Abstract
Background
Mycobacterium ulcerans causes necrotising infections of skin and soft tissue mediated by the polyketide exotoxin mycolactone that causes cell apoptosis and immune suppression. It has been postulated that infection can be eradicated before the development of clinical lesions but spontaneous resolution of clinical lesions has been rarely described.
Methodology/Principal findings
We report a case series of five Australian patients who achieved healing of small M. ulcerans lesions without antibiotics or surgery. The median age of patients was 47 years (IQR 30–68 years) and all patients had small ulcerative lesions (median size 144mm2, IQR 121-324mm2). The median duration of symptoms prior to diagnosis was 90 days (IQR 90–100 days) and the median time to heal from diagnosis without treatment was 68 days (IQR 63–105 days). No patients recurred after a median follow-up of 16.6 months (IQR 16.6–17.9 months) from the development of symptoms and no patients suffered long-term disability from the disease.
Conclusions
We have shown that healing without specific treatment can occur for small ulcerated M. ulcerans lesions suggesting that in selected cases a robust immune response alone can cure lesions. Further research is required to determine what lesion and host factors are associated with spontaneous healing, and whether observation alone is an effective and safe form of management for selected small M. ulcerans lesions.
Author summary
Mycobacterium ulcerans causes a destructive infection of skin and soft tissue known as Buruli ulcer that when severe can lead to serious long-term deformity and disability. It is currently not well documented whether people with Mycobacterium ulcerans disease can cure themselves without treatment. In our study we describe five people with small ulcers who cured their disease without specific medical or surgical treatment. This suggests that a proportion of people can develop an immune response sufficient enough to eradicate the disease without the help of medical intervention. This is an important step, as recognition of this possibility provides important further insights into the human immune response against the disease. It also opens the possibility to further studies that may determine characteristics of the organism and hosts that favour spontaneous healing of lesions. This knowledge may in turn improve efforts to prevent and control the disease which are currently lacking.
Introduction
Mycobacterium ulcerans causes a necrotising infection of skin and soft tissue known as Buruli ulcer (BU). If untreated it usually progresses, can result in major tissue destruction and be complicated by bone or joint infection.[1] In severe cases it may require plastic and reconstructive surgery and result in long-term disability.[2] The pathogenesis of M. ulcerans is mediated by a plasmid produced polyketide exotoxin called mycolactone which causes tissue destruction by inducing cell apoptosis.[3] It also allows persisting infection to develop by inhibiting dendritic cell function and secondarily T-cell activation,[4,5] as well as reducing the function of monocytes and macrophages by inhibiting cytokine production including tissue necrosis factors and gamma interferon.[6,7]
It has been postulated that infection can be eradicated before the development of clinical lesions,[8] and a partial protective effect of BCG in humans has been reported.[9] If true, these observations suggest that the hosts immune response can be protective against the development of BU, thought to be mediated via a protective T-helper-1 (TH1) cell mediated immune response.[10] Spontaneous resolution without medical or surgical treatment of clinical lesions in humans has been rarely reported.[11–13] Furthermore, in one of these studies involving five lesions from Africa the lesions were not bacteriologically confirmed to be M. ulcerans,[12] and in another study of a single lesion from Australia the lesion was surgically excised.[11] Recently Marion et al reported a case from Benin where a small nodular M. ulcerans lesion healed without medical or surgical intervention, as well as a small group of patients with active large M. ulcerans lesions who had separate scars suggestive of previously spontaneously healed large M. ulcerans lesions.[13] It is also unknown how often spontaneous resolution occurs and the factors associated with it. Therefore treatment is recommended for all M. ulcerans lesions.[14,15] The recommended first-line treatment is combination antibiotics for 8 weeks which is highly effective.[16,17] Wide surgical excision without antibiotics can be performed, with cure rates of greater than 90% if reserved for selected lesions with no risk factors for recurrence.[14,18] In a study from Africa, local heat application without antibiotics achieved high initial wound healing rates, but 18% of patients developed a recurrent lesion within 2 years.[19]
The endemic region of Victoria, Australia, is facing a worsening epidemic of M. ulcerans disease, with control efforts hampered by the limited understanding of transmission mechanisms to humans as well as the risk and mechanisms of disease development following exposure and infection.[20] Identifying that some patients can heal their disease without treatment, and the study of the factors that allowed them to do so, may provide insights that could aid the improved control of M. ulcerans disease. In this paper we report a case series of five Australian patients who achieved healing of their confirmed M. ulcerans lesions without recommended antibiotic regimens or surgery.
Methods
This was an observational study of routinely collected data from a clinical cohort of M. ulcerans patients managed at Barwon Health as previously described.[21] All patients were from the M. ulcerans endemic region of the Mornington and Bellarine Peninsulas in Victoria, Australia.[22] They were all diagnosed in 2017 on the basis of a positive IS2404 PCR for M. ulcerans.[23] The size of the lesion was determined by measuring with a ruler the diameter of induration in millimetres and calculating the surface area in millimetres squared. Patients were followed up on a 2 to 4 week basis until wound healing was achieved, and then at the end of the study period. Data on the type and frequency of wound dressings was not collected, although due to the small size of lesions, wound dressings were frequently not administered.
Results
The five cases of M. ulcerans disease all occurred in adults as single small ulcerative lesions ranging from 16 to 858 mm2 in size (median size 144mm2, IQR 121-324mm2). (Table 1). The median age of patients was 47 years (IQR 30–68 years) and there were 3 males and two females. No patients were known to be immune suppressed or have diabetes. HIV testing was not performed. The median duration of symptoms prior to diagnosis was 90 days (IQR 90–100 days). In one case an acid-fast bacilli (AFB) stain and culture for M. ulcerans were also positive.
Table 1. Characteristics of five M. ulcerans disease lesions that spontaneously healed.
| Patient Number | Age at diagnosis (years) | Duration of symptoms at diagnosis (days) | Gender | WHO Category | Lesion size at baseline (mm2) | Lesion site | Type of lesion | Diagnostic specimen | AFB Smear | PCR | Culture | Time to heal from diagnosis (days) | Follow-up time since symptoms developed (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | 90 | Male | 1 | 324 | ELBOW | ULCER | Swab | Positive | Positive | Positive | 105 | 22.3 |
| 2 | 28 | 100 | Male | 1 | 858 | BUTTOCK | ULCER | Biopsy | ND | Positive | ND | 63 | 17.9 |
| 3 | 69 | 90 | Male | 1 | 121 | LEG | ULCER | Swab | ND | Positive | ND | 68 | 16.6 |
| 4 | 47 | 120 | Female | 1 | 144 | FOOT | ULCER | Swab | Negative | Positive | Negative | 120 | 16.6 |
| 5 | 30 | 84 | Female | 1 | 16 | LEG | ULCER | Swab | ND | Positive | ND | 34 | 13.7 |
ND: Not done
Ethics statement
All patients gave informed oral consent to be managed with observation only and ethics approval for the study was provided by the Barwon Health Ethics Committee. All data were analysed anonymously.
Patient # 2 had an incisional biopsy but no other specific treatment. No other patients received recommended antibiotics or surgical treatment due to patient choice—in all 5 cases due to a reluctance to risk the toxicity of antibiotics or to undergo surgery in view of the small size of their M. ulcerans lesion. No patients were given heat treatment. The median time to heal from diagnosis was 68 days (IQR 63–105 days). (Figs 1 and 2) No patients had recurred after a median follow-up of 16.6 months (IQR 16.6–17.9 months) from the development of symptoms. No patients suffered long-term disability from the disease.
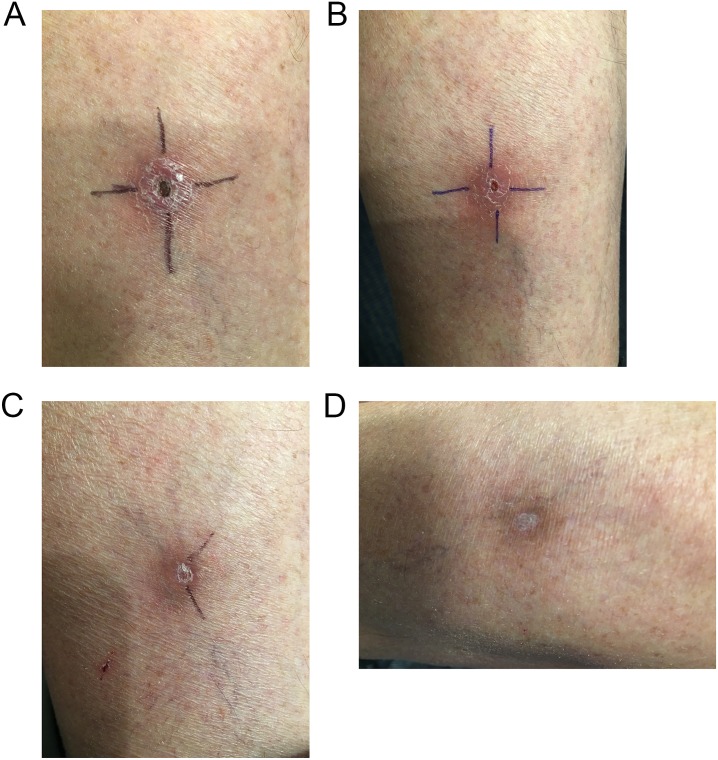
Fig 1. Spontaneous healing of M. ulcerans lesion left calf; a) at diagnosis, b) 2 months post diagnosis, c) 3 months post diagnosis, and d) 7 months post diagnosis.
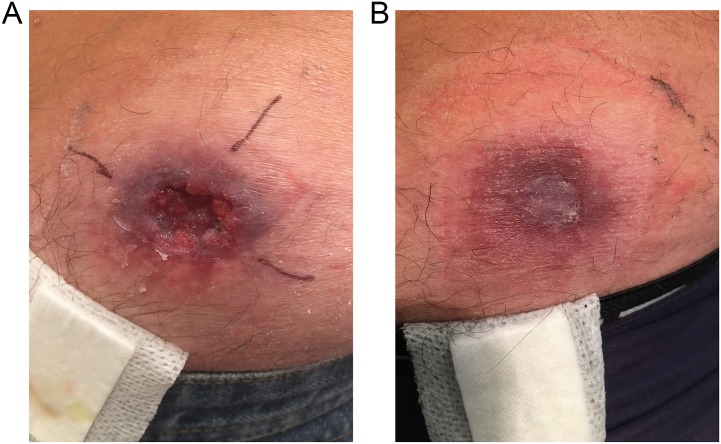
Fig 2. Spontaneous healing of M. ulcerans lesion buttock; a) at diagnosis, and b) 2 months post diagnosis.
One patient in the cohort, a 37 year old male diagnosed in 2017 by IS2404 PCR after 120 days of symptoms, was initially managed with observation alone but was changed to active treatment after 49 days of observation following an increase in size of his lesion from 154 mm2 to 340 mm2. He was subsequently cured with 4 weeks of rifampicin and clarithromycin antibiotic treatment combined with a surgical curette and did not suffer any long-term disability.
Discussion
This case series demonstrates that there are a proportion of patients with confirmed small ulcerative M. ulcerans lesions that spontaneously heal without specific antibiotic or surgical treatment. In our case series it is likely that all patients have been cured of their disease as they were followed for at least 14 months from the development of symptoms without evidence of relapse. We have previously demonstrated in Australian patients that disease relapse is rare more than 12 months following diagnosis and treatment.[24]
This suggests that in selected patients, the development of host immunity following the development of clinical disease may be effective in curing lesions. This presumably results from the host’s immune protection overcoming the immune suppressive effects of the mycolactone. This may relate to the development of a more robust TH1 immune response. The importance of the TH1 immune response in combating M. ulcerans disease is suggested by the fact that the expression level of gamma-interferon is inversely correlated with the severity of M. ulcerans lesions,[25] and gamma-interferon knockout mice developed more severe M. ulcerans disease with a greater numbers of organisms.[10]
It is notable that all our patients had symptoms for at least 84 days prior to diagnosis yet the lesions had remained small (<900mm2). This suggests lesions that have not progressed significantly in the first three months are exhibiting a degree of immune control that may allow spontaneous healing to occur. In addition, small lesions may have a lower number of organisms with less mycolactone production to inhibit the immune system, favouring the host’s immune response against the organism’s persistence. Observed factors in our cases series that may favour spontaneous resolution include small lesion size after at least approximately 90 days of symptoms, the lack of associated co-morbidities such as diabetes or malignancy that may impair the host’s immune response, and ulcerative lesions which allow the discharge of necrotic material that may contain live organisms and mycolactone. Our study is limited by the lack of further immunological testing of the host and biological testing of isolates and therefore we suggest further research be performed to examine host and pathogen factors associated with spontaneous healing of M. ulcerans disease. This will hopefully further enhance the understanding of human immune function against the organism which may in turn allow improved treatment of the disease. Furthermore, it may provide insights that allow the development of interventions that prevent disease post exposure, such as vaccination, an area for which the current lack of knowledge hampers disease control efforts.[20]
It is important to acknowledge that we have not performed a controlled trial comparing observation alone to active treatment of small ulcerative M. ulcerans lesions and therefore we cannot make conclusions about the safety and effectiveness of this approach as a mode of management. It is also important to understand that all patients had very small lesions that were not at risk of severe complications or disability without specific treatment—for larger lesions immediate antibiotic treatment is important to achieve optimal outcomes, and although spontaneous healing may be possible in severe lesions over a lengthy period, in most patients irreversible physical impairment occurs as a consequence.[13] Nevertheless, the recognition that some small lesions can resolve spontaneously suggests that further studies could be performed to determine the true prevalence of spontaneously healing small M. ulcerans lesions and whether there is the potential for treating clinicians to safely and effectively employ close observation for similar small lesions, rather than immediate antibiotic or surgical treatment. Additionally, it would be important to determine what lesion and host factors favour this approach. Observation alone has the advantage of avoiding the potential toxicity of antibiotic treatment which results in serious adverse effects in more than 20% of treated Australian patients.[26] Although surgery alone can be an effective option for small lesions[18] this usually involves a financial cost and is not always easily accessible. Importantly, observation alone as a mode of management for small lesions would need to be evaluated in settings with lower resources and more isolated populations where close monitoring may be less feasible, increasing the risk of undetected disease progression.
In conclusion, healing without specific treatment can occur for some small ulcerated M. ulcerans lesions in Australian patients suggesting that in selected cases a robust immune response alone can cure lesions. Further research is required to determine what lesion and host factors are associated with spontaneous healing, and whether observation alone is an effective and safe form of management for selected small M. ulcerans lesions.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.O’Brien DP, Athan E, Hughes A, Johnson PD (2008) Successful treatment of Mycobacterium ulcerans osteomyelitis with minor surgical debridement and prolonged rifampicin and ciprofloxacin therapy: a case report. J Med Case Rep 2: 123 10.1186/1752-1947-2-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asiedu K, Etuaful S (1998) Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am J Trop Med Hyg 59: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 3.Bieri R, Scherr N, Ruf MT, Dangy JP, Gersbach P, et al. (2017) The Macrolide Toxin Mycolactone Promotes Bim-Dependent Apoptosis in Buruli Ulcer through Inhibition of mTOR. ACS Chem Biol 12: 1297–1307. 10.1021/acschembio.7b00053 [DOI] [PubMed] [Google Scholar]
- 4.Coutanceau E, Decalf J, Martino A, Babon A, Winter N, et al. (2007) Selective suppression of dendritic cell functions by Mycobacterium ulcerans toxin mycolactone. J Exp Med 204: 1395–1403. 10.1084/jem.20070234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grotzke JE, Kozik P, Morel JD, Impens F, Pietrosemoli N, et al. (2017) Sec61 blockade by mycolactone inhibits antigen cross-presentation independently of endosome-to-cytosol export. Proc Natl Acad Sci U S A 114: E5910–E5919. 10.1073/pnas.1705242114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torrado E, Fraga AG, Logarinho E, Martins TG, Carmona JA, et al. (2010) IFN-gamma-dependent activation of macrophages during experimental infections by Mycobacterium ulcerans is impaired by the toxin mycolactone. J Immunol 184: 947–955. 10.4049/jimmunol.0902717 [DOI] [PubMed] [Google Scholar]
- 7.Simmonds RE, Lali FV, Smallie T, Small PL, Foxwell BM (2009) Mycolactone inhibits monocyte cytokine production by a posttranscriptional mechanism. J Immunol 182: 2194–2202. 10.4049/jimmunol.0802294 [DOI] [PubMed] [Google Scholar]
- 8.Yeboah-Manu D, Röltgen K, Opare W, Asan-Ampah K, Quenin-Fosu K, et al. (2012) Sero-Epidemiology as a Tool to Screen Populations for Exposure to Mycobacterium ulcerans. PLoS Negl Trop Dis 6: e1460 10.1371/journal.pntd.0001460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith PG, Revill WD, Lukwago E, Rykushin YP (1976) The protective effect of BCG against Mycobacterium ulcerans disease: a controlled trial in an endemic area of Uganda. Trans R Soc Trop Med Hyg 70: 449–457. [DOI] [PubMed] [Google Scholar]
- 10.Bieri R, Bolz M, Ruf MT, Pluschke G (2016) Interferon-gamma Is a Crucial Activator of Early Host Immune Defense against Mycobacterium ulcerans Infection in Mice. PLoS Negl Trop Dis 10: e0004450 10.1371/journal.pntd.0004450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon CL, Buntine JA, Hayman JA, Lavender CJ, Fyfe JA, et al. (2011) Spontaneous clearance of Mycobacterium ulcerans in a case of Buruli ulcer. PLoS Negl Trop Dis 5: e1290 10.1371/journal.pntd.0001290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Revill WD, Morrow RH, Pike MC, Ateng J (1973) A controlled trial of the treatment of Mycobacterium ulcerans infection with clofazimine. Lancet 2: 873–877. [DOI] [PubMed] [Google Scholar]
- 13.Marion E, Chauty A, Kempf M, Le Corre Y, Delneste Y, et al. (2016) Clinical Features of Spontaneous Partial Healing During Mycobacterium ulcerans Infection. Open Forum Infect Dis 3: ofw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien DP, Jenkin G, Buntine J, Steffen CM, McDonald A, et al. (2014) Treatment and prevention of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: guideline update. Med J Aust 200: 267–270. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organisation (2012) Treatment of Mycobacterium ulcerans disease (Buruli ulcer): guidance for health workers. Geneva, Switzerland. [Google Scholar]
- 16.Friedman ND, Athan E, Walton AL, O’Brien DP (2016) Increasing Experience with Primary Oral Medical Therapy for Mycobacterium ulcerans Disease in an Australian Cohort. Antimicrob Agents Chemother 60: 2692–2695. 10.1128/AAC.02853-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nienhuis WA, Stienstra Y, Thompson WA, Awuah PC, Abass KM, et al. (2010) Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375: 664–672. 10.1016/S0140-6736(09)61962-0 [DOI] [PubMed] [Google Scholar]
- 18.O’Brien DP, Callan P, Friedman ND, Athan E, Hughes A, et al. (2018) Mycobacterium ulcerans disease management in Australian patients: the re-emergence of surgery as an important treatment modality. ANZ J Surg. [DOI] [PubMed] [Google Scholar]
- 19.Vogel M, Bayi PF, Ruf MT, Bratschi MW, Bolz M, et al. (2016) Local Heat Application for the Treatment of Buruli Ulcer: Results of a Phase II Open Label Single Center Non Comparative Clinical Trial. Clin Infect Dis 62: 342–350. 10.1093/cid/civ883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien DP, Athan E, Blasdell K, De Barro P (2018) Tackling the worsening epidemic of Buruli ulcer in Australia in an information void: time for an urgent scientific response. Med J Aust 208: 287–289. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien DP, Friedman ND, McDonald A, Callan P, Hughes A, et al. (2018) Wound healing: Natural history and risk factors for delay in Australian patients treated with antibiotics for Mycobacterium ulcerans disease. PLoS Negl Trop Dis 12: e0006357 10.1371/journal.pntd.0006357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai AYC, Athan E, Friedman ND, Hughes A, Walton A, et al. (2018) Increased Severity and Spread of Mycobacterium ulcerans, Southeastern Australia. Emerg Infect Dis 24: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross BC, Marino L, Oppedisano F, Edwards R, Robins-Browne RM, et al. (1997) Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J Clin Microbiol 35: 1696–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynne JW, Stinear TP, Athan E, Michalski WP, O’Brien DP (2018) Low incidence of recurrent Buruli ulcers in treated Australian patients living in an endemic region. PLoS Negl Trop Dis 12: e0006724 10.1371/journal.pntd.0006724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prevot G, Bourreau E, Pascalis H, Pradinaud R, Tanghe A, et al. (2004) Differential production of systemic and intralesional gamma interferon and interleukin-10 in nodular and ulcerative forms of Buruli disease. Infect Immun 72: 958–965. 10.1128/IAI.72.2.958-965.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien DP, Friedman D, Hughes A, Walton A, Athan E (2017) Antibiotic complications during the treatment of Mycobacterium ulcerans disease in Australian patients. Internal Medicine Journal 47: 1011–1019. 10.1111/imj.13511 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.