Abstract
Human mast cells (HuMCs) are derived from CD34+ pluripotent hematopoietic cells which are KIT (CD117)+, FcεRI-, and lack lineage specific surface markers. Bone marrow and peripheral blood are the two readily available sources for obtaining CD34+ cells from which HuMCs can be cultured. CD34+ cells are isolated and enriched by magnetic separation columns and stored under specific conditions until ready for use. Alternatively, enriched CD34+ cells may be immediately cultured in serum free culture media containing recombinant human (rh) stem cell factor (SCF), rhIL-6 and rhIL-3 (added only during the first week). Weekly hemidepletions and removal of adherent cells and/or debris enables the investigator to obtain HuMC cultures, identified by Wright-Giemsa and acidic toluidine blue stains, by 8–10 weeks.
Keywords: Human mast cells, CD34+ cells, pluripotent hematopoietic cells, bone marrow, peripheral blood
1. Introduction
HuMCs are derived from CD34+ pluripotential hematopoietic cells which are KIT (CD117)+ and FcεRI- and lack T-cell (CD2), B-cell (CD19, CD20), macrophage (CD14) and eosinophil lineage surface markers (1). In addition to peripheral blood and bone marrow, HuMCs have been derived from CD34+ cells from cord blood (2–4), and fetal liver (5,6). In vitro studies have documented that the mature HuMC progeny will differ, depending on the tissue of origin. Furthermore, in the presence of rhSCF and rhIL-6, HuMCs require at least 8–10 wks in culture to fully mature. Monocytes and other lineages that appear in vitro are depleted with each weekly passage. This may prevent competition for growth factors, and release of inhibitory growth factors such as IFNγ (1) that may inhibit HuMC proliferation and maturation.
The use of peripheral blood leukapheresis to collect mononuclear cells, followed by immunomagnetic or affinity column enrichment of CD34+ cells, provides large numbers of CD34+ cells and significantly increases the HuMC yield. Laboratory methods detailing the isolation CD34+ cells from bone marrow or peripheral blood, and the growth of HuMCs from these progenitors are described.
2. Materials
MACS LS Separation Columns and MACS Separator (Miltenyi Biotec, San Diego, CA)
Anti-FITC (fluorescein isothiocyanate) MicroBeads (Miltenyi Biotec, San Siego, CA)
StemPro-34 serum-free medium (SFM) with nutrient supplement (Invitrogen, Carlsbad, CA). Complete media: StemPro-SFM media, nutrient supplement, 2 mM L-glutamine, 100 IU/mL penicillin, and 100 ug/mL streptomycin.
100X L-glutamine stock: 200 mM L-glutamine in sterile water
100X penicillin-streptomycin stock: 10,000 IU/ml penicillin, 10,000 ug/mL streptomycin (mediatech, Herndon, VA).
Ammonium chloride solution: 0.8% NH4Cl, 0.1 mM EDTA in ddH2O) (StemCell Technologies, Vancouver, Canada)
Recombinant human (rh) IL-3, rhIL-6, rhSCF (PeproTech, Rocky Hill, NJ)
75 cm2 tissue culture flasks (Fisher Scientific, Pittsburgh, PA)
5 mL polystyrene, 15 mL and 50 mL polypropylene tubes (Becton-Dickinson Labware, Franklin Lakes, NJ)
Blocking buffer: 1 x phosphate buffered saline (PBS) (pH 7.2), 0.5% bovine serum albumin (BSA), 2 mM ethylenediaminetetraacetic acid (EDTA). Prepare sterile or filter sterilize (0.22 μm).
FITC-conjugated anti-human CD34 (anti-HPCA2, Becton-Dickinson, San Jose, CA)
Toluidine blue (acidic): Add 0.5 g of toluidine blue to 30 mL of absolute ethanol. Bring the volume to 100 mL with distilled deionized water. Adjust to a pH <1.0 with 1N HCL. Store at room temperature (RT).
Mota’s fixative: Prepare in a 100 mL bottle with a magnetic stirrer by adding 4 g lead acetate (basic) to 50 mL of distilled deionized water. Stir at slow speed and add 2–4 mL of glacial acetic acid to dissolve the lead acetate and make the solution clear. Add 50 mL of absolute ethanol. Keep tightly closed and store at room temperature. Prepare fresh every 1–2 months.
Hematek-2000 Wright-Giemsa slide stainer (Bayer Corporation, Elkhart, IN)
Cytospin 3 (Shandon, Pittsburgh, PA)
M199 media: 1x with Earles’ salts, L-glutamine, sodium bicarbonate, HEPES buffer (Invitrogen,Carlsbad, CA)
Preservative-free heparin sodium (1000 Units/mL) (American Pharmaceutical Partners, Schaumburg, IL)
Lymphocyte separation media (ICN Biomedicals, Aurora, Ohio)
30 μm nylon net filter (Millipore, Bedford, MA)
Nalgene Cryo 1°C freezing container (Daigger, Vernon Hills, IL)
Nunc 1.8 mL SI (377267) cryotubes (Fisher Scientific, Pittsburgh, PA)
- Crypreservation solutions
- Solution A: Mix M199 media with dimethyl sulfoxide (DMSO) in a 4:1 v/v ratio. Aliquot in 15 mL tubes, and keep frozen at −20° C until use.
- Solution B: Add 30 U/mL preservative-free heparin to fetal bovine serum (FBS), aliquot in 15 mL tubes, and keep frozen at −20° C until use.
3. Methods
3.1. Preparation of bone marrow or peripheral blood for CD34+ selection
Collect CD34+ cells from either normal or patient donor bone marrow or peripheral blood. On average, bone marrow contains approximately 1% CD34+ cells, and peripheral blood contains 0.01–0.07% CD34+ cells (7), so yields will differ significantly by source.
Preload 10 or 50 mL syringes with 0.5 or 1 mL of preservative-free heparin sodium, respectively. Collect aspirated bone marrow in 10 mL syringes. Collect venipuncture-derived peripheral blood into 50 mL syringes. Mix cells and heparin by rotating the syringes for 1 min.
Prepare complete media containing, StemPro-SFM media, nutrient supplement, 2 mM L-glutamine, 100 IU/mL penicillin, and 100 ug/mL streptomycin. Complete media should be stored at 4°C and be remade fresh every 1–2 months.
Place a maximum of 10 mL of either heparinized bone marrow or peripheral blood into a 50 mL tube. Add 25 mL of complete media and resuspend the cells by gentle pipetting.
Place 14 mL of lymphocyte separation media into another 50 mL tube, and carefully overlay the cell suspension on top of the lymphocyte separation media. Centrifuge tubes at 675 x g for 20 min at room temperature. The red cells will collect below the separation media at the bottom of the tube. Identify the mononuclear cells in the interface layer, and pipette off the complete media just above the interface.
Using a 2 mL pipette, gently skim off and collect the mononuclear cells and transfer to a 50 mL tube (see Note 1). Discard the remaining red cell pellet and separation media. Add 25 mL of complete media and centrifuge the mononuclear cells at 300 x g for 10 min to remove debris. Remove the supernatant, and resuspend the pelleted mononuclear cells in 25 mL of media. Repeat twice.
Resuspend mononuclear cells in 5 mL of blocking buffer solution. Remove clumps, aggregates or particles by passing the cell suspension through a sterile 30 μm nylon net filter into a 15 mL tube (see Note 2). Count cells.
3.2. CD34+ cell selection and enrichment (see Note 3)
CD34+ purity is important for eliminating unwanted cells from cultures. Magnetic separation columns initially yield a CD34+ cell purity between 65–75%. A second CD34+ enrichment using a new column may be necessary to obtain purities of 90–95% CD34+ cells.
Resuspend 107 mononuclear cells in 100 μl of blocking buffer in a 5 mL tube. Add 10 μl of FITC-conjugated anti-human CD34 and incubate for 30 min at 37°C.
Add 2 mL of blocking buffer and centrifuge at 210 x g for 5 min. Remove the supernatant completely, and resuspend the cell pellet in 80 μl of blocking buffer. Add 20 μl of MACS anti-FITC microbeads per 107 cells, and incubate the cells for 15 min at 4–8°C.
Add 2 mL of blocking buffer and centrifuge at 210 x g for 5 min. Remove the supernatant completely, and resuspend the cells at a concentration up to 108 cells per 500 μl of blocking buffer.
Place the MACS LS column in the magnetic field, and run 3 mL of blocking buffer through the column. Pipette the cell suspension onto the column, and collect the effluent in a 15 mL tube as the negative fraction. Rinse the column with 3 mL of sterile blocking buffer three times. Remove the column from the magnetic cell separator, and place on a new 15 mL collection tube. Apply 5 mL of buffer onto the column, and flush out CD34+ cells by applying the plunger supplied with the column. Count cells.
3.3. Cryopreservation of CD34+ cells
A minimum of 5 × 106 CD34+ cells per mL of cryopreservative mixture is recommended for preservation and recovery (see Note 4). The cryopreservative mixture consists of two solutions.
To cryopreserve 5 – 10 × 106 cells, prepare 1 tube containing 0.5 mL of cold (4°C) solution A and 1 tube containing 0.5 mL of cold (4°C) solution B.
Add 2.5 – 5 × 106 cells into each tube, and keep on ice for several minutes.
Combine the 2 tubes into a total of 1 mL, and transfer into 1.8 mL cryotubes. Allow cells to equilibrate at 4°C for 30 min.
Transfer cells to a Nalgene Cryo 1°C freezing container, and place in a –70°C freezer overnight. After 24 hours, transfer cryotubes to liquid nitrogen.
3.4. CD34+ and HuMC cultures
Under ideal conditions, 5 ×106 CD34+ cells placed in culture for 7–10 weeks may give rise to 10–20 × 106 HuMCs with less than 5% contamination with other cell types, as determined by Wright-Giemsa and acidic toluidine blue staining.
Quick-thaw a vial of CD34+ cells at 37°C, resuspend in 10 mL of complete media and centrifuge at 450 x g for 5 min. Remove supernatant completely to prevent any DMSO carry over. Resuspend cells in 5–10 mL of complete media containing 100 ng/mL rhSCF, 100 ng/mL rhIL-6 and 30 ng/mL rhIL-3, Transfer Into a 175 mL flask and bring the final volume up to 30 mL. IL-3 is only used during the first week of culture. During subsequent weeks, complete media is supplemented with only 100 ng/mL rhSCF and 100 ng/mL rhIL-6. Incubate the flask for 1 week at 37°C, 5% CO2 (see Note 5).
After 1 week, add 30 mL of complete media containing 100 ng/mL rhSCF and 100 ng/mL rhIL-6 and transfer 30 mL of the diluted culture to another flask thus dividing the culture into two flasks. Repeat the same procedure after one more week in culture.
At the third week, pipette and transfer culture medium from each flask into a 50 mL tube and centrifuge at 450 x g for 5 min at room temperature. Remove 15 mL of the supernatant and resuspend the cells in the remaining medium. Transfer to a new 175 mL flask, and bring the final volume up to 30 mL with new complete medium containing 100 ng/mL rhSCF and 100 ng/mL rhIL-6. Repeat this procedure weekly. Check flasks weekly for adherent cells or debris. Monocytes and other cells will proliferate initially and compete for growth factors in suspension, resulting in adherent cells or debris from cell death. This extraneous material may have a deleterious effect on HuMC yields, and must be removed weekly. If adherent cells are present, gently transfer nonadherent HuMCs and growth media to a new flask. In the event of cell debris, pipette nonadherent HuMCs and growth media into a 50 mL tube, and centrifuge at slow speed (150 x g) for 5 min. Resuspend the cell pellet in 30 mL of fresh complete media with 100 ng/mL rhSCF and 100 ng/mL rhIL-6, and culture in new flasks (see Note 6).
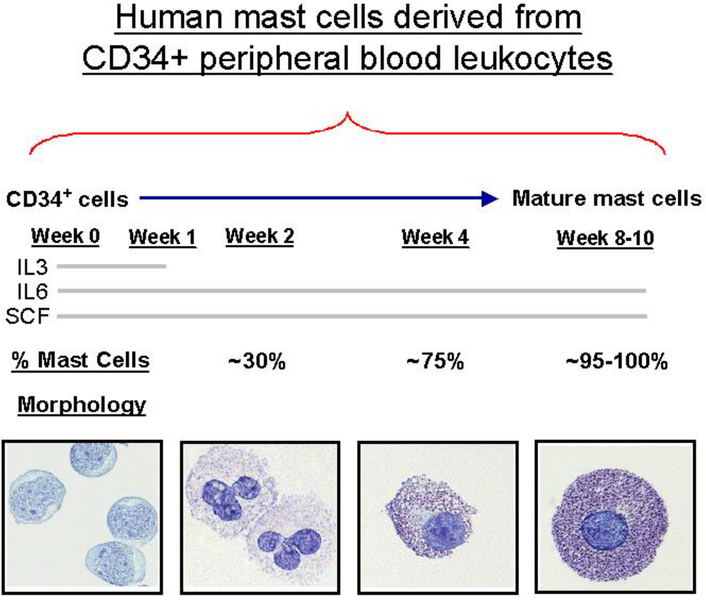
Continue to culture for 7–10 weeks at which point, mature mast cells are present. Check total and HuMC counts weekly by staining with Wright-Giemsa and acidic toluidine blue see below) (Fig. 1) (8).
Fig.1.
Human mast cells derived from CD34+ peripheral blood leukocytes. (Reproduced with the permission of ref 8).
3.5. HuMC histochemical stains
HuMC numbers are calculated by determining the percentage of acidic toluidine blue positive cells out of total Wright-Giemsa positive cells. Acidic toluidine blue positive HuMC numbers can be confirmed by tryptase staining.
3.5.1. Wright – Giemsa
-
1
Count cells directly out of flasks, and concentrate at 210 x g for 5 min to at least 2 × 105 cells per mL, for optimal cytospins.
-
2
Add 100 μl of cell suspension to cytospin sample chambers and clean slides. Spin slides at 400 rpm for 5 min. Let slides air dry, and place on an automated Hematek-2000 for Wright-Giemsa stain. Add 1–2 drops of Permount and mount with a coverslip.
3.5.2. Acidic toluidine blue
-
3
Fix cytospins by adding several drops of Mota’s fixative to cover the cells for 10 min. Mota’s evaporates quickly, so replenish drops once or twice to prevent crystal formation.
-
4
Slowly run water down the slides, not directly on cells, to remove fixative and blot any droplets. Do not disturb the cells.
-
5
Add 2–3 drops of acidic toluidine blue to the slide and let stain for 20 min. Run water down the slide to remove the excess stain, and blot dry. Add 1–2 drops of Permount and mount with a coverslip.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
The authors thank Dr. Alasdair Gilfillan for reviewing this manuscript.
Prior to skimming off of mononuclear cells, if clots are noted in the interface or below, suction clots with a 10 or 25 mL pipette placed directly on the clot. The interface is minimally disturbed and clots are avoided in the mononuclear cell suspension.
Red blood cells normally contaminate most preparations and will not affect HuMC yields if left in culture. For significant red cell contamination, lyse red blood cells by adding ammonium chloride to cells in a 4:1 ratio, incubate cells on ice for 10 min, and centrifuge at 300 x g for 5 min at 22°C. Resuspend the mononuclear cell pellet in complete media with growth factors.
MACS LS magnetic separation columns have a maximum capacity of 2 × 109 total cells and 108 magnetically labeled cells. De-gas buffer by applying a vacuum to the buffer at room temperature. Excessive gas in the buffer will form bubbles, and decrease the CD34+ cell yields. Use the column immediately after filling to avoid formation of air bubbles. Use a maximum cell concentration of 108 cells per 500 μl of buffer.
CD34+ cells generally survive cryopreservation well, with some variation between procedures. Cell loss due to crystallization can occur, and affect the overall yield of cells. Viability as measured by trypan blue dye exclusion may yield viabilities ranging between 75–90%. Remove cell debris from thawed CD34+ cells by centrifuging at 150 x g for at least 5 min. Resuspend CD34+ cells in complete media with growth factors.
CD34+ cells may initially proliferate 100 times or more the starting number of cells if rhSCF is combined with rhIL-3 and rhIL-6, so do not culture greater than 5 × 104 cells per mL. Luxurious growth is seen over the first 2–3 weeks, though debris will begin to accumulate due to non-HuMC lineage cell apoptosis and necrosis. Adherent macrophages also may start to proliferate by 2 weeks. Check cultures weekly and separate non-adherent HuMC committed progenitors from adherent cells and debris. Gently pipette and remove non-adherent cells to a new flask, or centrifuge non-adherent cells and culture media at 150 x g for 5 min, resuspend cells in complete media with growth factors and culture in a new flask. The four week time point appears to be a critical juncture, and cultures not properly cared may undergo significant HuMC loss. To counteract this, remove and replenish 95% of the media at 4 weeks.
SCF alone will give rise over 7–10 weeks to pure HuMC cultures, however HuMC numbers are less, and less cell debris is seen at all weeks in culture. IL-3 increases all cell lineages and is a basophil growth factor, but will not give rise to significant numbers of basophils if used only for the first week in the presence of rhSCF and rhIL-6. IL-6 helps supports HuMC growth and maturation, and prevents apoptosis.
5. References
- 1.Metcalfe DD (2008) Mast cells and mastocytosis. Blood 112, 946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee E, Min HK, Oskeritzian CA, Kambe N, Schwartz LB and Wook, Chang H (2003) Recombinant human (rh) stem cell factor and rhIL-4 stimulate differentiation and proliferation of CD3+ cells from umbilical cord blood and CD3+ cells enhance FcepsilonR1 expression on fetal liver-derived mast cells in the presence of rhIL-4. Cell Immunol. 226, 30–6. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T and Koike K (2003) IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J. Immunol 170, 3461–7. [DOI] [PubMed] [Google Scholar]
- 4.Piliponsky AM, Gleich GJ, Nagler A, Bar I and Levi-Schaffer F (2003) Non-IgE-dependent activation of human lung- and cord blood-derived mast cells is induced by eosinophil major basic protein and modulated by the membrane form of stem cell factor. Blood 101, 1898–904. [DOI] [PubMed] [Google Scholar]
- 5.Kambe N, Kambe M, Chang HW, Matsui A, Min HK, Hussein M, Oskerizian CA, Kochan J, Irani AA and Schwartz LB (2000) An improved procedure for the development of human mast cells from dispersed fetal liver cells in serum-free culture medium. J Immunol Methods 240, 101–10. [DOI] [PubMed] [Google Scholar]
- 6.Kambe M, Kambe N, Oskeritzian CA, Schechter N and Schwartz LB (2001) IL-6 attenuates apoptosis, while neither IL-6 nor IL-10 affect the numbers or protease phenotype of fetal liver- derived human mast cells. Clin Exp Allergy. 31, 1077–85. [DOI] [PubMed] [Google Scholar]
- 7.Anderson HB, Holm M, Hetland TE, Dahl C, Junker S, Schoitz PO, and Hoffman HJ (2008) Comparison of short term in vitro cultured human mast cells from different progenitors- Peripheral-blood derived progenitors generate highly mature and functional mast cells. J. Immunol. Methods 336, 166–174. [DOI] [PubMed] [Google Scholar]
- 8.Tkaczyk C, Okayama Y, Metcalfe DD and Gilfillan AM (2004) Fcε receptors on mast cells: activatory and inhibitory regulation of mediator release. Int. Arch. Allergy Immunol 1333, 305–315. Adapted with permission from S. Karger AG, Basel. [DOI] [PubMed] [Google Scholar]