Abstract
Objective
To determine whether altered metabolic profiles represent a link between atrial dysfunction and cardioembolic (CE) stroke, and thus whether underlying dysfunctional atrial substrate may contribute to thromboembolism risk in CE stroke.
Methods
A total of 144 metabolites were measured using liquid chromatography–tandem mass spectrometry in plasma samples collected within 9 hours of stroke onset in 367 acute stroke patients. Stroke subtype was assigned using the Causative Classification of Stroke System, and CE stroke (n = 181) was compared to non-CE stroke (n = 186). Markers of left atrial dysfunction included abnormal atrial function (P-wave terminal force in lead V1, PTFV1 >4,000 μV·ms), left atrial enlargement on echocardiography, and frank atrial fibrillation on ECG. Stroke recurrence risk was assessed using CHADS2 and CHA2DS2-VASc scores. Associations between metabolites and CE stroke, atrial dysfunction, and stroke recurrence risk were evaluated using logistic regression models.
Results
Three tricarboxylic acid metabolites—succinate (odds ratio [OR] 1.71, 95% confidence interval [CI] 1.36–2.15, p = 1.37 × 10−6), α-ketoglutarate (OR 1.62, 95% CI 1.29–2.04, p = 1.62 × 10−5), and malate (OR 1.58, 95% CI 1.26–1.97, p = 2.57 × 10−5)—were associated with CE stroke. Succinate (OR 1.36, 95% CI 1.31–1.98, p = 1.22 × 10−6), α-ketoglutarate (OR 2.14, 95% CI 1.60–2.87, p = 2.08 × 10−8), and malate (OR 2.02, 95% CI 1.53–2.66, p = 1.60 × 10−7) were among metabolites also associated with subclinical atrial dysfunction. Of these, succinate was also associated with left atrial enlargement (OR 1.54, 95% CI 1.23–1.94, p = 1.06 × 10−4) and stroke recurrence based on dichotomized CHADS2 (OR 2.63, 95% CI 1.68–4.13, p = 3.00 × 10−6) and CHA2DS2-VASc (OR 2.43, 95% CI 1.60–3.68, p = 4.25 × 10−6) scores.
Conclusions
Metabolite profiling identified changes in succinate associated with CE stroke, atrial dysfunction, and stroke recurrence, revealing a putative underlying link between CE stroke and energy metabolism.
Atrial fibrillation (AF) has a well-established link to stroke risk, where blood stasis in the left atrium leads to thrombus formation and subsequent embolism.1,2 However, recent evidence indicates that this model may not account for the entirety of elevated stroke risk, and underlying atrial dysfunction in the absence of frank fibrillation may be a contributing cause.1 Several atrial abnormalities modify the risk of stroke in AF, including atrial dilation, mechanical dysfunction of the left atrial appendage, fibrosis, endothelial dysfunction, and myocyte dysfunction.1,3 Some of these atrial abnormalities can lead to changes in the P-wave terminal force in lead V1 (PTFV1) on ECG. Recent studies have identified elevated PTFV1 as a marker of atrial dysfunction linked to stroke.1,4–7 Left atrial enlargement has also been shown to be independently associated with stroke.8–13 Furthermore, cardiovascular risk factors captured by the CHADS2 and CHA2DS2-VASc scores highlight comorbid risk factors that further augment stroke risk in patients with underlying AF.14–16
Atrial dysfunction is mediated by several processes—including atrial electrical dysfunction, neurohormonal change, and inflammation—all associated with shifts in atrial energy homeostasis.17 Accordingly, changes in myocardial energy metabolism have been directly linked to AF.18–21 These findings raise the possibility that altered energy metabolism may partially mediate the complex structural and physiologic changes underlying atrial dysfunction. However, the link between energy metabolites and cardioembolic (CE) stroke risk has not been previously investigated. In this study, we applied targeted metabolite profiling to test the hypothesis that altered energy metabolism may represent a link between atrial dysfunction and CE stroke.
Methods
Patient characteristics
Patients were enrolled in the Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) biorepository. The repository enrolled patients age 18 years or greater who presented with symptoms consistent with ischemic stroke within 9 hours of stroke onset between January 2007 and April 2010. For this nested case-control study, patients were eligible if the NIH Stroke Scale (NIHSS) score was ≥1 and they were enrolled at the Partners Healthcare SPOTRIAS sites (Massachusetts General Hospital and Brigham and Women's Hospital).
Standard protocol approvals, registrations, and patient consents
This study was approved by the Partners Healthcare Institutional Review Board. All patients or surrogates provided informed consent.
Stroke subtype
Stroke subtyping was based on the Causative Classification of Stroke System (CCS), which uses epidemiologic, diagnostic, and clinical data to determine classification of 5 different stroke subtypes (cardio-aortic embolism, small artery occlusion, large artery atherosclerosis, other causes, and undetermined causes).22
Atrial dysfunction assessment
PTFV1 was determined on admission ECGs as previously described.4,5,23 Briefly, PTFV1 was calculated as the product of the amplitude (in μV) and the duration (in ms) of the second p-wave deflection (p') in lead V1. The PTFV1 value was dichotomized at >4,000 μV·ms, which is the commonly defined threshold for left atrial abnormality.4,24,25 The presence of AF was assessed and recorded on ECGs. The size of the left atrium was obtained from transthoracic echocardiogram reports, from studies performed during the hospitalization for the index stroke. The anterior–posterior dimension was used to determine left atrial enlargement, which was defined as any value outside the echocardiography laboratory normative range, and corresponded to 39 mm or more.
Stroke recurrence risk
CHADS2 and CHA2DS2-Vasc scores were used to categorize risk of recurrent stroke in patients with AF14,15 and undetermined etiologies.16 CHADS2 was calculated as the sum of congestive heart failure, hypertension, age ≥75, diabetes mellitus (each 1 point), and ischemic stroke or TIA (2 points). CHA2DS2-Vasc was scored as the sum of congestive heart failure, hypertension, vascular disease (myocardial infarction or peripheral artery disease), diabetes mellitus (each 1 point), sex (female 1 point, male 0 points), age (65–74 one point, ≥75 two points), and ischemic stroke or TIA (2 points). We dichotomized CHADS2 into 0 vs ≥1 and CHA2DS2-VASc into 0–1 vs ≥2 based on prior studies.26,27 CHADS2 and CHA2DS2-VASc were scored and analyzed in patients with CE and undetermined etiologies (n = 249) since these scores have been validated for stroke recurrence risk only in these stroke subtypes.14–16
Metabolite profiling
EDTA blood samples were collected and immediately centrifuged to separate cellular material from plasma. Fasting blood samples were collected at admission (corresponding to 7.1 ± 3.3 hours after last seen well time), and collection occurred throughout the 24-hour cycle. Aliquots of plasma were frozen on dry ice and stored at −80°C until analysis. Thirty microliters of plasma was mixed with 70 μL of ice cold acetonitrile: methanol (75:25; v:v) containing deuterated internal standards (25 μM thymine-d4 [Sigma-Aldrich, St. Louis, MO], 10 μM inosine-15N4 [Cambridge Isotope Laboratories, Tewksbury, MA], 25 μM phenylalanine-d8 [Cambridge Isotope Laboratories], and 10 μM valine-d8 [Sigma-Aldrich]). After vortexing, the protein precipitates were centrifuged at 20,000g at 4°C for 15 minutes and the supernatants were transferred to glass vials with inserts (MicroSolv, Eatontown, NJ).
Plasma extracts were analyzed using a high-performance liquid chromatography (HPLC) system by hydrophilic interaction chromatography on a 2.1 × 100 mm 3.5 μm XBridge Amide column (Waters, Milford, MA) followed by targeted triple-quadrupole mass spectrometry (QQQ MS) detection in both positive and negative polarity modes, using our previously established method.28 Briefly, the chromatography system consisted of a 1290 Infinity autosampler and 2 1290 HLPC binary pumps, connected to a 6495 QQQ MS (Agilent, Santa Clara, CA). Mobile phase A was 95:5 (v:v) water: acetonitrile with 20 mM ammonium acetate and 20 mM ammonium hydroxide (pH 9.5). Mobile phase B was acetonitrile. Ammonium acetate, ammonium hydroxide, and HPLC grade solvents were purchased from Fisher Scientific (Hampton, NH).
Two XBridge Amide columns were run in parallel using alternating column regeneration, with one column devoted to metabolites with negative ionization character, hence negative polarity detection, and the second column for positive polarity detection. Peak integration for metabolite quantification was carried out using MassHunter QQQ Quantitative Analysis software (Agilent). Peaks were quality checked and normalized to human pooled plasma samples that were interspersed at regular intervals every 10 injections, using standard procedures.28,29
Statistical analysis
Baseline characteristics were expressed as mean ± SD for normally distributed continuous variables, or as median with interquartile range for ordinal variables or continuous variables showing deviation from normality. Binary variables were represented as frequency and percentage. Differences between binary variables were analyzed using χ2 testing as appropriate. Continuous variables were compared between groups using Student t test for normally distributed data and Wilcoxon rank-sum for nonparametric data.
To identify metabolites associated with CE stroke, cardio-aortic embolic etiology (based on CCS criteria) was compared to large vessel, small vessel, other, and undetermined stroke subtypes combined. A Bonferroni-corrected p value threshold of 3.47 × 10−4 was used to adjust for 144 metabolite associations. Raw metabolite peak area values were normalized to a human healthy pooled plasma reference standard, such that all values were converted to a ratio relative to healthy human pooled plasma. In order to standardize the effect sizes for the regression models, we log-transformed and unit standardized each metabolite separately. This allows the meaningful comparison of effect sizes (i.e., odds ratios [ORs]) between metabolites. Multivariable logistic regression models were then developed that adjusted for univariable significant metabolites (α-ketoglutarate and malate) and risk factors (age, sex, congestive heart failure, history of stroke, and NIHSS). We did not adjust for AF since this was one of our major outcomes of interest and AF is known to have a strong association with CE stroke.
Logistic regression models were also used to determine metabolites associated with severity of atrial dysfunction (i.e., PTFV1 <4,000 μV·ms, PTFV1 >4,000 μV·ms, ECG-identified AF, and echocardiogram-identified left atrial enlargement). Ordinal logistic regression models were examined for adherence to the proportional odds assumption where appropriate. CHADS2 and CHA2DS2-Vasc scores were each stratified into clinically relevant thresholds16 (CHADS2 ≥1 and CHA2DS2-Vasc ≥2), and metabolite associations were tested using logistic regression. Statistical analysis was performed using STATA SE 12.0 (College Station, TX).
Data availability
Investigators may request access to the data used in the analysis by contacting the corresponding author to submit a proposal. Primary data for reasonable requests will be made available in accordance with local institutional review board and institutional data transfer agreements.
Results
Clinical characteristics
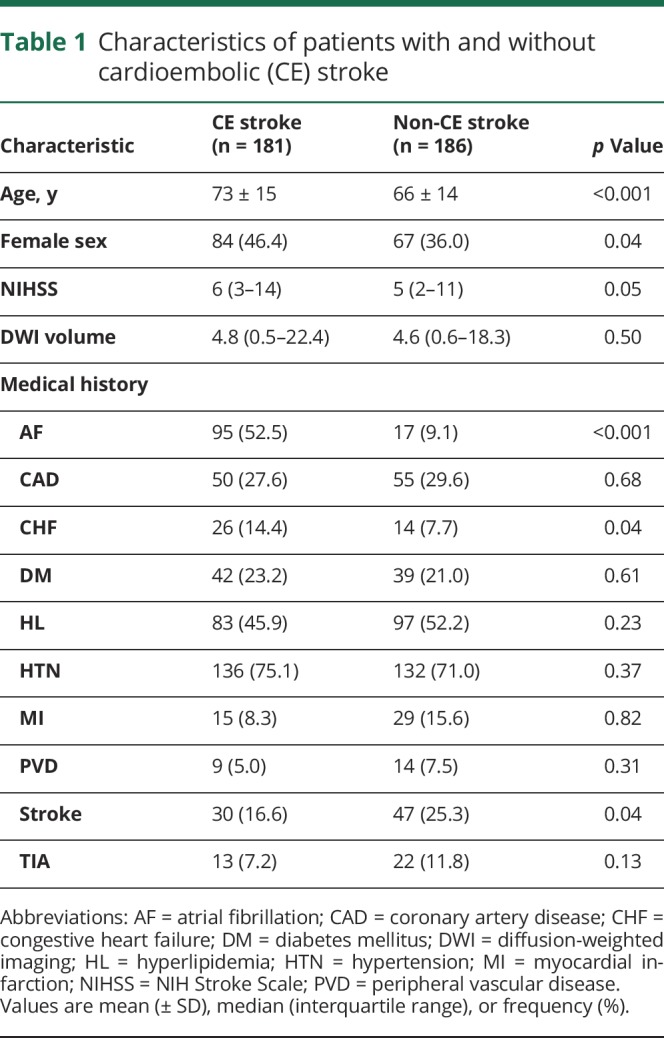
Of 522 enrolled patients, 141 patients were excluded because a baseline plasma sample was not available for analysis, and 14 patients were excluded due to incomplete CCS stroke subtyping information. The final study cohort consisted of 367 patients whose clinical characteristics are presented in table 1, comparing CE stroke subtype patients (n = 181) to other subtype etiologies (large artery: 70, small vessel: 29, undetermined: 68, and other: 19). The etiologic subtype assignments within the study cohort were similar to the main cohort. CE stroke patients were approximately 6 years older, more likely to be female, had a higher baseline NIHSS score, and were less likely to have a prior stroke compared to stroke patients without CE origin. As expected, patients with CE stroke were more likely to have a diagnosis of AF and congestive heart failure.
Table 1.
Characteristics of patients with and without cardioembolic (CE) stroke
Elevated TCA cycle intermediates are associated with CE stroke
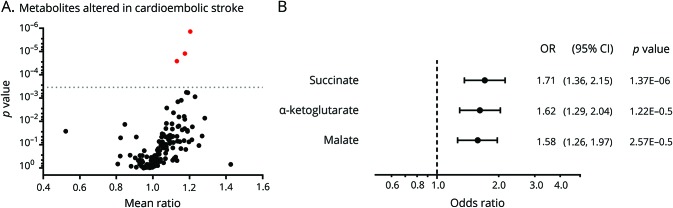
Admission plasma samples were analyzed by high-throughput metabolite profiling.28 Figure 1A summarizes the effect size for all analytes in relation to the individual p values. Following Bonferroni correction for multiple hypothesis testing, 3 metabolites were significantly associated with CE stroke, including succinate (OR 1.71, 95% confidence interval [CI] 1.36–2.15, p = 1.37 × 10−6), α-ketoglutarate (OR 1.62, 95% CI 1.29–2.04, p = 1.62 × 10−5), and malate (OR 1.58, 95% CI 1.26–1.97, p = 2.57 × 10−5; figure 1B). Table S1 (doi.org/10.5061/dryad.n08c0t7) provides a complete list of all metabolite associations including effect size and p values.
Figure 1. Comparisons among metabolites in cardioembolic and noncardioembolic stroke patients.
(A) The geometric mean ratio of each metabolite in cardioembolic cases vs noncardioembolic cases is plotted against the respective p value. The dotted line represents the Bonferroni-corrected p value threshold for 144 metabolites (p ≤ 3.47 × 10−4). The red dots highlight metabolites exceeding the Bonferroni threshold. (B) Odds ratios (ORs) and 95% confidence intervals (CIs) (in standardized units) for metabolites associated with cardioembolic stroke at the Bonferroni-corrected p value.
To determine which metabolites were independently associated with CE stroke, we developed multivariable models. The unadjusted OR of succinate for CE stroke was 1.71 (95% CI 1.36–2.15, p < 0.001) and 1.71 (95% CI 1.12–1.99, p = 0.008) after adjusting for the TCA cycle metabolites α-ketoglutarate and malate. Succinate was still an independent metabolite after adjusting for other variables significant in univariable analysis (age, sex, congestive heart failure, and history of stroke; adjusted OR [aOR] 1.48, 95% CI 1.11–1.97, p = 0.008). Following the addition of NIHSS to the model, succinate remained an independent predictor of CE stroke (aOR 1.36, 95% CI 1.01–1.83, p = 0.045). Examining receiver operating characteristic analysis, the area under the curve was 0.660 for succinate and CE stroke.
Atrial dysfunction and AF share a similar metabolite signature
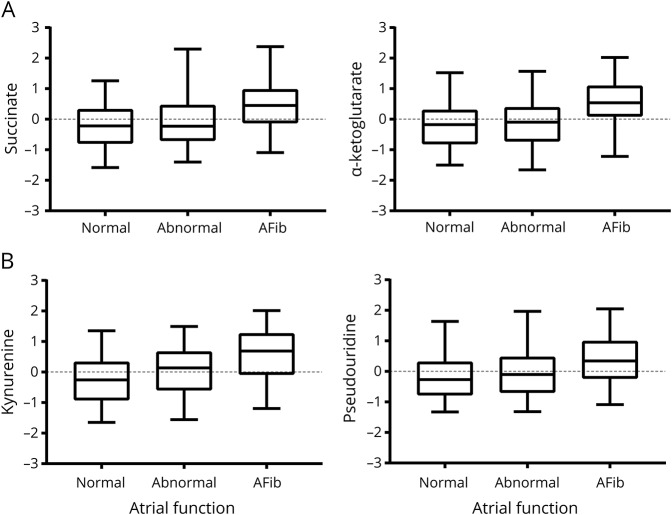
Emerging data suggest that impaired atrial function, or cardiopathy, predicts future AF30,31 and incident stroke.1,3 To further explore this hypothesis, we next assessed for metabolites significantly associated with atrial dysfunction (table 2), where atrial function was categorized into normal atrial function, abnormal atrial function, and frank AF. Significant metabolites included several TCA cycle intermediates, including α-ketoglutarate (OR 2.14, 95% CI 1.60–2.87, p = 2.08 × 10−8), aconitate (OR 2.13, 95% CI 1.52–2.99, p = 1.19 × 10−6), malate (OR 2.02, 95% CI 1.53–2.66, p = 1.60 × 10−7), and succinate (OR 1.86, 95% CI 1.42–2.41, p = 1.22 × 10−6). Some of these analytes did not demonstrate a proportional effect size (OR) across each level of atrial dysfunction, and accordingly, the increase was more pronounced in AF (figure 2A). In contrast, other metabolites including pseudouridine and kynurenine demonstrated a graded, stepwise increase across the spectrum of atrial dysfunction and AF (table 2 and figure 2B). A complete list of all metabolite associations is provided in table S2 (doi.org/10.5061/dryad.n08c0t7).
Table 2.
Metabolites associated with atrial dysfunction
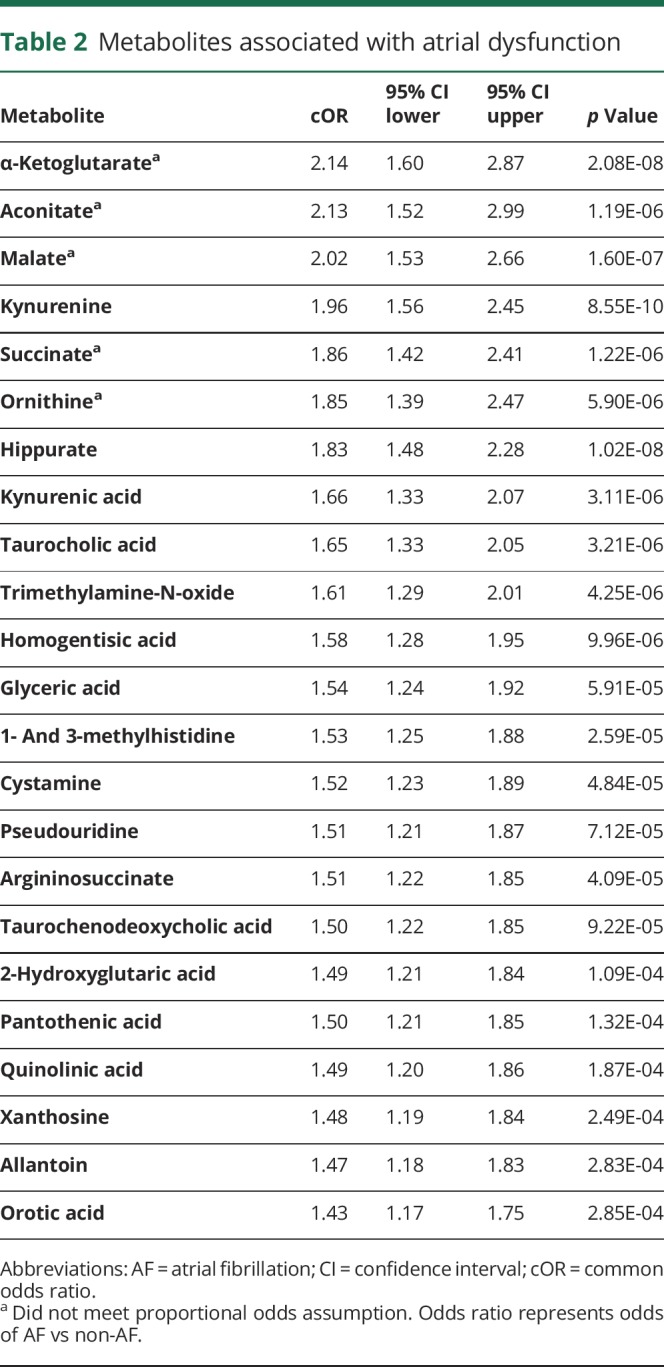
Figure 2. Metabolite differences in atrial dysfunction and atrial fibrillation.
(A) TCA cycle metabolites demonstrate increased accumulation with atrial fibrillation compared with normal and abnormal atrial function, including succinate (p < 1.22 × 10−5) and α-ketoglutarate (p < 2.08 × 10−8). Normal atrial function was defined as P-wave terminal force in lead V1 (PTFV1) <4,000 μV·ms and abnormal atrial function as PTFV1 >4,000 μV·ms. (B) Non-TCA cycle metabolites associated with cardioembolic stroke demonstrated a graded increase across the spectrum of atrial dysfunction, including kynurenine (p < 8.55 × 10−10) and pseudouridine (p < 7.12 × 10−5).
To confirm the associations were independent of age, multivariable logistic regression demonstrated that α-ketoglutarate (OR 1.97, 95% CI 1.46–2.65, p = 8.59 × 10−6), aconitate (OR 1.67, 95% CI 1.19–2.34, p = 2.61 × 10−3), malate (OR 1.73, 95% CI 1.29–2.31, p = 2.24 × 10−4), and succinate (OR 1.59, 95% CI 1.21–2.09, p = 8.69 × 10−4) were all associated with atrial dysfunction. To further confirm the link between energy metabolism and atrial dysfunction, we explored the relationship between TCA metabolites and left atrial enlargement, and found an association only with succinate (OR 1.54, 95% CI 1.23–1.94, p = 1.06 × 10−4), but not with α-ketoglutarate, aconitate, or malate (table S3, doi.org/10.5061/dryad.n08c0t7).
Succinate is associated with stroke risk scores
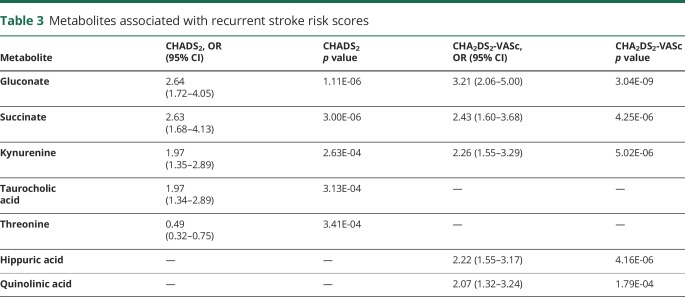
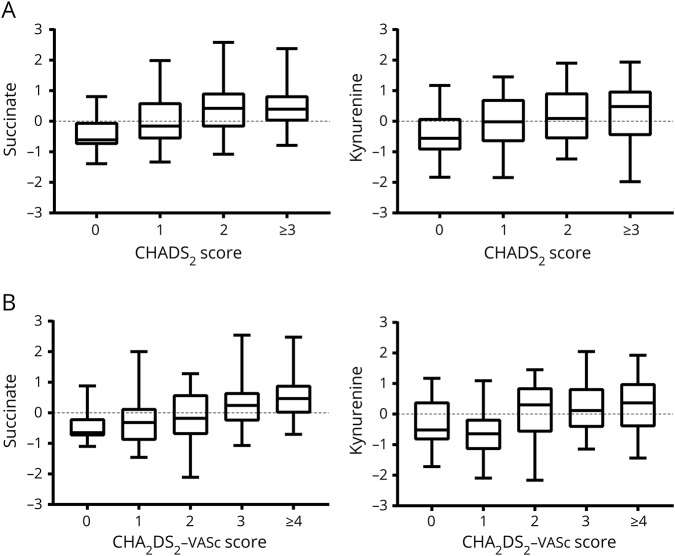
We reasoned that if candidate metabolites linked to atrial dysfunction were relevant to stroke risk, then the same metabolites should also be associated with CHADS2 and CHA2DS2-VASc scores, which are used to stratify the risk of stroke. Associations with dichotomized CHADS2 and CHA2DS2-VASc scores identified succinate, kynurenine, and gluconate as predictors of elevated stroke risk that were in common with both scores (table 3). After adjustment for age, both succinate and gluconate remained an independent predictor for both scores, but kynurenine did not. When examining the CHADS2 and CHA2DS2-VASc across the full range of the respective scales, there was a stepwise increase for each metabolite (figure 3), including succinate (common OR [cOR] 1.71, 95% CI 1.35–2.18, p = 3.85 × 10−6) and kynurenine (cOR 1.57, 95% CI 1.25–1.98, p = 1.02 × 10−4) for the CHADS2 scale and for the CHA2DS2-VASc scale (succinate: cOR 2.08, 95% CI 1.59–2.73, p = 8.32 × 10−9; kynurenine: cOR 1.58, 95% CI 1.24–2.00, p = 1.37 × 10−4). The full listing of metabolite associations with CHADS2 and CHA2DS2-VASc are presented in tables S4 and S5 (doi.org/10.5061/dryad.n08c0t7).
Table 3.
Metabolites associated with recurrent stroke risk scores
Figure 3. Metabolites altered in cardioembolic stroke and atrial dysfunction are also associated with stroke recurrence risk.
(A) Succinate and kynurenine demonstrated a dose–response relationship with CHADS2 score and (B) with CHA2DS2-VASc scores.
Discussion
In this study, we sought to identify circulating metabolites associated with CE stroke. We found that changes in TCA cycle intermediates were independently associated with CE stroke. We further identified TCA metabolites associated with markers of atrial dysfunction. Finally, we found an overlapping signature in association with stroke risk, as reflected by the CHADS2 and CHA2DS2-Vasc classification schema. Among these findings, succinate was the common alteration linking CE stroke, atrial dysfunction, and risk stratification.
Taken together, our findings have implications for understanding AF-associated thromboembolism. Notably, several lines of evidence suggest that rhythm alterations alone may not fully account for the risk of stroke attributed to AF.1 Accordingly, the causal relationship between AF and stroke may be more complex than originally appreciated. In support of this notion, rhythm-control therapy does not reduce the risk of stroke in AF,32 and conversely, lone AF in otherwise healthy individuals does not always impart an elevated risk of stroke.33 Recent evidence further highlights that AF episodes are often temporally dissociated from thromboembolism.34,35 These observations imply that AF-related blood stasis may not be the only mechanism that leads to CE stroke.1,36 Impaired underlying atrial function has been proposed as an alternative explanatory model for AF-associated stroke risk.1,3 In this context, our TCA metabolite findings in CE stroke and in atrial dysfunction are consistent with the concept that atrial cardiopathy may represent a spectrum of atrial dysfunction contributing to stroke risk. However, it is important to note that our findings do not specifically support a causal link between succinate and CE stroke or atrial dysfunction.
The TCA signature is also noteworthy given the metabolic demands of myocardium. In the healthy heart, mitochondrial oxidative phosphorylation provides the majority (>95%) of energy, with substrates provided by fatty acid oxidation and entry into the TCA cycle.37 In the diseased myocardium, including dilated cardiomyopathy, increased concentrations of TCA cycle intermediates have been identified in patients' plasma.38 Similar findings have been reported in a dog model of AF, where the propensity for sustained AF correlates with the cellular energetic state of the atrium.39 Moreover, in patients with AF after cardiac surgery, succinate dehydrogenase activity was significantly impaired relative to those who remained in sinus rhythm.18,19 Further, succinate dehydrogenase is the only enzyme shared by the electron transport chain and the TCA cycle; in cardiac myocytes, this link between substrate utilization and the electron transport chain has been reinforced by experiments showing that mitochondrial rate reserve respiratory capacity (the ability of mitochondria to increase ATP production as demand increases) was abolished by the absence of succinate dehydrogenase activity.40,41 Our metabolite findings in AF and stroke also converge on succinate as a leading candidate metabolite. We further acknowledge that while changes in energy metabolism are reported in other stroke mechanisms such as carotid plaque rupture and small vessel disease, alterations in those conditions do not involve the TCA cycle.42–44
Left atrial remodeling leads to progressive electrical and structural changes that further perpetuates AF, and is accompanied by changes in energy metabolism.21,45,46 While our findings corroborate a link between energy metabolism and AF, our analysis cannot distinguish whether AF or CE stroke causes TCA cycle alterations, whether TCA cycle changes contribute to atrial remodeling, or whether accumulation of TCA cycle intermediates is associated with other factors that cause AF. Nevertheless, our findings support the abnormal atrial substrate model of CE stroke.1 It is also notable that our findings are consistent with a biologically plausible gradient in atrial dysfunction, which raises the possibility that improving myocardial energetics may help attenuate CE stroke risk. In this regard, aggressive risk factor modification in patients with AF can reverse adverse atrial remodeling.47
The strengths of our study include the large number of patients, the detailed phenotyping, and measurement of metabolites using a recently developed metabolomics platform28; however, limitations of our study should be noted. We studied samples from stroke patients in the acute setting, and our results are not generalizable to community-dwelling individuals. Additional research would be needed to determine whether succinate or other TCA metabolites are predictors of incident CE stroke or of recurrent stroke events in a population-based study. We also did not have a separate cohort in which to validate our findings. In addition, we evaluated the risk of stroke recurrence in patients with CE and undetermined etiologies using the CHADS2 and CHA2DS2-Vasc scores; however, it should be noted that these widely validated scores have modest discriminating ability for ischemic stroke.48–54 Future study is necessary to determine whether succinate measurements would be useful in diagnosing occult or paroxysmal AF that would obviate the need for long-term cardiac monitoring. Whether succinate could help risk stratify embolic stroke of uncertain source would also require further study in future prospective cohorts.
Alterations in TCA cycle intermediates—and particularly succinate—suggest an underlying metabolic link among atrial dysfunction, CE stroke, and CE stroke risk. Future studies that focus on incident stroke risk are needed to confirm these findings and determine whether metabolite profiling may identify an important role for succinate in mediating or predicting stroke.
Glossary
- AF
atrial fibrillation
- aOR
adjusted odds ratio
- CCS
Causative Classification of Stroke System
- CE
cardioembolic
- CI
confidence interval
- cOR
crude odds ratio
- HPLC
high-performance liquid chromatography
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- PTFV1
P-wave terminal force in lead V1
- QQQ MS
triple-quadrupole mass spectrometry
- SPOTRIAS
Specialized Programs of Translational Research in Acute Stroke
Appendix. Authors
Study funding
This study was supported by NIH R01 DK081572 (R.E.G.), NIH K23 NS076597 (W.T.K.), AHA 14GRNT19060044 (W.T.K.), and NIH R01 NS099209 (W.T.K.).
Disclosure
S. Nelson, Z. Ament, and Z. Wolcott report no disclosures relevant to the manuscript. R. Gerszten is funded by NIH DK081572, DK112340, HL132320, and HL133870. W. Kimberly is funded by NIH NS099209 and AHA 17CSA33550004, has received research support from Remedy Pharmaceuticals, and receives research support from Biogen Idec. Go to Neurology.org/N for full disclosures.
References
- 1.Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke 2016;47:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nouh A, Hussain M, Mehta T, Yaghi S. Embolic strokes of unknown source and cryptogenic stroke: implications in clinical practice. Front Neurol 2016;7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberger JJ, Arora R, Green D, et al. Evaluating the atrial myopathy underlying atrial fibrillation: identifying the arrhythmogenic and thrombogenic substrate. Circulation 2015;132:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamel H, O'Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the Atherosclerosis Risk in Communities Study. Ann Neurol 2015;78:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamel H, Hunter M, Moon YP, et al. Electrocardiographic left atrial abnormality and risk of stroke: Northern Manhattan Study. Stroke 2015;46:3208–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamel H, Soliman EZ, Heckbert SR, et al. P-wave morphology and the risk of incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke 2014;45:2786–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Tse G, Korantzopoulos P, et al. P-wave indices and risk of ischemic stroke: a systematic review and meta-analysis. Stroke 2017;48:2066–2072. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death: the Framingham Heart Study. Circulation 1995;92:835–841. [DOI] [PubMed] [Google Scholar]
- 9.Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke 1999;30:2019–2024. [DOI] [PubMed] [Google Scholar]
- 10.Barnes ME, Miyasaka Y, Seward JB, et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc 2004;79:1008–1014. [DOI] [PubMed] [Google Scholar]
- 11.Karas MG, Devereux RB, Wiebers DO, et al. Incremental value of biochemical and echocardiographic measures in prediction of ischemic stroke: the Strong Heart Study. Stroke 2012;43:720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo C, Jin Z, Liu R, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. JACC Cardiovasc Imaging 2013;6:313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaghi S, Moon YP, Mora-McLaughlin C, et al. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke 2015;46:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 15.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 16.Ntaios G, Vemmos K, Lip GY, et al. Risk stratification for recurrence and mortality in embolic stroke of undetermined source. Stroke 2016;47:2278–2285. [DOI] [PubMed] [Google Scholar]
- 17.Calenda BW, Fuster V, Halperin JL, Granger CB. Stroke risk assessment in atrial fibrillation: risk factors and markers of atrial myopathy. Nat Rev Cardiol 2016;13:549–559. [DOI] [PubMed] [Google Scholar]
- 18.Mayr M, Yusuf S, Weir G, et al. Combined metabolomic and proteomic analysis of human atrial fibrillation. J Am Coll Cardiol 2008;51:585–594. [DOI] [PubMed] [Google Scholar]
- 19.Montaigne D, Marechal X, Lefebvre P, et al. Mitochondrial dysfunction as an arrhythmogenic substrate: a translational proof-of-concept study in patients with metabolic syndrome in whom post-operative atrial fibrillation develops. J Am Coll Cardiol 2013;62:1466–1473. [DOI] [PubMed] [Google Scholar]
- 20.De Souza AI, Cardin S, Wait R, et al. Proteomic and metabolomic analysis of atrial profibrillatory remodelling in congestive heart failure. J Mol Cel Cardiol 2010;49:851–863. [DOI] [PubMed] [Google Scholar]
- 21.Mihm MJ, Yu F, Carnes CA, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation 2001;104:174–180. [DOI] [PubMed] [Google Scholar]
- 22.Ay H, Benner T, Arsava EM, et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke 2007;38:2979–2984. [DOI] [PubMed] [Google Scholar]
- 23.Waggoner AD, Adyanthaya AV, Quinones MA, Alexander JK. Left atrial enlargement: echocardiographic assessment of electrocardiographic criteria. Circulation 1976;54:553–557. [DOI] [PubMed] [Google Scholar]
- 24.Morris JJ, Estes EH, Whalen RE, Thompson HK, Mcintosh HD. P-wave analysis in valvular heart disease. Circulation 1964;29:242–252. [DOI] [PubMed] [Google Scholar]
- 25.Jin L, Weisse AB, Hernandez F, Jordan T. Significance of electrocardiographic isolated abnormal terminal P-wave force (left atrial abnormality): an echocardiographic and clinical correlation. Arch Intern Med 1988;148:1545–1549. [PubMed] [Google Scholar]
- 26.Olesen JB, Lip GYH, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost 2011;106:739–749. [DOI] [PubMed] [Google Scholar]
- 27.Aakre CA, McLeod CJ, Cha SS, Tsang TS, Lip GY, Gersh BJ. Comparison of clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation. Stroke 2014;45:426–431. [DOI] [PubMed] [Google Scholar]
- 28.Kimberly WT, Sullivan JFO, Nath AK, et al. Metabolite profiling identifies anandamide as a biomarker of nonalcoholic steatohepatitis. JCI Insight 2017;2:e92989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunn WB, Broadhurst D, Begley P, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc 2011;6:1060–1083. [DOI] [PubMed] [Google Scholar]
- 30.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2009;40:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnani JW, Johnson VM, Sullivan LM, et al. P wave duration and risk of longitudinal atrial fibrillation in persons ≥ 60 years old (from the Framingham Heart Study). Am J Cardiol 2011;107:917–921.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 33.Chao TF, Liu CJ, Chen SJ, et al. Atrial fibrillation and the risk of ischemic stroke: does it still matter in patients with a CHA2DS2-VASc score of 0 or 1? Stroke 2012;43:2551–2555. [DOI] [PubMed] [Google Scholar]
- 34.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–2477. [DOI] [PubMed] [Google Scholar]
- 35.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 36.Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism. J Am Coll Cardiol 2015;65:2239–2251. [DOI] [PubMed] [Google Scholar]
- 37.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90:207–258. [DOI] [PubMed] [Google Scholar]
- 38.Alexander D, Lombardi R, Rodriguez G, Mitchell MM, Marian AJ. Metabolomic distinction and insights into the pathogenesis of human primary dilated cardiomyopathy. Eur J Clin Invest 2011;41:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cha YM, Dzeja PP, Shen WK, et al. Failing atrial myocardium: energetic deficits accompany structural remodeling and electrical instability. Am J Physiol Heart Circ Physiol 2003;284:H1313–H1320. [DOI] [PubMed] [Google Scholar]
- 40.Pfleger J, He M, Abdellatif M. Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival. Cell Death Dis 2015;6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhingra V, Kirshenbaum LA. Succinate dehydrogenase/complex II activity obligatorily links mitochondrial reserve respiratory capacity to cell survival in cardiac myocytes. Cel Death Dis 2015;6:e1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomas L, Edsfeldt A, Mollet IG, et al. Altered metabolism distinguishes high-risk from stable carotid atherosclerotic plaques. Eur Heart J 2018;39:2301–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vojinovic D, van der Lee SJ, van Duijn CM, et al. Metabolic profiling of intra- and extracranial carotid artery atherosclerosis. Atherosclerosis 2018;272:60–65. [DOI] [PubMed] [Google Scholar]
- 44.Gasparovic C, Prestopnik J, Thompson J, et al. 1H-MR spectroscopy metabolite levels correlate with executive function in vascular cognitive impairment. J Neurol Neurosurg Psychiatry 2013;84:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation 1995;92:1954–1968. [DOI] [PubMed] [Google Scholar]
- 46.Kopecky SL, Gersh BJ, McGoon MD, et al. The natural history of lone atrial fibrillation: a population-based study over three decades. N Engl J Med 1987;317:669–674. [DOI] [PubMed] [Google Scholar]
- 47.Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation 2017;136:583–596. [DOI] [PubMed] [Google Scholar]
- 48.Stroke Risk in Atrial Fibrillation Working Group. Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke 2008;39:1901–1910. [DOI] [PubMed] [Google Scholar]
- 49.Fang MC, Go AS, Chang Y, Borowsky L, Pomernacki NK, Singer DE. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am Coll Cardiol 2008;51:810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the congestive heart failure, hypertension, age >75, diabetes mellitus, and prior stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J 2008;156:57–64. [DOI] [PubMed] [Google Scholar]
- 51.Poli D, Antonucci E, Grifoni E, Abbate R, Gensini GF, Prisco D. Stroke risk in atrial fibrillation patients on warfarin: predictive ability of risk stratification schemes for primary and secondary prevention. Thromb Haemost 2009;101:367–372. [PubMed] [Google Scholar]
- 52.Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Staa TP, Setakis E, Di Tanna GL, Lane DA, Lip GYH. A comparison of risk stratification schemes for stroke in 79 884 atrial fibrillation patients in general practice. J Thromb Haemost 2011;9:39–48. [DOI] [PubMed] [Google Scholar]
- 54.Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Investigators may request access to the data used in the analysis by contacting the corresponding author to submit a proposal. Primary data for reasonable requests will be made available in accordance with local institutional review board and institutional data transfer agreements.