Abstract
Despite the common use of guinea pigs in investigations of the neural mechanisms of binaural and spatial hearing, their behavioral capabilities in spatial hearing tasks have surprisingly not been thoroughly investigated. To begin to fill this void, we tested the spatial hearing of adult male guinea pigs in several experiments using a paradigm based on the prepulse inhibition (PPI) of the acoustic startle response. In the first experiment, we presented continuous broadband noise from one speaker location and switched to a second speaker location (the “prepulse”) along the azimuth prior to presenting a brief, ~110 dB SPL startle-eliciting stimulus. We found that the startle response amplitude was systematically reduced for larger changes in speaker swap angle (i.e., greater PPI), indicating that using the speaker “swap” paradigm is sufficient to assess stimulus detection of spatially separated sounds. In a second set of experiments, we swapped low- and high-pass noise across the midline to estimate their ability to utilize interaural time- and level-difference cues, respectively. The results reveal that guinea pigs can utilize both binaural cues to discriminate azimuthal sound sources. A third set of experiments examined spatial release from masking using a continuous broadband noise masker and a broadband chirp signal, both presented concurrently at various speaker locations. In general, animals displayed a reduction in startle amplitude (i.e., greater PPI) when the masker was presented at speaker locations near the chirp signal. In summary, these results indicate that guinea pigs can: 1) discriminate changes in source location within a hemifield as well as across the midline, 2) discriminate sources of low- and high-pass sounds, demonstrating that they can effectively utilize both low-frequency interaural time and high-frequency level difference sound localization cues, and 3) utilize spatial release from masking to discriminate sound sources. This report confirms the guinea pig as a suitable spatial hearing model and reinforces prior estimates of guinea pig hearing ability from acoustical and physiological measurements.
Keywords: Guinea pig, sound localization, interaural level difference, interaural time difference, minimum audible angle
2. INTRODUCTION
Guinea pigs are a common model for studies of the auditory system. They are a good model for comparisons to human hearing as they have comparable hearing ranges (Heffner et al., 1971; Prosen et al., 1978) and are precocial, with functional (though not mature) peripheral and central auditory systems at birth (Sedlacek, 1976). Guinea pigs are commonly used for anatomical studies, where the anatomical projections within the auditory pathways are well characterized (e.g. Anderson et al., 2007; Caird et al., 1991; Coomes et al., 2004; Redies et al., 1989b; Schofield et al., 1991; Schofield et al., 1992; Schofield et al., 1996; Schofield et al., 1997; Shore et al., 1998; Smith, 1995; Zhao et al., 1996). There have been numerous physiological studies along the auditory pathway, including the auditory brainstem (e.g. Stabler et al., 1996; Winter et al., 1990), inferior colliculus (e.g. Hoa et al., 2008; McAlpine et al., 1996a; McAlpine et al., 1996b; Shackleton et al., 2010; Shackleton et al., 2003; Sterbing et al., 2003; Syka et al., 2000; Zohar et al., 2011), superior colliculus (e.g. Carlile et al., 1987a; King et al., 1983; King et al., 1985; Platt et al., 1998; Sterbing et al., 2002), thalamus (e.g. Jahnsen et al., 1984; Redies et al., 1991), and auditory cortex (e.g. Bakin et al., 1990; Grimsley et al., 2012; Montejo et al., 2015; Redies et al., 1989a; Rutkowski et al., 2000; Wallace et al., 2000a; Wallace et al., 2000b). Guinea pigs have been used extensively in studies of auditory disorders, including tinnitus (Berger et al., 2013; Dehmel et al., 2012a; Dehmel et al., 2012b), noise induced hearing loss (Furman et al., 2013; Lin et al., 2011; Mulders et al., 2013; Robertson et al., 2013; Shi et al., 2013), and otitis media (Gan et al., 2013; Guan et al., 2013; Wang et al., 2016). Furthermore, the acoustics of the guinea pig external ears, head, and torso have been assessed in several studies (Carlile et al., 1987b; Sinyor et al., 1973; Sterbing et al., 2003). In particular, our lab recently described the directional transfer functions (DTFs) of adult (Greene et al., 2014) and developing (Anbuhl et al., 2017a) guinea pigs. Acoustical measurements suggest that all three cues to sound location, including the monaural (i.e., spectral shapes) and binaural cues (interaural time [ITD] and level [ILD] differences), are available to the guinea pig and are likely utilized for sound localization behavior.
Surprisingly, despite this large body of literature investigating anatomy, physiology, and acoustics in the guinea pig, there lacks a comprehensive test of behavioral hearing ability, and in particular, spatial hearing ability. There are some studies that do describe different aspects of guinea pig hearing. Heffner et al. (1971) and Prosen et al. (1978) used classical conditioning methods to determine absolute and frequency-difference thresholds (i.e., a behavioral audiogram). Clements and Kelly (1978) found that normal hearing newborn (1–4 or 11–31 days old) guinea pigs could discriminate two targets separated by 90°, and that this level of performance is disrupted when animals are raised (for 21 days) with unilateral ear plugs. Thus far, it seems guinea pigs have not been good model for behavioral studies that require training on complex tasks, as they are often difficult to train (Jonson et al., 1975). To overcome this barrier, we propose to use an alternative, reflex-based method as they are known to elicit a robust startle response to a startle-eliciting stimulus (SES) (Avery, 1928a; Avery, 1928b; Dehmel et al., 2012a; Dodge et al., 1926; Rawdon-Smith et al., 1938). Here, we use a modification of the acoustic startle response (Young et al., 1983), initially described by Allen et al. (2010) for mice, to assess the spatial hearing capabilities of guinea pigs.
We use this technique to describe the ability of guinea pigs to: 1) discriminate changes in source location across the midline as well as within a hemifield, 2) discriminate sources of low- and high-pass sounds, demonstrating that they can effectively utilize both low-frequency interaural time (ITD) and high-frequency level difference (ILD) sound localization cues, and 3) utilize spatial release from masking to discriminate sound sources. The results of these experiments confirm that the guinea pig is a suitable model for studying binaural and spatial hearing, and reinforce prior acoustical and physiological measurements.
3. METHODS
3.1. Subjects
Fifteen adult male pigmented guinea pigs (Elm Hill Labs, Chelmsford, MA) were included in this study. Animals were individually or pair housed in stainless steel cages, and socialized in a larger group once a week by vivarium staff. The animals were provided with food and water ad-libitum, and supplemented with Timothy Hay except during testing. Animals were weighed before each recording session to assess the stress level and health during testing, and were regularly checked by vivarium and veterinary staff for injury or illness. Experimental procedures complied with the guidelines of the University of Colorado Anschutz Medical Campus Animal Care and Use Committees and the National Institutes of Health.
3.2. Apparatus
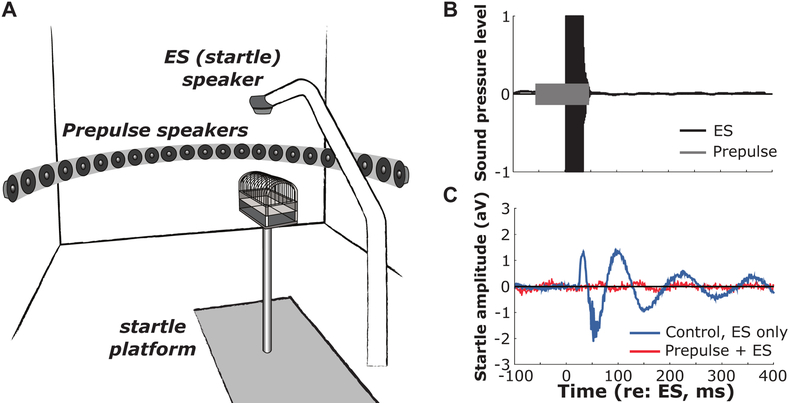
All experiments were conducted in a double-walled, sound-attenuating chamber (interior dimensions: ~3 × 3 × 3 m; IAC, Bronx, NY) lined with echo-attenuating acoustical foam. The animal was placed in a custom-built acoustically transparent aluminum wire cage mounted on a polyvinyl chloride (PVC) post anchored to a flexible polycarbonate platform (Figure 1A). The majority of the experiments described below require that the animal maintains a forward-facing orientation. Guinea pigs can turn their heads to either side somewhat; however, qualitative assessments revealed that head position was centered, on average, as long as the animal remained forward facing in the cage (i.e. did not turn around). To ensure the animal maintains this position, the cage, which comfortably housed the animal, was positioned such that the head of the animal was facing towards the center loudspeaker. The animal was prevented from turning around by reducing the cage width using moveable wire combs until they were pressed lightly against the sides of the animal. All animals were tested in the dark, and were visually monitored using a closed-circuit infrared (IR) camera to ensure the correct orientation. Prepulse stimuli were presented from 25 loudspeakers (Morel MDT-20) spaced along a 1m radius semicircular boom at 7.5° increments, from −90° (right) to +90° (left). The speaker boom was oriented horizontally (i.e. 0° elevation) for all experiments. Startle-eliciting stimuli were presented from a Faital Pro HF102 compression driver mounted ~25 cm above the animal and amplified with a Yamaha M-40 power amplifier. The startle response of the animal was captured using a cage-mounted accelerometer (Analog Devices ADXL335). An example startle response is shown in Figure 1C.
Figure 1.
Acoustic startle apparatus to assess spatial hearing ability. A: Illustration of startle apparatus. Experiments were performed in a double-walled, sound-attenuating chamber. Prepulse stimuli were presented from 25 loudspeakers (Morel MDT-20) attached to a semicircular arc (1m radius). The speakers were spaced in azimuth along the arc at 7.5˚ apart, from 90˚ left to 90˚ right. An additional loudspeaker providing the startle stimulus (SES) is positioned above the animal. The animal comfortably sits in a wire-mesh cage and always faces forward towards 0˚, 0˚. The cage is mounted on a post, which is secured onto a flexible platform. An accelerometer mounted to the base of the platform measures the startle response amplitude. B: Normalized, example sound pressure levels for the prepulse (gray) and SES (black) stimuli. C: Example startle response amplitudes for the control condition (blue, SES only) and for the prepulse condition (red).
Three Tucker-Davis Technologies (TDT) RP2.1 Real-time Processors controlled by custom written MATLAB (MathWorks) software generated the stimuli and recorded the startle response. Startle-eliciting stimuli (SES) were 20ms duration uniformly distributed broad-band noise bursts (rectangular-gated, 50 kHz bandwidth) dynamically generated by the first RP2.1, and presented at 110 dB SPL. Carrier stimuli (CS) consisted of uniformly distributed broad-band noise dynamically generated by the second RP2.1, and presented continuously (except when otherwise noted) during testing. In some experiments the broad-band noise CS was low-, high-, or band-pass filtered with a 100th order FIR filter that was designed in MATLAB and implemented in the second RP2.1. The CS was presented from one speaker at a time, controlled by switching the output from channel 1 to 2 on the third RP2.1 (which received the CS as an input). The speakers corresponding to these outputs were dynamically set by two sets of TDT PM2Relay power multiplexers, controlled by the first and third RP2.1. The motion of the polycarbonate plate resulting from the startle response was transduced by an accelerometer, and the voltage output sampled at 1 kHz by the first RP2.1. The startle response amplitude was calculated as the RMS of the accelerometer output in the first 100ms period after the delivery of the SES.
3.3. Experimental procedure
3.3.1. General
In all experiments, each condition was presented at least 11 times, and the first presentation of each condition within a session was excluded from analysis. Each experiment was split across several days due to the large number of conditions. Conditions were pseudo randomly assigned to each test session, along with appropriate controls, such that similar conditions were spread roughly evenly across days. Each test session was limited to ≤60 minutes. Stimulus presentation and response measurement was computer controlled.
3.3.2. Minimum audible angle
Minimum audible angle was assessed using the speaker-swap (SSwap) paradigm (Allen et al., 2010). The animal was oriented towards the 0° (center) or −45° (right of center) speaker, by rotating the wire cage, to test responses to sounds swapped across the midline and within a hemifield, respectively. Test sessions lasted approximately 60 minutes and sessions were separated by at least one rest day to minimize adaptation to the SES. Each test session started with a two-minute acclimatization period in the presence of continuous background noise specified by one (randomly chosen) experimental condition. The inter-trial interval (ITI) averaged 20s, and varied randomly between 15–25s in 1s increments. The prepulse in SSwap experiments was a change in the presentation location of the CS between two matched speakers with various separation angles, and the time-course of the effect was determined by varying the inter-stimulus interval (ISI) at which the prepulse was presented before the SES (i.e. both the swap angle and the ISI were varied across trials in each test session). An exception is the first SSwap experiment, where the swap angle was held constant and either the first or second speaker was silenced to test the effect of the rising (Noise Onset) and falling (Noise Offset) edges of the prepulse independently (see Figure 2). Control conditions, in which no speaker swap occurred, were presented for each speaker angle presented in a test session.
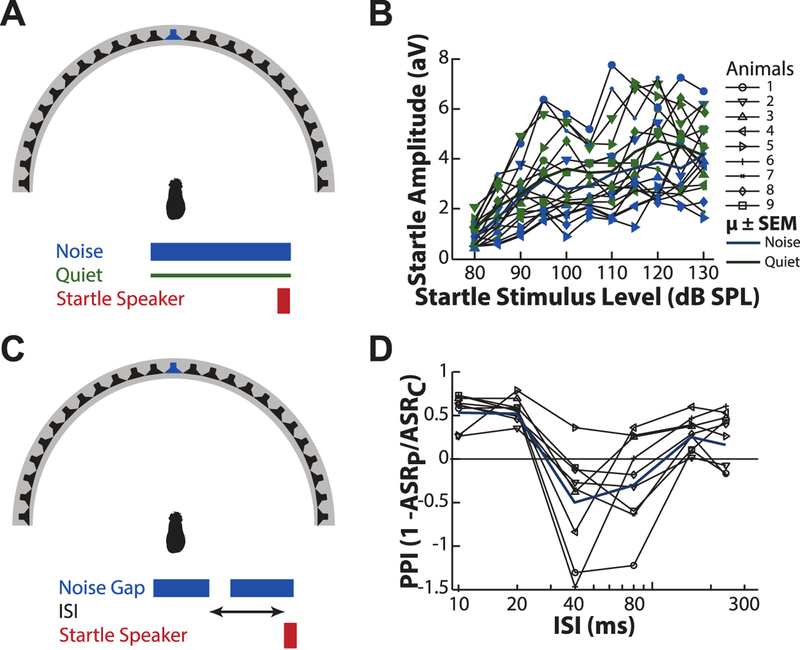
Figure 2.
Baseline tests: acoustic startle threshold and gap detection. A: Illustration of the experimental setup. Animals were placed in the center of the speaker array, in silence or in the presence of 70 dB SPL background noise presented from the 0° speaker (blue). The SES was presented from a speaker directly over the animal’s head. B: The evoked startle amplitude (ASR) is shown as a function of SES amplitude for both quiet (green) and noise (blue) background conditions. Thin lines with markers represent the mean performance of each animal, while thick lines ± shaded areas represent mean ± SEM across the population of animals tested. C: Gap detection task setup. A 10 ms duration gap in a 70 dB background noise was presented at a variable ISI preceding the SES (measured from the onset of the gap) from the 0° speaker (blue). D: Prepulse inhibition of the startle (PPI; 1 – ASRPrepulse/ASRControl) is shown as a function of the gap ISI. Note: positive PPI values represent a reduction in ASR, negative values represent increased ASR, and PPI = 0 represents no change relative to control ASR.
3.3.3. Spatial release from masking
Spatial release from masking (SRM) was similarly assessed by presenting a 12.5 ms duration broadband chirp, repeated eight times, from speakers located at 0°, 45°, and 90° (re: midline), at an ISI of 100 ms before the SES. Signals were presented at several sound levels in silence or in the presence of continuous 70 dB SPL broadband noise presented from one of several speakers (pseudorandomly varied from trial to trial) within the same hemifield as the signal. Speakers presenting the signal and noise masker were separated by 7.5° - 90°. The prepulse in SRM experiments was the broadband chirp acoustic stimulus.
3.4. Data analysis
The acoustic startle response (ASR) was assessed as the RMS output of the accelerometer, amplified by 25 dB, in the 100 ms following the startle stimulus presentation. The units of ASR are given as an arbitrary voltage (aV), which is consistent across recording sessions. The mean ASR was calculated for each animal, on each condition, with the first block excluded to minimize skew resulting from an initial adaptation to the SES. Prepulse inhibition is quantified as 1 minus the ratio of the mean ASR with the prepulse to the mean ASR with no prepulse (control) response recorded each test session:
Therefore, a positive PPI value represents a reduction in the startle amplitude, where a PPI of 1 indicates complete suppression of startle (i.e., detection of the speaker swap), a PPI value of 0 indicates the startle response is equal for both the control and speaker swap conditions (i.e., no detection of swap), and a negative value (< 0) indicates facilitation, or enhancement, of the startle response relative to the control response. Responses of the population of animals tested in each condition are summarized as the mean ± standard error of the mean (SEM) of the PPI, and were assessed with repeated-measures analysis of variance (ANOVA). Individual conditions in experiments that show a significant change across conditions in the ANOVA are further assessed for a change re: control (i.e. PPI ≠ 0) with Bonferroni corrected two-tailed Student’s t-tests.
4. RESULTS
The results are based on experiments conducted with 15 adult male guinea pigs. Animals were tested in three groups: the first group (n = 9) were tested on most conditions, whereas the second and third groups (n = 3 each) were tested on a limited set of the experiments refining the results obtained from the first group (described explicitly below). Animals were weighed at the beginning of each test session, which varied between 0.6 and 1.2 kg (generally increasing with age). Head and pinna dimensions were not routinely measured, but were stable and generally consistent with values measured during acoustical measurements on different groups of guinea pigs (Anbuhl et al., 2017a; Greene et al., 2014).
4.1. Baseline assessment of the startle reflex
4.1.1. Threshold of startle response
Initial testing was performed on each animal to determine the threshold of the startle response with increasing SES level in both quiet and in the presence of background noise. SES was presented, randomly ordered, between 80 and 130 dB SPL in 5 dB steps, first in silence and second in the presence of continuous 70dB broadband noise (presented from straight-ahead; Figure 2A). Here, no prepulse is presented. Figure 2B shows the recorded startle amplitude (mean ± SEM; black line ± gray area; aV units) shown as a function of startle amplitude (dB SPL) in quiet (green) and in the presence of noise (blue). In both conditions, startle amplitude increases with increasing SES intensity up to ~100 dB SPL, above which the response rises more slowly. Startle amplitude within this region was generally greater in the quiet than the background noise condition.
Comparisons of the startle response across startle level were assessed with a two-way ANOVA with SES level and repetition number as independent variables, and startle amplitude as the dependent variable. Results reveal significant main effects of SES level for both the quiet and 70 dB noise conditions (F10,880 = 15.64, p << 0.001, and F10,880 = 12.27, p << 0.001, respectively), a significant main effect for repetition in the 70 dB noise condition only (F9,880 = 3.97, p = 0.0001), and no significant interaction in either test. Post-hoc pair-wise comparisons with a Tukey HSD (honest significant difference, assessed at α = 0.05) test revealed several significant differences across SES levels, but most importantly a difference in startle amplitude between 80 dB and all startle levels 90 dB or greater in both quiet and noise conditions. Student’s t-tests reveal significantly different startle amplitudes than zero for all levels in both conditions (p<<0.001). The startle amplitude threshold was comparable to a previous report in guinea pig (Dehmel et al., 2012a), and generally plateaued for SES levels above ~100 dB SPL, thus the SES intensity was set at 110 dB SPL for the remainder of testing.
4.1.2. Verification of PPI
A second baseline test using a simple gap-detection task was used to establish that guinea pigs exhibit prepulse inhibition of the startle response. Startle amplitude was assessed in response to conditions containing a 10 ms gap (quiet time) in a 70 dB SPL broadband noise background (where the gap serves as the prepulse), presented from straight ahead, preceding the SES by 10, 20, 40, 80, 160, and 240 ms (Figure 2C), as well as two control conditions in which no gap was present (with and without background noise). Results are shown in Figure 2D, where startle amplitude (mean ± SEM; black line ± gray area) is shown as a function of ISI, the average startle response was suppressed by approximately 50% (PPI ~ 0.5) by a gap with a 10 or 20 ms ISI, somewhat facilitated (i.e. the startle response was greater than the control) by approximately 50% (PPI ~ −0.5) for gaps with a 40 ms and 80 ms ISI, and approached that of control (PPI ~ 0) for longer intervals.
Significance of these startle responses was assessed with a two-way ANOVA with ISI and repetition as independent variables, and PPI as the dependent variable. Significant main effects of each independent variable were observed (F5,480 = 27.5, p << 0.001, and F9,480 = 3.76, p = 0.0001, respectively), whereas no significant interaction was observed (F45,480 = 1.34, p = 0.077). Post-hoc pair-wise comparisons with a Tukey HSD (honest significant difference, assessed at α= 0.05) test revealed the first repetition was significantly different (lower PPI) than later repetitions (5–10). Bonferroni corrected Student’s t-tests (α = 0.0083) revealed that all PPIs are significantly different than zero (control) for all ISIs except 80 ms (p = 0.025). All nine animals (black lines) showed the same general trend, showing significant PPI and/or PPF for short ISIs, thus were included in further testing.
4.2. 180° Swap, stimulus onset, and stimulus offset
Base assessment of spatial hearing ability was tested by measuring the startle amplitude in response to a 180° speaker swap condition. As illustrated in Figure 3A, the speaker swap experiment consists of two simultaneous events: the offset of the initial speaker (speaker 1), and onset of the swap speaker (speaker 2). In order to assess the relative contributions of each, and verify that the responses to the speaker swap are not dominated by either a gap detection or noise burst detection, we tested the onset and offset events in isolation from one another, and compared the responses to the combination. That is, a set of trials in which speaker two is inactivated (thus only the noise offset from speaker one remains), and another set of trials in which speaker one is inactivated (thus only the noise onset from speaker two remains), is compared to the full speaker swap condition. The prepulses for these three conditions, therefore, are: the sound offset, the sound onset, and the sound source location change. The broadband noise was presented at 70 dB SPL, and was followed by the SES with ISIs of 2, 5, 10, 20, 40, 80, and 160 ms to track the temporal effects on the startle response. Two additional control conditions, (two trials) with and (one trial) without background noise, were also presented. Overall, a total of 24 conditions were tested over the course of three test sessions, presented 6 repetitions per session (15 repetitions are included in the final analysis after excluding the first repetition in each session).
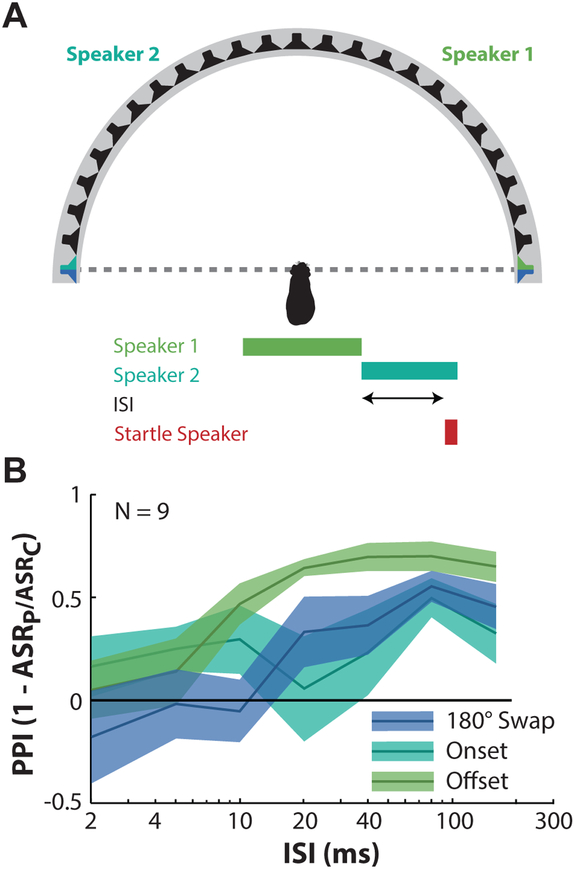
Figure 3.
180° speaker swap condition. A: Experimental setup: A 70 dB SPL broadband noise is initially presented from the speaker 90° to the right of midline, and is instantaneously swapped to the speaker 90° to the left of midline at a variable ISI preceding the SES. In two additional experiments one of the two speakers was turned off to determine the response to the prepulse offset of a noise (speaker 1 shuts off prior to SES) or the onset of a noise (speaker 2 turns on prior to SES). B: Mean (± SEM) PPI across the 9 animals tested as a function of ISI for each of the experimental conditions shown in A.
The results of the 180° speaker swap are shown in Figure 3B where PPI is represented on the ordinate, ISI on the abscissa, and the three conditions (mean ± SEM) are plotted independently. PPI was calculated based on comparisons to the control condition with a comparable background noise environment: the 180° swap and the noise offset condition were compared to the background noise control, and the noise onset condition was compared to the quiet background control condition. The 180° swap produced a substantial PPI compared to control (0.3–0.5) for ISIs >10 ms, and no inhibition for shorter intervals. The noise onset condition resulted in a similar PPI at long intervals, but was substantially more variable below 80 ms ISI. In contrast, the noise offset resulted in PPI with a greater magnitude (up to ~0.75), and at shorter ISIs (>5 ms vs >10 ms) than the swap condition.
Results were assessed via a two-way ANOVA with PPI magnitude as the dependent variable, and ISI and swap condition as independent variables. Significant main effects of both dependent variables were observed (ISI: F6,924 = 6.35, p << 0.001; Condition: F2,924 = 9.5, p = 0.0001). Additionally, a significant interaction between these variables was observed (F12,924 = 3.27, p = 0.0001). Post-hoc pair-wise comparisons with a Tukey HSD (honest significant difference, assessed at α= 0.05) test revealed PPIs for conditions with ISIs 20 ms and longer were significantly larger than for 2 ms (additionally, 80 ms is significantly different than 5 and 10 ms). Similarly, each swap condition was significantly different from one another.
These results suggest that the PPI produced by the 180° speaker swap contains two opposing effects. First, the offset of the noise produces a fast, strong inhibition in the startle response. Second the noise onset in the second speaker produces a slower and weaker inhibition. When combined, the resulting startle response to the 180° swap is intermediate between the two in both speed and amplitude, and it is unlikely that the startle response to the speaker swap is a result of the sound onset or offset alone. The speaker swap, therefore, appears to be a reasonable method to probe guinea pig spatial hearing abilities.
4.3. Discrimination across the midline
Minimum audible angle in the frontal field was assessed by swapping the location of a continuous broadband noise CS symmetrically across the midline in two sets of animals. The prepulse was the change in sound source location for all SSwap experiments (sections 4.3, 4.4, 4.5, and 4.7). The first set of animals performed a task in which the sound source location was swapped between speakers separated by 90°, 45°, 30°, 15°, and 7.5° (asymmetrically swapped between the 0° and 7.5° speakers due to setup constraints), preceding the SES with ISIs of 1, 2, 5, 10, 20, 30, 40, 50, 60, 100, 150, 200, and 300ms, as illustrated in Figure 4A. These 65 conditions, along with control conditions, were presented over the course of 6 sessions, with 15 conditions (14 on session 6) pseudorandomly assigned (such that each angle was presented each day) and presented 11 times per session (the first presentation excluded from analysis). Control conditions, in which no swap occurred, were presented from each of the four starting speaker locations (45°, 22.5°, 15°, and 7.5°), and were presented once each per repetition.
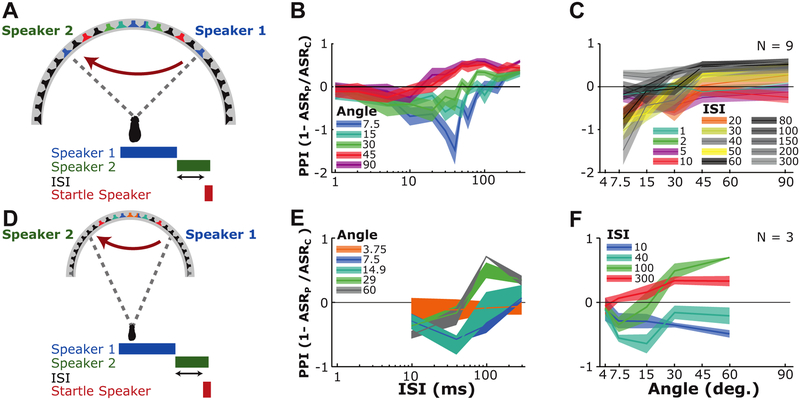
Figure 4.
Sound source discrimination across the midline. A(D): The experimental setups were largely similar to those shown in Figure 3, except in D the animal was moved backwards from the center of the arc to create smaller subtended angles. Note, due to the presence of a speaker on the midline, the center point of the narrowest angles tested were slightly offset to the right. B(E): mean (± SEM) PPI as a function of ISI for the five angles tested. C(F): mean (± SEM) PPI as a function of subtended angle for the 14(4) ISIs tested.
Figure 4B, C shows the results of varying speaker swap angle across the midline, where the PPI (mean ± SEM) for each angle condition is shown as a function of ISI. Consistent with the results from the 180° swap condition presented above, the average PPI was essentially zero for intervals shorter than 10 ms, and approached the maximum PPI observed at 180° (in Figure 3) for large ISIs (>100 ms), where the magnitude of that PPI correlated positively with swap angle. At intermediate intervals (~10 ms – 100 ms), however, responses differ substantially based upon the swap angle: large swap angles (>30°) elicited substantial PPI (0.25 – 0.5), whereas small swap angles elicited strong prepulse facilitation (PPF; as low as −1.5). PPF was inversely correlated with swap angle, such that the smallest swap angles elicited the greatest PPF magnitude. These results suggest that PPF is a substantial feature of guinea pig ASR, and may be more prominent than in other rodents (Plappert et al., 2004).
Results were assessed with an analysis of variance with ISI and angle as independent variables, and PPI as the dependent variable. Main effects of both variables, and their interaction, were evident (ISI: F4,1180 = 109, p << 0.001; Angle: F13,1180 = 33.0, p << 0.001; interaction: F12,1180 = 6.91, p << 0.001). Post-hoc analysis with a 2-tailed Student’s t-test (α = 0.05, α = 0.0007 after Bonferonni correction) revealed PPI was significantly different than zero (control) at several ISIs (5 ms or longer) for each angle, including 7.5° which was significantly different than zero for ISIs between 5–60 ms and ≥ 200 ms intervals. The presence of significant and substantial PPF in response to short intervals, and PPI to long intervals, in the 7.5° swap condition suggests that the guinea pig minimum audible angle is somewhat smaller than 7.5° for broadband noise.
In order to more carefully characterize the minimum angle that guinea pigs can discriminate, the second group animals (n = 3) was tested in a comparable experiment where the angle between the center speakers were adjusted from 7.5° to 3.75°. This was accomplished by doubling the distance between the cage and the center speakers, which, as result, approximately halved the subtended angle between the center speakers (Figure 4D). Note, the sound presentation was not altered thus the sound pressure level at the animal’s ears are expected to be reduced by ~6 dB for central speakers, and by ~1.5 dB for speakers at ±90°, due to the increased distance; however, this effect is not expected to significantly influence MAA assessments since: 1) the sounds are swapped symmetrically across the midline, 2) each assessment was compared to a control condition from the appropriate initial speaker, 3) sounds are presented well above threshold, and 4) the stimulus is a continuous broadband noise, interaural time differences are not expected to be affected by distance, and interaural level differences are not expected to vary for sources over 1m away (Jones et al., 2015; Kim et al., 2010). Results are shown in Figure 4E,F where PPI is shown as a function of swap angle (mean ± SEM).
Results were once again assessed with an analysis of variance with ISI and angle as independent variables, and PPI as the dependent variable. Main effects of both variables, and their interaction, were evident (ISI: F4,1180 = 12.05, p << 0.001; Angle: F3,1180 = 29.29, p = << 0.001; interaction: F12,1180 = 5.53, p << 0.001). Post-hoc analysis with a 2-tailed Student’s t-test (α = 0.05, α = 0.0025 after Bonferonni correction) revealed PPI was not significantly different from zero for any ISI tested at 3.75°, in contrast at least two ISIs were significant for 7.5° or larger swap angles, consistent with testing in the previous experimental group. These results thus suggest that guinea pig MAA is between 3.75° - 7.5°.
4.4. Discrimination within a hemifield
Laboratory rats, another common model used in the study of the auditory system, do not appear to discriminate between two sound sources within a hemifield, and show few deficits in sound localization ability following lesion of auditory cortex (Heffner et al., 1985; Heffner et al., 1994a; Kavanagh et al., 1986; Kelly, 1980; Kelly et al., 1986; Wesolek et al., 2010). We investigated the guinea pig’s ability to discriminate sound sources within a hemifield using a similar SSwap task as described above, using a broadband noise CS, but with the animal cage rotated 45° to the right (Figure 5A). The task thus requires discrimination of two sound sources entirely contained within one hemifield (0–90°). In order to reduce recording times, SSwaps across each angle (7.5, 15, 30, 45, and 90°) were tested with a more limited set of ISIs (5, 10, 20, 40, 80, 160, and 300 ms), for a total of 39 conditions tested across three recording sessions.
Figure 5.
Sound source discrimination within a hemifield. A: The experimental setup is similar to that described in Figure 4A, except the animal was rotated 45° to the right, thus all sound presentations were within the animal’s left hemifield, and the range of ISIs tested was shortened. B: Mean (± SEM) PPI as a function of ISI for the five angles tested.
The results of SSwap testing within a hemifield, as illustrated in Figure 5B, reveal a largely similar pattern of responses to the midline condition for most swap angles, where responses are comparable in magnitude to control (PPI ~0) for short ISI (5 ms), show strong PPF for moderate ISIs (< ~80 ms, angle dependent), and are comparable to the 180° swap in conditions with long ISIs (e.g. 300 ms). However, a substantial difference is observed at the smallest swap angle condition (7.5°), which does not deviate substantially (neither positively nor negatively) from control (PPI ~0) at any ISI, thus guinea pigs do not appear to be able to discriminate. MAA for 45° offset sound sources, therefore, appears to be between 7.5° and 15°. For larger angles, substantial PPF (an increase in startle amplitude of ~150% or PPI ~ −1.5) is observed for intermediate angles (15°, 30°), at intermediate ISIs (10–80 ms), and substantial PPI (startle amplitude decrease of ~1/2 or PPI ~ 0.5) is observed for large angles (ISI > ~ 40 ms).
Significance of these results was assessed with an analysis of variance with ISI and angle as independent variables, and PPI as the dependent variable. Main effects of both variables, and their interaction, were evident (ISI: F4,3565 = 52.92, p << 0.001; Angle: F6,3565 = 59.88, p << 0.001; interaction: F24, 3565 = 10.1, p << 0.001). Post-hoc analysis with a 2-tailed Student’s t-test (α = 0.05, α = 0.0014 after Bonferonni correction) revealed PPI was not significantly different for any ISI tested at 7.5°, in contrast at least four ISIs (longer than 5ms) were significant for 15° or larger swap angles, consistent with testing in the previous experimental group. In summary, while sound source discrimination ability within a hemifield appears broader than across the midline (<15° compared to 4–7.5°), guinea pigs appear comparable to other mammalian species in their ability to discriminate within the hemifield (reviewed further in the discussion), apart from the common laboratory rat which cannot discriminate within the hemifield.
4.5. Discrimination of band-limited noise
Azimuthal sound localization ability is largely mediated by interaural time differences (ITDs) for low frequencies, and by interaural level differences (ILDs) for high frequency sounds (Tollin, 2003). In order to estimate the minimum sensitivity to each of these cues, we performed a set of experiments using band-limited noise, two low-pass bands (<500 Hz and <2 kHz) and a high-pass (>4 kHz) noise CS. The angles and ISIs presented are identical to those tested in the 45° offset condition except that the animal was facing straight-ahead, thus the SSwap task was symmetric about the midline (Figure 6A). Testing for each CS was completed over the course of three sessions (9 sessions total).
Figure 6.
Discrimination of band-limited sound sources. A: The experimental setup is similar to that described in Figure 3A and 4A, except the sound source was 70 dB SPL high- or low-pass filtered noise. B: Mean (± SEM) PPI as a function of ISI for the five angles tested in response to noise high-pass filtered with a 4 kHz cutoff. C, D: Mean (± SEM) PPI as a function of ISI for the five angles tested in response to low-pass filtered noise with 2 kHz (C) and 500 Hz (D) cutoff frequencies.
PPI as a function of ISI for high-pass noise (>4 kHz) CS swapped across several angles is shown in Figure 6B. In general, responses showed substantially more PPI, and less PPF, than swapping a broadband noise, especially for large angles. Responses are comparable to control for small ISIs (i.e. 5 ms), and PPI tends to increase with increasing angle for all ISIs; however, large angles can reduce the response magnitude by more than half (PPI >0.5), even at relatively small ISIs (e.g. PPI ~0.5 at 10 ms ISI for the 90° SSwap). Similarly, small angles could show substantial PPF (PPI ~ −1.5), but over a limited range of ISIs, and the ISI showing maximum PPF (minimum PPI) varied with angle. Results of an ANOVA with ISI and angle as dependent variables, and PPI as the independent variable revealed main effects of both variables, and their interaction (ISI: F4,3565 = 80.16, p << 0.001; Angle: F6,3565 = 31.88, p << 0.001; interaction: F24, 3565 = 7.11, p << 0.001). Post-hoc analysis with a 2-tailed Student’s t-test (α = 0.05, α = 0.0014 after Bonferonni correction) revealed PPI was significantly different than zero for at least two ISIs tested (10 ms or longer) for all swap angles, suggesting MAA for high-pass noise (> 4 kHz) is at or lower than 7.5°.
Similarly, Figure 6C shows results of a low-pass noise (< 2 kHz) CS, which produced responses comparable to control for small ISIs (i.e. 5 ms), and responses that showed increasing reduction for larger ISIs, approaching a value somewhat lower (PPI ~0.25) than the broadband condition for large angles and large ISIs (i.e. 300 ms). Similarly, intermediate ISIs produced substantially increased responses (PPI ~−1) for small angles (i.e. 7.5°), that decreased with increasing angle. Furthermore, these response increases were present over the range of ISIs observed in general, responses to low-pass noise appear dominated by PPF, even at the largest ISIs where PPI dominates the response to broadband stimuli. Compared to high-pass noise, responses to low-pass noise elicited substantially less PPI, thus the response to a broadband CS appears intermediate between the two. The significance of these results was assessed with an analysis of variance with ISI and angle as dependent variables, and PPI as the independent variable. Main effects of both variables, and their interaction, were evident (ISI: F4,3565 = 26.5, p << 0.001; Angle: F6,3565 = 40.76, p << 0.001; interaction: F24, 3565 = 7.62, p << 0.001). Post-hoc analysis with a 2-tailed Student’s t-test (α = 0.05, α = 0.0014 after Bonferonni correction) revealed PPI was significantly different for several ISIs for 15°, 30°, and 90° for ISIs of 10ms and longer. Additionally, PPI was significantly different than zero for 7.5° swaps for 5 ms and 160 ms (t89 = 3.52 and 3.99; p = 0.0007 and 0.0001 respectively), though the distribution of PPI observed substantially overlapped with zero and may represent a statistical artifact (particularly the response to a 5 ms ISI). The MAA in response to a 2 kHz low pass noise thus appears to be somewhat larger than to the 4 kHz high pass noise and is in the range of 7.5°−15°.
We further refined our estimate of the guinea pig ability to discriminate low frequency sound sources by presenting low-pass noise filtered with a 500 Hz cutoff CS, thus further limiting the utility of the ILD cue in discriminating the sound sources. Responses are shown in Figure 6D, which once again show the general trend of responses comparable to control for small ISIs (i.e. 5 ms), and responses that showed increasing reduction for larger ISIs, approaching a value somewhat lower (PPI ~0.25) than the broadband condition for large angles and large ISIs (i.e. 300 ms). Similar to the 2 kHz low pass condition, intermediate ISIs produced substantial PPF, with a 7.5° swap elicited the largest change re: control. Significance of these results was assessed with an analysis of variance with ISI and angle as dependent variables, and PPI as the independent variable. Main effects of both variables, and their interaction, were evident (Angle: F4,1715 = 34.9, p << 0.001; ISI: F6,1715 = 21.61, p << 0.001; interaction: F24,1715 = 1.86, p = 0.0068). Post-hoc analysis with a 2-tailed Student’s t-test (α = 0.05, α = 0.0014 after Bonferonni correction) revealed PPI was significantly different than zero for several ISIs for 7.5°, 15°, and 90°, for ISIs of 10ms and longer (though curiously not for 30° or 45°, suggesting the separate inhibition and facilitation mechanisms counteract one another). The MAA in response to a 500 Hz low pass noise, thus appears to be comparable to both the 4 kHz high pass, and the 2 kHz low pass noise, and is in the range of 7.5°.
4.6. Spatial release from masking
To assess the ability of guinea pigs to utilize localization cues to aid in sound source segregation, we conducted a series of experiments assessing spatial release from masking. Prepulse stimuli were 12.5 ms broadband chirps, repeated eight times, and presented with a 100 ms ISI presented in quiet or in the presence of a 70 dB noise masker presented from another speaker in the array within the same hemifield as the signal (Figure 7A). An initial experiment was conducted in which the signal and noise masker were presented from adjacent speakers directly in front of the animal, and the sound level of the signal varied between 20–80 dB (attenuated from full scale). Figure 7B shows responses to the signal presentation in both quiet (0 dB) and in the presence of a continuous 70 dB broadband noise masker. Animals showed substantial PPI in response to loud (<24 dB attenuation) signal presentations for both background conditions; however, while the signal presentation produced substantial PPI down to ~60 dB attenuation in the quiet condition, substantial PPI was only observed down to ~30 dB attenuation in the 70 dB background noise condition. Subsequent testing thus proceeded using a 70 dB noise masker, and the signal levels indicated with colored circles in Figure 7B (24 to 42 dB attenuation in 6 dB steps).
Figure 7.
Spatial release from masking. A: Experimental setup. The animal was placed in the center of the speaker array, and the signal was presented from the left of the animal. The signal was eight repetitions of a 12.5ms duration chirp, presented with a 100 ms ISI. A broadband noise masker was presented at several angles away from the signal speaker. B: Mean (± SEM) PPI as a function of the signal level with the signal and masker presented from adjacent speakers on the midline. The noise masker was presented at both 0 dB (“Quiet”, dark grey) and 70 dB SPL (“Loud”, light grey). Subsequent testing was performed with a 70 dB SPL masker and signal levels between 24–42 dB attenuation. C: Mean (± SEM) PPI as a function of masker speaker angle when the signal speaker was located on the midline (left), 45° to the left (center), and 90° to the left (right). Lines ± areas represent signal levels. The location of the signal speaker is represented with a speaker icon on the x-axis.
Figure 7C shows the results of signal presentation at 0°, 45°, and 90° (left, center, and right panels; indicated with a speaker icon on each x-axis), with the noise masker located at adjacent and more distant speaker positions. In general, responses showed substantial inhibition for loud (24 dB attenuation; PPI ~0.5), and little inhibition for quiet (42 dB attenuation; PPI ~0) signal presentations that was independent of the speaker positions. For intermediate sound levels, and for all three signal locations, responses showed less PPI (i.e. ASR amplitudes were closer to control) for masker locations near the signal than for more distant sources. Specifically: for a signal attenuated by 30 dB and located at 0°, PPI is higher in magnitude when the masker is located at 15° or greater than at 7.5°; for a 45° signal source, PPI is higher for a 36 dB attenuated signal when the masker is presented at 15° or 75° than at 52.5°; and for a 90° source, PPI was higher for 30 dB and 36 dB attenuated signals when the masker was located at 60° or lower, and at 0° respectively, than at larger angles. Responses generally do not show this decrease in PPI at higher levels (24, 30, and 24 dB attenuation respectively), suggesting the chirp train was detected when played from speakers adjacent to the masking source at these higher levels. Depending upon the stimulus condition, therefore, increasing spatial separation from 7.5° to 15° or greater can yield a ~6 dB release from the masking signal.
4.7. The effect of binaural cues on sound source discrimination ability
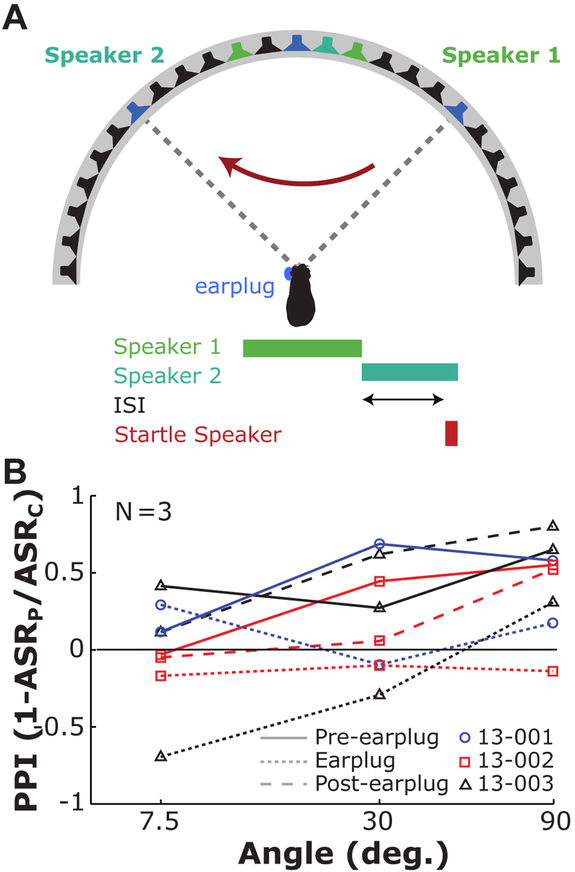
One final set of experiments was conducted on a third group of animals (n = 3), to assess the dependence of binaural hearing on sound source discrimination. Animals were tested using a broadband noise SSwap condition with angles of 7.5°, 30°, and 90°, and an ISI set at 300 ms. Responses of these animals were assessed before, during, and after a reversible unilateral hearing loss was induced using custom silicone earplugs inserted into the left ear canal. This was done to distort the binaural cues available to the animal to determine whether animals can discriminate sounds with one ear alone. Figure 8 shows these results, where symbol and color indicate the individual, and line-style indicates the experimental condition (solid: before insertion, dotted: while the earplug is in place, dashed: after the earplug was removed). All three animals showed robust PPI at 30° and 90° in the pre-earplug condition. Startle amplitudes shifted towards control (PPI = 0) at all angles tested in the plug condition. Finally, in the two animals for which data was collected, reversal of the conductive hearing loss through removal of the ear plug caused PPI to once again approach pre-earplug levels. Overall, performance deteriorated dramatically with a unilateral ear plug in place, suggesting that guinea pigs discriminate changes in sound source location using binaural cues.
Figure 8.
Speaker swap during unilateral earplug insertion. A: The experimental setup is similar to that described in Figure 4A, except tested for a limited set of angles and at a single ISI. B: mean PPI as a function of angle for each of the three animals tested for the three conditions tested (pre-earplug, during earplug, and post-earplug insertion).
5. DISCUSSION
The results of these experiments extend the limited data previously available on the spatial hearing ability of guinea pigs and reinforce its suitability as an animal model for auditory studies, particularly for studies of sound localization. In particular, the experiments described here show that prepulse inhibition of the acoustic startle response is a suitable metric for measuring guinea pig sound localization ability, and reveal that guinea pigs: can discriminate sounds both within and across hemifields, provide estimates of MAAs for broadband, low- , and high-pass noise, show that guinea pigs can use spatial information to unmask broadband stimuli, and suggest that binaural hearing is required to discriminate sound source locations. In the discussion that follows, we explore the relationships with the acoustically measured cues to sound localization, compare behavioral responses to physiological estimates of binaural cue sensitivity, PPI-based sensitivity and other measures, and PPI to PPF.
5.1. Relation to the acoustical cues to sound localization
The range of ILDs available to adult guinea pigs have been described previously (Greene et al., 2014), where we showed that ILD magnitudes are substantial for high frequencies (20–40 dB above ~4 kHz). Assuming that guinea pigs utilize ILDs to lateralize high frequency sounds, and following the methodology used in other studies (e.g. (Koka et al., 2008; Koka et al., 2011)), we can estimate ILD discrimination ability. Using the high-pass SSwap responses reported here (Section 4.5; found to be between 7.5°−15°), and the directional transfer function measurements described previously (Greene et al., 2014), where we computed the ILD vs azimuth slope for sources ±30° about the midline (dB/°) as a function of frequency for a population of adult guinea pigs, we find that the average acoustical ILD-azimuth slope for frequencies from between 4 kHz up to −30 kHz for adults was ~0.2 dB/°. In other words, for every spatial degree there is an average increment of 0.2 dB ILD. We then estimate the behavioral ILD sensitivity (i.e., the psychophysical ILD acuity) of the guinea pigs by multiplying the average ILD slope of 0.2 dB/°by the high-pass noise MAA of 7.5–15° to get a behavioral estimate of ~1.5–3 dB ILD. We also use similar assumptions to estimate behavioral ITD sensitivity, using the MAA from the low-pass noise speaker swap experiment (Section 4.5). Using acoustic directional transfer function measurements, we measured the mean ITD as a function of azimuthal sources ± 90° about the midline for a population of adult guinea pigs (Greene et al., 2014), calculating an average ITD slope for low frequencies (>2 kHz) of ~3 μs/° (ITD per degree). We estimate behavioral ITD sensitivity (i.e., the psychophysical ITD acuity) for the guinea pig as the product of the ITD slope ~3μs/° and low-pass noise MAA of 7.5–15° to obtain a behavioral estimate of ~22.5–45 μs ITD.
The estimated ILD/ITD sensitivity for the guinea pig described here are comparable to other similarly sized mammals used for sound localization studies (see Table 1 for a comparison across species). The guinea pig performs better than smaller animals like the gerbil and rat for both ILD and ITD cues (gerbil, mouse, rat: 3–4 dB ILD, 40–60 μs ITD vs 1.5–3 dB ILD, 23–45 μs ITD) and performs just as well as similarly-sized animals like the chinchilla and ferret. Larger species, like the cat and human, are somewhat more sensitive than the guinea pig to both cues (<1.7 dB ILD, <30 μs). Mammals, therefore, appear to vary in their sensitivity to ILD and ITDs. Heffner (2004) discusses this in great detail, hypothesizing certain evolutionary pressures shape sound localization acuity across mammals. The key factors examined were head size, limit of high-frequency hearing, and width of the field of best vision. Indeed, Table 1 does show the trend of increasing head size with increasing cue sensitivity. Heffner (2004) postulates that animals with larger heads could potentially benefit from the availability of a larger range of acoustical ILDs and ITDs to use in determining the source azimuth.
Table 1.
Cross-species comparisons of estimated ILD and ITD cue sensitivity. Species are ranked with increasing average head size. ILD and ITD sensitivity is estimated from the minimum audible angle (MAA) from high-pass noise and low-pass noise behavioral measures (respectively) and acoustical ILD and ITD ranges in azimuth. Mouse: (Allen et al., 2010; Behrens et al., 2016; Chen et al., 1995; Lauer et al., 2011). Gerbil: (Carney et al., 2011; Heffner et al., 1988a; Maier et al., 2006). Rat: (Heffner et al., 1994a; Kelly, 1980; Koka et al., 2008). Ferret: (Keating et al., 2013; Schnupp et al., 2003). Chinchilla: (Heffner et al., 1994b; Koka et al., 2011). Guinea Pig: (Greene et al., 2014). Rabbit: (Ebert et al., 2008; Kim et al., 2010). Cat: (Heffner et al., 1988b; May et al., 1996; Roth et al., 1980; Tollin et al., 2009; Tollin et al., 2005; Wakeford et al., 1974). Human: (Makous et al., 1990; Mills, 1960; Yost, 1974).
| Minimum Audible Angle (MAA) | Based on MAA and Acoustics | ||||
|---|---|---|---|---|---|
| Species | Average Head Diameter | High-Pass Noise | Low-Pass Noise | Estimated ILD Sensitivity | Estimated ITD Sensitivity |
| Gerbil | 15 mm | 27.4 – 30° | 20 – 30.3° | 4 dB | 40 – 66 μs |
| Mouse | 15.4 mm* *interaural axis | 22.5°–46° | --- | 7.5 – 15.3 dB | --- |
| Rat | 30 mm | 7 – 10° | ~20° | 2.8 – 4 dB | 54 μs |
| Ferret | 32 mm | 12° | 17° | 1.3 dB | 23 μs |
| Chinchilla | 35 mm | 13.7° | 15.7° | 4.1 dB | 55 μs |
| Guinea Pig | 45 mm | 7.5 – 15° | 7.5 – 7.5° | 1.5 – 3 dB | 23 – 45 μs |
| Rabbit | 56 mm | --- | 22° | --- | 50 – 60 μs |
| Cat | 60 – 70 mm | 3.1° | 1 – 6° | 1.7 dB | 30 μs |
| Human | 150 mm | 0.5 – 1° | 0.8° | 0.5 dB | 10 – 20 μs |
5.2. Relation to physiological JND estimates
There are two hypotheses for how neural JNDs give rise to psychophysical JNDs: either the behavioral performance is driven by the best performing neurons or by a pooling of neural responses, where evidence supporting both hypotheses is available in the literature. In support of the former, Skottun et al. (2001) argued that the very low ITD thresholds of human observers are reflected in the performance of single neurons having the greatest acuity. Shackleton et al. (2003) also supported this idea, showing that the lowest JNDs in the guinea pig were comparable with human JNDs, indicating that there is sufficient information in the firing responses of individual neurons to permit discrimination without obligatory pooling. In support of the latter hypothesis, previous studies have argued that pooling of responses from many neurons is required to achieve the very low psychophysical ITD thresholds in humans (Carr, 1993; Fitzpatrick et al., 1997; Gerstner et al., 1996; Hall, 1965). Many of these prior studies have attempted to compare small animal neural responses to human psychophysical responses, since both datasets are available in only a few species. The addition of the current data to the literature thus enables direct comparisons between neural to psychophysical responses within a well-studied mammalian species.
To compare our behavioral data to existing guinea pig physiological data, Figure 9A shows the estimated neural JNDs for ITD discrimination recorded from guinea pig central nucleus of the inferior colliculus (ICC) cells replotted from Shackleton et al. (2003). Importantly, the brainstem nuclei of the ascending auditory pathway through the ICC are critical components of the circuitry that mediates PPI (Koch, 1999). This dependence suggesting that the sensitivity of ICC cells to sound localization cues may determine the PPI elicited by a change in sound source location. Overlaid lines indicate the sound source separations that would produce each ITD (maximal), given a mean guinea pig head diameter of 45 mm (black, 7.5°; blue, 10°; red, 15°). Neural JNDs predict minimum ITD sensitivity of ~ 45 μs, which is somewhat longer than the behavioral estimates of 7.5°, which only produces a 22 μs ITD. However, they are closer to the behavioral estimates of 10–15° (40–55μs). While the neural and behavioral predictions are fairly similar, it should be noted that discrepancies could arise due to the number of neurons recorded, which is just a small sampling of the neural population. It is possible that this sample of neurons does not contain the best performing neurons. Nevertheless, our data supports the idea that behavioral performance is driven by the best performing neurons.
Figure 9.
ITD and ILD JNDs: comparing behavioral and neurophysiological estimates. A: ITD JNDs characterized by ICC neuron response type; data from (Shackleton et al., 2003). Lines indicate the sound source separations that would produce each ITD (maximal), given a mean guinea pig head diameter of 45 mm (black, 7.5°; blue, 10°; red, 15°). B: ILD JNDs from 103 ICC neurons recorded from the chinchilla; data from (Jones et al., 2015). Green shaded area indicates the estimated behavioral ILD sensitivity for the guinea pig.
We are unaware of any previous reports of ILD sensitivity in the guinea pig auditory system; however, data are available for the chinchilla, which is both similar in size and behavioral localization ability to the guinea pig (~ 15° MAA for high-pass noise; (Heffner et al., 1994b)). Figure 9B shows the estimated neural JNDs for ILD discrimination recorded from Chinchilla ICC cells replotted from Jones et al. (2015). The green shaded area indicate the range of estimated minimum discriminable ILDs given a mean guinea pig head diameter of 45 mm (~1.5–3 dB). Chinchilla neural JNDs predict a minimum ILD of ~2.5 dB, which is well within the range of guinea pig behavioral estimates (1.5–3 dB). Preliminary data recorded in the ICC of adult guinea pigs in our laboratory (Anbuhl et al., 2017b) suggest that the behavioral estimates are comparable to neural ILD sensitivity measurements: ILD JNDs calculated using methods described in (Brown et al., 2016) estimate the average ILD threshold is 2.8 ± 1.3 dB ILD (n=40). These preliminary data are consistent with the behavioral ILD sensitivity of ~1.5–3 dB ILD estimated here, and the ILD sensitivity of chinchilla reported above. Again, this supports the idea that the best performing neurons are driving behavioral performance.
5.3. Comparisons between PPI-based sensitivity and other measures
Spatial and binaural hearing studies have used a variety of different methods for assessing the auditory system, including reward-based operant conditioning (OC), reflex-based prepulse inhibition (PPI), and auditory brainstem response (ABR) procedures. When interpreting the sensitivity of PPI-based measures, it is important to consider the differences between the different methods. Behrens and Klump (2016) directly compared the sensitivity of C57BL/6 mice in OC and PPI procedures for detecting a switch in speaker location using broadband and narrowband noise stimuli and determined their minimum audible angle (MAA). They found that OC procedures result in a better performance of the subjects in the MAA task than PPI procedures, challenging the view that both procedures can be used interchangeably. Behrens argued that PPI-based performance can be misleading, and that a diminished response in the PPI procedure is not always conclusive evidence for a lack of perception. They highlight the possible limitation of this method for assessing the perception of some stimulus types.
On the other hand, (Longenecker et al., 2016) found that PPI-based audiometric functions were similar to both ABR and traditional operant conditioning audiograms in CBA/CaJ mice. They did highlight some advantages and limitations of PPI- and ABR-based assessments, and concluded that both methods are robust assessments of the auditory system that when combined, can provide valuable information regarding the functionality of the auditory system. Limitations aside, PPI-based procedures can provide useful assessments of the system particularly when detecting hearing related dysfunctions. This has been shown with studies of noise-induced hearing loss (Longenecker et al., 2016), tinnitus (Dehmel et al., 2012a; Middleton et al., 2011), as well as in genetically modified mice (Allen et al., 2012; Jalabi et al., 2013; Karcz et al., 2015). Overall, we show that PPI-based methods provide useful assessments of the auditory system, especially for the guinea pig.
5.4. PPI, PPF, and their relation to ISI
The results presented above reveal substantial decreases in startle amplitude with the presence of a prepulse compared to control trials in many conditions, but substantial increases in startle amplitude in others. These changes are quantified as substantial PPI for long ISIs (>~80 ms), and PPF for shorter ISIs (5–80 ms), and varied across specific experiments. Typically only inhibitory responses are considered (or responses are rectified thus discarding the distinction between PPI and PPF); however, similar facilitated responses have been observed in rats (Hoffman et al., 1980; Ison et al., 1973) and mice (Dirks et al., 2001; Plappert et al., 2004; Willott et al., 1995) for presentation of weak prepulse stimuli at short ISIs, as well as at much longer ISIs (>500 ms) for rats (Reijmers et al., 1994) and humans (Filion et al., 1993; Graham et al., 1975).
Note that while prior reports (e.g., Dehmel et al., 2012a) have not report observing PPF in guinea pigs, the range of conditions tested are generally much more limited, and in particular short ISIs, which would be expected to elicit facilitation based on the current results, have typically not been assessed. Anecdotally, PPF appears to coincide with a decrease in startle response latency and has been suggested as indicative of an alerting or orienting reaction within the auditory system for near-threshold stimuli (Hoffman et al., 1980; Ison et al., 1997).
Our results are generally consistent with these prior reports, in that PPI is elicited by prepulses presented with a moderate ISI (> ~100 ms), while shorter ISIs elicit PPF. Indeed, prior testing using the same technique in mice showed some degree of PPF for short ISIs (Allen et al., 2010); however, the range of ISIs over which these facilitated responses are observed were markedly different (<10 ms). Consistent with this result, Plappert and colleagues (2004) reported that mice exhibit PPI that was maximal for ISIs between 37.5–100 ms, and PPF that was maximal (and only observed) for an ISI of 12.5 ms. Similarly, rats exhibit PPF for ISIs up to ~20–40 ms (Hoffman et al., 1980; Ison et al., 1997), and reliably produce PPI for longer ISIs. The observation of PPF up to ISIs as long as ~80 ms thus represents a substantial difference between guinea pigs and other small rodents tested previously.
While the underlying PPI circuit and function is generally well characterized (Koch, 1999), our understanding of PPF and its source still remains unclear. It is thought that PPF might reflect a classical activating effect, indicating alerting or attention or automatically elicited generalized orienting. Ison et al (1997) suggested that PPF might be mediated by either an excitatory interaction between the sensory responses to the prepulse and to the startle stimulus or from a process of motor preparation elicited by the prepulse. Willott et al. (1995) found that PPF in mice was only present at an ISI shorter than 2 ms, and proposed that the cochlear nucleus may be a contributing source as this is the only central auditory structure that is capable of responding to a prepulse stimulus within this time course. However, we find that there is a substantially larger range of ISIs where PPF is observed in guinea pigs (ISIs: 5–80 ms). The time course for the cochlear nucleus is roughly 1–3 ms (using ABR wave I-II latency, Ferber et al. 2016; unpublished data; and Dum (1984) indicating the cochlear nucleus is not the primary source for PPF, at least in the guinea pig. One possible explanation is perhaps a slower processing or longer transmission time for modulation of the startle response. We also find a substantially larger PPF magnitudes compared to mice: Allen and Ison (2010) report PPF of ~−0.2, compared to our ~−2, a 10 fold difference in PPI between species. This disparity in magnitude between mice and guinea pigs highlights the possible species differences underlying the PPF circuit. In addition, we find that in general, low-pass noise produces PPF, and high-pass noise produces PPI. If PPF is indicative of a near-threshold response, this suggests that low-pass noise is more ‘difficult’ for the animal to discriminate (though they can nevertheless detect the stimulus).
6. CONCLUSIONS
Guinea pigs startle in response to a loud sound, and show PPI and PPF depending upon the ISI. Changing the position of a sound source allows for a reliable estimate of the minimum audible angle. MAA in guinea pig is bounded between 3.75–7.5° across the midline, and 7.5–15° when offset from the midline by 45°. The ability to discriminate high- and low-pass filtered noise sources within ~7.5° and ~15° suggests ILD and ITD sensitivity of 1.5–3 dB and 23–45 μs respectively. Finally, spatial separation between a sound source and a noise masker modulates the ability to detect that sound. These results suggest that the guinea pig is indeed a suitable animal model for the study of spatial hearing ability.
ACKNOWLEDGEMENTS
The authors thank Dr. J. Ison for guidance and discussion of the acoustic startle technique, Drs. A. Brown and V. Benichoux for comments on the manuscript, and Dr. M. Hall for assistance in the design and construction of the experimental apparatus. This work was supported by the National Institute of Deafness and Other Communication Disorders (NIDCD) grants R01-DC011555 (DJT), T32-DC012280 (NTG), F31-DC014219 (KLA), and F30-DC013932 (ATF).
9. REFERENCES
- Allen PD, Ison JR 2010. Sensitivity of the mouse to changes in azimuthal sound location: angular separation, spectral composition, and sound level. Behavioral neuroscience 124, 265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PD, Ison JR 2012. Kcna1 gene deletion lowers the behavioral sensitivity of mice to small changes in sound location and increases asynchronous brainstem auditory evoked potentials but does not affect hearing thresholds. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 2538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbuhl KL, Benichoux V, Greene NT, Brown AD, Tollin DJ 2017a. Development of the head, pinnae, and acoustical cues to sound location in a precocial species, the guinea pig (Cavia porcellus). Hearing research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbuhl KL, Brown AD, Benichoux V, Greene NT, Ferber AT, Tollin DJ 2017b. Duration of temporary hearing loss during development influences severity of behavioral and neural impairments to sound localization 40th Annual Midwinter Meeting of the Association for Research in Otolaryngology, Baltimore, MD. [Google Scholar]
- Anderson LA, Wallace MN, Palmer AR 2007. Identification of subdivisions in the medial geniculate body of the guinea pig. Hearing research 228, 156–67. [DOI] [PubMed] [Google Scholar]
- Avery GT 1928a. Responses of foetal guinea pigs prematurely delivered. Genetic Psychology Monograph 3, 248–331. [Google Scholar]
- Avery GT 1928b. Responses of foetal guinea pigs prematurely delivered. Genetic Psychology Monographs. [Google Scholar]
- Bakin JS, Weinberger NM 1990. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain research 536, 271–86. [DOI] [PubMed] [Google Scholar]
- Behrens D, Klump GM 2016. Comparison of mouse minimum audible angle determined in prepulse inhibition and operant conditioning procedures. Hearing research 333, 167–78. [DOI] [PubMed] [Google Scholar]
- Berger JI, Coomber B, Shackleton TM, Palmer AR, Wallace MN 2013. A novel behavioural approach to detecting tinnitus in the guinea pig. Journal of neuroscience methods 213, 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AD, Tollin DJ 2016. Slow Temporal Integration Enables Robust Neural Coding and Perception of a Cue to Sound Source Location. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 9908–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caird DM, Palmer AR, Rees A 1991. Binaural masking level difference effects in single units of the guinea pig inferior colliculus. Hearing research 57, 91–106. [DOI] [PubMed] [Google Scholar]
- Carlile S, Pettigrew AG 1987a. Distribution of frequency sensitivity in the superior colliculus of the guinea pig. Hearing research 31, 123–36. [DOI] [PubMed] [Google Scholar]
- Carlile S, Pettigrew AG 1987b. Directional properties of the auditory periphery in the guinea pig. Hearing research 31, 111–22. [DOI] [PubMed] [Google Scholar]
- Carney LH, Sarkar S, Abrams KS, Idrobo F 2011. Sound-localization ability of the Mongolian gerbil (Meriones unguiculatus) in a task with a simplified response map. Hearing research 275, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE 1993. Processing of temporal information in the brain. Annual review of neuroscience 16, 223–43. [DOI] [PubMed] [Google Scholar]
- Chen Q, Cain D, Jen P 1995. Sound pressure transformation at the pinna of Mus domesticus. Journal of Experimental Biology 198, 2007–2023. [DOI] [PubMed] [Google Scholar]
- Clements M, Kelly JB 1978. Auditory spatial responses of young guinea pigs (Cavia porcellus) during and after ear blocking. Journal of comparative and physiological psychology 92, 34–44. [DOI] [PubMed] [Google Scholar]
- Coomes DL, Schofield BR 2004. Projections from the auditory cortex to the superior olivary complex in guinea pigs. Eur J Neurosci 19, 2188–200. [DOI] [PubMed] [Google Scholar]
- Dehmel S, Eisinger D, Shore SE 2012a. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Frontiers in systems neuroscience 6, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Pradhan S, Koehler S, Bledsoe S, Shore S 2012b. Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus--possible basis for tinnitus-related hyperactivity? The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 1660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks A, Pattij T, Bouwknecht JA, Westphal TT, Hijzen TH, Groenink L, van der Gugten J, Oosting RS, Hen R, Geyer MA, Olivier B 2001. 5-HT1B receptor knockout, but not 5-HT1A receptor knockout mice, show reduced startle reactivity and footshock-induced sensitization, as measured with the acoustic startle response. Behavioural brain research 118, 169–78. [DOI] [PubMed] [Google Scholar]
- Dodge R, Louttit CM 1926. Modification of the patter of the guinea pig’s reflex response to noise. J. Comp. Psychol 3, 267–285. [Google Scholar]
- Dum N 1984. Postnatal development of the auditory evoked brainstem potentials in the guinea pig. Acta oto-laryngologica 97, 63–8. [DOI] [PubMed] [Google Scholar]
- Ebert CS, Blanks DA, Patel MR, Coffey CS, Marshall AF, Fitzpatrick DC 2008. Behavioral sensitivity to interaural time differences in the rabbit. Hearing research 235, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM 1993. Modification of the acoustic startle-reflex eyeblink: a tool for investigating early and late attentional processes. Biological psychology 35, 185–200. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Batra R, Stanford TR, Kuwada S 1997. A neuronal population code for sound localization. Nature 388, 871–4. [DOI] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC 2013. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. Journal of neurophysiology 110, 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan RZ, Nakmali D, Zhang X 2013. Dynamic properties of round window membrane in guinea pig otitis media model measured with electromagnetic stimulation. Hearing research 301, 125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner W, Kempter R, van Hemmen JL, Wagner H 1996. A neuronal learning rule for sub-millisecond temporal coding. Nature 383, 76–81. [DOI] [PubMed] [Google Scholar]
- Graham FK, Putnam LE, Leavitt LA 1975. Lead-stimulation effects of human cardiac orienting and blink reflexes. Journal of experimental psychology. Human perception and performance 104, 175–82. [PubMed] [Google Scholar]
- Greene NT, Anbuhl KL, Williams W, Tollin DJ 2014. The acoustical cues to sound location in the guinea pig (Cavia porcellus). Hearing research 316, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley JM, Shanbhag SJ, Palmer AR, Wallace MN 2012. Processing of communication calls in Guinea pig auditory cortex. PloS one 7, e51646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Gan RZ 2013. Mechanisms of tympanic membrane and incus mobility loss in acute otitis media model of guinea pig. Journal of the Association for Research in Otolaryngology : JARO 14, 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JL 2nd. 1965. Binaural Interaction in the Accessory Superior-Olivary Nucleus of the Cat. The Journal of the Acoustical Society of America 37, 814–23. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS 1985. Hearing in two cricetid rodents: wood rat (Neotoma floridana) and grasshopper mouse (Onychomys leucogaster). Journal of comparative psychology 99, 275–88. [PubMed] [Google Scholar]
- Heffner HE, Heffner RS, Contos C, Ott T 1994a. Audiogram of the hooded Norway rat. Hearing research 73, 244–7. [DOI] [PubMed] [Google Scholar]
- Heffner R, Heffner H, Masterton B 1971. Behavioral measurements of absolute and frequency-difference thresholds in guinea pig. The Journal of the Acoustical Society of America 49, 1888–95. [DOI] [PubMed] [Google Scholar]
- Heffner RS 2004. Primate hearing from a mammalian perspective. The Anatomical Record 281, 1111–1122. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE 1988a. Sound localization and use of binaural cues by the gerbil (Meriones unguiculatus). Behavioral neuroscience 102, 422–8. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE 1988b. Sound localization acuity in the cat: effect of azimuth, signal duration, and test procedure. Hearing research 36, 221–232. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE, Kearns D, Vogel J, Koay G 1994b. Sound localization in chinchillas. I: Left/right discriminations. Hearing research 80, 247–57. [DOI] [PubMed] [Google Scholar]
- Hoa M, Guan Z, Auner G, Zhang J 2008. Tonotopic responses in the inferior colliculus following electrical stimulation of the dorsal cochlear nucleus of guinea pigs. Otolaryngology--Head and Neck Surgery 139, 152–155. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR 1980. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychological review 87, 175–89. [PubMed] [Google Scholar]
- Ison JR, McAdam DW, Hammond GR 1973. Latency and amplitude changes in the acoustic startle reflex of the rat produced by variation in auditory prestimulation. Physiology & behavior 10, 1035–9. [DOI] [PubMed] [Google Scholar]
- Ison JR, Taylor MK, Bowen GP, Schwarzkopf SB 1997. Facilitation and inhibition of the acoustic startle reflex in the rat after a momentary increase in background noise level. Behavioral neuroscience 111, 1335–52. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R 1984. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. The Journal of physiology 349, 205–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalabi W, Kopp-Scheinpflug C, Allen PD, Schiavon E, DiGiacomo RR, Forsythe ID, Maricich SM 2013. Sound localization ability and glycinergic innervation of the superior olivary complex persist after genetic deletion of the medial nucleus of the trapezoid body. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 15044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HG, Brown AD, Koka K, Thornton JL, Tollin DJ 2015. Sound frequency-invariant neural coding of a frequency-dependent cue to sound source location. Journal of neurophysiology 114, 531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonson KM, Lyle JG, Edwards MJ, Penny RH 1975. Problems in behavioural research with the guinea pig: A selective review. Animal Behaviour 23, 632–639. [Google Scholar]
- Karcz A, Allen PD, Walton J, Ison JR, Kopp-Scheinpflug C 2015. Auditory deficits of Kcna1 deletion are similar to those of a monaural hearing impairment. Hearing research 321, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh GL, Kelly JB 1986. Midline and lateral field sound localization in the albino rat (Rattus norvegicus). Behavioral neuroscience 100, 200–5. [DOI] [PubMed] [Google Scholar]
- Keating P, Nodal FR, Gananandan K, Schulz AL, King AJ 2013. Behavioral sensitivity to broadband binaural localization cues in the ferret. Journal of the Association for Research in Otolaryngology 14, 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB 1980. Effects of auditory cortical lesions on sound localization by the rat. Journal of neurophysiology 44, 1161–74. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Kavanagh GL 1986. Effects of auditory cortical lesions on pure-tone sound localization by the albino rat. Behavioral neuroscience 100, 569–75. [DOI] [PubMed] [Google Scholar]
- Kim DO, Bishop B, Kuwada S 2010. Acoustic cues for sound source distance and azimuth in rabbits, a racquetball and a rigid spherical model. Journal of the Association for Research in Otolaryngology 11, 541–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Palmer AR 1983. Cells responsive to free-field auditory stimuli in guinea-pig superior colliculus: distribution and response properties. The Journal of physiology 342, 361–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Palmer AR 1985. Integration of visual and auditory information in bimodal neurones in the guinea-pig superior colliculus. Exp Brain Res 60, 492–500. [DOI] [PubMed] [Google Scholar]
- Koch M 1999. The neurobiology of startle. Progress in neurobiology 59, 107–28. [DOI] [PubMed] [Google Scholar]
- Koka K, Read HL, Tollin DJ 2008. The acoustical cues to sound location in the rat: measurements of directional transfer functions. The Journal of the Acoustical Society of America 123, 4297–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka K, Jones HG, Thornton JL, Lupo JE, Tollin DJ 2011. Sound pressure transformations by the head and pinnae of the adult Chinchilla (Chinchilla lanigera). Hearing research 272, 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Slee SJ, May BJ 2011. Acoustic basis of directional acuity in laboratory mice. Journal of the Association for Research in Otolaryngology : JARO 12, 633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC 2011. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. Journal of the Association for Research in Otolaryngology : JARO 12, 605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker RJ, Alghamdi F, Rosen MJ, Galazyuk AV 2016. Prepulse inhibition of the acoustic startle reflex vs. auditory brainstem response for hearing assessment. Hearing research 339, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JK, Klump GM 2006. Resolution in azimuth sound localization in the Mongolian gerbil (Meriones unguiculatus). The Journal of the Acoustical Society of America 119, 1029–36. [DOI] [PubMed] [Google Scholar]
- Makous JC, Middlebrooks JC 1990. Two‐dimensional sound localization by human listeners. The Journal of the Acoustical Society of America 87, 2188–2200. [DOI] [PubMed] [Google Scholar]
- May BJ, Huang AY 1996. Sound orientation behavior in cats. I. Localization of broadband noise. The Journal of the Acoustical Society of America 100, 1059–1069. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Jiang D, Palmer AR 1996a. Binaural masking level differences in the inferior colliculus of the guinea pig. The Journal of the Acoustical Society of America 100, 490–503. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Jiang D, Palmer AR 1996b. Interaural delay sensitivity and the classification of low best-frequency binaural responses in the inferior colliculus of the guinea pig. Hearing research 97, 136–52. [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T 2011. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proceedings of the National Academy of Sciences of the United States of America 108, 7601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AW 1960. Lateralization of High‐Frequency Tones. The Journal of the Acoustical Society of America 32, 132–134. [Google Scholar]
- Montejo N, Norena AJ 2015. Dynamic representation of spectral edges in guinea pig primary auditory cortex. Journal of neurophysiology 113, 2998–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders WH, Robertson D 2013. Development of hyperactivity after acoustic trauma in the guinea pig inferior colliculus. Hearing research 298, 104–8. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Pilz PK, Schnitzler HU 2004. Factors governing prepulse inhibition and prepulse facilitation of the acoustic startle response in mice. Behavioural brain research 152, 403–12. [DOI] [PubMed] [Google Scholar]
- Platt B, Withington DJ 1998. GABA-induced long-term potentiation in the guinea-pig superior colliculus. Neuropharmacology 37, 1111–22. [DOI] [PubMed] [Google Scholar]
- Prosen CA, Petersen MR, Moody DB, Stebbins WC 1978. Auditory thresholds and kanamycin-induced hearing loss in the guinea pig assessed by a positive reinforcement procedure. The Journal of the Acoustical Society of America 63, 559–66. [DOI] [PubMed] [Google Scholar]
- Rawdon-Smith AF, Carmichael L, Wellman B 1938. Electrical responses from the cochlea of the fetal guinea pig. Journal of Experimental Psychology 23, 531–535. [Google Scholar]
- Redies H, Brandner S 1991. Functional organization of the auditory thalamus in the guinea pig. Exp Brain Res 86, 384–92. [DOI] [PubMed] [Google Scholar]
- Redies H, Sieben U, Creutzfeldt OD 1989a. Functional subdivisions in the auditory cortex of the guinea pig. J Comp Neurol 282, 473–88. [DOI] [PubMed] [Google Scholar]
- Redies H, Brandner S, Creutzfeldt OD 1989b. Anatomy of the auditory thalamocortical system of the guinea pig. J Comp Neurol 282, 489–511. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Peeters BW 1994. Acoustic prepulses can facilitate the startle reflex in rats: discrepancy between rat and human data resolved. Brain research bulletin 35, 337–8. [DOI] [PubMed] [Google Scholar]
- Robertson D, Bester C, Vogler D, Mulders WH 2013. Spontaneous hyperactivity in the auditory midbrain: relationship to afferent input. Hearing research 295, 124–9. [DOI] [PubMed] [Google Scholar]
- Roth GL, Kochhar RK, Hind JE 1980. Interaural time differences: implications regarding the neurophysiology of sound localization. The Journal of the Acoustical Society of America 68, 1643–1651. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Wallace MN, Shackleton TM, Palmer AR 2000. Organisation of binaural interactions in the primary and dorsocaudal fields of the guinea pig auditory cortex. Hearing research 145, 177–89. [DOI] [PubMed] [Google Scholar]
- Schnupp JW, Booth J, King AJ 2003. Modeling individual differences in ferret external ear transfer functions. The Journal of the Acoustical Society of America 113, 2021–2030. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB 1991. Organization of the superior olivary complex in the guinea pig. I. Cytoarchitecture, cytochrome oxidase histochemistry, and dendritic morphology. J Comp Neurol 314, 645–70. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB 1992. Organization of the superior olivary complex in the guinea pig: II. Patterns of projection from the periolivary nuclei to the inferior colliculus. J Comp Neurol 317, 438–55. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB 1996. Projections from the ventral cochlear nucleus to the inferior colliculus and the contralateral cochlear nucleus in guinea pigs. Hearing research 102, 1–14. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB 1997. Ventral nucleus of the lateral lemniscus in guinea pigs: cytoarchitecture and inputs from the cochlear nucleus. J Comp Neurol 379, 363–85. [DOI] [PubMed] [Google Scholar]
- Sedlacek J 1976. Foetal and neonatal development of evoked responses in guinea-pig auditory cortex. Physiologia Bohemoslovaca 25, 13–21. [PubMed] [Google Scholar]
- Shackleton TM, Palmer AR 2010. The time course of binaural masking in the inferior colliculus of guinea pig does not account for binaural sluggishness. Journal of neurophysiology 104, 189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton TM, Skottun BC, Arnott RH, Palmer AR 2003. Interaural time difference discrimination thresholds for single neurons in the inferior colliculus of Guinea pigs. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Liu L, He T, Guo X, Yu Z, Yin S, Wang J 2013. Ribbon synapse plasticity in the cochleae of Guinea pigs after noise-induced silent damage. PloS one 8, e81566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Moore JK 1998. Sources of input to the cochlear granule cell region in the guinea pig. Hearing research 116, 33–42. [DOI] [PubMed] [Google Scholar]
- Sinyor A, Laszlo CA 1973. Acoustic behavior of the outer ear of the guinea pig and the influence of the middle ear. The Journal of the Acoustical Society of America 54, 916–21. [DOI] [PubMed] [Google Scholar]
- Skottun BC, Shackleton TM, Arnott RH, Palmer AR 2001. The ability of inferior colliculus neurons to signal differences in interaural delay. Proceedings of the National Academy of Sciences of the United States of America 98, 14050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH 1995. Structural and functional differences distinguish principal from nonprincipal cells in the guinea pig MSO slice. Journal of neurophysiology 73, 1653–67. [DOI] [PubMed] [Google Scholar]
- Stabler SE, Palmer AR, Winter IM 1996. Temporal and mean rate discharge patterns of single units in the dorsal cochlear nucleus of the anesthetized guinea pig. Journal of neurophysiology 76, 1667–88. [DOI] [PubMed] [Google Scholar]
- Sterbing SJ, Hartung K, Hoffmann KP 2002. Representation of sound source direction in the superior colliculus of the guinea pig in a virtual auditory environment. Exp Brain Res 142, 570–7. [DOI] [PubMed] [Google Scholar]
- Sterbing SJ, Hartung K, Hoffmann KP 2003. Spatial tuning to virtual sounds in the inferior colliculus of the guinea pig. Journal of neurophysiology 90, 2648–59. [DOI] [PubMed] [Google Scholar]
- Syka J, Popelar J, Kvasnak E, Astl J 2000. Response properties of neurons in the central nucleus and external and dorsal cortices of the inferior colliculus in guinea pig. Exp Brain Res 133, 254–66. [DOI] [PubMed] [Google Scholar]
- Tollin DJ 2003. The lateral superior olive: a functional role in sound source localization. The neuroscientist 9, 127–143. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Koka K 2009. Postnatal development of sound pressure transformations by the head and pinnae of the cat: binaural characteristics. The Journal of the Acoustical Society of America 126, 3125–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollin DJ, Populin LC, Moore JM, Ruhland JL, Yin TC 2005. Sound-localization performance in the cat: the effect of restraining the head. Journal of neurophysiology 93, 1223–1234. [DOI] [PubMed] [Google Scholar]
- Wakeford OS, Robinson DE 1974. Lateralization of tonal stimuli by the cat. The Journal of the Acoustical Society of America 55, 649–652. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Palmer AR 2000a. Identification and localisation of auditory areas in guinea pig cortex. Exp Brain Res 132, 445–56. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Shackleton TM, Palmer AR 2000b. Phase-locked responses to pure tones in guinea pig auditory cortex. Neuroreport 11, 3989–93. [DOI] [PubMed] [Google Scholar]
- Wang X, Guan X, Pineda M, Gan RZ 2016. Motion of tympanic membrane in guinea pig otitis media model measured by scanning laser Doppler vibrometry. Hearing research 339, 184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolek CM, Koay G, Heffner RS, Heffner HE 2010. Laboratory rats (Rattus norvegicus) do not use binaural phase differences to localize sound. Hearing research 265, 54–62. [DOI] [PubMed] [Google Scholar]
- Willott JF, Carlson S 1995. Modification of the acoustic startle response in hearing-impaired C57BL/6J mice: prepulse augmentation and prolongation of prepulse inhibition. Behavioral neuroscience 109, 396–403. [DOI] [PubMed] [Google Scholar]
- Winter IM, Palmer AR 1990. Responses of single units in the anteroventral cochlear nucleus of the guinea pig. Hearing research 44, 161–78. [DOI] [PubMed] [Google Scholar]
- Yost WA 1974. Discriminations of interaural phase differences. The Journal of the Acoustical Society of America 55, 1299–1303. [DOI] [PubMed] [Google Scholar]
- Young JS, Fechter LD 1983. Reflex inhibition procedures for animal audiometry: a technique for assessing ototoxicity. The Journal of the Acoustical Society of America 73, 1686–93. [DOI] [PubMed] [Google Scholar]
- Zhao HB, Liang ZA 1996. Processing of modulation frequency in the dorsal cochlear nucleus of the guinea pig: sinusoidal frequency-modulated tones. Hearing research 95, 120–34. [DOI] [PubMed] [Google Scholar]
- Zohar O, Shackleton TM, Nelken I, Palmer AR, Shamir M 2011. First spike latency code for interaural phase difference discrimination in the guinea pig inferior colliculus. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 9192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]