel study conducted in Montreal (Canada), Godri Pollitt et al. studied 70 school children with doctor-diagnosed asthma for 10 consecutive days (Godri Pollitt et al. 2016). The researchers collected data on daily personal exposure to PM2.5 and trace metals, and measured daily fractional exhaled nitric oxide levels to determine lung inflammation. They noted significant associations between the fractional exhaled nitric oxide and trace metals Ba and V, as well as Al and Fe. Concentrated ambient particles have been used in toxicological studies in which metals such as Al, Fe, V, and Ni in concentrated ambient particles caused significant increase in pulmonary inflammatory cells in dogs (Clarke et al. 2000), and oxidative stress in rats (Gurgueira et al. 2002). Few controlled human exposure studies have reported effects of metal constituents in PM. Huang et al. studied the inflammatory effects of water-soluble components of concentrated fine PM (size 0.1–2.5 μm, median concentration 72 μg/m3) on 37 healthy individuals exposed via inhalation for 2 hours in Chapel Hill, North Carolina (Huang et al. 2003). The researchers conducted principal component analysis and identified two factors that were highly correlated with CAP: sulfate/Fe/Se, a factor indicative of soil minerals, and Cu/Zn/V, a factor of various combustion processes. They reported significant associations between the sulfate/Fe/Se factor and increased percentage of neutrophils in bronchoalveolar lavage, and between the Cu/Zn/V factor and increased blood fibrinogen. Schaumann et al. conducted an experiment in which 100 μg of PM2.5 suspensions, collected simultaneously from a German metal smelter area and a non-industrialized area, were instilled through a bronchoscope into contralateral lung segments of 12 healthy volunteers (Schaumann et al. 2004). PM2.5 from the smelter area contained much higher concentrations of metals such as Cu, Cd, Cr, Fe, Ni and Zn compared to the non-industrialized area, and induced a significantly higher influx of monocytes. Instillation of metal-rich particles also resulted in a significant increase in oxidant radical production in bronchoalveolar lavage cells and proinflammatory cytokines IL-6 and tumor necrosis factor-α in the lavage. Our research findings are largely consistent with published reports demonstrating associations between exposure to metals of PM and biochemical and physiological changes in the body.
Reports on the adverse health effects of OP of ambient PM are still emerging. Associations of GSH depletion in personal PM2.5 samples were found with increased exhaled nitric oxide in school children with asthma in Montreal (Canada) (Maikawa et al. 2016), and associations of DTT oxidation in PM2.5 were noted with increased emergency department visits for asthma and congestive heart failure in Atlanta (USA) (Bates et al. 2015). Both of these studies have shown that OP of urban PM had significantly stronger associations with health outcomes than did PM mass concentrations. Tonne et al. investigated the effects of OP (GSH depletion) in PM10 on carotid intima-media thickness in a group of British participants (Tonne et al. 2012). They reported a significantly positive association between carotid intima-media thickness and the product term of PM10*OP, as well as PM10 mass, but not OP alone. Strak et al. studied a panel of healthy adult volunteers, in the Netherlands who, on separate occasions, spent time at a location with high traffic, an urban background location, an underground train station, and a farm (Strak et al. 2012). Air pollution concentrations including OP (GSH and AA depletion) of PM10 at these sites were measured. The researchers did not find significant associations between PM mass or OP and volunteers’ level of exhaled nitric oxide or lung function, although particle counts and nitrogen dioxide were significantly associated with adverse changes in lung inflammation and lung function. A birth cohort study in the Netherlands also reported significant associations between children’s exposure to OP (DTT oxidation) of PM2.5 and increased asthma incidence, prevalence of asthma symptoms and rhinitis, and decreased lung function (Yang et al. 2015a). Weichenthal et al. conducted a series of epidemiological studies using OP measured in PM2.5 as an exposure measurement. They studied emergency department visits for myocardial infarction (Weichenthal et al. 2016b) and respiratory diseases (Weichenthal et al. 2016a) between 2004 and 2011 in Canadian cities located in the province of Ontario using a case-crossover design. Their results show that between-city differences in OP levels (GSH but not AA depletion) in PM2.5 were significantly associated with increased emergency department visits for myocardial infarction, all respiratory illnesses, and asthma. These authors also investigated the influence of OP in PM2.5 on long-term mortality risk in Ontario (Canada) between 1991 and 2009 using Cox proportional hazard models adjusting for individual-level covariates and indirect-adjustment for cigarette smoking and obesity (Weichenthal et al. 2016a). They reported significant associations between GSH-related OP (but not AA-related OP) and cause-specific mortality risks, including lung cancer (12% increase per interquartile range of OP), cardio-metabolic mortality (3% increase) and total non-accidental death (3% increase). Interquartile range of PM2.5 concentration was associated with 5.0% increase in lung cancer mortality, a weaker association than for OP. In our present study, PM mass concentration in models adjusted for OP was significantly positively associated with blood MDA and urinary 8-OHdG, but not with other biomarkers, suggesting that PM mass, compared to OP, might have less effect on biomarkers of inflammation and neural function. To the best of our knowledge, the present controlled human exposure study appears to be the first to report the influence of OP on inflammatory and neural biomarkers. This work provides supporting evidence for the epidemiological literature on the adverse human health effects of oxidants found in ambient particulate matter.
There were some commonalities between OP and metals with regards to the effects on biomarkers: OP and some metals had significantly positive associations with blood VEGF (with Ni), UCHL1 (with Ba, K and Sn, marginally significant with Al and Fe), and negative associations with ET-1 (with Ag, Cu and V). On the other hand, significant effects on systemic stress markers VMA, HVA, and cortisol were only seen in metals, while significant effects on blood IL-6 and S100B were only seen for OP. The moderate correlation coefficients between OP and metals suggest that transition metals only partially contributed to the production of OP in these CAPs. These results suggest that metals may influence these stress biomarkers via a different mechanism(s), while other reactive chemical compounds in PM such as organic oxidants may also contribute to OP and its toxic potency.
One limitation of this study is that a subset of the PM filters was not available for OP measurements, which may have resulted in a reduced statistical power to detect effects of OP. The CAP concentrations used in this study (mean 198 μg/m3) were relatively high compared to ambient levels in countries such as Canada, the United States and western European countries. However, such concentrations are frequently experienced by a sizable population around the world. For example, Delhi (India) in 2014 had an annual average PM2.5 of 122 μg/m³, with daily spikes reaching above 600 μg/m³ per hour (Subramanian 2016). We studied the effects of 36 elements and 2 measures of OP on 16 biomarkers. As this large number of regression analyses could potentially introduce spurious statistically significant associations by multiple comparisons, we minimized the risk of false positive associations by reporting only consistent results in this paper. As discussed above, our results are in line with animal toxicological studies that reported significant associations between metal contents in CAP and systemic inflammatory and oxidative stress biomarkers (Chen and Lippmann 2009), as well as changes in cardiovascular physiology (Lippmann et al. 2013). Large scale epidemiological studies over 150 US cities/187 counties have shown risks of mortality and hospitalizations associated with elemental contents in PM2.5 (Bell and HEI Health Review Committee 2012; Lippmann et al. 2013). Nevertheless, given the exploratory nature of this study, we interpret the findings with caution. Further studies are warranted to verify these results.
In conclusion, this exploratory study shows that in a short period of exposure to concentrated ambient PM, some of the metals and OP measured in the PM were significantly associated with increased blood and urinary biomarkers indicative of systemic inflammation, oxidative stress, perturbations of blood-brain barrier integrity, and/or systemic stress in healthy volunteers. Metals may have partially contributed to the generation of OP in the particles and the adverse effects of OP, but they appear to have other unique properties which may explain their specific effects on biomarkers of systemic stress. These results provide supporting evidence to explain how metals and OP in ambient PM may affect disease development and mortality observed in epidemiological studies.
Supplementary Material
HIGHLIGHT.
Healthy volunteers were exposed to concentrated urban particles with varying metal contents and oxidative potential
After exposure, biomarkers in blood and urine samples were determined
Metals and oxidants in urban particles may influence systemic inflammation, oxidative stress, neural function, and stress
ACKNOWLEDGMENTS
The authors wish to thank the staff at the Division of Occupational and Environmental Health, University of Toronto, for their technical work on controlled exposures; Yasmin Dirieh at Health Canada for her work on biomarkers; Drs. Robert Dales and Scott Weichenthal and Ms. Julie Andrade of Health Canada for their wise critique on the manuscript.
Funding: This work was supported by Health Canada’s Clean Air Regulatory Agenda, USEPA RD-83241601, NIH Grant P30 ES005605, Environment and Climate Change Canada, and AllerGen Networks of Centres of Excellence. Infrastructure for concentrated ambient particle exposure facility was provided by SOCAAR through funding from the Canada Foundation for Innovation. The contents of this publication are solely the responsibility of the grantee and do not necessarily represent the official views of the funding agencies. The funding agencies do not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Anderson JL, Carlquist JF, Muhlestein JB, Horne BD, Elmer SP. 1998. Evaluation of C-reactive protein, an inflammatory marker, and infectious serology as risk factors for coronary artery disease and myocardial infarction. J Am Coll Cardio 32: 35–41. [DOI] [PubMed] [Google Scholar]
- Ayres JG, Borm P, Cassee F, Castranova V, Donaldson K, Ghio A, et al. 2008. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential--a workshop report and consensus statement. Inhal Toxicol 20: 75–99. [DOI] [PubMed] [Google Scholar]
- Baker MA, Cerniglia GJ, Zaman A. 1990. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem 190: 360–365. [DOI] [PubMed] [Google Scholar]
- Bates JT, Weber RJ, Abrams J, Verma V, Fang T, Klein M, et al. 2015. Reactive oxygen species generation linked to sources of atmospheric particulate matter and cardiorespiratory effects. Environ Sci Technol 49: 13605–13612. [DOI] [PubMed] [Google Scholar]
- Behbod B, Urch B, Speck M, Scott JA, Liu L, Poon R, et al. 2013. Endotoxin in concentrated coarse and fine ambient particles induce acute systemic inflammation in controlled human exposures. Occup Environ Med 70: 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, HEI Health Review Committee. 2012. Assessment of the health impacts of particulate matter characteristics. Res Rep Health Eff Inst 161: 5–38. [PubMed] [Google Scholar]
- Blyth BJ, Farahvar A, He H, Nayak A, Yang C, Shaw G, et al. 2011. Elevated serum ubiquitin carboxy-terminal hydrolase L1 is associated with abnormal blood-brain barrier function after traumatic brain injury. J Neurotrauma 28: 2453–2462. [DOI] [PubMed] [Google Scholar]
- Borm PJA, Kelly F, Künzli N, Schins RPF, Donaldson K. 2007. Oxidant generation by particulate matter: from biologically effective dose to a promising, novel metric. Occup Environ Med 64: 73–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, AV Diez Roux, et al. 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378. [DOI] [PubMed] [Google Scholar]
- Cakmak S, Dales R, Kauri LM, Mahmud M, Van Ryswyk K, Vanos J, et al. 2014. Metal composition of fine particulate air pollution and acute changes in cardiorespiratory physiology. Environmental Pollution 189: 201–214. [DOI] [PubMed] [Google Scholar]
- Chen LC, Lippmann M. 2009. Effects of metals within ambient air particulate matter (PM) on human health. Inhalation Toxicology 21: 1–31. [DOI] [PubMed] [Google Scholar]
- Clarke RW, Coull B, Reinisch U, Catalano P, Killingsworth CR, Koutrakis P, et al. 2000. Inhaled concentrated ambient particles are associated with hematologic and bronchoalveolar lavage changes in canines. Environ Health Perspect 108: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crobeddu B, Aragao-Santiago L, Bui LC, Boland S, Baeza Squiban A. 2017. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ Pollut 230: 125–133. [DOI] [PubMed] [Google Scholar]
- Daher N, Saliba NA, Shihadeh AL, Jaafar M, Baalbaki R, Shafer MM, et al. 2014. Oxidative potential and chemical speciation of size-resolved particulate matter (PM) at near-freeway and urban background sites in the greater Beirut area. Science of the Total Environment 470–471: 417–426. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser M, Lundberg U, Rauste von Wright M, von Wright J, Sedvall G. 1986. Urinary monoamine metabolites as indices of mental stress in healthy males and females. Pharmacol Biochem Behav 24: 1521–1525. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Hata A, Niwa S-I, Hiramatsu K-I, Honda H, Nakagome K, et al. 1996. Plasma vanillylmandelic acid level as an index of psychological stress response in normal subjects. Psychiatry Res 63: 7–16. [DOI] [PubMed] [Google Scholar]
- Godri Pollitt KJ, Maikawa CL, Wheeler AJ, Weichenthal S, Dobbin NA, Liu L, et al. 2016. Trace metal exposure is associated with increased exhaled nitric oxide in asthmatic children. Environmental Health 15: 94 10.1186/s12940-016-0173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgueira SA, Lawrence J, Coull B, Murthy GGK, Gonzalez-Flecha B. 2002. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect 110: 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberzettl P, Lee J, Duggineni D, McCracken J, Bollanowski D, O’Tool TE, et al. 2012. Exposure to ambient air fine particulate matter prevents VEGF-induced mobilization of endothelial progenitor cells from the bone marrow. Environ Health Perspect 120: 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes WG, Ferro CJ, O’Kane KP, Somerville D, Lomax CC, Webb DJ. 1996. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation 93: 1860–1870. [DOI] [PubMed] [Google Scholar]
- Huang Y-CT, Ghio AJ, Stonehuerner J, McGee J, Carter JD, Grambow SC, et al. 2003. The role of soluble components in ambient fine particles-induced changes in human lungs and blood. Inhalation Toxicology 15: 327–342. [DOI] [PubMed] [Google Scholar]
- Janero DR. 1990. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Rad Biol Med 9: 515–540. [DOI] [PubMed] [Google Scholar]
- Kelly F, Anderson HR, Armstrong B, Atkinson R, Barratt B, Beevers S, et al. 2011. The impact of the congestion charging scheme on air quality in London. Part 2. Analysis of the oxidative potential of particulate matter. Res Rep Health Eff Inst 155: 73–144. [PubMed] [Google Scholar]
- Lewis SB, Wolper R, Chi YY, Miralia L, Wang Y, Yang C, et al. 2010. Identification and preliminary characterization of ubiquitin C terminal hydrolase 1 (UCHL1) as a biomarker of neuronal loss in aneurysmal subarachnoid hemorrhage. J Neurosci Res 88: 1475–1484. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Chen LC, Gordon T, Ito K, Thurston GD. 2013. National Particle Component Toxicity (NPACT) Initiative: integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Res Rep Health Eff Inst 177: 5–13. [PubMed] [Google Scholar]
- Liu L, Urch B, Poon R, Szyszkowicz M, Speck M, Gold DR, et al. 2015. Effects of ambient coarse, fine, and ultrafine particles and their biological constituents on systemic biomarkers: A controlled human exposure study. Environ Health Perspect 123: 534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Urch B, Szyszkowicz M, Speck M, Leingartner K, Shutt R, et al. 2017. Influence of exposure to coarse, fine and ultrafine urban particulate matter and their biological constituents on neural biomarkers in a randomized controlled crossover study. Environ,International 101: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maikawa C, Weichenthal S, Wheeler AJ, Dobbin N, Smargiassi A, Evans GJ, et al. 2016. Particulate oxidative burden as a predictor of exhaled nitric oxide in children with asthma. Environ Health Perspect 124: 1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan S, Eyre HA, Maes M, Baune BT, Jacka FN, Berk M. 2013. Exercising the worry away: How inflammation, oxidative and nitrogen stress mediates the beneficial effect of physical activity on anxiety disorder symptoms and behaviours. Neuroscience and Biobehavioral Reviews 37: 573–584. [DOI] [PubMed] [Google Scholar]
- Nel A 2005. Air Pollution-Related Illness: Effects of Particles. Science 308: 804–806. [DOI] [PubMed] [Google Scholar]
- Nishimoto N, Kishimoto T. 2006. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol 2: 619–626. [DOI] [PubMed] [Google Scholar]
- Park J-W, Floyd RA. 1992. Lipid peroxidation products mediate the formation of 8-hydroxydeoxyguanosine in DNA. Free Rad Biol Med 12: 245–250. [DOI] [PubMed] [Google Scholar]
- Rastogi N, McWhinney RD, Akhtar US, Urch B, Fila M, Abbatt JPD, et al. 2012. Physical characterization of the University of Toronto coarse, fine, and ultrafine high-volume particle concentrator systems. Aerosol Science and Technology 46: 1015–1024. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. 2000. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocrine Reviews 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Schaumann F, Borm PJA, Herbrich A, Knoch J, Pitz M, Schins RPF, et al. 2004. Metal-rich ambient particles (particulate matter2.5) cause airway inflammation in healthy subjects. Am J Respir Crit Care Med 170: 898–903. [DOI] [PubMed] [Google Scholar]
- Strak M, Janssen NA, Godri KJ, Mudway IS, Cassee FR, Lebret E, et al. 2012. Respiratory health effects of airborne particulate matter: the role of particle size, composition, and oxidative potential-the RAPTES project. Environ Health Perspect 120: 1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian M 2016. Can Delhi save itself from its toxic air? Nature 534: 166–169. [DOI] [PubMed] [Google Scholar]
- Tonne C, Yanosky JD, Beevers S, Wilkinson P, Kelly FJ. 2012. PM mass concentration and PM oxidative potential in relation to carotid intima-media thickness. Epidemiol 23: 486–494. [DOI] [PubMed] [Google Scholar]
- Weichenthal S, Crouse DL, Pinault L, Godri-Pollitt K, Lavigne E, Evans G, et al. 2016a. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian Census Health and Environment Cohort (CanCHEC). Environ Res 146: 92–99. [DOI] [PubMed] [Google Scholar]
- Weichenthal S, Lavigne E, Evans G, Pollitt K, Burnett RT. 2016b. Ambient PM2.5 and risk of emergency room visits for myocardial infarction: impact of regional PM2.5 oxidative potential: a case-crossover study. Environ Health 15: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal S, Lavigne E, Evans GJ, Godri Pollitt KJ, Burnett RT. 2016c. Fine particulate matter and emergency room visits for respiratory illness. Effect modification by oxidative potential. Am J Respir Crit Care Med 194: 577–586. [DOI] [PubMed] [Google Scholar]
- Weichenthal S, Pollitt KG, Villeneuve PJ. 2013. PM2.5, oxidant defence and cardiorespiratory health: a review. Environ Health 12 (doi: 10.1186/1476-069X-12-40): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Zhao Y-H, Feng X, Wittmeier A. 1996. Application of inductively coupled plasma mass spectrometry for total metal determination in silicon-containing solid samples using the microwave-assisted nitric acid–hydrofluoric acid–hydrogen peroxide–boric acid digestion system. Journal of Analytical Atomic Spectrometry 11: 287–296. [Google Scholar]
- Yang A, Janssen NAH, Brunekreef B, Cassee FR, Hoek G, Gehring U. 2015a. Children’s respiratory health and oxidative potential of PM2.5: the PIAMA birth cohort study. Occup Envrion Med 73: 154–160. [DOI] [PubMed] [Google Scholar]
- Yang A, Wang M, Eeftens M, Beelen R, Dons E, Leseman DLAC, et al. 2015b. Spatial Variation and Land Use Regression Modeling of the Oxidative Potential of Fine Particles. Environ Health Perspect 123: 1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Smith DH, Blennow K. 2013. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol 9: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Urch B, Speck M, Coull BA, Koutrakis P, Thorne PS, et al. 2015. Endotoxin and ß-1,3-d-glucan in concentrated ambient particles induce rapid increase in blood pressure in controlled human exposures. Hypertension 66: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Ito K, Lall R, Lippmann M, Thurston G. 2011. Time-series analysis of mortality effects of fine particulate matter components in Detroit and Seattle. Environ Health Perspect 119: 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.